Pesticides Xenobiotics in Soil Ecosystem and Their Remediation Approaches
Abstract
:1. Introduction
2. Types of Pesticide Xenobiotics
3. Impacts of Pesticides on Ecosystem
- Pesticide transportation: The movement of pesticides from their point of origin to other parts of the environment is considered transportation [26]. It has been observed that most pesticides are applied through spraying, which can become part of the air through partial evaporation. Similarly, these contaminants can evaporate from the soil particles and plant body surface. After the application of pesticides and their reaction with colloidal particles, some of the contaminants do not get fixed with soil particles and become free to move to nearby watercourses and to the underground water sources, causing contamination in those bodies of water.
- Pesticide diffusion: The diffusion mechanism of pesticides between water, biota, soil, and atmosphere is also important regarding their movement in the environment [26]. Pesticides are mainly applied in solid and liquid formulations, but there are possibilities for their volatilization. Applied solid and liquid-phase pesticides are converted into a gaseous phase that will be part of the air [38,39]. With the help of air, these contaminants can move too far into areas from their point of application and can cause environmental pollution in large areas. Pesticide residues can get dissolved in water, which will move from soil to water bodies and will cause an accumulation of contaminants in watercourses. Residues can also be dissolved in rainwater and tend to leach into dissolved formulations. The leaching process is mainly dependent on the chemical properties of applied pesticides and the geological conditions of the area. Pesticide residues that are part of water, soil, and the atmosphere can easily be transferred to human beings and other living organisms. More information about the mechanisms of contaminant transfer in plant bodies and to microbial communities will be discussed further below.
- Pesticide conversion: Conversion is also an important mechanism that can influence the presence of pesticides in the environment [26]. Conversion of pesticides is mainly referred to as degradation of pesticides with the oxidation-reduction processes that can reduce their toxicity level. Pesticides applied to the environment can easily go through other conversion processes such as degradation. Upon application, the pesticide molecules encounter enzymes and microbes that break down their structure with the help of some chemicals and enzymatic activities. In addition to living organisms’ role in breaking down toxic substances, abiotic factors can also degrade pesticides. Photo-degradation can also take place upon exposure of these contaminants to sunlight. Microbial conversion is a highly influential remediation technique for the degradation of pesticide molecules. Further details will be discussed below as well.
4. Remediation of Pesticides Contaminated Soils
4.1. Bioremediation
4.2. Phytoremediation
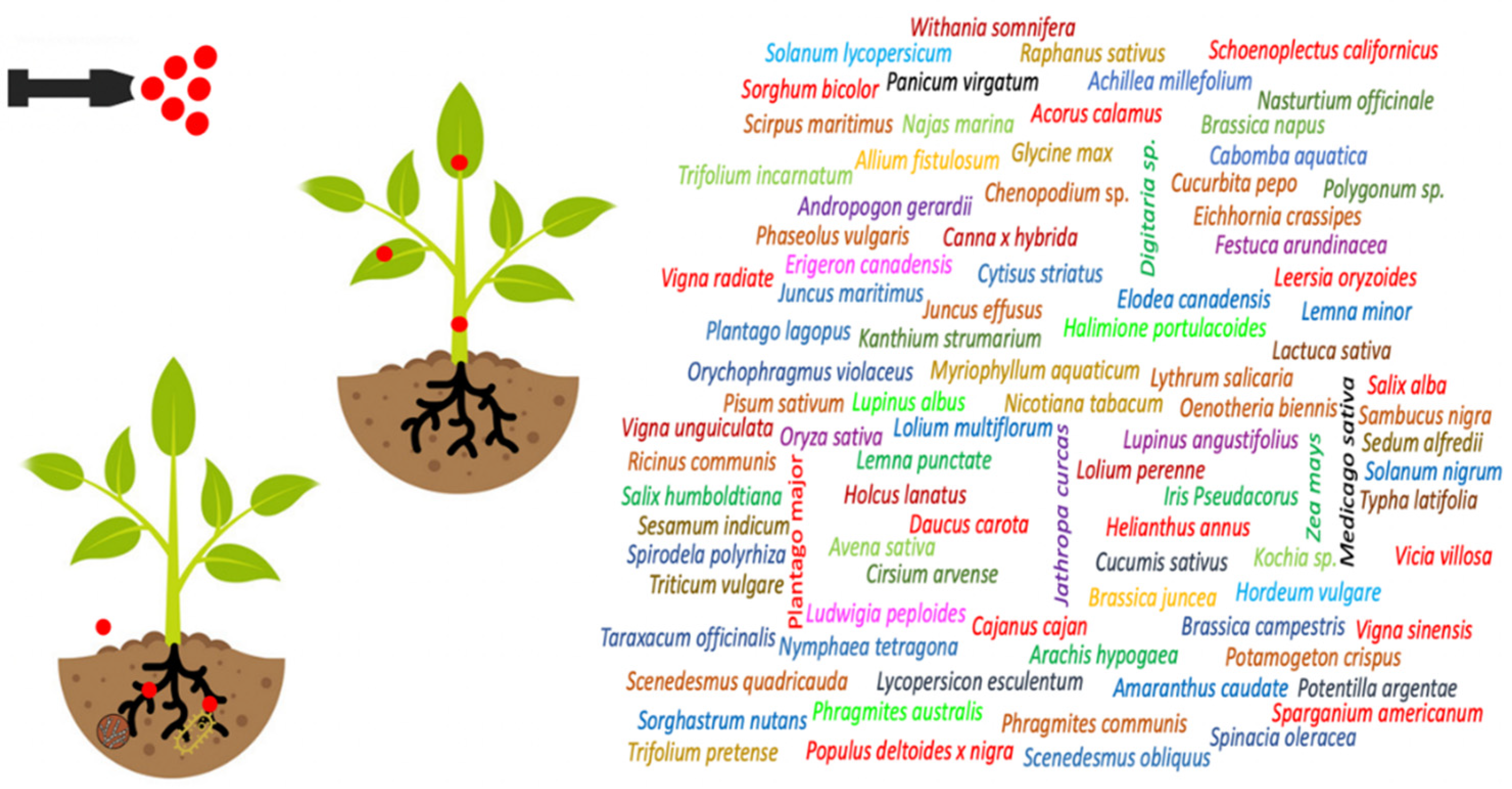
4.2.1. Phytoremediation Ability of Various Plants
4.2.2. Rhizoremediation Process
4.3. Use of Bioaugmentation
5. Natural Remediation Technologies: Associated Benefits and Risks
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Qureshi, M.A.; Jaskani, M.J.; Khan, A.S.; Ahmad, R. Influence of Endogenous Plant Hormones on Physiological and Growth Attributes of Kinnow Mandarin Grafted on Nine Rootstocks. J. Plant Growth Regul. 2021, 1–11. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal Communication: When I’m Calling You, Will You Answer Too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef] [PubMed]
- McKone, T.E.; Ryan, P.B. Human Exposures to Chemicals through Food Chains: An Uncertainty Analysis. Environ. Sci. Technol. 1989, 23, 1154–1163. [Google Scholar] [CrossRef]
- Sun, S.; Sidhu, V.; Rong, Y.; Zheng, Y. Pesticide Pollution in Agricultural Soils and Sustainable Remediation Methods: A Review. Curr. Pollut. Rep. 2018, 4, 240–250. [Google Scholar] [CrossRef]
- Karimi, H.; Mahdavi, S.; Asgari Lajayer, B.; Moghiseh, E.; Rajput, V.D.; Minkina, T.; Astatkie, T. Insights on the bioremediation technologies for pesticide-contaminated soils. Environ. Geochem. Health 2021, 1–26. [Google Scholar] [CrossRef]
- Baron, G.L.; Jansen, V.A.A.; Brown, M.J.F.; Raine, N.E. Pesticide reduces bumblebee colony initiation and increases probability of population extinction. Nat. Ecol. Evol. 2017, 1, 1308–1316. [Google Scholar] [CrossRef]
- Pascal-Lorber, S.; Laurent, F. Phytoremediation Techniques for Pesticide Contaminations. In Alternative Farming Systems, Biotechnology, Drought Stress and Ecological Fertilisation; Springer: Dordrecht, The Netherlands, 2011; Volume 6, pp. 77–105. [Google Scholar]
- Chaudhry, Q.; Blom-Zandstra, M.; Gupta, S.; Joner, E.J. Utilising the synergy between plants and rhizosphere microorganisms to enhance breakdown of organic pollutants in the environment. Environ. Sci. Pollut. Res. 2005, 12, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Eevers, N.; White, J.C.; Vangronsveld, J.; Weyens, N. Bio- and Phytoremediation of Pesticide-Contaminated Environments: A Review. Adv. Bot. Res. 2017, 83, 277–318. [Google Scholar] [CrossRef]
- MacKay, D.; Fraser, A. Bioaccumulation of persistent organic chemicals: Mechanisms and models. Environ. Pollut. 2000, 110, 375–391. [Google Scholar] [CrossRef]
- Berdowski, J.J.M.; Baas, J.; Bloos, J.P.J.; Visschedijk, A.J.H.; Zandveld, P.Y.J. The European Emission Inventory of Heavy Metals and Persistant Organic Pollutants for 1990; European Environment Agency: Kongens Nytorv, Denmark, 1997. [Google Scholar]
- Gavrilescu, M. Removal of heavy metals from the environment by biosorption. Eng. Life Sci. 2004, 4, 219–232. [Google Scholar] [CrossRef]
- Li, Y.F.; Scholtz, M.T.; Van Heyst, B.J. Global gridded emission inventories of α-hexachlorocyclohexane. J. Geophys. Res. Atmos. 2000, 105, 6621–6632. [Google Scholar] [CrossRef]
- Baruah, P.; Chaurasia, N. Recent Perspective on Bioremediation of Agrochemicals by Microalgae: Aspects and Strategies. In Environmental and Agricultural Microbiology; Wiley: Hoboken, NJ, USA, 2021; pp. 1–24. [Google Scholar]
- Sabarwal, A.; Kumar, K.; Singh, R.P. Hazardous effects of chemical pesticides on human health–Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 2018, 63, 103–114. [Google Scholar] [CrossRef]
- Kaufman, D.D. Fate Of Toxic Organic Compounds In Land-Applied Wastes. In Land Treatment of Hazardous Wastes; Noyes: Park Ridge, NJ, USA, 1983; pp. 77–151. [Google Scholar]
- Mörner, J.; Bos, R.; Fredrix, M. Reducing and eliminating the use of persistent organic perticides. UNEP Chem. Is Unit UNEP’s Technol. Ind. Econ. Div. 2002, 1–88. Available online: https://www.indiawaterportal.org/sites/default/files/iwp2/Reducing_and_eliminating_the_use_of_persistant_organic_pesticides_Johan_Morner__Robert_Bos__Marjon_Fredrix_Table_of_contents_WHO_2002.pdf (accessed on 27 December 2021).
- Wehtje, G.; Walker, R.H.; Shaw, J.N. Pesticide retention by inorganic soil amendments. Weed Sci. 2000, 48, 248–254. [Google Scholar] [CrossRef]
- Kempa, E.S. Hazardous wastes and economic risk reduction: Case study, Poland. Int. J. Environ. Pollut. 1997, 7, 231–248. [Google Scholar] [CrossRef]
- McGuinness, M.; Dowling, D. Plant-associated bacterial degradation of toxic organic compounds in soil. Int. J. Environ. Res. Public Health 2009, 6, 2226–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Yuan, X.; Xiong, T.; Wang, H.; Jiang, L. Bioremediation of co-contaminated soil with heavy metals and pesticides: Influence factors, mechanisms and evaluation methods. Chem. Eng. J. 2020, 398, 125657. [Google Scholar] [CrossRef]
- Glick, B.R. Phytoremediation: Synergistic use of plants and bacteria to clean up the environment. Biotechnol. Adv. 2003, 21, 383–393. [Google Scholar] [CrossRef]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Newman, L.; Vangronsveld, J. Exploiting plant–microbe partnerships to improve biomass production and remediation. Trends Biotechnol. 2009, 27, 591–598. [Google Scholar] [CrossRef]
- Rajmohan, K.S.; Chandrasekaran, R.; Varjani, S. A Review on Occurrence of Pesticides in Environment and Current Technologies for Their Remediation and Management. Indian J. Microbiol. 2020, 60, 125–138. [Google Scholar] [CrossRef]
- Ortiz-Hernández, M.L.; Sánchez-Salinas, E.; Dantán-González, E.; Castrejón-Godínez, M.L. Pesticide biodegradation: Mechanisms, genetics and strategies to enhance the process. In Biodegradation-Life of Science; IntechOpen: London, UK, 2013; pp. 251–287. [Google Scholar] [CrossRef] [Green Version]
- Gavrilescu, M. Fate of pesticides in the environment and its bioremediation. Eng. Life Sci. 2005, 5, 497–526. [Google Scholar] [CrossRef]
- Thomas, J.E.; Ou, L.T.; Al-Agely, A. DDE Remediation and Degradation. Rev. Environ. Contam. Toxicol. 2008, 194, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Lunney, A.I.; Zeeb, B.A.; Reimer, K.J. Uptake of weathered DDT in vascular plants: Potential for phytoremediation. Environ. Sci. Technol. 2004, 38, 6147–6154. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.M. Reproductive biology. Another DDT connection. Nature 1995, 375, 538–539. [Google Scholar] [CrossRef] [PubMed]
- Garmouma, M.; Teil, M.J.; Blanchard, M.; Chevreuil, M. Spatial and temporal variations of herbicide (triazines and phenylureas) concentrations in the catchment basin of the Marne river (France). Sci. Total Environ. 1998, 224, 93–107. [Google Scholar] [CrossRef]
- Forson, D.D.; Storfer, A. Atrazine Increases Ranavirus Susceptibility In The Tiger Salamander, Ambystoma Tigrinum. Ecol. Appl. 2006, 16, 2325–2332. [Google Scholar] [CrossRef] [Green Version]
- Hayes, T.; Haston, K.; Tsui, M.; Hoang, A.; Haeffele, C.; Vonk, A. Feminization of male frogs in the wild. Nature 2002, 419, 895–896. [Google Scholar] [CrossRef]
- Qiu, X.; Zhu, T.; Li, J.; Pan, H.; Li, Q.; Miao, G.; Gong, J. Organochlorine pesticides in the air around the Taihu Lake, China. Environ. Sci. Technol. 2004, 38, 1368–1374. [Google Scholar] [CrossRef]
- Bailey, R.E. Global hexachlorobenzene emissions. Chemosphere 2001, 43, 167–182. [Google Scholar] [CrossRef]
- Odukkathil, G.; Vasudevan, N. Toxicity and bioremediation of pesticides in agricultural soil. Rev. Environ. Sci. Bio/Technol. 2013, 12, 421–444. [Google Scholar] [CrossRef]
- Tang, Y.J.; Qi, L.; Krieger-Brockett, B. Evaluating factors that influence microbial phenanthrene biodegradation rates by regression with categorical variables. Chemosphere 2005, 59, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Andreu, V.; Picó, Y. Determination of pesticides and their degradation products in soil: Critical review and comparison of methods. TrAC Trends Anal. Chem. 2004, 23, 772–789. [Google Scholar] [CrossRef]
- Frazar, C. The Bioremediation and Phytoremediation of Pesticide-Contaminated Sites; US Environmental Protection Agency: Washington, DC, USA, 2000.
- Whitford, F.; Nelson, J.; Barrett, H.; Brichford, M. Pesticides and Water Quality: Principles, Policies, and Programs; Purdue University Cooperative Extension Service: West Lafayette, IN, USA, 1999. [Google Scholar]
- Bakshi, P.; Singh, A.D.; Kour, J.; Jan, S.; Ibrahim, M.; Mir, B.A.; Bhardwaj, R. Advanced Technologies for the Remediation of Pesticide-Contaminated Soils. In Handbook of Assisted and Amendment: Enhanced Sustainable Remediation Technology; Wiley: Hoboken, NJ, USA, 2021; pp. 331–353. [Google Scholar] [CrossRef]
- Morillo, E.; Villaverde, J. Advanced technologies for the remediation of pesticide-contaminated soils. Sci. Total Environ. 2017, 586, 576–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijkgraaf, E.; Vollebergh, H.R. Burn or bury? A social cost comparison of final waste disposal methods. Ecol. Econ. 2004, 50, 233–247. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Wang, Z.; Ran, S. Solid waste management in Macao: Practices and challenges. Waste Manag. 2006, 26, 1045–1051. [Google Scholar] [CrossRef]
- Hussain, S.; Siddique, T.; Arshad, M.; Saleem, M. Bioremediation and phytoremediation of pesticides: Recent advances. Crit. Rev. Environ. Sci. Technol. 2009, 39, 843–907. [Google Scholar] [CrossRef]
- Singh, D.K. Biodegradation and bioremediation of pesticide in soil: Concept, method and recent developments. Indian J. Microbiol. 2008, 48, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Megharaj, M.; Ramakrishnan, B.; Venkateswarlu, K.; Sethunathan, N.; Naidu, R. Bioremediation approaches for organic pollutants: A critical perspective. Environ. Int. 2011, 37, 1362–1375. [Google Scholar] [CrossRef]
- Singh, C.; Chowdhary, P.; Singh, J.S.; Chandra, R. Pulp and paper mill wastewater and coliform as health hazards: A review. Microbiol. Res. Int. 2016, 4, 28–39. [Google Scholar]
- Raffa, C.M.; Chiampo, F. Bioremediation of agricultural soils polluted with pesticides: A review. Bioengineering 2021, 8, 92. [Google Scholar] [CrossRef]
- Parween, T.; Bhandari, P.; Sharma, R.; Jan, S.; Siddiqui, Z.H.; Patanjali, P.K. Bioremediation: A sustainable tool to prevent pesticide pollution. In Modern Age Environmental Problems and Their Remediation; Springer: Cham, Switzerland, 2017; pp. 215–227. [Google Scholar] [CrossRef]
- Metting, F.B.; Marcel Dekker Inc.; Keyser, H.H.; Somasegaran, P.; Ben Bohloolt, B. Soil microbial Ecology, Applications in Agriculture and Environmental Management; Marcel Dekker Inc.: University Park, PA, USA, 1993. [Google Scholar]
- Karpouzas, D.G.; Fotopoulou, A.; Menkissoglu-Spiroudi, U.; Singh, B.K. Non-specific biodegradation of the organophosphorus pesticides, cadusafos and ethoprophos, by two bacterial isolates. FEMS Microbiol. Ecol. 2005, 53, 369–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam Gilani, R.; Rafique, M.; Rehman, A.; Farooq Hussain Munis, M.; ur Rehman, S.; Javed Chaudhary, H. Biodegradation of chlorpyrifos by bacterial genus Pseudomonas. J. Basic Microbiol. 2016, 56, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.; Wang, T.; Yu, J.; Qu, G.; Zhang, P.; Jia, H.; Sun, H. Remediation of organophosphorus pesticide polluted soil using persulfate oxidation activated by microwave. J. Hazard. Mater. 2021, 401, 123361. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Li, Y.; Zheng, W.; Peng, X.; Li, W.; Yan, Y. Mineralization of chlorpyrifos by co-culture of Serratia and Trichosporon spp. Biotechnol. Lett. 2007, 29, 1469–1473. [Google Scholar] [CrossRef]
- Yang, C.; Liu, N.; Guo, X.; Qiao, C. Cloning of mpd gene from a chlorpyrifos-degrading bacterium and use of this strain in bioremediation of contaminated soil. FEMS Microbiol. Lett. 2006, 265, 118–125. [Google Scholar] [CrossRef]
- Moklyachuk, L.; Gorodiska, I.; Slobodenyuk, O.; Petryshyna, V. Phytoremediation of Soil Polluted with Obsolete Pesticides in Ukraine. In Application of Phytotechnologies for Cleanup of Industrial, Agricultural, and Wastewater Contamination; Springer: Dordrecht, The Netherlands, 2010; pp. 113–124. [Google Scholar] [CrossRef]
- Nurzhanova, A.; Kulakow, P.; Rubin, E.; Rakhimbayev, I.; Sedlovskiy, A.; Zhambakin, K.; Kalugin, S.; Kolysheva, E.; Erickson, L. Obsolete Pesticides Pollution and Phytoremediation of Contaminated Soil in Kazakhstan. In Application of Phytotechnologies for Cleanup of Industrial, Agricultural, and Wastewater Contamination; Springer: Dordrecht, The Netherlands, 2010; pp. 87–111. [Google Scholar] [CrossRef]
- Fan, X.; Song, F. Bioremediation of atrazine: Recent advances and promises. J. Soils Sediments 2014, 14, 1727–1737. [Google Scholar] [CrossRef]
- Myresiotis, C.K.; Vryzas, Z.; Papadopoulou-Mourkidou, E. Biodegradation of soil-applied pesticides by selected strains of plant growth-promoting rhizobacteria (PGPR) and their effects on bacterial growth. Biodegradation 2012, 23, 297–310. [Google Scholar] [CrossRef]
- Singh, B.K.; Walker, A.; Wright, D.J. Bioremedial potential of fenamiphos and chlorpyrifos degrading isolates: Influence of different environmental conditions. Soil Biol. Biochem. 2006, 38, 2682–2693. [Google Scholar] [CrossRef]
- Bashir, M.A.; Liu, J.; Geng, Y.; Wang, H.; Pan, J.; Zhang, D.; Rehim, A.; Aon, M.; Liu, H. Co-culture of rice and aquatic animals: An integrated system to achieve production and environmental sustainability. J. Clean. Prod. 2020, 249, 119310. [Google Scholar] [CrossRef]
- Schroll, R.; Becher, H.H.; Dörfler, U.; Gayler, S.; Grundmann, S.; Hartmann, H.P.; Ruoss, J. Quantifying the effect of soil moisture on the aerobic microbial mineralization of selected pesticides in different soils. Environ. Sci. Technol. 2006, 40, 3305–3312. [Google Scholar] [CrossRef]
- Wu, C.; Wu, X.; Chen, S.; Wu, D. A Newly Discovered Humic-Reducing Bacterium, Pseudomonas geniculata PQ01, Isolated From Paddy Soil Promotes Paraquat Anaerobic Transformation. Front. Microbiol. 2020, 11, 2003. [Google Scholar] [CrossRef]
- Ferrell, J.A.; Witt, W.W.; Vencill, W.K. Sulfentrazone absorption by plant roots increases as soil or solution pH decreases. Weed Sci. 2003, 51, 826–830. [Google Scholar] [CrossRef]
- Briceño, G.; Palma, G.; Durán, N. Influence of Organic Amendment on the Biodegradation and Movement of Pesticides. Crit. Rev. Environ. Sci. Technol. 2007, 37, 233–271. [Google Scholar] [CrossRef]
- Zhang, P.; Sheng, G.; Feng, Y.; Miller, D.M. Role of Wheat-Residue-Derived Char in the Biodegradation of Benzonitrile in Soil: Nutritional Stimulation versus Adsorptive Inhibition. Environ. Sci. Technol. 2005, 39, 5442–5448. [Google Scholar] [CrossRef] [PubMed]
- Aronstein, B.N.; Calvlllo, Y.M.; Alexander, M. Effect of surfactants at low concentrations on the desorption and biodegradation of sorbed aromatic compounds in soil. Environ. Sci. Technol. 2002, 25, 1728–1731. [Google Scholar] [CrossRef]
- Sarker, A.; Nandi, R.; Kim, J.E.; Islam, T. Remediation of chemical pesticides from contaminated sites through potential microorganisms and their functional enzymes: Prospects and challenges. Environ. Technol. Innov. 2021, 23, 101777. [Google Scholar] [CrossRef]
- Scott, C.; Lewis, S.E.; Milla, R.; Taylor, M.C.; Rodgers, A.J.W.; Dumsday, G.; Brodie, J.E.; Oakeshott, J.G.; Russell, R.J. A free-enzyme catalyst for the bioremediation of environmental atrazine contamination. J. Environ. Manag. 2010, 91, 2075–2078. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, P.S.; Vo, D.V.N.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P.R. A review on catalytic-enzyme degradation of toxic environmental pollutants: Microbial enzymes. J. Hazard. Mater. 2021, 419, 126451. [Google Scholar] [CrossRef]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef]
- White, J.C. Inheritance of p,p′-DDE phytoextraction ability in hybridized cucurbita pepo cultivars. Environ. Sci. Technol. 2010, 44, 5165–5169. [Google Scholar] [CrossRef]
- Romeh, A.A.; Ibrahim Saber, R.A. Green nano-phytoremediation and solubility improving agents for the remediation of chlorfenapyr contaminated soil and water. J. Environ. Manag. 2020, 260, 110104. [Google Scholar] [CrossRef] [PubMed]
- Inna, R.; Olga, P.; Valentina, J. (Vorona) Bioremediation and phytoremediation of pesticide contaminated soil: Microbiological study. Lucr. Ştiinţifice Ser. Hortic. 2020, 63, 179–188. [Google Scholar]
- Mamirova, A.; Pidlisnyuk, V.; Amirbekov, A.; Ševců, A.; Nurzhanova, A. Phytoremediation potential of Miscanthus sinensis And. in organochlorine pesticides contaminated soil amended by Tween 20 and Activated carbon. Environ. Sci. Pollut. Res. 2021, 28, 16092–16106. [Google Scholar] [CrossRef] [PubMed]
- Tarla, D.N.; Erickson, L.E.; Hettiarachchi, G.M.; Amadi, S.I.; Galkaduwa, M.; Davis, L.C.; Nurzhanova, A.; Pidlisnyuk, V. Phytoremediation and Bioremediation of Pesticide-Contaminated Soil. Appl. Sci. 2020, 10, 1217. [Google Scholar] [CrossRef] [Green Version]
- Bouldin, J.L.; Farris, J.L.; Moore, M.T.; Smith, S.; Cooper, C.M. Hydroponic uptake of atrazine and lambda-cyhalothrin in Juncus effusus and Ludwigia peploides. Chemosphere 2006, 65, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Bromilow, R.H.; Chamberlain, K. Principles governing uptake and transport of chemicals. In Plant Contamination: Modeling and Simulation of Organic Chemical Processes; CRC Press: Boca Raton, FL, USA, 1995; pp. 37–68. [Google Scholar]
- Turgut, C. Uptake and modeling of pesticides by roots and shoots of parrotfeather (Myriophyllum aquaticum). Environ. Sci. Pollut. Res. Int. 2005, 12, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Trapp, S. Plant uptake and transport models for neutral and ionic chemicals. Environ. Sci. Pollut. Res. 2004, 11, 33–39. [Google Scholar] [CrossRef]
- Gredelj, A.; Polesel, F.; Trapp, S. Model-based analysis of the uptake of perfluoroalkyl acids (PFAAs) from soil into plants. Chemosphere 2020, 244, 125534. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Zhu, Y.G.; Lin, A.J.; Zhang, X.H. Interaction between cadmium and atrazine during uptake by rice seedlings (Oryza sativa L.). Chemosphere 2005, 60, 802–809. [Google Scholar] [CrossRef]
- Lee, W.Y.; Iannucci-Berger, W.A.; Eitzer, B.D.; White, J.C.; Mattina, M.J.I. Plant uptake and translocation of air-borne chlordane and comparison with the soil-to-plant route. Chemosphere 2003, 53, 111–121. [Google Scholar] [CrossRef]
- Xia, H.; Ma, X. Phytoremediation of ethion by water hyacinth (Eichhornia crassipes) from water. Bioresour. Technol. 2006, 97, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Lorber-Pascal, S.; Laurent, F. Fate of RDX and TNT in agronomic plants. Environ. Pollut. 2007, 148, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, S.; Paulose, B.; White, J.C.; Dhankher, O.P. Understanding the physiological and molecular mechanism of persistent organic pollutant uptake and detoxification in cucurbit species (zucchini and squash). Environ. Sci. Technol. 2010, 44, 7295–7301. [Google Scholar] [CrossRef] [PubMed]
- Burken, J.G.; Schnoor, J.L. Uptake and metabolism of atrazine by poplar trees. Environ. Sci. Technol. 1997, 31, 1399–1406. [Google Scholar] [CrossRef]
- Warsaw, A.L.; Thomas Fernandez, R.; Kort, D.R.; Cregg, B.M.; Rowe, B.; Vandervoort, C. Remediation of metalaxyl, trifluralin, and nitrate from nursery runoff using container-grown woody ornamentals and phytoremediation areas. Ecol. Eng. 2012, 47, 254–263. [Google Scholar] [CrossRef]
- Sandermann, H., Jr. Higher plant metabolism of xenobiotics: The “green liver” concept. Pharmacogenetics 1994, 4, 225–241. [Google Scholar] [CrossRef]
- Dupont, S.; Khan, S.U. Bound and extractable 14C residues in canola (Brassica napus) plants treated with radiolabelled atrazine. Weed Res. 1993, 33, 9–16. [Google Scholar] [CrossRef]
- Chen, W.M.; Tang, Y.Q.; Mori, K.; Wu, X.L. Distribution of culturable endophytic bacteria in aquatic plants and their potential for bioremediation in polluted waters. Aquat. Biol. 2012, 15, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.J.; Sun, J.Q.; Nie, Y.; Wu, X.L. Spirodela polyrhiza stimulates the growth of its endophytes but differentially increases their fenpropathrin-degradation capabilities. Chemosphere 2015, 125, 33–40. [Google Scholar] [CrossRef]
- Ryan, R.; Germaine, K.; Franks, A.; Ryan, D.; Dowling, D. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008, 278, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.C.; Ladha, J.K.; Tripathi, A.K. Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J. Biotechnol. 2001, 91, 127–141. [Google Scholar] [CrossRef]
- Wakelin, S.A.; Warren, R.A.; Harvey, P.R.; Ryder, M.H. Phosphate solubilization by Penicillium spp. closely associated with wheat roots. Biol. Fertil. Soils 2004, 40, 36–43. [Google Scholar] [CrossRef]
- Lee, S.; Flores-Encarnación, M.; Contreras-Zentella, M.; Garcia-Flores, L.; Escamilla, J.E.; Kennedy, C. Indole-3-acetic acid biosynthesis is deficient in Gluconacetobacter diazotrophicus strains with mutations in cytochrome c biogenesis genes. J. Bacteriol. 2004, 186, 5384–5391. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.M.; Loper, J.E. Characterization of siderophore production by the biological control agent Enterobacter cloacae. Mol. Plant-Microbe Interact. 1994, 7, 440–448. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Whipps, J. Carbon economy. In The Rhizosphere; Wiley & Son: Hoboken, NJ, USA, 1990; p. 59. [Google Scholar]
- Leigh, M.B.; Prouzová, P.; Macková, M.; Macek, T.; Nagle, D.P.; Fletcher, J.S. Polychlorinated biphenyl (PCB)-degrading bacteria associated with trees in a PCB-contaminated site. Appl. Environ. Microbiol. 2006, 72, 2331–2342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunney, A.I.; Rutter, A.; Zeeb, B.A. Effect of organic matter additions on uptake of weathered DDT by Cucurbita pepo ssp. pepo cv. Howden. Int. J. Phytoremediat. 2010, 12, 404–417. [Google Scholar] [CrossRef]
- Gerhardt, K.E.; Huang, X.D.; Glick, B.R.; Greenberg, B.M. Phytoremediation and rhizoremediation of organic soil contaminants: Potential and challenges. Plant Sci. 2009, 176, 20–30. [Google Scholar] [CrossRef]
- Kidd, P.S.; Prieto-Fernández, A.; Monterroso, C.; Acea, M.J. Rhizosphere microbial community and hexachlorocyclohexane degradative potential in contrasting plant species. Plant Soil 2008, 302, 233–247. [Google Scholar] [CrossRef]
- Wang, F.Y.; Tong, R.J.; Shi, Z.Y.; Xu, X.F.; He, X.H. Inoculations with Arbuscular mycorrhizal fungi increase vegetable yields and decrease phoxim concentrations in carrot and green onion and their soils. PLoS ONE 2011, 6, e16949. [Google Scholar] [CrossRef] [Green Version]
- Vacondio, B.; Birolli, W.G.; Ferreira, I.M.; Seleghim, M.H.R.; Gonçalves, S.; Vasconcellos, S.P.; Porto, A.L.M. Biodegradation of pentachlorophenol by marine-derived fungus Trichoderma harzianum CBMAI 1677 isolated from ascidian Didemnun ligulum. Biocatal. Agric. Biotechnol. 2015, 4, 266–275. [Google Scholar] [CrossRef]
- Jauregui, J.; Valderrama, B.; Albores, A.; Vazquez-Duhalt, R. Microsomal transformation of organophosphorus pesticides by white rot fungi. Biodegradation 2003, 14, 397–406. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; He, X.; Liu, J. Genetically Engineered Methanotroph as a Platform for Bioaugmentation of Chemical Pesticide Contaminated Soil. ACS Synth. Biol. 2021, 10, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Mawang, C.I.; Azman, A.S.; Fuad, A.S.M.; Ahamad, M. Actinobacteria: An eco-friendly and promising technology for the bioaugmentation of contaminants. Biotechnol. Reports 2021, 32, e00679. [Google Scholar] [CrossRef] [PubMed]
- El Fantroussi, S.; Agathos, S.N. Is bioaugmentation a feasible strategy for pollutant removal and site remediation? Curr. Opin. Microbiol. 2005, 8, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: A review. Chemosphere 2017, 172, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.; van der Gast, C.J.; Ciric, L.; Singer, A.; Thompson, I.P.; van der Gast, C.J.; Ciric, L.; Singer, A.C. Bioaugmentation for bioremediation: The challenge of strain selection. Environ. Microbiol. 2005, 7, 909–915. [Google Scholar] [CrossRef]
- Xiao, P.; Kondo, R. Biodegradation of dieldrin by Cordyceps fungi and detection of metabolites. Appl. Mech. Mater. 2013, 295–298, 30–34. [Google Scholar] [CrossRef]
- Ahmad, F.; Iqbal, S.; Anwar, S.; Afzal, M.; Islam, E.; Mustafa, T.; Khan, Q.M. Enhanced remediation of chlorpyrifos from soil using ryegrass (Lollium multiflorum) and chlorpyrifos-degrading bacterium Bacillus pumilus C2A1. J. Hazard. Mater. 2012, 237–238, 110–115. [Google Scholar] [CrossRef]
- Compernolle, T.; van Passel, S.; Weyens, N.; Vangronsveld, J.; Lebbe, L.; Thewys, T. Groundwater remediation and the cost effectiveness of phytoremediation. Int. J. Phytoremediation 2012, 14, 861–877. [Google Scholar] [CrossRef]
- Li, Z.; Jennings, A. Worldwide Regulations of Standard Values of Pesticides for Human Health Risk Control: A Review. Int. J. Environ. Res. Public Health 2017, 14, 826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USDA National Organic Program 2010–2011 Pilot Study Pesticide Residue Testing of Organic Produce USDA National Organic Program. 2012. Available online: https://www.ams.usda.gov/reports/2010-2011-pilot-study-pesticide-residue-testing-organic-produce (accessed on 27 December 2021).
- Ma, J.; Pan, L.B.; Yang, X.Y.; Liu, X.L.; Tao, S.Y.; Zhao, L.; Qin, X.-P.; Sun, Z.-J.; Hou, H.; Zhou, Y.-Z. DDT, DDD, and DDE in soil of Xiangfen County, China: Residues, sources, spatial distribution, and health risks. Chemosphere 2016, 163, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Joko, T.; Anggoro, S.; Sunoko, H.R.; Rachmawati, S. Pesticides Usage in the Soil Quality Degradation Potential in Wanasari Subdistrict, Brebes, Indonesia. Appl. Environ. Soil Sci. 2017, 2017, 5896191. [Google Scholar] [CrossRef]


| Pesticides | Groups |
|---|---|
| DDT, HCH, Cyhalothrin, Cypermethrin, Chlorpyrifos, Fenpropathrin, Carbaryl, Ethion, Cyanophos, Thiamethoxam, Bifenthrin, Methylparathion, Monocrotophos, Phorate, DDE, Fenamiphos, Naphtalene, Coumaphos, Diazonin, Parathion, Aldrin, Dieldrin, Heptachlor, Heptachlor epoxide, Fenitrothion, Permethrin, Fenvalerate, Dimethoate, Malathion, Endosulfan, Quinalphos, Profenos, Triazophos, Monocrotophos, Chlordane, Methoxychlor, Profenofos, Pentachlorophenol, Azinphosmethyl, Phosmet, Terbufos, Aldicarb, Phoxim, Endosulfan sulphate, DDD, Endrin, Gamma-cyhalothrin | Insecticides |
| Atrazine, Metolachlor, Butachlor, Metribuzin, 2,4-Dichlorophenoxyacetic acid, Napropamide, Trifluralin, Sulfentrazone, Alachlor, Acetochlor, Tertbutryn Propisochlor, Glyphosate, Simazine, Linuron, Tribufos, Clofibric acid, Diclofop-methyl, Pendimethalin, Terbuthylazine, Flazasulfuron, Picloram, Isoproturon, Cycloxidim, Napropamide | Herbicides |
| Acibenzolar-S-methyl, Propamocarb hydrochloride, Azoxystrobin, Difenoconazole, Copper sulphate, Dimethomorph, Dodemorph, Tridemorph, Hexachlorbenzene, Metalaxyl, Pyrimethanil | Fungicides |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Sial, M.U.; Bashir, M.A.; Bilal, M.; Raza, Q.-U.-A.; Ali Raza, H.M.; Rehim, A.; Geng, Y. Pesticides Xenobiotics in Soil Ecosystem and Their Remediation Approaches. Sustainability 2022, 14, 3353. https://doi.org/10.3390/su14063353
Wang X, Sial MU, Bashir MA, Bilal M, Raza Q-U-A, Ali Raza HM, Rehim A, Geng Y. Pesticides Xenobiotics in Soil Ecosystem and Their Remediation Approaches. Sustainability. 2022; 14(6):3353. https://doi.org/10.3390/su14063353
Chicago/Turabian StyleWang, Xingwen, Muhammad Umair Sial, Muhammad Amjad Bashir, Muhammad Bilal, Qurat-Ul-Ain Raza, Hafiz Muhammad Ali Raza, Abdur Rehim, and Yucong Geng. 2022. "Pesticides Xenobiotics in Soil Ecosystem and Their Remediation Approaches" Sustainability 14, no. 6: 3353. https://doi.org/10.3390/su14063353
APA StyleWang, X., Sial, M. U., Bashir, M. A., Bilal, M., Raza, Q.-U.-A., Ali Raza, H. M., Rehim, A., & Geng, Y. (2022). Pesticides Xenobiotics in Soil Ecosystem and Their Remediation Approaches. Sustainability, 14(6), 3353. https://doi.org/10.3390/su14063353







