NiO-Based Electronic Flexible Devices
Abstract
:1. Introduction
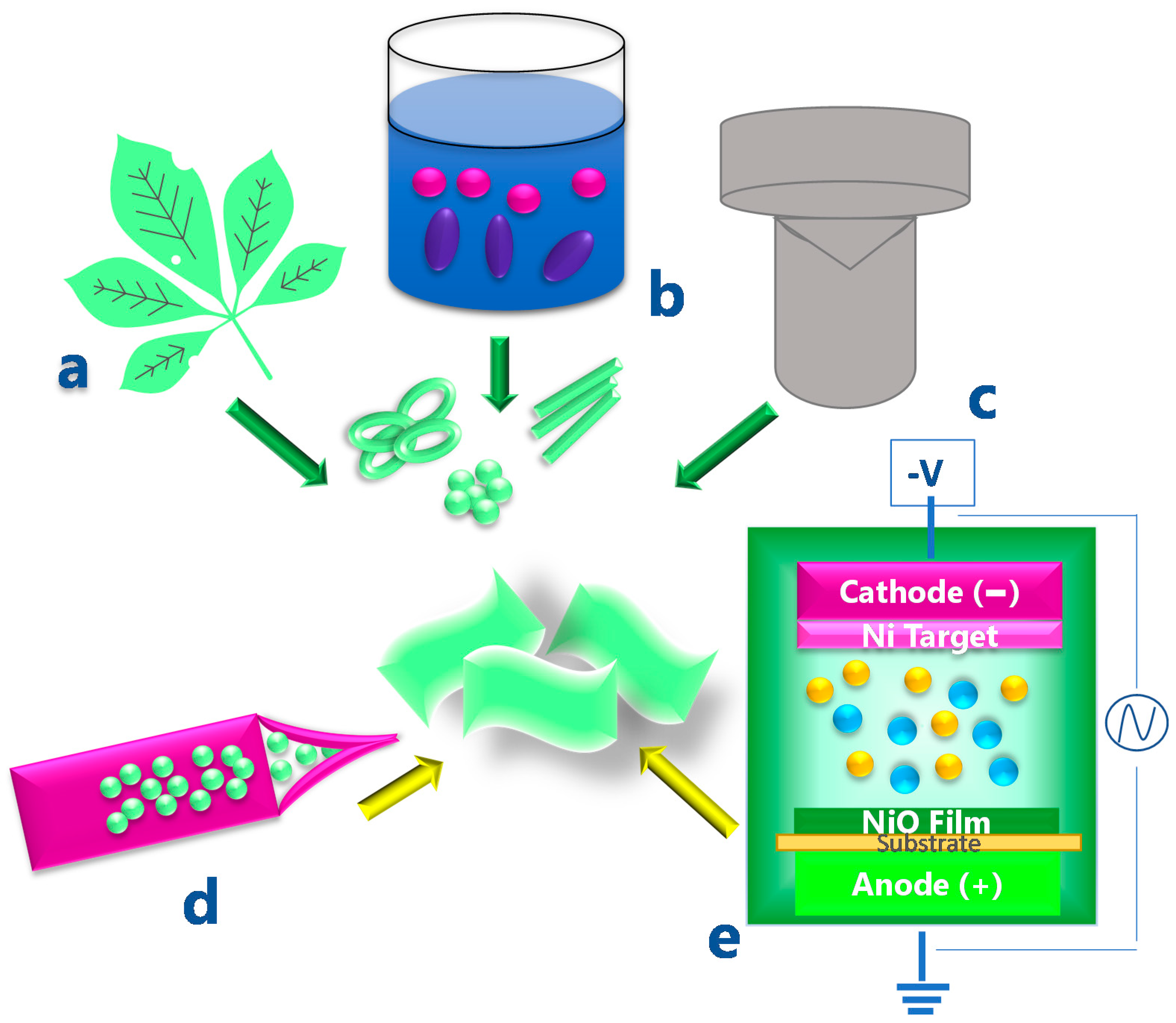
2. Synthesis and Properties of NiO Nanoparticles and Thin Films
3. NiO-Based Flexible Devices
3.1. Supercapacitors and Electrodes
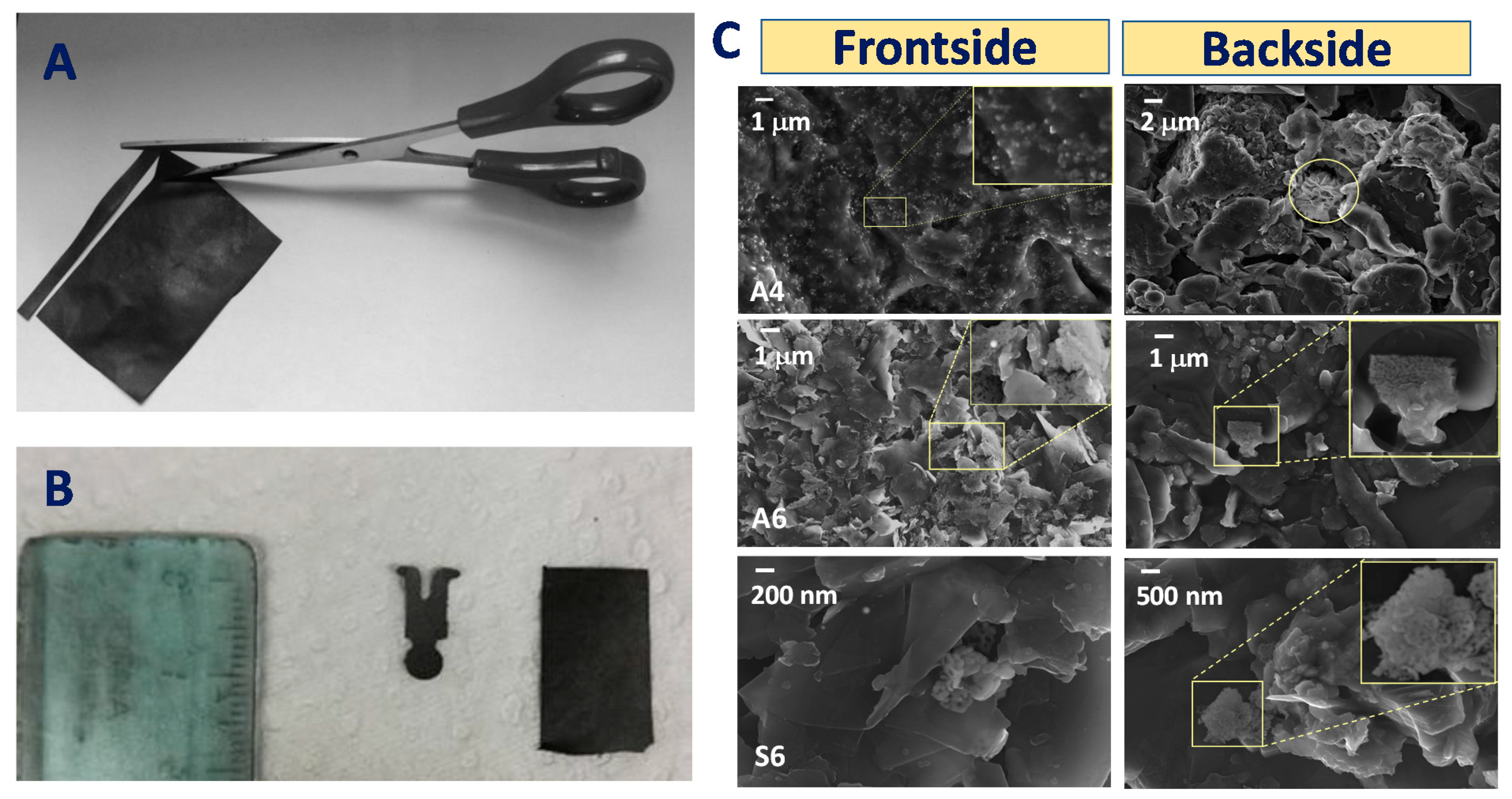
NiO-Plastisol Electrodes
3.2. Resistive Random Access Memory
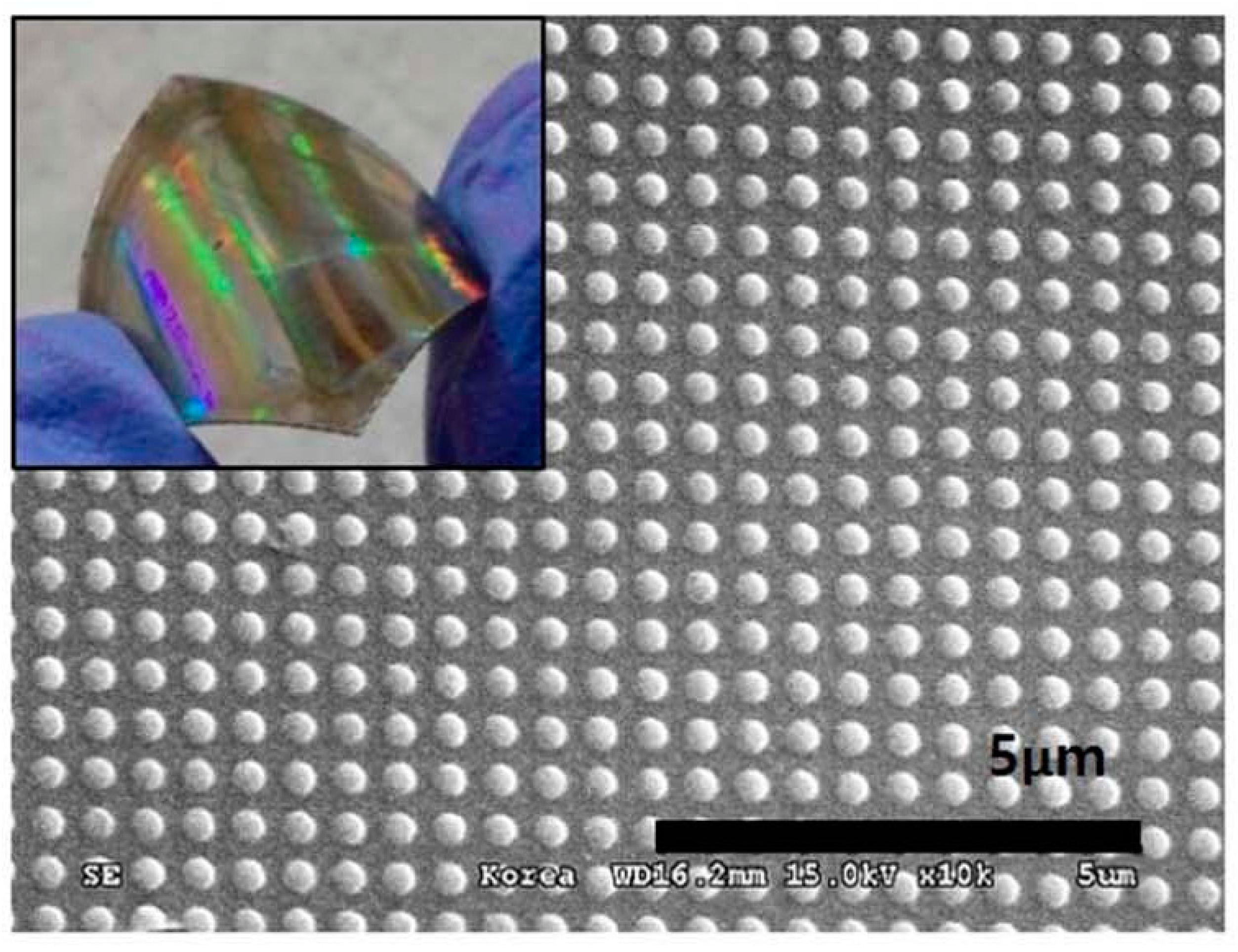
3.3. Electrochromic Devices
3.4. Temperature Devices
3.5. Solar Cells
4. Electronic Flexible Devices Based on Other Oxides
5. Future Perspectives and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mannsfeld, S.C.; Tee, B.C.; Stoltenberg, R.M.; Chen, C.V.; Barman, S.; Muir, B.V.O.; Sokolov, A.N.; Reese, C.; Bao, Z. Highly sensitive flexible pressure sensors with microstructured rubber dielectric layers. Nat. Mater. 2010, 9, 859. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A stretchable carbon nanotube strain sensor for human-motion detection. Nat. Nanotechnol. 2011, 6, 296. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 2019, 48, 1465–1491. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.; Tee, B.C.K.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef] [PubMed]
- Choong, C.L.; Shim, M.B.; Lee, B.S.; Jeon, S.; Ko, D.-S.; Kang, T.-H.; Bae, J.; Lee, S.-H.; Byun, K.-E.; Im, J.; et al. Highly stretchable resistive pressure sensors using a conductive elastomeric composite on a micropyramid array. Adv. Mater. 2014, 26, 3451. [Google Scholar] [CrossRef] [PubMed]
- Trung, T.Q.; Lee, N.E. Flexible and stretchable physical sensor integrated platforms for wearable human-activity monitoring and personal healthcare. Adv. Mater. 2016, 28, 4338–4372. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, X.H.; Liang, J.; Lv, H.; Tong, H.; Ma, L.; Hu, Y.; Zhu, G.; Zhang, T.; Tie, Z.; et al. Versatile electronic skins for motion detection of joints enabled by aligned few-walled carbon nanotubes in flexible polymer composites. Adv. Funct. Mater. 2017, 27, 1606604. [Google Scholar] [CrossRef]
- Kim, D.H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal electronics. Science 2011, 333, 838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenry, J.C.Y.; Lim, C.T. Emerging flexible and wearable physical sensing platforms for healthcare and biomedical applications. Microsyst. Nanoeng. 2016, 2, 16043. [Google Scholar] [CrossRef] [PubMed]
- Lacour, S.P.; Benmerah, S.; Tarte, E.; FitzGerald, J.; Serra, J.; McMahon, S.; Fawcett, J.; Graudejus, O.; Yu, Z.; Morrison, B. Flexible and stretchable micro-electrodes for in vitro and in vivo neural interfaces. Med. Biolog. Eng. Comput. 2010, 48, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Kim, M.K.; Cheng, H.; Yeo, W.-H.; Huang, X.; Liu, Y.; Zhang, Y.; Huang, Y.; Rogers, J.A. Capacitive epidermal electronics for electrically safe, long-term electrophysiological measurements. Adv. Healthc. Mater. 2014, 3, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, X.; Zou, X.; Wu, S.; Wan, D.; Cao, A.; Liao, L.; Yuan, Q.; Duan, X. Ultrafine graphene nanomesh with large on/off ratio for high-performance flexible biosensors. Adv. Funct. Mater. 2017, 27, 1604096. [Google Scholar] [CrossRef]
- Zou, L.; Ge, Z.C.; Wang, J.; Cretu, E.; Li, X. Novel Tactile Sensor Technology and Smart Tactile Sensing Systems: A Review. Sensors 2017, 17, 2653. [Google Scholar] [CrossRef] [PubMed]
- Eh, A.L.-S.; Tan, A.W.M.; Cheng, X.; Magdassi, S.; Lee, P.S. Recent Advances in Flexible Electrochromic Devices: Prerequisites, Challenges, and Prospects. Energy Technol. 2018, 6, 33–45. [Google Scholar] [CrossRef]
- Chen, Z.; To, J.W.F.; Wang, C.; Lu, Z.; Liu, N.; Chortos, A.; Pan, L.; Wei, F.; Cui, Y.; Bao, Z. A three-dimensionally interconnected carbon nanotube-conducting polymer hydrogel network for high-performance flexible battery electrodes. Adv. Energy Mater. 2014, 4, 1400207. [Google Scholar] [CrossRef]
- Lin, H.; Weng, W.; Ren, J.; Qiu, L.; Zhang, Z.; Chen, P.; Chen, X.; Deng, J.; Wang, Y.; Peng, H. Twisted aligned carbon nanotube/silicon composite fiber anode for flexible wire-shaped lithium-ion battery. Adv. Mater. 2014, 26, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Gwon, H.; Hong, J.; Kim, H.; Seo, D.-H.; Jeon, S.; Kang, K. Recent progress on flexible lithium rechargeable batteries. Energy Environm. Sci. 2014, 7, 538–551. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, G.; Liu, Z.; Ma, X.; Chen, J.; Zhang, Z.; Ma, X.; Li, F.; Cheng, H.-M.; Ren, W. Scalable clean exfoliation of high-quality few-layer black phosphorus for a flexible lithium ion battery. Adv. Mater. 2016, 28, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Rooney, D.; Sun, K.; Yang, H.Y. 3D nitrogen-doped graphene foam with encapsulated germanium/nitrogen-doped graphene yolk-she, ll nanoarchitecture for high-performance flexible Li ion battery. Nat. Commun. 2017, 8, 13949. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, P.; Wu, Z.; Luo, D.; Yu, H.-Y.; Lu, Z.-H. Review and perspective of materials for flexible solar cells. Mater. Rep. Energy 2021, 1, 100001. [Google Scholar] [CrossRef]
- Dong, S.; Xi, J.; Wu, Y.; Liu, H.; Fu, C.; Liu, H.; Xiao, F. High loading MnO2 nanowires on graphene paper: Facile electrochemical synthesis and use as flexible electrode for tracking hydrogen peroxide secretion in live cells. Anal. Chim. Acta 2015, 853, 200–206. [Google Scholar] [CrossRef]
- Nagaraju, G.; Raju, G.S.R.; Ko, Y.H.; Yu, J.S. Hierarchical Ni–Co layered double hydroxide nanosheets entrapped on conductive textile fibers: A cost-effective and flexible electrode for high-performance pseudocapacitors. Nanoscale 2016, 8, 812–825. [Google Scholar] [CrossRef]
- Pu, J.; Yomogida, Y.; Liu, K.-K.; Li, L.-J.; Iwasa, Y.; Takenobu, T. Highly flexible MoS2 thin film transistors with ion gel dielectrics. Nano Lett. 2012, 12, 4013–4017. [Google Scholar] [CrossRef]
- Lee, G.H.; Yu, Y.J.; Cui, X.; Petrone, N.; Lee, C.-H.; Choi, M.S.; Lee, D.-Y.; Lee, C.; Yoo, W.J.; Watanabe, K.; et al. Flexible and transparent MoS2 field-effect transistors on hexagonal boron nitride-graphene heterostructures. ACS Nano 2013, 7, 7931–7936. [Google Scholar] [CrossRef]
- Chortos, A.; Lim, J.; To, J.W.F.; Vosgueritchian, M.; Dusseault, T.J.; Kim, T.H.; Hwang, S.; Bao, Z. Highly stretchable transistors using a microcracked organic semiconductor. Adv. Mater. 2014, 26, 4253–4259. [Google Scholar] [CrossRef]
- Park, S.; Shin, S.H.; Yogeesh, M.N.; Lee, A.L.; Rahimi, S.; Akinwande, D. Extremely high-frequency flexible graphene thin-film transistors. IEEE Electron Device Lett. 2016, 37, 512–515. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, E.Y.; Lee, H.H.; Seo, S. Characteristics of reduced graphene oxide quantum dots for a flexible memory thin film transistor. ACS Appl. Mater. Interfaces 2017, 9, 16375–16380. [Google Scholar] [CrossRef]
- Liang, J.; Li, L.; Niu, X.; Pei, Q. Elastomeric polymer light-emitting devices and displays. Nat. Photonics 2013, 7, 817–824. [Google Scholar] [CrossRef]
- Jung, M.; Kim, J.; Noh, J.; Lim, N.; Lim, C.; Lee, G.; Kim, J.; Kang, H.; Jung, K.; Leonard, A.D.; et al. All-printed and roll-to-roll-printable 13.56-MHz-operated 1-bit RF tag on plastic foils. IEEE Trans. Electron Devices 2010, 57, 571. [Google Scholar] [CrossRef]
- Gupta, V.; Kapur, S.; Saurabh, S.; Grover, A. Resistive Random Access Memory: A Review of Device Challenges. IETE Tech. Rev. 2020, 37, 377–390. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Zanoni, R.; Carbone, M.; Piancastelli, M.N.; Aballe, L.; Weiss, K.; Horn, K. Ethylene adsorption on Si(100)2 × 1: A high-resolution photoemission study. Phys. Rev. B 2000, 62, 17128–17133. [Google Scholar] [CrossRef]
- Kim, J.W.; Carbone, M.; Tallarida, J.H.; Dil, M.; Horn, K.; Casaletto, M.P.; Flammini, R.; Piancastelli, M.N. Adsorption of 2,3-butanediol on Si(100). Surf. Sci. 2004, 559, 179–185. [Google Scholar] [CrossRef]
- Carbone, M.; Caminiti, R. Adsorption states and site conversions of phenylacetylene on Si(100)2 × 1 calculated by DFT. J. Theor. Comput. Chem. 2012, 11, 1089–1099. [Google Scholar] [CrossRef]
- Carbone, M.; Piancastelli, M.N.; Zanoni, R.; Comtet, G.; Dujardin, G.; Hellner, L. Methanol adsorption on Si(111)-(7 × 7), investigated by core-line photoemission and mass spectrometry of photodesorbed ions. Surf. Sci. 1997, 370, L179–L184. [Google Scholar] [CrossRef]
- Carbone, M.; Zanoni, R.; Piancastelli, M.N.; Comtet, G.; Hellner, L.; Mayne, A. Adsorption of ethylene on Si(111)7 × 7 by synchrotron radiation photoemission. J. Electron Spectrosc. 1995, 76, 271–276. [Google Scholar] [CrossRef]
- Carbone, M.; Piancastelli, M.N.; Casaletto, M.P.; Zanoni, R.; Besnard-Ramage, M.J.; Comtet, G.; Dujardin, G.; Hellner, L. Phenol adsorption on Si (111)7 × 7 studied by synchrotron radiation photoemission and photodesorption. Surf. Sci. 1999, 419, 114–119. [Google Scholar] [CrossRef]
- Flammini, R.; Cecchetti, D.; Tagliatesta, P.; Carbone, M. Multiple options for phenol on Si(111)7 × 7 revealed by high resolution photoemission. Surf. Sci. 2020, 692, 121510. [Google Scholar] [CrossRef]
- Carbone, M.; Zanoni, R.; Piancastelli, M.N.; Comtet, G.; Dujardin, G.; Hellner, L. Synchrotron radiation photoemission and photostimulated desorption of deuterated methanol on Si(111)7 × 7 and Si(100)2 × 1. Surf. Sci. 1996, 352–354, 391–395. [Google Scholar] [CrossRef]
- Carbone, M.; Piancastelli, M.N.; Paggel, J.J.; Weindel, C.; Horn, K. A high-resolution photoemission study of ethanol adsorption on Si(111)-(7 × 7). Surf. Sci. 1998, 412–413, 441–446. [Google Scholar] [CrossRef]
- Carbone, M.; Caminiti, R. Fragmentation pathways of acetic acid upon adsorption on Si(100)2 × 1. Surf. Sci. 2008, 602, 852–858. [Google Scholar] [CrossRef]
- Carbone, M.; Meloni, S.; Caminiti, R. Dissociative versus molecular adsorption of phenol on Si (100) 2 × 1: A first-principles calculation. Phys. Rev. B 2007, 76, 085332. [Google Scholar] [CrossRef]
- Hammock, M.L.; Chortos, A.; Tee, B.C.K.; Tok, J.B.-H.; Bao, Z. 25th anniversary article: The evolution of electronic skin (e-skin): A brief history, design considerations, and recent progress. Adv. Mater. 2013, 25, 5997. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.A.; Someya, T.; Huang, Y. Materials and mechanics for stretchable electronics. Science 2010, 327, 1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, M.; Bonifas, A.P.; Lu, N.; Su, Y.; Li, R.; Cheng, H.; Ameen, A.; Huang, Y.; Rogers, J.A. Silicon nanomembranes for fingertip electronics. Nanotechnology 2012, 23, 344004. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Ma, Y.; Yuan, X.; Hu, Y.; Liu, J.; Jin., Z. Flexible devices: From materials, architectures to applications. J. Semicond. 2018, 39, 011010. [Google Scholar] [CrossRef]
- Valentini, F.; Roscioli, D.; Carbone, M.; Conte, V.; Floris, B.; Palleschi, G.; Flammini, R.; Bauer, E.M.; Nasillo, G.; Caponetti, E. Oxidized graphene in ionic liquids for assembling chemically modified electrodes: A structural and electrochemical characterization study. Anal. Chem. 2012, 84, 5823–5831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentini, F.; Roscioli, D.; Carbone, M.; Conte, V.; Floris, B.; Bauer, E.M.; Ditaranto, N.; Sabbatini, L.; Caponetti, E.; Chillura-Martino, D. Graphene and ionic liquids new gel paste electrodes for caffeic acid quantification. Sens. Actuat. B Chem. 2015, 212, 248–255. [Google Scholar] [CrossRef]
- Valentini, F.; Carbone, M.; Palleschi, G. Graphene oxide nanoribbons (GNO), reduced graphene nanoribbons (GNR), and multi-layers of oxidized graphene functionalized with ionic liquids (GO-IL) for assembly of miniaturized electrochemical devices. Anal. Bioanal. Chem. 2013, 405, 3449–3474. [Google Scholar] [CrossRef]
- Han, T.-H.; Kim, H.; Kwon, S.-J.; Lee, T.-W. Graphene-based flexible electronic devices. Mater. Sci. Eng. R Rep. 2017, 118, 1–43. [Google Scholar] [CrossRef]
- Lee, S.-M.; Kim, J.-H.; Ahn, J.-H. Graphene as a flexible electronicmaterial: Mechanical limitations by defectformation and efforts to overcome. Mater. Today 2015, 18, 336–344. [Google Scholar] [CrossRef]
- Petrone, N.; Meric, I.; Chari, T.; Shepard, K.L.; Hone, J. Graphene Field-Effect Transistors for Radio-Frequency Flexible Electronics. IEEE J. Electron Devices Soc. 2015, 3, 44–48. [Google Scholar] [CrossRef]
- Carbone, M.; Missori, M.; Micheli, L.; Tagliatesta, P.; Bauer, E.M. NiO pseudocapacitance and optical properties: Does the shape win? Materials 2020, 13, 1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillén, C.; Herrero, J. Influence of Acceptor Defects on the Structural, Optical and Electrical Properties of Sputtered NiO Thin Films. Phys. Status Solidi A 2021, 218, 2100237. [Google Scholar] [CrossRef]
- Mallick, G.; Labh, J.; Giri, L.; Pandey, A.C.; Karna, S.P. Facile synthesis and electron transport properties of NiO nanostructures investigated by scanning tunneling microscopy. AIP Adv. 2017, 7, 085007. [Google Scholar] [CrossRef] [Green Version]
- Kwon, U.; Kim, B.-G.; Nguyen, D.C.; Park, J.-H.; Ha, N.Y.; Kim, S.-J.; Ko, S.H.; Lee, S.; Lee, D.; Park, H.J. Solution-Processible Crystalline NiO Nanoparticles for HighPerformance Planar Perovskite Photovoltaic Cells. Sci. Rep. 2016, 6, 30759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donia, D.; Bauer, E.M.; Missori, M.; Roselli, L.; Cecchetti, D.; Tagliatesta, P.; Gontrani, L.; Carbone, M. Room Temperature Syntheses of ZnO and Its Structure. Symmetry 2021, 13, 733. [Google Scholar] [CrossRef]
- Donia, D.T.; Carbone, M. Fate of the nanoparticles in environmental cycles. Int. J. Environ. Sci. Technol. 2019, 16, 583–600. [Google Scholar] [CrossRef]
- Carbone, M. Cu-Zn-Co nanosized mixed oxides prepared from hydroxycarbonate precursors. J. Alloys Compd. 2016, 688, 202–209. [Google Scholar] [CrossRef]
- Carbone, M.; Nesticò, A.; Bellucci, N.; Micheli, L.; Palleschi, G. Enhanced performances of sensors based on screen printed electrodes modified with nanosized NiO particles. Electrochim. Acta 2017, 246, 580–587. [Google Scholar] [CrossRef]
- Carbone, M.; Briancesco, R.; Bonadonna, L. Antimicrobial power of Cu/Zn mixed oxide nanoparticles to Escherichia coli. Environ. Nanotech. Monitor. Manag. 2017, 7, 97–102. [Google Scholar] [CrossRef]
- Carbone, M. Zn defective ZnCo2O4 nanorods as high capacity anode for lithium ion batteries. J. Electroanal. Chem. 2018, 815, 151–157. [Google Scholar] [CrossRef]
- Carbone, M.; Sabbatella, G.; Antonaroli, S.; Remita, H.; Orlando, V.; Biagioni, S.; Nucara, A. Exogenous control over intracellular acidification: Enhancement via proton caged compounds coupled to gold nanoparticles. Biochim. Biophys. Acta Gen.-Subj. 2015, 1850, 2304–2307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbatella, G.; Antonaroli, S.; Diociauti, M.; Nucara, A.; Carbone, M. Synthesis of proton caged disulphide compounds for gold nanoparticle functionalization. New J. Chem. 2015, 39, 2489–2496. [Google Scholar] [CrossRef]
- Carbone, M.; Bauer, E.M.; Micheli, L.; Missori, M. NiO morphology dependent optical and electrochemical properties. Colloids Surf. A 2017, 532, 178–182. [Google Scholar] [CrossRef]
- Nakate, U.T.; Ahmad, R.; Patil, P.; Yud, Y.T.; Hahn, Y.-B. Ultra thin NiO nanosheets for high performance hydrogen gas sensor device. Appl. Surf. Sci. 2020, 506, 144971. [Google Scholar] [CrossRef]
- Carbone, M.; Tagliatesta, P. NiO grained-flowers and nanoparticles for ethanol sensing. Materials 2020, 13, 1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokoena, T.P.; Swart, H.C.; Motaung, D.E. A review on recent progress of p-type nickel oxide based gas sensors: Future perspectives. J. Alloys Compd. 2019, 805, 267–294. [Google Scholar] [CrossRef]
- Carbone, M. CQDs@NiO: An efficient tool for CH4 sensing. Appl. Sci. 2020, 10, 6251. [Google Scholar] [CrossRef]
- Dhas, S.D.; Maldar, P.S.; Patil, M.D.; Nagare, A.B.; Waikar, M.R.; Sonkawade, R.G.; Moholkar, A.V. Synthesis of NiO nanoparticles for supercapacitor application as an efficient electrode material. Vacuum 2020, 181, 109646. [Google Scholar] [CrossRef]
- Xiong, S.; Jiang, S.; Wang, J.; Lin, H.; Lin, M.; Weng, S.; Liu, S.; Jiao, Y.; Xu, Y.; Chen, J. A high-performance hybrid supercapacitor with NiO derived NiO@Ni-MOF composite electrodes. Electrochim. Acta 2020, 340, 135956. [Google Scholar] [CrossRef]
- Xiao, H.; Yao, S.; Liu, H.; Qu, F.; Zhang, X.; Wun, X. NiO nanosheet assembles for supercapacitor electrode materials. Progr. Nat. Sci. 2016, 26, 271–275. [Google Scholar] [CrossRef] [Green Version]
- Di Girolamo, D.; Di Giacomo, F.; Matteocci, F.; Marrani, A.G.; Dini, D.; Abate, A. Progress, highlights and perspectives on NiO in perovskite photovoltaics. Chem. Sci. 2020, 11, 7746–7759. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhang, Z.; Yu, L.; Yuan, H.; Zhang, J.; Liu, X.; Hu, Z.; Zhu, Y. Synthesis of well dispersed NiO ink for efficient perovskite solar cells. J. Alloys Compd. 2021, 860, 157889. [Google Scholar] [CrossRef]
- Ielmini, D.; Nardi, F.; Cagli, C. Physical models of size-dependent nanofilament formation and rupture in NiO resistive switching memories. Nanotechnology 2011, 22, 254022. [Google Scholar] [CrossRef] [PubMed]
- Ielmini, D.; Nardi, F.; Cagli, C.; Lacaita, A.L. Size-Dependent Retention Time in NiO-Based Resistive-Switching Memories. IEEE Electr. Device Lett. 2010, 31, 353–355. [Google Scholar] [CrossRef]
- Lee, E.M.; Ahn, Y.; Son, J.Y. Electric field control of magnetization reversal in conducting filament nanostructures in NiO resistive random access memory. J. Alloys Compd. 2020, 840, 155748. [Google Scholar] [CrossRef]
- Lang, X.; Hirata, A.; Fujita, T.; Chen, M. Nanoporous metal/oxide hybrid electrodes for electrochemical supercapacitors. Nat. Nanotechnol. 2011, 6, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.V.; Ahamed, A.J.; Karthikeyan, M. Synthesis and characterization of NiO nanoparticles by chemical as well as green routes and their comparisons with respect to cytotoxic effect and toxicity studies in microbial and MCF-7 cancer cell models. SN Appl. Sci. 2019, 1, 1083. [Google Scholar] [CrossRef] [Green Version]
- Ariyanta, H.A.; Ivandini, T.A.; Yulizar, Y. Novel NiO nanoparticles via phytosynthesis method: Structural, morphological and optical properties. J. Mol. Struct. 2021, 1227, 129543. [Google Scholar] [CrossRef]
- Ukoba, K.O.; Eloka-Eboka, A.C.; Inambao, F.L. Review of nanostructured NiO thin film deposition using the spray pyrolysis Technique. Renew. Sust. Energ. Rev. 2018, 82, 2900–2915. [Google Scholar] [CrossRef]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 45, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Meher, S.K.; Justin, P.; Rao, G.R. Nanoscale morphology dependent pseudocapacitance of NiO: Influence of intercalating anions during synthesis. Nanoscale 2011, 3, 683. [Google Scholar] [CrossRef] [PubMed]
- Brus, L.E. Electronic wave functions in semiconductor clusters: Experiment and theory. J. Phys. Chem. 1986, 90, 2555–2560. [Google Scholar] [CrossRef]
- Raj, B.G.S.; Natesan, B.; Asiri, A.M.; Wu, J.J.; Anandan, S. Pseudocapacitive properties of nickel oxide nanoparticles synthesized via ultrasonication approach. Ionics 2019, 26, 953–960. [Google Scholar]
- Sun, W.; Xiao, L.; Wu, X. Facile synthesis of NiO nanocubes for photocatalysts and supercapacitor electrodes. J. Alloys Compd. 2019, 772, 465–471. [Google Scholar] [CrossRef]
- Vidhyadharan, B.; Zain, N.K.M.; Misnon, I.I.; Aziz, R.A.; Ismail, J.; Yusoff, M.M.; Jose, R. High performance supercapacitor electrodes from electrospun nickel oxide nanowires. J. Alloys Compd. 2014, 610, 143–150. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Hou, L.; Yang, L.; Shen, L.; Zhang, X. Facile growth of hexagonal NiO nanoplatelet arrays assembled by mesoporous nanosheets on Ni foam towards high-performance electrochemical capacitors. Electrochim. Acta 2012, 78, 532–538. [Google Scholar] [CrossRef]
- Yang, H.; Zou, J. Controllable preparation of hierarchical NiO hollow microspheres with high pseudo-capacitance. Trans. Nonferrous Met. Soc. China 2018, 28, 1808–1818. [Google Scholar] [CrossRef]
- Xu, K.; Zou, R.; Li, W.; Xue, Y.; Song, G.; Liu, Q.; Liu, X.; Hu, J. Self-assembling hybrid NiO/Co3O4 ultrathin and mesoporous nanosheets into flower-like architectures for pseudocapacitance. J. Mater. Chem. A 2013, 1, 9107–9113. [Google Scholar] [CrossRef]
- Hakamada, M.; Abe, T.; Mabuchi, M. Electrodes from carbon nanotubes/NiO nanocomposites synthesized in modified Watts bath for supercapacitors. J. Power Sources 2016, 325, 670–674. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Wang, Q.; Zhang, W.; Wang, Y.; Chen, W. Nano nickel oxide coated graphene/polyaniline composite film with high electrochemical performance for flexible supercapacitor. Electrochim. Acta 2016, 211, 1066–1075. [Google Scholar] [CrossRef]
- Qiu, D.; Ma, X.; Zhang, J.; Lin, Z.; Zhao, B. In situ synthesis of mesoporous NiO nanoplates embedded in a flexible graphene matrix for supercapacitor electrodes. Mater. Lett. 2018, 232, 163–166. [Google Scholar] [CrossRef]
- Ran, F.; Yang, H.; Wu, Y.; Zhao, X.; Tan, Y.; Liu, Y.; Niu, X.; Chen, Y.; Kong, L.; Kang, L. Facile preparation of porous nickel oxide membrane for flexible supercapacitors electrode via phase-separation method of polymer. Mater. Res. Bull. 2018, 103, 25–31. [Google Scholar] [CrossRef]
- Buxton, S.; Garman, E.; Heim, K.E.; Lyons-Darden, T.; Schlekat, C.E.; Taylor, M.D.; Oller, A.R. Concise Review of Nickel Human Health Toxicology and Ecotoxicology. Inorganics 2019, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Horie, M.; Stowe, M.; Tabei, M.; Kuroda, E. Metal ion release of manufactured metal oxide nanoparticles is involved in the allergic response to inhaled ovalbumin in mice. Occup. Dis. Environ. Med. 2016, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Oller, A.R. Respiratory carcinogenicity assessment of soluble nickel compounds. Environ. Health Perspect. 2002, 110, 841–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, C.A.; Soares, H.M.V.M.; Soares, E.V. Toxic effects of nickel oxide (NiO) nanoparticles on the freshwater alga Pseudokirchneriella subcapitata. Aquat. Toxicol. 2018, 204, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zheng, D.; Hu, Q.; Zhang, X.; Wang, Z.; Li, Y.; Zhu, J.; Ou, J.Z.; Yang, C.; Wang, Y. Flexible integrated metallic glass-based sandwich electrodes for high-performance wearable all-solid-state supercapacitors. App. Mater. Today 2020, 19, 100539. [Google Scholar] [CrossRef]
- Wang, Z.F.; Zhang, X.M.; Liu, X.L.; Wang, Y.C.; Zhang, Y.G.; Li, Y.Y.; Zhao, W.M.; Qin, C.L.; Mukanova, A.; Bakenov, Z. Bimodal nanoporous NiO@Ni–Si network prepared by dealloying method for stable Li-ion storage. J. Power Sources 2020, 449, 227550. [Google Scholar] [CrossRef]
- Wang, Z.F.; Fei, P.Y.; Xiong, H.Q.; Qin, C.L.; Zhao, W.M.; Liu, X.Z. CoFe2O4 nanoplates synthesized by dealloying method as high performance Li-ion battery anodes. Electrochim. Acta 2017, 252, 295–305. [Google Scholar] [CrossRef]
- Zhao, F.; Zheng, D.; Liu, Y.; Pan, F.; Deng, Q.; Qin, C.; Li, Y.; Wang, Z. Flexible Co(OH)2/NiOxHy@Ni hybrid electrodes for high energy density supercapacitors. Chem. Eng. J. 2021, 415, 18871. [Google Scholar] [CrossRef]
- Marsilia, M.; Susmel, S. Free-standing Plastic electrodes: Formulation, electrochemical characterization and application to dopamine detection. Sensor. Actuat. B Chem. 2018, 255, 1087–1096. [Google Scholar] [CrossRef]
- Carbone, M.; Aneggi, E.; Figueredo, F.; Susmel, S. NiO-nanoflowers decorating a plastic electrode for the non-enzymatic amperometric detection of H2O2 in milk: Old issue, new challenge. Food Control 2022, 132, 108549. [Google Scholar] [CrossRef]
- Kima, S.-J.; Lee, H.; Hong, S.-H. Solution-processed flexible NiO resistive random access memory device. Solid State Electron. 2018, 142, 56–61. [Google Scholar] [CrossRef]
- Rakibuddin, M.; Shinde, M.A.; Kim, H. Sol-gel fabrication of NiO and NiO/WO3 based electrochromic device on ITO and flexible substrate. Ceram. Int. 2020, 46, 8631–8639. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, T.-G.; Nahm, S.; Kim, D.H.; Yang, D.J.; Han, S.H. Investigation of all-solid-state electrochromic devices with durability enhanced tungsten-doped nickel oxide as a counter electrode. J. Alloys Compd. 2020, 815, 152399. [Google Scholar] [CrossRef]
- Appiagyei, A.B.; Banua, J.; Han, J.I. Flexible and patterned-free Ni/NiO-based temperature device on cylindrical PET fabricated by RF magnetron sputtering: Bending and washing endurance tests. J. Ind. Eng. Chem. 2021, 100, 372–382. [Google Scholar] [CrossRef]
- Cao, B.; Yang, L.; Jiang, S.; Lin, H.; Wang, N.; Li, X. Flexible Quintuple Cation Perovskite Solar Cells with High Efficiency. J. Mater. Chem. A 2019, 7, 4960–4970. [Google Scholar] [CrossRef]
- Jo, J.W.; Seo, M.-S.; Park, M.; Kim, J.-Y.; Park, J.S.; Han, I.K.; Ahn, H.; Jung, J.W.; Sohn, B.-H.; Ko, M.J.; et al. Improving Performance and Stability of Flexible Planar-Heterojunction Perovskite Solar Cells Using Polymeric Hole-Transport Material. Adv. Funct. Mater. 2016, 26, 4464–4471. [Google Scholar] [CrossRef]
- Yin, X.; Chen, P.; Que, M.; Xing, Y.; Que, W.; Niu, C.; Shao, J. Highly Efficient Flexible Perovskite Solar Cells Using Solution-Derived NiOx Hole Contacts. ACS Nano 2016, 10, 3630–3636. [Google Scholar] [CrossRef]
- Wang, Z.; Rong, X.; Wang, L.; Wang, W.; Lin, H.; Li, X. Dual Role of Amino-Functionalized Graphene Quantum Dots in NiOx Films for Efficient Inverted Flexible Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 8342–8350. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Bi, H. Electrodes for Flexible Integrated Supercapacitors, from Flexible Supercapacitor Nanoarchitectonics. Scrivener Publ. LLC 2021, Chapter 1, 1–26. [Google Scholar]
- Shi, S.; Xu, C.; Yang, C.; Li, J.; Du, H.; Li, B.; Kang, F. Flexible supercapacitors. Particuology 2013, 11, 371–377. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Wang, Y.; Cheng, T.; Lai, W.-Y.; Pang, H.; Huang, W. Flexible supercapacitors based on paper substrates: A new paradigm for low-cost energy storage. Chem. Soc. Rev. 2015, 44, 5181–5199. [Google Scholar] [CrossRef] [PubMed]
- Chee, W.; Lim, H.; Zainal, Z.; Harrison, I.; Huang, N.; Andou, Y.; Chong, K.; Pandikumar, A. Electrospun nanofiber membrane as ultrathin flexible supercapacitors. RSC Adv. 2017, 7, 12033–12040. [Google Scholar] [CrossRef] [Green Version]
- Zhai, S.; Jin, K.; Zhou, M.; Fan, Z.; Zhao, H.; Li, X.; Zhao, Y.; Ge, F.; Cai, Z. A novel high performance flexible supercapacitor based on porous carbonized cotton/ZnO nanoparticle/CuS micro-sphere. Colloids Surf. A Physicochem. Eng. Asp. 2020, 584, 124025. [Google Scholar] [CrossRef]
- Khattak, A.M.; Yin, H.; Ghazi, Z.A.; Liang, B.; Iqbal, A.; Khan, N.A.; Gao, Y.; Li, L.; Tang, Z. Three dimensional iron oxide/graphene aerogel hybrids as all-solid- state flexible supercapacitor electrodes. RSC Adv. 2016, 6, 58994–59000. [Google Scholar] [CrossRef]
- Fu, W.; Zhao, E.; Ma, R.; Sun, Z.; Yang, Y.; Sevilla, M.; Fuertes, A.B.; Magasinski, A.; Yushin, G. Anatase TiO2 confined in carbon nanopores for high-energy Li-Ion hybrid supercapacitors operating at high rates and subzero temperatures. Adv. Energy Mater. 2020, 10, 1902993. [Google Scholar] [CrossRef]
- Gwo-Bin, L.; Fu-Chun, H.; Chia-Yen, L.; Jiun-Jih, M. A new fabrication process for a flexible skin with temperature sensor array and its applications. Acta Mech. Sin. 2003, 20, 27–32. [Google Scholar] [CrossRef]
- Yu, C.; Wang, Z.; Yu, H.; Jiang, H. A stretchable temperature sensor based on elastically buckled thin film devices on elastomeric substrates. Appl. Phys. Lett. 2009, 95, 141912. [Google Scholar] [CrossRef] [Green Version]
- Dankoco, M.D.; Tesfay, G.Y.; Benevent, E.; Bendahan, M. Temperature sensor realized by inkjet printing process on flexible substrate. Mater. Sci. Eng. B 2015, 205, 1–5. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, B.; Ma, X.; Hao, Y.; Chen, X. Physically Transient Resistive Switching Memory Based on Silk Protein. Small 2016, 12, 2715–2719. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, B.; Wang, H.; Ma, X.; Hao, Y.; Chen, X. Ultra-Lightweight Resistive Switching Memory Devices Based on Silk Fibroin. Small 2016, 12, 3360–3365. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Yu, H.C.; Huang, C.Y.; Chung, W.L.; Wu, S.L.; Su, Y.K. Nonvolatile Bio-Memristor Fabricated with Egg Albumen Film. Sci. Rep. 2014, 5, 1002201–1002212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Banerjee, W.; Cao, J.; Ji, Z.; Li, L.; Liu, M. Improvement of durability and switching speed by incorporating nanocrystals in the HfOx based resistive random access memory devices. Appl. Phys. Lett. 2018, 113, 023105. [Google Scholar] [CrossRef]
- Hu, Q.; Park, M.R.; Abbas, H.; Kang, T.S.; Yoon, T.S.; Kang, C.J. Forming-free resistive switching characteristics in tantalum oxide and manganese oxide based crossbar array structure. Microelectron. Eng. 2018, 190, 7–10. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chang, Y.F.; Wu, X.; Zhou, F.; Guo, M.; Lin, C.Y.; Hsieh, C.-C.; Fowler, B.; Chang, T.-C.; Lee, J.C. Dynamic conductance characteristics in HfO2 based resistive random access memory. RSC Adv. 2017, 7, 12984–12989. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.W.; Baik, S.J.; Kang, S.J.; Lim, K.S. Characteristics of ZnO thin film for the resistive random access memory. MRS Online Proc. Libr. Arch. 2010, 1250. [Google Scholar] [CrossRef]
- Mondal, S.; Chueh, C.-H.; Pan, T.-M. High-Performance Flexible Ni/Sm2O3/ITO ReRAM Device for Low-Power Nonvolatile Memory Applications. IEEE Electron Dev. Lett. 2013, 9, 1145–1147. [Google Scholar] [CrossRef]
- Raeis-Hosseini, N.; Rho, J. Solution-Processed Flexible Biomemristor Based on Gold-Decorated Chitosan. ACS Appl. Mater. Interfaces 2021, 13, 5445–5450. [Google Scholar] [CrossRef]
- Li, R.; Ma, X.; Li, J.; Cao, J.; Gao, H.; Li, T.; Zhang, X.; Wang, L.; Zhang, Q.; Wang, G.; et al. Flexible and high-performance electrochromic devices enabled by self-assembled 2D TiO2/MXene heterostructures. Nat. Commun. 2021, 12, 1587. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.; Zhang, Z.; Xi, J.; Huang, Y.; Xiao, F.; Wang, S.; Liu, Y. Freestanding Graphene Paper Supported Three-Dimensional Porous Graphene−Polyaniline Nanocomposite Synthesized by Inkjet Printing and in Flexible All-Solid-State Supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 16312–16319. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Zhang, L.; Huang, Y.; Wang, S.; Pan, G.; Li, L. Directly writing flexible temperature sensor with graphene nanoribbons for disposable healthcare devices. RSC Adv. 2020, 10, 22222–22229. [Google Scholar] [CrossRef]
- Hao, C.; Wen, F.; Xiang, J.; Yuan, S.; Yang, B.; Li, L.; Wang, W.; Zeng, Z.; Wang, L.; Liu, Z.; et al. Liquid-Exfoliated Black Phosphorous Nanosheet Thin Films for Flexible Resistive Random Access Memory Applications. Adv. Funct. Mater. 2016, 13, 2448–2455. [Google Scholar] [CrossRef]
- Aziz, T.; Wei, S.; Sun, Y.; Ma, L.-P.; Pei, S.; Dong, S.; Ren, W.; Liu, Q.; Cheng, H.-M.; Sun, D.-M. High-performance flexible resistive random access memory devices based on graphene oxidized with a perpendicular oxidation gradient. Nanoscale 2021, 254, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Shinde, M.A.; Kim, H. Flexible electrochromic device with simple solution processed stable silver nanowire based transparent conductive electrodes. Synth. Met. 2019, 254, 97–105. [Google Scholar] [CrossRef]

| NiO Morphology | Capacitance | Reference |
|---|---|---|
| Nanoparticles | 423 F/g at 0.5 mA/cm2 | [84] |
| Nanoparticles | 1012 mF/cm2 at 1 mA/cm2 | [85] |
| Nanowires | 670 F/g at 1 A/g | [86] |
| Nanoplates array | 1124 F/g at 2 A/g | [87] |
| Microspheres | 1482 F/g at 0.5 A/g | [88] |
| NiO Composites | ||
| NiO@CeO2 (5 wt%) Flower-like microspheres | 2155.6 F/g at 1 A/g | [89] |
| NiO@CNT Particles | 2480 F/g at 0.5 A/g | [90] |
| NiO-GNS/PANI | 1409 F/g | [91] |
| NiO/GNS | 1050 F/g | [92] |
| M-NiO-G//AC | 154.0 F/g | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbone, M. NiO-Based Electronic Flexible Devices. Appl. Sci. 2022, 12, 2839. https://doi.org/10.3390/app12062839
Carbone M. NiO-Based Electronic Flexible Devices. Applied Sciences. 2022; 12(6):2839. https://doi.org/10.3390/app12062839
Chicago/Turabian StyleCarbone, Marilena. 2022. "NiO-Based Electronic Flexible Devices" Applied Sciences 12, no. 6: 2839. https://doi.org/10.3390/app12062839







