Nanocluster-Based Drug Delivery and Theranostic Systems: Towards Cancer Therapy
Abstract
1. Introduction
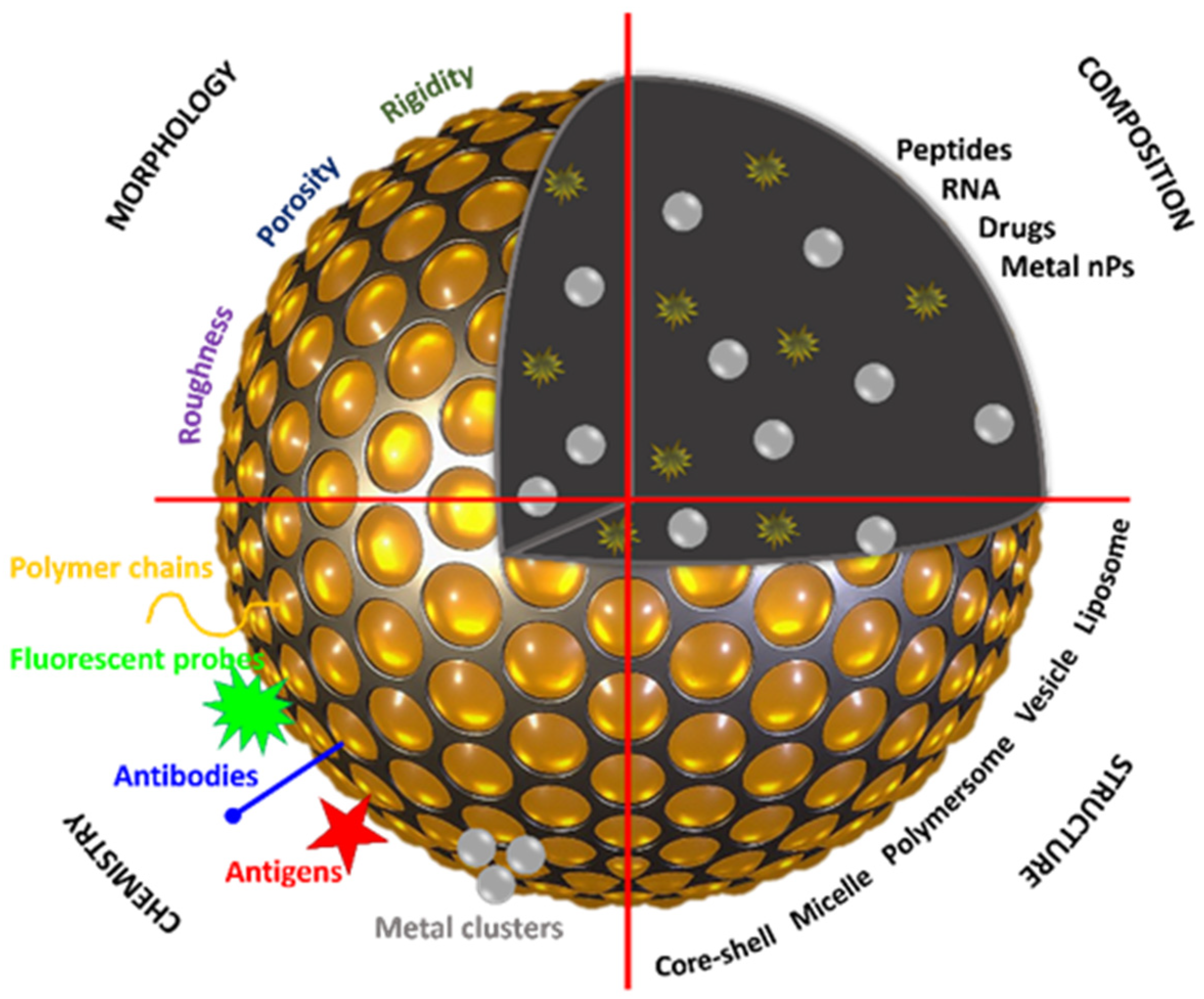
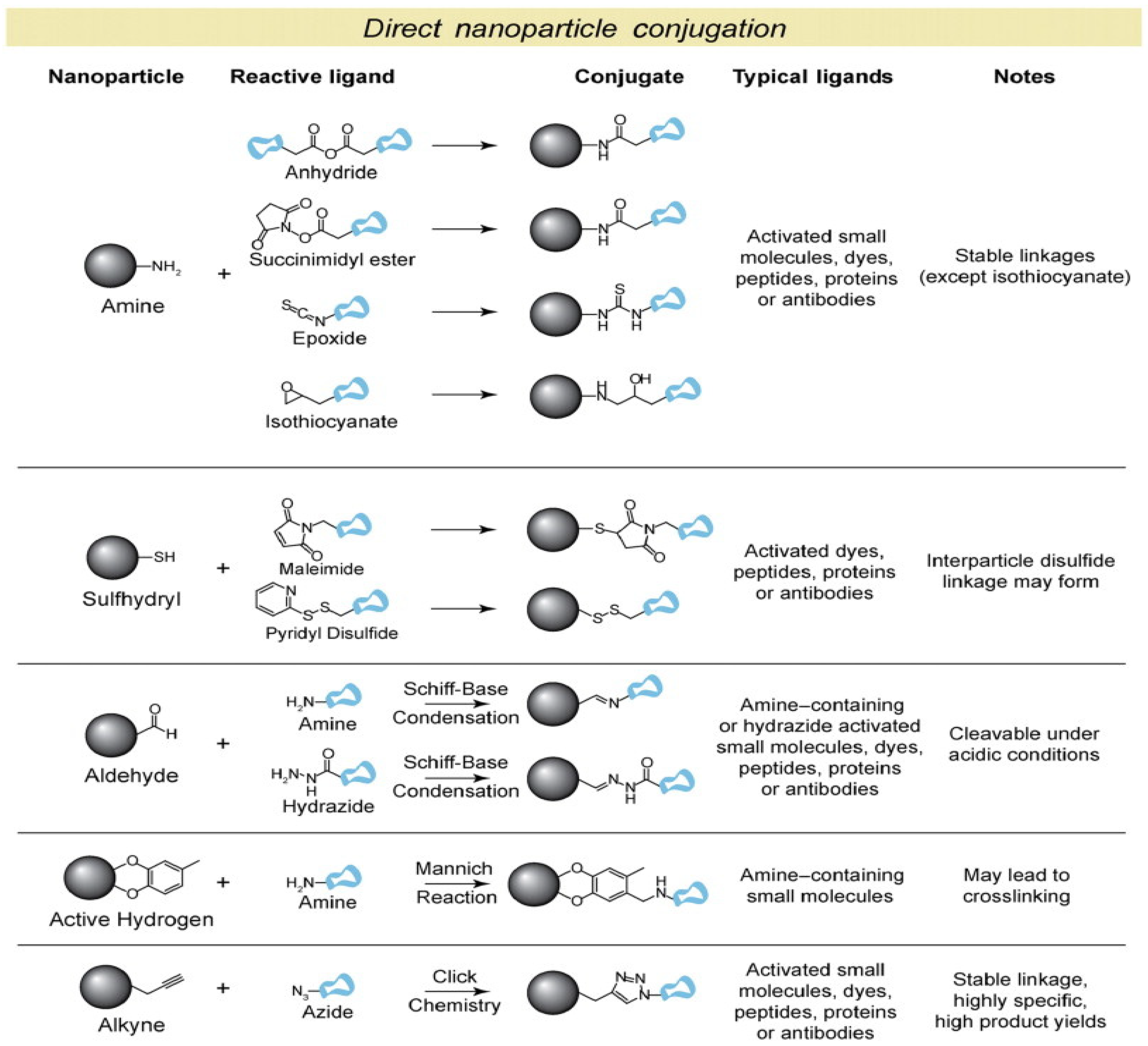
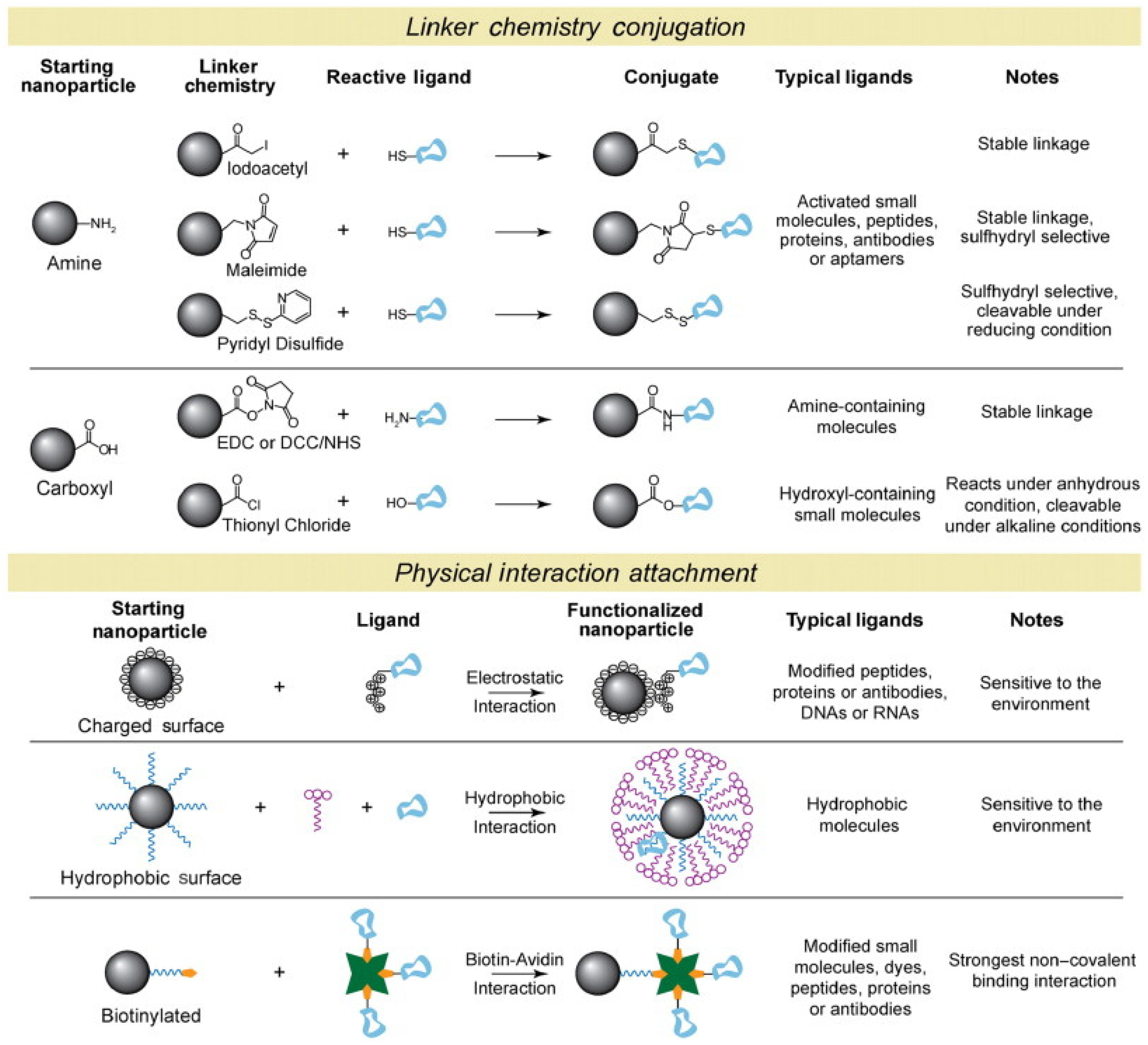
2. Nano-Based Building Blocks
2.1. Polymeric Nanocarriers as Building Blocks
2.2. Inorganic Nanoparticles
2.2.1. Gold Nanoparticles
2.2.2. Silver Nanoparticles
| Size of Ag NPs (nm) | Anticancer Drug | Cancer Cells | Ref. |
|---|---|---|---|
| 30–50 | DOX | Human lung carcinoma (A549) | [152] |
| Breast cancer (MCF-7) | |||
| 32 | Plumbagin | Human cervical cancer cells | [172] |
| 23 | Dexamethasone | HeLa cervical cancer cells | [173] |
| Osteocytic cells (MLO-Y4) | |||
| 20 | Imatinib | Breast cancer cells (MCF-7) | [174] |
2.2.3. Superparamagnetic Iron Oxide Nanoparticles
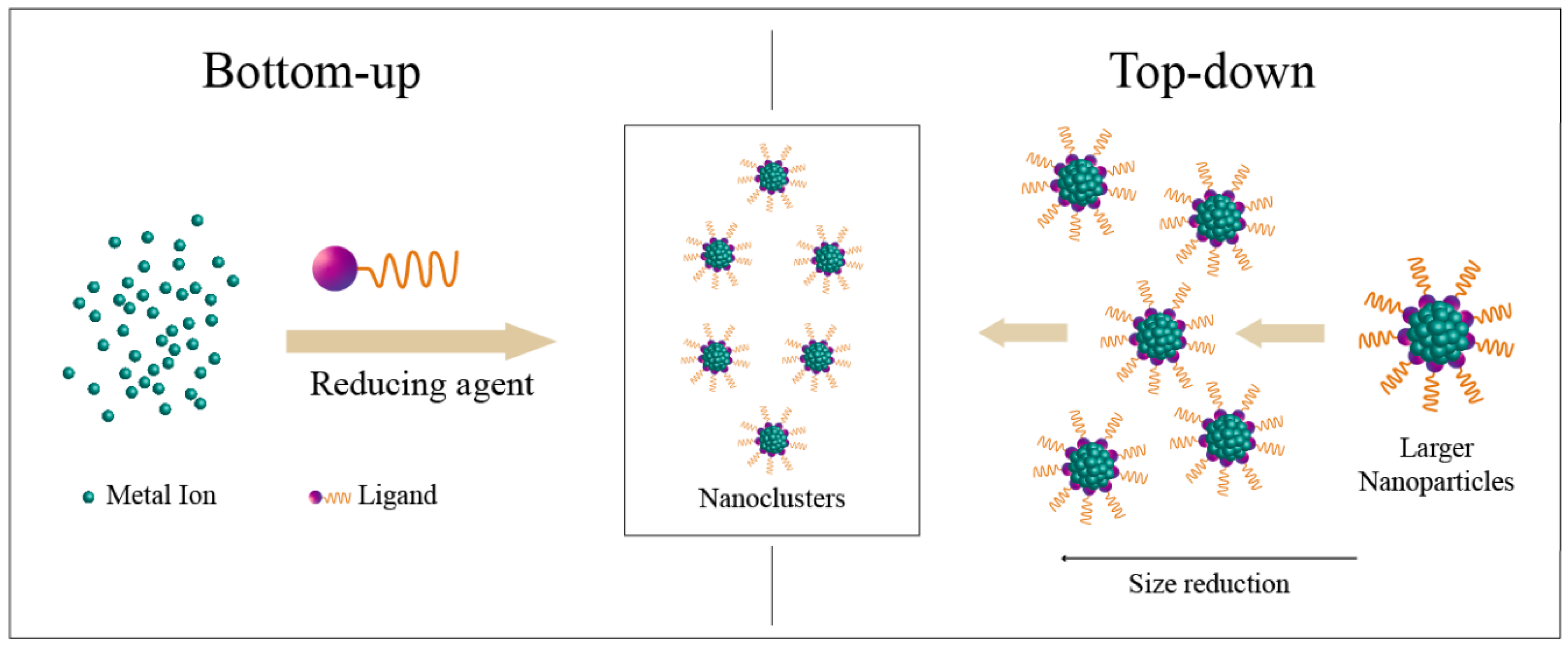
2.3. Design towards Nanoclusters as Drug Delivery Systems and Theranostics
2.3.1. Metallic Nanoclusters
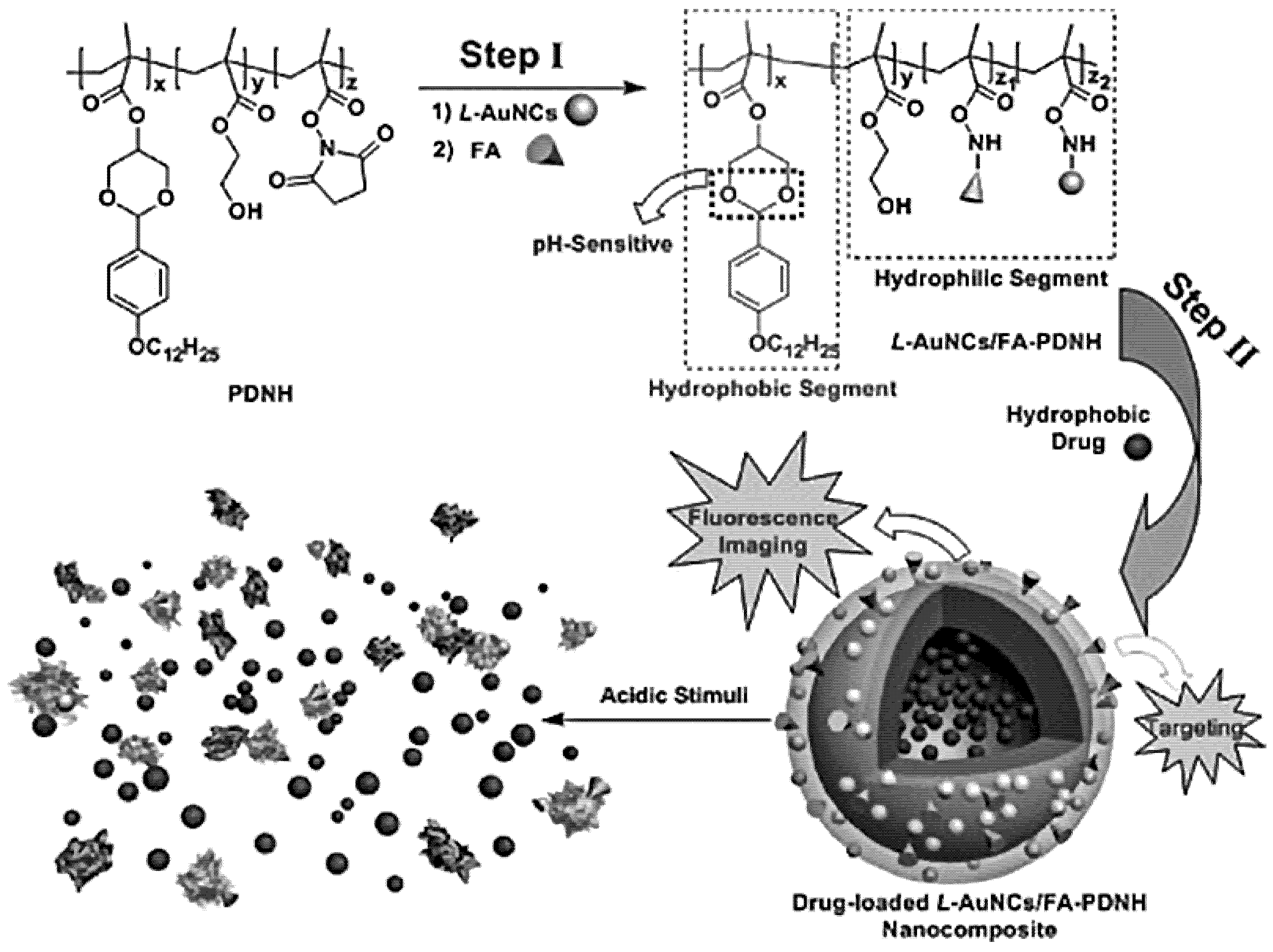
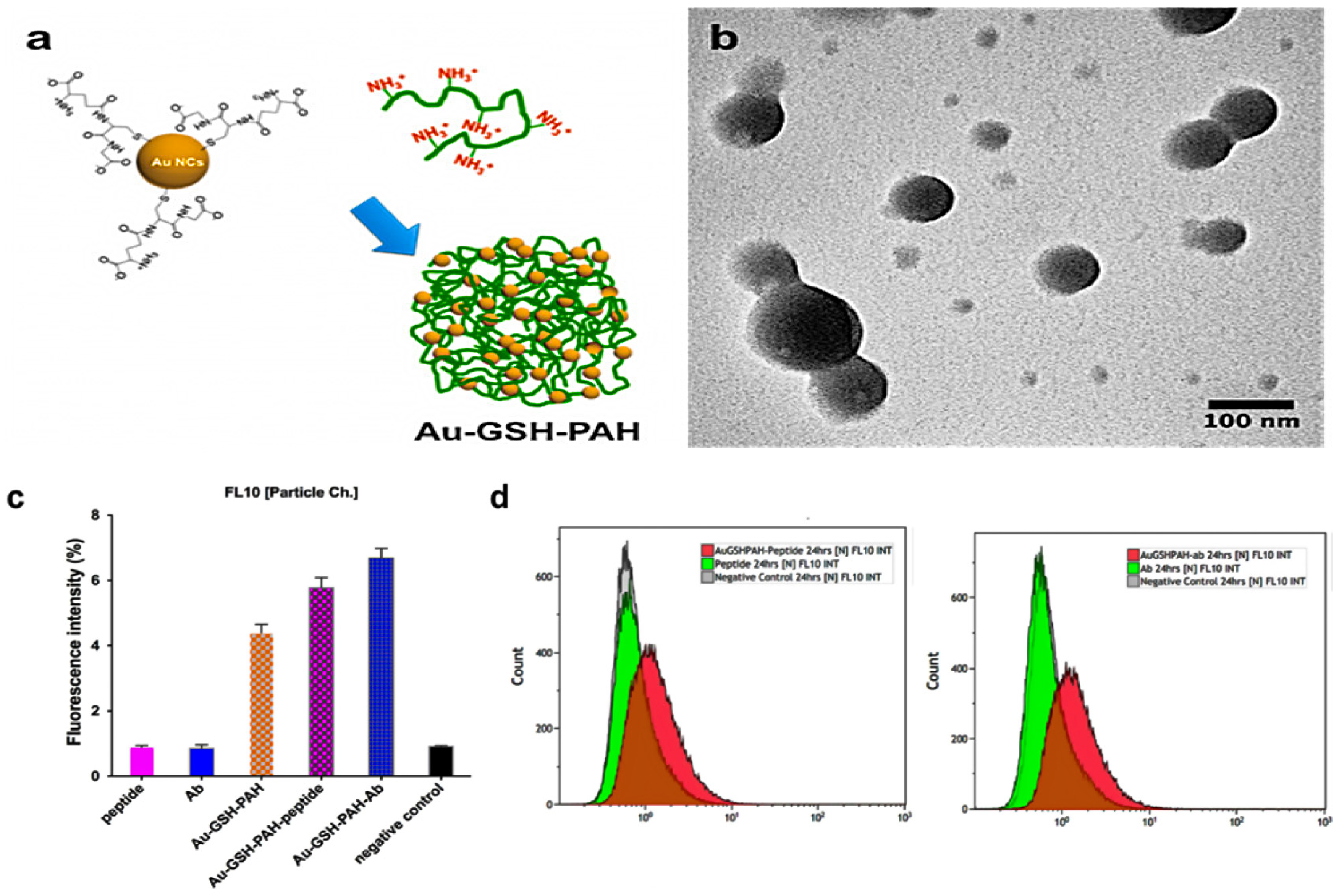
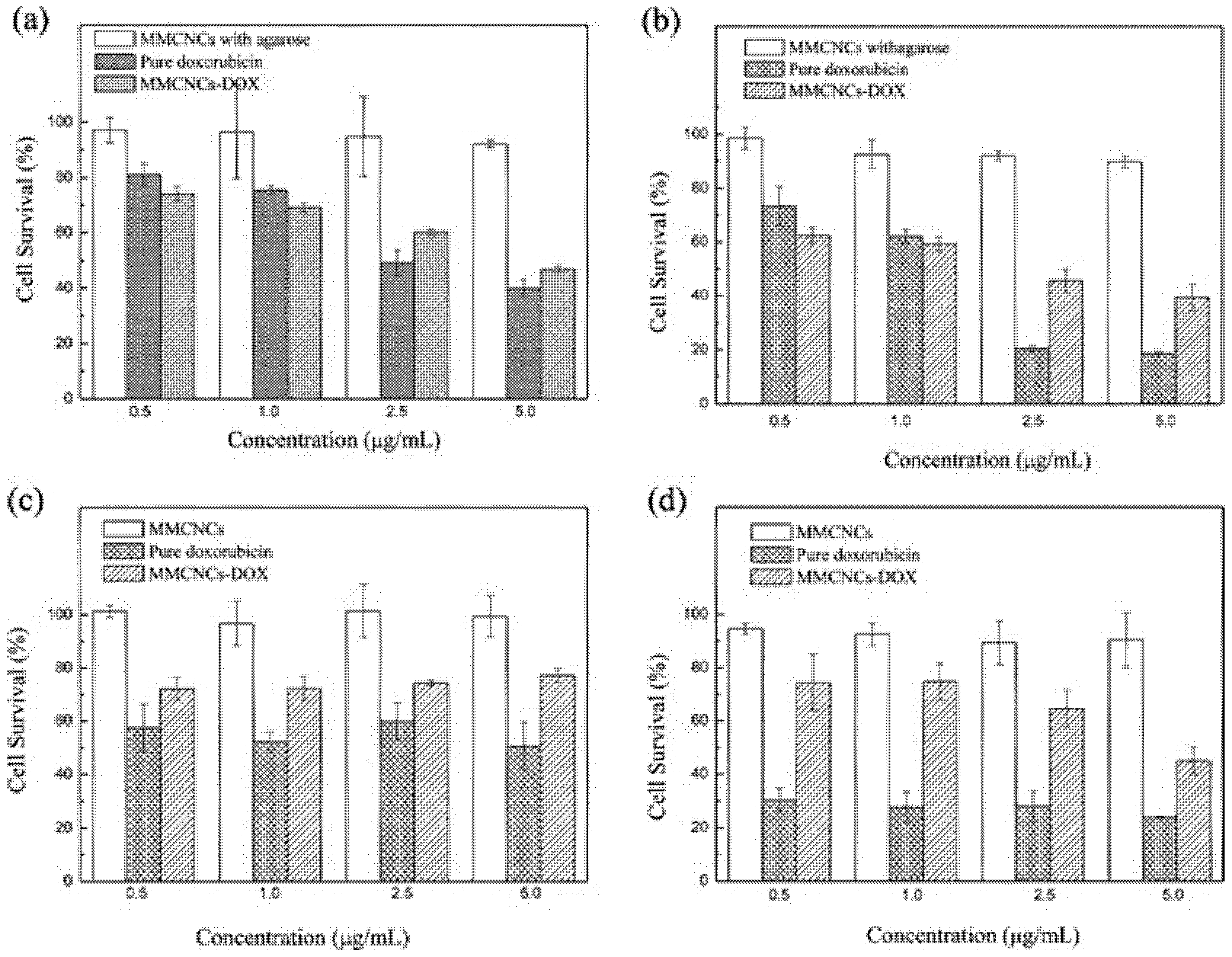
2.3.2. Multicomponent and Multifunctional Nanoclusters
MNCs—Based Multifunctional Nanoclusters
Supramolecular Assembly of Building Blocks into Multifunctional Nanoclusters
3. Summary and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cancer Research UK. Worlwide Cancer Incidence Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer/incidence (accessed on 27 February 2022).
- Misra, R.; Acharya, S.; Sahoo, S.K. Cancer nanotechnology: Application of nanotechnology in cancer therapy. Drug Discov. Today 2010, 15, 842–850. [Google Scholar] [CrossRef] [PubMed]
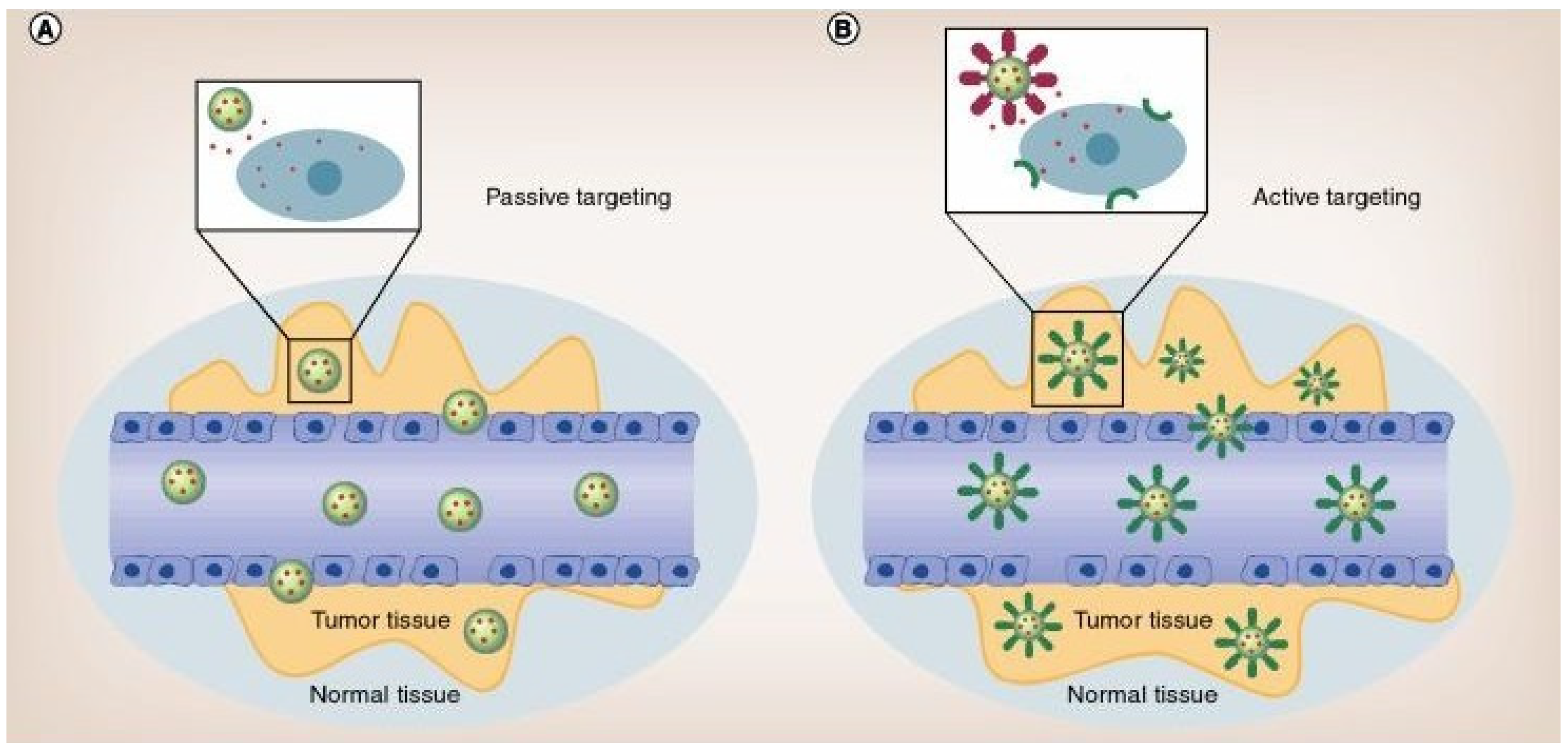
- Danhier, F.; Feron, O.; Preat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Estanqueiro, M.; Amaral, M.H.; Conceicao, J.; Sousa Lobo, J.M. Nanotechnological carriers for cancer chemotherapy: The state of the art. Colloids Surf. B 2015, 126, 631–648. [Google Scholar] [CrossRef] [PubMed]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Koo, O.M.; Rubinstein, I.; Onyuksel, H. Role of nanotechnology in targeted drug delivery and imaging: A concise review. Nanomedicine 2005, 1, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed]
- Doane, T.L.; Burda, C. The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem. Soc. Rev. 2012, 41, 2885–2911. [Google Scholar] [CrossRef] [PubMed]
- Moxley, J.H., III; De Vita, V.T.; Brace, K.; Frei, E., III. Intensive Combination Chemotherapy and X-irradiation in Hodgkin’s Disease. Cancer Res. 1967, 27, 1258–1263. [Google Scholar] [PubMed]
- Sadighian, S.; Rostamizadeh, K.; Hosseini-Monfared, H.; Hamidi, M. Doxorubicin-conjugated core-shell magnetite nanoparticles as dual-targeting carriers for anticancer drug delivery. Colloids Surf. B Biointerfaces 2014, 117, 406–413. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zhang, L.; Chen, H.; Shi, J. MBG/PLGA composite microspheres with prolonged drug release. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Song, X.; Jin, W.; Wu, F.; Zhang, Q.; Zhang, M.; Zhou, N.; Shen, J. Image-guided cancer therapy using aptamer-functionalized cross-linked magnetic-responsive Fe3O4@carbon nanoparticles. Anal. Chim. Acta 2019, 1056, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.; Vicent, M.J. Combination therapy: Opportunities and challenges for polymer-drug conjugates as anticancer nanomedicines. Adv. Drug Deliv. Rev. 2009, 61, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Park, G.Y.; Oh, K.T.; Oh, N.M.; Kwag, D.S.; Youn, Y.S.; Oh, Y.T.; Park, J.W.; Lee, E.S. Multifunctional poly (lactide-co-glycolide) nanoparticles for luminescence/magnetic resonance imaging and photodynamic therapy. Int. J. Pharm. 2012, 434, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.R., Jr. Dendrimer-based nanoparticles for cancer therapy. Hematol. Am. Soc. Hematol. Educ. Program 2009, 2009, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, H.; Liu, A.Y.; Shen, J.J.; Shah, V.; Zhang, C.; Hong, J.; Ding, Y. Gold conjugate-based liposomes with hybrid cluster bomb structure for liver cancer therapy. Biomaterials 2016, 74, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, A.; Baldi, A.; Kumar Narang, R. Nanoparticles as carriers for drug delivery in cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Shi, H.; Ma, G.; Luo, L.; Tang, Z. Ultrasmall Au and Ag Nanoclusters for Biomedical Applications: A Review. Front. Bioeng. Biotechnol. 2020, 8, 1019. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, H.; Li, S.; Zhang, X.; Cheng, Q.; Ding, Y.; Wang, L.H.; Wang, R. Polymeric Nanomedicine with “Lego” Surface Allowing Modular Functionalization and Drug Encapsulation. ACS Appl. Mater. Interfaces 2018, 10, 25090–25098. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Stroh, M.; Zimmer, J.P.; Duda, D.G.; Levchenko, T.S.; Cohen, K.S.; Brown, E.B.; Scadden, D.T.; Torchilin, V.P.; Bawendi, M.G.; Fukumura, D.; et al. Quantum dots spectrally distinguish multiple species within the tumor milieu in vivo. Nat. Med. 2005, 11, 678–682. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.; Wang, L.; Guo, T.; Wu, J.; Wan, J.; Zhou, L.; Li, H.; Li, Z.; Jiang, D.; et al. New Generation Nanomedicines Constructed from Self-Assembling Small-Molecule Prodrugs Alleviate Cancer Drug Toxicity. Cancer Res. 2017, 77, 6963–6974. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ding, J.; Wang, Y.; Cheng, J.; Ji, S.; Zhuang, X.; Chen, X. Sequentially Responsive Shell-Stacked Nanoparticles for Deep Penetration into Solid Tumors. Adv. Mater. 2017, 29, 1701170. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yan, B.; Gao, L.; Dong, C.; Zhong, J.; D’Ortenzio, M.; Nguyen, B.; Seong Lee, S.; Hu, X.; Liang, F. Targeted Delivery of Bleomycin: A Comprehensive Anticancer Review. Curr. Cancer Drug Targets 2016, 16, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Sadat, S.M.A.; Jahan, S.T.; Haddadi, A. Effects of Size and Surface Charge of Polymeric Nanoparticles on In Vitro and In Vivo Applications. J. Biomater. Nanobiotechnol. 2016, 7, 91–108. [Google Scholar] [CrossRef]
- McNeeley, K.M.; Karathanasis, E.; Annapragada, A.V.; Bellamkonda, R.V. Masking and triggered unmasking of targeting ligands on nanocarriers to improve drug delivery to brain tumors. Biomaterials 2009, 30, 3986–3995. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Rammohan, R.; Weissig, V.; Levchenko, T.S. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc. Natl. Acad. Sci. USA 2001, 98, 8786–8791. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Fujimoto, K. A reduction-triggered delivery by a liposomal carrier possessing membrane-permeable ligands and a detachable coating. Colloids Surf. B 2006, 49, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.K.; Patel, A.P. Toxicity of Nanomaterials on the Liver, Kidney, and Spleen. In Biointeractions of Nanomaterials; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Fernandez-Fernandez, A.; Manchanda, R.; Carvajal, D.A.; Lei, T.; Srinivasan, S.; McGoron, A.J. Covalent IR820-PEG-diamine nanoconjugates for theranostic applications in cancer. Int. J. Nanomed. 2014, 9, 4631–4648. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duong, T.; Li, X.; Yang, B.; Schumann, C.; Albarqi, H.A.; Taratula, O.; Taratula, O. Phototheranostic nanoplatform based on a single cyanine dye for image-guided combinatorial phototherapy. Nanomedicine 2017, 13, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Qiu, X.; Tang, X.; Liu, W.; Wu, J.; Hu, Y. Self-assembled PEG-IR-780-C13 micelle as a targeting, safe and highly-effective photothermal agent for in vivo imaging and cancer therapy. Biomaterials 2015, 51, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Palao-Suay, R.; Martin-Saavedra, F.M.; Rosa Aguilar, M.; Escudero-Duch, C.; Martin-Saldana, S.; Parra-Ruiz, F.J.; Rohner, N.A.; Thomas, S.N.; Vilaboa, N.; San Roman, J. Photothermal and photodynamic activity of polymeric nanoparticles based on alpha-tocopheryl succinate-RAFT block copolymers conjugated to IR-780. Acta Biomater. 2017, 57, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Yu, M.; Wang, J.; Tan, F.; Li, N. Smart IR780 Theranostic Nanocarrier for Tumor-Specific Therapy: Hyperthermia-Mediated Bubble-Generating and Folate-Targeted Liposomes. ACS Appl. Mater. Interfaces 2015, 7, 20556–20567. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Song, L.; Chen, S.; Gao, J.; Zhao, P.; Du, J. A superparamagnetic polymersome with extremely high T2 relaxivity for MRI and cancer-targeted drug delivery. Biomaterials 2017, 114, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, H.; Yi, W.; Chen, J.; Liang, B. Acid-triggered core cross-linked nanomicelles for targeted drug delivery and magnetic resonance imaging in liver cancer cells. Int. J. Nanomed. 2013, 8, 3019–3031. [Google Scholar] [CrossRef][Green Version]
- Yang, X.; Grailer, J.J.; Rowland, I.J.; Javadi, A.; Hurley, S.A.; Matson, V.Z.; Steeber, D.A.; Gong, S. Multifunctional stable and pH-responsive polymer vesicles formed by heterofunctional triblock copolymer for targeted anticancer drug delivery and ultrasensitive MR imaging. ACS Nano 2010, 4, 6805–6817. [Google Scholar] [CrossRef]
- Qin, J.; Liu, Q.; Zhang, J.; Chen, J.; Chen, S.; Zhao, Y.; Du, J. Rationally Separating the Corona and Membrane Functions of Polymer Vesicles for Enhanced T(2) MRI and Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 14043–14052. [Google Scholar] [CrossRef] [PubMed]
- Schleich, N.; Sibret, P.; Danhier, P.; Ucakar, B.; Laurent, S.; Muller, R.N.; Jerome, C.; Gallez, B.; Preat, V.; Danhier, F. Dual anticancer drug/superparamagnetic iron oxide-loaded PLGA-based nanoparticles for cancer therapy and magnetic resonance imaging. Int. J. Pharm. 2013, 447, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.H.; Min, H.S.; Li, L.; Tran, T.H.; Lee, Y.K.; Kwon, I.C.; Choi, K.; Kim, K.; Huh, K.M. Cancer cell-specific photoactivity of pheophorbide a-glycol chitosan nanoparticles for photodynamic therapy in tumor-bearing mice. Biomaterials 2013, 34, 6454–6463. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Di, J.; Sun, Y.; Fu, J.; Wei, Z.; Matsui, H.; del C. Alonso, A.; Zhou, S. Biocompatible PEG-Chitosan@Carbon Dots Hybrid Nanogels for Two-Photon Fluorescence Imaging, Near-Infrared Light/pH Dual-Responsive Drug Carrier, and Synergistic Therapy. Adv. Funct. Mater. 2015, 25, 5537–5547. [Google Scholar] [CrossRef]
- Tan, L.; Wan, A.; Li, H. Ag2S quantum dots conjugated chitosan nanospheres toward light-triggered nitric oxide release and near-infrared fluorescence imaging. Langmuir 2013, 29, 15032–15042. [Google Scholar] [CrossRef] [PubMed]
- Avramovic, N.; Mandic, B.; Savic-Radojevic, A.; Simic, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Szczech, M.; Szczepanowicz, K. Polymeric Core-Shell Nanoparticles Prepared by Spontaneous Emulsification Solvent Evaporation and Functionalized by the Layer-by-Layer Method. Nanomaterials 2020, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Bechnak, L.; Khalil, C.; Kurdi, R.E.; Khnayzer, R.S.; Patra, D. Curcumin encapsulated colloidal amphiphilic block co-polymeric nanocapsules: Colloidal nanocapsules enhance photodynamic and anticancer activities of curcumin. Photochem. Photobiol. Sci. 2020, 19, 1088–1098. [Google Scholar] [CrossRef]
- Douglas, D. Pharmaceutical Nanotechnology: A Therapeutic Revolution. Int. J. Pharm. Sci. Dev. Res. 2020, 6, 009–011. [Google Scholar] [CrossRef]
- Zielinska, A.; Carreiro, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Bukchin, A.; Sanchez-Navarro, M.; Carrera, A.; Teixidó, M.; Carcaboso, A.M.; Giralt, E.; Sosnik, A. Amphiphilic Polymeric Nanoparticles Modified with a Retro-Enantio Peptide Shuttle Target the Brain of Mice. Chem. Mater. 2020, 32, 7679–7693. [Google Scholar] [CrossRef]
- Şenel, B.; Öztürk, A.A. New approaches to tumor therapy with siRNA-decorated and chitosan-modified PLGA nanoparticles. Drug Dev. Ind. Pharm. 2019, 45, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Liu, T.I.; Chuang, C.L.; Chen, H.H.; Chiang, W.H.; Chiu, H.C. Alendronate/folic acid-decorated polymeric nanoparticles for hierarchically targetable chemotherapy against bone metastatic breast cancer. J. Mater. Chem. B 2020, 8, 3789–3800. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Gai, W.; Wang, L.; Zhao, J.; Sun, D.; Yang, F.; Jiang, H.; Feng, Y. 5-Boronopicolinic acid-functionalized polymeric nanoparticles for targeting drug delivery and enhanced tumor therapy. Mater. Sci. Eng. C 2021, 119, 111553. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Shen, S.; Xu, C.F.; Li, H.J.; Liu, Y.; Cao, Z.T.; Yang, X.Z.; Xia, J.X.; Wang, J. Tumor Acidity-Sensitive Polymeric Vector for Active Targeted siRNA Delivery. J. Am. Chem. Soc. 2015, 137, 15217–15224. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.-B.; Jang, Y.; Fan, Q.-Q.; Chang, S.-H.; Xing, L.; Cui, P.-F.; He, Y.-J.; Lee, S.; Hwang, S.; Cho, M.-H.; et al. Aerosol delivery of biocompatible dihydroergotamine-loaded PLGA-PSPE polymeric micelles for efficient lung cancer therapy. Polym. Chem. 2017, 8, 1540–1554. [Google Scholar] [CrossRef]
- Zhu, J.J.; Zhang, X.X.; Miao, Y.Q.; He, S.F.; Tian, D.M.; Yao, X.S.; Tang, J.S.; Gan, Y. Delivery of acetylthevetin B, an antitumor cardiac glycoside, using polymeric micelles for enhanced therapeutic efficacy against lung cancer cells. Acta Pharmacol. Sin. 2017, 38, 290–300. [Google Scholar] [CrossRef]
- Ma, Y.; Fan, X.; Li, L. pH-sensitive polymeric micelles formed by doxorubicin conjugated prodrugs for co-delivery of doxorubicin and paclitaxel. Carbohydr. Polym. 2016, 137, 19–29. [Google Scholar] [CrossRef]
- Boateng, F.; Ngwa, W. Delivery of Nanoparticle-Based Radiosensitizers for Radiotherapy Applications. Int. J. Mol. Sci. 2019, 21, 273. [Google Scholar] [CrossRef]
- Kim, S.; Im, S.; Park, E.Y.; Lee, J.; Kim, C.; Kim, T.I.; Kim, W.J. Drug-loaded titanium dioxide nanoparticle coated with tumor targeting polymer as a sonodynamic chemotherapeutic agent for anti-cancer therapy. Nanomedicine 2020, 24, 102110. [Google Scholar] [CrossRef]
- Li, S.; Saw, P.E.; Lin, C.; Nie, Y.; Tao, W.; Farokhzad, O.C.; Zhang, L.; Xu, X. Redox-responsive polyprodrug nanoparticles for targeted siRNA delivery and synergistic liver cancer therapy. Biomaterials 2020, 234, 119760. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Chung, S.-J.; Cho, H.-J.; Kim, D.-D. Phenylboronic Acid-Decorated Chondroitin Sulfate A-Based Theranostic Nanoparticles for Enhanced Tumor Targeting and Penetration. Adv. Funct. Mater. 2015, 25, 3705–3717. [Google Scholar] [CrossRef]
- Sun, W.; Fan, J.; Wang, S.; Kang, Y.; Du, J.; Peng, X. Biodegradable Drug-Loaded Hydroxyapatite Nanotherapeutic Agent for Targeted Drug Release in Tumors. ACS Appl. Mater. Interfaces 2018, 10, 7832–7840. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Park, J.; Willis, K.; Park, J.E.; Lyle, L.T.; Lee, W.; Yeo, Y. Surface modification of polymer nanoparticles with native albumin for enhancing drug delivery to solid tumors. Biomaterials 2018, 180, 206–224. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; He, W.; Yan, S.; Niu, F.; Liu, T.; Ma, B.; Shao, Y.; Yan, Y.; Yang, G.; Lu, W.; et al. Self-Assembled Peptide-Lanthanide Nanoclusters for Safe Tumor Therapy: Overcoming and Utilizing Biological Barriers to Peptide Drug Delivery. ACS Nano 2018, 12, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Heckert, B.; Banerjee, T.; Sulthana, S.; Naz, S.; Alnasser, R.; Thompson, D.; Normand, G.; Grimm, J.; Perez, J.M.; Santra, S. Design and Synthesis of New Sulfur-Containing Hyperbranched Polymer and Theranostic Nanomaterials for Bimodal Imaging and Treatment of Cancer. ACS Macro Lett. 2017, 6, 235–240. [Google Scholar] [CrossRef]
- Jin, C.; Wang, K.; Oppong-Gyebi, A.; Hu, J. Application of Nanotechnology in Cancer Diagnosis and Therapy—A Mini-Review. Int. J. Med. Sci. 2020, 17, 2964–2973. [Google Scholar] [CrossRef]
- Busseron, E.; Ruff, Y.; Moulin, E.; Giuseppone, N. Supramolecular self-assemblies as functional nanomaterials. Nanoscale 2013, 5, 7098–7140. [Google Scholar] [CrossRef]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Park, T.G.; Jeong, J.H.; Kim, S.W. Current status of polymeric gene delivery systems. Adv. Drug Deliv. Rev. 2006, 58, 467–486. [Google Scholar] [CrossRef]
- Dinarvand, R.; Sepehri, N.; Manoochehri, S.; Rouhani, H.; Atyabi, F. Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int. J. Nanomed. 2011, 6, 877–895. [Google Scholar] [CrossRef] [PubMed]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Kim, S.; Yun, G.; Khan, S.; Kim, J.; Murray, J.; Lee, Y.M.; Kim, W.J.; Lee, G.; Kim, S.; Shetty, D.; et al. Cucurbit[6]uril-based polymer nanocapsules as a non-covalent and modular bioimaging platform for multimodal in vivo imaging. Mater. Horiz. 2017, 4, 450–455. [Google Scholar] [CrossRef]
- Park, K.M.; Suh, K.; Jung, H.; Lee, D.W.; Ahn, Y.; Kim, J.; Baek, K.; Kim, K. Cucurbituril-based nanoparticles: A new efficient vehicle for targeted intracellular delivery of hydrophobic drugs. Chem. Commun. 2009, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Lee, D.W.; Sarkar, B.; Jung, H.; Kim, J.; Ko, Y.H.; Lee, K.E.; Jeon, H.; Kim, K. Reduction-sensitive, robust vesicles with a non-covalently modifiable surface as a multifunctional drug-delivery platform. Small 2010, 6, 1430–1441. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, D.; Jung, H.; Lee, J.; Paul, S.; Selvapalam, N.; Yang, Y.; Lim, N.; Park, C.G.; Kim, K. Inside Cover: Facile, Template-Free Synthesis of Stimuli-Responsive Polymer Nanocapsules for Targeted Drug Delivery (Angew. Chem. Int. Ed. 26/2010). Angew. Chem. Int. Ed. Engl. 2010, 49, 4316. [Google Scholar] [CrossRef]
- Huang, H.C.; Barua, S.; Sharma, G.; Dey, S.K.; Rege, K. Inorganic nanoparticles for cancer imaging and therapy. J. Control. Release 2011, 155, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A. Application of gold nanoparticles in biomedical and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Page Faulk, W.; Malcolm Taylor, G. Communication to the editors. Immunochemistry 1971, 8, 1081–1083. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Gold Nanoparticles for Drug Delivery and Cancer Therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Kong, F.Y.; Zhang, J.W.; Li, R.F.; Wang, Z.X.; Wang, W.J.; Wang, W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [PubMed]
- Hauser, E.A. Experiments in Colloid Chemistry; Hauser, E.A., Lynn, J.E., Eds.; McGraw-Hill: New York, NY, USA, 1940. [Google Scholar]
- Herizchi, R.; Abbasi, E.; Milani, M.; Akbarzadeh, A. Current methods for synthesis of gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Leff, D.V.; Brandt, L.; Heath, J.R. Synthesis and Characterization of Hydrophobic, Organically-Soluble Gold Nanocrystals Functionalized with Primary Amines. Langmuir 1996, 12, 4723–4730. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatized gold nanoparticles in a twophase liquid-liquid system. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Shao, Y.; Jin, Y.; Dong, S. Synthesis of gold nanoplates by aspartate reduction of gold chloride. Chem. Commun. 2004, 1104–1105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Black, K.C.; Luehmann, H.; Li, W.; Zhang, Y.; Cai, X.; Wan, D.; Liu, S.Y.; Li, M.; Kim, P.; et al. Comparison study of gold nanohexapods, nanorods, and nanocages for photothermal cancer treatment. ACS Nano 2013, 7, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Vigderman, L.; Khanal, B.P.; Zubarev, E.R. Functional gold nanorods: Synthesis, self-assembly, and sensing applications. Adv. Mater. 2012, 24, 4811–4841, 5014. [Google Scholar] [CrossRef] [PubMed]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-C.; Shen, C.-M.; Xiao, C.-W.; Yang, T.-Z.; Zhang, H.-R.; Li, J.-Q.; Li, H.-L.; Gao, H.-J. Wet chemical synthesis of gold nanoparticles using silver seeds: A shape control from nanorods to hollow spherical nanoparticles. Nanotechnology 2007, 18, 115608. [Google Scholar] [CrossRef]
- Pang, B.; Yang, X.; Xia, Y. Putting gold nanocages to work for optical imaging, controlled release and cancer theranostics. Nanomedicine 2016, 11, 1715–1728. [Google Scholar] [CrossRef]
- Beik, J.; Khateri, M.; Khosravi, Z.; Kamrava, S.K.; Kooranifar, S.; Ghaznavi, H.; Shakeri-Zadeh, A. Gold nanoparticles in combinatorial cancer therapy strategies. Coord. Chem. Rev. 2019, 387, 299–324. [Google Scholar] [CrossRef]
- Lin, L.; Fan, Y.; Gao, F.; Jin, L.; Li, D.; Sun, W.; Li, F.; Qin, P.; Shi, Q.; Shi, X.; et al. UTMD-Promoted Co-Delivery of Gemcitabine and miR-21 Inhibitor by Dendrimer-Entrapped Gold Nanoparticles for Pancreatic Cancer Therapy. Theranostics 2018, 8, 1923–1939. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.S.C.; Rodrigues, C.F.; Moreira, A.F.; Correia, I.J. Strategies to improve the photothermal capacity of gold-based nanomedicines. Acta Biomater. 2020, 116, 105–137. [Google Scholar] [CrossRef] [PubMed]
- Okoampah, E.; Mao, Y.; Yang, S.; Sun, S.; Zhou, C. Gold nanoparticles-biomembrane interactions: From fundamental to simulation. Colloids Surf. B 2020, 196, 111312. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.A.; Pillai, B.; Das, T.K.; Singh, Y.; Maiti, S. Molecular effects of uptake of gold nanoparticles in HeLa cells. Chembiochem Eur. J. Chem. Biol. 2007, 8, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Park, S.; Lee, J.H.; Jeong, Y.Y.; Jon, S. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. J. Am. Chem. Soc. 2007, 129, 7661–7665. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Khan, S.; Imran, M.; Sohail, M.; Shah, S.W.A.; de Matas, M. PEGylation: A promising strategy to overcome challenges to cancer-targeted nanomedicines: A review of challenges to clinical transition and promising resolution. Drug Deliv. Transl. Res. 2019, 9, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Cho, M.; Jeong, J.; Choi, M.; Cho, H.Y.; Han, B.S.; Kim, S.H.; Kim, H.O.; Lim, Y.T.; Chung, B.H.; et al. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol. Appl. Pharmacol. 2009, 236, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef] [PubMed]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. How toxic are gold nanoparticles? The state-of-the-art. Nano Res. 2015, 8, 1771–1799. [Google Scholar] [CrossRef]
- Lopez-Chaves, C.; Soto-Alvaredo, J.; Montes-Bayon, M.; Bettmer, J.; Llopis, J.; Sanchez-Gonzalez, C. Gold nanoparticles: Distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomedicine 2018, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-P.; Ma, B.-Y.; Wei, X.-W.; Qian, Z.-Y. The in vitro and in vivo toxicity of gold nanoparticles. Chin. Chem. Lett. 2017, 28, 691–702. [Google Scholar] [CrossRef]
- Murphy, C.J.; Gole, A.M.; Stone, J.W.; Sisco, P.N.; Alkilany, A.M.; Goldsmith, E.C.; Baxter, S.C. Gold nanoparticles in biology: Beyond toxicity to cellular imaging. Acc. Chem. Res. 2008, 41, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Salmaso, S.; Caliceti, P.; Amendola, V.; Meneghetti, M.; Magnusson, J.P.; Pasparakis, G.; Alexander, C. Cell up-take control of gold nanoparticles functionalized with a thermoresponsive polymer. J. Mater. Chem. 2009, 19, 1608–1615. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, B.; Rozynek, Z.; Xie, Z.; Fossum, J.O.; Yu, X.; Raaen, S. Minute synthesis of extremely stable gold nanoparticles. Nanotechnology 2009, 20, 505606. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.C.; Giljohann, D.A.; Daniel, W.L.; Zheng, D.; Prigodich, A.E.; Mirkin, C.A. Scavenger receptors mediate cellular uptake of polyvalent oligonucleotide-functionalized gold nanoparticles. Bioconjug. Chem. 2010, 21, 2250–2256. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Babiuch, K.; Lu, H.; Dag, A.; Gottschaldt, M.; Stenzel, M.H. Fructose-coated nanoparticles: A promising drug nanocarrier for triple-negative breast cancer therapy. Chem. Commun. 2014, 50, 15928–15931. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, D.; Zhang, L.; Zhai, J.; Wang, E. One-step synthesis of folic acid protected gold nanoparticles and their receptor-mediated intracellular uptake. Chemistry 2009, 15, 9868–9873. [Google Scholar] [CrossRef] [PubMed]
- Melancon, M.P.; Lu, W.; Yang, Z.; Zhang, R.; Cheng, Z.; Elliot, A.M.; Stafford, J.; Olson, T.; Zhang, J.Z.; Li, C. In vitro and in vivo targeting of hollow gold nanoshells directed at epidermal growth factor receptor for photothermal ablation therapy. Mol. Cancer Ther. 2008, 7, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Gibson, J.D.; Khanal, B.P.; Zubarev, E.R. Paclitaxel-functionalized gold nanoparticles. J. Am. Chem. Soc. 2007, 129, 11653–11661. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Shah, N.; Visaria, R.; Paciotti, G.F.; Bischof, J.C. Biodistribution of TNF-alpha-coated gold nanoparticles in an in vivo model system. Nanomedicine 2009, 4, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Kim, H.; Yu, J.; Yu, S.H.; Ban, S.; Oh, S.; Jeong, D.; Im, J.; Baek, M.J.; Kim, T.H. Doxorubicin-loaded oligonucleotide conjugated gold nanoparticles: A promising in vivo drug delivery system for colorectal cancer therapy. Eur. J. Med. Chem. 2017, 142, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Tsai, C.Y.; Huang, P.Y.; Chang, M.Y.; Cheng, P.C.; Chou, C.H.; Chen, D.H.; Wang, C.R.; Shiau, A.L.; Wu, C.L. Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model. Mol. Pharm. 2007, 4, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Xuan, M.; Shao, J.; Dai, L.; Li, J.; He, Q. Macrophage Cell Membrane Camouflaged Au Nanoshells for in Vivo Prolonged Circulation Life and Enhanced Cancer Photothermal Therapy. ACS Appl. Mater. Interfaces 2016, 8, 9610–9618. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; You, Q.; Yang, L.; Li, P.; Lu, Q.; Wang, S.; Tan, F.; Ji, Y.; Li, N. Rodlike MSN@Au Nanohybrid-Modified Supermolecular Photosensitizer for NIRF/MSOT/CT/MR Quadmodal Imaging-Guided Photothermal/Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 6777–6788. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Clare, S.E.; Halas, N.J. Nanoshell-enabled photothermal cancer therapy: Impending clinical impact. Acc. Chem. Res. 2008, 41, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, X. Gold nanoparticles for photoacoustic imaging. Nanomedicine 2015, 10, 299–320. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Han, Y.; Gao, S.; Yan, H.; Cao, L.; Li, Z.; Liang, X.J.; Zhang, J. Ultrasmall gold nanoparticles in cancer diagnosis and therapy. Theranostics 2020, 10, 4944–4957. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: Applications in oral cancer. Nano Lett. 2005, 5, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, K.; Follen, M.; Aaron, J.; Pavlova, I.; Malpica, A.; Lotan, R.; Richards-Kortum, R. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003, 63, 1999–2004. [Google Scholar] [PubMed]
- Dixit, V.; Van den Bossche, J.; Sherman, D.M.; Thompson, D.H.; Andres, R.P. Synthesis and grafting of thioctic acid-PEG-folate conjugates onto Au nanoparticles for selective targeting of folate receptor-positive tumor cells. Bioconjug. Chem. 2006, 17, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S. Theranostics: Are we there yet? Mol. Pharm. 2013, 10, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Pitsillides, C.M.; Joe, E.K.; Wei, X.; Anderson, R.R.; Lin, C.P. Selective Cell Targeting with Light-Absorbing Microparticles and Nanoparticles. Biophys. J. 2003, 84, 4023–4032. [Google Scholar] [CrossRef]
- Kang, S.; Bhang, S.H.; Hwang, S.; Yoon, J.K.; Song, J.; Jang, H.K.; Kim, S.; Kim, B.S. Mesenchymal Stem Cells Aggregate and Deliver Gold Nanoparticles to Tumors for Photothermal Therapy. ACS Nano 2015, 9, 9678–9690. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, N.; Yu, W.; Xu, H.; Li, X.; Li, M.; Peng, C.; Wang, Q.; Zhu, M.; Chen, Z. In situ growth of Au nanoparticles on natural melanin as biocompatible and multifunctional nanoagent for efficient tumor theranostics. J. Mater. Chem. B 2019, 7, 133–142. [Google Scholar] [CrossRef]
- Guo, J.; Rahme, K.; He, Y.; Li, L.L.; Holmes, J.D.; O’Driscoll, C.M. Gold nanoparticles enlighten the future of cancer theranostics. Int. J. Nanomed. 2017, 12, 6131–6152. [Google Scholar] [CrossRef]
- Huang, P.; Lin, J.; Li, W.; Rong, P.; Wang, Z.; Wang, S.; Wang, X.; Sun, X.; Aronova, M.; Niu, G.; et al. Biodegradable gold nanovesicles with an ultrastrong plasmonic coupling effect for photoacoustic imaging and photothermal therapy. Angew. Chem. Int. Ed. Engl. 2013, 52, 13958–13964. [Google Scholar] [CrossRef] [PubMed]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in Cancer Treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef] [PubMed]
- Kumthekar, P.; Rademaker, A.; Ko, C.; Dixit, K.; Schwartz, M.A.; Sonabend, A.M.; Sharp, L.; Lukas, R.V.; Stupp, R.; Horbinski, C.; et al. A phase 0 first-in-human study using NU-0129: A gold base spherical nucleic acid (SNA) nanoconjugate targeting BCL2L12 in recurrent glioblastoma patients. J. Clin. Oncol. 2019, 37, 3012. [Google Scholar] [CrossRef]
- Barillo, D.J.; Marx, D.E. Silver in medicine: A brief history BC 335 to present. Burns 2014, 40 (Suppl. S1), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Li, Q.; Wang, M.; Ouyang, J.; Li, X.; Xing, M.M. Nanosilver particles in medical applications: Synthesis, performance, and toxicity. Int. J. Nanomed. 2014, 9, 2399–2407. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Syafiuddin, A.; Salmiati; Salim, M.R.; Beng Hong Kueh, A.; Hadibarata, T.; Nur, H. A Review of Silver Nanoparticles: Research Trends, Global Consumption, Synthesis, Properties, and Future Challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Goebl, J.; Lu, Z.; Yin, Y. A systematic study of the synthesis of silver nanoplates: Is citrate a “magic” reagent? J. Am. Chem. Soc. 2011, 133, 18931–18939. [Google Scholar] [CrossRef] [PubMed]
- Roldán, M.V.; Pellegri, N.; de Sanctis, O. Electrochemical Method for Ag-PEG Nanoparticles Synthesis. J. Nanopar. 2013, 2013, 524150. [Google Scholar] [CrossRef]
- Shirtcliffe, N.; Nickel, U.; Schneider, S. Reproducible Preparation of Silver Sols with Small Particle Size Using Borohydride Reduction: For Use as Nuclei for Preparation of Larger Particles. J. Colloid Interface Sci. 1999, 211, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Pinto, V.V.; Ferreira, M.J.; Silva, R.; Santos, H.A.; Silva, F.; Pereira, C.M. Long time effect on the stability of silver nanoparticles in aqueous medium: Effect of the synthesis and storage conditions. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 19–25. [Google Scholar] [CrossRef]
- Dong, X.; Ji, X.; Wu, H.; Zhao, L.; Li, J.; Yang, W. Shape Control of Silver Nanoparticles by Stepwise Citrate Reduction. J. Phys. Chem. C 2009, 113, 6573–6576. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, H.; Huang, W.; Zhou, Y.; Yan, D. Facile preparation and characterization of highly antimicrobial colloid Ag or Au nanoparticles. J. Colloid Interface Sci. 2008, 325, 371–376. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Tan, J.J.; Liew, K.Y.; Liu, H. Synthesis of size controlled Ag nanoparticles. J. Mol. Catal. A Chem. 2004, 221, 121–126. [Google Scholar] [CrossRef]
- Chakraborty, B.; Pal, R.; Ali, M.; Singh, L.M.; Shahidur Rahman, D.; Kumar Ghosh, S.; Sengupta, M. Immunomodulatory properties of silver nanoparticles contribute to anticancer strategy for murine fibrosarcoma. Cell. Mol. Immunol. 2016, 13, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Asanithi, P.; Chaiyakun, S.; Limsuwan, P. Growth of Silver Nanoparticles by DC Magnetron Sputtering. J. Nanomater. 2012, 2012, 963609. [Google Scholar] [CrossRef]
- Tien, D.-C.; Tseng, K.-H.; Liao, C.-Y.; Huang, J.-C.; Tsung, T.-T. Discovery of ionic silver in silver nanoparticle suspension fabricated by arc discharge method. J. Alloys Compd. 2008, 463, 408–411. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Mukherjee, S.; Barui, A.K.; Ganguly, A.; Sreedhar, B.; Patra, C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C 2015, 53, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; He, D.; Qian, Y.; Guan, B.; Gao, S.; Cui, Y.; Yokoyama, K.; Wang, L. Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int. J. Mol. Sci. 2012, 13, 466–476. [Google Scholar] [CrossRef]
- Mourato, A.; Gadanho, M.; Lino, A.R.; Tenreiro, R. Biosynthesis of crystalline silver and gold nanoparticles by extremophilic yeasts. Bioinorg. Chem. Appl. 2011, 2011, 546074. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kalishwaralal, K.; Vaidyanathan, R.; Venkataraman, D.; Pandian, S.R.; Muniyandi, J.; Hariharan, N.; Eom, S.H. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf. B 2009, 74, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef]
- Lengke, M.F.; Fleet, M.E.; Southam, G. Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver(I) nitrate complex. Langmuir 2007, 23, 2694–2699. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, K.; Suresh Babu, R.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. B 2008, 65, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Jeyaraj, M.; Kim, J.H. Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. Biomed. Res. Int. 2013, 2013, 535796. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Zhu, L.; Huang, Z.; Zhou, H.; Ge, Y.; Ma, W.; Wu, J.; Zhang, X.; Zhou, X.; Zhang, Y.; et al. Anti-leukemia activity of PVP-coated silver nanoparticles via generation of reactive oxygen species and release of silver ions. Biomaterials 2013, 34, 7884–7894. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.; Sethu, S.; Lim, H.K.; Balaji, G.; Valiyaveettil, S.; Hande, M.P. Differential regulation of intracellular factors mediating cell cycle, DNA repair and inflammation following exposure to silver nanoparticles in human cells. Genome Integr. 2012, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef]
- Zuberek, M.; Wojciechowska, D.; Krzyzanowski, D.; Meczynska-Wielgosz, S.; Kruszewski, M.; Grzelak, A. Glucose availability determines silver nanoparticles toxicity in HepG2. J. Nanobiotechnol. 2015, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Park, J.H.; Han, J.W.; Kim, J.H. Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: Targeting p53 for anticancer therapy. Int. J. Nanomed. 2015, 10, 4203–4222. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.-T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 033001. [Google Scholar] [CrossRef]
- Locatelli, E.; Broggi, F.; Ponti, J.; Marmorato, P.; Franchini, F.; Lena, S.; Franchini, M.C. Lipophilic silver nanoparticles and their polymeric entrapment into targeted-PEG-based micelles for the treatment of glioblastoma. Adv. Healthc. Mater. 2012, 1, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S.; B, V.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 2014, 4, 316–335. [Google Scholar] [CrossRef] [PubMed]
- Farrag, N.S.; El-Sabagh, H.A.; Al-Mahallawi, A.M.; Amin, A.M.; AbdEl-Bary, A.; Mamdouh, W. Comparative study on radiolabeling and biodistribution of core-shell silver/polymeric nanoparticles-based theranostics for tumor targeting. Int. J. Pharm. 2017, 529, 123–133. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Leporatti, S. Silver Nanoparticles: Synthetic Routes, In Vitro Toxicity and Theranostic Applications for Cancer Disease. Nanomaterials 2018, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef]
- Franco-Molina, M.A.; Mendoza-Gamboa, E.; Sierra-Rivera, C.A.; Gomez-Flores, R.A.; Zapata-Benavides, P.; Castillo-Tello, P.; Alcocer-Gonzalez, J.M.; Miranda-Hernandez, D.F.; Tamez-Guerra, R.S.; Rodriguez-Padilla, C. Antitumor activity of colloidal silver on MCF-7 human breast cancer cells. J. Exp. Clin. Cancer Res. 2010, 29, 148. [Google Scholar] [CrossRef]
- Appadurai, P.; Rathinasamy, K. Plumbagin-silver nanoparticle formulations enhance the cellular uptake of plumbagin and its antiproliferative activities. IET Nanobiotechnol. 2015, 9, 264–272. [Google Scholar] [CrossRef]
- Mahmood, M.; Casciano, D.A.; Mocan, T.; Iancu, C.; Xu, Y.; Mocan, L.; Iancu, D.T.; Dervishi, E.; Li, Z.; Abdalmuhsen, M.; et al. Cytotoxicity and biological effects of functional nanomaterials delivered to various cell lines. J. Appl. Toxicol. JAT 2010, 30, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Sadat Shandiz, S.A.; Shafiee Ardestani, M.; Shahbazzadeh, D.; Assadi, A.; Ahangari Cohan, R.; Asgary, V.; Salehi, S. Novel imatinib-loaded silver nanoparticles for enhanced apoptosis of human breast cancer MCF-7 cells. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wahajuddin; Arora, S. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef] [PubMed]
- Demirer, G.S.; Okur, A.C.; Kizilel, S. Synthesis and design of biologically inspired biocompatible iron oxide nanoparticles for biomedical applications. J. Mater. Chem. B 2015, 3, 7831–7849. [Google Scholar] [CrossRef] [PubMed]
- Bayda, S.; Hadla, M.; Palazzolo, S.; Riello, P.; Corona, G.; Toffoli, G.; Rizzolio, F. Inorganic Nanoparticles for Cancer Therapy: A Transition from Lab to Clinic. Curr. Med. Chem. 2018, 25, 4269–4303. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.; Hyeon, T. Chemical design of biocompatible iron oxide nanoparticles for medical applications. Small 2013, 9, 1450–1466. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- LaMer, V.K.; Dinegar, R.H. Theory, Production and Mechanism of Formation of Monodispersed Hydrosols. J. Am. Chem. Soc. 2002, 72, 4847–4854. [Google Scholar] [CrossRef]
- Inouye, K.; Endo, R.; Otsuka, Y.; Miyashiro, K.; Kaneko, K.; Ishikawa, T. Oxygenation of ferrous ions in reversed micelle and reversed microemulsion. J. Phys. Chem. 2002, 86, 1465–1469. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Bae, C.J.; Park, J.G.; Noh, H.J.; Park, J.H.; Hyeon, T. Large-Scale Synthesis of Uniform and Crystalline Magnetite Nanoparticles Using Reverse Micelles as Nanoreactors under Reflux Conditions. Adv. Funct. Mater. 2005, 15, 503–509. [Google Scholar] [CrossRef]
- Zhi, J.; Wang, Y.; Lu, Y.; Ma, J.; Luo, G. In situ preparation of magnetic chitosan/Fe3O4 composite nanoparticles in tiny pools of water-in-oil microemulsion. React. Funct. Polym. 2006, 66, 1552–1558. [Google Scholar] [CrossRef]
- Chen, D.; Xu, R. Hydrothermal synthesis and characterization of nanocrystalline Fe3O4 powders. MRS Bull. 1998, 33, 1015–1021. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Simchi, A.; Imani, M.; Shokrgozar, M.A.; Milani, A.S.; Hafeli, U.O.; Stroeve, P. A new approach for the in vitro identification of the cytotoxicity of superparamagnetic iron oxide nanoparticles. Colloids Surf. B Biointerfaces 2010, 75, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Simchi, A.; Imani, M.; Milani, A.S.; Stroeve, P. An in vitro study of bare and poly(ethylene glycol)-co-fumarate-coated superparamagnetic iron oxide nanoparticles: A new toxicity identification procedure. Nanotechnology 2009, 20, 225104. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Jenkins, G.J.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef] [PubMed]
- Stroh, A.; Zimmer, C.; Gutzeit, C.; Jakstadt, M.; Marschinke, F.; Jung, T.; Pilgrimm, H.; Grune, T. Iron oxide particles for molecular magnetic resonance imaging cause transient oxidative stress in rat macrophages. Free Radic. Biol. Med. 2004, 36, 976–984. [Google Scholar] [CrossRef]
- Sadeghiani, N.; Barbosa, L.S.; Silva, L.P.; Azevedo, R.B.; Morais, P.C.; Lacava, Z.G.M. Genotoxicity and inflammatory investigation in mice treated with magnetite nanoparticles surface coated with polyaspartic acid. J. Magn. Magn. Mater. 2005, 289, 466–468. [Google Scholar] [CrossRef]
- Hafeli, U.O.; Riffle, J.S.; Harris-Shekhawat, L.; Carmichael-Baranauskas, A.; Mark, F.; Dailey, J.P.; Bardenstein, D. Cell uptake and in vitro toxicity of magnetic nanoparticles suitable for drug delivery. Mol. Pharm. 2009, 6, 1417–1428. [Google Scholar] [CrossRef]
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, W.; Kumbhar, A.; Wiemann, J.; Fang, J.; Carpenter, E.E.; O’Connor, C.J. Gold-Coated Iron (Fe@Au) Nanoparticles: Synthesis, Characterization, and Magnetic Field-Induced Self-Assembly. J. Solid State Chem. 2001, 159, 26–31. [Google Scholar] [CrossRef]
- Caro, C.; Gamez, F.; Quaresma, P.; Paez-Munoz, J.M.; Dominguez, A.; Pearson, J.R.; Pernia Leal, M.; Beltran, A.M.; Fernandez-Afonso, Y.; De la Fuente, J.M.; et al. Fe3O4-Au Core-Shell Nanoparticles as a Multimodal Platform for In Vivo Imaging and Focused Photothermal Therapy. Pharmaceutics 2021, 13, 416. [Google Scholar] [CrossRef]
- Schellenberger, E.A.; Weissleder, R.; Josephson, L. Optimal modification of annexin V with fluorescent dyes. Chembiochem Eur. J. Chem. Biol. 2004, 5, 271–274. [Google Scholar] [CrossRef] [PubMed]
- von Maltzahn, G.; Ren, Y.; Park, J.H.; Min, D.H.; Kotamraju, V.R.; Jayakumar, J.; Fogal, V.; Sailor, M.J.; Ruoslahti, E.; Bhatia, S.N. In vivo tumor cell targeting with “click” nanoparticles. Bioconjug. Chem. 2008, 19, 1570–1578. [Google Scholar] [CrossRef]
- Hein, C.D.; Liu, X.M.; Wang, D. Click chemistry, a powerful tool for pharmaceutical sciences. Pharm. Res. 2008, 25, 2216–2230. [Google Scholar] [CrossRef] [PubMed]
- Steitz, B.; Hofmann, H.; Kamau, S.W.; Hassa, P.O.; Hottiger, M.O.; von Rechenberg, B.; Hofmann-Amtenbrink, M.; Petri-Fink, A. Characterization of PEI-coated superparamagnetic iron oxide nanoparticles for transfection: Size distribution, colloidal properties and DNA interaction. J. Magn. Magn. Mater. 2007, 311, 300–305. [Google Scholar] [CrossRef]
- McBain, S.C.; Yiu, H.H.P.; El Haj, A.; Dobson, J. Polyethyleneimine functionalized iron oxide nanoparticles as agents for DNA delivery and transfection. J. Mater. Chem. 2007, 17, 2561. [Google Scholar] [CrossRef]
- Gunn, J.; Wallen, H.; Veiseh, O.; Sun, C.; Fang, C.; Cao, J.; Yee, C.; Zhang, M. A multimodal targeting nanoparticle for selectively labeling T cells. Small 2008, 4, 712–715. [Google Scholar] [CrossRef]
- Lübbe, A.S.; Bergemann, C.; Huhnt, W.; Fricke, T.; Riess, H.; Brock, J.W.; Huhn, D. Preclinical experiences with magnetic drug targeting: Tolerance and efficacy. Cancer Res. 1996, 56, 4694–4701. [Google Scholar] [PubMed]
- Johannsen, M.; Thiesen, B.; Wust, P.; Jordan, A. Magnetic nanoparticle hyperthermia for prostate cancer. Int. J. Hyperth. 2010, 26, 790–795. [Google Scholar] [CrossRef]
- Ulbrich, K.; Hola, K.; Subr, V.; Bakandritsos, A.; Tucek, J.; Zboril, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neuro-Oncol. 2011, 103, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, M.; Zhu, Y.; Mao, C. Metallic Nanoclusters for Cancer Imaging and Therapy. Curr. Med. Chem. 2018, 25, 1379–1396. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, M.; Ren, J.; Qu, X. Metal nanoclusters: Novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 2015, 44, 8636–8663. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Gonzalez, B.; Monguzzi, A.; Caputo, M.; Villa, C.; Prato, M.; Santambrogio, C.; Torrente, Y.; Meinardi, F.; Brovelli, S. Metal Nanoclusters with Synergistically Engineered Optical and Buffering Activity of Intracellular Reactive Oxygen Species by Compositional and Supramolecular Design. Sci. Rep. 2017, 7, 5976. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Wan, A. Luminescent gold nanoclusters for in vivo tumor imaging. Analyst 2020, 145, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Liang, M.; Sun, J.; Liu, X.; Wang, F. Lighting Up Fluorescent Silver Clusters via Target-Catalyzed Hairpin Assembly for Amplified Biosensing. Langmuir 2018, 34, 14851–14857. [Google Scholar] [CrossRef] [PubMed]
- Romeo, M.V.; López-Martínez, E.; Berganza-Granda, J.; Goñi-de-Cerio, F.; Cortajarena, A.L. Biomarker sensing platforms based on fluorescent metal nanoclusters. Nanoscale Adv. 2021, 3, 1331–1341. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, W. Sub-nanometre sized metal clusters: From synthetic challenges to the unique property discoveries. Chem. Soc. Rev. 2012, 41, 3594–3623. [Google Scholar] [CrossRef]
- Shang, L.; Dong, S.; Nienhaus, G.U. Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today 2011, 6, 401–418. [Google Scholar] [CrossRef]
- Higaki, T.; Zeng, C.; Chen, Y.; Hussain, E.; Jin, R. Controlling the crystalline phases (FCC, HCP and BCC) of thiolate-protected gold nanoclusters by ligand-based strategies. CrystEngComm 2016, 18, 6979–6986. [Google Scholar] [CrossRef]
- Adhikari, B.; Banerjee, A. Facile Synthesis of Water-Soluble Fluorescent Silver Nanoclusters and HgIISensing. Chem. Mater. 2010, 22, 4364–4371. [Google Scholar] [CrossRef]
- Negishi, Y.; Nobusada, K.; Tsukuda, T. Glutathione-protected gold clusters revisited: Bridging the gap between gold(I)-thiolate complexes and thiolate-protected gold nanocrystals. J. Am. Chem. Soc. 2005, 127, 5261–5270. [Google Scholar] [CrossRef]
- Han, B.; Wang, E. DNA-templated fluorescent silver nanoclusters. Anal. Bioanal. Chem. 2012, 402, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. DNA-stabilized, fluorescent, metal nanoclusters for biosensor development. TrAC Trends Anal. Chem. 2014, 58, 99–111. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Irudayaraj, J. Nuclear targeting dynamics of gold nanoclusters for enhanced therapy of HER2+ breast cancer. ACS Nano 2011, 5, 9718–9725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Chen, J.; Luo, Z.; Wu, D.; Shen, X.; Song, S.S.; Sun, Y.M.; Liu, P.X.; Zhao, J.; Huo, S.; et al. Enhanced tumor accumulation of sub-2 nm gold nanoclusters for cancer radiation therapy. Adv. Healthc. Mater. 2014, 3, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Geng, J.; Ong, E.Y.; Chellappan, V.; Tan, Y.N. Bovine Serum Albulmin Protein-Templated Silver Nanocluster (BSA-Ag13): An Effective Singlet Oxygen Generator for Photodynamic Cancer Therapy. Adv. Healthc. Mater. 2016, 5, 2528–2535. [Google Scholar] [CrossRef]
- Goswami, N.; Luo, Z.; Yuan, X.; Leong, D.T.; Xie, J. Engineering gold-based radiosensitizers for cancer radiotherapy. Mater. Horiz. 2017, 4, 817–831. [Google Scholar] [CrossRef]
- Song, X.R.; Goswami, N.; Yang, H.H.; Xie, J. Functionalization of metal nanoclusters for biomedical applications. Analyst 2016, 141, 3126–3140. [Google Scholar] [CrossRef]
- Zhang, X.D.; Luo, Z.; Chen, J.; Shen, X.; Song, S.; Sun, Y.; Fan, S.; Fan, F.; Leong, D.T.; Xie, J. Ultrasmall Au10-12(SG)10-12 nanomolecules for high tumor specificity and cancer radiotherapy. Adv. Mater. 2014, 26, 4565–4568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Luo, Z.; Chen, J.; Song, S.; Yuan, X.; Shen, X.; Wang, H.; Sun, Y.; Gao, K.; Zhang, L.; et al. Ultrasmall glutathione-protected gold nanoclusters as next generation radiotherapy sensitizers with high tumor uptake and high renal clearance. Sci. Rep. 2015, 5, 8669. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Rius, A.; Ivask, A.; Das, S.; Penya-Auladell, N.; Fabregas, L.; Fletcher, N.L.; Houston, Z.H.; Thurecht, K.J.; Voelcker, N.H. Gold Nanocluster-Mediated Cellular Death under Electromagnetic Radiation. ACS Appl. Mater. Interfaces 2017, 9, 41159–41167. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, M.; Park, H.S.; Shin, U.S.; Gong, M.S.; Kim, H.W. Size-dependent cellular toxicity of silver nanoparticles. J. Biomed. Mater. Res. A 2012, 100, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Mironava, T.; Hadjiargyrou, M.; Simon, M.; Jurukovski, V.; Rafailovich, M.H. Gold nanoparticles cellular toxicity and recovery: Effect of size, concentration and exposure time. Nanotoxicology 2010, 4, 120–137. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, T.D.; Pearson, J.R.; Leal, M.P.; Torres, M.J.; Blanca, M.; Mayorga, C.; Le Guevel, X. Intracellular accumulation and immunological properties of fluorescent gold nanoclusters in human dendritic cells. Biomaterials 2015, 43, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ivleva, E.A.; Obraztsova, E.A.; Pavlova, E.R.; Morozova, O.V.; Ivanov, D.G.; Kononikhin, A.S.; Klinov, D.V. Albumin-stabilized fluorescent metal nanoclusters: Fabrication, physico-chemical properties and cytotoxicity. Mater. Des. 2020, 192, 108771. [Google Scholar] [CrossRef]
- Cui, L.; Li, C.; Chen, B.; Huang, H.; Xia, Q.; Li, X.; Shen, Z.; Ge, Z.; Wang, Y. Surface functionalized red fluorescent dual-metallic Au/Ag nanoclusters for endoplasmic reticulum imaging. Mikrochim. Acta 2020, 187, 606. [Google Scholar] [CrossRef] [PubMed]
- Zohrabi, T.; Hosseinkhani, S. Ternary Nanocomplexes of Metallic Nanoclusters and Recombinant Peptides for Fluorescence Imaging and Enhanced Gene Delivery. Mol. Biotechnol. 2020, 62, 495–507. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, M.; Zhao, Z.; Zhu, L.; Yuan, S. A multicomponent-based microemulsion for boosting ovarian cancer therapy through dual modification with transferrin and SA-R6H4. Drug Deliv. Transl. Res. 2021, 11, 1969–1982. [Google Scholar] [CrossRef]
- Key, J.; Park, K. Multicomponent, Tumor-Homing Chitosan Nanoparticles for Cancer Imaging and Therapy. Int. J. Mol. Sci. 2017, 18, 594. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Monteiro, P.F.; Gurnani, P.; Stolnik, S.; Ungaro, F.; Quaglia, F.; Clarke, P.; Grabowska, A.; Kavallaris, M.; Alexander, C. Multi-component bioresponsive nanoparticles for synchronous delivery of docetaxel and TUBB3 siRNA to lung cancer cells. Nanoscale 2021, 13, 11414–11426. [Google Scholar] [CrossRef] [PubMed]
- Kutsevol, N.; Kuziv, Y.; Bezugla, T.; Virych, P.; Marynin, A.; Borikun, T.; Lukianova, N.; Virych, P.; Chekhun, V. Application of new multicomponent nanosystems for overcoming doxorubicin resistance in breast cancer therapy. Appl. Nanosci. 2021, 12, 427–437. [Google Scholar] [CrossRef]
- Peiris, P.M.; Toy, R.; Abramowski, A.; Vicente, P.; Tucci, S.; Bauer, L.; Mayer, A.; Tam, M.; Doolittle, E.; Pansky, J.; et al. Treatment of cancer micrometastasis using a multicomponent chain-like nanoparticle. J. Control. Release 2014, 173, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Bothun, G.D.; Lelis, A.; Chen, Y.; Scully, K.; Anderson, L.E.; Stoner, M.A. Multicomponent folate-targeted magnetoliposomes: Design, characterization, and cellular uptake. Nanomedicine 2011, 7, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Matai, I.; Sachdev, A.; Gopinath, P. Multicomponent 5-fluorouracil loaded PAMAM stabilized-silver nanocomposites synergistically induce apoptosis in human cancer cells. Biomater. Sci. 2015, 3, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, B.; Zhao, R.; Zhang, Q.; Kong, X. Multifunctional nanoparticles as photosensitizer delivery carriers for enhanced photodynamic cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 111099. [Google Scholar] [CrossRef] [PubMed]
- Nan, X.; Zhang, X.; Liu, Y.; Zhou, M.; Chen, X.; Zhang, X. Dual-Targeted Multifunctional Nanoparticles for Magnetic Resonance Imaging Guided Cancer Diagnosis and Therapy. ACS Appl. Mater. Interfaces 2017, 9, 9986–9995. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xu, S.; Zhao, T.; Zhu, L.; Wei, D.; Li, Y.; Zhang, H.; Zhao, C. Gold nanocluster-conjugated amphiphilic block copolymer for tumor-targeted drug delivery. ACS Appl. Mater. Interfaces 2012, 4, 5766–5774. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Li, J.; Ga, L.; Yun, G.; Xu, L.; Wang, E. Multifunctional near-infrared fluorescent nanoclusters for simultaneous targeted cancer imaging and photodynamic therapy. Sens. Actuators B 2016, 222, 918–922. [Google Scholar] [CrossRef]
- Chen, D.; Luo, Z.; Li, N.; Lee, J.Y.; Xie, J.; Lu, J. Amphiphilic Polymeric Nanocarriers with Luminescent Gold Nanoclusters for Concurrent Bioimaging and Controlled Drug Release. Adv. Funct. Mater. 2013, 23, 4324–4331. [Google Scholar] [CrossRef]
- Khandelia, R.; Bhandari, S.; Pan, U.N.; Ghosh, S.S.; Chattopadhyay, A. Gold Nanocluster Embedded Albumin Nanoparticles for Two-Photon Imaging of Cancer Cells Accompanying Drug Delivery. Small 2015, 11, 4075–4081. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Feng, B.; Yu, H.; Wang, D.; Wang, T.; Liu, J.; Meng, Q.; Wang, S.; Zhang, P.; Zhang, Z.; et al. Cisplatin Prodrug-Conjugated Gold Nanocluster for Fluorescence Imaging and Targeted Therapy of the Breast Cancer. Theranostics 2016, 6, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Yahia-Ammar, A.; Sierra, D.; Merola, F.; Hildebrandt, N.; Le Guevel, X. Self-Assembled Gold Nanoclusters for Bright Fluorescence Imaging and Enhanced Drug Delivery. ACS Nano 2016, 10, 2591–2599. [Google Scholar] [CrossRef]
- Li, M.; Huang, L.; Wang, X.; Song, Z.; Zhao, W.; Wang, Y.; Liu, J. Direct generation of Ag nanoclusters on reduced graphene oxide nanosheets for efficient catalysis, antibacteria and photothermal anticancer applications. J. Colloid Interface Sci. 2018, 529, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Shi, S.; Feng, L.; Chen, F.; Graves, S.A.; Ehlerding, E.B.; Goel, S.; Sun, H.; England, C.G.; Nickles, R.J.; et al. Long circulating reduced graphene oxide-iron oxide nanoparticles for efficient tumor targeting and multimodality imaging. Nanoscale 2016, 8, 12683–12692. [Google Scholar] [CrossRef] [PubMed]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- Dash, B.S.; Jose, G.; Lu, Y.J.; Chen, J.P. Functionalized Reduced Graphene Oxide as a Versatile Tool for Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 2989. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, C.; Zhang, S.; He, L.; Wang, M.; Peng, D.; Tian, J.; Fang, S. Carbon-based nanocomposites with aptamer-templated silver nanoclusters for the highly sensitive and selective detection of platelet-derived growth factor. Biosens. Bioelectron. 2017, 89, 735–742. [Google Scholar] [CrossRef]
- Wong, X.Y.; Quesada-Gonzalez, D.; Manickam, S.; Muthoosamy, K. Fluorescence “turn-off/turn-on” biosensing of metal ions by gold nanoclusters, folic acid and reduced graphene oxide. Anal. Chim. Acta 2021, 1175, 338745. [Google Scholar] [CrossRef]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lei, C.; Yu, C. Mesoporous Silica Nanoparticles for Protein Protection and Delivery. Front. Chem. 2019, 7, 290. [Google Scholar] [CrossRef] [PubMed]
- Mulikova, T.; Abduraimova, A.; Molkenova, A.; Em, S.; Duisenbayeva, B.; Han, D.-W.; Atabaev, T.S. Mesoporous silica decorated with gold nanoparticles as a promising nanoprobe for effective CT X-ray attenuation and potential drug delivery. Nano-Struct. Nano-Objects 2021, 26, 100712. [Google Scholar] [CrossRef]
- Guo, D.; Bao, Y.; Zhang, Y.; Yang, H.; Chen, L. Reduction-responsive Au decorated mesoporous silica-based nanoplatform for photodynamic-chemotherapy. Microporous Mesoporous Mater. 2020, 292, 109729. [Google Scholar] [CrossRef]
- Ong, C.; Cha, B.G.; Kim, J. Mesoporous Silica Nanoparticles Doped with Gold Nanoparticles for Combined Cancer Immunotherapy and Photothermal Therapy. ACS Appl. Bio Mater. 2019, 2, 3630–3638. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Qi, C.; Maynadier, M.; Cattoen, X.; Wong Chi Man, M.; Raehm, L.; Mongin, O.; Blanchard-Desce, M.; Garcia, M.; Gary-Bobo, M.; et al. Multifunctional Gold-Mesoporous Silica Nanocomposites for Enhanced Two-Photon Imaging and Therapy of Cancer Cells. Front. Mol. Biosci. 2016, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Lin, J.; Wang, S.; Zhou, Z.; Li, Z.; Wang, Z.; Zhang, C.; Yue, X.; Niu, G.; Yang, M.; et al. Photosensitizer-conjugated silica-coated gold nanoclusters for fluorescence imaging-guided photodynamic therapy. Biomaterials 2013, 34, 4643–4654. [Google Scholar] [CrossRef] [PubMed]
- Zumaya, A.L.V.; Martynek, D.; Bautkinová, T.; Šoóš, M.; Ulbrich, P.; Raquez, J.-M.; Dendisová, M.; Merna, J.; Vilčáková, J.; Kopecký, D.; et al. Self-assembly of poly(L-lactide-co-glycolide) and magnetic nanoparticles into nanoclusters for controlled drug delivery. Eur. Polym. J. 2020, 133, 109795. [Google Scholar] [CrossRef]
- Zumaya, A.L.V.; Ulbrich, P.; Vilčáková, J.; Dendisová, M.; Fulem, M.; Šoóš, M.; Hassouna, F. Comparison between two multicomponent drug delivery systems based on PEGylated-poly (l-lactide-co-glycolide) and superparamagnetic nanoparticles: Nanoparticulate versus nanocluster systems. J. Drug Deliv. Sci. Technol. 2021, 64, 102643. [Google Scholar] [CrossRef]
- Hasa, J.; Hanus, J.; Stepanek, F. Magnetically Controlled Liposome Aggregates for On-Demand Release of Reactive Payloads. ACS Appl. Mater. Interfaces 2018, 10, 20306–20314. [Google Scholar] [CrossRef]
- Codari, F.; Moscatelli, D.; Furlan, M.; Lattuada, M.; Morbidelli, M.; Soos, M. Synthesis of hetero-nanoclusters: The case of polymer-magnetite systems. Langmuir 2014, 30, 2266–2273. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Sun, C.; Guo, J.; Xu, K.; Wang, C. Biopolymer-directed synthesis of high-surface-area magnetite colloidal nanocrystal clusters for dual drug delivery in prostate cancer. J. Mater. Chem. 2012, 22, 19067–19075. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Deng, Y.; Zou, Y.; Li, C.; Guo, X.; Xiong, L.; Gao, Y.; Li, F.; Zhao, D. Highly water-dispersible biocompatible magnetite particles with low cytotoxicity stabilized by citrate groups. Angew. Chem. Int. Ed. Engl. 2009, 48, 5875–5879. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tang, J.; Wei, C.; Guo, J.; Wang, S.; Chaudhary, D.; Wang, C. Doxorubicin-conjugated mesoporous magnetic colloidal nanocrystal clusters stabilized by polysaccharide as a smart anticancer drug vehicle. Small 2012, 8, 2690–2697. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Guo, W.; Bae, J.H.; Kim, S.H.; Ha, C.S. Highly porous, water-soluble, superparamagnetic, and biocompatible magnetite nanocrystal clusters for targeted drug delivery. Chemistry 2011, 17, 12802–12808. [Google Scholar] [CrossRef] [PubMed]

| Polymer | PNPs Size (nm) | Therapeutic/Ligand | Role | Cancer Type | Ref. |
|---|---|---|---|---|---|
| Polyethers (e.g., PEG-based) | 40–160 | Photothermal agent (IR-780), folic acid (FA) | Imaging diagnostics therapy | Ovarian, colon, breast, lung | [35,36,37,38,39] |
| Polyesters (PCL, PLA, PLGA and block-copolymers) | 60–150 | FA, doxorubicin (DOX), paclitaxel, SPION, antibodies (Ab), Indocyanine green (ICG), metal NPs | cervical cancer, HeLa cells, carcinoma (SCC7) | [40,41,42,43,44] | |
| Polysaccharides (CHIT) | 100–200 | Subcutaneous tumors, prostate | [41,45,46,47] |
| Vector | Name | Formulation | Treatment | Clinical Approve | Ref. |
|---|---|---|---|---|---|
| Au NPs | CYT-6091 (Aurimune) | PEGylated colloidal Au-TNF | Solid tumors | Phase I completed | [115] |
| Non-small lung cancer | undergoing Phase II | ||||
| AuroLase | Silica core coated with Au shell | Head and neck cancer | Completed pilot study | [134] | |
| NU-0129 | Spherical nucleic acid formulation conjugated to Au NPs | Glioblastoma multiform | Phase 0 completed | [135] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villela Zumaya, A.L.; Mincheva, R.; Raquez, J.-M.; Hassouna, F. Nanocluster-Based Drug Delivery and Theranostic Systems: Towards Cancer Therapy. Polymers 2022, 14, 1188. https://doi.org/10.3390/polym14061188
Villela Zumaya AL, Mincheva R, Raquez J-M, Hassouna F. Nanocluster-Based Drug Delivery and Theranostic Systems: Towards Cancer Therapy. Polymers. 2022; 14(6):1188. https://doi.org/10.3390/polym14061188
Chicago/Turabian StyleVillela Zumaya, Alma Lucia, Rosica Mincheva, Jean-Marie Raquez, and Fatima Hassouna. 2022. "Nanocluster-Based Drug Delivery and Theranostic Systems: Towards Cancer Therapy" Polymers 14, no. 6: 1188. https://doi.org/10.3390/polym14061188
APA StyleVillela Zumaya, A. L., Mincheva, R., Raquez, J.-M., & Hassouna, F. (2022). Nanocluster-Based Drug Delivery and Theranostic Systems: Towards Cancer Therapy. Polymers, 14(6), 1188. https://doi.org/10.3390/polym14061188








