Biomaterials as Haemostatic Agents in Cardiovascular Surgery: Review of Current Situation and Future Trends
Abstract
1. Introduction
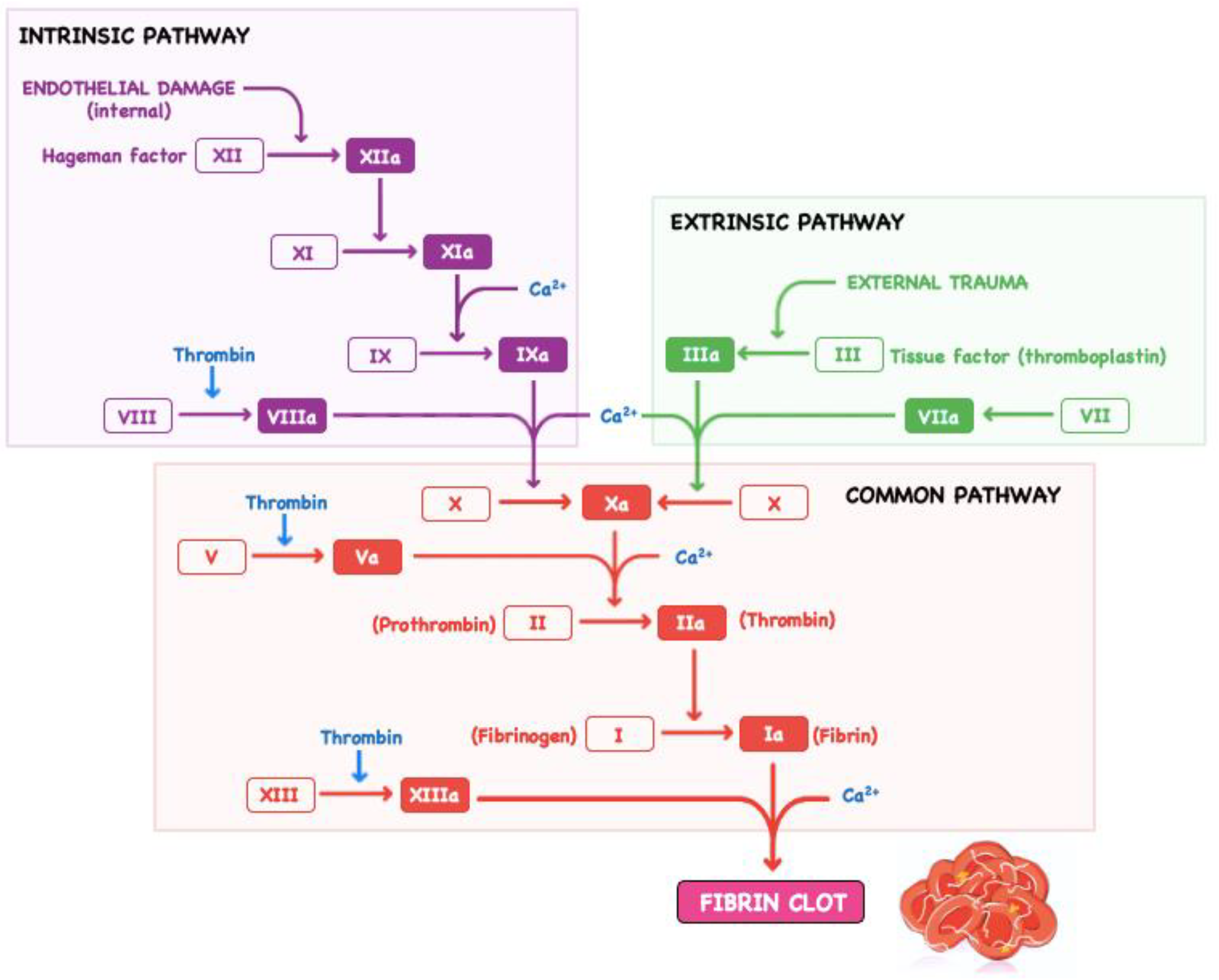
2. Specific Aspects Related to Bleeding in Cardiovascular Surgery
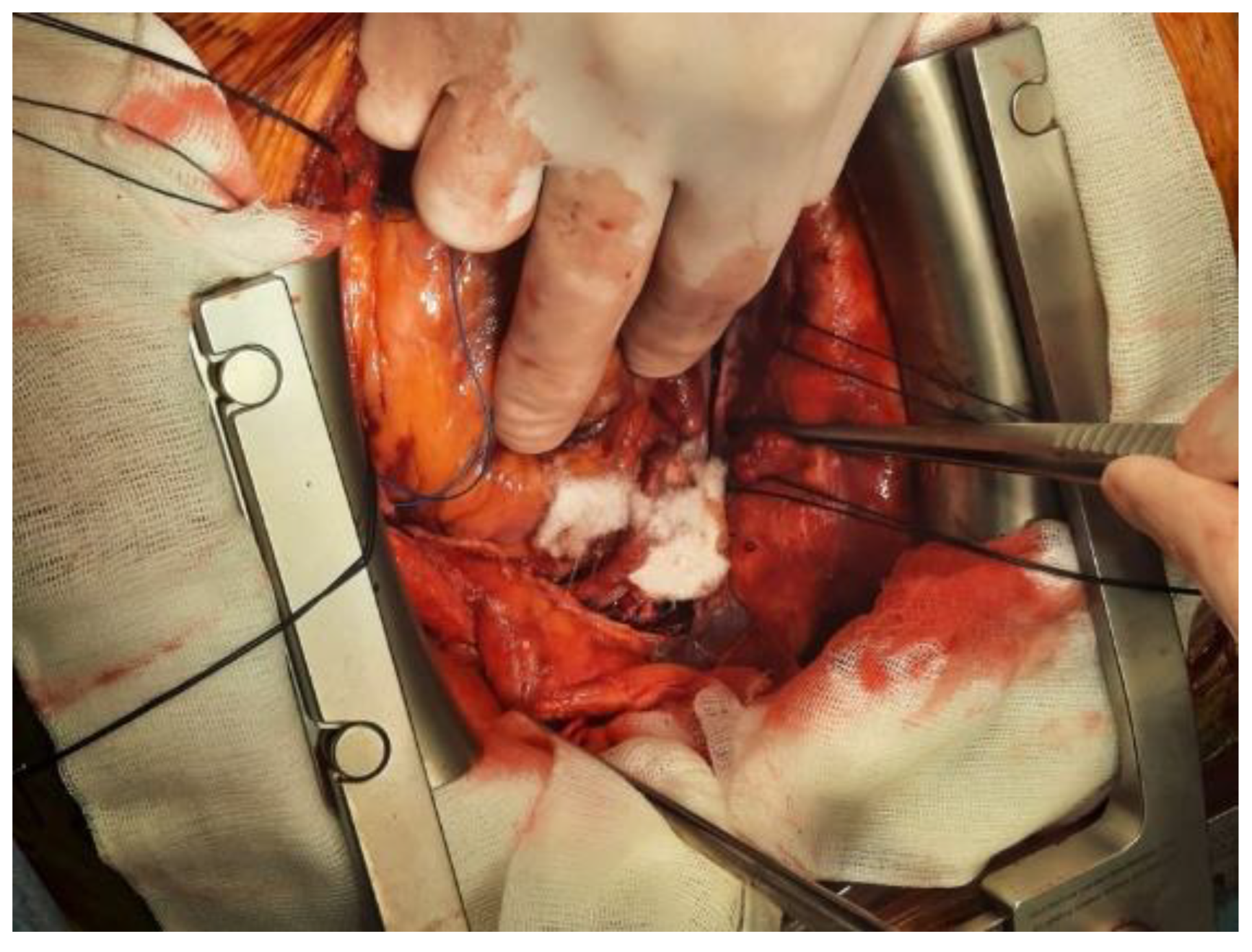
3. Current Haemostatic Agents and Their Clinical Use in Cardiovascular Surgery
3.1. Conventional Haemostatics in Cardiovascular Surgery
3.2. Haemostatic Agents with Active Mechanisms in Cardiovascular Surgery
3.2.1. Thrombin Sealants
3.2.2. Fibrin Sealants and Fibrin Patches
3.3. Haemostatic Agents with Nonactive Mechanisms in Cardiovascular Surgery
3.3.1. Gelatine Sponges
3.3.2. Microfibrillar Collagens
3.3.3. Oxidised Regenerated Cellulose
3.3.4. Microporous Polysaccharide Hemispheres
3.4. Haemostatic Agents with Active and Nonactive Mechanisms
3.4.1. Gelatine–Thrombin Matrix Sealant
3.4.2. Composite Collagen and Thrombin Sealant
3.5. Tissue Sealants
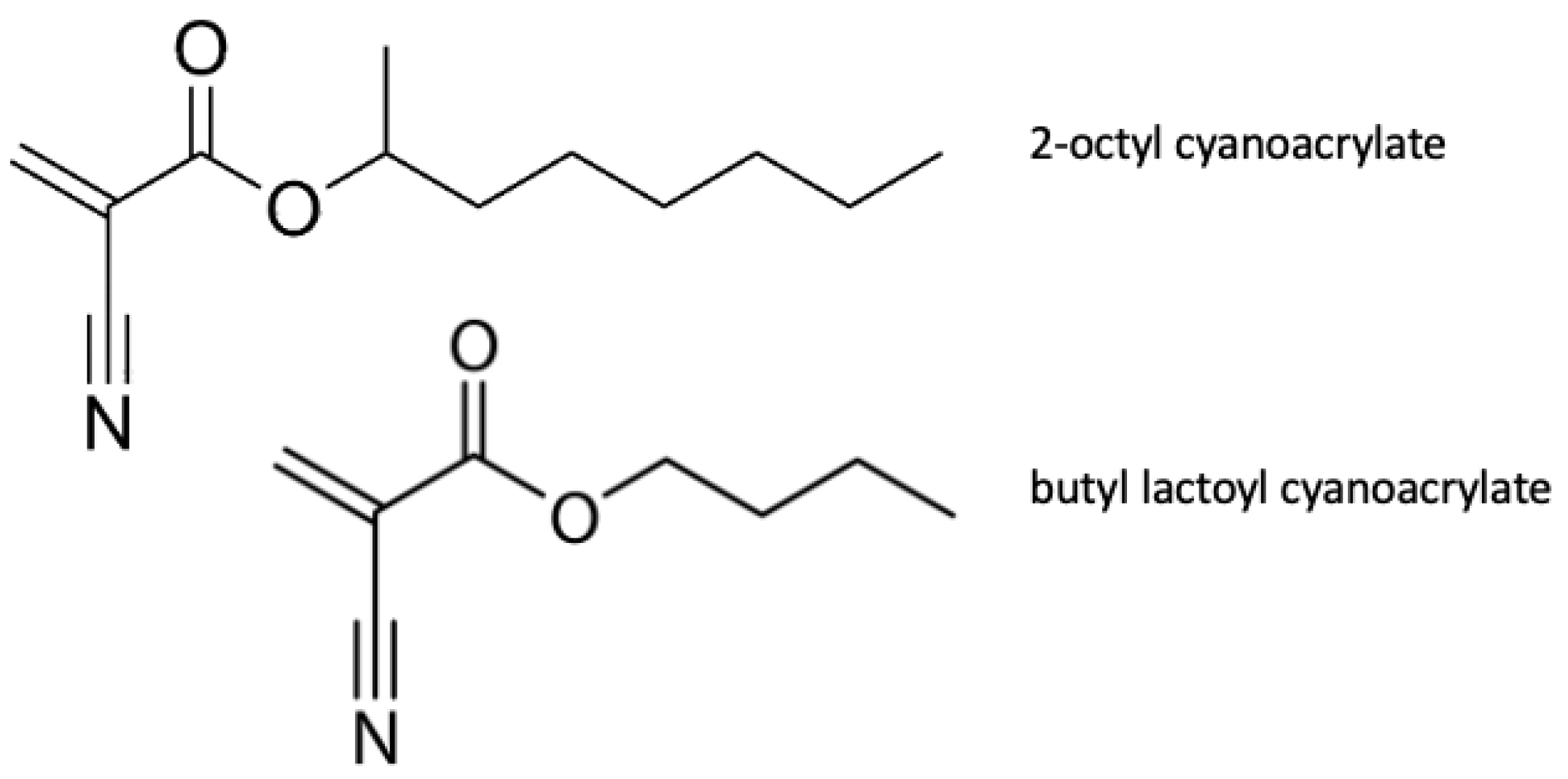
3.5.1. Absorbable Cyanoacrylate
3.5.2. Glutaraldehyde/Bovine Albumin
3.5.3. Chitin and Chitosan-Based Haemostatic Agents (HemCon, Closure, Chitoseal, Celox)
3.5.4. Polyethylene Glycol Polymers (CoSeal)
4. Current Novelties in Research on Biomaterials for Haemostatics
4.1. Literature Overview Regarding Nanotechnologies and Novel Biomaterial for Haemostatics
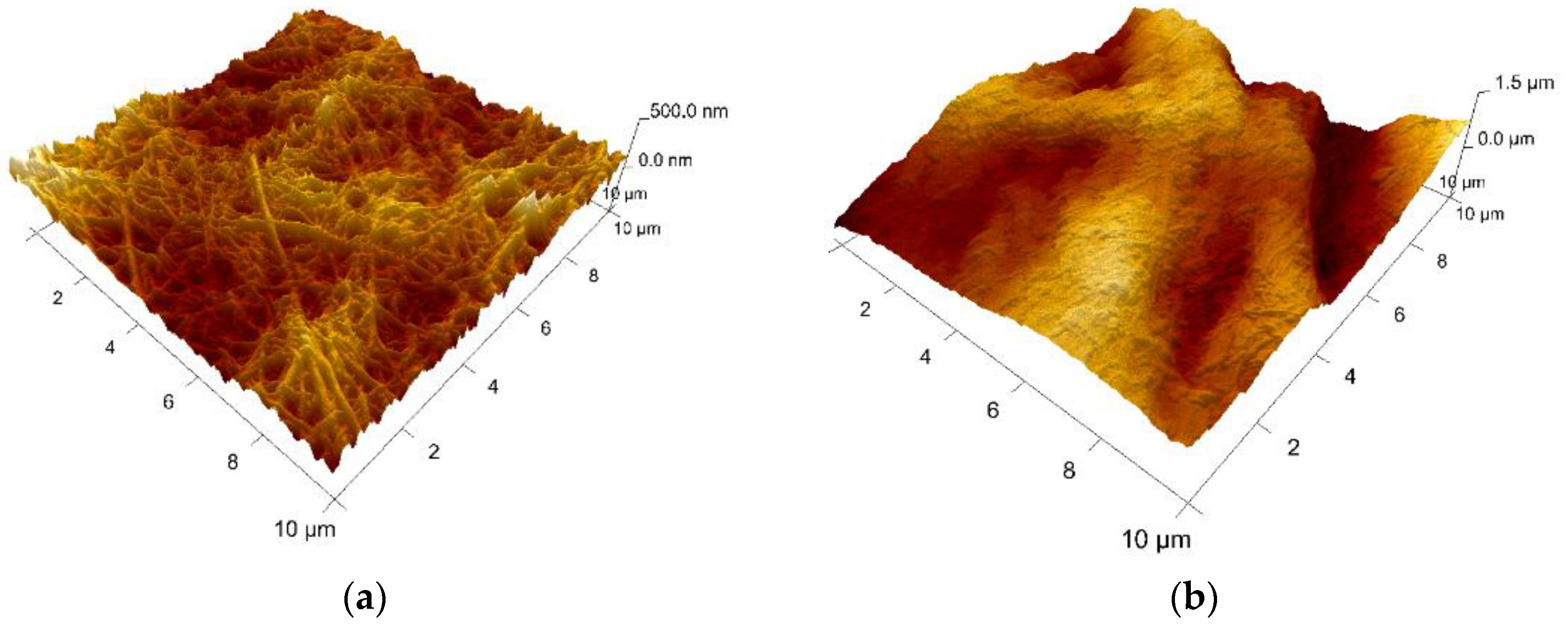
4.2. Collagen Loaded with Gelatin Microspheres as a New Composite Biomaterial for Haemostatics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dyke, C.; Aronson, S.; Dietrich, W.; Hofmann, A.; Karkouti, K.; Levi, M.; Murphy, G.J.; Sellke, F.W.; Shore-Lesserson, L.; von Heymann, C.; et al. Universal definition of perioperative bleeding in adult cardiac surgery. J. Thorac. Cardiovasc. Surg. 2013, 147, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, K.L.; Rauer, L.J.; Mortensen, P.E.; Kjeldsen, B.J. Reoperation for bleeding in cardiac surgery. Interact. Cardiovasc. Thorac. Surg. 2012, 14, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Elassal, A.A.; Al-Ebrahim, K.E.; Debis, R.S.; Ragab, E.S.; Faden, M.S.; Fatani, M.A.; Allam, A.R.; Abdulla, A.H.; Bukhary, A.M.; Noaman, N.A.; et al. Re-exploration for bleeding after cardiac surgery: Revaluation of urgency and factors promoting low rate. J. Cardiothorac. Surg. 2021, 16, 166. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Chen, Z.-Y.; Zhang, W.-L.; Guo, L.; Sun, Y.-Q.; Cui, H.-Z.; Bu, J.-Q.; Cai, J.-H. Severe bleeding following off-pump coronary artery bypass grafting: Predictive factors and risk model. J. Geriatr. Cardiol. 2021, 18, 449–461. [Google Scholar] [CrossRef]
- Al-Attar, N.; Johnston, S.; Jamous, N.; Mistry, S.; Ghosh, E.; Gangoli, G.; Danker, W.; Etter, K.; Ammann, E. Impact of bleeding complications on length of stay and critical care utilization in cardiac surgery patients in England. J. Cardiothorac. Surg. 2019, 14, 64. [Google Scholar] [CrossRef]
- Stokes, M.E.; Ye, X.; Shah, M.; Mercaldi, K.; Reynolds, M.W.; Rupnow, M.F.T.; Hammond, J. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv. Res. 2011, 11, 135. [Google Scholar] [CrossRef]
- Ligon, R.A.; Downey, L.; Gruenewald, D.L.; Bauser-Heaton, H.; Kim, D.W.; Roman, M.F.; Vincent, R.N.; Petit, C.J. Risk Factors for Red Blood Cell Transfusions in Children Undergoing Cardiac Catheterization. J. Pediatr. 2019, 217, 25–32.e4. [Google Scholar] [CrossRef]
- Pagano, D.; Milojevic, M.; Meesters, M.I.; Benedetto, U.; Bolliger, D.; von Heymann, C.; Jeppsson, A.; Koster, A.; Osnabrugge, R.L.; Ranucci, M.; et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. The Task Force on Patient Blood Management for Adult Cardiac Surgery of the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Cardiothoracic Anaesthesiology (EACTA). Eur. J. Cardio-Thorac. Surg. 2018, 53, 79–111. [Google Scholar]
- Dutta, T.K.; Verma, S.P. Rational Use of Recombinant Factor VIIa in Clinical Practice. Indian J. Hematol. Blood Transfus. 2014, 30, 85–90. [Google Scholar] [CrossRef][Green Version]
- Sang, Y.Q.; Roest, M.; de Laat, B.; de Groot, P.G.; Huskens, D. Interplay between platelets and coagulation. Blood Rev. 2021, 46, 100733. [Google Scholar] [CrossRef]
- Tompeck, A.J.; Gajdhar, A.U.R.; Dowling, M.; Johnson, S.B.; Barie, P.S.; Winchell, R.J.; King, D.; Scalea, T.M.; Britt, L.; Narayan, M. A comprehensive review of topical hemostatic agents: The good, the bad, and the novel. J. Trauma Acute Care Surg. 2019, 88, e1–e21. [Google Scholar] [CrossRef] [PubMed]
- Bracey, A.; Shander, A.; Aronson, S.; Boucher, B.A.; Calcaterra, D.; Chu, M.W.; Culbertson, R.; Jabr, K.; Kehlet, H.; Lattouf, O.; et al. The Use of Topical Hemostatic Agents in Cardiothoracic Surgery. Ann. Thorac. Surg. 2017, 104, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Kozar, R.; Zhang, J.; Dong, J. Diverse activities of von Willebrand factor in traumatic brain injury and associated coagulopathy. J. Thromb. Haemost. 2020, 18, 3154–3162. [Google Scholar] [CrossRef] [PubMed]
- Stevens, H.; McFadyen, J.D. Platelets as Central Actors in Thrombosis—Reprising an Old Role and Defining a New Character. Semin. Thromb. Hemost. 2019, 45, 802–809. [Google Scholar] [CrossRef]
- Green, D. Coagulation cascade. Hemodial. Int. 2006, 10 (Suppl. S2), S2–S4. [Google Scholar] [CrossRef]
- Williams, B.; Wehman, B.; Mazzeffi, M.A.; Odonkor, P.; Harris, R.L.; Kon, Z.; Tanaka, K.A. Acute Intracardiac Thrombosis and Pulmonary Thromboembolism After Cardiopulmonary Bypass: A Systematic Review of Reported Cases. Anesth. Analg. 2018, 126, 425–434. [Google Scholar] [CrossRef]
- Rawish, E.; Nording, H.; Münte, T.; Langer, H.F. Platelets as Mediators of Neuroinflammation and Thrombosis. Front. Immunol. 2020, 11, 548631. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Rezaei, S.; Seiri, P.; Ryzhikov, M.; Hashemy, S.I.; Hassanian, S.M. Factor Xa Signaling Contributes to the Pathogenesis of Inflammatory Diseases. J. Cell. Physiol. 2016, 232, 1966–1970. [Google Scholar] [CrossRef]
- Guo, B.L.; Dong, R.N.; Bang, Y.P.; Li, M. Haemostatic materials for wound healing applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef]
- Samudrala, S. Topical Hemostatic Agents in Surgery: A Surgeon’s Perspective. AORN J. 2008, 88, S2–S11. [Google Scholar] [CrossRef]
- Gabay, M.; Boucher, B.A. An Essential Primer for Understanding the Role of Topical Hemostats, Surgical Sealants, and Adhesives for Maintaining Hemostasis. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2013, 33, 935–955. [Google Scholar] [CrossRef] [PubMed]
- Hickman, D.A.; Pawlowski, C.L.; Sekhon, U.D.S.; Marks, J.; Gupta, A.S. Biomaterials and Advanced Technologies for Hemostatic Management of Bleeding. Adv. Mater. 2018, 30, 1700859. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, B.A.; Blau, L.N.; Rodriguez, L.; Suarez, E.E.; Ngo, U.Q.; Reardon, M.J.; Loebe, M. Microporous polysaccharide hemosphere absorbable hemostat use in cardiothoracic surgical procedures. J. Cardiothorac. Surg. 2014, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, S.; Sarkar, P.; Rigby, K.; Maan, A.; Mukherjee, S.; Crawford, K.E.; Mukhopadhyay, K. Polymeric Materials for Hemostatic Wound Healing. Pharmaceutics 2021, 13, 2127. [Google Scholar] [CrossRef]
- Azadani, A.N.; Matthews, P.B.; Ge, L.; Shen, Y.; Jhun, C.-S.; Guy, T.S.; Tseng, E.E. Mechanical Properties of Surgical Glues Used in Aortic Root Replacement. Ann. Thorac. Surg. 2009, 87, 1154–1160. [Google Scholar] [CrossRef]
- Spotnitz, W.D.; Burks, S. State-of-the-Art Review: Hemostats, Sealants, and Adhesives II: Update as Well as How and When to Use the Components of the Surgical Toolbox. Clin. Appl. Thromb. 2010, 16, 497–514. [Google Scholar] [CrossRef]
- Spotnitz, W.D. Hemostats, sealants, and adhesives: A practical guide for the surgeon. Am. Surg. 2012, 78, 1305–1321. [Google Scholar] [CrossRef]
- Schonauer, C.; Tessitore, E.; Barbagallo, G.M.V.; Albanese, V.; Moraci, A. The use of local agents: Bone wax, gelatin, collagen, oxidized cellulose. Eur. Spine J. 2004, 13 (Suppl. S1), S89–S96. [Google Scholar] [CrossRef]
- Gan, E.C.; Alsaleh, S.; Manji, J.; Habib, A.-R.R.; Amanian, A.; Javer, A.R. Hemostatic effect of hot saline irrigation during functional endoscopic sinus surgery: A randomized controlled trial. Int. Forum Allergy Rhinol. 2014, 4, 877–884. [Google Scholar] [CrossRef]
- Barnard, J.; Millner, R. A Review of Topical Hemostatic Agents for Use in Cardiac Surgery. Ann. Thorac. Surg. 2009, 88, 1377–1383. [Google Scholar] [CrossRef]
- Micovic, S.; Everts, P.; Calija, B.; Strugarevic, E.; Grubor, N.; Boricic, M.; Lesanovic, J.; Box, H.; Abazovic, D. Novel autologous, high concentrated fibrin as advanced hemostatic agent for coronary surgery. Transfus. Apher. Sci. 2021, 60, 103171. [Google Scholar] [CrossRef] [PubMed]
- Chapman, W.C.; Singla, N.; Genyk, Y.; McNeil, J.W.; Renkens, K.L.; Reynolds, T.C.; Murphy, A.; Weaver, F.A. A Phase 3, Randomized, Double-Blind Comparative Study of the Efficacy and Safety of Topical Recombinant Human Thrombin and Bovine Thrombin in Surgical Hemostasis. J. Am. Coll. Surg. 2007, 205, 256–265. [Google Scholar] [CrossRef]
- Schoenecker, J.G.; Hauck, R.K.; Mercer, M.C.; Parker, W.; Lawson, J.H. Exposure to Topical Bovine Thrombin During Surgery Elicits a Response Against the Xenogeneic Carbohydrate Galactose α1-3Galactose. J. Clin. Immunol. 2000, 20, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Matras, H.; Dinges, H.P.; Lassmann, H.; Mamoli, B. Suture-free interfascicular nerve transplantation in animal experiments. Wien. Med. Wochenschr. 1972, 122, 517–523. [Google Scholar] [PubMed]
- Daud, S.A.; Kaur, B.; McClure, G.R.; Belley-Cote, E.P.; Harlock, J.; Crowther, M.; Whitlock, R.P. Fibrin and Thrombin Sealants in Vascular and Cardiac Surgery: A Systematic Review and Meta-analysis. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Tredree, R.; Beierlein, W.; Debrix, I.; Eisert, A.; Goffredo, F.; Gòmez de Salazar, E.; Rambourg, P.; Saint-Remy, J.M.; Sturm, C.; Watson, N. Evaluating the difference between fibrin sealants: Recommendations from an international advisory panel of hospital pharmacists. Eur. J. Hosp. Pharm. Sci. 2006, 12, 3–9. [Google Scholar]
- Navarro, A.; Brooks, A. Use of local pro-coagulant haemostatic agents for intra-cavity control of haemorrhage after trauma. Eur. J. Trauma Emerg. Surg. 2014, 41, 493–500. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.; Martinez, E.E.; Zhu, J.; Wang, T.; Shi, S.; Shin, S.R.; Hassan, S.; Guo, C. Emerging Biopolymer-Based Bioadhesives. Macromol. Biosci. 2021, 22, 2100340. [Google Scholar] [CrossRef]
- Heher, P.; Mühleder, S.; Mittermayr, R.; Redl, H.; Slezak, P. Fibrin-based delivery strategies for acute and chronic wound healing. Adv. Drug Deliv. Rev. 2018, 129, 134–147. [Google Scholar] [CrossRef]
- Codispoti, M.; Mankad, P. Significant merits of a fibrin sealant in the presence of coagulopathy following paediatric cardiac surgery: Randomised controlled trial. Eur. J. Cardio-Thorac. Surg. 2002, 22, 200–205. [Google Scholar] [CrossRef]
- Lamm, P.; Adelhard, K.; Juchem, G.; Weitkunat, R.; Milz, S.; Kilger, E.; Götz, A.; Reichart, B. Fibrin glue in coronary artery bypass grafting operations: Casting out the Devil with Beelzebub? Eur. J. Cardio-Thorac. Surg. 2007, 32, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Fiss, I.; Danne, M.; Stendel, R. Use of Gelatin-Thrombin Matrix Hemostatic Sealant in Cranial Neurosurgery. Neurol. Med. Chir. 2007, 47, 462–467. [Google Scholar] [CrossRef]
- Das, D.; Acharya, D.; Singh, J.; Pramanik, S. A detailed review of management of coronary perforations by gelfoam closure. J. Pract. Cardiovasc. Sci. 2021, 7, 230–235. [Google Scholar] [CrossRef]
- Sirlak, M.; Eryilmaz, S.; Yazicioglu, L.; Kiziltepe, U.; Eyileten, Z.; Durdu, S.; Tasoz, R.; Eren, N.T.; Aral, A.; Kaya, B.; et al. Comparative study of microfibrillar collagen hemostat (Colgel) and oxidized cellulose (Surgicel) in high transfusion-risk cardiac surgery. J. Thorac. Cardiovasc. Surg. 2003, 126, 666–670. [Google Scholar] [CrossRef]
- Robicsek, F. Microfibrillar collagen hemostat in cardiac surgery. J. Thorac. Cardiovasc. Surg. 2004, 127, 1228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Chen, S.; Zhang, X.; Ma, J.; He, J. Oxidized cellulose-based hemostatic materials. Carbohydr. Polym. 2020, 230, 115585. [Google Scholar] [CrossRef]
- Sabel, M.; Stummer, W. The use of local agents: Surgicel and surgifoam. Eur. Spine J. 2004, 13, S97–S101. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, A.; Öner, G.; Taş, M.; Çınaroğlu, S. The Effects of an Absorbable Hemostat Produced From Oxidized Regenerated Cellulose on Adhesion Formation in a Rat Mode. Med. J. Bakirkoy 2021, 17, 142–148. [Google Scholar] [CrossRef]
- Spangler, D.; Rothenburger, S.; Nguyen, K.; Jampani, H.; Weiss, S.; Bhende, S. In Vitro Antimicrobial Activity of Oxidized Regenerated Cellulose Against Antibiotic-Resistant Microorganisms. Surg. Infect. 2003, 4, 255–262. [Google Scholar] [CrossRef]
- Vamvakas, E.C.; Blajchman, M.A. Transfusion-related mortality: The ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood 2009, 113, 3406–3417. [Google Scholar] [CrossRef]
- Shander, A.; Hofmann, A.; Ozawa, S.; Theusinger, O.M.; Gombotz, H.; Spahn, D.R. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion 2010, 50, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, H.; Gheorghita, D.; Antoniac, I.; Gheorghe, D.; Fiori, F.; Mohan, A.; Raftu, G.; Ionel, C.; Costache, V. Bioadhesives Used in Cardiovascular Surgery. Rev. Chim. 2018, 10, 2799–2803. [Google Scholar] [CrossRef]
- Oz, M.C.; Cosgrove, D.M., 3rd; Badduke, B.R.; Hill, J.D.; Flannery, M.R.; Palumbo, R.; Topic, N.; Fusion Matrix Study Group. Controlled clinical trial of a novel hemostatic agent in cardiac surgery. Ann. Thorac. Surg. 2000, 69, 1376–1382. [Google Scholar] [CrossRef]
- Bowman, L.J.; Anderson, C.D.; Chapman, W.C. Topical Recombinant Human Thrombin in Surgical Hemostasis. Semin. Thromb. Hemost. 2010, 36, 477–484. [Google Scholar] [CrossRef] [PubMed]
- CoStasis Multi-Center Collaborative Writing Committee. A novel collagen-based composite offers effective hemostasis for multiple surgical indications: Results of a randomized controlled trial. Surgery 2001, 129, 445–450. [Google Scholar] [CrossRef]
- Franchini, M.; Lippi, G. Acquired factor V inhibitors: A systematic review. J. Thromb. Thrombolysis 2011, 31, 449–457. [Google Scholar] [CrossRef]
- Lumsden, A.B.; Heyman, E.R. Prospective randomized study evaluating an absorbable cyanoacrylate for use in vascular reconstructions. J. Vasc. Surg. 2006, 44, 1002–1009. [Google Scholar] [CrossRef]
- Bhamidipati, C.M.; Coselli, J.S.; LeMaire, S.A. BioGlue in 2011: What Is Its Role in Cardiac Surgery? J. Extra-Corpor. Technol. 2012, 44, P6–P12. [Google Scholar]
- Coselli, J.S.; Bavaria, J.E.; Fehrenbacher, J.; Stowe, C.L.; Macheers, S.K.; Gundry, S.R. Prospective randomized study of a protein-based tissue adhesive used as a hemostatic and structural adjunct in cardiac and vascular anastomotic repair procedures. J. Am. Coll. Surg. 2003, 197, 243–252. [Google Scholar] [CrossRef]
- LeMaire, S.A.; Ochoa, L.N.; Conklin, L.D.; Schmittling, Z.C.; Ündar, A.; Clubb, F.J.; Wang, X.L.; Coselli, J.S.; Fraser, C.D. Nerve and Conduction Tissue Injury Caused by Contact with BioGlue. J. Surg. Res. 2007, 143, 286–293. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Antonopoulou, A.; Ntanasis-Stathopoulos, I.; Patrini, D.; Papagiannopoulos, K.; Lawrence, D.; Panagiotopoulos, N. The role of BioGlue in thoracic surgery: A systematic review. J. Thorac. Dis. 2017, 9, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Gabrijelčič, T. Blockage of a Mechanical Aortic Valve Leaflet with BioGlue: A Case Report. Hear. Surg. Forum 2012, 15, E310–E312. [Google Scholar] [CrossRef] [PubMed]
- Allotey, J.K.; King, A.H.; Kumins, N.H.; Wong, V.L.; Harth, K.C.; Cho, J.S.; Kashyap, V.S. Systematic review of hemostatic agents used in vascular surgery. J. Vasc. Surg. 2020, 73, 2189–2197. [Google Scholar] [CrossRef] [PubMed]
- Devbhandari, M.P.; Chaudhery, Q.; Duncan, A.J. Acute intraoperative malfunction of aortic valve due to surgical glue. Ann. Thorac. Surg. 2006, 81, 1499–1500. [Google Scholar] [CrossRef] [PubMed]
- Costache, V.S.; Moldovan, H.; Arsenescu, C.; Costache, A. Aortic valve surgery of the 21st century: Sutureless avr versus tavi. Minerva Cardioangiol. 2018, 66, 191–197. [Google Scholar] [CrossRef]
- Ngaage, D.L.; Edwards, W.D.; Bell, M.R.; Sundt, T.M. A cautionary note regarding long-term sequelae of biologic glue. J. Thorac. Cardiovasc. Surg. 2005, 129, 937–938. [Google Scholar] [CrossRef] [PubMed]
- Shruthi, P.; Venkatesan, K. Application of chitosan and its derivatives in drug delivery. Res. J. Biotechnol. 2021, 16, 161–172. [Google Scholar]
- Hiranpattanakul, P.; Jongjitpissamai, T.; Aungwerojanawit, S.; Tachaboonyakiat, W. Fabrication of a chitin/chitosan hydrocolloid wound dressing and evaluation of its bioactive properties. Res. Chem. Intermed. 2018, 44, 4913–4928. [Google Scholar] [CrossRef]
- Millner, R.W.; Lockhart, A.S.; Bird, H.; Alexiou, C. A New Hemostatic Agent: Initial Life-Saving Experience With Celox (Chitosan) in Cardiothoracic Surgery. Ann. Thorac. Surg. 2009, 87, e13–e14. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, R.C.; Safi, H.J.; Sabik, J.; Conte, J.; Block, J.E. Improved intraoperative management of anastomotic bleeding during aortic reconstruction: Results of a randomized controlled trial. Am. Surg. 2004, 70, 307–311. [Google Scholar]
- Glickman, M.; Gheissari, A.; Money, S.; Martin, J.; Ballard, J.L. A Polymeric Sealant Inhibits Anastomotic Suture Hole Bleeding More Rapidly Than Gelfoam/ThrombinResults of a Randomized Controlled Trial. Arch. Surg. 2002, 137, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Mujahid, M. A review on recent advances in chitosan based composite for hemostatic dressings. Int. J. Biol. Macromol. 2018, 124, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Liu, C.-C.; Cherng, J.-H.; Lin, C.-S.; Chang, S.-J.; Hong, Z.-J.; Liu, C.-C.; Chiu, Y.-K.; Hsu, S.-D.; Chang, H.; et al. Biological Effects of Chitosan-Based Dressing on Hemostasis Mechanism. Polymers 2019, 11, 1906. [Google Scholar] [CrossRef] [PubMed]
- Gu, R.; Sun, W.; Zhou, H.; Wu, Z.; Meng, Z.; Zhu, X.; Tang, Q.; Dong, J.; Dou, G. The performance of a fly-larva shell-derived chitosan sponge as an absorbable surgical hemostatic agent. Biomaterials 2010, 31, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, E.E.; Kang, N.; Hamad, M.A.; Wooley, K.L.; Elsabahy, M. Absorbable hemostatic hydrogels comprising composites of sacrificial templates and honeycomb-like nanofibrous mats of chitosan. Nat. Commun. 2019, 10, 2307. [Google Scholar] [CrossRef]
- Deprés-Tremblay, G.; Chevrier, A.; Tran-Khanh, N.; Nelea, M.; Buschmann, M.D. Chitosan inhibits platelet-mediated clot retraction, increases platelet-derived growth factor release, and increases residence time and bioactivity of platelet-rich plasma in vivo. Biomed. Mater. 2018, 13, 015005. [Google Scholar] [CrossRef]
- Hangge, P.; Stone, J.; Albadawi, H.; Zhang, Y.S.; Khademhosseini, A.; Oklu, R. Hemostasis and nanotechnology. Cardiovasc. Diagn. Ther. 2017, 7, S267–S275. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Dai, C.; Tong, T.; Wu, J. Hemostatic nanotechnologies for external and internal hemorrhage management. Biomater. Sci. 2020, 8, 4396–4412. [Google Scholar] [CrossRef]
- Lashof-Sullivan, M.M.; Shoffstall, E.; Atkins, K.T.; Keane, N.; Bir, C.; VandeVord, P.; Lavik, E.B. Intravenously administered nanoparticles increase survival following blast trauma. Proc. Natl. Acad. Sci. USA 2014, 111, 10293–10298. [Google Scholar] [CrossRef]
- Biranje, S.; Madiwale, P.V.; Patankar, K.C.; Chhabra, R.; Dandekar-Jain, P.; Adivarekar, R.V. Hemostasis and anti-necrotic activity of wound-healing dressing containing chitosan nanoparticles. Int. J. Biol. Macromol. 2018, 121, 936–946. [Google Scholar] [CrossRef]





| Commercial Haemostatic Agents | ||
|---|---|---|
| I. Without Human or Bovine Component | Commercial name | Specifications/Composition |
| Surgicel | Oxidized cellulose polymer | |
| Oxycel | ||
| Omnex | Synthetic tissue adhesive (Cyanoacrylates) | |
| Coseal | Combination of two polyethylene glycol polymers | |
| Arista | Microporous polysaccharide hemispheres | |
| Hem Con | Freeze-dried chitosan derived from shrimp shellContains chitosan | |
| Chitoseal | ||
| Celox | ||
| Recothrom | Recombinant thrombin (topical use) | |
| II. With Bovine Component | BioGlue | Bovine albumin, and glutaraldehyde |
| CoStasis | Bovine collagen, bovine thrombin, and calcium chloride mixed with autologous plasma at the time of surgery | |
| Thrombin | Bovine protein | |
| Avitene | Water-insoluble acid salts of bovine collagen | |
| Colgel | ||
| Helitene | ||
| III. With Human Plasma Component | Tisseel | Human thrombin and fibrinogen, synthetic aprotinin |
| Vivostat | Combination of platelets and autologous fibrin (prepare on site) | |
| Crosseal | Tranexamic acid, human plasma protein, thrombin | |
| Quixil | ||
| IV. With Bovine and Human plasma component | Floseal | Human thrombin and bovine gelatine |
| Beriplast | Human thrombin and fibrinogen, bovine aprotinin | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, H.; Antoniac, I.; Gheorghiță, D.; Safta, M.S.; Preda, S.; Broască, M.; Badilă, E.; Fronea, O.; Scafa-Udrişte, A.; Cacoveanu, M.; et al. Biomaterials as Haemostatic Agents in Cardiovascular Surgery: Review of Current Situation and Future Trends. Polymers 2022, 14, 1189. https://doi.org/10.3390/polym14061189
Moldovan H, Antoniac I, Gheorghiță D, Safta MS, Preda S, Broască M, Badilă E, Fronea O, Scafa-Udrişte A, Cacoveanu M, et al. Biomaterials as Haemostatic Agents in Cardiovascular Surgery: Review of Current Situation and Future Trends. Polymers. 2022; 14(6):1189. https://doi.org/10.3390/polym14061189
Chicago/Turabian StyleMoldovan, Horațiu, Iulian Antoniac, Daniela Gheorghiță, Maria Sabina Safta, Silvia Preda, Marian Broască, Elisabeta Badilă, Oana Fronea, Alexandru Scafa-Udrişte, Mihai Cacoveanu, and et al. 2022. "Biomaterials as Haemostatic Agents in Cardiovascular Surgery: Review of Current Situation and Future Trends" Polymers 14, no. 6: 1189. https://doi.org/10.3390/polym14061189
APA StyleMoldovan, H., Antoniac, I., Gheorghiță, D., Safta, M. S., Preda, S., Broască, M., Badilă, E., Fronea, O., Scafa-Udrişte, A., Cacoveanu, M., Molnar, A., Costache, V. S., & Zaharia, O. (2022). Biomaterials as Haemostatic Agents in Cardiovascular Surgery: Review of Current Situation and Future Trends. Polymers, 14(6), 1189. https://doi.org/10.3390/polym14061189










