Regional Variation of Chemical Characteristics in Young Marselan (Vitis vinifera L.) Red Wines from Five Regions of China
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Wine Samples
2.3. Meteorological Data
2.4. Colorimetric Measurement
2.5. HS–SPME–GC–MS Analysis of Aroma Compounds
2.6. HPLC–QqQ–MS/MS Analysis of Phenolic Compounds
2.7. Descriptive Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Meteorological Data
3.2. Basic Wine Compositions
3.3. Aroma Compounds
3.3.1. Terpenes
3.3.2. Norisoprenoids
3.3.3. Fermentation Aroma Compounds
3.4. Chromatic Parameters and Phenolic Compounds
3.5. Sensory Profiles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, J.; Harding, J.; Vouillamoz, J. Wine Grapes: A Complete Guide to 1368 Vine Varieties, Including Their Origins and Flavours; Penguin UK: London, UK, 2013. [Google Scholar]
- Wang, S.; Xuelei, M.A.; Ma, Y.; Zhang, Z.; Yang, X. Cultivation performance and research progress on ‘Marselan’ grapevine in china. Sino-Overseas Grapevine Wine 2019, 3, 66–70. [Google Scholar]
- Shi, P.B.; Yue, T.X.; Ai, L.L.; Cheng, Y.F.; Meng, J.F.; Li, M.H.; Zhang, Z.W. Phenolic compound profiles in grape skins of Cabernet Sauvignon, Merlot, Syrah and Marselan cultivated in the Shacheng area (China). S. Afr. J. Enol. Vitic. 2016, 37, 132–138. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.X.; Pan, Q.H.; Yan, G.L.; He, J.J.; Duan, C.Q. Changes of flavan-3-ols with different degrees of polymerization in seeds of ‘Shiraz’, ‘Cabernet Sauvignon’ and ‘Marselan’ grapes after veraison. Molecules 2010, 15, 7763–7774. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yang, S.; Mao, J.; Li, W.; Li, W.; Zuo, C.; Chu, M.; Zhao, X.; Zhou, Q.; Chen, B. Effects of shading on the synthesis of volatile organic compounds in ‘Marselan’ grape berries (Vitis vinifera L.). J. Plant Growth Regul. 2021, 40, 679–693. [Google Scholar] [CrossRef]
- Liu, M.; Song, Y.; Liu, H.; Tang, M.; Yao, Y.; Zhai, H.; Gao, Z.; Du, Y. Effects of flower cluster tip removal on phenolics and antioxidant activity of grape berries and wines. Am. J. Enol. Vitic. 2021, 72, 298–306. [Google Scholar] [CrossRef]
- Xi, X.; Xin, A.; You, Y.; Huang, W.; Zhan, J. Increased varietal aroma diversity of Marselan wine by mixed fermentation with indigenous non-saccharomyces yeasts. Fermentation 2021, 7, 133. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, F.; Wang, W.; Liu, Y.; Wang, J.; Sun, J.; Mu, J.; Gao, Z. Effects of spontaneous fermentation on the microorganisms diversity and volatile compounds during ‘Marselan’ from grape to wine. LWT 2020, 134, 110193. [Google Scholar] [CrossRef]
- Pozzatti, M.; Guerra, C.; Martins, G.; dos Santos, I.; Wagner, R.; Ferrão, M.; Manfroi, V. Effects of winemaking on ‘Marselan’ red wines: Volatile compounds and sensory aspects. Ciênc. Téc. Vitivinic. 2020, 35, 63–75. [Google Scholar] [CrossRef]
- Lyu, J.; Ma, Y.; Xu, Y.; Nie, Y.; Tang, K. Characterization of the key aroma compounds in Marselan wine by gas chromatography-olfactometry, quantitative measurements, aroma recombination, and omission tests. Molecules 2019, 24, 2978. [Google Scholar] [CrossRef]
- Yang, L. Analysis of Color and Aroma of ‘Cabernet Sauvignon’ and ‘Marselan’ Red Wine from Different Regions. Master’s Thesis, China Agricultural University, Beijing, China, 2020. [Google Scholar]
- Green, J.A.; Parr, W.V.; Breitmeyer, J.; Valentin, D.; Sherlock, R. Sensory and chemical characterisation of Sauvignon Blanc wine: Influence of source of origin. Food Res. Int. 2011, 44, 2788–2797. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Roby, J.P.; de Rességuier, L. Soil-related terroir factors: A review. OENO One 2018, 52, 173–188. [Google Scholar] [CrossRef]
- Ziółkowska, A.; Wąsowicz, E.; Jeleń, H.H. Differentiation of wines according to grape variety and geographical origin based on volatiles profiling using SPME-MS and SPME-GC/MS methods. Food Chem. 2016, 213, 714–720. [Google Scholar] [CrossRef] [PubMed]
- China Meteorological Administration. Available online: http://www.cma.gov.cn/ (accessed on 17 August 2021).
- Science Data Bank. Available online: https://www.scidb.cn/ (accessed on 29 August 2021).
- Ayala, F.; Echávarri, J.F.; Negueruela, A.I. A new simplified method for measuring the color of wines. I. Red and rose wines. Am. J. Enol. Vitic. 1997, 48, 357–363. [Google Scholar]
- Cai, J.; Zhu, B.Q.; Wang, Y.H.; Lu, L.; Lan, Y.B.; Reeves, M.J.; Duan, C.Q. Influence of pre-fermentation cold maceration treatment on aroma compounds of Cabernet Sauvignon wines fermented in different industrial scale fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Zhu, B.Q.; Reeves, M.J.; Duan, C.Q. Phenolic analysis and theoretic design for Chinese commercial wines’ authentication. J. Food Sci. 2018, 83, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; He, F.; Zhu, B.Q.; Xing, R.R.; Reeves, M.J.; Duan, C.Q. A systematic analysis strategy for accurate detection of anthocyanin pigments in red wines. Rapid Commun. Mass Spectrom. 2016, 30, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.B.; Xiang, X.F.; Qian, X.; Wang, J.M.; Ling, M.Q.; Zhu, B.Q.; Liu, T.; Sun, L.B.; Shi, Y.; Reynolds, A.G.; et al. Characterization and differentiation of key odor-active compounds of ‘Beibinghong’ icewine and dry wine by gas chromatography-olfactometry and aroma reconstitution. Food Chem. 2019, 287, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Heymann, H.; Noble, A.C. Descriptive analysis of commercial Cabernet Sauvignon wines from California. Am. J. Enol. Vitic. 1987, 38, 41–44. [Google Scholar]
- World Maps of Köppen-Geiger Climate Classification. Available online: http://koeppen-geiger.vu-wien.ac.at/ (accessed on 9 November 2021).
- Li, H.; Wang, H.; Fang, Y.; Huo, X. Study on the viticultural climatic zoning in china (I). Sci. Technol. Rev. 2007, 25, 63–68. [Google Scholar]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Chaves, M.; Zea, L.; Moyano, L.; Medina, M. Changes in color and odorant compounds during oxidative aging of Pedro Ximenez sweet wines. J. Agric. Food Chem. 2007, 55, 3592–3598. [Google Scholar] [CrossRef] [PubMed]
- Zea, L.; Moyano, L.; Moreno, J.A.; Medina, M. Aroma series as fingerprints for biological ageing in fino sherry-type wines. J. Sci. Food Agric. 2007, 87, 2319–2326. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Zea, L.; Moyano, L.; Moreno, J.; Cortes, B.; Medina, M. Discrimination of the aroma fraction of Sherry wines obtained by oxidative and biological ageing. Food Chem. 2001, 75, 79–84. [Google Scholar] [CrossRef]
- Culleré, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas chromatography−olfactometry and chemical quantitative study of the aroma of six premium quality Spanish aged red wines. J. Agric. Food Chem. 2004, 52, 1653–1660. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, L. Intensity prediction of typical aroma characters of Cabernet Sauvignon wine in Changli County (China). LWT 2010, 43, 1550–1556. [Google Scholar] [CrossRef]
- Engel, K.H.; Flath, R.A.; Buttery, R.G.; Mon, T.R.; Ramming, D.W.; Teranishi, R. Investigation of volatile constituents in nectarines. 1. Analytical and sensory characterization of aroma components in some nectarine cultivars. J. Agric. Food Chem. 1988, 36, 549–553. [Google Scholar] [CrossRef]
- Zhang, L.; Tao, Y.S.; Wen, Y.; Wang, H. Aroma evaluation of young Chinese Merlot wines with denomination of origin. S. Afr. J. Enol. Vitic. 2013, 34, 46–53. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Peinado, R.A.; Mauricio, J.C.; Moreno, J. Aromatic series in sherry wines with gluconic acid subjected to different biological aging conditions by Saccharomyces cerevisiae var. capensis. Food Chem. 2006, 94, 232–239. [Google Scholar] [CrossRef]
- La Guerche, S.; Dauphin, B.; Pons, M.; Blancard, D.; Darriet, P. Characterization of some mushroom and earthy off-odors microbially induced by the development of rot on grapes. J. Agric. Food Chem. 2006, 54, 9193–9200. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhang, Z. Volatile compounds of young wines from Cabernet Sauvignon, Cabernet Gernischet and Chardonnay varieties grown in the loess plateau region of China. Molecules 2010, 15, 9184–9196. [Google Scholar] [CrossRef] [PubMed]
- Zoecklein, B.W.; Wolf, T.K.; Marcy, J.E.; Jasinski, Y. Effect of fruit zone leaf thinning on total glycosides and selected aglycone concentrations of Riesling (Vitis vinifera L.) grapes. Am. J. Enol. Vitic. 1998, 49, 35–43. [Google Scholar]
- Black, C.A.; Parker, M.; Siebert, T.E.; Capone, D.L.; Francis, I.L. Terpenoids and their role in wine flavour: Recent advances. Aust. J. Grape Wine Res. 2015, 21, 582–600. [Google Scholar] [CrossRef]
- Jiang, B.; Xi, Z.; Luo, M.; Zhang, Z. Comparison on aroma compounds in Cabernet Sauvignon and Merlot wines from four wine grape-growing regions in China. Food Res. Int. 2013, 51, 482–489. [Google Scholar] [CrossRef]
- Wen, Y.Q.; Zhong, G.Y.; Gao, Y.; Lan, Y.B.; Duan, C.Q.; Pan, Q.H. Using the combined analysis of transcripts and metabolites to propose key genes for differential terpene accumulation across two regions. BMC Plant Biol. 2015, 15, 240. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Wardle, D.A.; Dever, M. Vine performance, fruit composition, and wine sensory attributes of Gewürztraminer in response to vineyard location and canopy manipulation. Am. J. Enol. Vitic. 1996, 47, 77–92. [Google Scholar]
- Feng, H.; Yuan, F.; Skinkis, P.A.; Qian, M.C. Influence of cluster zone leaf removal on Pinot Noir grape chemical and volatile composition. Food Chem. 2015, 173, 414–423. [Google Scholar] [CrossRef]
- Marais, J.; Versini, G.; Van Wyk, C.J.; Rapp, A. Effect of region on free and bound monoterpene and C13-norisoprenoid concentrations in Weisser Riesling wines. S. Afr. J. Enol. Vitic. 1992, 13, 71–77. [Google Scholar]
- Chen, W.K.; Yu, K.J.; Liu, B.; Lan, Y.B.; Sun, R.Z.; Li, Q.; He, F.; Pan, Q.H.; Duan, C.Q.; Wang, J. Comparison of transcriptional expression patterns of carotenoid metabolism in ‘Cabernet Sauvignon’ grapes from two regions with distinct climate. J. Plant Physiol. 2017, 213, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Seo, M.J.; Riu, M.; Cotta, J.P.; Block, D.E.; Dokoozlian, N.K.; Ebeler, S.E. Vine microclimate and norisoprenoid concentration in Cabernet Sauvignon grapes and wines. Am. J. Enol. Vitic. 2007, 58, 291–301. [Google Scholar]
- Bindon, K.A.; Dry, P.R.; Loveys, B.R. Influence of plant water status on the production of C13-norisoprenoid precursors in Vitis vinifera L. cv. Cabernet Sauvignon grape berries. J. Agric. Food Chem. 2007, 55, 4493–4500. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Shellie, K.C.; Wang, H.; Qian, M.C. Influence of deficit irrigation and kaolin particle film on grape composition and volatile compounds in Merlot grape (Vitis vinifera L.). Food Chem. 2012, 134, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Lafon-Lafourcade, S. Wine and Brandy. In Food and Feed Production with Microorganisms (Biotechnology); Rehm, H.J., Fleet, G.H., Eds.; Harwood Academic Publishers: Chur, Switzerland, 1983; pp. 81–163. [Google Scholar]
- Ribereau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Biochemistry of alcoholic fermentation and metabolic pathways of wine yeasts. In Handbook of Enology, The Microbiology of Wine and Vinifications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 1, pp. 74–77. [Google Scholar]
- Pigeau, G.M.; Inglis, D.L. Upregulation of ALD3 and GPD1 in Saccharomyces cerevisiae during icewine fermentation. J. Appl. Microbiol. 2005, 99, 112–125. [Google Scholar] [CrossRef]
- Bowen, A.J.; Reynolds, A.G. Odor potency of aroma compounds in Riesling and Vidal Blanc table wines and icewines by gas chromatography-olfactometry-mass spectrometry. J. Agric. Food Chem. 2012, 60, 2874–2883. [Google Scholar] [CrossRef]
- Genovese, A.; Gambuti, A.; Piombino, P.; Moio, L. Sensory properties and aroma compounds of sweet Fiano wine. Food Chem. 2007, 103, 1228–1236. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Ye, D.; Duan, L.; Duan, C.; Yan, G. Effect of the addition of branched-chain amino acids to non-limited nitrogen synthetic grape must on volatile compounds and global gene expression during alcoholic fermentation. Aust. J. Grape Wine Res. 2018, 24, 197–205. [Google Scholar] [CrossRef]
- Liu, L.; Gregan, S.; Winefield, C.; Jordan, B. From UVR 8 to flavonol synthase: UV-B-induced gene expression in Sauvignon Blanc grape berry. Plant Cell Environ. 2015, 38, 905–919. [Google Scholar] [CrossRef]
- Li, Q.; He, F.; Zhu, B.Q.; Liu, B.; Sun, R.Z.; Duan, C.Q.; Reeves, M.J.; Wang, J. Comparison of distinct transcriptional expression patterns of flavonoid biosynthesis in Cabernet Sauvignon grapes from east and west China. Plant Physiol. Biochem. 2014, 84, 45–56. [Google Scholar] [CrossRef]
- Li, Z.; Pan, Q.; Jin, Z.; Mu, L.; Duan, C. Comparison on phenolic compounds in Vitis vinifera cv. Cabernet Sauvignon wines from five wine-growing regions in China. Food Chem. 2011, 125, 77–83. [Google Scholar] [CrossRef]
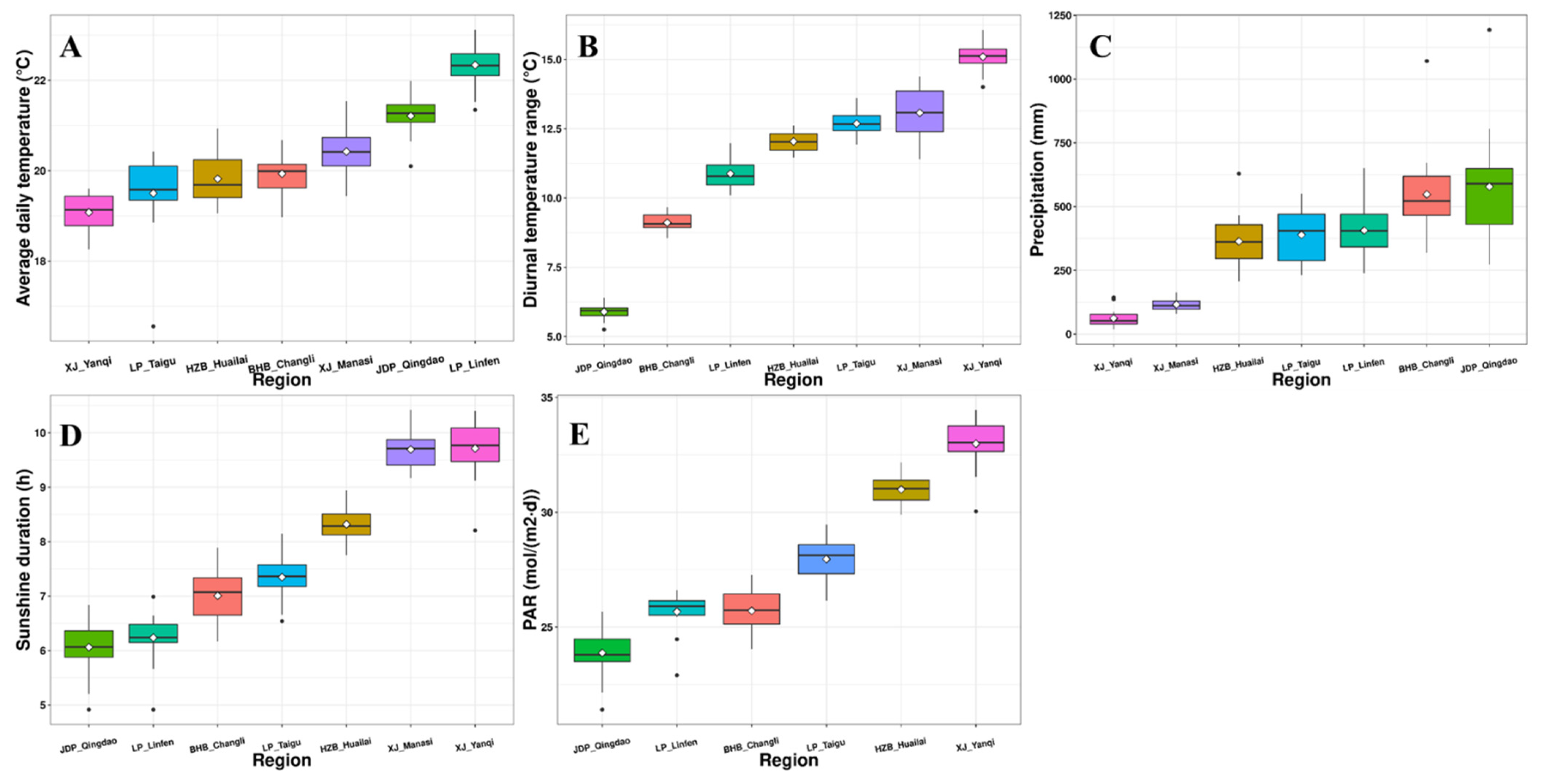
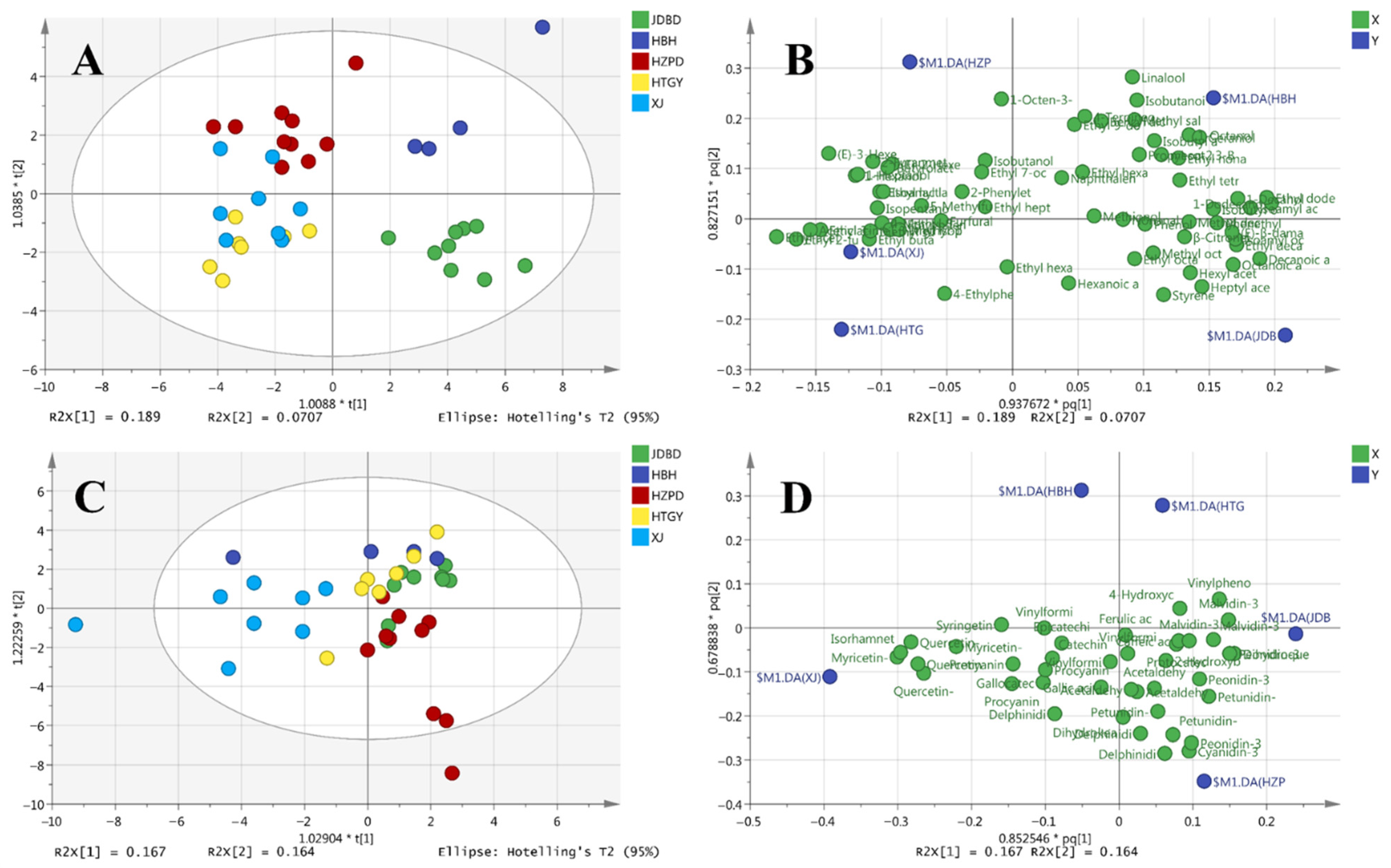
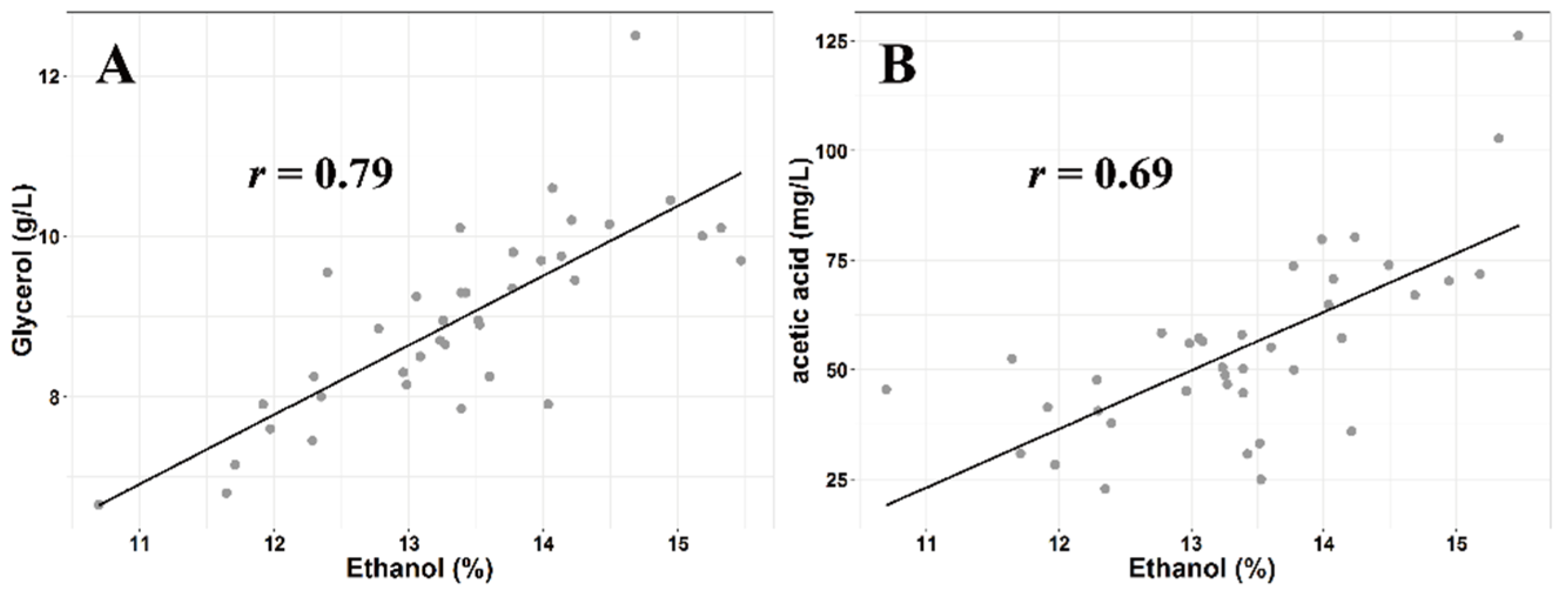
| Region | Alcohol (%) | Reducing Sugar (g/L) | Total Acidity (g/L) | pH | Glycerol (g/L) |
|---|---|---|---|---|---|
| Jiaodong Peninsula (JDP) | 12.75 ± 0.70 b | 2.93 ± 0.81 c | 4.87 ± 0.53 b | 3.69 ± 0.07 a | 8.31 ± 0.57 bc |
| Bohai Bay (BHB) | 12.45 ± 0.79 b | 3.46 ± 1.04 bc | 5.18 ± 0.75 ab | 3.85 ± 0.09 a | 7.78 ± 0.85 c |
| Huaizhuo Basin (HZB) | 13.54 ± 0.80 ab | 3.46 ± 0.50 bc | 5.84 ± 0.80 a | 3.63 ± 0.18 a | 9.57 ± 1.21 a |
| Loess Plateau (LP) | 13.52 ± 1.53 ab | 4.11 ± 0.41 ab | 5.71 ± 1.17 ab | 3.74 ± 0.32 a | 9.07 ± 1.43 ab |
| Xinjiang (XJ) | 14.24 ± 0.77 a | 4.36 ± 0.92 a | 5.70 ± 0.35 ab | 3.77 ± 0.21 a | 9.49 ± 0.94 a |
| Compound | Threshold † | Jiaodong Peninsula | Bohai Bay | Huaizhuo Basin | Loess Plateau | Xinjiang |
|---|---|---|---|---|---|---|
| Ethyl acetate | 7500 [25] | 53,131.62 ± 12,669.67 c | 70,487.82 ± 24,192.58 bc | 88,681.25 ± 27,710.30 ab | 120,690.88 ± 39,741.90 a | 116,549.20 ± 30,546.87 a |
| Isobutyl acetate | 1600 [26] | 9.48 ± 6.33 ab | 18.86 ± 13.76 a | 9.21 ± 13.26 ab | 7.24 ± 5.06 b | 6.67 ± 4.70 b |
| Isoamyl acetate | 30 [25] | 1648.58 ± 1016.73 a | 2050.30 ± 1503.37 a | 632.94 ± 349.79 b | 663.18 ± 211.84 b | 841.42 ± 344.28 b |
| Hexyl acetate | 1000 [27] | 71.17 ± 87.43 a | 33.40 ± 20.60 ab | 8.85 ± 4.20 b | 11.30 ± 5.05 b | 9.26 ± 4.20 b |
| Heptyl acetate | - | 0.40 ± 0.44 a | 0.09 ± 0.08 b | 0.00 ± 0.01 b | 0.00 ± 0.00 b | 0.01 ± 0.02 b |
| Phenethyl acetate | 250 [25] | 66.68 ± 45.04 a | 58.00 ± 51.97 ab | 32.50 ± 14.62 ab | 23.61 ± 3.02 b | 37.79 ± 19.14 ab |
| Acetic acid (mg/L) | 200,000 [25] | 37.31 ± 9.83 c | 45.96 ± 13.08 bc | 53.17 ± 12.05 bc | 61.16 ± 17.52 ab | 75.99 ± 26.30 a |
| Isobutanoic acid | 20,000 [28] | 2901.63 ± 794.67 abc | 3506.68 ± 1230.91 ab | 3807.84 ± 2048.22 a | 1572.45 ± 651.55 c | 2102.83 ± 560.75 bc |
| Hexanoic acid | 420 [29] | 1654.44 ± 405.83 a | 1555.85 ± 192.78 a | 1409.18 ± 175.31 a | 1647.64 ± 359.27 a | 1391.26 ± 347.36 a |
| Octanoic acid | 500 [29] | 2065.59 ± 257.39 a | 1996.64 ± 181.01 ab | 1789.24 ± 62.88 c | 1821.23 ± 118.35 bc | 1810.82 ± 141.07 bc |
| Decanoic acid | 1000 [29] | 2526.69 ± 174.48 a | 2469.04 ± 98.39 a | 2363.42 ± 27.35 b | 2347.84 ± 40.13 b | 2358.52 ± 46.73 b |
| Styrene | - | 3.26 ± 3.22 a | 0.13 ± 0.26 b | 0.35 ± 0.61 b | 0.60 ± 1.07 b | 0.40 ± 1.13 b |
| Naphthalene | - | 0.20 ± 0.29 b | 1.78 ± 2.60 a | 0.41 ± 0.34 b | 0.44 ± 0.19 b | 0.41 ± 0.16 b |
| 1-Hexanol | 8000 [25] | 2696.95 ± 1012.55 b | 2392.31 ± 909.25 b | 4636.94 ± 1454.32 a | 3712.82 ± 1413.24 ab | 2890.52 ± 1048.65 b |
| (E)-3-Hexen-1-ol | 1000 [30] | 23.28 ± 21.82 c | 46.41 ± 13.36 bc | 82.05 ± 33.15 a | 70.21 ± 25.95 ab | 54.18 ± 37.32 abc |
| (E)-2-Hexen-1-ol | - | 2.47 ± 2.36 b | 13.66 ± 4.69 a | 7.91 ± 4.18 ab | 14.18 ± 5.97 a | 9.34 ± 8.82 a |
| Nonanal | 15 [31] | 3.16 ± 0.91 ab | 3.73 ± 1.28 a | 2.63 ± 0.53 b | 2.86 ± 0.82 ab | 3.06 ± 0.84 ab |
| Ethyl lactate | 100,000 [27] | 79,455.87 ± 52,034.26 b | 144,101.07 ± 112,565.14 ab | 147,259.23 ± 59,523.25 ab | 161,621.62 ± 69,605.82 ab | 178,206.48 ± 96,658.57 a |
| Ethyl 9-decenoate | 100 [32] | 60.60 ± 10.18 b | 108.33 ± 57.16 a | 73.59 ± 12.51 b | 71.48 ± 10.31 b | 68.47 ± 11.99 b |
| Ethyl 3-methylbutanoate | 3 [25] | 53.68 ± 101.47 b | 60.06 ± 55.18 b | 180.82 ± 81.76 ab | 205.26 ± 183.23 ab | 299.00 ± 197.91 a |
| Ethyl 7-octenoate | - | 14.41 ± 0.61 c | 17.06 ± 2.28 a | 14.96 ± 0.68 bc | 14.98 ± 0.78 bc | 15.74 ± 1.15 b |
| Ethyl 2-furoate | - | 207.82 ± 166.39 b | 164.16 ± 55.48 b | 561.80 ± 164.48 a | 663.56 ± 463.01 a | 533.49 ± 348.75 a |
| Diethyl succinate | 100,000 [33] | 4388.69 ± 4118.95 b | 7575.93 ± 5683.54 ab | 9159.18 ± 7346.40 ab | 9219.71 ± 6041.19 ab | 16,445.07 ± 12,604.95 a |
| Ethyl phenylacetate | 650 [34] | 4.86 ± 3.43 a | 3.53 ± 1.88 a | 7.84 ± 4.95 a | 7.10 ± 5.55 a | 7.42 ± 5.20 a |
| Ethyl butanoate | 20 [25] | 1258.88 ± 272.63 b | 1696.14 ± 319.91 ab | 1545.42 ± 319.48 ab | 1999.73 ± 507.79 a | 1835.93 ± 1002.26 ab |
| Ethyl hexanoate | 5 [25] | 788.79 ± 231.88 a | 795.16 ± 107.19 a | 702.95 ± 127.01 a | 861.11 ± 221.67 a | 756.81 ± 282.59 a |
| Ethyl heptanoate | - | 2.04 ± 0.97 a | 1.74 ± 0.16 a | 2.33 ± 0.94 a | 1.81 ± 0.27 a | 1.90 ± 0.29 a |
| Ethyl octanoate | 580 [35] | 2466.88 ± 424.25 a | 2472.94 ± 218.44 a | 2105.96 ± 127.39 a | 2210.65 ± 237.12 a | 2303.16 ± 448.24 a |
| Ethyl nonanoate | - | 2.50 ± 0.73 a | 2.56 ± 0.23 a | 2.11 ± 0.24 a | 0.59 ± 1.01 b | 1.75 ± 1.48 a |
| Ethyl decanoate | 200 [29] | 1481.79 ± 683.54 a | 1445.06 ± 285.06 a | 804.51 ± 165.77 b | 706.14 ± 215.70 b | 906.88 ± 404.60 b |
| Ethyl dodecanoate | 1500 [32] | 92.08 ± 25.05 a | 111.85 ± 48.88 a | 57.43 ± 8.96 b | 54.43 ± 10.64 b | 62.44 ± 22.28 b |
| Ethyl tetradecanoate | - | 44.49 ± 1.74 b | 47.35 ± 2.86 a | 42.81 ± 1.05 b | 42.57 ± 1.59 b | 44.43 ± 2.27 b |
| Ethyl hexadecanoate | - | 50.08 ± 3.24 bc | 55.41 ± 4.70 a | 48.94 ± 2.32 c | 46.97 ± 1.64 c | 53.70 ± 4.87 ab |
| Furfural | 14,100 [29] | 380.26 ± 399.68 a | 208.00 ± 185.12 a | 477.23 ± 415.17 a | 425.47 ± 514.34 a | 411.58 ± 545.35 a |
| 5-Methylfurfural | - | 21.57 ± 26.66 a | 9.25 ± 4.05 a | 63.57 ± 67.52 a | 24.35 ± 33.22 a | 49.07 ± 102.85 a |
| Butyrolactone | 100,000 [27] | 7867.33 ± 2764.38 c | 7670.68 ± 2714.28 c | 14,149.21 ± 6046.98 ab | 8700.18 ± 2291.87 bc | 15,995.18 ± 7491.72 a |
| 2-Furanmethanol | 15,000 [36] | 6.70 ± 6.80 b | 32.11 ± 19.72 ab | 132.67 ± 170.21 a | 65.29 ± 62.63 ab | 20.87 ± 38.25 ab |
| Isobutanol | 40,000 [25] | 65,080.39 ± 11,267.73 a | 57,566.81 ± 11,746.28 a | 93,732.62 ± 76,667.05 a | 72,284.92 ± 32,943.52 a | 70,926.97 ± 30,974.16 a |
| Isopentanol | 65,000 [27] | 155,154.36 ± 29,340.28 ab | 127,002.67 ± 16,205.86 b | 186,864.22 ± 48,011.05 ab | 172,232.42 ± 43,205.62 ab | 208,170.03 ± 82,626.75 a |
| 4-Methyl-1-pentanol | 50,000 [29] | 8.94 ± 2.24 ab | 5.91 ± 1.34 b | 10.09 ± 2.40 a | 8.69 ± 2.80 ab | 12.28 ± 4.72 a |
| 3-Methyl-1-pentanol | 500 [32] | 86.99 ± 34.75 ab | 39.98 ± 26.43 b | 104.69 ± 53.17 ab | 86.93 ± 70.03 ab | 128.39 ± 73.99 a |
| 1-Octen-3-ol | 20 [37] | 9.55 ± 3.11 b | 18.28 ± 15.21 a | 17.45 ± 6.84 a | 10.91 ± 2.75 ab | 12.64 ± 3.51 ab |
| 1-Heptanol | 200 [35] | 24.19 ± 9.23 b | 19.87 ± 1.94 b | 46.40 ± 17.53 a | 36.41 ± 5.39 a | 22.49 ± 4.01 b |
| meso-2,3-Butanediol (mg/L) | 150,000 [36] | 35.18 ± 25.13 a | 22.42 ± 14.13 ab | 35.47 ± 30.17 a | 0.25 ± 0.67 b | 1.39 ± 1.98 b |
| 1-Octanol | 800 [36] | 24.42 ± 7.02 b | 32.06 ± 7.72 a | 24.01 ± 5.12 b | 18.72 ± 2.87 b | 19.64 ± 6.45 b |
| 1-Decanol | 400 [29] | 15.03 ± 3.00 a | 14.77 ± 2.39 a | 12.78 ± 1.67 ab | 11.27 ± 1.41 b | 12.12 ± 1.61 b |
| Benzyl alcohol | 900,000 [36] | 382.85 ± 113.74 b | 1258.33 ± 1172.10 a | 527.11 ± 254.85 b | 544.30 ± 265.40 b | 436.11 ± 172.33 b |
| 2-Phenylethanol | 10,000 [25] | 22,642.31 ± 7850.73 a | 13,017.55 ± 2461.30 a | 28,433.94 ± 14,819.99 a | 17,845.87 ± 7045.54 a | 30,107.99 ± 23,591.23 a |
| 1-Dodecanol | - | 2.84 ± 0.74 a | 2.73 ± 0.56 ab | 1.81 ± 0.73 bc | 0.86 ± 1.09 cd | 0.57 ± 1.06 d |
| (E)-β-damascenone | 0.05 [25] | 15.31 ± 5.79 a | 16.59 ± 4.27 a | 10.08 ± 2.68 b | 10.41 ± 2.82 b | 9.47 ± 1.70 b |
| Methyl octanoate | 200 [38] | 9.86 ± 4.62 ab | 11.87 ± 1.94 a | 5.86 ± 2.06 b | 8.17 ± 3.68 ab | 7.63 ± 4.15 ab |
| Propyl octanoate | - | 8.80 ± 6.61 abc | 13.21 ± 0.22 a | 11.56 ± 4.06 ab | 5.61 ± 7.00 bc | 3.27 ± 6.05 c |
| Isobutyl octanoate | - | 2.82 ± 0.81 a | 2.89 ± 0.29 a | 2.20 ± 0.73 ab | 1.81 ± 0.82 b | 1.62 ± 1.06 b |
| Isoamyl lactate | - | 144.81 ± 114.54 a | 223.67 ± 144.04 a | 273.75 ± 126.53 a | 275.98 ± 87.64 a | 333.73 ± 251.57 a |
| Methyl decanoate | - | 73.20 ± 16.38 a | 73.76 ± 7.39 a | 59.18 ± 5.67 ab | 50.78 ± 22.96 b | 62.68 ± 8.81 ab |
| Isoamyl octanoate | 125 [29] | 19.78 ± 10.96 a | 18.66 ± 3.70 a | 9.47 ± 2.55 b | 8.34 ± 2.78 b | 10.19 ± 5.23 b |
| Methyl salicylate | - | 19.13 ± 4.49 b | 140.69 ± 232.51 a | 29.85 ± 14.90 b | 19.66 ± 4.49 b | 14.78 ± 7.84 b |
| Ethyl isopentyl succinate | - | 169.46 ± 136.97 a | 174.63 ± 93.52 a | 232.41 ± 146.88 a | 190.51 ± 76.28 a | 329.11 ± 194.12 a |
| Methionol | 500 [25] | 1697.09 ± 716.09 a | 907.62 ± 99.15 a | 1330.56 ± 825.61 a | 952.80 ± 449.08 a | 1157.88 ± 923.19 a |
| Linalool | 15 [25] | 2.58 ± 0.15 c | 4.70 ± 2.47 a | 3.63 ± 0.37 b | 0.00 ± 0.00 d | 3.20 ± 0.89 bc |
| 4-Terpineol | 5000 [30] | 1.03 ± 0.29 b | 2.20 ± 2.36 a | 1.57 ± 0.54 ab | 1.12 ± 0.80 b | 0.99 ± 0.66 b |
| β-Citronellol | 100 [25] | 11.72 ± 4.79 a | 12.15 ± 6.29 a | 6.68 ± 3.76 b | 6.26 ± 5.34 b | 6.81 ± 2.10 b |
| Geraniol | 30 [25] | 24.83 ± 10.43 a | 33.92 ± 10.10 a | 22.74 ± 2.82 ab | 12.03 ± 15.29 bc | 8.95 ± 12.42 c |
| Phenol | - | 43.74 ± 25.48 a | 43.35 ± 16.63 a | 36.00 ± 5.01 ab | 42.48 ± 9.70 a | 20.49 ± 3.90 b |
| 4-Ethylphenol | 440 [31] | 70.12 ± 4.50 b | 66.12 ± 1.13 b | 59.02 ± 20.75 b | 182.44 ± 115.00 a | 49.09 ± 89.82 b |
| CIELab | Jiaodong Peninsula | Bohai Bay | Huaizhuo Basin | Loess Plateau | Xinjiang |
|---|---|---|---|---|---|
| L | 66.32 ± 13.31 a | 61.54 ± 20.24 ab | 42.58 ± 10.02 c | 48.90 ± 8.62 bc | 39.11 ± 12.07 c |
| a | 34.74 ± 11.95 b | 34.49 ± 14.90 b | 49.98 ± 8.76 a | 45.64 ± 9.33 ab | 52.52 ± 12.07 a |
| b | 6.94 ± 5.50 a | 7.10 ± 1.58 a | 7.91 ± 3.79 a | 9.22 ± 4.43 a | 9.07 ± 7.95 a |
| Hab | 10.78 ± 5.62 a | 13.70 ± 8.28 a | 9.76 ± 6.27 a | 11.46 ± 5.12 a | 11.18 ± 8.74 a |
| Cab | 35.62 ± 12.56 b | 35.39 ± 14.41 b | 50.85 ± 8.02 a | 46.72 ± 9.43 ab | 53.93 ± 11.42 a |
| Compound | Jiaodong Peninsula | Bohai Bay | Huaizhuo Basin | Loess Plateau | Xinjiang |
|---|---|---|---|---|---|
| Myricetin-3-O-galactoside | 0.04 ± 0.09 a | 0.08 ± 0.19 a | 0.05 ± 0.11 a | 0.07 ± 0.09 a | 0.17 ± 0.30 a |
| Myricetin-3-O-glucoside | 2.38 ± 1.31 b | 1.97 ± 4.19 b | 2.71 ± 2.73 b | 1.62 ± 1.59 b | 14.68 ± 3.83 a |
| Dihydroquercetin | 1.40 ± 0.66 a | 0.95 ± 0.50 ab | 1.00 ± 0.45 ab | 0.82 ± 0.50 ab | 0.63 ± 0.38 b |
| Quercetin-3-O-glucuronide | 0.53 ± 0.46 c | 1.40 ± 2.45 bc | 1.68 ± 1.44 bc | 3.04 ± 2.36 ab | 5.10 ± 2.26 a |
| Quercetin-3-O-galactoside | 0.20 ± 0.19 b | 0.27 ± 0.57 b | 0.29 ± 0.32 b | 0.38 ± 0.28 ab | 0.84 ± 0.61 a |
| Quercetin-3-O-glucoside | 0.00 ± 0.00 b | 0.02 ± 0.04 b | 0.08 ± 0.26 b | 0.00 ± 0.00 b | 4.66 ± 4.13 a |
| Dihydrokeampferol | 0.44 ± 0.10 a | 0.52 ± 0.24 a | 0.63 ± 0.21 a | 0.51 ± 0.26 a | 0.53 ± 0.28 a |
| Syringetin-3-O-glucoside | 2.65 ± 0.62 b | 3.36 ± 0.76 b | 2.72 ± 0.94 b | 4.69 ± 1.22 a | 4.39 ± 0.89 a |
| Isorhamnetin-3-O-glucoside | 0.08 ± 0.13 b | 0.19 ± 0.48 b | 0.14 ± 0.34 b | 0.07 ± 0.19 b | 1.58 ± 1.04 a |
| Procyanin B1 | 32.91 ± 9.91 ab | 36.35 ± 27.27 ab | 35.66 ± 8.64 ab | 25.87 ± 19.83 b | 44.06 ± 15.36 a |
| Gallocatechin | 4.26 ± 2.02 ab | 2.25 ± 0.54 c | 2.85 ± 1.06 bc | 2.36 ± 1.34 c | 4.36 ± 0.55 a |
| Catechin | 24.57 ± 8.43 a | 28.26 ± 14.46 a | 20.83 ± 4.14 a | 19.29 ± 10.50 a | 28.05 ± 12.67 a |
| Procyanin C1 | 5.38 ± 3.55 ab | 6.50 ± 6.57 a | 4.88 ± 2.36 ab | 2.14 ± 3.50 b | 8.13 ± 6.91 a |
| Procyanin B2 | 12.71 ± 5.04 ab | 15.62 ± 11.53 a | 13.49 ± 3.88 ab | 8.98 ± 7.70 b | 18.31 ± 7.84 a |
| Epicatechin | 39.99 ± 16.27 ab | 43.69 ± 23.87 a | 30.09 ± 8.80 ab | 25.55 ± 17.65 b | 42.53 ± 17.50 ab |
| Gallic acid | 19.95 ± 5.87 bc | 12.95 ± 9.55 c | 31.93 ± 9.98 a | 25.30 ± 15.63 ab | 25.30 ± 5.92 ab |
| Protocatechuic acid | 1.38 ± 0.57 a | 1.61 ± 1.11 a | 2.69 ± 3.41 a | 2.17 ± 1.35 a | 0.89 ± 0.36 a |
| 2-Hydroxybenzoic acid | 0.12 ± 0.10 ab | 0.08 ± 0.16 ab | 0.24 ± 0.29 a | 0.12 ± 0.13 ab | 0.00 ± 0.00 b |
| Caffeic acid | 10.00 ± 5.14 a | 12.06 ± 9.27 a | 10.59 ± 10.10 a | 8.58 ± 8.30 a | 6.38 ± 8.37 a |
| 4-Hydroxycinnamic acid | 4.85 ± 2.64 a | 5.57 ± 1.90 a | 3.83 ± 3.04 a | 4.31 ± 3.12 a | 3.00 ± 2.69 a |
| Ferulic acid | 0.05 ± 0.06 a | 0.21 ± 0.18 a | 0.10 ± 0.17 a | 0.03 ± 0.07 a | 0.09 ± 0.12 a |
| Delphinidin-3-O-glucoside | 6.56 ± 2.97 ab | 3.91 ± 1.26 b | 10.94 ± 7.24 a | 3.78 ± 2.59 b | 6.75 ± 3.13 ab |
| Cyanidin-3-O-glucoside | 0.44 ± 0.14 b | 0.39 ± 0.10 b | 1.24 ± 0.96 a | 0.40 ± 0.25 b | 0.43 ± 0.13 b |
| Petunidin-3-O-glucoside | 4.66 ± 1.88 ab | 3.07 ± 0.96 b | 6.33 ± 4.09 a | 3.12 ± 2.11 b | 4.33 ± 2.51 ab |
| Peonidin-3-O-glucoside | 2.47 ± 0.79 ab | 1.73 ± 0.69 b | 3.73 ± 2.62 a | 1.74 ± 1.12 b | 1.95 ± 0.99 ab |
| Malvidin-3-O-glucoside | 156.24 ± 43.75 a | 110.71 ± 45.35 a | 111.27 ± 58.38 a | 120.86 ± 105.00 a | 99.42 ± 51.61 a |
| Delphinidin-3-O-acetylglucoside | 2.09 ± 1.39 ab | 1.60 ± 0.33 b | 2.71 ± 1.37 a | 1.35 ± 0.53 b | 2.06 ± 1.17 ab |
| Petunidin-3-O-acetylglucoside | 1.96 ± 1.70 a | 1.14 ± 0.43 a | 2.04 ± 1.45 a | 1.03 ± 0.89 a | 1.60 ± 1.16 a |
| Peonidin-3-O-acetylglucoside | 1.59 ± 0.75 a | 1.34 ± 0.84 a | 1.49 ± 1.12 a | 1.34 ± 1.29 a | 1.03 ± 0.72 a |
| Malvidin-3-O-acetylglucoside | 78.57 ± 34.30 a | 54.57 ± 26.21 a | 43.45 ± 26.85 a | 43.10 ± 43.35 a | 46.43 ± 30.73 a |
| Delphinidin-3-O-coumaroylglucoside (cis + trans) | 0.85 ± 0.14 a | 0.71 ± 0.09 a | 0.91 ± 0.27 a | 0.70 ± 0.14 a | 0.95 ± 0.37 a |
| Petunidin-3-O-coumaroylglucoside (cis + trans) | 0.49 ± 0.27 a | 0.20 ± 0.17 a | 0.38 ± 0.37 a | 0.21 ± 0.24 a | 0.25 ± 0.22 a |
| Peonidin-3-O-coumaroylglucoside (cis + trans) | 1.23 ± 0.57 a | 0.83 ± 0.66 a | 0.84 ± 0.77 a | 0.81 ± 0.93 a | 0.52 ± 0.50 a |
| Malvidin-3-O-coumaroylglucoside (cis + trans) | 32.38 ± 14.09 a | 18.51 ± 14.84 ab | 13.86 ± 11.34 b | 17.04 ± 20.98 ab | 11.96 ± 9.07 b |
| Vinylformic acid adduct of Malvidin-3-O-glucoside (Vitisin A) | 11.26 ± 8.00 a | 12.44 ± 9.31 a | 16.81 ± 6.19 a | 15.68 ± 4.54 a | 14.66 ± 8.01 a |
| Vinylformic acid adduct of Malvidin-3-O-acetylglucoside | 7.88 ± 10.34 a | 6.05 ± 4.36 a | 7.07 ± 3.75 a | 5.64 ± 2.79 a | 6.34 ± 3.18 a |
| Vinylformic acid adduct of Malvidin-3-O-coumaroylglucoside | 2.82 ± 1.39 b | 5.12 ± 6.09 a | 3.10 ± 1.00 ab | 2.70 ± 1.00 b | 3.57 ± 1.61 ab |
| Acetaldehyde adduct of Malvidin-3-O-glucoside (Vitisin B) | 24.11 ± 21.10 a | 9.16 ± 4.63 a | 32.86 ± 43.65 a | 39.29 ± 51.29 a | 23.01 ± 19.80 a |
| Acetaldehyde adduct of Malvidin-3-O-acetylglucoside | 10.64 ± 9.17 a | 4.58 ± 2.11 a | 11.85 ± 16.27 a | 15.77 ± 22.79 a | 9.62 ± 6.73 a |
| Acetaldehyde adduct of Malvidin-3-O-coumaroylglucoside | 7.74 ± 7.27 a | 2.26 ± 0.49 a | 7.68 ± 11.05 a | 7.11 ± 9.55 a | 5.60 ± 6.11 a |
| Vinylphenol adduct of Malvidin-3-O-glucoside | 22.23 ± 18.87 a | 23.63 ± 12.48 a | 15.60 ± 11.72 a | 21.14 ± 8.83 a | 11.61 ± 10.23 a |
| Attributes | Jiaodong Peninsula | Bohai Bay | Huaizhuo Basin | Loess Plateau | Xinjiang |
|---|---|---|---|---|---|
| Hue | 9.58 ± 0.04 a | 9.3 ± 0.29 a | 9.14 ± 0.57 a | 9.83 ± 0.06 a | 9.41 ± 0.18 a |
| Color intensity | 7.93 ± 0.20 c | 8.91 ± 0.76 ab | 8.42 ± 0.10 bc | 9.44 ± 0.03 a | 9.21 ± 0.23 a |
| Floral | 6.57 ± 0.66 a | 6.51 ± 0.86 a | 4.26 ± 0.67 b | 5.87 ± 0.23 a | 5.62 ± 0.54 a |
| Fruity | 7.9 ± 0.02 a | 7.23 ± 0.47 a | 6.26 ± 0.34 b | 7.24 ± 0.37 a | 7.50 ± 0.44 a |
| Hebaceous | 5.23 ± 0.84 a | 4.56 ± 0.24 a | 3.82 ± 1.03 a | 4.14 ± 0.33 a | 4.3 ± 0.89 a |
| Acidiy | 7.44 ± 0.53 a | 7.21 ± 0.83 ab | 6.41 ± 0.11 ab | 7.28 ± 0.04 ab | 5.99 ± 0.88 b |
| Astringency | 5.32 ± 0.56 a | 5.44 ± 0.59 a | 5.85 ± 0.62 a | 6.39 ± 0.38 a | 6.46 ± 0.32 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Y.; Liu, M.; Zhang, X.; Li, S.; Shi, Y.; Duan, C. Regional Variation of Chemical Characteristics in Young Marselan (Vitis vinifera L.) Red Wines from Five Regions of China. Foods 2022, 11, 787. https://doi.org/10.3390/foods11060787
Lan Y, Liu M, Zhang X, Li S, Shi Y, Duan C. Regional Variation of Chemical Characteristics in Young Marselan (Vitis vinifera L.) Red Wines from Five Regions of China. Foods. 2022; 11(6):787. https://doi.org/10.3390/foods11060787
Chicago/Turabian StyleLan, Yibin, Min Liu, Xinke Zhang, Siyu Li, Ying Shi, and Changqing Duan. 2022. "Regional Variation of Chemical Characteristics in Young Marselan (Vitis vinifera L.) Red Wines from Five Regions of China" Foods 11, no. 6: 787. https://doi.org/10.3390/foods11060787
APA StyleLan, Y., Liu, M., Zhang, X., Li, S., Shi, Y., & Duan, C. (2022). Regional Variation of Chemical Characteristics in Young Marselan (Vitis vinifera L.) Red Wines from Five Regions of China. Foods, 11(6), 787. https://doi.org/10.3390/foods11060787









