Structural and Physicochemical Properties of Tunisian Quercus suber L. Starches for Custard Formulation: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starch Analysis
2.2.1. Chemical Composition
2.2.2. Solubility, Swelling Power, Water Absorption Capacity, Refrigeration, and Freezing Stability
2.2.3. Fourier Transform Infrared Spectroscopy
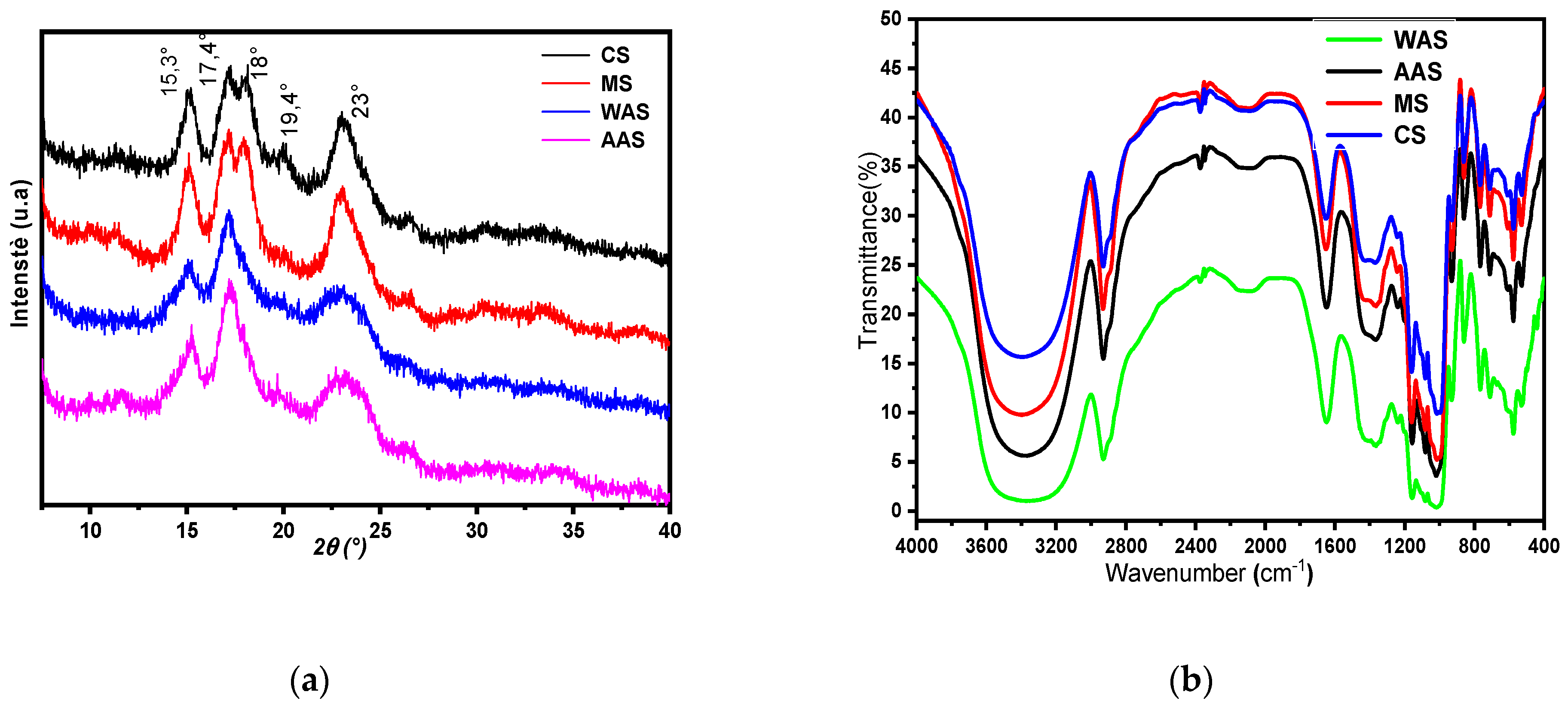
2.2.4. X-ray Diffraction (XRD)
2.2.5. Thermogravimetric (TGA) Analysis
2.2.6. Thermal Properties
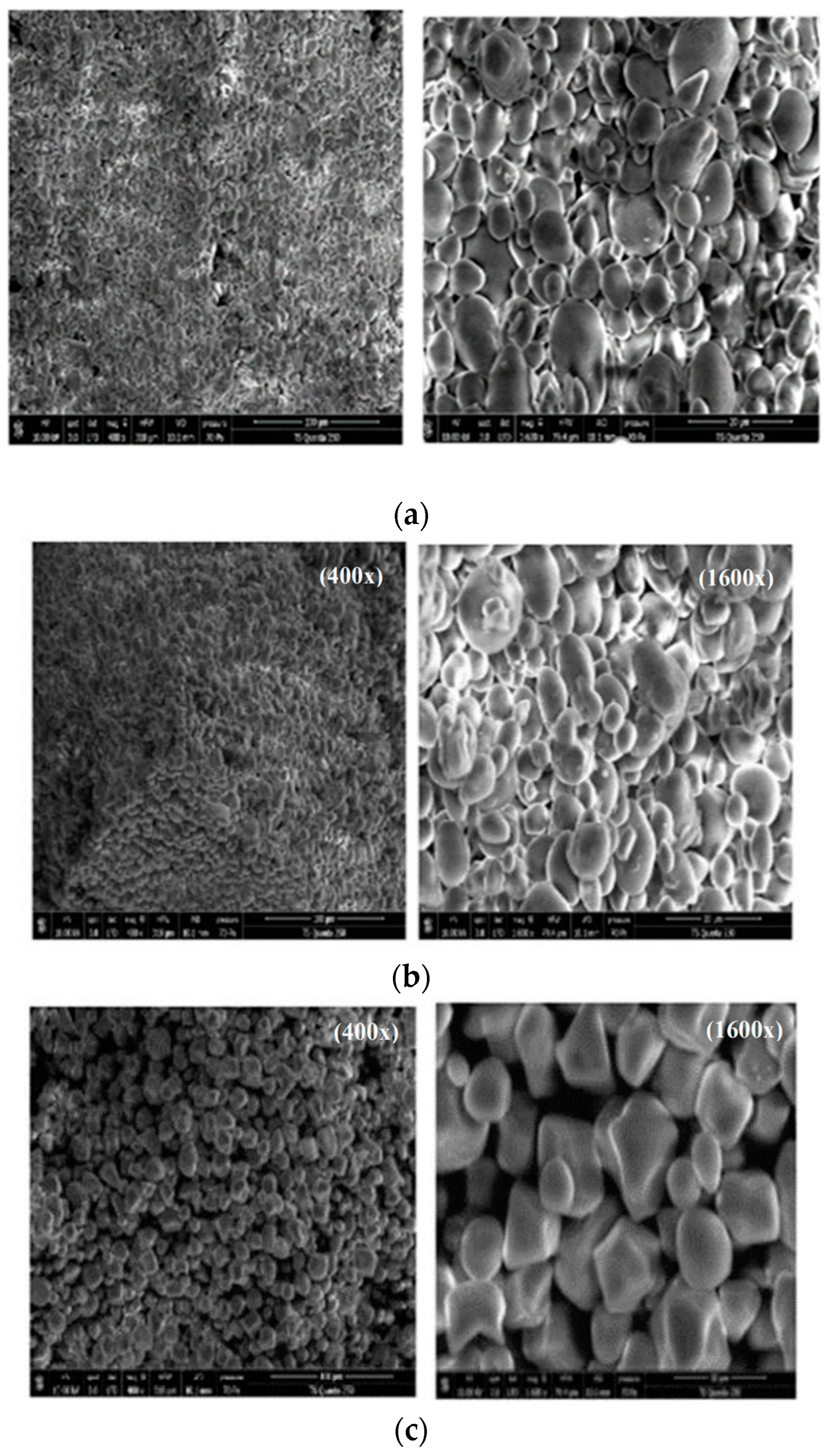
2.2.7. Scanning Electron Microscopy (SEM)
2.3. Custard Preparation and Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Acorn Starch Characterization
3.1.1. Physicochemical Characterization of Starches
3.1.2. Swelling Power, Solubility, and Water Absorption of Starches
3.1.3. Refrigeration and Freezing Stability of Starches
3.1.4. Thermal and Retrogradation Properties
3.1.5. X-ray Diffraction and FTIR Spectral Analysis
3.1.6. Thermogravimetric (TGA) Analysis
3.1.7. Microstructure of Starch Samples
3.2. Characterization of New Custard
3.2.1. Physicochemical Composition of the Formulated Custard
3.2.2. Syneresis of the Formulated Custard
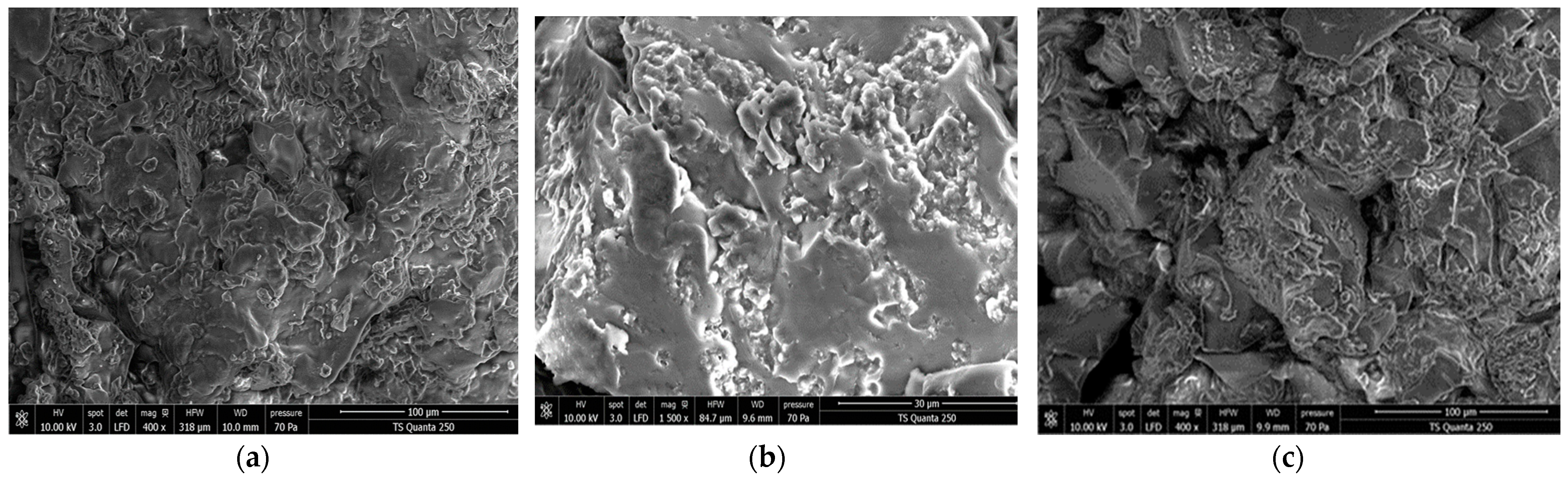
3.2.3. Microstructure of the Formulated Custard
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Taib, M.; Bouyazza, L. Composition, Physicochemical Properties, and Uses of Acorn Starch. J. Chem. 2021, 2021, 9988570. [Google Scholar] [CrossRef]
- Korus, J.; Witczak, M.; Ziobro, R.; Juszczak, L. The influence of acorn flour on rheological properties of gluten free dough and physical characteristics of the bread. Eur. Food Res. Technol. 2015, 240, 1135–1143. [Google Scholar] [CrossRef]
- Jdaidi, N.; Chaabane, A.; Toumi, L.; Hasnaoui, B. Influence of the sanitary state of acorns on the regeneration of Quercus suber in Tunisia. Revue d’Ecologie (Terre Vie) 2018, 73, 71–79. [Google Scholar]
- Lin, Y.; Lu, Y.; Song, Z.; Huang, D. Characterizations of the endogenous starch hydrolase inhibitors in acorns of Quercus fabri hance. Food Chem. 2018, 258, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, X.; Yue, J.; Sun, Y.; Zhang, X.; Zhao, Y. Polyphenols from acorn leaves (quercus liaotungensis) protect pancreatic beta cells and their inhibitory activity against α-glucosidase and protein tyrosine phosphatase 1B. Molecules 2018, 23, 2167. [Google Scholar] [CrossRef] [PubMed]
- Vinha, A.F.; Barreira, J.C.M.; Costa, A.S.; Oliveira, M.B.P.P. A New Age for Quercus spp. Fruits: Review on Nutritional and Phytochemical Composition and Related Biological Activities of Acorns. Compr. Rev. Food Sci. Food Saf. 2016, 15, 947–981. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Shi, X.; Li, L. Analysis on basic physicochemical properties and antioxidant activities of the starch from acorn. Hans J. Food Nutr. Sci. 2019, 8, 195–207. [Google Scholar] [CrossRef]
- Foster, J.F. Physical Properties of Amylose and Amylopectin. In Starch Chemistry and Technology, 1st ed.; Whistler, R.L., Paschall, E.F., Eds.; Academic Press: New York, NY, USA, 1965; pp. 349–392. [Google Scholar]
- Pascoal, A.M.; Di-Medeiros, M.C.B.; Batista, K.A.; Leles, M.I.G.; Lião, L.M.; Fernandes, K.F. Extraction and chemical characterization of starch from S. lycocarpum fruits. Carbohydr. Polym. 2013, 98, 1304–1310. [Google Scholar] [CrossRef]
- Boukhelkhal, M.; Moulai-Mostefa, N. Physicochemical Characterization of Starch Isolated from Soft Acorns of Holm Oak (Quercus Ilex Subsp. Ballota (Desf.) Samp.) Grown in Algeria. J. Food Meas. Charact. 2017, 11, 1995–2005. [Google Scholar] [CrossRef]
- Peroni, F.H.G.; Rocha, T.S.; Franco, C.M.L. Some structural and physicochemical characteristics of tuber and root starches. Food Sci. Technol. Int. 2006, 12, 505–513. [Google Scholar] [CrossRef]
- Rondán-Sanabria, G.G.; Finardi-Filho, F. Physiscalchemical and functional properties of maca root starch (Lepidium meyenii Walpers). Food Chem. 2009, 114, 492–498. [Google Scholar] [CrossRef]
- Zarroug, Y.; Boulares, M.; Mejri, J.; Slimi, B.; Hamdaoui, G.; Djebi, S.; Nasri, H.; Sfayhi, D.; Kharrat, M. Extraction and characterization of Tunisian Quercus ilex starch and its effect on fermented dairy product quality. Int. J. Anal. Chem. 2020, 2020, 8868673. [Google Scholar] [CrossRef]
- Özünlü, O.; Ergezer, H.; Gökçe, R. Improving physicochemical, antioxidative and sensory quality of raw chicken meat by using acorn extracts. LWT 2018, 98, 477–484. [Google Scholar] [CrossRef]
- Godbillot, L.; Dole, L.; Joly, C.; Roge, B.; Mathlouthi, M. Analysis of water binding in starch plasticized films. Food Chem. 2006, 96, 380–386. [Google Scholar] [CrossRef]
- Franklin, M.E.E.; Pushpadass, H.A.; Kumar, B.; Kulkarni, S.; Muthurayappa, M.; Kandasamy, R.; Vellingiri, P. Physicochemical, thermal, pasting and microstructural characterization of commercial Curcuma angustifolia starch. Food Hydrocoll. 2017, 67, 27–36. [Google Scholar] [CrossRef]
- Onem, E.; Gulumser, G.; Akay, S.; Yesil-Celiktas, O. Optimization of tannin isolation from acorn and application in leather processing. Ind. Crops Prod. 2014, 53, 16. [Google Scholar] [CrossRef]
- Jiang, Q.; Liang, S.; Zeng, Y.; Lin, W.; Ding, F.; Li, Z.; Cao, M.; Li, Y.; Ma, M.; Wu, Z. MicroRNAs and angiogenesis: A new era for the management of colorectal cancer. Int. J. Biol. Macromol. 2019, 125, 1147. [Google Scholar] [CrossRef]
- Markom, M.; Hasan, M.; Daud, W.R.W.; Singh, H.; Jahim, J.M. Extraction of hydrolysable tannins from Phyllanthus niruri Linn.: Effects of solvents and extraction methods. Sep. Purif. Technol. 2007, 52, 487. [Google Scholar] [CrossRef]
- Zhang, Z.; Saleh, A.S.M.; Wu, H.; Gou, M.; Liu, Y.; Jing, L.; Zhao, K.; Su, C.; Zhang, B.; Li, W. Effect of Starch Isolation Method on Structural and Physicochemical Properties of Acorn Kernel Starch. Food Sci. Technol. 2019, 72, 1900122. [Google Scholar] [CrossRef]
- Pérez-Pachecoa, E.; Moo-Huchin, V.M.; Estrada-León, R.J.; Ortiz-Fernández, A.; May Hernández, L.H.; Ríos-Soberanis, C.R.; Betancur-Ancona, D. Isolation and characterization of starch obtained from Brosimum alicastrum Swarts Seeds. Carbohydr. Polym. 2014, 101, 101920–101927. [Google Scholar] [CrossRef]
- Singh, J.; Singh, N. Studies on the morphological, thermal and rheological properties of starch separated from some Indian potato cultivars. Food Chem. 2001, 75, 67–77. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of The Association of the Official Analytical Chemists, 18th ed.; AOAC: Arlingto, VA, USA, 2005. [Google Scholar]
- Achri, A.; Sukeri, M.; Yusof, M.; Suzeren Jamil, M.; Abdullah, A.; Fairus, S.; Yusoff, M.; Arip, M.N.M.; Mat Lazim, A. Physicochemical characterization of starch extracted from Malaysian wild yam (Dioscorea hispida Dennst.). Emir. J. Food Agric. 2014, 652–658. [Google Scholar] [CrossRef]
- Lan, W.; Hihua, Y.; Yun, Z.; Bijun, X.; Zhida, S. Morpho-logical, physicochemical and textural properties of starch separatedfrom Chinese water chestnut. Starch-Starke 2008, 60, 181–191. [Google Scholar] [CrossRef]
- Ben Moussa, O.; Rouissi, E.; Boulares, M.; Hassouna, M. Effects of chia seed levels on quality and bio-functional profile of stirred yoghurt. Acta Aliment. 2020, 49, 398–405. [Google Scholar] [CrossRef]
- Irinislimane, H.; Belhaneche-Bensemra, N. Extraction and Characterization of Starch from Oak Acorn, Sorghum, and Potato and Adsorption Application for Removal of Maxilon Red GRL from Wastewater. Chem. Eng. Commun. 2017, 204, 897–906. [Google Scholar] [CrossRef]
- Correia, P.R.; Leitao, A.E.; Beirao-da-Costa, M.L. Effect of drying temperatures on chemical and morphological properties of acorn flours. Int. J. Food Sci. Technol. 2009, 44, 1729–1736. [Google Scholar] [CrossRef]
- Oyeyinka, S.; Singh, S.; Adebola, P.; Gerrano, A.; Amonsou, E. Physicochemical properties of starches with variable amylose contents extracted from bambara groundnut genotypes. Carbohydr. Polym. 2015, 6, 100. [Google Scholar] [CrossRef]
- Shadrack, M.C.; Tilahun, S.W.; Geremew, B.; Mark, L. Characterization of physicochemical properties of starches from improved cassava varieties grown in Zambia. AIMS Agric. Food 2019, 4, 939–966. [Google Scholar] [CrossRef]
- Kormin, S.; Kormin, F.; Beg, M.D.H. Physical and mechanical properties of LDPE incorporated with different starch sources. IOP Conf. Ser. Mater. Sci. Eng. 2017, 226, 012157. [Google Scholar] [CrossRef]
- Sharlina, M.E.; Yaacob, W.A.; Lazim, A.M.; Fazry, S.; Lim, S.J.; Abdullah, S.; Kumaran, M. Physicochemical properties of starch from Dioscorea pyrifolia tubers. Food Chem. 2017, 220, 225–232. [Google Scholar] [CrossRef]
- Usman, M.; Ishfaq, T.; Malik, S.R.; Iqbal, M.; Ishfaq, B. Alkaline Extraction of Starch from Broken Rice of Pakistan. Int. J. Innov. Appl. Stud. 2014, 7, 146–152. [Google Scholar]
- Awoyale, W.; Sanni, L.O.; Shittu, T.A.; Adebowale, A.A.; Adegunwa, M.O. Development of an Optimized Cassava Starch-Based Custard Powder. J. Culin. Sci. Technol. 2017, 17, 1–23. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Hoover, R.; Donner, E.; Liu, Q. Retrogradation Characteristics of Pulse Starches. Food Res Int. 2013, 54, 203–212. [Google Scholar] [CrossRef]
- Singh, J.; McCarthy, O.; Singh, H. Starch–A Potential Biomaterial for Biomedical Applications. Carbohydr. Polym. 2006, 64, 569–581. [Google Scholar] [CrossRef]
- Correia, P.R.; Maria, L.B. Chestnut and acorn starch properties affected by isolation methods. Starch-Stärke 2010, 62, 421–428. [Google Scholar] [CrossRef]
- Correia, P.R.; Nunes, C.; Beirão-da-Costa, M.L. The effect of starch isolation method on physical and functional properties of Portuguese nuts starches. I. Chestnuts (Castanea sativa Mill. var. Martainha and Longal) fruits. Food Hydrocoll. 2012, 27, 10. [Google Scholar] [CrossRef]
- Jiang, Q.; Gao, W.; Li, X.; Xia, Y.; Wang, H.; Wu, S.; Huang, L.; Liu, C.; Xiao, P. Characterizations of starches isolated from five different Dioscorea, L. species. Food Hydrocoll. 2012, 29, 35–41. [Google Scholar] [CrossRef]
- Cruz, B.R.; Abraao, A.S.; Lemos, A.M.; Correia, P.R.; Nunes, M.C.; Beirão-da-Costa, M.L. The effect of starch isolation method on physical and functional properties of Portuguese nut starches. II. Q. rotundifolia lam. and Q. suber lam. acorns starches. Food Hydrocoll. 2013, 30, 448–455. [Google Scholar]
- Kaur, K.; Singh, N.; Singh, H. Studies on the effect of skim milk powder, sprouted wheat flour, and pH on rheological and baking properties of flour. Int. J. Food Prop. 2005, 5, 13–24. [Google Scholar] [CrossRef]
- Molavi, H.; Razavi, S.M.A.; Farhoosh, R. Impact of hydrothermal modifications on the physicochemical, morphology, crystallinity, pasting and thermal properties of acorn starch. Food Chem. 2018, 245, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Ascheri, J.L.R.; Zamudio, L.H.B.; Carvalho, C.W.P.; Arevalo, A.M.; Fontoura, L.M. Extraction and Characterization of Starch Fractions of Five Phenotypes Pachyrhizus tuberosus (Lam.) Spreng. Food Nutr. Sci. 2014, 5, 1875–1885. [Google Scholar] [CrossRef]
- Moorthy, S.N. Physicochemical and Functional Properties of Tropical Tuber 219 Starches. A Review. Starch-Stärke 2002, 54, 559–592. [Google Scholar] [CrossRef]
- Ascheri, J.L.R. Characterization of Job’s Tears Starch. II. Pasting Characteristics. Alimentaria 1996, 96, 101–104. [Google Scholar]
- Teixeira, M.A.V.; Ciacco, C.F.; Tavares, D.Q.; Bonezzi, A.N. Ocorrência e caracterização do amido resistente em amidos de milho e de banana. Ciênc. Tecnol. Aliment. Camp. 1998, 18, 246. [Google Scholar] [CrossRef]
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the Crystallinity Degree of Starch Having Different Types of Crystal Structure after Mechanical Pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef]
- Yoo, S.H.; Lee, C.S.; Kim, B.S.; Shin, M. The properties and molecular structures of gusiljatbam starch compared to those of acorn and chestnut starches. Starch-Starke 2012, 64, 339–347. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of irradiated starches by uisng FT-Raman and FTIR spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef]
- Miao, M.; Jiang, H.; Jiang, B.; Cui, S.W.; Jin, Z.; Zhang, T. Structure and functional properties of starches from Chinese ginkgo (Ginkgo biloba L.) nuts. Food Res. Int. 2012, 49, 303–310. [Google Scholar] [CrossRef]
- Liu, H.; Yu, L.; Tong, Z.; Chen, L. Retrogradation of waxy cornstarch studied by DSC. Starch–Stärke 2010, 62, 524–529. [Google Scholar] [CrossRef]
- Ali, A.; Wani, T.A.; Wani, I.A.; Masoodi, F.A. Comparative study of the physico-chemical properties of rice and corn starches grown in Indian temperate climate. J. Saudi Soc. Agric. Sci. 2016, 15, 75–82. [Google Scholar] [CrossRef]
- Liu, C.; Wang, S.; Chang, X.; Wang, S. Structural and functional properties of starches from Chinese chestnuts. Food Hydrocoll. 2015, 43, 568–576. [Google Scholar] [CrossRef]
- Hernández-Medina, M.; Torruco-Uco, J.G.; Chel-Guerrero, L.; Betancur-Ancona, D. Caracterización Fisicoquímica de Almidones de Tubérculos Cultivados en Yucatán, México. Cienc. Tecnol. Aliment. 2008, 28, 718–726. [Google Scholar] [CrossRef]
- Singh, N.; Singh, J.; Kaur, L.; Sodhi, S.N.; Gill, S.B. Morphological, Thermal and Rheological Properties of Starches from Different Botanical Sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Salami, H.O.; Olorunlambe, A.A.; Boluwatife, O.A.; Akinwande, F.F.; Ahmed ElImam, A.M.; Samson, A.O. Physicochemical and sensory properties of corn starch custard soured with tamarind, soursop and lime. Croat. J. Food Technol. Biotechnol. Nutr. 2019, 14, 91–97. [Google Scholar] [CrossRef]
- Senadeera, S.S.; Prasanna, P.H.P.; Jayawardana, N.W.I.A.; Gunasekara, D.C.S.; Senadeera, P.; Chandrasekara, A. Antioxidant, physicochemical, microbiological, and sensory properties of probiotic yoghurt incorporated with various Annona species pulp. Heliyon 2018, 4, 955. [Google Scholar] [CrossRef]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Physicochemical, textural and rheological properties of probiotic yogurt fortified with fibre-rich pineapple peel powder during refrigerated storage. LWT-Food Sci. Technol. 2016, 65, 978–986. [Google Scholar] [CrossRef]
- Tarakci, Z. Influence of kiwi marmalade on the rheology characteristics, color values and sensorial acceptability of fruit yogurt. Kafkas. Univ. Vet. Fak. Derg. 2010, 16, 173–178. [Google Scholar]
- Vélez-Ruiz, J.F.; Hernando, I.; González-Tomás, L.; Pérez-Munuera, I.; Quiles, A.; Tárrega, A.; Lluch, M.A.; Costell, E. Rheology and microstructure of custard model systems with cross-linked waxy maize starch. Flavour Frag. J. 2006, 21, 30–36. [Google Scholar] [CrossRef]



| Components | WAS | AAS | CS | MS |
|---|---|---|---|---|
| Yield (%) | 48.32 ± 0.007 a | 48.1 ± 0.07 b | - | - |
| Moisture (%) | 12.20 ± 0.16 b | 12.41 ± 0.13 b | 9.16 ± 0.45 c | 7.95 ± 0.22 a |
| Fat (%) | 0.44 ± 0.007 a | 0.61 ± 0.028 d | 0.35 ± 0.014 b | 0.47 ± 0.01 c |
| Protein (%) | 0.30 ± 0.01 d | 0.27 ± 0.01 c | 0.14 ± 0.01 b | 0.12 ± 0.01 a |
| Ash (%) | 0.35 ± 0.02 c | 0.24 ± 0.04 b | 0.17 ± 0.005 d | 0.09 ± 0.02 a |
| Carbohydrates (%) | 86.71 ± 0.01 a | 86.47 ± 0.02 b | 90.18 ± 0.01 c | 91.37 ± 0.02 d |
| pH | 5.66 ± 0.05 b | 5.85 ± 0.01 a | 6.61 ± 0.02 c | 6.81 ± 0.01 d |
| L* | 55.76 ± 1.70 a | 60.41 ± 0.23 a | 87.8 ± 0.46 c | 92.72 ± 0.31 b |
| a* | 1.47 ± 0.234 d | 0.89 ± 0.02 c | −0.27 ± 0.04 b | −1.98 ± 0.12 a |
| b* | 15.81 ± 0.04 a | 15.18 ± 0.64 a | −1.15 ± 0.13 a | 3.36 ± 0.22 a |
| Transmittance (%) | 56.10 ± 0.75 c | 40.80 ± 0.45 a | 15.67 ± 0.62 d | 23.67 ± 0.99 b |
| Solubility (%) | Swelling Power (g Water/g Starch) | Water Absorption (g Water/g Starch) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | 60 °C | 70 °C | 80 °C | 90 °C | 60 °C | 70 °C | 80 °C | 90 °C | 60 °C | 70 °C | 80 °C | 90 °C |
| WAS | 0.19 ± 0.01 a | 3.37 ± 0.01 c | 5.37 ± 0.02 c | 7.27 ± 0.01 c | 3.01 ± 0.01 a | 8.51 ± 0.01 c | 10.06 ± 0.01 c | 20.7 ± 0.01 c | 3.18 ± 0.01 c | 7.36 ± 0.01 c | 11.75 ± 0.01 d | 13.97 ± 0.01 c |
| AAS | 0.33 ± 0.01 c | 4.44 ± 0.01 d | 7.19 ± 0.01 d | 13.3 ± 0.14 d | 6.05 ± 0.02 d | 11.3 ± 0.14 d | 14.03 ± 0.01 a | 22.51 ± 0.01 d | 4.32 ± 0.02 d | 8.44 ± 0.01 d | 11.21 ± 0.01 c | 14 ± 0.01 d |
| MS | 1.89 ± 0.01 d | 0.14 ± 0.01 a | 0.47 ± 0.01 a | 4.34 ± 0.21 a | 4.83 ± 0.01 c | 3.17 ± 0.01 a | 3.36 ± 0.01 d | 3.68 ± 0.01 a | 1.88 ± 0.01 a | 4.14 ± 0.01 a | 5.34 ± 0.01 a | 5.8 ± 0.01 a |
| CS | 0.2 ± 0.01 b | 0.3 ± 0.01 b | 3.19 ± 0.01 b | 6.15 ± 0.00 b | 3.82 ± 0.01 b | 8.44 ± 0.08 b | 8.59 ± 0.01 b | 11.29 ± 0.01 b | 2 ± 0.01 b | 6.02 ± 0.01 b | 6.28 ± 0.02 b | 8.31 ± 0.01 b |
| Syneresis to Refrigeration at 4 °C (%) | Syneresis to Refrigeration at −20 °C (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Time (h) | WAS | AAS | MS | CS | WAS | AAS | MS | CS |
| 24 | 40.4 ± 0.01 b | 42.82 ± 0.01 c | 46.57 ± 0.01 d | 39.62 ± 0.01 a | 54.97 ± 0.01 c | 46.66 ± 0.01 b | 35.44 ± 0.01 a | 56.04 ± 0.01 d |
| 48 | 45.85 ± 0.01 c | 44.46 ± 0.01 b | 62.58 ± 0.01 d | 43.34 ± 0.01 a | 59.96 ± 0.01 d | 47.62 ± 0.01 c | 37.32 ± 0.01 a | 56.27 ± 0.01 b |
| 72 | 46.06 ± 0.01 c | 44.79 ± 0.01 b | 72.32 ± 0.01 d | 43.6 ± 0.01 a | 64.01 ± 0.01 a | 55.79 ± 0.01 c | 42.02 ± 0.01 b | 58.92 ± 0.01 d |
| 96 | 46.93 ± 0.01 b | 45.98 ± 0.02 a | 79.16 ± 0.01 d | 47.55 ± 0.01 c | 64.73 ± 0.01 d | 61.5 ± 0.01 b | 44.17 ± 0.01 a | 62.01 ± 0.01 c |
| 120 | 62.99 ± 0.03 b | 46.07 ± 0.01 a | 92.6 ± 0.01 d | 73.03 ± 0.01 c | 69.77 ± 0.01 d | 63.04 ± 0.01 c | 61.4 ± 0.01 a | 62.36 ± 0.01 b |
| Samples | Thermal Properties | ||||
|---|---|---|---|---|---|
| T0 (°C) | TP (°C) | Tc (°C) | GR (°C) | ΔHG (J/g) | |
| AAS | 45.18 ± 0.45 a | 84.66 ± 0.36 c | 118.92 ± 0.20 a | 78.96 ± 0.26 a | 23.40 ± 0.09 a |
| WAS | 41.67 ± 0.25 b | 78.36 ± 0.83 d | 117.41 ± 0.80 a | 75.38 ± 0.86 b | 17.67 ± 0.04 b |
| CS | 40.53 ± 0.60 b | 76.75 ± 0.40 b | 116.83 ± 0.7 a | 70.44 ± 0.91 c | 18.19 ± 0.27 c |
| MS | 39.32 ± 0.74 c | 73.72 ± 0.57 a | 115.35 ± 0.20 a | 68.80 ± 0.44 d | 19.79 ± 0.94 d |
| Samples | Moisture (%) | Fat (%) | Protein (%) | Ash (%) | Carbohydrates (%) | pH | L* | a* | b* | Syneresis (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | ||||||||||
| CC | 77.44 ± 0.27 c | 1.38 ± 0.01 a | 2.70 ± 0.01 a | 0.71 ± 0.06 a | 18 ± 0.01 c | 6.64 ± 0.02 a | 52.94 ± 0.02 c | 3.07 ± 0.01 a | 34.67 ± 0.01 c | 3.32 ± 0.01 a | 8.34 ± 0.01 b | 12.59 ± 0.01 b | 22.32 ± 0.01 a |
| AASC | 77.23 ± 0.11 b | 3.01 ± 0.01 c | 3.65 ± 0.01 c | 0.72 ± 0.03 a | 15.39 ± 0.01 a | 6.99 ± 0.02 c | 37.16 ± 0.01 b | 4.06 ± 0.01 b | 29.48 ± 0.01 a | 3.42 ± 0.01 b | 5.98 ± 0.01 a | 18.45 ± 0.01 a | 23.88 ± 0.01 b |
| WASC | 76.76 ± 0.64 a | 2.01 ± 0.01 b | 3.05 ± 0.01 b | 0.72 ± 0.03 a | 17.45 ± 0.01 b | 6.98 ± 0.01 b | 29.38 ± 0.01 a | 7.41 ± 0.01 c | 29.72 ± 0.01 b | 14.28 ± 0.01 c | 21.91 ± 0.01 c | 28.34 ± 0.01 c | 32.34 ± 0.01 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarroug, Y.; Boulares, M.; Sfayhi, D.; Slimi, B.; Stiti, B.; Zaieni, K.; Nefissi, S.; Kharrat, M. Structural and Physicochemical Properties of Tunisian Quercus suber L. Starches for Custard Formulation: A Comparative Study. Polymers 2022, 14, 556. https://doi.org/10.3390/polym14030556
Zarroug Y, Boulares M, Sfayhi D, Slimi B, Stiti B, Zaieni K, Nefissi S, Kharrat M. Structural and Physicochemical Properties of Tunisian Quercus suber L. Starches for Custard Formulation: A Comparative Study. Polymers. 2022; 14(3):556. https://doi.org/10.3390/polym14030556
Chicago/Turabian StyleZarroug, Youkabed, Mouna Boulares, Dorra Sfayhi, Bechir Slimi, Bouthaina Stiti, Kamel Zaieni, Sirine Nefissi, and Mohamed Kharrat. 2022. "Structural and Physicochemical Properties of Tunisian Quercus suber L. Starches for Custard Formulation: A Comparative Study" Polymers 14, no. 3: 556. https://doi.org/10.3390/polym14030556
APA StyleZarroug, Y., Boulares, M., Sfayhi, D., Slimi, B., Stiti, B., Zaieni, K., Nefissi, S., & Kharrat, M. (2022). Structural and Physicochemical Properties of Tunisian Quercus suber L. Starches for Custard Formulation: A Comparative Study. Polymers, 14(3), 556. https://doi.org/10.3390/polym14030556






