Bioengineered Living Bone Grafts—A Concise Review on Bioreactors and Production Techniques In Vitro
Abstract
:1. Introduction
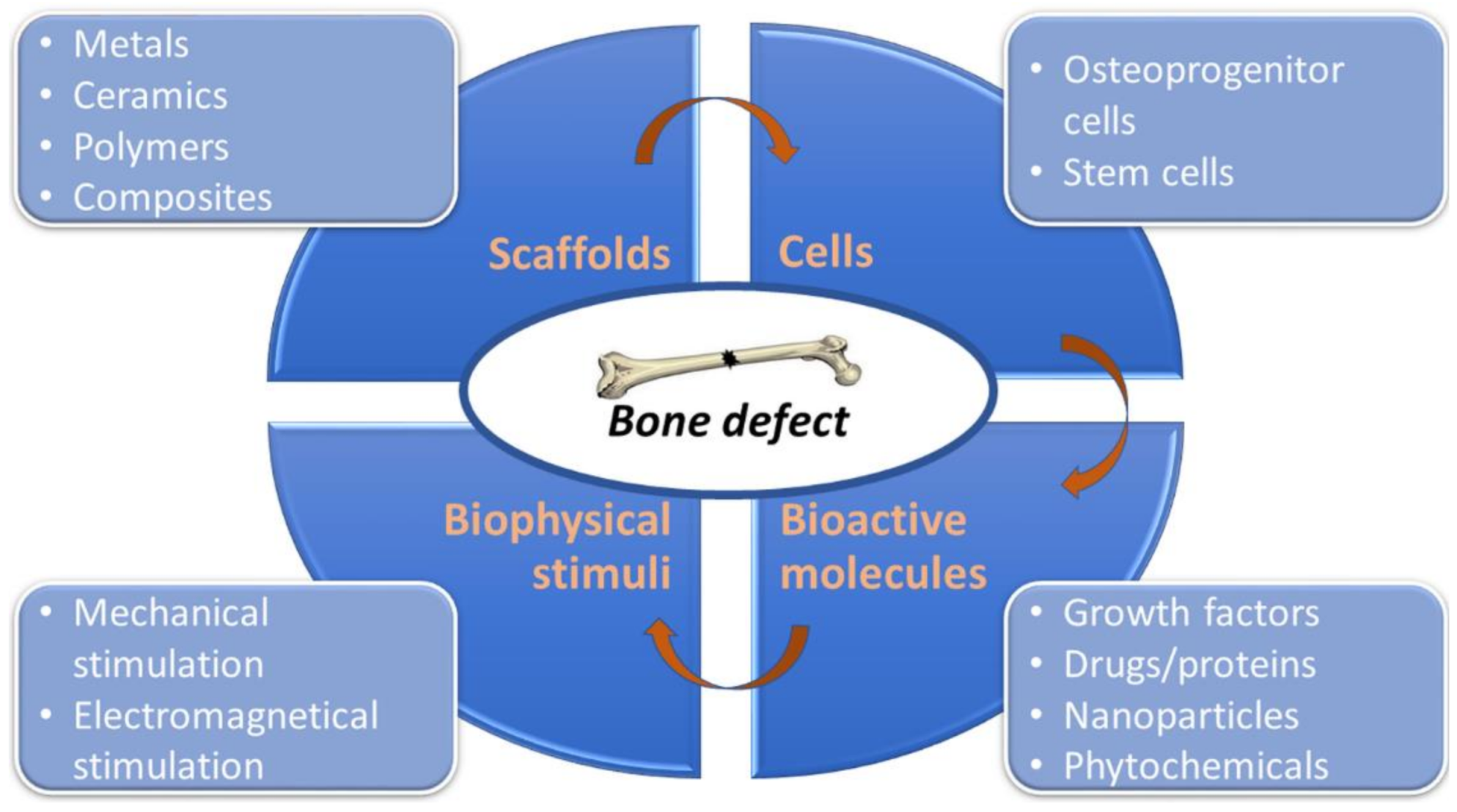
2. Current Concept of Bone Tissue Engineering
2.1. Biomaterials
2.2. Cells
2.3. Three-Dimensional Bioprinting
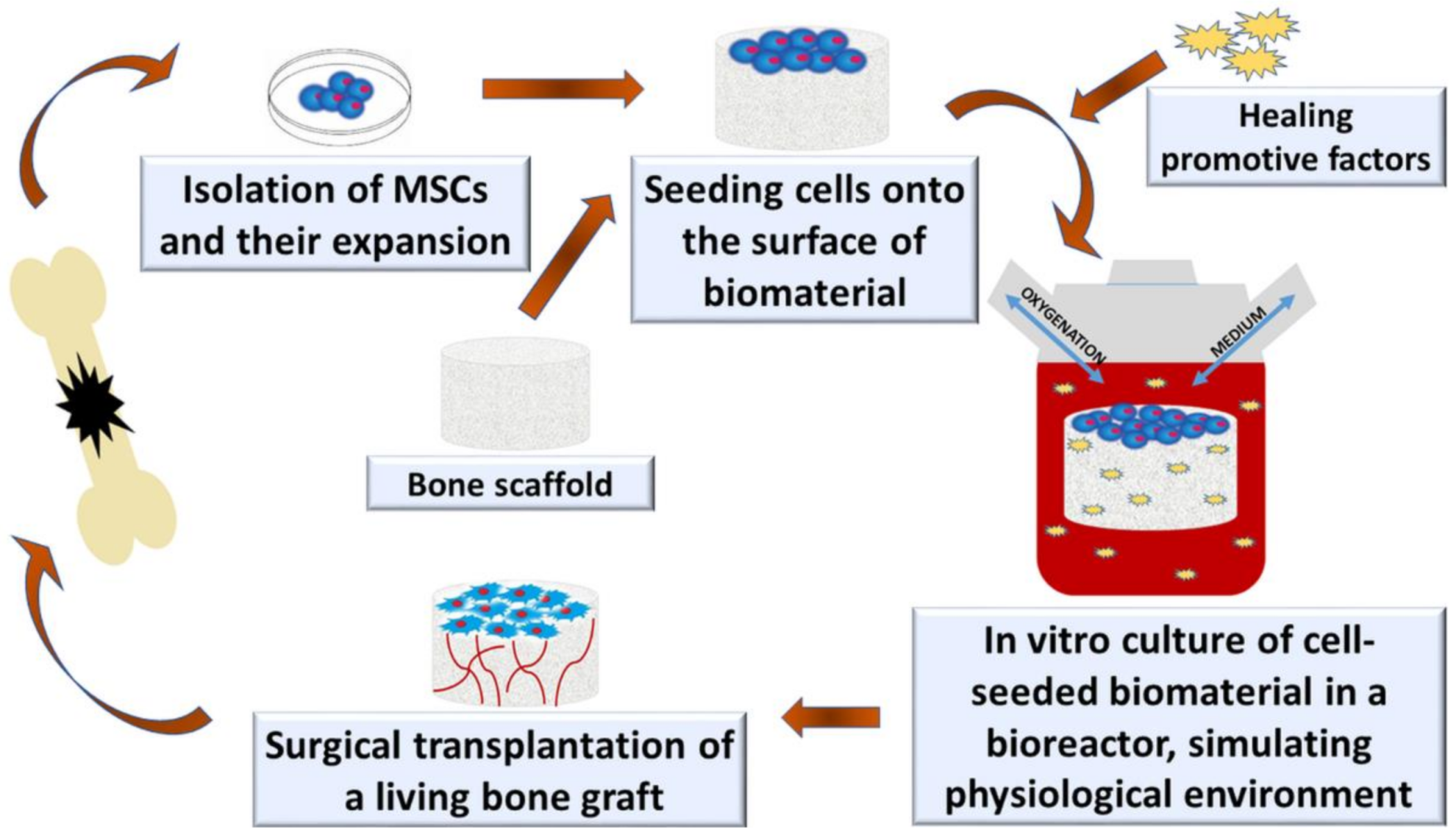
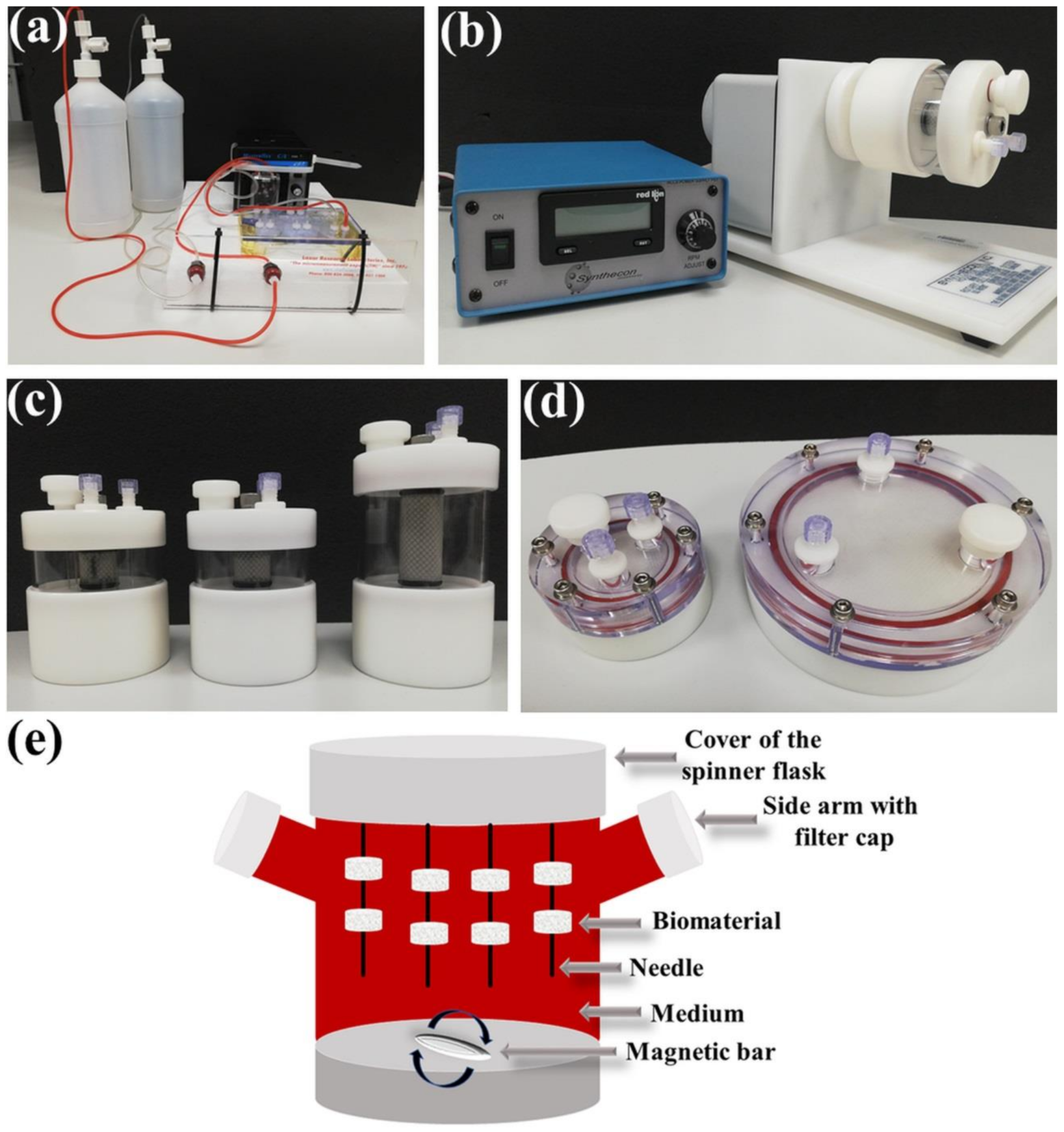
3. Bioreactor Systems
3.1. Perfusion Bioreactors
3.2. Rotating Bioreactors
3.3. Spinner Flask Bioreactors
3.4. Pulsed Electromagnetic Fields-Based Bioreactors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velasco, M.A.; Narváez-Tovar, C.A.; Garzón-Alvarado, D.A. Design, Materials, and Mechanobiology of Biodegradable Scaffolds for Bone Tissue Engineering. BioMed. Res. Int. 2015, 2015, 1–21. [Google Scholar] [CrossRef]
- Codrea, C.I.; Croitoru, A.-M.; Baciu, C.C.; Melinescu, A.; Ficai, D.; Fruth, V.; Ficai, A. Advances in Osteoporotic Bone Tissue Engineering. J. Clin. Med. 2021, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Prasadh, S.; Wong, R.C.W. Unraveling the Mechanical Strength of Biomaterials Used as a Bone Scaffold in Oral and Maxillofacial Defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Tournis, S.; Dede, A.D. Osteogenesis Imperfecta–A Clinical Update. Metabolism 2018, 80, 27–37. [Google Scholar] [CrossRef]
- Sozen, T.; Ozisik, L.; Basaran, N.C. An Overview and Management of Osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Tiefenbach, M.; Scheel, M.; Maier, A.; Gehlen, M.; Schwarz-Eywill, M.; Werner, M.; Siebers-Renelt, U.; Hammer, M. Osteomalacia—Clinical Aspects, Diagnostics and Treatment. Z. Rheumatol. 2018, 77, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Papalia, R.; Zampogna, B.; Torre, G.; Albo, E.; Denaro, V. The Management of Osteomyelitis in the Adult. Surgeon 2016, 14, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, L.; Xiong, Q.; Gao, Y.; Ge, W.; Tang, P. Bench-to-Bedside Strategies for Osteoporotic Fracture: From Osteoimmunology to Mechanosensation. Bone Res. 2019, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone Fracture Healing in Mechanobiological Modeling: A Review of Principles and Methods. Bone Rep. 2017, 6, 87–100. [Google Scholar] [CrossRef]
- Al-Aql, Z.S.; Alagl, A.S.; Graves, D.T.; Gerstenfeld, L.C.; Einhorn, T.A. Molecular Mechanisms Controlling Bone Formation during Fracture Healing and Distraction Osteogenesis. J. Dent. Res. 2008, 87, 107–118. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone Regenerative Medicine: Classic Options, Novel Strategies, and Future Directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassi, F.R.; Grassi, R.; Vivarelli, L.; Dallari, D.; Govoni, M.; Nardi, G.M.; Kalemaj, Z.; Ballini, A. Design Techniques to Optimize the Scaffold Performance: Freeze-Dried Bone Custom-Made Allografts for Maxillary Alveolar Horizontal Ridge Augmentation. Materials 2020, 13, 1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the Art and New Perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Vangsness, C.T.; Wagner, P.P.; Moore, T.M.; Roberts, M.R. Overview of Safety Issues Concerning the Preparation and Processing of Soft-Tissue Allografts. J. Arthrosc. Relat. Surg. 2006, 22, 1351–1358. [Google Scholar] [CrossRef]
- Govoni, M.; Vivarelli, L.; Mazzotta, A.; Stagni, C.; Maso, A.; Dallari, D. Commercial Bone Grafts Claimed as an Alternative to Autografts: Current Trends for Clinical Applications in Orthopaedics. Materials 2021, 14, 3290. [Google Scholar] [CrossRef]
- Orapiriyakul, W.; Young, P.S.; Damiati, L.; Tsimbouri, P.M. Antibacterial Surface Modification of Titanium Implants in Orthopaedics. J. Tissue Eng. 2018, 9, 2041731418789838. [Google Scholar] [CrossRef] [Green Version]
- Miller, P.D. Management of Severe Osteoporosis. Expert Opin. Pharmacother. 2015, 17, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.R.; Kouroupis, D.; Li, D.J.; Best, T.M.; Kaplan, L.; Correa, D. Tissue Engineering and Cell-Based Therapies for Fractures and Bone Defects. Front. Bioeng. Biotechnol. 2018, 6, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashwin, B.; Abinaya, B.; Prasith, T.; Chandran, S.V.; Yadav, L.R.; Vairamani, M.; Patil, S.; Selvamurugan, N. 3D-Poly (Lactic Acid) Scaffolds Coated with Gelatin and Mucic Acid for Bone Tissue Engineering. Int. J. Biol. Macromol. 2020, 162, 523–532. [Google Scholar] [CrossRef]
- Diaz-Rodriguez, P.; Sánchez, M.; Landin, M. Drug-Loaded Biomimetic Ceramics for Tissue Engineering. Pharmaceutics 2018, 10, 272. [Google Scholar] [CrossRef] [Green Version]
- Walmsley, G.G.; McArdle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, P.H.; Longaker, M.T.; et al. Nanotechnology in Bone Tissue Engineering. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1253–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentino, A.; Di Cristo, F.; Bosetti, M.; Amaghnouje, A.; Bousta, D.; Conte, R.; Calarco, A. Bioactivity and Delivery Strategies of Phytochemical Compounds in Bone Tissue Regeneration. Appl. Sci. 2021, 11, 5122. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arvidson, K.; Abdallah, B.; Applegate, L.A.; Baldini, N.; Cenni, E.; Gomez-Barrena, E.; Granchi, D.; Kassem, M.; Konttinen, Y.T.; Mustafa, K.; et al. Bone Regeneration and Stem Cells. J. Cell. Mol. Med. 2010, 15, 718–746. [Google Scholar] [CrossRef]
- Przekora, A. The Summary of the most Important Cell-Biomaterial Interactions that Need to Be Considered during in Vitro Biocompatibility Testing of Bone Scaffolds for Tissue Engineering Applications. Mater. Sci. Eng. C 2019, 97, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Zivic, F.; Affatato, S.; Trajanovic, M.; Schnabelrauch, M.; Grujovic, N.; Choy, K.L. Biomaterials in Clinical Practice. In Advances in Clinical Research and Medical Devices; Springer International Publishing AG: Cham, Switzerland, 2018; ISBN 978. [Google Scholar]
- Fousová, M.; Vojtěch, D.; Kubásek, J.; Jablonská, E.; Fojt, J. Promising Characteristics of Gradient Porosity Ti-6Al-4V Alloy Prepared by SLM Process. J. Mech. Behav. Biomed. Mater. 2017, 69, 368–376. [Google Scholar] [CrossRef]
- Gao, C.; Deng, Y.; Feng, P.; Mao, Z.; Li, P.; Yang, B.; Deng, J.; Cao, Y.; Shuai, C.; Peng, S. Current Progress in Bioactive Ceramic Scaffolds for Bone Repair and Regeneration. Int. J. Mol. Sci. 2014, 15, 4714–4732. [Google Scholar] [CrossRef]
- Ioan, S.; Buruiana, L.I. Biodegradable Polymers in Tissue Engineering. In Handbook of Composites from Renewable Materials; Thakur, V.K., Thakur, M.K., Kessler, M.R., Eds.; Scrivener Publishing: Hoboken, NJ, USA, 2017; pp. 145–182. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D Bioactive Composite Scaffolds for Bone Tissue Engineering. Bioact. Mater. 2017, 3, 278–314. [Google Scholar] [CrossRef] [Green Version]
- Sachot, N.; Engel, E.; Castano, O. Hybrid Organic-Inorganic Scaffolding Biomaterials for Regenerative Therapies. Curr. Org. Chem. 2014, 18, 2299–2314. [Google Scholar] [CrossRef] [Green Version]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Przekora, A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Joseph, J.S.; Malindisa, S.T.; Ntwasa, M. Two-Dimentional (2D) and Three-Dimensional (3D) Cell Culturing in Drug Discovery. In Cell Culture; Mehanna, R.A., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Subia, B.; Kundu, J.; Kundu, S.C. Biomaterial Scaffold Fabrication Techniques for Potential Tissue Engineering Applications. In Tissue Engineering; Eberli, D., Ed.; InTech: London, UK, 2010; ISBN 978–953–307–079–7. [Google Scholar]
- Iaquinta, M.R.; Mazzoni, E.; Bononi, I.; Rotondo, J.C.; Mazziotta, C.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Adult Stem Cells for Bone Regeneration and Repair. Front. Cell Dev. Biol. 2019, 7, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazimierczak, P.; Syta, E.; Przekora, A.; Ginalska, G. Comparison of Osteogenic Differentiation Ability between Bone Marrow-Derived Mesenchymal Stem Cells and Adipose Tissue-Derived Mesenchymal Stem Cells. Med. Ogólna Nauk. Zdrowiu 2018, 24, 101–106. [Google Scholar] [CrossRef]
- Kuhn, L.T.; Liu, Y.; Boyd, N.L.; Dennis, J.E.; Jiang, X.; Xin, X.; Charles, L.F.; Wang, L.; Aguila, H.L.; Rowe, D.W.; et al. Developmental-Like Bone Regeneration by Human Embryonic Stem Cell-Derived Mesenchymal Cells. Tissue Eng. Part A 2014, 20, 365–377. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Goulart, V.; Ferreira, L.B.; Duarte, C.A.; de Lima, I.L.; Ferreira, E.R.; de Oliveira, B.C.; Vargas, L.N.; de Moraes, D.D.; Silva, I.B.B.; Faria, R.D.O.; et al. Mesenchymal Stem Cells from Human Adipose Tissue and Bone Repair: A Literature Review. Biotechnol. Res. Innov. 2018, 2, 74–80. [Google Scholar] [CrossRef]
- Csobonyeiova, M.; Polák, S.; Zamborsky, R.; Danisovic, L. iPS Cell Technologies and their Prospect for Bone Regeneration and Disease Modeling: A Mini Review. J. Adv. Res. 2017, 8, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Chan, Y.-H.; Hsieh, S.-C.; Lew, W.-Z.; Feng, S.-W. Comparing the Osteogenic Potentials and Bone Regeneration Capacities of Bone Marrow and Dental Pulp Mesenchymal Stem Cells in a Rabbit Calvarial Bone Defect Model. Int. J. Mol. Sci. 2019, 20, 5015. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.-W.; Su, Y.-H.; Lin, Y.-K.; Wu, Y.-C.; Huang, Y.-H.; Yang, F.-H.; Chiang, H.-J.; Yen, Y.; Wang, P.D.-Y. Small Blood Stem Cells for Enhancing Early Osseointegration Formation on Dental Implants: A Human Phase I Safety Study. Stem Cell Res. Ther. 2021, 12, 380. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Chahla, J.; Mannava, S.; Cinque, M.E.; Geeslin, A.G.; Codina, D.; LaPrade, R.F. Bone Marrow Aspirate Concentrate Harvesting and Processing Technique. Arthrosc. Tech. 2017, 6, e441–e445. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, F.S.M.; Shams, S.; Silva, E.A.; Stilhano, R.S. PRP and BMAC for Musculoskeletal Conditions via Biomaterial Carriers. Int. J. Mol. Sci. 2019, 20, 5328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genova, T.; Roato, I.; Carossa, M.; Motta, C.; Cavagnetto, D.; Mussano, F. Advances on Bone Substitutes through 3D Bioprinting. Int. J. Mol. Sci. 2020, 21, 7012. [Google Scholar] [CrossRef]
- Genova, T.; Petrillo, S.; Zicola, E.; Roato, I.; Ferracini, R.; Tolosano, E.; Altruda, F.; Carossa, S.; Mussano, F.; Munaron, L. The Crosstalk Between Osteodifferentiating Stem Cells and Endothelial Cells Promotes Angiogenesis and Bone Formation. Front. Physiol. 2019, 10, 1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salah, M.; Tayebi, L.; Moharamzadeh, K.; Naini, F.B. Three-Dimensional Bio-Printing and Bone Tissue Engineering: Technical Innovations and Potential Applications in Maxillofacial Reconstructive Surgery. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar] [CrossRef]
- Udofia, E.N.; Zhou, W. Microextrusion Based 3D Printing-A Review. In Proceedings of the 29th Annual. International. Solid Freedom. Fabrication Symposium-An Additive Manufaxturing. Conference, Austin, TX, USA, 13–15 August 2018; pp. 2033–2060. [Google Scholar]
- Guillotin, B.; Ali, M.; Ducom, A.; Catros, S.; Keriquel, V.; Souquet, A.; Remy, M.; Fricain, J.-C.; Guillemot, F. Laser-Assisted Bioprinting for Tissue Engineering. In Biofabrication; Elsevier: Amsterdam, The Netherlands, 2013; pp. 95–118. [Google Scholar] [CrossRef]
- Li, N.; Guo, R.; Zhang, Z.J. Bioink Formulations for Bone Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 44. [Google Scholar] [CrossRef]
- Amler, A.-K.; Dinkelborg, P.; Schlauch, D.; Spinnen, J.; Stich, S.; Lauster, R.; Sittinger, M.; Nahles, S.; Heiland, M.; Kloke, L.; et al. Comparison of the Translational Potential of Human Mesenchymal Progenitor Cells from Different Bone Entities for Autologous 3D Bioprinted Bone Grafts. Int. J. Mol. Sci. 2021, 22, 796. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Miller, L.; Cross, L.M.; Jaiswal, M.K.; Singh, I.; Gaharwar, A.K. Nanoengineered Osteoinductive Bioink for 3D Bioprinting Bone Tissue. ACS Appl. Mater. Interfaces 2020, 12, 15976–15988. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and Bioactive Porous Polymer/Inorganic Composite Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Lei, X.; Cheng, P.; Song, Y.; Gao, Y.; Hu, J.; Wang, C.; Zhang, S.; Li, D.; et al. 3D-Bioprinted Functional and Biomimetic Hydrogel Scaffolds Incorporated with Nanosilicates to Promote Bone Healing in Rat Calvarial Defect Model. Mater. Sci. Eng. C 2020, 112, 110905. [Google Scholar] [CrossRef] [PubMed]
- Kosik-Kozioł, A.; Costantini, M.; Mróz, A.; Idaszek, J.; Heljak, M.; Jaroszewicz, J.; Kijeńska, E.; Szöke, K.; Frerker, N.; Barbetta, A.; et al. 3D Bioprinted Hydrogel Model Incorporating β-Tricalcium Phosphate for Calcified Cartilage Tissue Engineering. Biofabrication 2019, 11, 035016. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Kim, W.J.; Ahn, J.Y.; Lee, J.; Ko, D.W.; Park, S.; Kim, J.Y.; Jang, C.H.; Lim, J.M.; Kim, G.H. New Bioink Derived from Neonatal Chicken Bone Marrow Cells and Its 3D-Bioprinted Niche for Osteogenic Stimulators. ACS Appl. Mater. Interfaces 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Alcala-Orozco, C.R.; Mutreja, I.; Cui, X.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B. Hybrid Biofabrication of 3D Osteoconductive Constructs Comprising Mg-Based Nanocomposites and Cell-Laden Bioinks for Bone Repair. Bone 2021, 154, 116198. [Google Scholar] [CrossRef]
- Abu Awwad, H.A.-D.M.; Thiagarajan, L.; Kanczler, J.; Amer, M.; Bruce, G.; Lanham, S.; Rumney, R.; Oreffo, R.; Dixon, J.E. Genetically-Programmed, Mesenchymal Stromal Cell-Laden & Mechanically Strong 3D Bioprinted Scaffolds for Bone Repair. J. Control. Release 2020, 325, 335–346. [Google Scholar] [CrossRef]
- Wang, Z.; Hui, A.; Zhao, H.; Ye, X.; Zhang, C.; Wang, A.; Zhang, C. A Novel 3D-Bioprinted Porous Nano Attapulgite Scaffolds with Good Performance for Bone Regeneration. Int. J. Nanomed. 2020, 15, 6945–6960. [Google Scholar] [CrossRef]
- Jeong, J.E.; Park, S.Y.; Shin, J.Y.; Seok, J.M.; Byun, J.H.; Oh, S.H.; Kim, W.D.; Lee, J.H.; Park, W.H.; Park, S.A. 3D Printing of Bone-Mimetic Scaffold Composed of Gelatin/β-Tri-Calcium Phosphate for Bone Tissue Engineering. Macromol. Biosci. 2020, 20, 2000256. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D Printed Scaffolds for Promoting Bone Regeneration. Biomaterials 2018, 190–191, 97–110. [Google Scholar] [CrossRef]
- Li, L.; Yu, F.; Shi, J.; Shen, S.; Teng, H.; Yang, J.; Wang, X.; Jiang, Q. In Situ Repair of Bone and Cartilage Defects Using 3D Scanning and 3D Printing. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Shi, J.; Ma, K.; Jin, J.; Wang, P.; Liang, H.; Cao, Y.; Wang, X.; Jiang, Q. Robotic in Situ 3D Bio-Printing Technology for Repairing Large Segmental Bone Defects. J. Adv. Res. 2020, 30, 75–84. [Google Scholar] [CrossRef]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-Dimensional Cell Culture: A Powerful Tool in Tumor Research and Drug Discovery (Review). Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef] [Green Version]
- Justice, B.A.; Badr, N.A.; Felder, R.A. 3D Cell Culture Opens New Dimensions in Cell-Based Assays. Drug Discov. Today 2009, 14, 102–107. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Rauh, J.; Milan, F.; Günther, K.-P.; Stiehler, M. Bioreactor Systems for Bone Tissue Engineering. Tissue Eng. Part B Rev. 2011, 17, 263–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bin Hassan, M.N.F.; Yazid, M.D.; Yunus, M.H.M.; Chowdhury, S.R.; Lokanathan, Y.; Idrus, R.B.H.; Ng, A.M.H.; Law, J.X. Large-Scale Expansion of Human Mesenchymal Stem Cells. Stem Cells Int. 2020, 2020, 1–17. [Google Scholar] [CrossRef]
- Egger, D.; Tripisciano, C.; Weber, V.; Dominici, M.; Kasper, C. Dynamic Cultivation of Mesenchymal Stem Cell Aggregates. Bioengineering 2018, 5, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravichandran, A.; Liu, Y.; Teoh, S.-H. Review: Bioreactor Design towards Generation of Relevant Engineered Tissues: Focus on Clinical Translation. J. Tissue Eng. Regen. Med. 2017, 12, e7–e22. [Google Scholar] [CrossRef]
- Lovecchio, J.; Gargiulo, P.; Vargas Luna, J.L.; Giordano, E.; Sigurjónsson, Ó.E. A Standalone Bioreactor System to Deliver Compressive Load under Perfusion Flow to hBMSC-Seeded 3D Chitosan-Graphene Templates. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Gardel, L.S.; Serra, L.A.; Reis, R.L.; Gomes, M. Use of Perfusion Bioreactors and Large Animal Models for Long Bone Tissue Engineering. Tissue Eng. Part B Rev. 2014, 20, 126–146. [Google Scholar] [CrossRef] [Green Version]
- Ng, J.; Spiller, K.; Bernhard, J.; Vunjak-Novakovic, G. Biomimetic Approaches for Bone Tissue Engineering. Tissue Eng. Part B Rev. 2017, 23, 480–493. [Google Scholar] [CrossRef]
- Panek, M.; Antunović, M.; Pribolšan, L.; Ivković, A.; Gotić, M.; Vukasović, A.; Mihalić, K.C.; Pušić, M.; Jurkin, T.; Marijanović, I. Bone Tissue Engineering in a Perfusion Bioreactor Using Dexamethasone-Loaded Peptide Hydrogel. Materials 2019, 12, 919. [Google Scholar] [CrossRef] [Green Version]
- Ressler, A.; Antunović, M.; Teruel-Biosca, L.; Ferrer, G.G.; Babić, S.; Urlić, I.; Ivanković, M.; Ivanković, H. Osteogenic Differentiation of Human Mesenchymal Stem Cells on Substituted Calcium Phosphate/Chitosan Composite Scaffold. Carbohydr. Polym. 2021, 277, 118883. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Lipphaus, A.; Ergin, M.; Salehi, S.; Gehweiler, D.; Rudert, M.; Hansmann, J.; Herrmann, M. Modeling of the Human Bone Environment: Mechanical Stimuli Guide Mesenchymal Stem Cell–Extracellular Matrix Interactions. Materials 2021, 14, 4431. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Yassin, M.A.; Schwarz, T.; Hansmann, J.; Mustafa, K. Induction of Osteogenic Differentiation of Bone Marrow Stromal Cells on 3D Polyester-Based Scaffolds Solely by Subphysiological Fluidic Stimulation in a Laminar Flow Bioreactor. J. Tissue Eng. 2021, 12, 20417314211019375. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.K.; Kao, S.-W.; Roux, B.M.; Rodriguez, R.A.; Tang, S.-J.; Fisher, J.P.; Cheng, M.-H.; Brey, E.M. Perfusion Bioreactor Culture of Bone Marrow Stromal Cells Enhances Cranial Defect Regeneration. Plast. Reconstr. Surg. 2019, 143, 993e–1002e. [Google Scholar] [CrossRef]
- Bhaskar, B.; Owen, R.; Bahmaee, H.; Rao, P.S.; Reilly, G.C. Design and Assessment of a Dynamic Perfusion Bioreactor for Large Bone Tissue Engineering Scaffolds. Appl. Biochem. Biotechnol. 2017, 185, 555–563. [Google Scholar] [CrossRef]
- Salifu, A.A.; Obayemi, J.D.; Uzonwanne, V.O.; Soboyejo, W.O. Mechanical Stimulation Improves Osteogenesis and the Mechanical Properties of Osteoblast-Laden RGD-Functionalized Polycaprolactone/Hydroxyapatite Scaffolds. J. Biomed. Mater. Res. Part A 2020, 108, 2421–2434. [Google Scholar] [CrossRef]
- Han, H.; Jun, I.; Seok, H.; Lee, K.; Lee, K.; Witte, F.; Mantovani, D.; Kim, Y.; Glyn-Jones, S.; Edwards, J.R. Biodegradable Magnesium Alloys Promote Angio-Osteogenesis to Enhance Bone Repair. Adv. Sci. 2020, 7, 2000800. [Google Scholar] [CrossRef]
- Liu, X.; Jakus, A.E.; Kural, M.; Qian, H.; Engler, A.; Ghaedi, M.; Shah, R.; Steinbacher, D.M.; Niklason, L.E. Vascularization of Natural and Synthetic Bone Scaffolds. Cell Transplant. 2018, 27, 1269–1280. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, A.; Wen, F.; Lim, J.; Chong, M.S.K.; Chan, J.K.; Teoh, S. Biomimetic Fetal Rotation Bioreactor for Engineering Bone Tissues—Effect of Cyclic Strains on Upregulation of Osteogenic Gene Expression. J. Tissue Eng. Regen. Med. 2018, 12, e2039–e2050. [Google Scholar] [CrossRef]
- Demir, A.K.; Elçin, A.E.; Elçin, Y.M. Osteogenic Differentiation of Encapsulated Rat Mesenchymal Stem Cells Inside a Rotating Microgravity Bioreactor: In Vitro and in Vivo Evaluation. Cytotechnology 2018, 70, 1375–1388. [Google Scholar] [CrossRef]
- Li, Y.; He, L.; Pan, S.; Zhang, L.; Zhang, W.; Yi, H.; Niu, Y. Three-Dimensional Simulated Microgravity Culture Improves the Proliferation and Odontogenic Differentiation of Dental Pulp Stem Cell in PLGA Scaffolds Implanted in Mice. Mol. Med. Rep. 2016, 15, 873–878. [Google Scholar] [CrossRef] [Green Version]
- Nokhbatolfoghahaei, H.; Paknejad, Z.; Bohlouli, M.; Rad, M.R.; Aminishakib, P.; Derakhshan, S.; Amirabad, L.M.; Nadjmi, N.; Khojasteh, A. Fabrication of Decellularized Engineered Extracellular Matrix through Bioreactor-Based Environment for Bone Tissue Engineering. ACS Omega 2020, 5, 31943–31956. [Google Scholar] [CrossRef] [PubMed]
- Nokhbatolfoghahaei, H.; Bohlouli, M.; Paknejad, Z.; Rad, M.R.; Amirabad, L.M.; Salehi-Nik, N.; Khani, M.M.; Shahriari, S.; Nadjmi, N.; Ebrahimpour, A.; et al. Bioreactor Cultivation Condition for Engineered Bone Tissue: Effect of Various Bioreactor Designs on Extra Cellular Matrix Synthesis. J. Biomed. Mater. Res.-Part A 2020, 108, 1662–1672. [Google Scholar] [CrossRef]
- Tsai, H.-H.; Yang, K.-C.; Wu, M.-H.; Chen, J.-C.; Tseng, C.-L. The Effects of Different Dynamic Culture Systems on Cell Proliferation and Osteogenic Differentiation in Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 4024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado, C.L.; Barrias, C.C.; Monteiro, F.J.M. Clarifying the Tooth-Derived Stem Cells Behavior in a 3D Biomimetic Scaffold for Bone Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2020, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nadine, S.; Patrício, S.G.; Correia, C.R.; Mano, J.F. Dynamic Microfactories Co-Encapsulating Osteoblastic and Adipose-Derived Stromal Cells for the Biofabrication of Bone Units. Biofabrication 2019, 12, 015005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, M.; Ye, Z.; Zhou, Y.; Tan, W.-S. Fabrication of Viable and Functional Pre-Vascularized Modular Bone Tissues by Coculturing MSCs and HUVECs on Microcarriers in Spinner Flasks. Biotechnol. J. 2017, 12, 1700008. [Google Scholar] [CrossRef]
- Gaspar, D.; Gomide, V.; Monteiro, F. The Role of Perfusion Bioreactors in Bone Tissue Engineering. Biomatter 2012, 2, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Birru, B.; Mekala, N.K.; Parcha, S.R. Mechanistic role of perfusion culture on bone regeneration. J. Biosci. 2019, 44, 23. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Bloise, N.; Vercellino, M.; Battaglia, R.; Morgante, L.; Gabriella Cusella De Angelis, M.; Imbriani, M.; Visai, L. In Vitro Osteogenesis of Human Stem Cells by Using a Three-Dimensional Perfusion Bioreactor Culture System: A Review. Recent Pat. Drug Deliv. Formul. 2013, 7, 29–38. [Google Scholar] [CrossRef]
- Zhao, F.; Van Rietbergen, B.; Ito, K.; Hofmann, S. Fluid Flow-Induced Cell Stimulation in Bone Tissue Engineering Changes due to Interstitial Tissue Formation in Vitro. Int. J. Numer. Methods Biomed. Eng. 2020, 36, e3342. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, G.N.; Sikavitsas, V.I.; Mikos, A.G. Technical Note: Design of a Flow Perfusion Bioreactor System for Bone Tissue-Engineering Applications. Tissue Eng. 2003, 9, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Seddiqi, H.; Saatchi, A.; Amoabediny, G.; Helder, M.N.; Ravasjani, S.A.; Aghaei, M.S.H.; Jin, J.; Zandieh-Doulabi, B.; Klein-Nulend, J. Inlet Flow Rate of Perfusion Bioreactors Affects Fluid Flow Dynamics, but Not Oxygen Concentration in 3D-Printed Scaffolds for Bone Tissue Engineering: Computational Analysis and Experimental Validation. Comput. Biol. Med. 2020, 124, 103826. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Born, G.; Nikolova, M.; Scherberich, A.; Treutlein, B.; García-García, A.; Martin, I. Engineering of Fully Humanized and Vascularized 3D Bone Marrow Niches Sustaining Undifferentiated Human Cord Blood Hematopoietic Stem and Progenitor Cells. J. Tissue Eng. 2021, 12, 20417314211044855. [Google Scholar] [CrossRef]
- Ismail, T.; Lunger, A.; Haumer, A.; Todorov, A.; Menzi, N.; Schweizer, T.; Bieback, K.; Bürgin, J.; Schaefer, D.J.; Martin, I.; et al. Platelet-Rich Plasma and Stromal Vascular Fraction Cells for the Engineering of Axially Vascularized Osteogenic Grafts. J. Tissue Eng. Regen. Med. 2020, 14, 1908–1917. [Google Scholar] [CrossRef]
- Mazzoleni, G.; Boukhechba, F.; Steimberg, N.; Boniotti, J.; Bouler, J.M.; Rochet, N. Impact of Dynamic Culture in the RCCSTM Bioreactor on a Three-Dimensional Model of Bone Matrix Formation. Procedia Eng. 2011, 10, 3662–3667. [Google Scholar] [CrossRef] [Green Version]
- Hansmann, J.; Groeber, F.; Kahlig, A.; Kleinhans, C.; Walles, H. Bioreactors in Tissue Engineering-Principles, Applications and Commercial Constraints. Biotechnol. J. 2012, 8, 298–307. [Google Scholar] [CrossRef]
- Facer, S.R.; Zaharias, R.S.; Andracki, M.E.; Lafoon, J.; Hunter, S.; Schneider, G.B. Rotary Culture Enhances Pre-Osteoblast Aggregation and Mineralization. J. Dent. Res. 2005, 84, 542–547. [Google Scholar] [CrossRef]
- Penolazzi, L.; Lolli, A.; Sardelli, L.; Angelozzi, M.; Lambertini, E.; Trombelli, L.; Ciarpella, F.; Vecchiatini, R.; Piva, R. Establishment of a 3D-Dynamic Osteoblasts–Osteoclasts Co-Culture Model to Simulate the Jawbone Microenvironment in Vitro. Life Sci. 2016, 152, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Egli, M.; Krüger, M.; Riwaldt, S.; Corydon, T.J.; Kopp, S.; Wehland, M.; Wise, P.; Infanger, M.; Mann, V.; et al. Tissue Engineering Under Microgravity Conditions–Use of Stem Cells and Specialized Cells. Stem Cells Dev. 2018, 27, 787–804. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, V.; Bechtel, G.; Tchantchaleishvili, V. Artificial Tissue Creation under Microgravity Conditions: Considerations and Future Applications. Artif. Organs 2021, 45, 1446–1455. [Google Scholar] [CrossRef]
- Yeatts, A.; Fisher, J.P. Bone Tissue Engineering Bioreactors: Dynamic Culture and the Influence of Shear Stress. Bone 2011, 48, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Xu, Z.; Wang, X.; Wang, Y.; Li, H.; Chen, T.; Hu, Y.; Chen, R.; Huang, K.; Chen, C.; et al. Biophysical Stimuli as the Fourth Pillar of Bone Tissue Engineering. Front. Cell Dev. Biol. 2021, 9, 790050. [Google Scholar] [CrossRef]
- Hatefi, S.; Alizargar, J.; Le Roux, F.; Hatefi, K.; Sh, M.E.; Davids, H.; Hsieh, N.-C.; Smith, F.; Abou-El-Hossein, K. Review of Physical Stimulation Techniques for Assisting Distraction Osteogenesis in Maxillofacial Reconstruction Applications. Med. Eng. Phys. 2021, 91, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.L.; Syed, I.; Smith, T.L.; Harrison, B.S. The Regenerative Effects of Electromagnetic Field on Spinal Cord Injury. Electromagn. Biol. Med. 2017, 36, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Caliogna, L.; Medetti, M.; Bina, V.; Brancato, A.; Castelli, A.; Jannelli, E.; Ivone, A.; Gastaldi, G.; Annunziata, S.; Mosconi, M.; et al. Pulsed Electromagnetic Fields in Bone Healing: Molecular Pathways and Clinical Applications. Int. J. Mol. Sci. 2021, 22, 7403. [Google Scholar] [CrossRef]
- Yuan, J.; Xin, F.; Jiang, W. Underlying Signaling Pathways and Therapeutic Applications of Pulsed Electromagnetic Fields in Bone Repair. Cell. Physiol. Biochem. 2018, 46, 1581–1594. [Google Scholar] [CrossRef]
- Wang, T.; Yang, L.; Jiang, J.; Liu, Y.; Fan, Z.; Zhong, C.; He, C. Pulsed Electromagnetic Fields: Promising Treatment for Osteoporosis. Osteoporos. Int. 2019, 30, 267–276. [Google Scholar] [CrossRef]
- Varani, K.; Vincenzi, F.; Pasquini, S.; Blo, I.; Salati, S.; Cadossi, M.; De Mattei, M. Pulsed Electromagnetic Field Stimulation in Osteogenesis and Chondrogenesis: Signaling Pathways and Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 809. [Google Scholar] [CrossRef]
- Leone, L.; Podda, M.V.; Grassi, C. Impact of Electromagnetic Fields on Stem Cells: Common Mechanisms at the Crossroad between Adult Neurogenesis and Osteogenesis. Front. Cell. Neurosci. 2015, 9, 228. [Google Scholar] [CrossRef] [Green Version]
- Ross, C.L.; Siriwardane, M.; Almeida-Porada, G.; Porada, C.D.; Brink, P.; Christ, G.J.; Harrison, B.S. The Effect of Low-Frequency Electromagnetic Field on Human Bone Marrow Stem/Progenitor Cell Differentiation. Stem Cell Res. 2015, 15, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Ross, C.L.; Ang, D.C.; Almeida-Porada, G. Targeting Mesenchymal Stromal Cells/Pericytes (MSCs) With Pulsed Electromagnetic Field (PEMF) Has the Potential to Treat Rheumatoid Arthritis. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Galli, C.; Pedrazzi, G.; Guizzardi, S. The Cellular Effects of Pulsed Electromagnetic Fields on Osteoblasts: A Review. Bioelectromagnetics 2019, 40, 211–233. [Google Scholar] [CrossRef]
- Fini, M.; Giavaresi, G.; Setti, S.; Martini, L.; Torricelli, P.; Giardino, R. Current Trends in the Enhancement of Biomaterial Osteointegration: Biophysical Stimulation. Int. J. Artif. Organs 2004, 27, 681–690. [Google Scholar] [CrossRef]
- Parmaksiz, M.; Lalegül-Ülker, Ö.; Vurat, M.T.; Elçin, A.E.; Elçin, Y.M. Magneto-Sensitive Decellularized Bone Matrix with or without Low Frequency-Pulsed Electromagnetic Field Exposure for the Healing of a Critical-Size Bone Defect. Mater. Sci. Eng. C 2021, 124, 112065. [Google Scholar] [CrossRef]
- Tsai, M.-T.; Chang, W.H.-S.; Chang, K.; Hou, R.-J.; Wu, T.-W. Pulsed Electromagnetic Fields Affect Osteoblast Proliferation and Differentiation in Bone Tissue Engineering. Bioelectromagnetics 2007, 28, 519–528. [Google Scholar] [CrossRef]
- Nerem, R.M. Tissue Engineering: The Hope, the Hype, and the Future. Tissue Eng. 2006, 12, 1143–1150. [Google Scholar] [CrossRef]
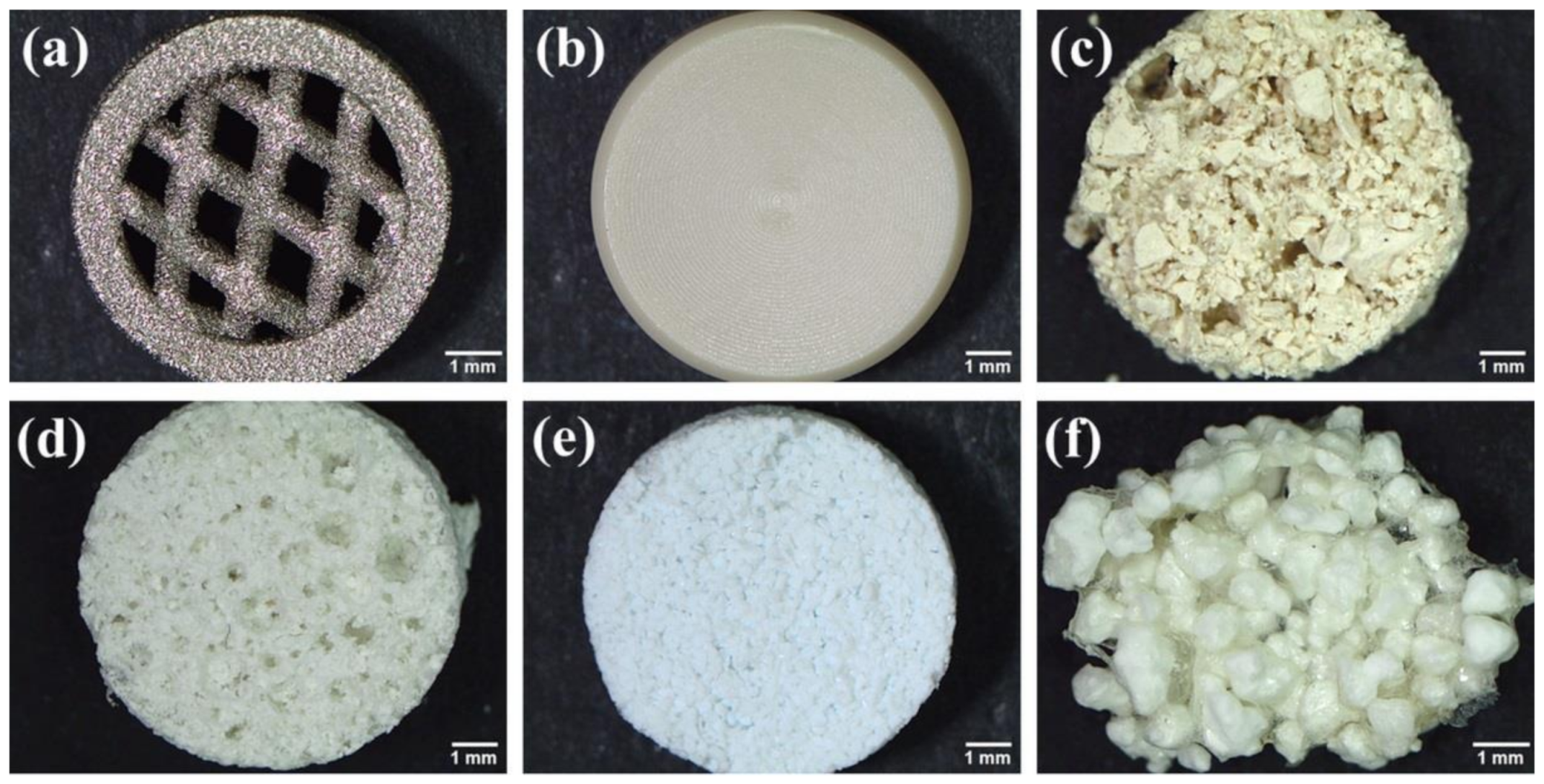
| Biomaterial Type | General Features | Ref. |
|---|---|---|
| Metallic | Very high biomechanical load capacity and high Young’s modulus, causing stress-shielding effect, corrosion resistance, poor biodegradability, and biocompatibility | [26,32] |
| Ceramic | Low mechanical strength, high brittleness, slow resorption rate, biocompatibility, bioactivity, osteoconductivity, and osteoinductivity | [18,32] |
| Polymeric | Poor mechanical properties, low stiffness, biodegradability, biocompatibility, and low immunogenicity | [13,29,32] |
| Composite | Biomimetic properties, good mechanical strength, biocompatibility, osteoconductivity, osteoinductivity, bioactivity, and biodegradability | [18,31,33] |
| Bioprinting Technique | Bioink | Results | Ref. |
|---|---|---|---|
| Micro-extrusion | Gelatin methacryloyl, kappa-carrageenan, nanosilicates, and human BMDSCs | In vitro (human BMDSCs): stimulated endochondral differentiation and increased ECM mineralization | [54] |
| Micro-extrusion | Gelatin, alginate, nanosilicates, and rat BMDSCs | In vitro (rat BMDSCs): increased ALP activity and ECM mineralization, and supported expression of osteogenesis-related genes (RUNX2, Osterix, OCN, OPN, and COL 1) In vivo (rat model): supported bone formation | [56] |
| Micro-extrusion | Gelatin methacrylamide, alginate, β-TCP, and human BMDSCs | In vitro (human BMDSCs): increased expression of osteogenesis-related genes (ALP and BGLAP) | [57] |
| Micro-extrusion | Collagen, chicken BMDSCs-conditioned medium, and human ADSCs | In vitro (human ADSCs): increased ALP activity, ECM mineralization, and expression of osteogenesis-related genes (RUNX2, COL 1, ALP, BMP-2, OCN, and OPN) In vivo (rat model): stimulated bone formation | [58] |
| Micro-extrusion | PCL, magnesium hydroxide nanoparticles, Sr-gelatin methacrylamide, and human BMDSCs | In vitro (human BMDSCs): increased ECM mineralization and expression of COL 1 and OCN | [59] |
| Micro-extrusion | PLGA, PEG, GET-RUNX, and human MSCs | In vitro (human MSCs): increased osteogenic differentiation In vivo (mouse model): supported bone formation | [60] |
| Micro-extrusion | Natural nano-attapulgite with polyvinyl alcohol as binder | In vitro (human BMDSCs): induced expression of osteogenesis-related genes (BMP-2 and RUNX2) In vivo (rat model): supported bone formation | [61] |
| Micro-extrusion | Gelatin and β-TCP | In vitro (mouse preosteoblast, MC3T3-E1 cell line): supported cell migration, proliferation, and osteogenic differentiation In vivo (rat model): stimulated bone formation | [62] |
| Micro-extrusion | PCL | In vitro (rat BMDSCs): increased ECM mineralization and expression of osteogenesis-related genes (RUNX2, Osterix, OCN, OPN, and COL 1) In vivo (rat model): supported vascular ingrowth and bone regeneration | [63] |
| Bioreactor System | Applied Physical Stimuli | Biomaterial | Cells | Results | Ref. |
|---|---|---|---|---|---|
| Perfusion | 1 mL/min medium flow rate; dynamic compression (1% strain at 1 Hz) | Chitosan-graphene scaffold | Human BMDSCs | Increased cell viability and enhanced ECM mineralization | [73] |
| Perfusion | 0.1 mL/min medium flow rate | DEX-loaded RADA 16-I scaffold | Human BMDSCs | Increased ECM mineralization and expression of osteogenesis-related genes (ALP, OCN, and COL 1) | [76] |
| Perfusion | 1.7 mL/min medium flow rate | Calcium phosphate (substituted with Mg2+, Zn2+ and SeO32−)/chitosan composite scaffold | Human BMDSCs | Supported COL 1 synthesis and ECM mineralization | [77] |
| Perfusion | 1.7 mL/min medium flow rate; dynamic compression (10% strain at 1 Hz) | Human femoral head-derived decellularized bone scaffold | Human BMDSCs | Increased cell proliferation and ECM synthesis | [78] |
| Perfusion | 1.6 mL/min medium flow rate | Poly(L-lactide-co-trimethylene carbonate) lactide (LTMC) scaffold | Rat BMDSCs | Decreased cell proliferation and increased expression of osteogenesis-related genes (RUNX2, ALP, SP7, BSP, OPN, and OCN) | [79] |
| Perfusion | 10 mL/min medium flow rate | Fibrin beads | Rat BMDSCs | Increased expression of osteogenesis-related genes (OPN, RUNX2, and VEGF) | [80] |
| Perfusion | 3.47 mL/min medium flow rate | Polyurethane scaffold | Human embryonic stem cell-derived mesenchymal progenitors | Increased ALP activity and cell number | [81] |
| Perfusion | 1.6 mL/min medium flow rate; shear stress of 3.93 mPa | Polycaprolactone/hydroxyapatite (PCL/HA) scaffold functionalized with RGD–C (arginine–glycine–aspartate–cysteine) | Human fetal osteoblasts (hFOB 1.19) | Decreased cell proliferation as well as increased ALP activity and ECM mineralization | [82] |
| Perfusion | 0.3 mL/min medium flow rate | Mg-based alloy/HA scaffold | Human fetal osteoblasts (hFOB 1.19) | Increased COL 1, ALP, OCN, and OPN synthesis | [83] |
| Perfusion | 1 mL/min medium flow rate | Porcine decellularized native bone | Human smooth muscle cells (hSMCs) and human umbilical vein endothelial cells (HUVECs) | Improved cellular density and increased microvascular networks | [84] |
| Rotating | 5 rpm rotation rate | Polycaprolactone–β-tricalcium phosphate (PCL-TCP) scaffold | Human BMDSCs | Increased expression of osteogenesis-related genes (ALP, OC, OCN, and COL 1) | [85] |
| Rotating | Not provided | Chitosan/hydroxyapatite microbeads | Rat BMDSCs | Increased OC and OPN synthesis | [86] |
| Rotating | Not provided | Poly(lactic-co-glycolic acid; PLGA) scaffold | Human dental pulp-derived mesenchymal stem cells | Increased COL 1 synthesis and ECM mineralization | [87] |
| Rotating and perfusion | 1 rpm rotation rate; 1−2 mL/min medium flow rate | Gelatin-coated β-tricalcium phosphate scaffold | Buccal fat pad tissue-derived mesenchymal stem cells | Supported ECM protein synthesis | [88] |
| Rotating and perfusion | 1 rpm rotation rate; 1−2 mL/min medium flow rate | Gelatin-coated β-tricalcium phosphate scaffold | Buccal fat pad tissue-derived mesenchymal stem cells | Increased expression of osteogenesis-related genes (RUNX2, ALP, OC, and COL 1) | [89] |
| Spinner flask | 30 rpm stirred rate | Fibra-Cel® Disk (Eppendorf) | Human BMDSCs | Increased ALP activity and decreased ECM mineralization | [90] |
| Spinner flask | 50 rpm stirred rate | Collagen/nanohydroxyapatite/phosphoserine scaffold | Human dental pulp-derived mesenchymal stem cells and human dental follicle-derived mesenchymal stem cells | Increased ALP activity and higher osteogenic gene expression (OC and BMP-2) | [91] |
| Spinner flask | 50 rpm stirred rate | Polycaprolactone (PCL) microparticles | Human ADSCs and human osteoblasts | Enhanced ECM mineralization | [92] |
| Spinner flask | 50 rpm stirred rate | CultiSpher S microcarriers | Human amnion-derived MSCs and HUVECs | Downregulated ALP activity, ECM mineralization, and gene expression (COL I, RUNX2, and OC) | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazimierczak, P.; Przekora, A. Bioengineered Living Bone Grafts—A Concise Review on Bioreactors and Production Techniques In Vitro. Int. J. Mol. Sci. 2022, 23, 1765. https://doi.org/10.3390/ijms23031765
Kazimierczak P, Przekora A. Bioengineered Living Bone Grafts—A Concise Review on Bioreactors and Production Techniques In Vitro. International Journal of Molecular Sciences. 2022; 23(3):1765. https://doi.org/10.3390/ijms23031765
Chicago/Turabian StyleKazimierczak, Paulina, and Agata Przekora. 2022. "Bioengineered Living Bone Grafts—A Concise Review on Bioreactors and Production Techniques In Vitro" International Journal of Molecular Sciences 23, no. 3: 1765. https://doi.org/10.3390/ijms23031765
APA StyleKazimierczak, P., & Przekora, A. (2022). Bioengineered Living Bone Grafts—A Concise Review on Bioreactors and Production Techniques In Vitro. International Journal of Molecular Sciences, 23(3), 1765. https://doi.org/10.3390/ijms23031765







