Enzymatic Characterization of Purified β-Glucosidase from Non-Saccharomyces Yeasts and Application on Chardonnay Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Media
2.2. β-Glucosidase Purification and Enzyme Activity Determination
2.3. Enzymatic Characterization
2.3.1. Measurement of Optimal Temperature and pH Value
2.3.2. Effects of Metal Ions and Enzyme Inhibitors on Enzyme Activity
2.3.3. Effect of Various Sugars on Enzyme Activity
2.3.4. Effect of Ethanol on Enzyme Activity
2.4. Enzyme Treatment of Chardonnay Young Wines
2.5. Volatile Aroma Compounds Identification and Quantification
2.6. Sensory Evaluation
2.7. Data and Statistical Analysis
3. Results
3.1. Purification of β-Glucosidase from M. guilliermondii NM218 and H. uvarum BF345
3.2. Enzymatic Characterization
3.3. Analysis of Volatile Aroma Compounds in Chardonnay Wines
3.4. Sensory Evaluation of Wines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebeler, S.E. Analytical chemistry: Unlocking the secrets of wine flavor. Food Rev. Int. 2001, 17, 45–64. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, Q.; Yan, G.; Duan, C. Using headspace solid phase microextraction for analysis of aromatic compounds during alcoholic fermentation of red wine. Food Chem. 2011, 125, 743–749. [Google Scholar] [CrossRef]
- Lee, S.J.; Noble, A.C. Characterization of odor-active compounds in californian chardonnay wines using GC-Olfactometry and GC-Mass spectrometry. J. Agric. Food Chem. 2014, 51, 8036. [Google Scholar] [CrossRef]
- Van, R.P.; Pretorius, I.S. Enzymes in winemaking, harnessing natural catalysts for efficient biotransformations—A review. Am. J. Enol. Vitic. 2000, 21, 52–73. [Google Scholar]
- Günata, Z.; Dugelay, I.; Sapis, J.C.; Baumes, R.L.; Bayonove, C.L. Role of enzyme in the use of the flavor potential from grape glycosides in winemaking. In Progress in Flavor Precursor Studies; Schreier, P., Winterhalter, P., Eds.; Allured Publishing Corporation: Carol Stream, IL, USA, 1993; pp. 219–234. [Google Scholar]
- Gallifuoco, A.; D’Ercole, L.; Alfani, F.; Cantarella, M.; Spagna, G.; Pifferi, P.G. On the use of chitosan-immobilized β-glucosidase in wine-making: Kinetics and enzyme inhibition. Process Biochem. 1998, 33, 163–168. [Google Scholar] [CrossRef]
- Gallifuoco, A.; Alfani, F.; Cantarella, M.; Spagna, G.; Pifferi, P.G. Immobilized β-glucosidase for the winemaking industry: Study of biocatalyst operational stability in laboratory-scale continuous reactors. Process Biochem. 1999, 35, 179–185. [Google Scholar] [CrossRef]
- Palmeri, R.; Spagna, G. Beta-glucosidase in cellular and acellular form for wine-making application. Enzym. Microb. Technol. 2007, 40, 382–389. [Google Scholar] [CrossRef]
- Fia, G.; Olivier, V.; Cavaglioni, A.; Canuti, V.; Zanoni, B. Side activities of commercial enzyme preparations and their influence on the hydroxycinnamic acids, volatile compounds and nitrogenous components of white wine: Collateral effects of enzyme treatment on white wine. Aust. J. Grape Wine Res. 2016, 22, 366–375. [Google Scholar] [CrossRef]
- Mateo, J.J.; Peris, L.; Ibañez, C.; Maicas, S. Characterization of glycolytic activities from non-Saccharomyces yeasts isolated from Bobal musts. J. Ind. Microbiol. Biot. 2011, 38, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Maturano, P.; Assof, M.; Fabani, M.P.; Nally, M.C.; Jofré, V.; Rodríguez Assaf, L.A.; Vazquez, F. Enzymatic activities produced by mixed Saccharomyces and non- Saccharomyces cultures: Relationship with wine volatile composition. Antonie van Leeuwenhoek. 2015, 108, 1239–1256. [Google Scholar] [CrossRef]
- Zhang, W.; Zhuo, X.; Hu, L.; Zhang, X. Effects of crude β-glucosidases from Issatchenkia terricola, Pichia kudriavzevii, Metschnikowia pulcherrima on the flavor complexity and characteristics of wines. Microorganisms 2020, 8, 953. [Google Scholar] [CrossRef] [PubMed]
- López, M.C.; Mateo, J.J.; Maicas, S. Screening of β-glucosidase and β-xylosidase activities in four non-Saccharomyces yeast isolates: Glycosidasic activities from wine yeasts. J. Food Sci. 2015, 80, C1696–C1704. [Google Scholar] [CrossRef]
- Belancic, A.; Gunata, Z.; Vallier, M.J.; Agosin, E. β-Glucosidase from the grape native yeast Debaryomyces vanrijiae: Purification, characterization, and its effect on monoterpene content of a Muscat grape juice. J. Agric. Food Chem. 2003, 51, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Sereni, A.; Phan, Q.; Osborne, J.; Tomasino, E. Impact of the timing and temperature of malolactic fermentation on the aroma composition and mouthfeel properties of Chardonnay wine. Foods 2020, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Gambetta, J.M.; Bastian, S.E.P.; Cozzolino, D.; Jeffery, D.W. Factors influencing the aroma composition of Chardonnay wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef]
- Gcab, C.; Kcab, C.; Plta, B. Use of oak wood during malolactic fermentation and ageing: Impact on Chardonnay wine character-sciencedirect. Food Chem. 2019, 278, 460–468. [Google Scholar]
- Cid, A.G.; Goldner, M.C.; Daz, M.; Ellenrieder, G. The effect of endozym β-split, a commercial enzyme preparation used for aroma release, on Tannat wine glycosides. S. Afr. J. Enol. Vitic. 2012, 33, 51–57. [Google Scholar] [CrossRef][Green Version]
- Cai, L.N.; Xu, S.N.; Lu, T.; Lin, D.Q.; Yao, S.J. Directed expression of halophilic and acidophilic β-glucosidases by introducing homologous constitutive expression cassettes in marine Aspergillus niger. J. Biotechnol. 2019, 292, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Baffi, M.A.; Martin, N.; Tobal, T.M.; Ferrarezi, A.L.; Lago, J.H.G.; Boscolo, M.; Gomes, E.; Da-Silva, R. Purification and characterization of an ethanol-tolerant β-glucosidase from Sporidiobolus pararoseus and Its potential for hydrolysis of wine aroma precursors. Appl. Biochem. Biotechnol. 2013, 171, 1681–1691. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.J.; Xu, Y.H.; Yin, H.; Tao, Y.S. Wine aroma response to different participation of selected Hanseniaspora uvarum in mixed fermentation with Saccharomyces cerevisiae. Food Res. Int. 2018, 108, 119–127. [Google Scholar] [CrossRef]
- Mallek-Fakhfakh, H.; Belghith, H. Physicochemical properties of thermotolerant extracellular β-glucosidase from Talaromyces thermophilus and enzymatic synthesis of cello-oligosaccharides. Carbohydr. Res. 2016, 419, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, N.; Xu, J.; Qi, Y.; Fan, M. Homology analysis of 35 β-glucosidases in oenococcus oeni and biochemical characterization of a novel β-glucosidase bgl0224. Food Chem. 2020, 334, 127593. [Google Scholar] [CrossRef] [PubMed]
- Ovalle, S.D.; Brena, B.; González-Pombo, P. Influence of beta-glucosidases from native yeast on the aroma of Muscat and Tannat wines. Food Chem. 2021, 346, 128899. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Li, A.H.; Dizy, M.; Ullah, N.; Sun, W.X.; Tao, Y.S. Evaluation of aroma enhancement for ‘‘Ecolly’’ dry white wines by mixed inoculation of selected Rhodotorula mucilaginosa and Saccharomyces cerevisiae. Food Chem. 2017, 228, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.P.; Zhu, B.Q.; Song, R.R.; Zhang, B.; Lan, Y.B.; Zhu, X.; Duan, C.Q.; Han, S.Y. Volatile composition and aromatic attributes of wine made with Vitisvinifera L.cv Cabernet Sauvignon grapes in the Xinjiang region of China: Effect of different commercial yeasts. Int. J. Food Prop. 2018, 21, 1423–1441. [Google Scholar] [CrossRef]
- Tao, Y.; Li, H.; Wang, H.; Zhang, L. Volatile compounds of young Cabernet Sauvignon red wine from Changli County (China). J. Food Compos. Anal. 2008, 21, 689–694. [Google Scholar] [CrossRef]
- Arcari, S.G.; Caliari, V.; Sganzerla, M.; Godoy, H.T. Volatile composition of Merlot red wine and its contribution to the aroma: Optimization and validation of analytical method. Talanta 2017, 174, 752–766. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.; Cmara, J.S. Madeira wine volatile profile. A platform to establish Madeira wine aroma descriptors. Molecules 2019, 24, 3028. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Peng, C.T.; Wen, Y.; Tao, Y.S.; Lan, Y.Y. Modulating the formation of Meili wine aroma by prefermentative freezing process. J. Agric. Food Chem. 2013, 61, 1542–1553. [Google Scholar] [CrossRef]
- Tao, Y.S.; Li, H. Active volatiles of Cabernet Sauvignon wine from Changli County. Health 2009, 1, 176–182. [Google Scholar] [CrossRef]
- Boido, E.; Medina, K.; Fariña, L.; Carrau, F.; Versini, G.; Dellacassa, E. The effect of bacterial strain and aging on the secondary volatile metabolites produced during malolactic fermentation of Tannat red wine. J. Agric. Food Chem. 2009, 57, 6271–6278. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, D.P.; Maret, D.T.; Nieuwoudt, H.; Marieta, V.D.R.; Martin, K.; Neil, J. Effect of Saccharomyces, Non-Saccharomyces yeasts and malolactic fermentation strategies on fermentation kinetics and flavor of Shiraz wines. Fermentation 2017, 3, 64. [Google Scholar]
- Hu, K.; Qin, Y.; Tao, Y.S.; Zhu, X.L.; Ullah, N. Potential of glycosidase from non-Saccharomyces isolates for enhancement of wine aroma. J. Food Sci. 2016, 814, 935–943. [Google Scholar] [CrossRef]
- Baffi, M.A.; Tobal, T.M.; Lago, J.H.G.; Boscolo, M.; Gomes, E.; Da-Silva, R. Wine aroma improvement using a β-lucosidase preparation from Aureobasidium pullulans. Appl. Biochem. Biotechnol. 2013, 69, 493–501. [Google Scholar] [CrossRef]
- González-Pombo, P.; Fariña, L.; Carrau, F.; Batista-Viera, F.; Brena, B.M. A novel extracellular β-glucosidase from Issatchenkia terricola: Isolation, immobilization and application for aroma enhancement of white Muscat wine. Process Biochem. 2011, 46, 385–389. [Google Scholar] [CrossRef]
- Romero, A.M.; Mateo, J.J.; Maicas, S. Characterization of an ethanol tolerant β-xylosidase produced by Pichia membranifaciens. Lett. Appl. Microbiol. 2012, 55, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.R.; da Conceição, P.J.P.; de Menezes, C.L.A.; de Oliveira Nascimento, C.E.; Bertelli, M.M.; Júnior, A.P.; de Souza, G.M.; da Silva, R.; Gomes, E. Biochemical characteristics and potential application of a novel ethanol and glucose-tolerant β-glucosidase secreted by Pichia guilliermondii G1.2. J. Biotechnol. 2019, 294, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Pyeon, H.M.; Lee, Y.S.; Choi, Y.L. Cloning, purification, and characterization of GH3 β-glucosidase, MtBgl85, from Microbulbifer thermotolerans DAU221. PeerJ 2019, 7, e7106. [Google Scholar] [CrossRef]
- Cristiane, A.U.; Gaku, T.; Hirofumi, W.; Katsuhiko, K.; Manabu, A. A novel glucose-tolerant β-glucosidase from the salivary gland of the termite Nasutitermes takasagoensis. J. Gen. Appl. Microbio. 2013, 59, 141–145. [Google Scholar]
- González-Pombo, P.; Pérez, G.; Carrau, F.; Guisán, J.M.; Batista-Viera, F.; Brena, B. One-step purification and characterization of an intracellular β-glucosidase from Metschnikowia pulcherrima. Biotechnol. Lett. 2008, 30, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Yang, Y.; Zhang, X.; Yin, Q.; Zhang, X.; Wang, X.; Xiao, Y. Improve ethanol tolerance of β-glucosidase Bgl1A by semi-rational engineering for the hydrolysis of soybean isoflavone glycosides. J. Biotechnol. 2016, 227, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Skouroumounis, G.K.; Massy-Westropp, R.A.; Sefton, M.A.; Williams, P.J. Precursors of damascenone in fruit juices. Tetrahedron Lett. 1992, 33, 3533–3536. [Google Scholar] [CrossRef]
- Francis, I.L.; Tate, M.E.; Williams, P.J. The effect of hydrolysis conditions on the aroma released from Semillon grape glucosides. Aust. J. Grape Wine Res. 1996, 2, 270–276. [Google Scholar] [CrossRef]
- Codresi, C.; Rapeanu, G.; Alexe, P. Effect of beta-glucosidases in the making of Chardonnay wines. In Annals of the University Dunarea de Jos of Galati Fascicle VI Food Technology; Galati University Press: Galati, Romania, 2012; p. 36. [Google Scholar]
- Vázquez, L.C.; Pérez-Coello, M.S.; Cabezudo, M.D. Effects of enzyme treatment and skin extraction on varietal volatiles in Spanish wines made from Chardonnay, Muscat, Airén, and Macabeo grapes. Anal. Chim. Acta 2002, 458, 39–44. [Google Scholar] [CrossRef]
- Gunata, Y.Z.; Bayonove, C.; Baumes, R.; Cordonnier, R.E. The aroma of grapes. Extraction and determination of free and glycosidically bound fractions of some grape aroma composants. J. Chromatogr. A 1985, 331, 83–90. [Google Scholar] [CrossRef]
- Mateo, J.J.; Jimenez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: A review. Appl. Microbiol. Biot. 2005, 67, 322–335. [Google Scholar] [CrossRef]
- Ferreira, V.; Fernández, P.; Peña, C.; Escudero, A.; Cacho, J.F. Investigation on the role played by fermentation esters in the aroma of young Spanish wines by multivariate analysis. J. Sci. Food Agric. 1995, 67, 381–392. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, R.; Sirisena, S.; Gan, R.; Fang, Z. Beta-glucosidase activity of wine yeasts and its impacts on wine volatiles and phenolics: A mini-review. Food Microbiol. 2021, 100, 103859. [Google Scholar] [CrossRef]
- Wang, J.; Capone, D.L.; Wilkinson, K.L.; Jeffery, D.W. Chemical and sensory profiles of rosé wines from Australia. Food Chem. 2016, 196, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Jin, G.J.; Mei, W.C.; Li, T.; Tao, Y.S. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef] [PubMed]
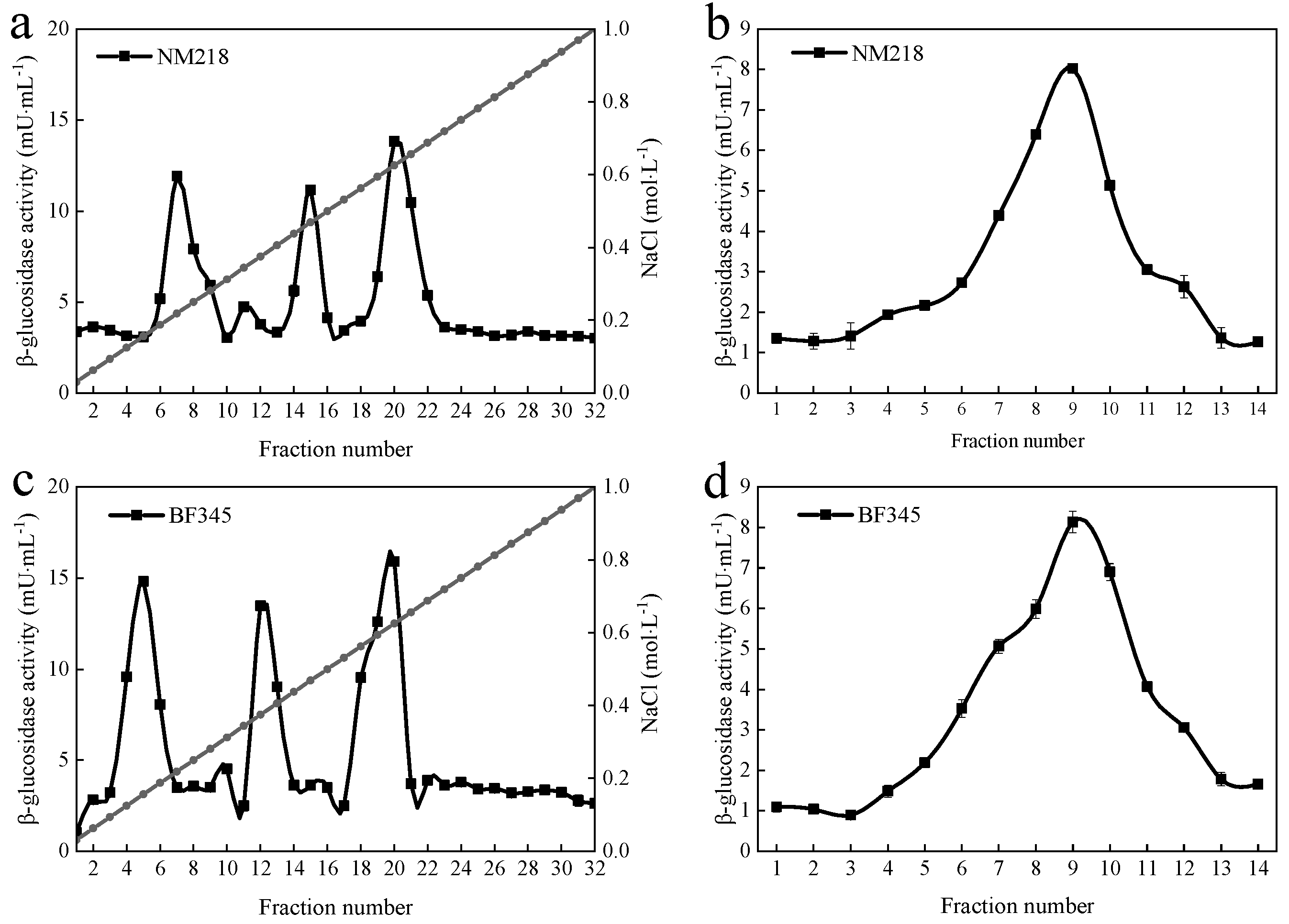
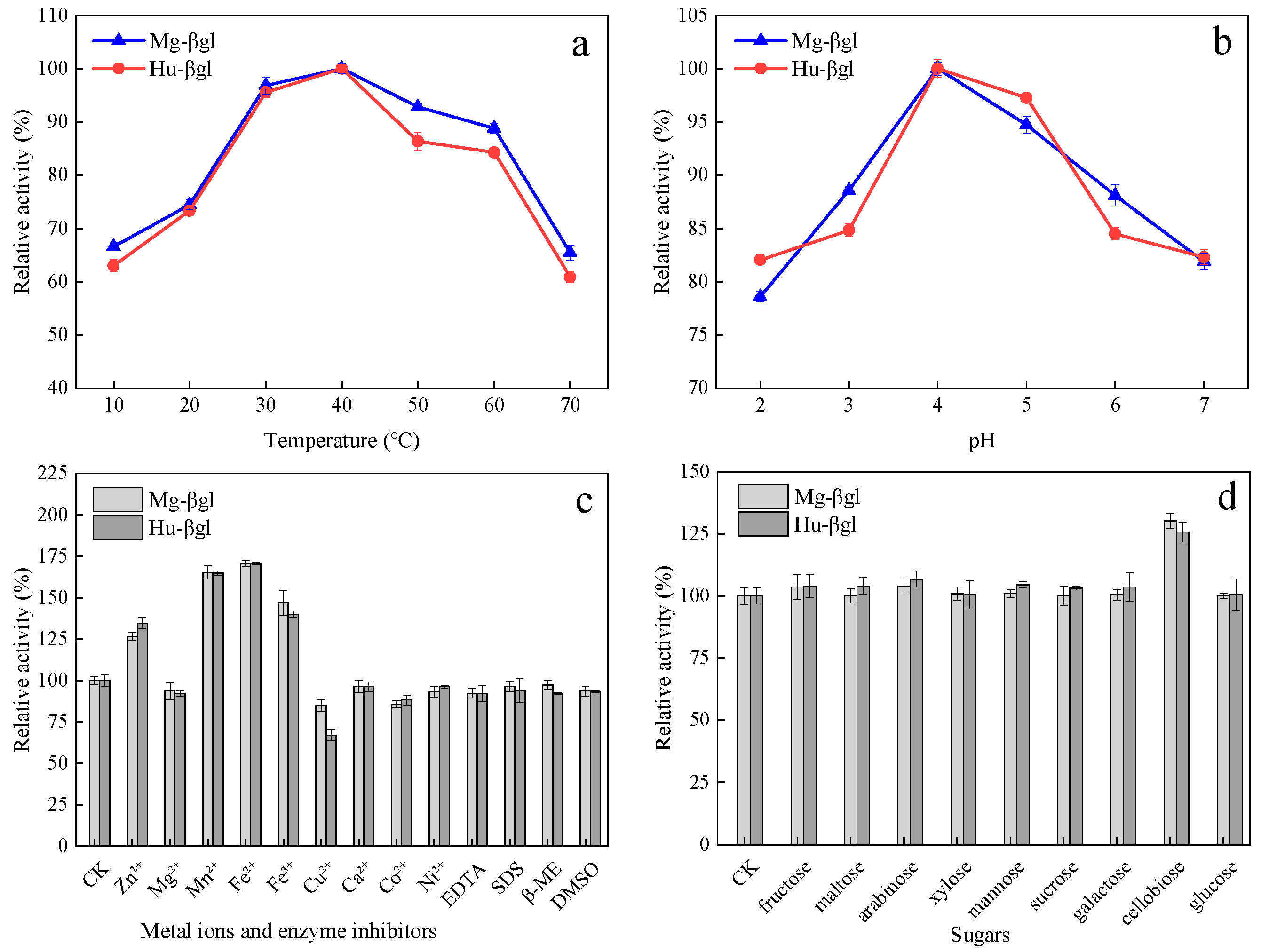
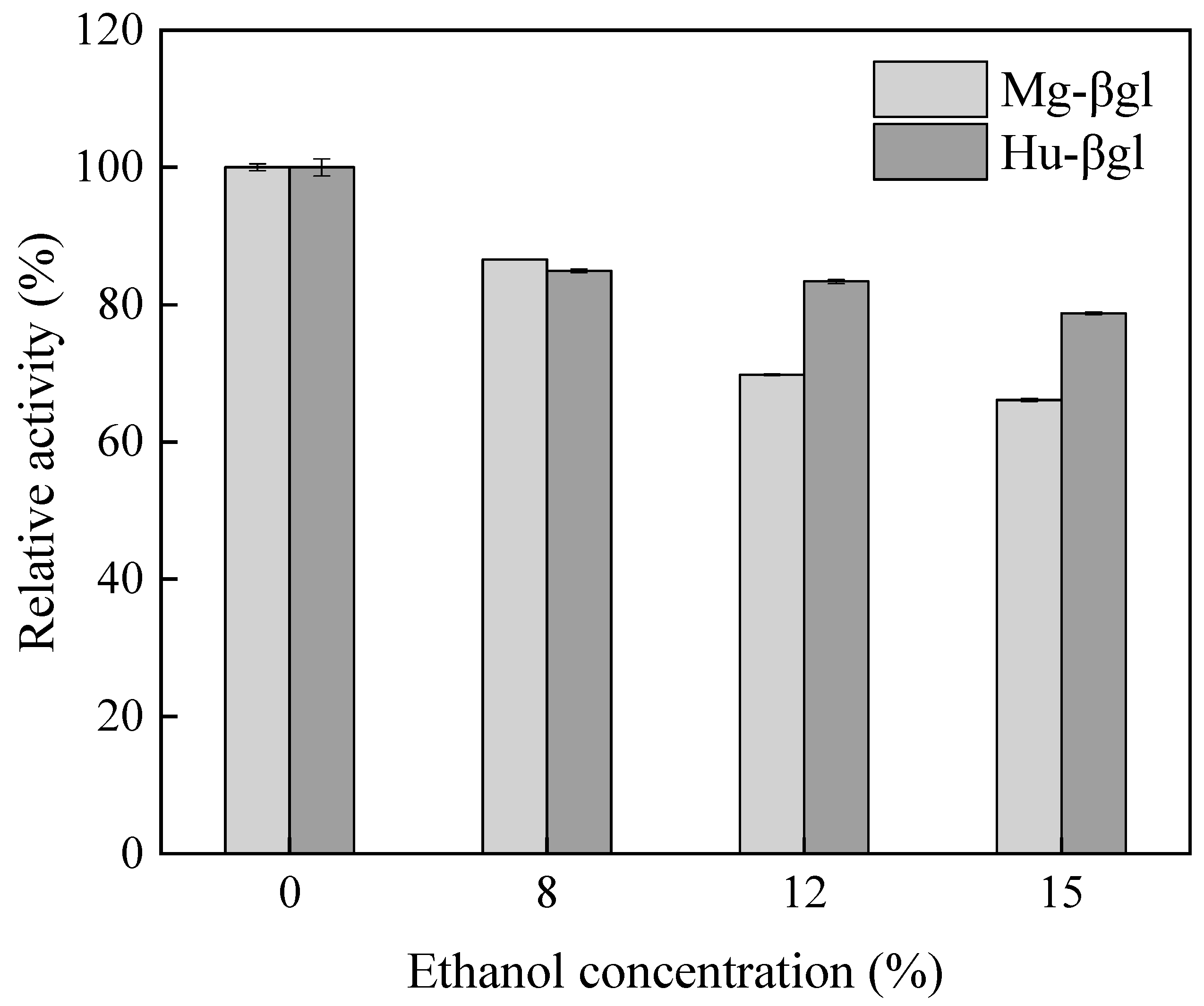
| Purification Steps | Total Activity/(U) | Total Protein/(mg) | Specific Activity/(U·mg−1) | Purification/(Fold) |
|---|---|---|---|---|
| M. guilliermondii NM218 | ||||
| Enzymatic extract | 22.41 | 138.61 | 0.16 | 1 |
| 80% ammonium sulfate | 17.46 | 59.37 | 0.29 | 1.8 |
| DEAE-Sepharose-FF | 3.54 | 10.43 | 0.34 | 2.1 |
| Sephacry1 S-200 | 1.77 | 0.91 | 1.95 | 12.2 |
| H. uvarum BF345 | ||||
| Enzymatic extract | 24.68 | 149.2 | 0.17 | 1 |
| 80% ammonium sulfate | 19.06 | 65.76 | 0.29 | 1.7 |
| DEAE-Sepharose-FF | 4.62 | 10.32 | 0.45 | 2.7 |
| Sephacry1 S-200 | 1.86 | 0.88 | 2.11 | 12.4 |
| Aroma Compound | Compound Concentration (mg/L) | Threshold (mg/L) | Odor Description | |||
|---|---|---|---|---|---|---|
| Hu-βgl | Mg-βgl | An-βgl | CK | |||
| Terpenes | ||||||
| Geraniol | NA | NA | 0.010 ± 0.002 | NA | 0.02 [28] | Lemon, peach, rose |
| Citronellol | 0.010 ± 0.000 b | 0.011 ± 0.001 b | 0.015 ± 0.003 b | 0.008 ± 0.001 a | 0.03 [29] | Grassy, lilac, rose |
| Linalool | 0.023 ± 0.001 b | 0.023 ± 0.001 b | 0.014 ± 0.001 a | 0.012 ± 0.001 a | 0.015 [30] | Rose, citrus, fruity, sweet |
| Total | 0.033 ± 0.001 b | 0.034 ± 0.002 b | 0.039 ± 0.006 b | 0.020 ± 0.002 a | ||
| C13-norisoprenoids | ||||||
| Damastone | 0.016 ± 0.001 a | 0.026 ± 0.001 b | 0.030 ± 0.007 b | NA | 0.05 [28] | Bark, canned peaches, baked apples, plums |
| Geranyl acetone | NA | NA | 0.004 ± 0.001 | NA | 0.06 [31] | Light sweet fragrance, rose |
| Total | 0.016 ± 0.001 a | 0.026 ± 0.001 b | 0.034 ± 0.008 c | |||
| C6 Compounds | ||||||
| Hexanol | 0.116 ± 0.004 b | 0.095 ± 0.005 b | 0.070 ± 0.005 a | 0.074 ± 0.002 a | 8 [32] | Grass |
| 2-Ethylhexanol | 0.024 ± 0.006 c | 0.013 ± 0.004 b | 0.004 ± 0.000 a | 0.010 ± 0.003 b | ||
| Total | 0.140 ± 0.010 c | 0.108 ± 0.009 b | 0.074 ± 0.005 a | 0.084 ± 0.005 b | ||
| Alcohols | ||||||
| Benzyl alcohol | NA | NA | 0.005 ± 0 | NA | 200 [32] | Toasted, fruity |
| 2-Phenylethanol | 5.412 ± 0.458 b | 5.810 ± 0.650 b | 4.122 ± 0.567 a | 4.568 ± 0.531 a | 1.4 [33] | Rose, honey |
| Isobutanol | 0.051 ± 0.002 c | 0.043 ± 0.003 b | 0.028 ± 0.003 a | 0.042 ± 0.002 b | 40 [34] | Solvent, raw green |
| Total | 5.463 ± 0.460 c | 5.853 ± 0.653 c | 4.155 ± 0.570 a | 4.610 ± 0.533 b | ||
| Esters | ||||||
| Phenethyl acetate | 0.608 ± 0.07 a | 0.738 ± 0.03 c | 0.605 ± 0.12 a | 0.696 ± 0.052 b | 0.25 [32] | Rose, jasmine |
| Ethyl caproate | 0.555 ± 0.054 b | 0.503 ± 0.038 a | 0.539 ± 0.017 ab | 0.571 ± 0.021 b | 0.014 [34] | Banana, green apple, strawberry, anise |
| Ethyl octanoate | 2.130 ± 0.193 d | 1.482 ± 0.132 c | 0.008 ± 0.002 a | 1.226 ± 0.042 b | 0.005 [25] | Rose fragrance, neroli oil, cool fruity |
| Ethyl heptanoate | 0.093 ± 0.009 d | 0.019 ± 0.001 b | 0.003 ± 0.000 a | 0.056 ± 0.002 c | 0.22 [31] | Banana, green apple, strawberry |
| Ethyl caprate | 0.723 ± 0.166 d | 0.380 ± 0.014 b | 0.118 ± 0.019 a | 0.523 ± 0.029 c | 0.2 [31] | Coconut fruit |
| Ethyl butyrate | 0.031 ± 0.003 a | 0.024 ± 0.003 a | 0.020 ± 0.001 a | 0.031 ± 0.001 a | 0.02 [32] | Banana, pineapple, strawberry |
| Total | 4.140 ± 0.495 c | 3.146 ± 0.218 b | 1.293 ± 0.159 a | 3.103 ± 0.147 b | ||
| Fatty acids | ||||||
| Hexanoic acid | 0.106 ± 0.009 b | 0.092 ± 0.006 b | 0.069 ± 0.046 a | 0.055 ± 0.039 a | 0.42 [32] | Fatty, cheesy |
| Octanoic acid | 0.046 ± 0.005 a | 0.059 ± 0.024 a | 0.105 ± 0.029 c | 0.072 ± 0.046 b | 0.5 [33] | Putrid, pungent, cheesy |
| Total | 0.152 ± 0.014 b | 0.151 ± 0.004 b | 0.174 ± 0.023 c | 0.127 ± 0.043 a | ||
| Triangle Test | Number of Trials | Number of Correct Answers | Significance Level | Odor Descriptors |
|---|---|---|---|---|
| Mg-βgl vs. CK | 16 | 14 | p ≤ 0.001 | Sweet, floral, fruity, banana, medicinal |
| Hu-βgl vs. CK | 16 | 14 | p ≤ 0.001 | Sweet, floral, honey, pomelo, banana |
| An-βgl vs. CK | 16 | 12 | p ≤ 0.001 | Sweet, floral, fruity |
| Mg-βgl vs. An-βgl | 16 | 13 | p ≤ 0.001 | Sweet, floral, fruity, banana |
| Hu-βgl vs. An-βgl | 16 | 13 | p ≤ 0.001 | Floral, fruity, toasty |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.; Sam, F.E.; Zhang, B.; Peng, S.; Li, M.; Wang, J. Enzymatic Characterization of Purified β-Glucosidase from Non-Saccharomyces Yeasts and Application on Chardonnay Aging. Foods 2022, 11, 852. https://doi.org/10.3390/foods11060852
Gao P, Sam FE, Zhang B, Peng S, Li M, Wang J. Enzymatic Characterization of Purified β-Glucosidase from Non-Saccharomyces Yeasts and Application on Chardonnay Aging. Foods. 2022; 11(6):852. https://doi.org/10.3390/foods11060852
Chicago/Turabian StyleGao, Pingping, Faisal Eudes Sam, Bo Zhang, Shuai Peng, Min Li, and Jing Wang. 2022. "Enzymatic Characterization of Purified β-Glucosidase from Non-Saccharomyces Yeasts and Application on Chardonnay Aging" Foods 11, no. 6: 852. https://doi.org/10.3390/foods11060852
APA StyleGao, P., Sam, F. E., Zhang, B., Peng, S., Li, M., & Wang, J. (2022). Enzymatic Characterization of Purified β-Glucosidase from Non-Saccharomyces Yeasts and Application on Chardonnay Aging. Foods, 11(6), 852. https://doi.org/10.3390/foods11060852








