Broad-Spectrum Theranostics and Biomedical Application of Functionalized Nanomaterials
Abstract
:1. Introduction
2. Types of Nanoparticles
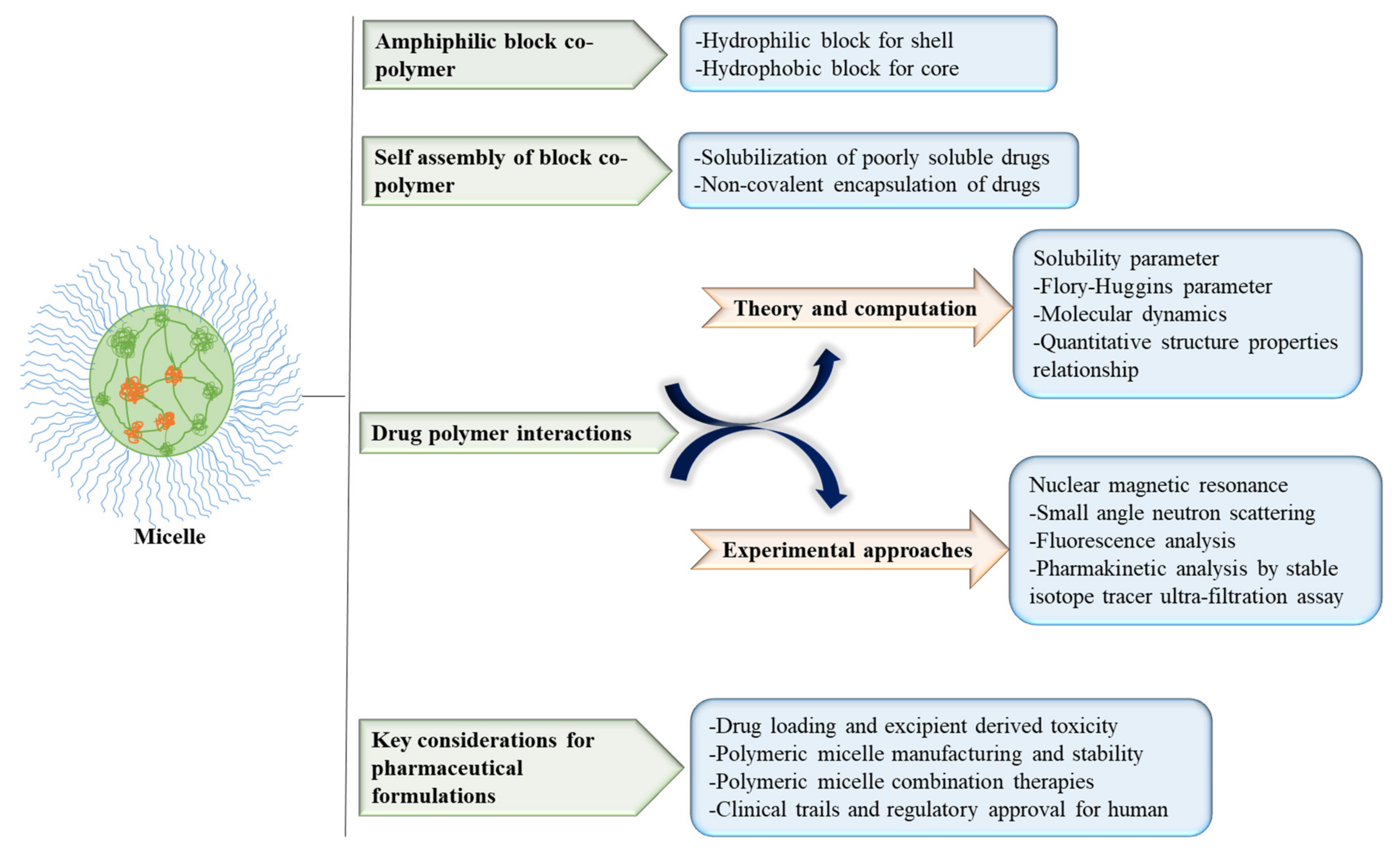
2.1. Micelles
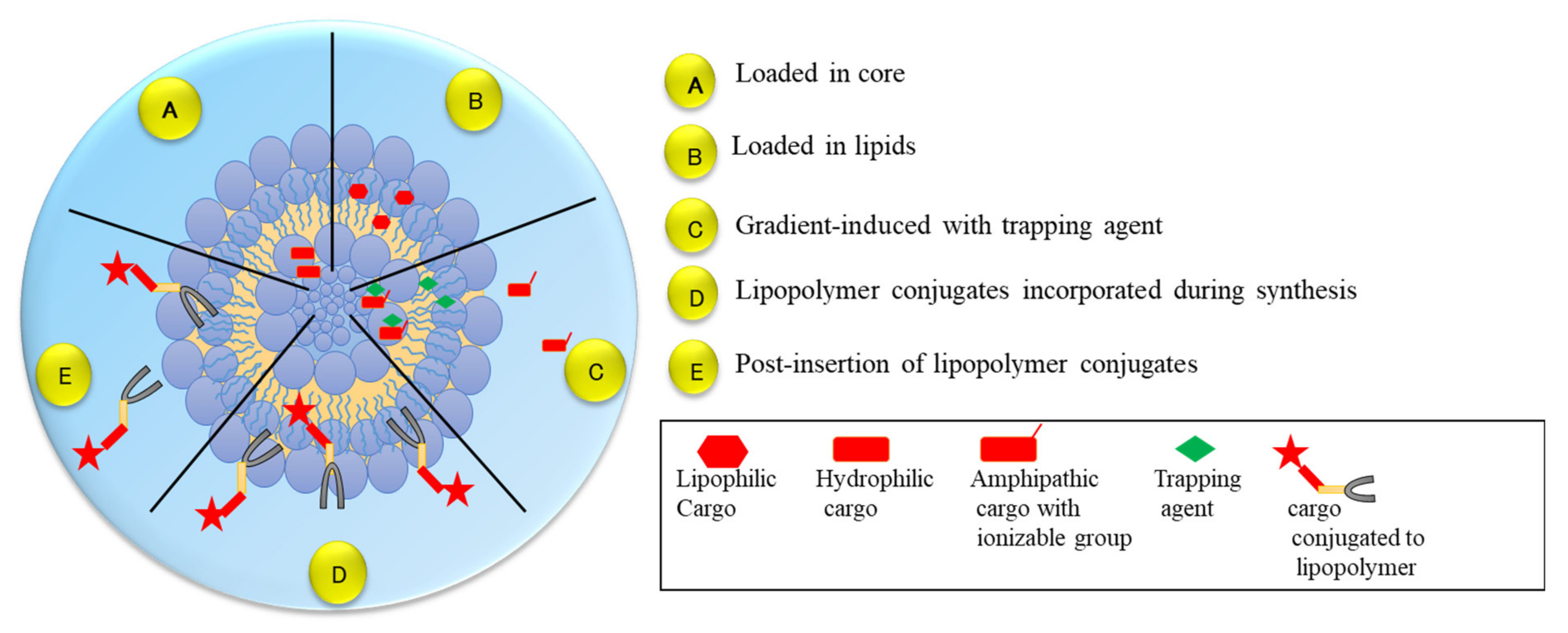
2.2. Liposomes
2.3. Dendrimers
2.4. Carbon Nanotubes
2.5. Metallic Nanoparticles
2.6. Quantum Dots
2.7. Nanospheres
3. Bioconjugation Process and Nanoparticles
4. Nanoparticles Cytotoxicity
5. Nanomaterials and Their Clinical Application
5.1. Biomedical Imaging
5.2. Drug Targeting and Delivery
5.3. Biosensors
5.4. Tissue Engineering
5.5. Immunoassays
5.6. Medical Devices
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Liu, L.H.; Ramström, O.; Yan, M. Engineering nanomaterial surfaces for biomedical applications. Exp. Biol. Med. 2009, 234, 1128–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, D.; England, C.G.; Cai, W. DNA nanomaterials for preclinical imaging and drug delivery. J. Control Release Off. J. Control Release Soc. 2016, 239, 27–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Lee, N.; Hyeon, T. Recent development of nanoparticles for molecular imaging. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2017, 375, 20170022. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.; Sim, S. Nanotechnology and its use in imaging and drug delivery (Review). Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Allhoff, F.; Patrick, L.; Daniel, M. What Is Nanotechnology and Why Does It Matter? From Science to Ethics; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 3–5. [Google Scholar]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol. 2015, 88, 20150207. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Tessmer, I.; Kaur, P.; Lin, J.; Wang, H. Investigating bioconjugation by atomic force microscopy. J. Nanobiotechnol. 2013, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Shiraishi, K.; Sanada, Y.; Mochizuki, S.; Kawano, K.; Maitani, Y.; Sakurai, K.; Yokoyama, M. Determination of polymeric micelles’ structural characteristics, and effect of the characteristics on pharmacokinetic behaviors. J. Control Release 2015, 203, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 714–729. [Google Scholar] [CrossRef]
- Gothwal, A.; Khan, I.; Gupta, U. Polymeric micelles: Recent Advancements in the Delivery of Anticancer Drugs. Pharm. Res. 2016, 33, 18–39. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Jiang, Y.; Cheng, R.; Meng, F.; Zhong, Z. Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: Promises, progress and prospects. Nano Today 2012, 7, 467–480. [Google Scholar] [CrossRef]
- Bisht, R.; Jaiswal, J.K.; Chen, Y.S.; Jin, J.; Rupenthal, I.D. Light-responsive in situ forming injectable implants for effective drug delivery to the posterior segment of the eye. Expert Opin. Drug Deliv. 2016, 13, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, H.M.; Lavasanifar, A. Polymeric micelles for drug delivery. Expert Opin. Drug Deliv. 2006, 3, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Zhong, W.; Ren, X.; Sha, X.; Fang, X. Matrix metalloproteinases-2/9-sensitive peptide-conjugated polymer micelles for site-specific release of drugs and enhancing tumor accumulation: Preparation and in vitro and in vivo evaluation. Int. J. Nanomed. 2016, 11, 1643–1661. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.Y.; Li, Y.J.; Liu, X.Y.; Cai, J.X.; Hu, X.B.; Wang, J.M.; Tang, T.T.; Xiang, D.X. 3,5,4′-trimethoxy-trans-stilbene loaded PEG-PE micelles for the treatment of colon cancer. Int. J. Nanomed. 2019, 14, 7489–7502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Li, Y.; Chen, K.; Zhang, L.; Qiao, S.; Tan, G.; Chen, F.; Pan, W. Dual receptor-targeted and redox-sensitive polymeric micelles self-assembled from a folic acid-hyaluronic acid-SS-vitamin E succinate polymer for precise cancer therapy. Int. J. Nanomed. 2020, 15, 2885–2902. [Google Scholar] [CrossRef] [Green Version]
- Niu, J.; Yuan, M.; Chen, C.; Wang, L.; Tang, Z.; Fan, Y.; Liu, X.; Ma, Y.J.; Gan, Y. Berberine-Loaded Thiolated Pluronic F127 Polymeric Micelles for Improving Skin Permeation and Retention. Int. J. Nanomed. 2020, 15, 9987–10005. [Google Scholar] [CrossRef]
- Zhu, W.T.; Liu, S.Y.; Wu, L.; Xu, H.L.; Wang, J.; Ni, G.X.; Zeng, Q.B. Delivery of curcumin by directed self-assembled micelles enhances therapeutic treatment of non-small-cell lung cancer. Int. J. Nanomed. 2017, 12, 2621–2634. [Google Scholar] [CrossRef] [Green Version]
- Martins, Y.A.; Fonseca, M.; Pavan, T.Z.; Lopez, R. Bifunctional therapeutic application of low-frequency ultrasound associated with zinc phthalocyanine-loaded micelles. Int. J. Nanomed. 2020, 15, 8075–8095. [Google Scholar] [CrossRef]
- Liu, Y.; Scrivano, L.; Peterson, J.D.; Fens, M.; Hernández, I.B.; Mesquita, B.; Toraño, J.S.; Hennink, W.E.; van Nostrum, C.F.; Oliveira, S. EGFR-targeted nanobody functionalized polymeric micelles loaded with mTHPC for selective photodynamic therapy. Mol. Pharm. 2020, 17, 1276–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Wang, Y.; Shi, H.; Dong, M.; Han, H.; Li, Q. Nucleolin-targeting AS1411 aptamer-modified micelle for the co-delivery of doxorubicin and miR-519c to Improve the therapeutic efficacy in hepatocellular carcinoma treatment. Int. J. Nanomed. 2021, 16, 2569–2584. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Seo, M.J.; Deci, M.B.; Weil, B.R.; Canty, J.M.; Nguyen, J. Effect of CCR2 inhibitor-loaded lipid micelles on inflammatory cell migration and cardiac function after myocardial infarction. Int. J. Nanomed. 2018, 13, 6441–6451. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Xu, C.; Li, N.; Zhang, S.; Fu, L.; Chu, X.; Hua, H.; Zeng, X.; Zhao, Y. Folate receptor-targeted mixed polysialic acid micelles for combating rheumatoid arthritis: In vitro and in vivo evaluation. Drug Deliv. 2018, 25, 1182–1191. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, W.; Ma, B.; Hu, J.; Jiang, J.; Li, G.; Yang, L.; Wang, Y. Two-photon AIE luminogen labeled multifunctional polymeric micelles for theranostics. Theranostics 2019, 9, 6618–6630. [Google Scholar] [CrossRef]
- Almeida, B.; Nag, O.K.; Rogers, K.E.; Delehanty, J.B. Recent Progress in Bioconjugation Strategies for Liposome-Mediated Drug Delivery. Molecules 2020, 25, 5672. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, P.; Pal, P.; Das, A.K.; Ray, S.; Bhattacharjee, A.; Mazumder, B. Nano lipid-drug conjugate: An integrated review. Int. J. Pharm. 2017, 529, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozafari, M.R. Liposomes: An overview of manufacturing techniques. Cell. Mol. Biol. Lett. 2005, 10, 711–719. [Google Scholar]
- Yan, Y.; Li, X.Q.; Duan, J.L.; Bao, C.J.; Cui, Y.N.; Su, Z.B.; Xu, J.R.; Luo, Q.; Chen, M.; Xie, Y.; et al. Nanosized functional miRNA liposomes and application in the treatment of TNBC by silencing Slug gene. Int. J. Nanomed. 2019, 14, 3645–3667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.T.; Tang, W.; Jiang, Y.; Wang, X.M.; Wang, Y.H.; Cheng, L.; Meng, X.S. Multifunctional targeting vinorelbine plus tetrandrine liposomes for treating brain glioma along with eliminating glioma stem cells. Oncotarget 2016, 7, 24604–24622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alaarg, A.; Jordan, N.Y.; Verhoef, J.J.; Metselaar, J.M.; Storm, G.; Kok, R.J. Docosahexaenoic acid liposomes for targeting chronic inflammatory diseases and cancer: An in vitro assessment. Int. J. Nanomed. 2016, 11, 5027–5040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sine, J.; Urban, C.; Thayer, D.; Charron, H.; Valim, N.; Tata, D.B.; Schiff, R.; Blumenthal, R.; Joshi, A.; Puri, A. Photo activation of HPPH encapsulated in “Pocket” liposomes triggers multiple drug release and tumor cell killing in mouse breast cancer xenografts. Int. J. Nanomed. 2014, 10, 125–145. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, L.; Jiang, W.; Yang, Z.; Gan, Z.; Yu, C.; Tao, R.; Chen, H. In vitro and in vivo evaluation of liposomes modified with polypeptides and red cell membrane as a novel drug delivery system for myocardium targeting. Drug Deliv. 2020, 27, 599–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Hong, S.S.; Lee, M.; Lee, E.H.; Rhee, I.; Chang, S.Y.; Lim, S.J. Krill oil-incorporated liposomes as an effective nanovehicle to ameliorate the inflammatory responses of DSS-induced colitis. Int. J. Nanomed. 2020, 14, 8305–8320. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.C.; Lin, C.C. Rescuing apoptotic neurons in Alzheimer’s disease using wheat germ agglutinin-conjugated and cardiolipin-conjugated liposomes with encapsulated nerve growth factor and curcumin. Int. J. Nanomed. 2015, 10, 2653–2672. [Google Scholar] [CrossRef] [Green Version]
- Vanić, Ž.; Rukavina, Z.; Manner, S.; Fallarero, A.; Uzelac, L.; Kralj, M.; Amidžić Klarić, D.; Bogdanov, A.; Raffai, T.; Virok, D.P.; et al. Azithromycin-liposomes as a novel approach for localized therapy of cervicovaginal bacterial infections. Int. J. Nanomed. 2019, 14, 5957–5976. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Fernandez, S.; Pujol-Autonell, I.; Brianso, F.; Perna-Barrull, D.; Cano-Sarabia, M.; Garcia-Jimeno, S.; Villalba, A.; Sanchez, A.; Aguilera, E.; Vazquez, F.; et al. Phosphatidylserine-liposomes promote tolerogenic features on dendritic cells in human type 1 diabetes by apoptotic mimicry. Front. Immunol. 2018, 9, 253. [Google Scholar] [CrossRef] [Green Version]
- Dias, A.P.; da Silva Santos, S.; da Silva, J.V.; Parise-Filho, R.; Ferreira, E.I.; El Seoud, O.; Giarolla, J. Dendrimers in the context of nanomedicine. Int. J. Pharm. 2020, 573, 118814. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.J.; Bugno, J.; Lee, S.R.; Hong, S. Dendrimer-based nanocarriers: A versatile platform for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Srinageshwar, B.; Peruzzaro, S.; Andrews, M.; Johnson, K.; Hietpas, A.; Clark, B.; McGuire, C.; Petersen, E.; Kippe, J.; Stewart, A.; et al. PAMAM Dendrimers Cross the Blood-Brain Barrier When Administered through the Carotid Artery in C57BL/6J Mice. Int. J. Mol. Sci. 2017, 18, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vacas-Córdoba, E.; Galán, M.; de la Mata, F.J.; Gómez, R.; Pion, M.; Muñoz-Fernández, M.Á. Enhanced activity of carbosilane dendrimers against HIV when combined with reverse transcriptase inhibitor drugs: Searching for more potent microbicides. Int. J. Nanomed. 2014, 9, 3591–3600. [Google Scholar] [CrossRef] [Green Version]
- Somani, S.; Laskar, P.; Altwaijry, N.; Kewcharoenvong, P.; Irving, C.; Robb, G.; Pickard, B.S.; Dufès, C. PEGylation of polypropylenimine dendrimers: Effects on cytotoxicity, DNA condensation, gene delivery and expression in cancer cells. Sci. Rep. 2018, 8, 9410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulhari, H.; Pooja, D.; Shrivastava, S.; Kuncha, M.; Naidu, V.; Bansal, V.; Sistla, R.; Adams, D.J. Trastuzumab-grafted PAMAM dendrimers for the selective delivery of anticancer drugs to HER2-positive breast cancer. Sci. Rep. 2016, 6, 23179. [Google Scholar] [CrossRef]
- Akhtar, S.; Al-Zaid, B.; El-Hashim, A.Z.; Chandrasekhar, B.; Attur, S.; Yousif, M.H.; Benter, I.F. Cationic polyamidoamine dendrimers as modulators of EGFR signaling in vitro and in vivo. PLoS ONE 2015, 10, e0132215. [Google Scholar] [CrossRef]
- Michlewska, S.; Ionov, M.; Szwed, A.; Rogalska, A.; Sanz Del Olmo, N.; Ortega, P.; Denel, M.; Jacenik, D.; Shcharbin, D.; de la Mata, F.J.; et al. Ruthenium Dendrimers against Human Lymphoblastic Leukemia 1301 Cells. Int. J. Mol. Sci. 2020, 21, 4119. [Google Scholar] [CrossRef] [PubMed]
- Günther, S.C.; Maier, J.D.; Vetter, J.; Podvalnyy, N.; Khanzhin, N.; Hennet, T.; Stertz, S. Antiviral potential of 3′-sialyllactose- and 6′-sialyllactose-conjugated dendritic polymers against human and avian influenza viruses. Sci. Rep. 2020, 10, 768. [Google Scholar] [CrossRef] [Green Version]
- Kambhampati, S.P.; Clunies-Ross, A.J.; Bhutto, I.; Mishra, M.K.; Edwards, M.; McLeod, D.S.; Kannan, R.M.; Lutty, G. Systemic and Intravitreal Delivery of Dendrimers to Activated Microglia/Macrophage in Ischemia/Reperfusion Mouse Retina. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4413–4424. [Google Scholar] [CrossRef] [Green Version]
- Wrobel, D.; Kolanowska, K.; Gajek, A.; Gomez-Ramirez, R.; de la Mata, J.; Pedziwiatr-Werbicka, E.; Klajnert, B.; Waczulikova, I.; Bryszewska, M. Interaction of cationic carbosilane dendrimers and their complexes with siRNA with erythrocytes and red blood cell ghosts. Biochim. Biophys. Acta 2014, 1838, 882–889. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.H.; Bryant, H.; Shapsa, A.; Street, H.; Mani, V.; Fayad, Z.A.; Frank, J.A.; Tsimikas, S.; Briley-Saebo, K.C. Manganese G8 dendrimers targeted to oxidation-specific epitopes: In vivo MR imaging of atherosclerosis. J. Magn. Reson. Imaging JMRI 2015, 41, 797–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Yang, S.; Wang, L.; Feng, H. Use of poly (amidoamine) dendrimer for dentinal tubule occlusion: A preliminary study. PLoS ONE 2015, 10, e0124735. [Google Scholar] [CrossRef] [PubMed]
- Tegos, G.P.; Demidova, T.N.; Arcila-lopez, D.; Lee, H.; Wharton, T.; Gali, H.; Hamblin, M.R. Cationic fullerenes are effective and selective antimicrobial photosensitizers. NIH Public Access 2011, 12, 1127–1135. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Yang, Z.; Jiang, W.; Geng, C.; Gong, M.; Xiao, H.; Wang, Z.; Cheng, L. Antiviral activity of nano carbon fullerene lipidosome against influenza virus in vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 2008, 28, 243–246. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon nanotube—A review on Synthesis, Properties and plethora of applications in the field of biomedical science. Sens. Int. 2020, 1, 1–10. [Google Scholar] [CrossRef]
- Tangboriboon, N. Carbon and carbon nanotube drug delivery and its characterization, properties, and applications. Nanocarriers Drug Deliv. 2019, 2019, 451–467. [Google Scholar]
- Fujita, K.; Fukuda, M.; Endoh, S.; Maru, J.; Kato, H.; Nakamura, A.; Shinohara, N.; Uchino, K.; Honda, K. Size effects of single-walled carbon nanotubes on in vivo and in vitro pulmonary toxicity. Inhal. Toxicol. 2015, 27, 207–223. [Google Scholar] [CrossRef] [Green Version]
- Komane, P.P.; Kumar, P.; Choonara, Y.E. Atrial Natriuretic Peptide Antibody-Functionalised, PEGylated Multiwalled Carbon Nanotubes for Targeted Ischemic Stroke Intervention. Pharmaceutics 2021, 13, 1357. [Google Scholar] [CrossRef]
- Romano-Feinholz, S.; Salazar-Ramiro, A.; Muñoz-Sandoval, E.; Magaña-Maldonado, R.; Hernández Pedro, N.; Rangel López, E.; González Aguilar, A.; Sánchez García, A.; Sotelo, J.; Pérez de la Cruz, V.; et al. Cytotoxicity induced by carbon nanotubes in experimental malignant glioma. Int. J. Nanomed. 2017, 12, 6005–6026. [Google Scholar] [CrossRef] [Green Version]
- Komane, P.P.; Kumar, P.; Marimuthu, T.; Toit, L.; Kondiah, P.; Choonara, Y.E.; Pillay, V. Dexamethasone-loaded, PEGylated, vertically Aligned, multiwalled carbon nanotubes for potential ischemic stroke intervention. Molecules 2018, 23, 1406. [Google Scholar] [CrossRef] [Green Version]
- Huzil, J.T.; Saliaj, E.; Ivanova, M.V.; Gharagozloo, M.; Loureiro, M.J.; Lamprecht, C.; Korinek, A.; Chen, D.W.; Foldvari, M. Selective nuclear localization of siRNA by metallic versus semiconducting single wall carbon nanotubes in keratinocytes. Future Sci. OA 2015, 1, FSO17. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, S.; Zhao, G.; Fu, X.; Xie, X.; Huang, Y.; Cheng, X.; Wei, J.; Liu, H.; Lai, Z. Long-term intravenous administration of carboxylated single-walled carbon nanotubes induces persistent accumulation in the lungs and pulmonary fibrosis via the nuclear factor-kappa B pathway. Int. J. Nanomed. 2016, 12, 263–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Zhu, Z.; Xiao, W.; Li, L. Multi-walled carbon nanotubes promote cementoblast differentiation and mineralization through the TGF-β/Smad signaling pathway. Int. J. Mol. Sci. 2015, 16, 3188–3201. [Google Scholar] [CrossRef] [Green Version]
- Polimeni, M.; Gulino, G.R.; Gazzano, E.; Kopecka, J.; Marucco, A.; Fenoglio, I.; Cesano, F.; Campagnolo, L.; Magrini, A.; Pietroiusti, A.; et al. Multi-walled carbon nanotubes directly induce epithelial-mesenchymal transition in human bronchial epithelial cells via the TGF-β-mediated Akt/GSK-3β/SNAIL-1 signalling pathway. Part. Fibre Toxicol. 2016, 13, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangamuthu, M.; Gabriel, W.E.; Santschi, C.; Martin, O. Electrochemical sensor for bilirubin detection using screen printed electrodes functionalized with carbon nanotubes and graphene. Sensors 2018, 18, 800. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R. The use of gold nanoparticles in diagnostics and detection. Chem. Soc. Rev. 2008, 37, 2028–2045. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Shahid, S.; Ayaz, A.; Alkahtani, J.; Elshikh, M.S.; Riaz, T. Phytomolecules-coated NiO nanoparticles synthesis using Abutilon indicum leaf extract: Antioxidant, antibacterial, and anticancer activities. Int. J. Nanomed. 2021, 16, 1757–1773. [Google Scholar] [CrossRef]
- Majoumouo, M.S.; Sharma, J.R.; Sibuyi, N.; Tincho, M.B.; Boyom, F.F.; Meyer, M. Synthesis of biogenic gold nanoparticles from Terminalia mantaly extracts and the evaluation of their in vitro cytotoxic effects in cancer cells. Molecules 2020, 25, 4469. [Google Scholar] [CrossRef]
- Khandel, P.; Shahi, S.K.; Soni, D.K.; Yadaw, R.K.; Kanwar, L. Alpinia calcarata: Potential source for the fabrication of bioactive silver nanoparticles. Nano Converg. 2018, 5, 37. [Google Scholar] [CrossRef]
- Kamli, M.R.; Srivastava, V.; Hajrah, N.H.; Sabir, J.; Hakeem, K.R.; Ahmad, A.; Malik, M.A. Facile bio-fabrication of Ag-Cu-Co trimetallic nanoparticles and its fungicidal activity against Candida auris. J. Fungi 2021, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Adewale, O.B.; Egbeyemi, K.A.; Onwuelu, J.O.; Potts-Johnson, S.S.; Anadozie, S.O.; Fadaka, A.O.; Osukoya, O.A.; Aluko, B.T.; Johnson, J.; Obafemi, T.O.; et al. Biological synthesis of gold and silver nanoparticles using leaf extracts of Crassocephalum rubens and their comparative in vitro antioxidant activities. Heliyon 2020, 6, e05501. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, I.A.; Usman, A.I.; Shittu, F.B.; Ismail, N.Z.; Arsad, H.; Muftaudeen, T.K.; Samian, M.R. Boswellia dalzielii-mediated silver nanoparticles inhibited acute myeloid leukemia (AML) kasumi-1 cells by inducing cell cycle arrest. Bioinorg. Chem. Appl. 2020, 2020, 8898360. [Google Scholar] [CrossRef]
- El-Zayat, M.M.; Eraqi, M.M.; Alrefai, H.; El-Khateeb, A.Y.; Ibrahim, M.A.; Aljohani, H.M.; Aljohani, M.M.; Elshaer, M.M. The Antimicrobial, Antioxidant, and Anticancer Activity of Greenly Synthesized Selenium and Zinc Composite Nanoparticles Using Ephedra aphylla Extract. Biomolecules 2021, 11, 470. [Google Scholar] [CrossRef]
- Ren, S.X.; Zhan, B.; Lin, Y.; Ma, D.S.; Yan, H. Selenium nanoparticles dispersed in phytochemical exert anti-inflammatory activity by modulating catalase, GPx1, and COX-2 gene expression in a rheumatoid arthritis rat model. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 991–1000. [Google Scholar] [CrossRef]
- Amina, M.; Al Musayeib, N.M.; Alarfaj, N.A.; El-Tohamy, M.F.; Al-Hamoud, G.A. Antibacterial and immunomodulatory potentials of biosynthesized Ag, Au, Ag-Au bimetallic alloy nanoparticles using the Asparagus racemosus root extract. Nanomaterials 2020, 10, 2453. [Google Scholar] [CrossRef] [PubMed]
- Nellore, J.; Pauline, C.; Amarnath, K. Bacopa monnieri phytochemicals mediated synthesis of platinum nanoparticles and its neurorescue effect on 1-methyl 4-phenyl 1,2,3,6 tetrahydropyridine-induced experimental Parkinsonism in Zebrafish. J. Neurodegener. Dis. 2013, 2013, 972391. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.; Jin, J.; Wang, Z.; Wang, Y.; Gao, Q.; Zhao, J. The metal nanoparticle-induced inflammatory response is regulated by SIRT1 through NF-κB deacetylation in aseptic loosening. Int. J. Nanomed. 2017, 12, 3617–3636. [Google Scholar] [CrossRef] [Green Version]
- Wan, R.; Mo, Y.; Feng, L.; Chien, S.; Tollerud, D.J.; Zhang, Q. DNA damage caused by metal nanoparticles: Involvement of oxidative stress and activation of ATM. Chem. Res. Toxicol. 2012, 25, 1402–1411. [Google Scholar] [CrossRef] [Green Version]
- Ashton, J.R.; Castle, K.D.; Qi, Y.; Kirsch, D.G.; West, J.L.; Badea, C.T. Dual-energy CT imaging of tumor liposome delivery after gold nanoparticle-augmented radiation therapy. Theranostics 2018, 8, 1782–1797. [Google Scholar] [CrossRef]
- Lu, L.; Li, K.; Mao, Y.H.; Qu, H.; Yao, B.; Zhong, W.W.; Ma, B.; Wang, Z.Y. Gold-chrysophanol nanoparticles suppress human prostate cancer progression through inactivating AKT expression and inducing apoptosis and ROS generation in vitro and in vivo. Int. J. Oncol. 2017, 51, 1089–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Sarkar, T.; Choudhury, B.K.; Barman, A.; Bhattacharjya, D.; Srivastava, A.; Baishya, D.; Edinur, H.A.; et al. Green synthesis of gold nanoparticles using plant extracts as beneficial prospect for cancer theranostics. Molecules 2021, 26, 6389. [Google Scholar] [CrossRef] [PubMed]
- Boomi, P.; Ganesan, R.; Prabu Poorani, G.; Jegatheeswaran, S.; Balakumar, C.; Gurumallesh Prabu, H.; Anand, K.; Marimuthu Prabhu, N.; Jeyakanthan, J.; Saravanan, M. Phyto-engineered gold nanoparticles (AuNPs) with potential antibacterial, antioxidant, and wound healing activities under in vitro and in vivo conditions. Int. J. Nanomed. 2020, 15, 7553–7568. [Google Scholar] [CrossRef] [PubMed]
- Abdulrehman, T.; Qadri, S.; Skariah, S.; Sultan, A.; Mansour, S.; Azzi, J.; Haik, Y. Boron doped silver-copper alloy nanoparticle targeting intracellular S. aureus in bone cells. PLoS ONE 2020, 15, e0231276. [Google Scholar] [CrossRef]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 8013–8024. [Google Scholar] [CrossRef] [Green Version]
- Ullah, I.; Khalil, A.T.; Ali, M.; Iqbal, J.; Ali, W.; Alarifi, S.; Shinwari, Z.K. Green-Synthesized Silver Nanoparticles Induced Apoptotic Cell Death in MCF-7 Breast Cancer Cells by Generating Reactive Oxygen Species and Activating Caspase 3 and 9 Enzyme Activities. Oxidative Med. Cell. Longev. 2020, 2020, 1215395. [Google Scholar] [CrossRef]
- Vinzant, N.; Scholl, J.L.; Wu, C.M.; Kindle, T.; Koodali, R.; Forster, G.L. Iron Oxide Nanoparticle Delivery of Peptides to the Brain: Reversal of Anxiety during Drug Withdrawal. Front. Neurosci. 2017, 11, 608. [Google Scholar] [CrossRef] [Green Version]
- Hauser, A.K.; Anderson, K.W.; Hilt, J.Z. Peptide conjugated magnetic nanoparticles for magnetically mediated energy delivery to lung cancer cells. Nanomedicine 2016, 11, 1769–1785. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.K.; Kim, T.J.; Kim, Y.J.; Kang, L.; Kim, J.; Lee, N.; Hyeon, T.; Lim, M.S.; Mo, H.J.; Shin, J.H.; et al. Targeted Delivery of Iron Oxide Nanoparticle-Loaded Human Embryonic Stem Cell-Derived Spherical Neural Masses for Treating Intracerebral Hemorrhage. Int. J. Mol. Sci. 2020, 21, 3658. [Google Scholar] [CrossRef]
- He, H.; Zou, Z.; Wang, B.; Xu, G.; Chen, C.; Qin, X.; Yu, C.; Zhang, J. Copper oxide nanoparticles induce oxidative DNA damage and cell death via copper ion-mediated P38 MAPK activation in vascular endothelial cells. Int. J. Nanomed. 2020, 15, 3291–3302. [Google Scholar] [CrossRef]
- Kim, H.; Beack, S.; Han, S.; Shin, M.; Lee, T.; Park, Y.; Kim, K.S.; Yetisen, A.K.; Yun, S.H.; Kwon, W.; et al. Multifunctional photonic nanomaterials for diagnostic, therapeutic, and theranostic applications. Adv. Mater. 2018, 30, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Kania, K.D.; Wagner, W.; Pułaski, Ł. CdSe/ZnS core-shell-type quantum dot nanoparticles disrupt the cellular homeostasis in cellular blood-brain barrier models. Int. J. Mol. Sci. 2021, 22, 1068. [Google Scholar] [CrossRef]
- Gorshkov, K.; Susumu, K.; Chen, J.; Xu, M.; Pradhan, M.; Zhu, W.; Hu, X.; Breger, J.C.; Wolak, M.; Oh, E. Quantum dot-conjugated SARS-CoV-2 spike pseudo-virions enable tracking of angiotensin converting enzyme 2 binding and endocytosis. ACS Nano 2020, 14, 12234–12247. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, M. Dysfunction of various organelles provokes multiple cell death after quantum dot exposure. Int. J. Nanomed. 2018, 13, 2729–2742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundrotas, G.; Karabanovas, V.; Pleckaitis, M.; Juraleviciute, M.; Steponkiene, S.; Gudleviciene, Z.; Rotomskis, R. Uptake and distribution of carboxylated quantum dots in human mesenchymal stem cells: Cell growing density matters. J. Nanobiotechnol. 2019, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; Jeong, H.Y.; Kang, S.J.; Choi, M.J.; You, Y.M.; Im, C.S.; Lee, T.S.; Song, I.H.; Lee, C.G.; Rhee, K.J.; et al. Cancer-targeted Nucleic Acid Delivery and Quantum Dot Imaging Using EGF Receptor Aptamer-conjugated Lipid Nanoparticles. Sci. Rep. 2017, 7, 9474. [Google Scholar] [CrossRef] [Green Version]
- Shao, D.; Li, J.; Guan, F.; Pan, Y.; Xiao, X.; Zhang, M.; Zhang, H.; Chen, L. Selective inhibition of liver cancer growth realized by the intrinsic toxicity of a quantum dot-lipid complex. Int. J. Nanomed. 2014, 9, 5753–5769. [Google Scholar] [CrossRef] [Green Version]
- Wahab, R.; Kaushik, N.; Khan, F.; Kaushik, N.K.; Lee, S.J.; Choi, E.H.; Al-Khedhairy, A.A. Gold quantum dots impair the tumorigenic potential of glioma stem-like cells via β-catenin downregulation in vitro. Int. J. Nanomed. 2019, 14, 1131–1148. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Yin, X.; Cai, Y.; Xu, W.; Song, C.; Wang, Y.; Zhang, J.; Kang, A.; Wang, Z.; Han, W. Antitumor effect of a Pt-loaded nanocomposite based on graphene quantum dots combats hypoxia-induced chemoresistance of oral squamous cell carcinoma. Int. J. Nanomed. 2018, 13, 1505–1524. [Google Scholar] [CrossRef] [Green Version]
- Liang, G.; Li, Y.; Feng, W.; Wang, X.; Jing, A.; Li, J.; Ma, K. Polyethyleneimine-coated quantum dots for miRNA delivery and its enhanced suppression in HepG2 cells. Int. J. Nanomed. 2016, 11, 6079–6088. [Google Scholar] [CrossRef] [Green Version]
- Boltnarova, B.; Kubackova, J.; Skoda, J.; Stefela, A.; Smekalova, M.; Svacinova, P.; Pavkova, I.; Dittrich, M.; Scherman, D.; Zbytovska, J.; et al. PLGA Based Nanospheres as a Potent Macrophage-Specific Drug Delivery System. Nanomaterials 2021, 11, 749. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, N.; Hillaireau, H.; Vergnaud, J.; Tsapis, N.; Pallardy, M.; Kerdine-Römer, S.; Fattal, E. Surface coating mediates the toxicity of polymeric nanoparticles towards human-like macrophages. Int. J. Pharm. 2015, 482, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Gwak, S.J.; Yun, Y.; Yoon, D.H.; Kim, K.N.; Ha, Y. Therapeutic use of 3β-[N-(N′,N′-dimethylaminoethane) carbamoyl] cholesterol-modified PLGA nanospheres as gene delivery vehicles for spinal cord injury. PLoS ONE 2016, 11, e0147389. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.H.; Xu, S.Y.; Shan, Y.H.; Wei, W.; Liu, S.; Zhang, C.Z.; Wu, J.H.; Liang, W.Q.; Gao, J.Q. Sequential release of salidroside and paeonol from a nanosphere-hydrogel system inhibits ultraviolet B-induced melanogenesis in guinea pig skin. Int. J. Nanomed. 2014, 9, 1897–1908. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Deng, G.; Ji, A.; Yao, J.; Meng, X.; Wang, J.; Wang, Q.; Wang, Q.; Wang, R. Porous Se@SiO2 nanospheres treated paraquat-induced acute lung injury by resisting oxidative stress. Int. J. Nanomed. 2017, 12, 7143–7152. [Google Scholar] [CrossRef] [Green Version]
- Yoshimoto, Y.; Jo, J.I.; Tabata, Y. Preparation of antibody-immobilized gelatin nanospheres incorporating a molecular beacon to visualize the biological function of macrophages. Regen. Ther. 2020, 14, 11–18. [Google Scholar] [CrossRef]
- Rezaei, R.; Safaei, M.; Mozaffari, H.R.; Moradpoor, H.; Karami, S.; Golshah, A.; Salimi, B.; Karami, H. The Role of Nanomaterials in the Treatment of Diseases and Their Effects on the Immune System. Open Access Maced. J. Med. Sci. 2019, 7, 1884–1890. [Google Scholar] [CrossRef] [Green Version]
- Wuang, S.C.; Neoh, K.G.; Kang, E.T.; Pack, D.W.; Leckband, D.E. HER-2-mediated endocytosis of magnetic nanospheres and the implications in cell targeting and particle magnetization. Biomaterials 2008, 29, 2270–2279. [Google Scholar] [CrossRef] [Green Version]
- Portilho, F.A.; Cavalcanti, C.E.; Miranda-Vilela, A.L.; Estevanato, L.L.; Longo, J.P.; Almeida Santos, M.; Bocca, A.L.; Martins, O.P.; Simioni, A.R.; Morais, P.C.; et al. Antitumor activity of photodynamic therapy performed with nanospheres containing zinc-phthalocyanine. J. Nanobiotechnol. 2013, 11, 41. [Google Scholar] [CrossRef] [Green Version]
- Herrán, E.; Requejo, C.; Ruiz-Ortega, J.A.; Aristieta, A.; Igartua, M.; Bengoetxea, H.; Ugedo, L.; Pedraz, J.L.; Lafuente, J.V.; Hernández, R.M. Increased antiparkinson efficacy of the combined administration of VEGF- and GDNF-loaded nanospheres in a partial lesion model of Parkinson’s disease. Int. J. Nanomed. 2014, 9, 2677–2687. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Deng, G.; Qi, C.; Xu, Y.; Liu, X.; Zhao, Z.; Zhang, Z.; Chu, Y.; Wu, H.; Liu, J. Porous Se@SiO2 nanospheres attenuate ischemia/reperfusion (I/R)-induced acute kidney injury (AKI) and inflammation by antioxidative stress. Int. J. Nanomed. 2018, 14, 215–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Xu, X.; Jiang, L.; Ji, H.; Zhu, F.; Jin, B.; Han, J.; Dong, X.; Yang, F.; Li, B. Targeted antitumor mechanism of C-PC/CMC-CD55sp nanospheres in HeLa cervical cancer cells. Front. Pharmacol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Afzali, E.; Eslaminejad, T.; Yazdi Rouholamini, S.E.; Shahrokhi-Farjah, M.; Ansari, M. Cytotoxicity effects of curcumin loaded on chitosan alginate nanospheres on the KMBC-10 spheroids cell line. Int. J. Nanomed. 2021, 16, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Di, Y.; Jin, C.; Fu, D.; Yang, F.; Jiang, Y.; Yao, L.; Hao, S.; Wang, X.; Subedi, S.; et al. Gemcitabine-loaded albumin nanospheres (GEM-ANPs) inhibit PANC-1 cells in vitro and in vivo. Nanoscale Res. Lett. 2013, 8, 176. [Google Scholar] [CrossRef] [Green Version]
- Mulikova, T.; Abduraimova, A.; Molkenova, A.; Em, S.; Duisenbayeva, B.; Han, D.W.; Atabaev, T.S. Mesoporous silica decorated with gold nanoparticles as a promising nanoprobe for effective CT X-ray attenuation and potential drug delivery. Nano-Struct. Nano-Objects 2021, 26, 100712. [Google Scholar] [CrossRef]
- Monem, A.S.; Elbialy, N.; Mohamed, N. Mesoporous silica coated gold nanorods loaded doxorubicin for combined chemo-photothermal therapy. Int. J. Pharm. 2014, 470, 1–7. [Google Scholar] [CrossRef]
- Yudasaka, M.; Ajima, K.; Suenaga, K.; Ichihashi, T.; Hashimoto, A.; Iijima, S. Nano-extraction and nano-condensation for C60 incorporation into single-wall carbon nanotubes in liquid phases. Chem. Phys. Lett. 2003, 380, 42–46. [Google Scholar] [CrossRef]
- Lai, P.-S.; Lou, P.-J.; Peng, C.-L.; Pai, C.-L.; Yen, W.-N.; Huang, M.-Y.; Young, T.-H.; Shieh, M.-J. Doxorubicin delivery by polyamidoamine dendrimer conjugation and photochemical internalization for cancer therapy. J. Control Release 2007, 122, 39–46. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugation Techniques; Academic Press: London, UK, 2008. [Google Scholar]
- Capehart, S.L.; ElSohly, A.M.; Obermeyer, A.C.; Francis, M.B. Bioconjugation of gold nanoparticles through the oxidative coupling of ortho-aminophenols and anilines. Bioconjugate Chem. 2014, 25, 1888–1892. [Google Scholar] [CrossRef]
- Politi, J.; De Stefano, L.; Rea, I.; Gravagnuolo, A.M.; Giardina, P.; Methivier, C.; Casale, S.; Spadavecchia, J. One-pot synthesis of a gold nanoparticle–Vmh2 hydrophobin nanobiocomplex for glucose monitoring. Nanotechnology 2016, 27, 195701. [Google Scholar] [CrossRef]
- Mwai, L.M.; Kyama, M.C.; Ngugi, C.W.; Walong, E. Bioconjugation of AuNPs with HPV 16/18 E6 antibody through physical adsorption technique. J. Nanotechnol. Nanomater. 2020, 1, 16–22. [Google Scholar]
- Bloemen, M.; Van Stappen, T.; Willot, P.; Lammertyn, J.; Koeckelberghs, G.; Geukens, N.; Gils, A.; Verbiest, T. Heterobifunctional PEG ligands for bioconjugation reactions on iron oxide nanoparticles. PLoS ONE 2014, 9, e109475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Żuk, M.; Gawęda, W.; Majkowska-Pilip, A.; Osial, M.; Wolski, M.; Bilewicz, A.; Krysiński, P. Hybrid radiobioconjugated superparamagnetic iron oxide-based nanoparticles for multimodal cancer therapy. Pharmaceutics 2021, 13, 1843. [Google Scholar] [CrossRef] [PubMed]
- Busch, R.T.; Karim, F.; Weis, J.; Sun, Y.; Zhao, C.; Vasquez, E.S. Optimization and structural stability of gold nanoparticle-antibody bioconjugates. ACS Omega 2019, 4, 15269–15279. [Google Scholar] [CrossRef] [Green Version]
- Shevtsov, M.A.; Nikolaev, B.P.; Ryzhov, V.A.; Yakovleva, L.Y.; Dobrodumov, A.V.; Marchenko, Y.Y.; Margulis, B.A.; Pitkin, E.; Mikhrina, A.L.; Guzhova, I.V.; et al. Detection of experimental myocardium infarction in rats by MRI using heat shock protein 70 conjugated superparamagnetic iron oxide nanoparticle. Nanomedicine 2016, 12, 611–621. [Google Scholar] [CrossRef]
- Kulkarni, A.; Rao, P.; Natarajan, S.; Goldman, A.; Sabbisetti, V.S.; Khater, Y.; Korimerla, N.; Chandrasekar, V.; Mashelkar, R.A.; Sengupta, S. Reporter nanoparticle that monitors its anticancer efficacy in real time. Proc. Natl. Acad. Sci. USA 2016, 113, E2104–E2113. [Google Scholar] [CrossRef] [Green Version]
- Stawicki, C.M.; Rinker, T.E.; Burns, M.; Tonapi, S.S.; Galimidi, R.P.; Anumala, D.; Robinson, J.K.; Klein, J.S.; Mallick, P. Modular fluorescent nanoparticle DNA probes for detection of peptides and proteins. Sci. Rep. 2021, 11, 19921. [Google Scholar] [CrossRef]
- Gu, W.; Song, G.; Li, S.; Shao, C.; Yan, C.; Ye, L. Chlorotoxin-conjugated, PEGylated Gd2O3 nanoparticles as a glioma-specific magnetic resonance imaging contrast agent. RSC Adv. 2014, 4, 50254–50260. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, Z.; Zhou, R.; Su, X. Synthesis of gold nanoshuttles as efficient surface-enhanced raman scattering substrate for malachite green detection. Nanosci. Nanotechnol. Lett. 2016, 8, 135–139. [Google Scholar] [CrossRef]
- Aly, M.A.; Domig, K.J.; Kneifel, W.; Reimhult, E. Immunogold Nanoparticles for Rapid Plasmonic Detection of C. sakazakii. Sensors 2018, 18, 2028. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Fournier, P.; Mendoza-Lavaniegos, V.; Sengar, P.; Guerra-Olvera, F.M.; Iñiguez, E.; Kretzschmar, T.G.; Hirata, G.A.; Juárez, P. Functionalized rare earth-doped nanoparticles for breast cancer nanodiagnostic using fluorescence and CT imaging. J. Nanobiotechnol. 2018, 16, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkitt, S.; Mehraein, M.; Stanciauskas, R.K.; Campbell, J.; Fraser, S.; Zavaleta, C. Label-free visualization and tracking of gold nanoparticles in vasculature using multiphoton luminescence. Nanomaterials 2020, 10, 2239. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J.R.; Gottlin, E.B.; Patz, E.F., Jr.; West, J.L.; Badea, C.T. A comparative analysis of EGFR-targeting antibodies for gold nanoparticle CT imaging of lung cancer. PLoS ONE 2018, 13, e0206950. [Google Scholar] [CrossRef] [Green Version]
- Rayavarapu, R.G.; Petersen, W.; Ungureanu, C.; Post, J.N.; van Leeuwen, T.G.; Manohar, S. Synthesis and bioconjugation of gold nanoparticles as potential molecular probes for light-based imaging techniques. Int. J. Biomed. Imaging 2007, 2007, 29817. [Google Scholar] [CrossRef]
- Sakpakdeejaroen, I.; Somani, S.; Laskar, P.; Mullin, M.; Dufès, C. Regression of melanoma following intravenous injection of plumbagin entrapped in transferrin-conjugated, lipid-polymer hybrid nanoparticles. Int. J. Nanomed. 2021, 16, 2615–2631. [Google Scholar] [CrossRef]
- Baganizi, D.R.; Nyairo, E.; Duncan, S.A.; Singh, S.R.; Dennis, V.A. Interleukin-10 Conjugation to Carboxylated PVP-Coated Silver Nanoparticles for Improved Stability and Therapeutic Efficacy. Nanomaterials 2017, 7, 165. [Google Scholar] [CrossRef] [Green Version]
- Abd Ellah, N.H.; Abouelmagd, S.A. Surface functionalization of polymeric nanoparticles for tumor drug delivery: Approaches and challenges. Expert Opin. Drug Deliv. 2017, 14, 201–214. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Gindl-Altmutter, W.; Bacher, M.; Edler, M.; Griesser, T. Surface chemical functionalization of cellulose nanocrystals by 3-aminopropyltriethoxysilane. Int. J. Biol. Macromol. 2018, 106, 1288–1296, Erratum in Int. J. Biol. Macromol. 2018, 113, 1316. [Google Scholar] [CrossRef]
- Abdelkhaliq, A.; van der Zande, M.; Punt, A.; Helsdingen, R.; Boeren, S.; Vervoort, J.J.M.; Rietjens, I.M.C.M.; Bouwmeester, H. Impact of nanoparticle surface functionalization on the protein corona and cellular adhesion, uptake and transport. J. Nanobiotechnol. 2018, 16, 70. [Google Scholar] [CrossRef] [Green Version]
- Debnath, K.; Mandal, K.; Jana, N.R. Phase transfer and surface functionalization of hydrophobic nanoparticle using amphiphilic poly(amino acid). Langmuir 2016, 32, 2798–2807. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, Y.; Xia, H.; Gao, Q. Surface chemical functionalization of starch nanocrystals modified by 3-aminopropyl triethoxysilane. Int. J. Biol. Macromol. 2019, 126, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Pannuzzo, M.; Esposito, S.; Wu, L.P.; Key, J.; Aryal, S.; Celia, C.; di Marzio, L.; Moghimi, S.M.; Decuzzi, P. Overcoming nanoparticle-mediated complement activation by surface PEG pairing. Nano Lett. 2020, 20, 4312–4321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, X.; Kang, X.; Yang, H.; Guo, W.; Guan, L.; Wu, H.; Du, L. Surface functionalization of pegylated gold nanoparticles with antioxidants suppresses nanoparticle-induced oxidative stress and neurotoxicity. Chem. Res. Toxicol. 2020, 33, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, T.D.; Travassos, L.R.; Arruda, D.C.; Tada, D.B. Intracellular targeting of poly lactic-co-glycolic acid nanoparticles by surface functionalization with peptides. J. Biomed. Nanotechnol. 2021, 17, 1320–1329. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacurari, M.; Yin, X.J.; Zhao, J.; Ding, M.; Leonard, S.; Schwegler-Berry, D.; Ducatman, B.S.; Sbarra, D.; Hoover, M.D.; Castranova, V.; et al. Raw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-kB, and Akt in normal and malignant human mesothelial cells. Environ. Health Perspect. 2008, 116, 1211–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, X.; Wang, J.Y.; Bai, X.; Xu, F.; Liu, H.; Yang, J.; Jing, Y.; Liu, L.; Xue, X.; Dai, H.; et al. Black phosphorus quantum dot induced oxidative stress and toxicity in living cells and mice. ACS Appl. Mater. Interfaces 2017, 9, 20399–20409. [Google Scholar] [CrossRef]
- Quevedo, A.C.; Lynch, I.; Valsami-Jones, E. Silver nanoparticle induced toxicity and cell death mechanisms in embryonic zebrafish cells. Nanoscale 2021, 13, 6142–6161. [Google Scholar] [CrossRef]
- Ryvolova, M.; Chomoucka, J.; Drbohlavova, J.; Kopel, P.; Babula, P.; Hynek, D.; Adam, V.; Eckschlager, T.; Hubalek, J.; Stiborova, M.; et al. Modern micro and nanoparticle-based imaging techniques. Sensors 2012, 12, 14792–14820. [Google Scholar] [CrossRef]
- Aziz, F.; Ihsan, A.; Nazir, A.; Ahmad, I.; Bajwa, S.Z.; Rehman, A.; Diallo, A.; Khan, W.S. Novel route synthesis of porous and solid gold nanoparticles for investigating their comparative performance as contrast agent in computed tomography scan and effect on liver and kidney function. Int. J. Nanomed. 2017, 12, 1555–1563. [Google Scholar] [CrossRef] [Green Version]
- Sillerud, L.O.; Yang, Y.; Yang, L.Y.; Duval, K.B.; Thompson, J.; Yang, Y. Longitudinal monitoring of microglial/macrophage activation in ischemic rat brain using Iba-1-specific nanoparticle-enhanced magnetic resonance imaging. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2020, 40, S117–S133. [Google Scholar] [CrossRef]
- Jamburidze, A.; Huerre, A.; Baresch, D.; Poulichet, V.; De Corato, M.; Garbin, V. Nanoparticle-coated microbubbles for combined ultrasound imaging and drug delivery. Langmuir 2019, 35, 10087–10096. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.V.D.; Jacobsen, M.C.; Damasco, J.A.; Melancon, A.; Huang, S.Y.; Layman, R.R.; Melancon, M.P. Optimization of the differentiation and quantification of high-Z nanoparticles incorporated in medical devices for CT-guided interventions. Med. Phys. 2021, 48, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.A.; Webster, T.J. Antimicrobial selenium nanoparticle coatings on polymeric medical devices. Nanotechnology 2013, 24, 155101. [Google Scholar] [CrossRef] [PubMed]
- Rocca, D.M.; Aiassa, V.; Zoppi, A.; Silvero Compagnucci, J.; Becerra, M.C. Nanostructured gold coating for prevention of biofilm development in medical devices. J. Endourol. 2020, 34, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Meddahi-Pellé, A.; Legrand, A.; Marcellan, A.; Louedec, L.; Letourneur, D.; Leibler, L. Organ repair, hemostasis, and in vivo bonding of medical devices by aqueous solutions of nanoparticles. Angew. Chem. Int. Ed. Engl. 2014, 53, 6369–6373. [Google Scholar] [CrossRef] [Green Version]
- Seo, M.; Gorelikov, I.; Williams, R.; Matsuura, N. Microfluidic assembly of monodisperse, nanoparticle-incorporated perfluorocarbon microbubbles for medical imaging and therapy. Langmuir 2010, 26, 13855–13860. [Google Scholar] [CrossRef]
- Parracino, A.; Gajula, G.P.; di Gennaro, A.K.; Neves-Petersen, M.T.; Rafaelsen, J.; Petersen, S.B. Towards nanoscale biomedical devices in medicine: Biofunctional and spectroscopic characterization of superparamagnetic nanoparticles. J. Fluoresc. 2011, 21, 663–672. [Google Scholar] [CrossRef]
- Seeni, R.Z.; Yu, X.; Chang, H.; Chen, P.; Liu, L.; Xu, C. Iron oxide nanoparticle-powered micro-optical coherence tomography for in situ imaging the penetration and swelling of polymeric microneedles in the skin. ACS Appl. Mater. Interfaces 2017, 9, 20340–20347. [Google Scholar] [CrossRef]
- Piktel, E.; Suprewicz, Ł.; Depciuch, J.; Chmielewska, S.; Skłodowski, K.; Daniluk, T.; Król, G.; Kołat-Brodecka, P.; Bijak, P.; Pajor-Świerzy, A.; et al. Varied-shaped gold nanoparticles with nanogram killing efficiency as potential antimicrobial surface coatings for the medical devices. Sci. Rep. 2021, 11, 12546. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Greco, J.B.; Uluer, M.C.; Zhang, Z.; Zhang, Z.; Fishbein, K.W.; Spencer, R.G. Magnetic resonance imaging of chondrocytes labeled with superparamagnetic iron oxide nanoparticles in tissue-engineered cartilage. Tissue Eng. Part A 2009, 15, 3899–3910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.W.; Liou, G.G.; Pan, H.B.; Tseng, H.H.; Hung, Y.T.; Chou, C.P. Specific detection of CD133-positive tumor cells with iron oxide nanoparticles labeling using noninvasive molecular magnetic resonance imaging. Int. J. Nanomed. 2015, 10, 6997–7018. [Google Scholar] [CrossRef] [Green Version]
- Evertsson, M.; Kjellman, P.; Cinthio, M.; Andersson, R.; Tran, T.A.; In’t Zandt, R.; Grafström, G.; Toftevall, H.; Fredriksson, S.; Ingvar, C.; et al. Combined Magnetomotive ultrasound, PET/CT, and MR imaging of 68Ga-labelled superparamagnetic iron oxide nanoparticles in rat sentinel lymph nodes in vivo. Sci. Rep. 2017, 7, 4824. [Google Scholar] [CrossRef] [PubMed]
- Keliher, E.J.; Ye, Y.X.; Wojtkiewicz, G.R.; Aguirre, A.D.; Tricot, B.; Senders, M.L.; Groenen, H.; Fay, F.; Perez-Medina, C.; Calcagno, C.; et al. Polyglucose nanoparticles with renal elimination and macrophage avidity facilitate PET imaging in ischaemic heart disease. Nat. Commun. 2017, 8, 14064. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shields, A.F.; Siegel, B.A.; Miller, K.D.; Krop, I.; Ma, C.X.; LoRusso, P.M.; Munster, P.N.; Campbell, K.; Gaddy, D.F.; et al. 64Cu-MM-302 positron emission tomography quantifies variability of enhanced permeability and retention of nanoparticles in relation to treatment response in patients with metastatic breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 4190–4202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Qu, Y.; Wang, K.; Zhang, X.; Zha, J.; Song, T.; Bao, C.; Liu, H.; Wang, Z.; Wang, J.; et al. In vivo nanoparticle-mediated radiopharmaceutical-excited fluorescence molecular imaging. Nat. Commun. 2015, 6, 7560. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lan, M.; Shen, D.; Fang, K.; Zhu, L.; Liu, Y.; Hao, L.; Li, P. Targeted nanobubbles carrying indocyanine green for ultrasound, photoacoustic and fluorescence imaging of prostate cancer. Int. J. Nanomed. 2020, 15, 4289–4309. [Google Scholar] [CrossRef]
- Yu, S.; Yao, T.; Liu, Y.; Yuan, B. In vivo ultrasound-switchable fluorescence imaging using a camera-based system. Biomed. Opt. Express 2020, 11, 1517–1538. [Google Scholar] [CrossRef]
- Kumari, A.; Kumar, V.; Yadav, S. Nanotechnology: A tool to enhance therapeutic values of natural plant products. Trends Med. Res. 2012, 7, 34–42. [Google Scholar]
- Hu, G.; Chun, X.; Wang, Y.; He, Q.; Gao, H. Peptide mediated active targeting and intelligent particle size reduction-mediated enhanced penetrating of fabricated nanoparticles for triple-negative breast cancer treatment. Oncotarget 2015, 6, 41258–41274. [Google Scholar] [CrossRef] [Green Version]
- Adesina, S.K.; Holly, A.; Kramer-Marek, G.; Capala, J.; Akala, E.O. Polylactide-based paclitaxel-loaded nanoparticles fabricated by dispersion polymerization: Characterization, evaluation in cancer cell lines, and preliminary biodistribution studies. J. Pharm. Sci. 2014, 103, 2546–2555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Luo, S.; Du, Z.; Zhou, M.; Li, P.; Fu, Y.; Sun, X.; Huang, Y.; Zhang, Z. Targeted delivery of celastrol to mesangial cells is effective against mesangioproliferative glomerulonephritis. Nat. Commun. 2017, 8, 878. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Ruan, S.B.; Zhang, Q.Y.; He, Q.; Gao, H.L. Lapatinib-incorporated lipoprotein-like nanoparticles: Preparation and a proposed breast cancer-targeting mechanism. Acta Pharmacol. Sin. 2014, 35, 846–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, Y.; Bai, M.; Sun, Y.; Wang, Q.; Li, F.; Xing, J.; Du, L.; Gong, T.; Duan, Y. Enhanced delivery of PEAL nanoparticles with ultrasound targeted microbubble destruction mediated siRNA transfection in human MCF-7/S and MCF-7/ADR cells in vitro. Int. J. Nanomed. 2015, 10, 5447–5457. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.H.; Cui, W.; Wang, G.L.; Meng, S.; Liu, Y.C.; Jin, H.W.; Zhang, L.R.; Xie, Y. Combined computational and experimental studies of molecular interactions of albuterol sulfate with bovine serum albumin for pulmonary drug nanoparticles. Drug Des. Dev. Ther. 2016, 10, 2973–2987. [Google Scholar] [CrossRef] [Green Version]
- Amreddy, N.; Babu, A.; Panneerselvam, J.; Srivastava, A.; Muralidharan, R.; Chen, A.; Zhao, Y.D.; Munshi, A.; Ramesh, R. Chemo-biologic combinatorial drug delivery using folate receptor-targeted dendrimer nanoparticles for lung cancer treatment. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, J.; Li, G.; Zhang, H.; Wang, L.; Li, D.; Ding, J. Spermidine-mediated poly(lactic-co-glycolic acid) nanoparticles containing fluorofenidone for the treatment of idiopathic pulmonary fibrosis. Int. J. Nanomed. 2017, 12, 6687–6704. [Google Scholar] [CrossRef] [Green Version]
- Soorya, V.C.; Berchmans, S. Flower like Bi structures on Pt surface facilitating effective cholesterol Biosensing. Mater. Sci. Eng. C. 2016, 64, 183–189. [Google Scholar]
- Giri, A.K.; Charan, C.; Saha, A.; Shahi, V.K.; Panda, A.B. An amperometric cholesterol biosensor with excellent sensitivity and limit of detection based on an enzyme-immobilized microtubular ZnO@ZnS heterostructure. J. Mater. Chem. A 2014, 2, 16997–17004. [Google Scholar] [CrossRef]
- Sharma, D.; Lim, Y.; Lee, Y.; Shin, H. Glucose sensor based on redox-cycling between selectively modified and unmodified combs of carbon interdigitated array nanoelectrodes. Anal. Chim. Acta 2015, 889, 194–202. [Google Scholar] [CrossRef]
- Sharma, D.; Lee, J.; Seo, J.; Shin, H. Development of a Sensitive Electrochemical Enzymatic Reaction-Based Cholesterol Biosensor Using Nano-Sized Carbon Interdigitated Electrodes Decorated with Gold Nanoparticles. Sensors 2017, 17, 2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.; Ondrake, J.; Cui, T. A conductometric indium oxide semiconducting nanoparticle enzymatic biosensor array. Sensors 2011, 11, 9300–9312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.; Jeong, H.; Park, S.K.; Park, S.; Lee, J.S. Interdigitated Electrode Biosensor Based on Plasma-Deposited TiO2 Nanoparticles for Detecting DNA. Biosensors 2021, 11, 212. [Google Scholar] [CrossRef]
- Narwal, V.; Yadav, N.; Thakur, M.; Pundir, C.S. An amperometric H2O2 biosensor based on hemoglobin nanoparticles immobilized on to a gold electrode. Biosci. Rep. 2017, 37, BSR20170194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toubanaki, D.K.; Margaroni, M.; Prapas, A.; Karagouni, E. Development of a nanoparticle-based lateral flow strip biosensor for visual detection of whole nervous necrosis virus particles. Sci. Rep. 2020, 10, 6529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Li, X.; Zou, R.; Wu, H.; Shi, H.; Yu, S.; Liu, Y. Multifunctional glucose biosensors from Fe3O4 nanoparticles modified chitosan/graphene nanocomposites. Sci. Rep. 2015, 5, 11129. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, Z.; Xie, H.; Sun, R.; Cao, T.; Paudyal, N.; Fang, W.; Song, H. Development of a magnetic nanoparticles-based screen-printed electrodes (MNPs-SPEs) biosensor for the quantification of ochratoxin a in cereal and feed samples. Toxins 2018, 10, 317. [Google Scholar] [CrossRef] [Green Version]
- Hakimian, F.; Ghourchian, H.; Hashemi, A.S.; Arastoo, M.R.; Behnam Rad, M. Ultrasensitive optical biosensor for detection of miRNA-155 using positively charged Au nanoparticles. Sci. Rep. 2018, 8, 2943. [Google Scholar] [CrossRef] [Green Version]
- Nadzirah, S.H.; Azizah, N.; Hashim, U.; Gopinath, S.C.; Kashif, M. Titanium dioxide nanoparticle-based interdigitated electrodes: A novel current to voltage dna biosensor recognizes E. coli O157:H7. PLoS ONE 2015, 10, e0139766. [Google Scholar] [CrossRef]
- Heredia, F.L.; Resto, P.J.; Parés-Matos, E.I. Fast Adhesion of Gold Nanoparticles (AuNPs) to a Surface Using Starch Hydrogels for Characterization of Biomolecules in Biosensor Applications. Biosensors 2020, 10, 99. [Google Scholar] [CrossRef]
- Mandala, S.; Liu, T.J.; Chen, C.M.; Liu, K.K.; Januar, M.; Chang, Y.F.; Lai, C.S.; Chang, K.H.; Liu, K.C. Enhanced plasmonic biosensor utilizing paired antibody and label-free Fe3O4 nanoparticles for highly sensitive and selective detection of Parkinson’s α-Synuclein in serum. Biosensors 2021, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Fiérrez, F.; González-Sánchez, M.I.; Jiménez-Pérez, R.; Iniesta, J.; Valero, E. Glucose biosensor based on disposable activated carbon electrodes modified with platinum nanoparticles electrodeposited on poly(Azure A). Sensors 2020, 20, 4489. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H.E. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef] [Green Version]
- Mostafavi, E.; Medina-Cruz, D.; Kalantari, K.; Taymoori, A.; Soltantabar, P.; Webster, T.J. Electroconductive nanobiomaterials for tissue engineering and regenerative medicine. Bioelectricity 2020, 2, 120–149. [Google Scholar] [CrossRef] [PubMed]
- De Witte, T.M.; Wagner, A.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Degradable Poly(Methyl Methacrylate)-co-methacrylic acid nanoparticles for controlled delivery of growth factors for bone regeneration. Tissue Eng. Part A 2020, 26, 1226–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.Q.; Kankala, R.K.; Chen, A.Z.; Yang, D.Z.; Cheng, X.X.; Jiang, N.N.; Zhu, K.; Wang, S.B. Investigation of silk fibroin nanoparticle-decorated poly(l-lactic acid) composite scaffolds for osteoblast growth and differentiation. Int. J. Nanomed. 2017, 12, 1877–1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parizek, M.; Douglas, T.E.; Novotna, K.; Kromka, A.; Brady, M.A.; Renzing, A.; Voss, E.; Jarosova, M.; Palatinus, L.; Tesarek, P.; et al. Nanofibrous poly(lactide-co-glycolide) membranes loaded with diamond nanoparticles as promising substrates for bone tissue engineering. Int. J. Nanomed. 2012, 7, 1931–1951. [Google Scholar] [CrossRef] [Green Version]
- Costard, L.S.; Kelly, D.C.; Power, R.N.; Hobbs, C.; Jaskaniec, S.; Nicolosi, V.; Cavanagh, B.L.; Curtin, C.M.; O’Brien, F.J. Layered double hydroxide as a potent non-viral vector for nucleic acid delivery using gene-activated scaffolds for tissue regeneration applications. Pharmaceutics 2020, 12, 1219. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Z.; Guo, Y.; Wang, P.; Yang, Z.; Han, L.; Sun, J.; Xia, Y. Enhanced osteoinduction of electrospun scaffolds with assemblies of hematite nanoparticles as a bioactive interface. Int. J. Nanomed. 2019, 14, 1051–1068. [Google Scholar] [CrossRef] [Green Version]
- Tayebi, T.; Baradaran-Rafii, A.; Hajifathali, A.; Rahimpour, A.; Zali, H.; Shaabani, A.; Niknejad, H. Biofabrication of chitosan/chitosan nanoparticles/polycaprolactone transparent membrane for corneal endothelial tissue engineering. Sci. Rep. 2021, 11, 7060. [Google Scholar] [CrossRef]
- Rui, Y.; Wilson, D.R.; Choi, J.; Varanasi, M.; Sanders, K.; Karlsson, J.; Lim, M.; Green, J.J. Carboxylated branched poly(β-amino ester) nanoparticles enable robust cytosolic protein delivery and CRISPR-Cas9 gene editing. Sci. Adv. 2019, 5, eaay3255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gluck, J.M.; Rahgozar, P.; Ingle, N.P.; Rofail, F.; Petrosian, A.; Cline, M.G.; Jordan, M.C.; Roos, K.P.; Maclellan, W.R.; Shemin, R.J.; et al. Hybrid coaxial electrospun nanofibrous scaffolds with limited immunological response created for tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 99, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Zhang, W.; Qian, H.; Lim, P.N.; Thian, E.S.; Lei, P.; Hu, Y.; Wang, Z. Silicon-incorporated nanohydroxyapatite-reinforced poly(ε-caprolactone) film to enhance osteogenesis for bone tissue engineering applications. Colloids Surf. B Biointerfaces 2020, 187, 110714. [Google Scholar] [CrossRef] [PubMed]
- Mehrasa, M.; Asadollahi, M.A.; Ghaedi, K.; Salehi, H.; Arpanaei, A. Electrospun aligned PLGA and PLGA/gelatin nanofibers embedded with silica nanoparticles for tissue engineering. Int. J. Biol. Macromol. 2015, 79, 687–695. [Google Scholar] [CrossRef]
- Singh, N.; Dahiya, B.; Radhakrishnan, V.S.; Prasad, T.; Mehta, P.K. Detection of Mycobacterium tuberculosis purified ESAT-6 (Rv3875) by magnetic bead-coupled gold nanoparticle-based immuno-PCR assay. Int. J. Nanomed. 2018, 13, 8523–8535. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.Q.; Ji, C.F.; Yang, X.Q.; Wang, R.; Yang, S.; Zhang, H.Q.; Zhang, J.G. An improved gold nanoparticle probe-based assay for HCV core antigen ultrasensitive detection. J. Virol. Methods 2017, 243, 142–145. [Google Scholar] [CrossRef]
- Potůčková, L.; Franko, F.; Bambousková, M.; Dráber, P. Rapid and sensitive detection of cytokines using functionalized gold nanoparticle-based immuno-PCR, comparison with immuno-PCR and ELISA. J. Immunol Methods 2011, 371, 38–47. [Google Scholar] [CrossRef] [Green Version]
- You, S.M.; Luo, K.; Jung, J.Y.; Jeong, K.B.; Lee, E.S.; Oh, M.H.; Kim, Y.R. Gold nanoparticle-coated starch magnetic beads for the separation, concentration, and SERS-based detection of E. coli O157:H7. ACS Appl. Mater. Interfaces 2020, 12, 18292–18300. [Google Scholar] [CrossRef]
- Stegurová, L.; Dráberová, E.; Bartos, A.; Dráber, P.; Rípová, D.; Dráber, P. Gold nanoparticle-based immuno-PCR for detection of tau protein in cerebrospinal fluid. J. Immunol. Methods 2014, 406, 137–142. [Google Scholar] [CrossRef]
- Dahiya, B.; Sharma, S.; Khan, A.; Kamra, E.; Mor, P.; Sheoran, A.; Sreenivas, V.; Varma-Basil, M.; Gupta, K.B.; Gupta, M.C.; et al. Detection of mycobacterial CFP-10 (Rv3874) protein in tuberculosis patients by gold nanoparticle-based real-time immuno-PCR. Future Microbiol. 2020, 15, 601–612. [Google Scholar] [CrossRef]
- Yin, H.Q.; Jia, M.X.; Shi, L.J.; Liu, J.; Wang, R.; Lv, M.M.; Ma, Y.Y.; Zhao, X.; Zhang, J.G. Evaluation of a novel ultra-sensitive nanoparticle probe-based assay for ricin detection. J. Immunotoxicol. 2013, 11, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, S.V.; Gupta, S.; Chaubey, K.K.; Stephan, B.J.; Sohal, J.S.; Dutta, M. ‘Nano-immuno test’ for the detection of live Mycobacterium avium subspecies Paratuberculosis bacilli in the milk samples using magnetic nano-particles and chromogen. Vet. Res. Commun. 2018, 42, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.H.; Irudayaraj, J. In-situ immuno-gold nanoparticle network ELISA biosensors for pathogen detection. Int. J. Food Microbiol. 2013, 164, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, W.; Wang, P.; Su, X. A paper-based competitive lateral flow immunoassay for multi β-agonist residues by using a single monoclonal antibody labelled with red fluorescent nanoparticles. Mikrochim. Acta 2018, 185, 191. [Google Scholar] [CrossRef] [Green Version]
- Pietschmann, J.; Spiegel, H.; Krause, H.J.; Schillberg, S.; Schröper, F. Sensitive aflatoxin b1 detection using nanoparticle-based competitive magnetic immunodetection. Toxins 2020, 12, 337. [Google Scholar] [CrossRef]
- Talan, A.; Mishra, A.; Eremin, S.A.; Narang, J.; Kumar, A.; Gandhi, S. Ultrasensitive electrochemical immuno-sensing platform based on gold nanoparticles triggering chlorpyrifos detection in fruits and vegetables. Biosens. Bioelectron. 2018, 105, 14–21. [Google Scholar] [CrossRef]
- Lima, R.S.; Li, H.; Khor, K.A.; Marple, B.R. Biocompatible nanostructured high velocity oxy-fuel (HVOF) sprayed titania coating: Deposition, characterization, and mechanical properties. J. Therm. Spray Technol. 2006, 15, 623–627. [Google Scholar] [CrossRef] [Green Version]
- Sevostyanov, M.; Baikin, A.; Sergienko, K.; Shatova, L.; Kirsankin, A.; Baymler, I.; Shkirin, A.; Gudkov, S. Biodegradable stent coatings on the basis of PLGA polymers of different molecular mass, sustaining a steady release of the thrombolityc enzyme streptokinase. React. Funct. Polym. 2020, 150, 104550. [Google Scholar] [CrossRef]
- Chong, H.; Lou, J.; Bogie, K.M.; Zorman, C.A.; Majerus, S. Vascular pressure-flow measurement using CB-PDMS flexible strain sensor. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 1451–1461. [Google Scholar] [CrossRef]
- Gorzelanny, C.; Kmeth, R.; Obermeier, A.; Bauer, A.T.; Halter, N.; Kümpel, K.; Schneider, M.F.; Wixforth, A.; Gollwitzer, H.; Burgkart, R.; et al. Silver nanoparticle-enriched diamond-like carbon implant modification as a mammalian cell compatible surface with antimicrobial properties. Sci. Rep. 2016, 6, 22849. [Google Scholar] [CrossRef] [Green Version]
- Peiris, P.M.; Bauer, L.; Toy, R.; Tran, E.; Pansky, J.; Doolittle, E.; Schmidt, E.; Hayden, E.; Mayer, A.; Keri, R.A.; et al. Enhanced delivery of chemotherapy to tumors using a multicomponent nanochain with radio-frequency-tunable drug release. ACS Nano 2012, 6, 4157–4168. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| S. No. | Nanoparticles | Disease | In Vitro or In Vivo Studies | Mechanism | References |
|---|---|---|---|---|---|
| 1. | NiO nanoparticles coated with phytomolecules using Abutilon indicum leaf extract. | Cervical cancer | HeLa cancer cells, Fibroblast cells. | Cytotoxicity andbiocompatibility; protects normal cells through their antioxidant potential. | [69] |
| 2. | Gold nanoparticles using Terminalia mantaly extract | Colorectal cancer, breast and liver cancer | Caco-2, MCF-7 and HepG2, KMST-6 cells | Selective toxicity to cancer. | [70] |
| 3. | Silver nanoparticles using Alpinia calcarata leaf extract | Bacterial infection | E. coli, P. aeruginosa (Gram negative) and S. aureus (Gram positive). | Antioxidant and antibacterial effect. | [71] |
| 4. | Trimetallic Ag-Cu-Co nanoparticles using Salvia officinalis aqueous extract | Fungal infection | Candida auris | Induced apoptosis, stimulated cell cycle arrest (G2/M phase). | [72] |
| 5. | Gold and silver nanoparticle using Crassocephalum rubens | Oxidative stress | In vitro determination | Scavenges free radicals and inhibits lipid peroxidation. | [73] |
| 6. | Silver nanoparticles with Boswellia dalzielii | Leukemia | Kasumi-1 cells | Cell cycle arrest, antioxidant potential by scavenging free radicals. | [74] |
| 7. | Se and Zn nanoparticles using Ephedra aphylla extract | Cancer | HePG-2 cells, HCT-116 cells, HeLa cells, WI-3 cell line | Cytotoxic effect. Antioxidant and antibacterial potential. | [75] |
| 8. | Selenium dispersed in P-Coumaric acid | Rheumatoid arthritis | Wistar albino rats | Decreased lipid peroxidation, decreased TNF-α, IL-6, and MCP-1) upregulated MnSOD, Cu/ZnSOD, ECSOD, CAT, and GPx1. | [76] |
| 9. | Ag–Au nanoparticles using Asparagus racemosus root extract | Inflammation | THP1 and NK92 cells | Decreased IL-1β, IL-6, and TNF-α IFN-γ. | [77] |
| 10. | Bacopa monnieri phytochemicals, mediated synthesis of platinum nanoparticles | Parkinson’s disease | Zebrafish | Increased dopamine, reduced glutathione, glutathione peroxidase, catalase, SOD, reduced levels of malondialdehyde with enhanced locomotor activity. | [78] |
| S. No. | Nanoparticles | Purpose | In Vitro or In Vivo Studies | Mechanism | References |
|---|---|---|---|---|---|
| 1. | Hsp70: superparamagnetic iron-oxide nanoparticle | Detection of experimental myocardium infarction | Male Wistar rats | Underwent sequential MRI scanning, accumulated in acture infarct. | [127] |
| 2. | Reporter nanoparticle | Monitoring antitumor activity in real time |
Breast cancer cells, ovarian cancer cells, and lung cancer cells. 4T1 breast cancer and Lewis lung carcinoma xenogratft Balb/c mouse model. |
Nanoparticle entered into tumor via EPR effect. Upon release, drug stimulated apoptosis via caspase3 enzyme that cleaved the DEVD peptide and unquenched the fluorescent signal. | [128] |
| 3. | DNA aptamer probe linked with azide–PEG nanoparticle | Detection of peptides and proteins | Plate-based assays |
Signal brightness and stability higher compared to other labeling techniques, biocompatible, high affinity for targets, with less non-specific binding. | [129] |
| 4. | Functionalized lanthanide oxide (Ln2O3) nanoparticles | MRI imaging | BALB/C-nude mice |
Biocompatible, effective glioma-specific contrast agent | [130] |
| 5. | PDDA and Au nanoparticle (AuNP) | Electrochemical immunosensor for interferon-gamma | Fabricated disposable ITO electrode and multiplexed electrochemical immunosensor | High sensitivity with a detection limit of 0.048 pg/mL | [16] |
| 6. | Gold nanoshuttle | Detection | Immobilized into filter paper |
Stronger extinction intensity at surface plasmon resonance peak, and exhibits much higher SERS activity. High SERS sensitivity in the detection of malachite green. | [131] |
| 7. | Immunogold nanoparticle | Detection of C. sakazakii | C. sakazakii | Detection limit 10 CFU/mL. | [132] |
| 8. | Folic acid-conjugated Gd2O3:Eu3+ nanoparticles | Detecting breast cancer |
Breast cancer cells T-47D tumor xenograft |
Less cell cytotoxic, get accumulated in the tumor tissue and used to detect breast cancer. | [133] |
| 9. | Gold nanoparticle | Visualizing unlabeled gold nanoparticles | NU/NU (Crl:NU-Foxn1nu) nude mice | Identification and tracking of Au nanoparticles in vasculature (in real-time). | [134] |
| 10. | Gold nanoparticle | Detecting lung cancer | A431 cells C57BL/6 mice and in nude mice (subcutaneous tumor) |
Increased binding affinity with targeting ligands, increased tumor accumulation compared tothe circulation. | [135] |
| S. No. | Nanomaterials | Surface Functionalization Agents | Purpose | References |
|---|---|---|---|---|
| 1. | Cellulose nanocrystal | 3-aminopropyltriethoxysilane | Exerts good thermal stability and a greater amount of residual char was formed at 500 °C. | [140] |
| 2. | Negatively charged polystyrene nanoparticles | Sulfone or carboxyl groups, | Increased intestinal transport efficiency in caco2 cells. | [141] |
| 3. | Hydrophobic nanoparticle | Amphiphilic polyaspartimide | Less than 12 nm hydrodynamic size, high colloidal stability, and biocompartibility. | [142] |
| 4. | Starch nanocrystal | 3-aminopropyl triethoxysilane | Uniform dispersion, improved hydrophobicity | [143] |
| 5. | Poly(lactic-co-glycolic acid) nanoparticle | CarboxyPEG2000 and methoxyPEG550 | Longevity in the blood and macrophage uptake. | [144] |
| 6. | Gold nanoparticle | PEG | Biocompatability, biosafety | [145] |
| 7. | Poly lactic-co-glycolic acid nanoparticle | peptide AC-1001 H3 (GQYGNLWFAY) | Enhance drug delivery | [146] |
| S. No. | Nanoparticles | Purpose | Studies | Mechanism | References |
|---|---|---|---|---|---|
| 1. | Nanoparticle-coated microbubble | Ultrasound imaging | Releasing of nanoparticles using ultrasound-driven | The formation of stable nanoparticle-coated bubbles and controlled nanoparticle release using ultrasound. | [154] |
| 2. | High-Z nanoparticles (ultrasmall bismuth nanoparticles) | Incorporated in medical devices (inferior vena cava filters (IVCFs)) | Implanted adult domestic pig | Maintained increased contrast in high-energy single energy computer tomography for quantification. Coating the IVCF with bismuth NPs showed greater radiopacity than that of an uncoated IVCF. Able to differentiate between BiNP-IVCF and iodine contrast while injecting into the inferior vena cava of the pig. | [155] |
| 3. | Selenium nanoparticle | Polymeric medical device coating | Se-coated substrate | Inhibited bacterial growth on PVC, PU and silicone and also reduced its function without using antibiotics. | [156] |
| 4. | Nanostructured gold coating | Coating on medical devices | In situ studies | Antibiofouling potential Preventing the biofilm formation, clinical isolates and ATCC strains on medical devices | [157] |
| 5. | Aqueous Stöber silica or iron oxide NPs | Fixing of polymermembrane to tissues | Wistar rats | Rapid closure and healing of deep wounds in skin and liver Inspite of blood flow, NPs can fix membranes to tissues. | [158] |
| 6. | Np-incorporated perfluorocarbon microbubbles (MB) | Medical imaging | Fabrication of microfluidic devices | NP-incorporated MB can be detected using low-pressure ultrasound, and the monodispersed MB platform can be used for in vivo (10(6) MB/sec). | [159] |
| 7. | Au-surface modified superparamagnetic core-shell NPs | Biosensor application | Biofunctional and spectroscopic characterization of superparamagnetic NPs | For bioseparation, NP can be directed using external magnetic fields | [160] |
| 8. | FeO NP-Powered Micro-OCT | Imaging | In situ studies | Increased contrast, imaging and visualizing the real-time swelling process of polymeric MNs in biological samples using micro OCT Achievement of the best contrast-to-noise ratio using μOCT | [161] |
| 9. | Varied-shaped gold nanoparticles | Coating for medical devices | Tested against Candida albicans, Pseudomonas aeruginosa, Staphylococcus aureus uropathogenic Escherichia coli | Increased bactericidal efficiency at nanogram doses, less toxicity can be coated on urological catheters | [162] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshamrani, M. Broad-Spectrum Theranostics and Biomedical Application of Functionalized Nanomaterials. Polymers 2022, 14, 1221. https://doi.org/10.3390/polym14061221
Alshamrani M. Broad-Spectrum Theranostics and Biomedical Application of Functionalized Nanomaterials. Polymers. 2022; 14(6):1221. https://doi.org/10.3390/polym14061221
Chicago/Turabian StyleAlshamrani, Meshal. 2022. "Broad-Spectrum Theranostics and Biomedical Application of Functionalized Nanomaterials" Polymers 14, no. 6: 1221. https://doi.org/10.3390/polym14061221
APA StyleAlshamrani, M. (2022). Broad-Spectrum Theranostics and Biomedical Application of Functionalized Nanomaterials. Polymers, 14(6), 1221. https://doi.org/10.3390/polym14061221






