1. Introduction
Bone tissue engineering allows the development of alternative strategies to repair bone defects caused by disease or trauma that cannot be healed spontaneously in the living tissue. This approach emerged due to the limitations of the conventional therapies that usually includes transplantation, surgical reconstitution, and artificial prosthesis. Bone tissue engineering requires the incorporation of osteoblasts on 3D scaffolds for the adhesion, proliferation and differentiation of cells, as well as nutrient and oxygen availability [
1,
2,
3].
There are several requirements that should be considered to obtain an efficient 3D scaffold. In the initial stage, the biocompatibility and bioactivity of the support have a major impact on cell attachment, and an appropriate macro and micro porosity and shape allow the tissue in-growth and delivery of nutrients and oxygen to the cells. Furthermore, the scaffold should have sufficient mechanical strength to provide the structural requirements of the substituted tissue, and the degradation rate must be gradual for cell growth and proliferation to promote the creation of a new bone tissue [
2,
4,
5].
The fabrication of 3D scaffolds with high resolution and control on micro/nano level structure are facilitated using 3D printing technologies. Extrusion-based techniques consist in a computer-controlled layer-by-layer deposition where polymer filament fused is extruded through a nozzle. This technology has been used to process various biomaterials, mainly thermoplastics, with polycaprolactone (PCL), polylactic acid (PLA), and poly(lactic-co-glycolic) acid (PLGA) being the most frequently used for bone tissue engineering applications [
6,
7,
8,
9,
10,
11,
12,
13]. However, due to their low bioactivity, the development of composite scaffolds has been especially explored by combining polymers with calcium-phosphate-based materials [
14]. The composites are comprised of two or more materials, aiming at the development of more efficient scaffolds by combining regenerative properties of more than one biomaterial [
15]. In addition, composites integrating calcium phosphates and polymers combine good mechanical properties with good biocompatibility, reaching to a 3D substitute that mimics the heterogeneity and hierarchical structure typical of the native extracellular bone matrix [
16,
17]. Therefore, the use of hydroxyapatite (HA) can be justified because of its chemical similarity to the natural mineral component of bone tissue. Additionally, HA has the ability to integrate into bone structures and support its growth without breaking or dissolving the tissue (bioactive properties) [
18,
19]. HA has been previously blended with PCL, a polymer considered safe by the Food and Drug Administration (FDA) [
20]. PCL, through its melt processing, allows 3D porous scaffolds with highly interconnected porous network and with high reproducibility to be obtained by using commercialised [
21] and novel biomanufacturing systems [
5,
8,
9,
10,
11,
12,
22,
23,
24]. PCL-based scaffolds have been produced by these newly developed equipments and have been tested in vitro and in vivo with good cell viability, osteogenic differentiation promotion and improved bone tissue regeneration outcomes, both in vitro and in vivo [
5,
8,
11,
25,
26,
27,
28,
29,
30,
31].
With respect to the production of the composite structures, their properties are mainly dependent on the nature of the materials and preparation method. In the present study, one of the objectives considered is to investigate how the solvent can influence the resultant composite mixture and its properties. Concerning this topic, a few relevant studies regarding solvents’ impact in the solvent casting technique have been reported. Patlolla et al. [
32] conducted a study where 20% HA/80% β-TCP and PCL composites were produced using either methylene chloride (MC) or a combination of MC and dimethylformamide (MC + DMF) solvents. This study demonstrates that the produced structures presented uniform fibres and homogeneous ceramic dispersions, and the solvent or solvent combination used to produce the composites proved to be a determining factor to define its properties, thus affecting cell growth kinetics. In another study, Choudhury and colleagues [
33] investigated the effect of different solvents, 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), dichloromethane (DCM) and chloroform (CF), to fabricate PLA scaffolds. PLA/DCM scaffolds presented more thermal stability and a stiffer base compared with PLA/HFIP and PLA/CF scaffolds. However, the PLA/CF scaffold showed higher porosity against the PLA/HFIP scaffold, which is beneficial for the requirements for bone tissue engineering.
Altogether, it has been established that different solvents interact with the polymer quite differently. These findings appeared to be caused by (I) proton conductivity [
34,
35,
36], (II) solvent volatility [
33,
34], which has been found to be better with a less volatile solvent, combined with (III) stronger solvent–polymer interactions [
34], to address the uniformity requirements of the scaffold/structure and the regularity of polymer surface. Additionally, the choice of solvent can impact the dispersion of particles within a polymeric matrix, and all these features can affect composite structure, morphology and properties, which may, in turn, affect cell behaviour and tissue in-growth and regeneration [
32].
In this work, in addition to the incorporation of a ceramic amount of 20 and 40 wt% on PCL, we analyse the influence of the two mixture techniques (solvent casting (SC) and melt blending (MB)) on the properties of PCL and PCL/HA composites. Following this, the six different groups considered (PCL SC, PCL MB, PCL/HA 80:20 SC, PCL/HA 80:20 MB, PCL/HA 60:40 SC and PCL/HA 60:40 MB) are 3D printed through an extrusion-based technique, using a previously developed biomanufacturing system [
8,
9,
10,
12,
22,
24,
25,
26,
27,
28,
29], and their physicochemical properties are further analysed. Further in vitro assays are taken, applying mesenchymal stem cells so as to evaluate the regenerative potential of the produced scaffolds.
3. Discussion
Three-dimensional porous biodegradable scaffolds were explored using various techniques in the interest of being suitable as bone substitutes for bone repair and reconstruction. The research on the production processes for the dispersion of nanomaterials in polymer matrices and the design of 3D printing scaffolds, as well the combination of these features, plays a critical role in tissue engineering. These processes contribute to achieve the requirements of 3D scaffolds and the appropriate physical inter-connections for an efficient cell permeation and colonization. The current work is focused on the processing of PCL and PCL/HA composites prepared by MB and SC methods, using an extrusion-based technique for the development of 3D substitutes for bone tissue engineering. PCL was selected as the major component of scaffolds because it is easy to process by 3D printing, is biodegradable and biocompatible, and possesses adequate mechanical strength. However, the hydrophobic surface and lack of osteoconductivity of the PCL matrix represents some disadvantages, which can impair cells adhesion and proliferation [
42,
43]. Therefore, HA incorporation was considered to enhance scaffolds’ performance, providing more strength and improving cellular activities, including cell attachment, proliferation, and differentiation [
17,
44]. Moreover, in human body nearly 70% of hard tissues are composed of HA and are clinically used for orthopaedic and dental repairs [
45]. Considering that usually the amount of ceramic remains under 40% of the final material weight, and the most used polymer/ceramic weight ratio is 80/20, the weight ratio of polymer/ceramic was defined at 80/20 and 60/40 wt% [
14]. These proportions allow to evaluate and correlate this work outcomes with other previous works and also to prevent 3D printing difficulties, such as nozzle clogging and nonuniform deposition of the composite material filaments. Regarding the mixture techniques to produce PCL and PCL/HA composites, MB and SC methods are widely used for this purpose, as they allow an easy way to produce composites and provide good dispersion of fillers into the polymer matrix. While the MB method depends on high temperatures to mix the two materials, the SC implies the use of an organic solvent to promote polymer dissolution and improve HA nanoparticles dispersion. The macroporosity of the scaffolds was designed using an extrusion-based 3D printing technique to obtain well-defined and interconnected pores for efficient cell colonization and infiltration, and consequently cell adhesion and in-growth. Following previous valuable works on PCL/HA scaffolds with promising outcomes [
13,
44,
46,
47,
48,
49], this work intended to further analyse the impact of different proportions of HA content, as well as the application of two different fabrication processes for the incorporation of HA nanoparticles in PCL matrices, on the mechanical, physical, chemical, and in vitro outcomes of the scaffolds. Moreover, it is also important to highlight the aim of achieving controlled, reproducible and well-defined 3D structures fabricated by a previously developed 3D printing system [
8,
9,
10,
12,
22,
24,
25,
26,
27,
28,
29].
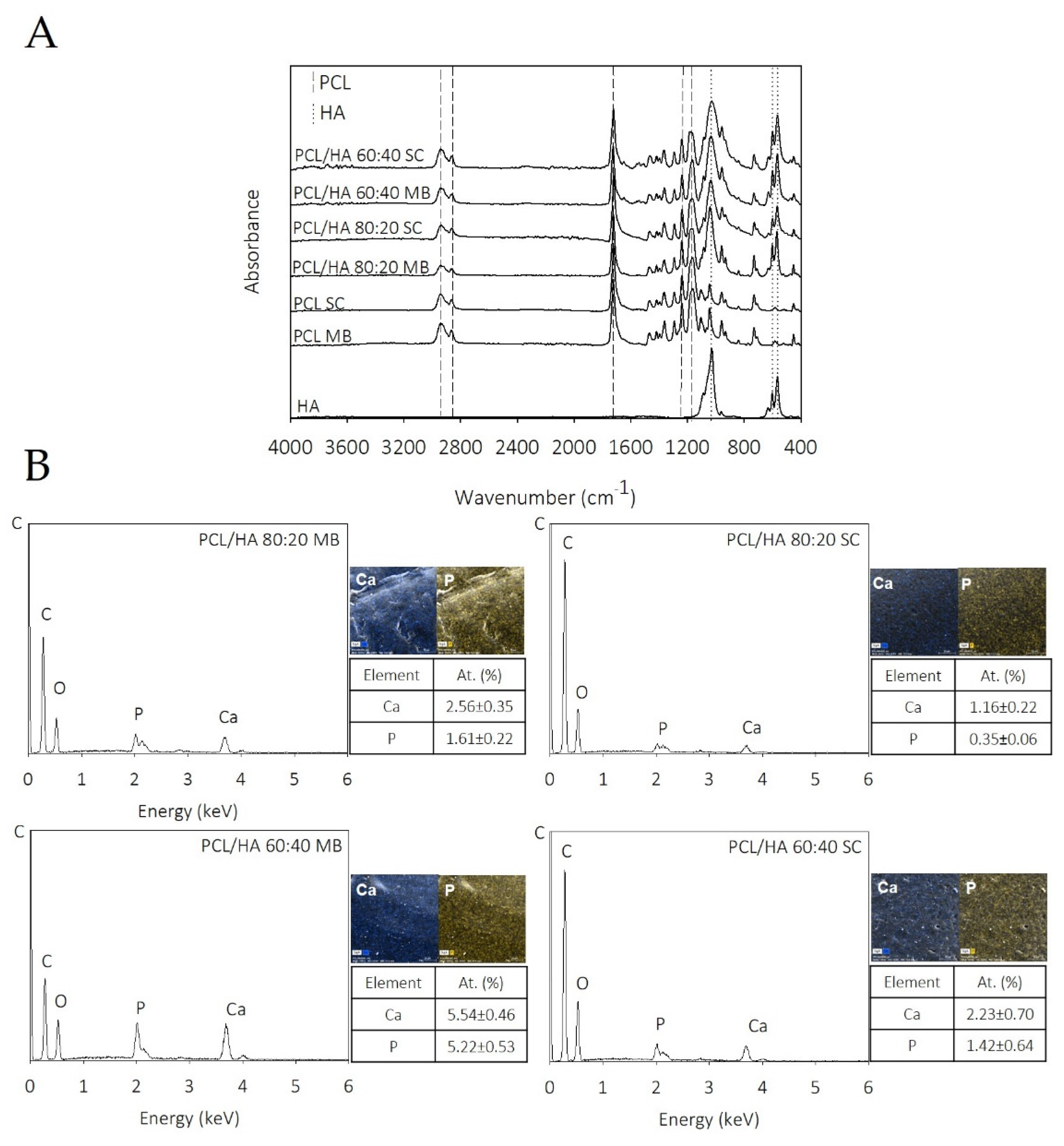
The chemical composition of the composites was analysed by FTIR to investigate the functional groups of the PCL/HA composite scaffolds and any chemical interactions among the components of PCL and HA. The spectra of the PCL/HA composite scaffolds presented all characteristic bands of PCL and HA, and only differed in terms of intensity, confirming the lack of chemical interaction. These results are consistent with those reported by others works [
50,
51]. Chemical analysis indicates that MB and SC composites have been properly prepared and successfully 3D printed with the integration of the characteristic peaks of the individual components.
Regarding EDX mapping data (
Figure 1B), the HA was detected as distributed throughout the filaments of composites produced from both preparation methods. Comparing the EDX spectra of scaffolds obtained by the different mixture techniques, it is evident that, in the MB method, bioceramic particles are less embedded on PCL matrix and consequently more elements of the HA are detected on these samples’ surface. The Ca/P ratios of the scaffolds are also presented in
Figure 1B. The results show that the Ca/P ratio is different among the produced scaffolds. The samples produced by MB present a Ca/P ratio closer to the stochiometric HA (1.67) [
52]. The authors hypothesise these differences to be related with the blending processes, as well as to the presence of some HA particles exposed on the filament surface. These results also support the higher mechanical properties obtained in the samples produced by MB.
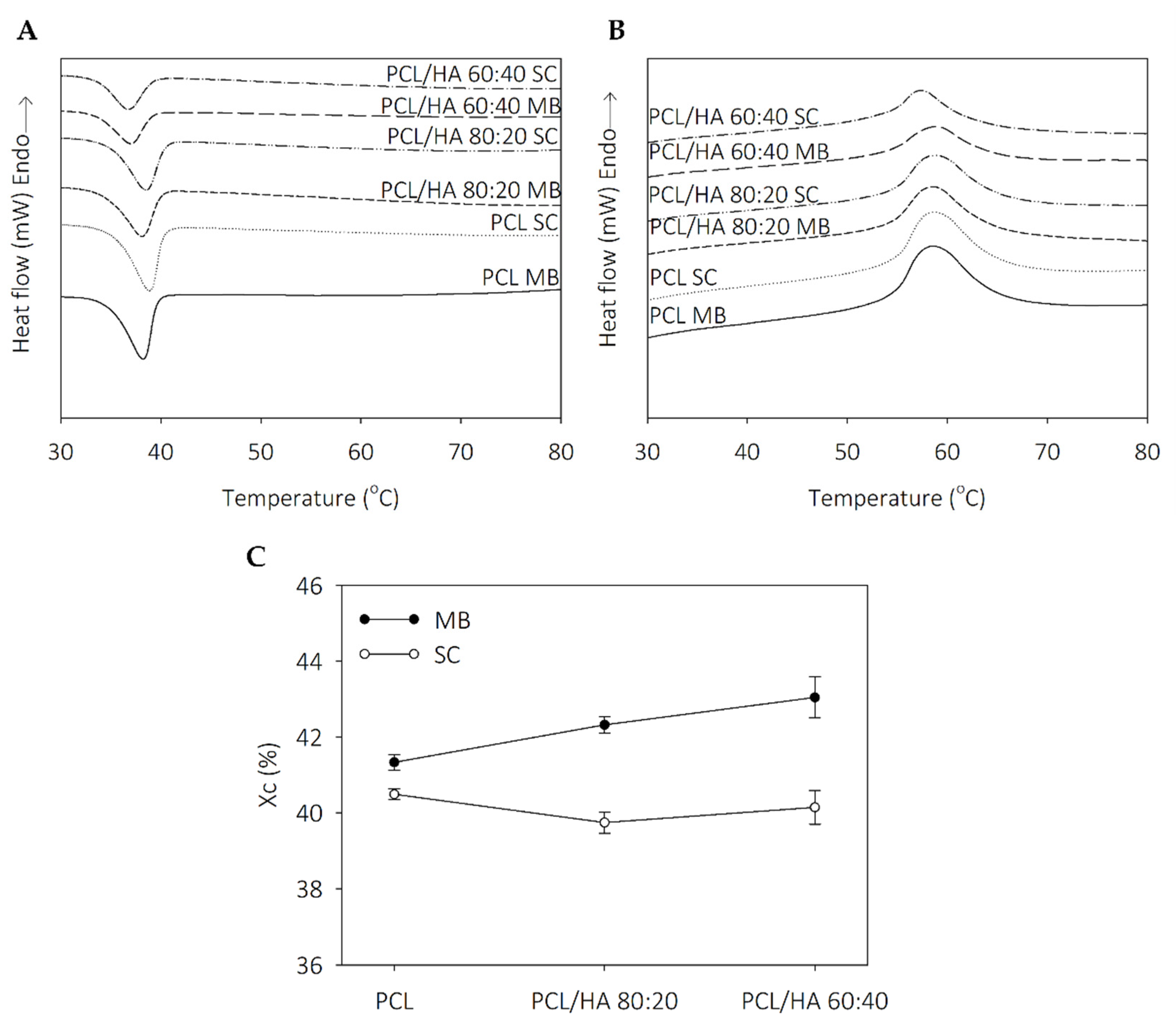
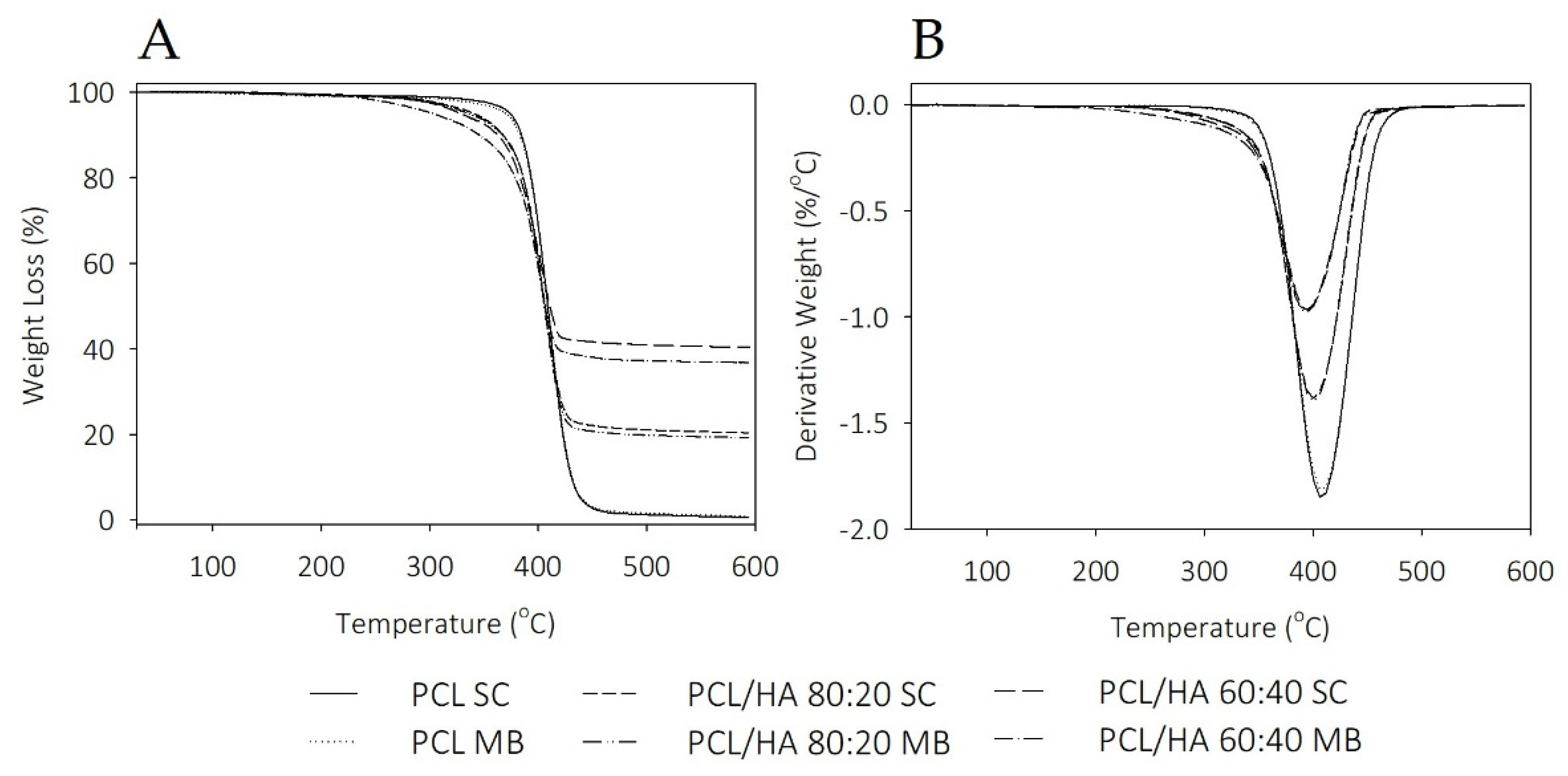
Thermal analysis revealed that the thermal behavior of the scaffolds is influenced by solvent addition and the dispersion characteristics of the ceramic in polymer. The degrees of crystallinity in the SC samples are similar, with an evident increase in the MB composites. The addition of HA promotes a decrease in the endothermic melting enthalpies. Koupaei and Karkhaneh [
53] and Pedrosa et al. [
54] reported the same behaviour in PCL/HA scaffolds and in PCL/HA membranes, respectively. These results can be explained due to the high crystallinity of HA that may alter the crystalline properties of the polymer and accelerate the nucleation of the PCL chain segments. Furthermore, extrusion-based 3D printing technique also induces oriented crystallisation due to the formation of row nuclei, enhanced by the flow stress applied to the molten polymer [
55,
56]. Polymer crystallinity determines the mechanical properties of the produced scaffolds and is a crucial point when considering bone tissue engineering applications [
53,
55]. TGA thermograms reveal thermal stability in all samples at the processing temperatures used for composites preparation and scaffolds fabrication. Therefore, SC and MB are confirmed as viable methods for mixture preparation and the extrusion-based technique maintains the integrity of the composites.
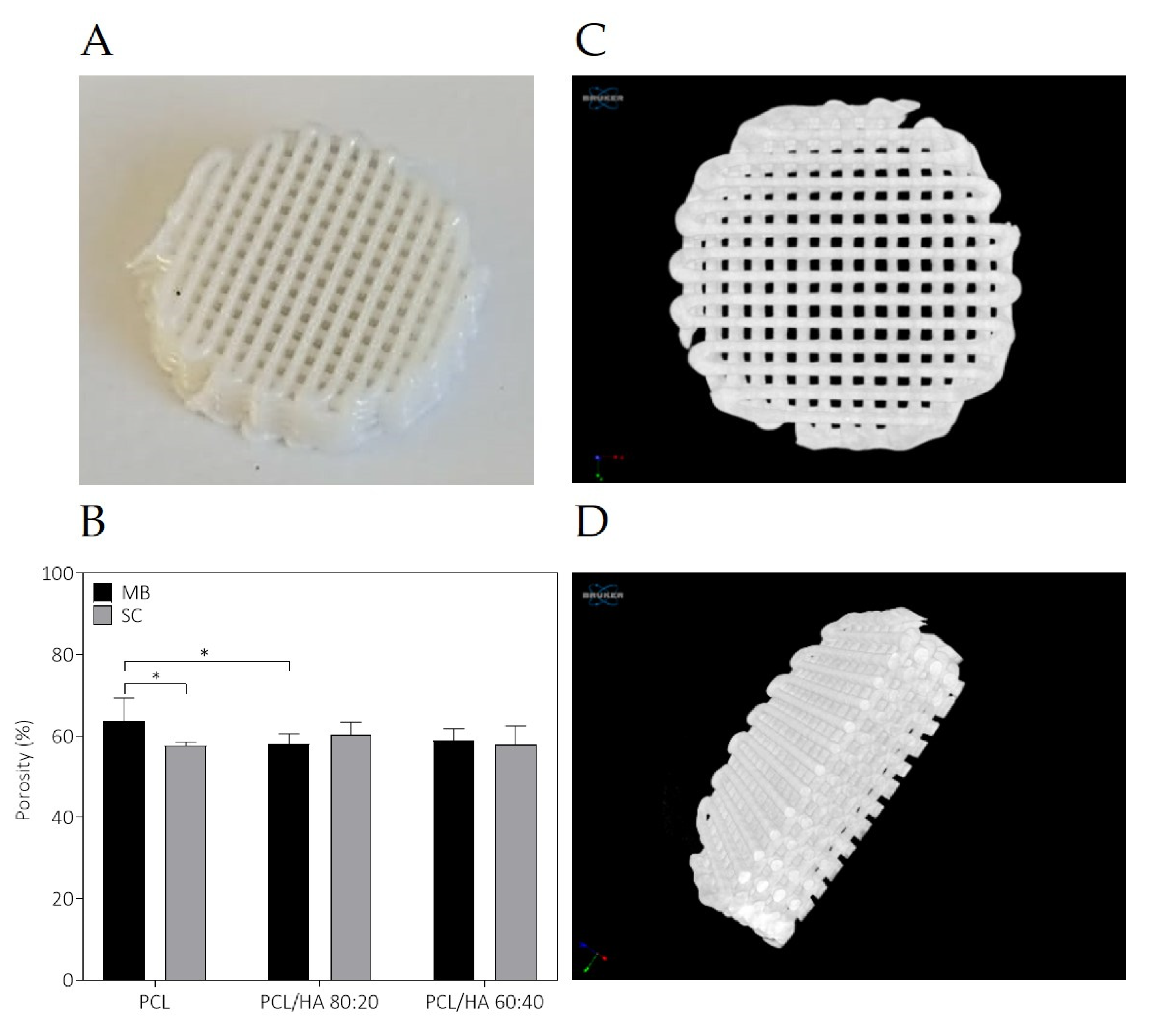
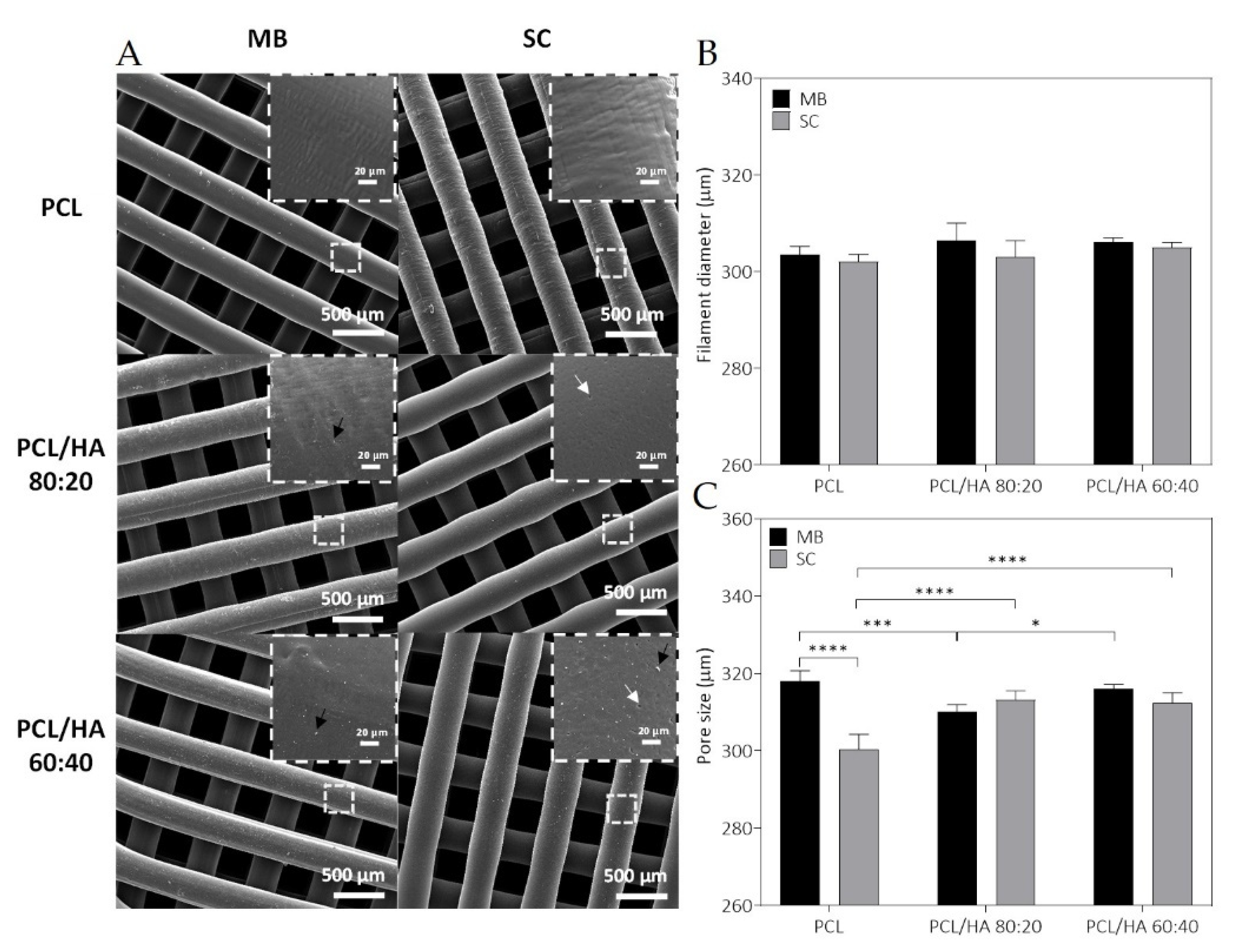
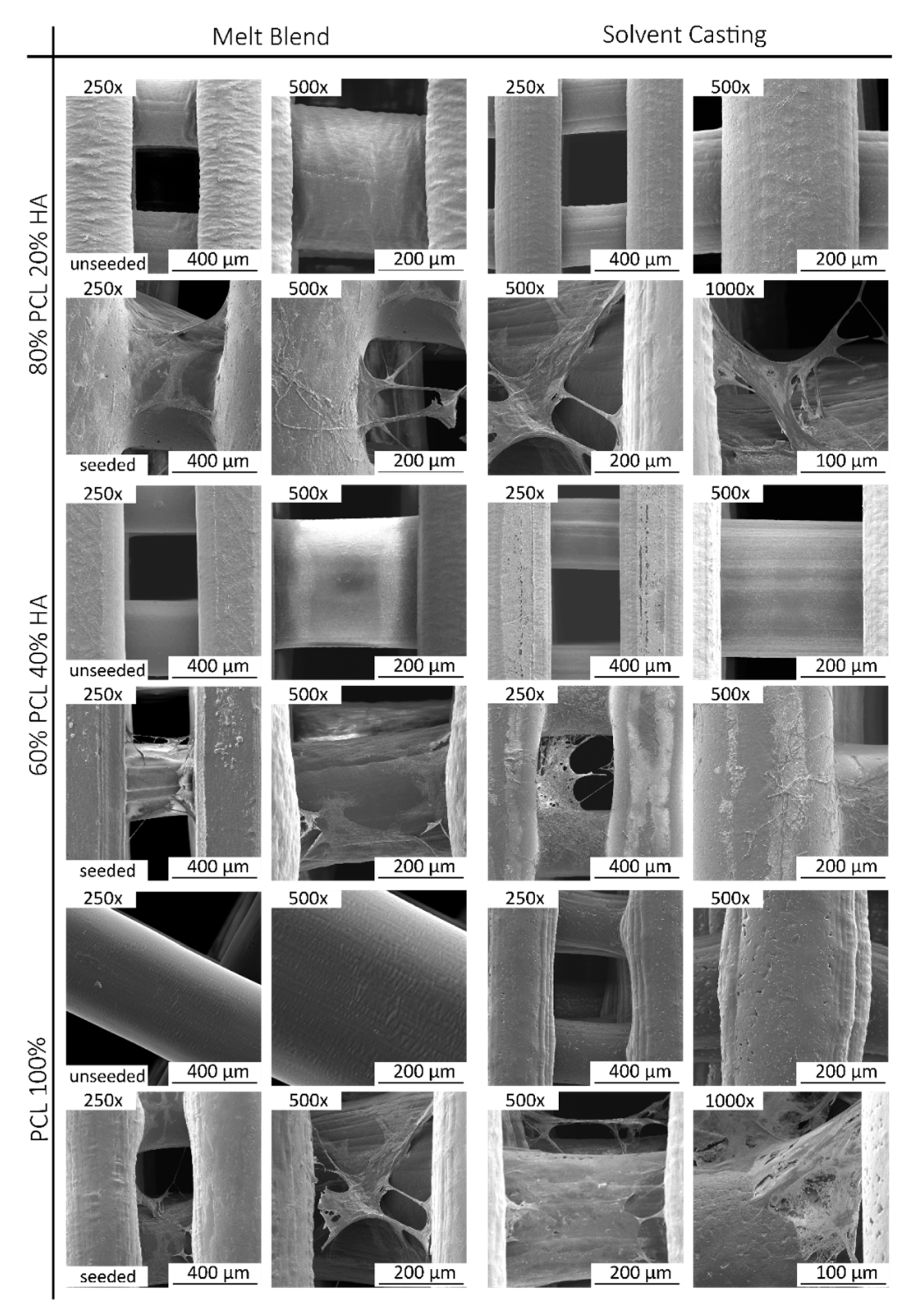
The fabricated scaffolds present a homogeneous 3D structure and similar porosity. Micro-CT does not allow a precise analysis of HA particles as the spatial resolution limit is between 6–30 µm. Nevertheless, with this analysis, it was possible to verify that in both mixture methods no aggregates above this size are formed. For this reason, and to compare the morphological characteristics of the fabricated scaffolds, in particular filament and pore size, additionally studies of the scaffolds’ surface were conducted by SEM. As for the qualitative analysis of the images, SEM analysis revealed a homogeneous distribution of the HA on the polymer matrix, with some particles exposed on the filament surface and some particles agglomerations. Cestari et al. demonstrate that ceramic filler creates a certain roughness on the surface of the material, which could improve cell adhesion [
57]. The solvent addition in the SC method promotes microporosity and this surface morphology is also corroborated in other works [
58,
59,
60]. DMF is a high boiling point solvent that evaporates slowly. The micropores in the filament surface can be related with these chemical properties, as well as the high polarity. However, as other authors reported, the cause for this distinct architecture is not evident [
59,
61]. As for the semi-quantitative analysis of the SEM images, the 3D printed structures present interconnected pores and uniform pore sizes. The filament and pore size were measured to analyse the structural characteristics of the proposed scaffolds and results are consistent with those of the design parameters with pore size ~310 µm. The scaffolds’ 3D structure and pore size have a great effect on cell attachment and proliferation, and it has been reported that pore size above 300 μm improved vascularisation and bone ingrowth [
59,
62,
63,
64].
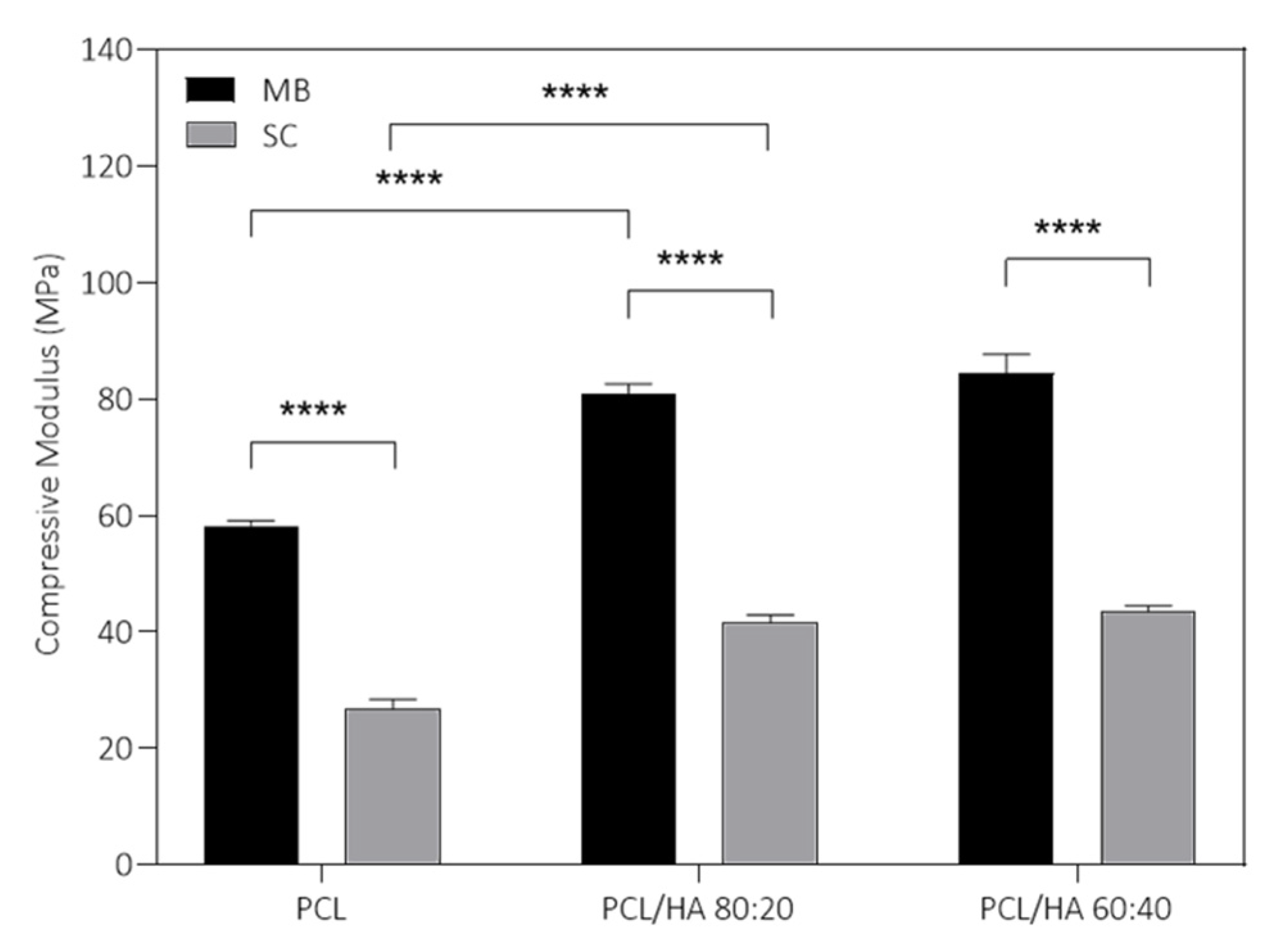
Regarding the results presented in
Figure 6, the incorporation of HA in the PCL polymer matrix contributes to the increase in the mechanical properties. However, they do not increase proportionally to the increase in the ceramic proportion of the composite. In fact, differences between PCL/HA 80:20 and 60:40 are not statistically significant. The lack of difference between the HA incorporated groups can be explained due to the stabilization on the compressive modulus of a certain concentration value of HA. Furthermore, the MB method presented for all groups better outcomes regarding mechanical properties, when compared to the SC groups. Finally, the MB method, combined with the addition of HA, provides the best mechanical response of the scaffolds (compressive modulus and maximum stress). Furthermore, according to the Micro-CT and SEM results, the differences in the mechanical properties are not caused by the porosity, since it is very similar for all the produced scaffolds. However, the micropores observed in the surface of the SC scaffolds may justify the decrease in the compressive strength of the scaffolds. The addition of a fraction of nucleating agents, in this case HA, has a positive influence on crystallinity and mechanical properties. Aliotta et al. investigated this correlation using various nucleating agents and a semicrystalline polymer [
58]. The evaluated 3D structures present compressive strength and modulus within the same range of human cancellous bone, between 2–12 MPa and 0.01–2 GPa, respectively [
15,
60,
61]. Therefore, the PCL/HA scaffolds present adequate mechanical support to be applied as bone tissue substitutes. Additionally, the mechanical properties of the scaffolds can be adjusted as a function of mixture method and HA content, depending on the characteristics of the bone defect. These analyses indicate the MB method as a promising choice for producing scaffolds envisioning bone tissue engineering, combined with the incorporation of HA.
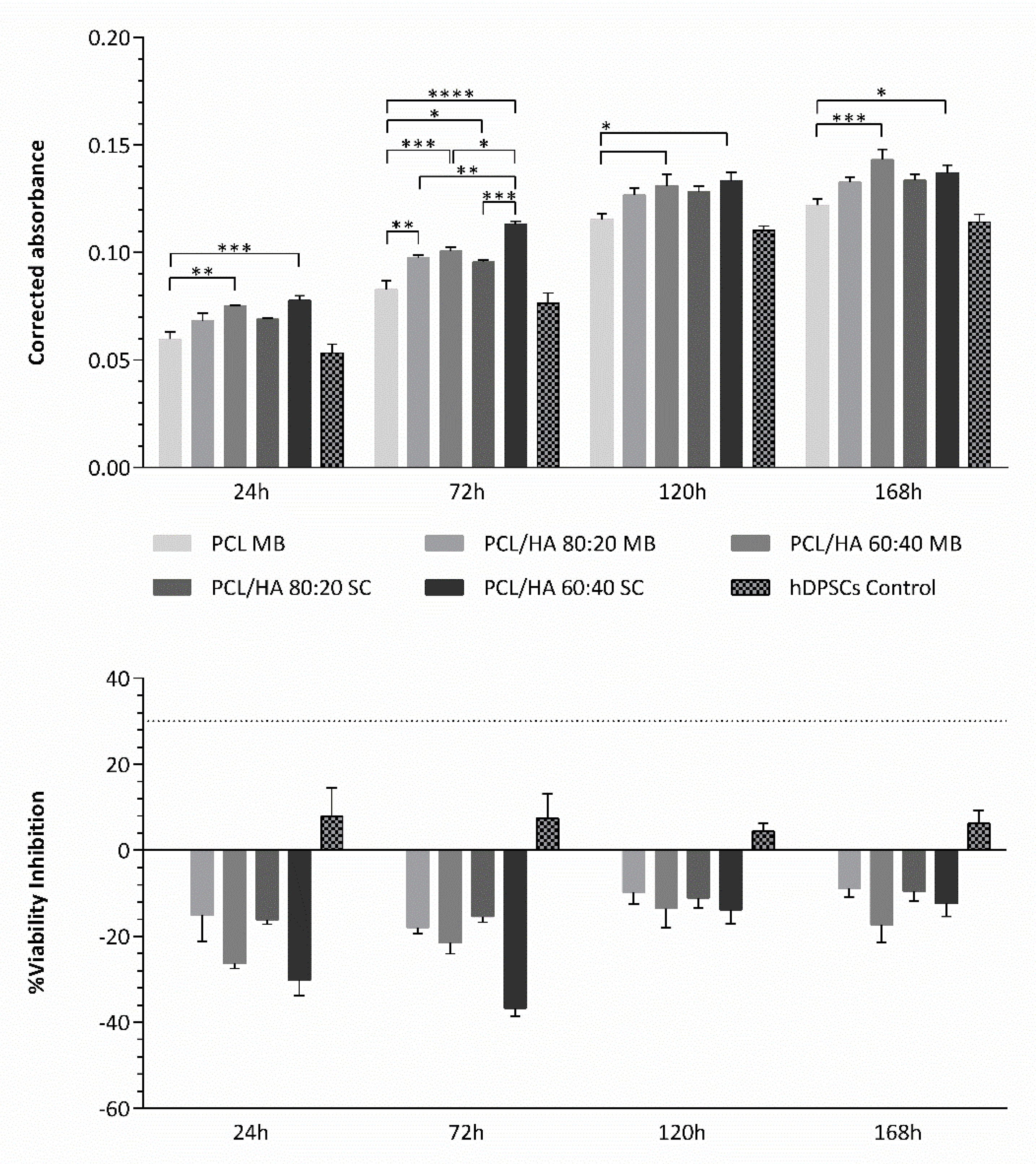
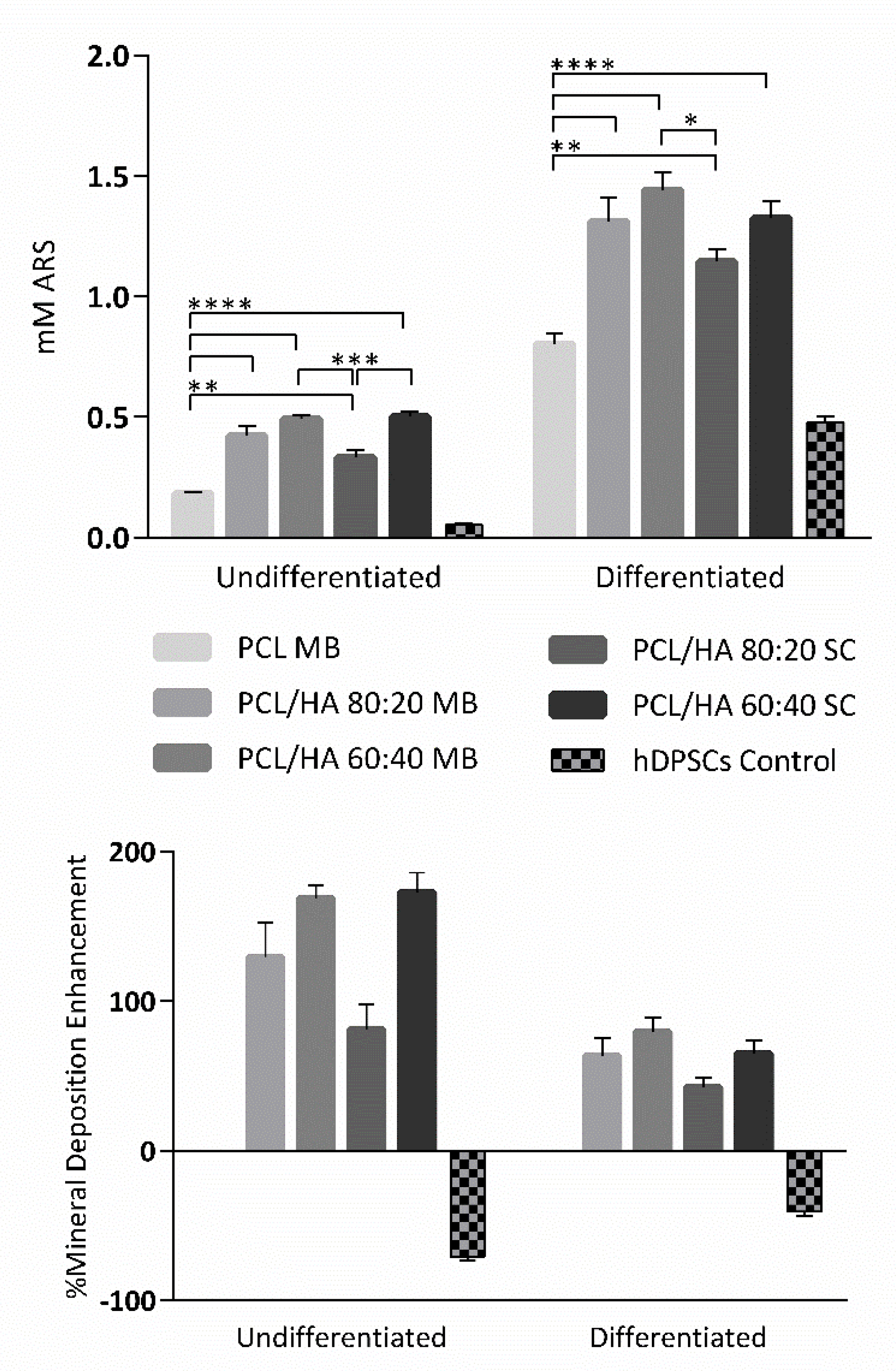
Regarding the cytocompatibility assessment, outcomes for cell viability show that this assay can be considered viable, as the cell population presented normal growth and proliferation in culture, considering the hDPSCs control group. The preliminary assay compared the cytocompatibility of PCL MB and PCL SC, both presenting similar results with no statistically significant differences. However, during most of the assay, PCL MB presented slightly better outcomes, in agreement with the previous characterization assays outcomes. Thus, from this point forward, the PCL MB group was employed as a scaffold control group for further in vitro studies where different PCL/HA formulations were evaluated. Furthermore, data were analysed according to manufacturing instructions and results were interpreted following ISO 10993-5:2009 “Biological evaluation of medical devices”—Part 5—“Test for in vitro cytotoxicity” guidelines. The PrestoBlue
TM assay was used, as it allows live-cell evaluations [
65]. Thus, the same cell population and the same scaffolds can be analysed throughout the duration of the experiment. According to the guidelines (annex C), a viability inhibition superior to 30% is considered a cytotoxic effect. None of the observed groups presented a viability inhibition superior to 30% and, therefore, can be considered cytocompatible. Overall, the PCL/HA scaffolds outperformed the PCL MB scaffolds in terms of cellular viability with higher HA content, presenting increasing viability outcomes. Similar results have been previously reported by other groups [
15,
50,
66,
67,
68,
69,
70,
71]. Regarding blending techniques, differences between the respective SC and MB groups are not statistically significant, with both MB and SC groups presenting positive hDPSCs viability outcomes. Finally, with this assay, the cytocompatibility of all the scaffolds could be confirmed. As for the osteodifferentiation assay, it demonstrated the effect on the osteogenic extension promoted by the incorporation of HA in the PCL scaffolds. All PCL/HA groups presented superior mineral deposition detection, when compared to the PCL MB group. Furthermore, considering the undifferentiated group, the PCL/HA groups were capable of inducing osteogenesis, thus suggesting the incorporation of HA to induce spontaneous osteogenesis [
72]. The differences between PCL/HA groups are not relevant, with very similar results between groups. The PCL alone was also capable of inducing, although to a lesser extent, intrinsic osteogenesis, in the undifferentiated group, as other groups have reported before [
73,
74]. As for the control group, the direct comparison with the scaffolds group should not be considered, as this group consisted of a 2D culture condition, in contrast with the 3D culture condition from the scaffolds. It has been widely accepted that 3D cultures present superior differentiation ability, when comparing with 2D cultures [
75,
76]. This control group, similarly, to the previous assay, was considered as a control of the cell population health and normal behaviour in culture. hDPSCs were selected for this assay due to their pre-established potential towards the osteogenic line and consequent aptitude towards bone tissue regeneration [
37,
38]. A previous work compared the differentiation potential of PCL and PCL/HA scaffolds with hDPCs, human umbilical cord mesenchymal stem cells (MSCs) and human bone marrow derived MSCs, with the hDPSCs presenting superior osteogenic outcomes [
66]. Other have been successfully applying hDPSCs for the evaluation of PCL/HA scaffolds for bone tissue regeneration [
71,
77,
78].