Polymer-Based Wound Dressing Materials Loaded with Bioactive Agents: Potential Materials for the Treatment of Diabetic Wounds
Abstract
:1. Introduction
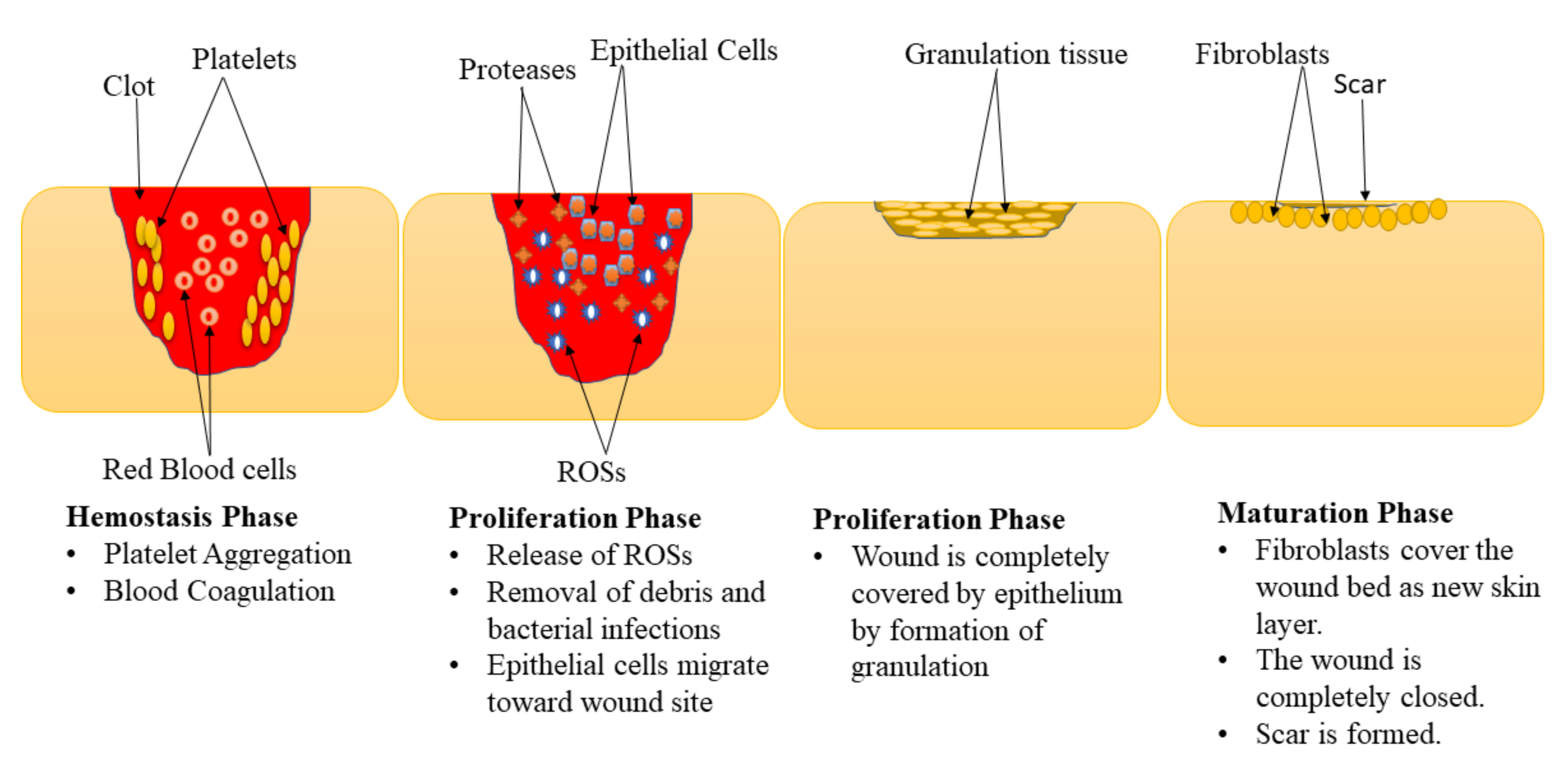
2. Classification of Wounds and Phases of Healing Process in Diabetic Wounds
3. Factors That Impede the Healing of Diabetic Wounds
3.1. Vasculopathy
3.2. Neuropathy
3.3. Infections
3.4. Immune System Deficiency
3.5. Interrupted Growth Factor Activity
3.6. Cellular Dysfunction
3.7. Poor Oxygenation
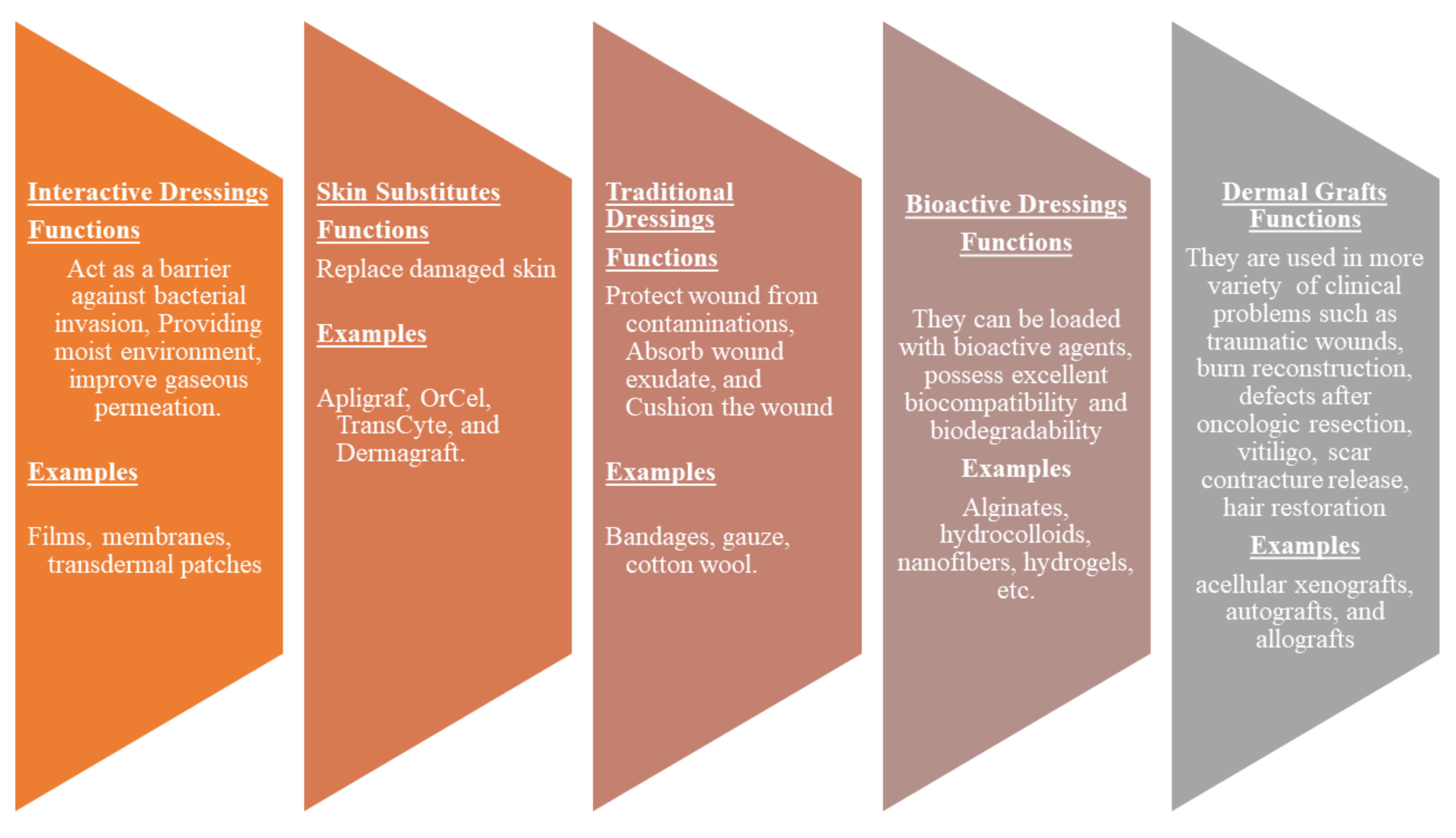
4. Classification of Wound Dressings
5. Polymer-Based Dressings Loaded with Bioactive Agents for Diabetic Wound Management
5.1. Nanofibers
5.2. Films and Membranes
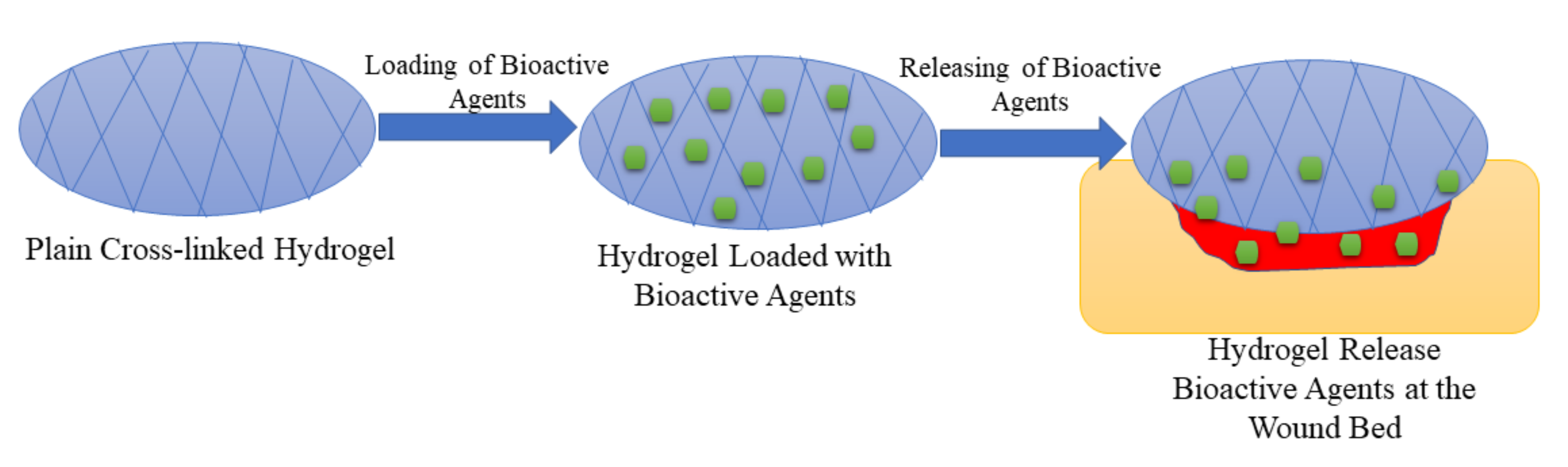
5.3. Hydrogels
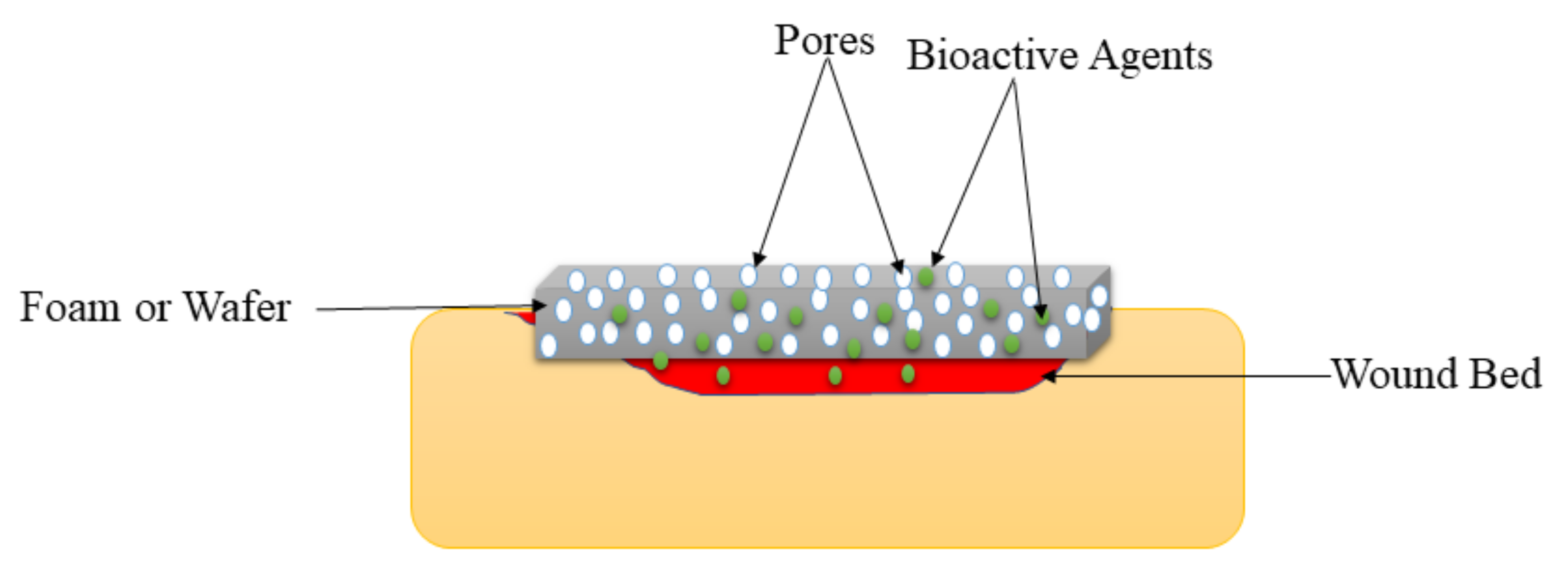
5.4. Foams and Wafers
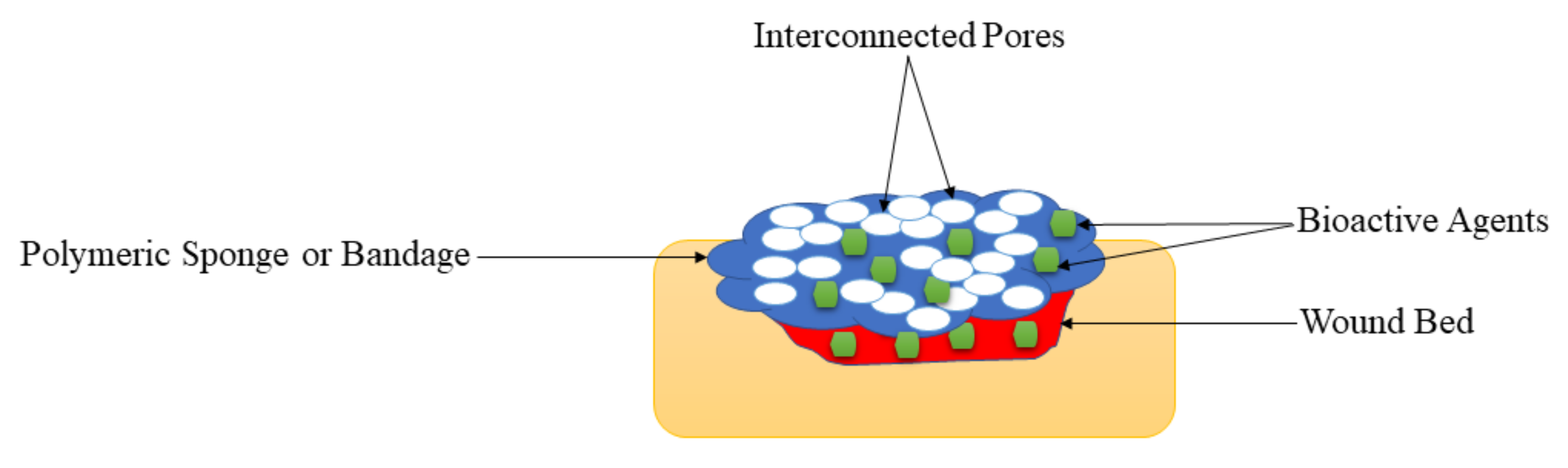
5.5. Sponges and Bandages
6. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kakkar, R.; Madgula, K.; Nehru, Y.V.S.; Kakkar, J. Polyvinyl alcohol-melamine formaldehyde films and coatings with silver nanoparticles as wound dressings in diabetic foot disease. Eur. Chem. Bull. 2015, 4, 98–105. [Google Scholar]
- Liu, Y.; Zhou, S.; Gao, Y.; Zhai, Y. Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer. Asian J. Pharm. Sci. 2019, 14, 130–143. [Google Scholar] [CrossRef]
- Moura, L.I.F.; Dias, A.M.A.; Carvalho, E.; De Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef] [Green Version]
- Venault, A.; Lin, K.H.; Tang, S.H.; Dizon, G.V.; Hsu, C.H.; Maggay, I.V.B.; Chang, Y. Zwitterionic electrospun PVDF fibrous membranes with a well-controlled hydration for diabetic wound recovery. J. Memb. Sci. 2020, 598, 117648. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; De Matas, M.; Sikstone, V.; Hussain, V.; Abbasi, M.; Kousar, M. Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef]
- Majd, S.A.; Khorasgani, M.R.; Moshtaghian, S.J.; Talebi, A.; Khezri, M. Application of Chitosan/PVA Nano fiber as a potential wound dressing for streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2016, 92, 1162–1168. [Google Scholar] [CrossRef]
- Lin, H.; Venault, A.; Chang, Y. Zwitterionized chitosan based soft membranes for diabetic wound healing. J. Memb. Sci. 2019, 591, 117319. [Google Scholar] [CrossRef]
- Akturk, A.; Van Netten, J.J.; Scheer, R.; Vermeer, M.; Van Baal, J.G. Ulcer-free surviv-al days and ulcer healing in patients with diabetic foot ulcers: A prospective cohort study. Int. Wound J. 2019, 16, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; You, Y.; Ma, Y.; Huang, W.; Liang, X.; Zhang, A.; Lin, Y. Bi-layer supramolecular polydimethylsiloxane elastomer film: Synthesis, characterization, and application in wound dressing on normal and diabetic rat. React. Funct. Polym. 2019, 141, 21–32. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Guo, C.; Qin, H.; Yu, X. A promising wound dressing material with excellent cytocompatibility and proangiogenesis action for wound healing: Strontium loaded Silk fibroin/Sodium alginate (SF/SA) blend films. Int. J. Biol. Macromol. 2017, 104, 969–978. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Kahya, N.; Adımcılar, V.; Kaygusuz, H.; Torlak, E.; Akin-Evingue, G.; Erim, F.B. Antibacterial nano cerium oxide/chitosan/cellulose acetate composite fi lms as potential wound dressing. Eur. Polym. J. 2020, 133, 109777. [Google Scholar] [CrossRef]
- Basu, P.; Narendrakumar, U.; Arunachalam, R.; Devi, S.; Manjubala, I. Characterization and Evaluation of Carboxymethyl Cellulose-Based Films for Healing of Full-Thickness Wounds in Normal and Diabetic Rats. ACS Omega 2018, 3, 12622–12632. [Google Scholar] [CrossRef] [Green Version]
- Hussain, Z.; Thu, H.E.; Shuid, A.N.; Katas, H.; Hussain, F. Recent Advances in Polymer-based Wound Dressings for the Treatment of Diabetic Foot Ulcer: An Overview of State-of-the-art. Curr. Drug Targets 2017, 18, 527–550. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Aderibigbe, B.A. Fabrication of Hybrid Nanofibers from Biopolymers and Poly (Vinyl Alcohol)/Poly (ɛ-Caprolactone) for Wound Dressing Applications. Polymers 2021, 13, 2104. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Nan, K.; Li, L.; Zhang, Z.; Chen, H. In vivo evaluation of curcumin nanoformulation loaded methoxy poly ( ethylene glycol ) -graft-chitosan composite film for wound healing application. Carbohydr. Polym. 2012, 88, 84–90. [Google Scholar] [CrossRef]
- Alven, S.; Nqoro, X.; Aderibigbe, B.A. Polymer-Based Materials Loaded with Curcumin for Wound Healing Application. Polymers 2020, 12, 2286. [Google Scholar] [CrossRef]
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.; Lee, B. Curcumin incorporation into an oxidized cellulose nano fi ber-polyvinyl alcohol hydrogel system promotes wound healing. Mater. Des. 2020, 186, 108313. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings, and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Massee, M.; Chinn, K.; Lim, J.J.; Godwin, L.; Young, C.S.; Koob, T.J. Type I and II Diabetic Adipose-Derived Stem Cells Respond In Vitro to Dehydrated Human Amnion/Chorion Membrane Allograft Treatment by Increasing Proliferation, Migration, and Altering Cytokine Secretion. Adv. Wound Care 2015, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.; Srivastava, S.; Rawat, M.; Singh, D. Preparation and optimization of chitosan-gelatin films for sustained delivery of lupeol for wound healing. Int. J. Biol. Macromol. 2018, 107, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Mannino, F.; Vaccaro, M.; Arcoraci, V.; Aliquò, F.; Minutoli, L.; Colonna, M.R.; et al. Activation of the EPOR- β common receptor complex by cibinetide ameliorates impaired wound healing in mice with genetic diabetes. BBA Mol. Basis Dis. 2018, 1864, 632–639. [Google Scholar] [CrossRef]
- Rezvanian, M.; Ahmad, N.; Cairul, M.; Mohd, I.; Ng, S. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef]
- Kim, J.O.; Noh, J.-K.; Thapa, R.K.; Hasan, N.; Choi, M.; Kim, J.H.; Lee, J.-H.; Ku, S.K.; Yoo, J.-W. Nitric oxide-releasing chitosan film for enhanced antibacterial and in vivo wound-healing efficacy. Int. J. Biol. Macromol. 2015, 79, 217–225. [Google Scholar] [CrossRef]
- Galkowska, H.; Wojewodzka, U.; Olszewski, W.L. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair Regen. 2006, 14, 558–565. [Google Scholar] [CrossRef]
- Elshazly, N.; Khalil, A.; Saad, M.; Patruno, M.; Chakraborty, J.; Marei, M. Efficacy of Bioactive Glass Nanofibers Tested for Oral Mucosal Regeneration in Rabbits with Induced Diabeties. Materials 2020, 13, 2603. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the treatment of chronic wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.A.; DiPietro, S.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.A.; Birch, N.P.; Schi, J.D. Designing electrospun nano fiber mats to promote wound healing—A review. J. Mater. Chem. B 2013, 1, 4531–4541. [Google Scholar] [CrossRef] [Green Version]
- Oro, F.B.; Sikka, R.S.; Wolters, B.; Graver, R.; Boyd, J.L.; Nelson, B.; Swiontkowski, M.F. Autograft versus allograft: An economic cost comparison of anterior cruciate ligament reconstruction. Arthroscopy 2011, 27, 219–1225. [Google Scholar] [CrossRef] [PubMed]
- Karri, V.V.S.R.; Kuppusamy, G.; Talluri, S.V.; Yamjala, K.; Mannemala, S.S.; Malayandi, R. Current and emerging therapies in the management of diabetic foot ulcers. Curr. Med. Res. Opin. 2016, 32, 519–542. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.P. Focusing on Metabolomic Dysregulation and Modulation of Retinal Metabolism to Develop Novel Therapeutic Strategies for Diabetic Retinopathy. Ph.D. Thesis, Universidade de Lisboa, Lisabon, Portugal, 2015. [Google Scholar]
- Decker, P.; Muller, S. Modulating poly (ADP-ribose) polymerase activity: Potential for the prevention and therapy of pathogenic situations involving DNA damage and oxidative stress. Curr. Pharm. Biotechnol. 2002, 3, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.C.; Steed, D.L.; Franz, M.G. Wound healing: Biologic features and approaches tomaximize healing trajectories. Curr. Probl. Surg. 2001, 2, 72–140. [Google Scholar] [CrossRef]
- Patel, V.; Chivukula, I.V.; Roy, S.; Khanna, S.; He, G.; Ojha, N.; Mehrotra, A.; Dias, L.M.; Hunt, T.K.; Sen, C.K. Oxygen: From the benefits of inducing VEGF expression to managing the risk of hyperbaric stress. Antioxid. Redox Signal. 2005, 7, 1377–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancement in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Inter. J. Bio. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef]
- Jude, E.B. Transforming growth factor-beta 1, 2, 3 and receptor type I and II in diabetic foot ulcers. Diabetes Med. 2002, 19, 440–447. [Google Scholar] [CrossRef]
- Liang, L.; Stone, R.C.; Stojadinovic, O.; Ramirez, H.; Pastar, I.; Maione, A.G.; Smith, A.; Yanez, V.; Veves, A.; Kirsner, R.S.; et al. Integrative analysis of miRNA and mRNA paired expression profiling of primary fibroblast derived from diabetic foot ulcers reveals multiple impaired cellular functions. Wound Repair Regen. 2016, 24, 943–953. [Google Scholar] [CrossRef]
- Iacob, A.-T.; Dragan, M.; Ionescu, O.-M.; Profire, L.; Ficai, A.; Andronescu, E.; Confedarat, L.G.; Lupascu, D. An Overview of Biopolymeric Electrospun Nanofibers Based on Polysaccharides for Wound Healing Management. Pharmaceutics 2020, 12, 983. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef]
- Dhivya, S.; Vijaya, V.; Santhini, E. Review article Wound dressings—A review. BioMedicine 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Sharma, S.; Dua, A.; Malik, A. Third generation materials for wound dressings. Int. J. Pharm. Sci. Res. 2014, 6, 2113–2124. [Google Scholar]
- Felgueiras, H.P.; Amorim, M.T.P. Functionalization of electrospun polymeric wound dressings with antimicrobial peptides. Coll. Surf. B Biointer. 2017, 156, 133–148. [Google Scholar] [CrossRef]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 1, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nano fibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Shimizu, R.; Kishi, K. Skin Graft. Plastic Surg. Int. 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naseri-nosar, M.; Maria, Z. Wound dressings from naturally-occurring polymers: A review on homopolysaccharide-based composites. Carbohydr. Polym. 2018, 189, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Fahimirad, S.; Ajalloueian, F. Naturally-derived electro- spun wound dressings for target delivery of bioactive agents. Int. J. Pharm. 2019, 566, 307–328. [Google Scholar] [CrossRef]
- Tort, S.; Acartürk, F.; Be, A. Evaluation of three-layered doxycycline-collagen loaded nano fi ber wound dressing. Int. J. Pharm. 2017, 529, 642–653. [Google Scholar] [CrossRef]
- Hajilou, H.; Farahpour, M.R.; Hamishehkar, H. Polycaprolactone nano fi ber coated with chitosan and Gamma oryzanol functionalized as a novel wound dressing for healing infected wounds. Int. J. Biol. Macromol. 2020, 164, 2358–2369. [Google Scholar] [CrossRef]
- Mulholland, E.J.; Ali, A.; Robson, T.; Dunne, N.J.; Mccarthy, H.O. Delivery of RALA/siFKBPL nanoparticles via electrospun bilayer nano fi bres: An innovative angiogenic therapy for wound repair. J. Control. Rel. 2019, 16, 53–65. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, Y.; Wang, H.; Chen, Y.; Jin, S.; Chen, S. Preparation of Nanofibers with Renewable Polymers and Their Application in Wound Dressing. Int. J. Polym. Sci. 2016, 2016, 17. [Google Scholar] [CrossRef] [Green Version]
- Chao, F.C.; Wu, M.H.; Chen, L.C.; Lin, H.L.; Liu, D.Z.; Ho, H.O.; Sheu, M.T. Preparation and characterization of chemically TEMPO-oxidized and mechanically disintegrated sacchachitin nanofibers (SCNF) for enhanced diabetic wound healing. Carbohydr. Polym. 2020, 229, 115507. [Google Scholar] [CrossRef]
- Liu, M.; Duan, X.P.; Li, Y.M.; Yang, D.P.; Long, Y.Z. Electrospun nanofibers for wound healing. Mater. Sci. Eng. C 2017, 76, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Heydari, P.; Varshosaz, J.; Kharazi, A.; Karbasi, S. Preparation and evaluation of poly glycerol sebacate/poly hydroxy butyrate core—Shell electrospun nanofibers with sequentially release of ciprofloxacin and simvastatin in wound dressings. Polym. Advaced Technol. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Cam, M.E.; Crabbe-Mann, M.; Alenezi, H.; Hazar-Yavuz, A.N.; Ertas, B.; Ekentok, C.; Ozcan, G.S.; Topal, F.; Guler, E.; Yazir, Y.; et al. The comparision of glybenclamide and metformin-loaded bacterial cellulose/gelatin nano fi bres produced by a portable electrohydrodynamic gun for diabetic wound healing. Eur. Polym. J. 2020, 134, 109844. [Google Scholar] [CrossRef]
- Choi, J.S.; Leong, K.W.; Yoo, H.S. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials 2008, 29, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Cam, M.E.; Crabbe-Mann, M.; Alenezi, H.; Hazar-Yavuz, A.N.; Ertas, B.; Ekentok, C.; Ozcan, G.S.; Topal, F.; Guler, E.; Yazir, Y.; et al. Polylactide nanofibers delivering doxycycline for chronic wound treatment. Mater. Sci. Eng. C 2019, 104, 109745. [Google Scholar]
- Ranjbar-mohammadi, M.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.T.; Moayer, F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly (ε -caprolactone ) electrospun nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Almasian, A.; Naja, F.; Eftekhari, M.; Ardekani, M.R.S.; Sharifzadeh, M.; Khanavi, M. Polyurethane/carboxymethylcellulose nanofibers containing Malva sylvestris extract for healing diabetic wounds: Preparation, characterization, in vitro and in vivo studies. Mater. Sci. Eng. C 2020, 114, 111039. [Google Scholar] [CrossRef] [PubMed]
- Grip, J.; Engstad, R.E.; Skjæveland, I.; Škalko-Basnet, N.; Isaksson, J.; Basnet, P.; Holsæter, A.M. Beta-glucan-loaded nanofiber dressing improves wound healing in diabetic mice. Eur. J. Pharm. Sci. 2018, 121, 269–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Zhou, W.; Zha, K.; Liu, G.; Yang, S.; Ye, S.; Liu, Y.; Xiong, Y.; Wu, Y.; Cao, F. Treatment of chronic ulcer in diabetic rats with self assembling nanofiber gel encapsulated-polydeoxyribonucleotide. Am. J. Transl. Res 2016, 8, 3067–3076. [Google Scholar] [PubMed]
- Kanj, S.; Manjusri, D.; Jo, M.; Agga, R.; Sudarshana, M.S. Nanofiber-expanded human CD34+ cells heal cutaneous wounds in streptozotocin-induced diabetic mice. Sci. Rep. 2019, 9, 8415. [Google Scholar] [CrossRef] [Green Version]
- Pinzón-García, A.D.; Cassini-Vieira, P.; Ribeiro, C.C.; De Matos Jensen, C.E.; Barcelos, L.S.; Cortes, M.E.; Sinisterra, R.D. Efficient cutaneous wound healing using bixin-loaded PCL nanofibers in diabetic mice. J. Biomed. Mater. Res. Part B 2017, 105, 1938–1949. [Google Scholar] [CrossRef]
- Lee, C.H.; Liu, K.S.; Cheng, C.W.; Chan, E.C.; Hung, K.C.; Hsieh, M.J.; Chang, S.H.; Fu, X.; Juang, J.H.; Hsieh, I.C.; et al. Co-delivery of sustainable anti-microbial agents and platelet-derived growth factor via biodegradable nanofibers for repair of diabetic infectious wounds. ACS Infect. Dis. 2020, 6, 2688–2697. [Google Scholar] [CrossRef] [PubMed]
- Zehra, M.; Zubairi, W.; Hasan, A.; Butt, H.; Ramzan, A.; Azam, M.; Mehmood, A.; Falahati, M.; Chaudhry, A.A.; Rehman, I.U.; et al. Oxygen Generating Polymeric Nano Fibers That Stimulate Angiogenesis and Show Ef fi cient Wound Healing in a Diabetic Wound Model. Int. J. Nanomed. 2020, 15, 3511–3522. [Google Scholar] [CrossRef] [PubMed]
- Fhalaby, T.; Fekry, N.; Sodfy, A.; Sheredy, A.; Moustafa, M. Preparation and characterisation of antibacterial silver-containing nanofibres for wound healing in diabetic mice. Int. J. Nano. 2015, 8, 82–98. [Google Scholar]
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.L.; Nair, S. Curcumin loaded poly (ε-caprolactone) nanofibers: Diabetic wound dressing with antioxidant and anti-inflammatory properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149–1156. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Hung, K.C.; Hsieh, M.J.; Chang, S.H.; Juang, J.H.; Hsieh, I.C.; Wen, M.S.; Liu, S.J. Core-shell insulin-loaded nanofibrous scaffolds for repairing diabetic wounds. Nanomed. Nanotech. Biol. Med. 2020, 24, 102123. [Google Scholar] [CrossRef]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Khanam, P.N.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nano fi brous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef]
- Cam, M.E.; Yildiz, S.; Alenezi, H.; Cesur, S.; Ozcan, G.S.; Erdemir, G.; Edirisinghe, U.; Akakin, D.; Kuruca, D.S.; Kabasakal, L.; et al. Evaluation of burst release and sustained release of pioglitazone-loaded fibrous mats on diabetic wound healing: An in vitro and in vivo comparison study. J. Royal Soc. Inter. 2020, 17, 20190712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, B.; Agarwal, R.; Alam, M. Textile-based smart wound dressings. Indian J. Fibre Text. Res. 2010, 35, 174–184. [Google Scholar]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound dressings and comparative effectiveness data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, W.S.; Arulselvan, P.; Ng, S.; Norma, C.; Taib, M.; Sarian, M.N. Improvement of diabetic wound healing by topical application of Vicenin-2 hydrocolloid film on Sprague Dawley rats. BMC Complement. Altern. Med. 2019, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Colobatiu, L.; Gavan, A.; Potarniche, A.V.; Rus, V.; Diaconeasa, Z.; Mocan, A.; Tomuta, I.; Mirel, S.; Mihaiu, M. Evaluation of bioactive compounds-loaded chitosan fi lms as a novel and potential diabetic wound dressing material. React. Funct. Polym. 2019, 145, 104369. [Google Scholar] [CrossRef]
- Mizuno, K.; Yamamura, K.; Yano, K.; Osada, T.; Saeki, S.; Takimoto, N.; Sakurai, T.; Nimura, Y. Effect of chitosan film containing basic fibroblast growth factor on wound healing in genetically diabetic mice. J. Biomed. Mater. Res. Part A 2003, 64, 177–181. [Google Scholar] [CrossRef]
- Voss, G.T.; Gularte, M.S.; Vogt, A.G.; Giongo, J.L.; Vaucher, R.A.; Echenique, J.V.; Soares, M.P.; Luchese, C.; Wilhelm, E.A.; Fajardo, A.R. Polysaccharide-based fi lm loaded with vitamin C and propolis: A promising device to accelerate diabetic wound healing. Int. J. Pharm. 2018, 52, 340–351. [Google Scholar] [CrossRef]
- Inpanya, P.; Faikrua, A.; Ounaroon, A.; Sittichokechaiwut, A.; Viyoch, J. Effects of the blended fibroin/aloe gel film on wound healing in streptozotocin-induced diabetic rats. Biomed. Mater. 2012, 7, 035008. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Jiao, Y.P.; Xiao, L.L.; Li, M.M.; Liu, H.W.; Li, S.H.; Liao, X.; Chen, Y.T.; Li, J.X.; Zhang, Y. Experimental Study on Effects of Adipose-Derived Stem Cell—Seeded Silk Fibroin Chitosan Film on Wound Healing of a Diabetic Rat Model. Ann. Plast. Surg. 2018, 80, 572–580. [Google Scholar] [CrossRef] [Green Version]
- Tong, W.Y.; Bin Abdullah, A.Y.K.; Binti Rozman, N.A.S.; Bin Wahid, M.I.A.; Hossain, M.S.; Ring, L.C.; Lazim, Y.; Tan, W.N. Antimicrobial wound dressing film utilizing cellulose nanocrystal as drug delivery system for curcumin. Cellulose 2018, 25, 631–638. [Google Scholar] [CrossRef]
- Arantes, V.T.; Faraco, A.A.; Ferreira, F.B.; Oliveira, C.A.; Martins-Santos, E.; Cassini-Vieira, P.; Barcelos, L.S.; Ferreira, L.A.; Goulart, G.A. Retinoic acid-loaded solid lipid nanoparticles surrounded by chitosan fi lm support diabetic wound healing in in vivo study. Coll. Surf.B Biointer. 2020, 188, 110749. [Google Scholar] [CrossRef] [PubMed]
- Arul, V.; Kartha, R.; Jayakumar, R. A therapeutic approach for diabetic wound healing using biotinylated GHK incorporated collagen matrices. Life Sci. 2007, 80, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kim, K.S.; Seo, Y.G.; Lee, B.J.; Park, Y.J.; Youn, Y.S.; Kim, J.O.; Yong, C.S.; Jin, S.G.; Choi, H.G. Novel sodium fusidate-loaded fi lm-forming hydrogel with easy application and excellent wound healing. Int. J. Pharm. 2015, 495, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Song, S.H.; Kim, J.E.; Koh, E.K.; Sung, J.E.; Lee, H.A.; Yun, W.B.; Hong, J.T.; Hwang, D.Y. Selenium-loaded cellulose film derived from Styela clava tunic accelerates the healing process of cutaneous wounds in streptozotocin-induced diabetic Sprague–Dawley rats. J. Dermatolog. Treat. 2018, 29, 606–616. [Google Scholar] [CrossRef]
- Benskin, L.L. Evidence for Polymeric Membrane Dressings as a Unique Dressing Subcategory, Using Pressure Ulcers as an Example. Adv. Wound Care 2018, 7, 419–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustine, R.; Hasan, A.; Patan, N.K.; Dalvi, Y.B.; Varghese, R.; Antony, A.; Unni, R.N.; Sandhyarani, N.; Moustafa, A.E.A. Cerium Oxide Nanoparticle Incorporated Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Membranes for Diabetic Wound Healing Applications. ACS Biomater. Sci. Eng. 2019, 6, 58–70. [Google Scholar] [CrossRef]
- Augustine, R.; Zahid, A.A.; Hasan, A.; Wang, M.; Webster, T.J. CTGF loaded electrospun dual porous core-shell membrane for diabetic wound healing. Int. J. Nanomed. 2019, 14, 8573–8588. [Google Scholar] [CrossRef] [Green Version]
- Lobmann, R.; Pittasch, D.; Mühlen, I.; Lehnert, H. Autologous human keratinocytes cultured on membranes composed of benzyl ester of hyaluronic acid for grafting in nonhealing diabetic foot lesions: A pilot study. J. Diabetes Compl. 2003, 17, 199–204. [Google Scholar] [CrossRef]
- Lee, C.H.; Chang, S.H.; Chen, W.J.; Hung, K.C.; Lin, Y.H.; Liu, S.J.; Hsieh, M.J.; Pang, J.H.S.; Juang, J.H. Augmentation of diabetic wound healing and enhancement of collagen content using nanofibrous glucophage-loaded collagen/PLGA scaffold membranes. J. Coll. Inter.Sci. 2015, 439, 88–97. [Google Scholar] [CrossRef]
- Lee, C.H.; Hsieh, M.J.; Chang, S.H.; Lin, Y.H.; Liu, S.J.; Lin, T.Y.; Hung, K.C.; Pang, J.H.S.; Juang, J.H. Enhancement of diabetic wound repair using biodegradable nanofibrous metformin-eluting membranes: In vitro and in vivo. ACS Appl. Mater. Inter. 2014, 6, 3979–3986. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Han, Y.; Wang, J.; Jiang, Y.; Yi, Z.; Xu, H.; Ke, Q. An aligned porous electrospun fibrous membrane with controlled drug delivery—An efficient strategy to accelerate diabetic wound healing with improved angiogenesis. Acta Biomater. 2018, 70, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, X.; Wang, L.; Yan, X.; Ma, D.; Liu, Z.; Liu, X. Sesamol incorporated cellulose acetate-zein composite nano fi ber membrane: An ef fi cient strategy to accelerate diabetic wound healing. Int. J. Biol. Macromol. 2020, 149, 627–638. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, Y.; Huang, W.; Mo, Y.; Lan, Y.; Guo, R.; Cheng, B. Neurotensin-loaded PLGA/CNC composite nanofiber membranes accelerate diabetic wound healing. Artif. Cells Nanomed. Biotechnol. 2018, 46, S493–S501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francesko, A.; Petkova, P.; Tzanov, T. Hydrogel dressings for advanced wound management. Curr. Med. Chem. 2018, 25, 5782–5797. [Google Scholar] [CrossRef]
- Graça, M.F.P.; Miguela, S.P.; Cabrala, C.S.D.; Correia, I.J. Hyaluronic acid—Based wound dressings. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef]
- Sun, G.; Shen, Y.I.; Harmon, J.W. Engineering pro-regenerative hydrogels for scar-less wound healing. Adv. Health Mater. 2018, 7, 1800016. [Google Scholar] [CrossRef]
- Weller, C.; Weller, C.; Team, V. Interactive dressings and their role in moist wound management. Adv. Text. Wound Care 2019, 105–134. [Google Scholar]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef]
- Xu, Q.; Sigen, A.; Gao, Y.; Guo, L.; Creagh-Flynn, J.; Zhou, D.; Greiser, U.; Dong, Y.; Wang, F.; Tai, H.; et al. A hybrid injectable hydrogel from hyperbranched PEG macromer as a stem cell delivery and retention platform for diabetic wound healing. Acta Biomater. 2018, 75, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Niu, L.; Liang, H.; Tan, H.; Liu, C.; Zhu, F. pH and Glucose Dual-Responsive Injectable Hydrogels with Insulin and Fibroblasts as Bioactive Dressings for Diabetic Wound Healing. ACS Appl. Mater. Interf. 2017, 9, 37563–37574. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.P.; Santos, T.C.; Rodrigues, D.B.; Pirraco, R.P.; Cerqueira, M.T.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Stem Cell-Containing Hyaluronic Acid-Based Spongy Hydrogels for Integrated Diabetic Wound Healing. J. Investig. Dermatol. 2017, 137, 1541–1551. [Google Scholar] [CrossRef] [Green Version]
- Yoon, D.S.; Lee, Y.; Ryu, H.A.; Jang, Y.; Lee, K.M.; Choi, Y.; Choi, W.J.; Lee, M.; Park, K.M.; Park, K.D.; et al. Cell recruiting chemokine-loaded sprayable gelatin hydrogel dressings for diabetic wound healing. Acta Biomater. 2016, 38, 59–68. [Google Scholar] [CrossRef]
- Lai, J.C.Y.; Lai, H.Y.; Rao, N.K.; Ng, S.F. Treatment for diabetic ulcer wounds using a fern tannin optimized hydrogel formulation with antibacterial and antioxidative properties. J. Ethnopharmacol. 2016, 189, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Kaisang, L.; Siyu, W.; Lijun, F.; Daoyan, P.; Xian, C.J.; Jie, S. Adipose-derived stem cells seeded in Pluronic F-127 hydrogel promotes diabetic wound healing. J. Surg. Res. 2017, 217, 63–74. [Google Scholar] [CrossRef]
- Moon, K.C.; Suh, H.S.; Kim, K.B.; Han, S.K.; Young, K.W.; Lee, J.W.; Kim, M.H. Potential of allogeneic adipose-derived stem cell–hydrogel complex for treating diabetic foot ulcers. Diabetes 2019, 68, 837–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Ke, Q.F.; Tao, S.C.; Guo, S.C.; Rui, B.Y.; Guo, Y.P. Fabrication of hydroxyapatite/chitosan composite hydrogels loaded with exosomes derived from miR-126-3p overexpressed synovial mesenchymal stem cells for diabetic chronic wound healing. J. Mater. Chem. B 2016, 4, 6830–6841. [Google Scholar] [CrossRef]
- Zhu, Y.; Hoshi, R.; Chen, S.; Yi, J.; Duan, C.; Galiano, R.D.; Zhang, H.F.; Ameer, G.A. Sustained release of stromal cell derived factor-1 from an antioxidant thermoresponsive hydrogel enhances dermal wound healing in diabetes. J. Control. Rel. 2016, 238, 114–122. [Google Scholar] [CrossRef]
- Veerasubramanian, P.K.; Thangavel, P.; Kannan, R.; Chakraborty, S.; Ramachandran, B.; Suguna, L.; Muthuvijayan, V. An investigation of konjac glucomannan-keratin hydrogel scaffold loaded with Avena sativa extracts for diabetic wound healing. Coll. Surf. B Biointerf. 2018, 165, 92–102. [Google Scholar] [CrossRef]
- Thangavel, P.; Ramachandran, B.; Chakraborty, S.; Kannan, R.; Lonchin, S.; Muthuvijayan, V. Accelerated Healing of Diabetic Wounds Treated with L-Glutamic acid Loaded Hydrogels Through Enhanced Collagen Deposition and Angiogenesis: An in Vivo Study. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Wang, J.; Li, R.; Li, T.; Chang, M.; Yan, F.; Wang, Y. Encapsulation of Curcumin Nanoparticles with MMP9-Responsive and Thermos-Sensitive Hydrogel Improves Diabetic Wound Healing. ACS Appl. Mater. Interf. 2018, 10, 16315–16326. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Chen, S.; Yi, J.; Zhang, H.F.; Ameer, G.A. A Cooperative Copper Metal–Organic Framework-Hydrogel System Improves Wound Healing in Diabetes. Adv. Funct. Mater. 2017, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bohl Masters, K.S.; Leibovich, S.J.; Belem, P.; West, J.L.; Poole-Warren, L.A. Effects of nitric oxide releasing poly(vinyl alcohol) hydrogel dressings on dermal wound healing in diabetic mice. Wound Repair Regen. 2002, 10, 286–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokatlian, T.; Cam, C.; Segura, T.; Angeles, L. Porous Hyaluronic Acid Hydrogels for Localized Non-Viral DNA Delivery. Adv Health Mater. 2016, 4, 1084–1091. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Ma, Y.; Pan, X.; Chen, S.; Zhuang, H.; Wang, S. A composite hydrogel of chitosan/heparin/poly (γ-glutamic acid) loaded with superoxide dismutase for wound healing. Carbohydr. Poly. 2018, 180, 168–174. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Rao, Z.-F.; Liu, Y.-J.; Liu, X.-S.; Liu, Y.-F.; Xu, L.-X.; Wang, Z.-Q.; Guo, J.-Y.; Zhang, L.; Dong, Y.-S.; et al. Multifunctional Injectable Hydrogel Loaded with Cerium-Containing Bioactive Glass Nanoparticles for Diabetic. Wound Healing. Biomolecules 2021, 11, 702. [Google Scholar] [CrossRef]
- Shi, G.; Chen, W.; Zhang, Y.; Dai, X.; Zhang, X.; Wu, Z. An Antifouling Hydrogel Containing Silver Nanoparticles for Modulating the Therapeutic Immune Response in Chronic Wound Healing. Langmuir 2019, 35, 1837–1845. [Google Scholar] [CrossRef]
- Morgan, D. Wounds- what should a dressing formulary include? Hosp. Pharm. 2002, 9, 216–261. [Google Scholar]
- Ramos-e-Silva, M.; Ribeiro de Castro, M.C. New dressings, including tissue-enginnered living skin. Clin. Dermatol. 2002, 6, 715–723. [Google Scholar]
- Pyun, D.G.; Choi, H.J.; Yoon, H.S.; Thambi, T.; Lee, D.S. Polyurethane foam containing rhEGF as a dressing material for healing diabetic wounds: Synthesis, characterization, in vitro and in vivo studies. Collo. Surf. B Biointerf. 2015, 135, 699–706. [Google Scholar] [CrossRef]
- Coutts, P.M.; Ryan, J.; Sibbald, R.G. Case series of lower-extremity chronic wounds managed with an antibacterial foam dressing bound with gentian violet and methylene blue. Adv. Ski. Wound Care 2014, 27, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.; Choi, R.; Han, S. Influence of Silver-Containing Dressings on Epithelialization of Wounds in Diabetic Patients. J. Korean Wound Manag. Soc. 2012, 8, 61–67. [Google Scholar]
- Choi, H.J.; Thambi, T.; Yang, Y.H.; Bang, S.I.; Kim, B.S.; Pyun, D.G.; Lee, D.S. AgNP and rhEGF-incorporating synergistic polyurethane foam as a dressing material for scar-free healing of diabetic wounds. RSC Adv. 2017, 7, 13714. [Google Scholar] [CrossRef] [Green Version]
- Günal, Ö.; Tuncel, U.; Turan, A.; Barut, S.; Kostakoglu, N. The Use of Vacuum-Assisted Closure and GranuFoam Silver® Dressing in the Management of Diabetic Foot Ulcer. Surg. Infect. 2015, 16, 558–565. [Google Scholar] [CrossRef]
- Bai, M.-Y.; Chen, M.-C.; Yu, W.-C.; Lin, J.-Y. Foam dressing incorporating herbal extract: An all-natural dressing for potential use in wound healing. J. Bioact. Compat. Polym. 2017, 32, 293–308. [Google Scholar] [CrossRef]
- Moura, L.I.F.; Dias, A.M.A.; Leal, E.C.; Carvalho, L.; De Sousa, H.C.; Carvalho, E. Chitosan-based dressings loaded with neurotensin—An efficient strategy to improve early diabetic wound healing. Acta Biomater. 2014, 10, 843–857. [Google Scholar] [CrossRef] [Green Version]
- Tong, J. Case reports on the use of antimicrobial (silver impregnated) soft silicone foam dressing on infected diabetic foot ulcers. Tools 2009, 6, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Hoey, C. Topical antimicrobial therapy for treating chronic wounds. Clin. Infect. Dis. 2009, 49, 1541–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, K.H.; Stevens, H.N.E.; Auffret, A.D.; Humphrey, M.J.; Eccleston, G.M. Formulation, stabulity and thermal analysis of lyophilised wound healing wafers containing an insoluble MMP-3 inhibitor and non-ionic surfactant. Int. J. Pharm. 2008, 356, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Getti, G.; Boateng, J. Ciprofloxacin-loaded calcium alginate wafers prepared by freeze-drying technique for potential healing of chronic diabetic foot ulcers. Drug Deliv. Transl. Res. 2018, 8, 1751–1768. [Google Scholar] [CrossRef] [PubMed]
- Gadad, P.C.; Matthews, K.H.; Knott, R.M. Silymarin released from sterile wafers restores glucose impaired endothelial cell migration. Int. J. Pharm. 2013, 457, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Atia, N.M.; Hazzah, H.A.; Gaafar, P.M.E.; Abdallah, O.Y. Diosmin Nanocrystal–Loaded Wafers for Treatment of Diabetic Ulcer: In Vitro and In Vivo Evaluation. J. Pharm. Sci. 2019, 108, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, W.; Xi, G.; Wang, M.; Liang, B.; Shi, Y.; Feng, Y.; Ren, X.; Shi, C. Fabricating antimicrobial peptideimmobilized starch sponges for hemorrhage control and antibacterial treatment. Carbohydr. Polym. 2019, 222, 115012. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Zhang, Q.; Yan, S.; Guo, Y.; Li, M.; You, R. Mechanically robust and flexible silk protein/polysaccharide composite sponges for wound dressing. Carbohydr. Polym. 2019, 216, 17–24. [Google Scholar] [CrossRef]
- Chen, C.; Liu, L.; Huang, T.; Wang, Q.; Fang, Y. Bubble template fabrication of chitosan/poly (vinyl alcohol) sponges for wound dressing applications. Int. J. Biol. Macromol. 2013, 62, 188–193. [Google Scholar] [CrossRef]
- Wang, W.; Lin, S.; Xiao, Y.; Huang, Y.; Tan, Y.; Cai, L.; Li, X. Acceleration of diabetic wound healing with chitosan-crosslinked collagen sponge containing recombinant human acidic fibroblast growth factor in healing-impaired STZ diabetic rats. Life. Sci. 2008, 82, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Anisha, B.S.; Biswas, R.; Chennazhi, K.P.; Jayakumar, R. Chitosan–hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic wounds. Int. J. Biol. Macromol. 2013, 62, 310–320. [Google Scholar] [CrossRef]
- Xia, G.; Zhai, D.; Sun, Y.; Hou, L.; Guo, X.; Wang, L.; Li, Z.; Wang, F. Preparation of a novel asymmetric wettable chitosan-based sponge and its role in promoting chronic wound healing. Carbohydr. Polym. 2020, 27, 115296. [Google Scholar] [CrossRef]
- Mohandas, A.; Anisha, B.S.; Chennazhi, K.P.; Jayakumar, R. Chitosan–hyaluronic acid/VEGF loaded fibrin nanoparticles composite sponges for enhancing angiogenesis in wounds. Coll. Surf. B Biointerf. 2015, 127, 105–113. [Google Scholar] [CrossRef]
- Kondo, S.; Niiyama, H.; Yu, A.; Kuroyanagi, Y. Evaluation of a wound dressing composed of hyaluronic acid and collagen sponge containing epidermal growth factor in diabetic mice. J. Biomater. Sci. Polym. 2012, 23, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Kuss, M.; Edmonds, M.; Reyzelman, A.; Sigal, F. Topical application of a gentamicin-collagen sponge combined with systemic antibiotic therapy for the treatment of diabetic foot infections of moderate severity: A randomized, controlled, multicenter clinical trial. J. Am. Pod. Med. Assoc. 2012, 102, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Qian, Z.; Liu, D.; Sun, J.; Wang, X.; Liu, H.; Xu, J.; Guo, X. GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front. Physiol. 2017, 8, 904. [Google Scholar] [CrossRef] [PubMed]
- Momin, M.; Kurhade, S.; Khanekar, P.; Mhatre, S. Novel biodegradable hydrogel sponge containing curcumin and honey for wound healing. J. Wound Care 2016, 25, 364–372. [Google Scholar] [CrossRef]
- Ti, D.; Hao, H.; Xia, L.; Tong, C.; Liu, J.; Dong, L.; Xu, S.; Zhao, Y.; Liu, H.; Fu, X.; et al. Controlled release of thymosin beta 4 using a collagen–chitosan sponge scaffold augments cutaneous wound healing and increases angiogenesis in diabetic rats with hindlimb ischemia. Tissue. Eng. Part 2 2015, 21, 541–549. [Google Scholar] [CrossRef]
- Kumar, P.T.S.; Lakshmanan, V.K.; Anilkumar, T.V.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.G.; Nair, S.V.; Jayakumar, R. Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interf. 2012, 4, 2618–2629. [Google Scholar] [CrossRef]
- Mohanty, C.; Pradhan, J. A human epidermal growth factor-curcumin bandage bioconjugate loaded with mesenchymal stem cell for in vivo diabetic wound healing. Mater. Sci. Eng. 2020, 111, 110751. [Google Scholar] [CrossRef]
- Kumar, P.T.; Lakshmanan, V.K.; Biswas, R.; Nair, S.V.; Jayakumar, R. Synthesis and biological evaluation of chitin hydrogel/nano ZnO composite bandage as antibacterial wound dressing. J. Biomed. Nanotechnol. 2012, 8, 891–900. [Google Scholar] [CrossRef]
- Raveendran, N.T.; Mohandas, A.; Menon, R.R.; Menon, A.S.; Biswas, R.; Jayakumar, R. Ciprofloxacin-and Fluconazole-Containing Fibrin-Nanoparticle-Incorporated Chitosan Bandages for the Treatment of Polymicrobial Wound Infections. ACS Appl. Bio Mater. 2018, 2, 243–254. [Google Scholar] [CrossRef]


| Types of Wound Dressing | Used Polymers | Loaded Bioactive Agents | Results | Ref |
|---|---|---|---|---|
| Nanofiber | Gelatin and cellulose | Glybenclamide and metformin | Accelerated wound healing process and good biocompatibility | [57] |
| Nanofiber | PEG and PCL | EGF | Superior wound healing process | [58] |
| Nanofiber | Polylactide | Doxycycline | Excellent mechanical performance, antibacterial effects, and excellent diabetic wound healing properties | [59] |
| Nanofiber | PCL and gum tragacanth | Curcumin | Bead-free morphology and full wound closure on day 15. | [60] |
| Nanofiber | PU and carboxymethylcellulose | Malva sylvestris plant extract | Good diabetic wound healing rate | [61] |
| Nanofiber | Hydroxypropyl methylcellulose and PEO | beta-glucan | Non-toxic and accelerated wound closure. | [62] |
| Nanofiber | poly-N-acetyl glucosamine | polydeoxyribonucleotide | High rate of cell proliferation and angiogenesis. | [63] |
| Nanofiber | polyethersulfone | henceforth CD34+ cells | The fast diabetic wound healing process | [64] |
| Nanofiber | PCL | Bixin | Sustained drug release and accelerated wound healing. | [65] |
| Nanofiber | PLGA | PDGF, vancomycin, and gentamicin | Sustained drug release and accelerated wound healing. | [66] |
| Nanofiber | PCL | Sodium percarbonate | Superior vascularization. | [67] |
| Nanofiber | Cellulose acetate | Ag nanoparticles | High antibacterial efficacy and accelerated diabetic wound contraction. | [68] |
| Nanofiber | PCL | Curcumin | Excellent biocompatibility and increased rate of wound reduction | [69] |
| Nanofiber | PLGA | Insulin | Good mechanical performance and prolong drug release | [70] |
| Nanofibers | Chitosan and PVA | ZnO | Excellent antibacterial effects and accelerated diabetic wounds | [71] |
| Nanofibers | PVP and PCL | Pioglitazone | Non-toxicity and sustained drug release. | [72] |
| Film | Sodium alginate | Vicenin-2 | Faster diabetic wound recovery | [76] |
| Film | Chitosan | Alcoholic extracts | Excellent biocompatibility | [77] |
| Film | Chitosan | Fibroblast growth factors | High diabetic wound contraction rate | [78] |
| Film | Cellulose and PVA | Propolis and vitamin C | High swelling rate, controlled drug release, and accelerated diabetic wound healing | [79] |
| Film | Fibroin | Aloe gel | Excellent mechanical properties and fibroblast distribution and collagen fiber organization. | [80] |
| Film | Fibroin and chitosan | ADSCs | Good diabetic wound closure. | [81] |
| Film | PVA and cellulose | Curcumin | Good antibacterial effects and significantly diabetic wound closure. | [82] |
| Film | Chitosan | Retinoic acid | Increased wound reduction rate. | [83] |
| Film | Collagen | Biotinylated GHK peptide | Accelerated wound healing | [84] |
| Film | PVP and PVA | Sodium fusidate | Excellent mechanical performance | [85] |
| Film | Cellulose | Selenium | Fast diabetic wound healing rate | [86] |
| Membrane | PHBV | Cerium Oxide nanoparticles | Significant enhancement in cell infiltration and granulation tissue formation | [88] |
| Membrane | PVA and PLA | GFs | Excellent cell migration and proliferation | [89] |
| Membrane | HA | Human keratinocytes | The good clinical wound healing process | [90] |
| Membrane | PLGA and collagen | Glucophage | The faster wound healing process | [91] |
| Membrane | PLGA | Metformin | Enhanced the wound healing and re-epithelialization in diabetic rats | [92] |
| Membrane | PLLA | Dimethyloxalylglycine | Burst drug released followed by sustained drug release. | [93] |
| Membrane | Cellulose acetate | Sesamol | Improved diabetic wound healing | [94] |
| Membrane | PLGA and cellulose | Neurotensin | Sustained drug release and faster wound healing process | [95] |
| Hydrogel | Poly-ε-L-lysine, HA, and pluronic | Adipose mesenchymal stem cells | Increased diabetic wound rate | [100] |
| Hydrogel | HA and PEG | Stem cell | Good mechanical properties and faster diabetic wound healing. | [101] |
| Hydrogel | PEG and PVA | Fibroblasts and insulin | Accelerated wound repair | [102] |
| Hydrogel | HA | Human adipose stem cells | Improved wound closure rate | [103] |
| Hydrogel | Gelatin | Chemotactic cytokines | Accelerated wound healing | [104] |
| Hydrogel | Sodium carboxymethylcellulose | B. orientale | Fast wound recovery | [105] |
| Hydrogel | Pluronic F-127 | ADSCs | Accelerated wound healing | [106] |
| Hydrogel | PU | AASCs | Fast diabetic wound | [107] |
| Hydrogel | Chitosan | Exosomes | Accelerate angiogenesis and wound surface re-epithelialization | [108] |
| Hydrogel | PPCN | SDF-1 | Improved epithelial maturation and granulation tissue production | [109] |
| Hydrogel | Konjac glucomannan | Avena sativa | Support collagen expression, keratinocyte migration, fibroblast attachment, and proliferation | [110] |
| Hydrogel | Chitosan | L-glutamic acid | Promotes collagen deposition and accelerates vascularization | [111] |
| Hydrogel | Gelatin | Curcumin | Good cell migration | [112] |
| Hydrogel | Chitosan and PEG | Ag nanoparticles | Controlled drug release and diabetic wound stimulation. | [113] |
| Hydrogel | poly-(polyethyleneglycol citrate-co-N-isopropylacrylamide) | Copper metal-organic framework | Enhanced dermal cell migration and improved wound closure rates | [114] |
| Hydrogel | PVA | Nitric Oxide | Enhance diabetic wound healing | [115] |
| Hydrogel | HA | DNA | Enhanced development of granulation tissue | [116] |
| Hydrogel | poly (γ-glutamic acid) and chitosan | Superoxide dismutase | Good cytocompatibility and accelerated wound healing process | [117] |
| Hydrogel | Gelatin | Cerium-containing bioactive glass nanoparticles | Good antibacterial effects | [118] |
| Hydrogel | chitosan-dextran | Ag nanoparticles | Broad-spectrum and long-lasting antibacterial activity | [119] |
| Foam | PU | RhEGF | Moderate WVTR and good biocompatibility | [122] |
| Foam | PVA | Gentian violet and methylene blue | High wound reduction rate | [123] |
| Foam | PU | Ag nanoparticle | Fast wound healing rate | [124] |
| Foam | PU | Ag nanoparticle | Good antibacterial efficacy | [125] |
| Foam | PU | Ag | Good diabetic wound closure | [126] |
| Foam | Silk fibroin | Gastrodia elata and tea tree oil | High porosity and excellent biocompatibility | [127] |
| Foam | Chitosan | Neurotensin | High wound healing reduction | [128] |
| Foam | Silicone | Silver | Positive diabetic wound closure and reduction in size | [129] |
| Wafer | Calcium alginate | Ciprofloxacin | High porosity and burst drug release followed the sustained release with good antibacterial efficacy | [132] |
| Wafer | Xanthan gum | Silymarin | Good cell migration | [133] |
| Wafer | Sodium alginate and gelatin | Diosmin nanocrystals | Sustained drug release and well-developed granulation tissue, well-organized dermal layers, complete re-epithelialization, and mature collagen bundles in diabetic wounds. | [134] |
| Sponges | Chitosan and collagen | Recombinant human acidic fibroblast growth factors | Enhanced diabetic wound healing | [138] |
| Sponge | HA and chitosan | Ag nanoparticle | Good antibacterial effects and good cytocompatibility | [139] |
| Sponge | Chitosan | TMC nanoparticles | Faster diabetic wound healing | [140] |
| Sponge | Chitosan and HA | VEGFs | Burst release of GFs followed by sustained release. | [141] |
| Sponge | HA and collagen | EGF | Promoted blood vascular formation and granulation tissue development. | [142] |
| Sponge | Chitosan and silk | GMSC-derived exosomes | Enhanced deposition, re-epithelialization, and remodeling of ECM | [144] |
| Sponge | Collagen | Gementacin | Good pathogen eradication in diabetic wound | [143] |
| Sponge | Chitosan and alginate | Curcumin and honey | Sustained drug release and faster wound healing | [145] |
| Sponge | Chitosan and collagen | Thymosin beta 4 | Enhanced diabetic cutaneous wound healing | [146] |
| Bandages | Chitosan | ZnO nanoparticles | Good cytocompatibility and antibacterial effects. | [147] |
| Bandage | Chitin | ZnO nanoparticles | excellent antibacterial activity and high cell adhesion and migration | [149] |
| Bandage | Sodium alginate | EGF and curcumin | Non-toxicity and good biocompatibility | [148] |
| Bandage | Chitosan | Fluconazole and ciprofloxacin | High porosity and sustained drug release with good antimicrobial effects | [150] |
| Types of Wound Dressings | Advantages | Disadvantages | Highlights |
|---|---|---|---|
| Nanofibers | They possess a structure that mimics ECM, making them suitable for skin wound healing and regeneration. They are frequently formulated using efficient and easily employed electrospinning techniques. | It is not easy to produce nanofibers less than 10 nm in diameter. | The SEM micrographs of nanofibers loaded with bioactive agents display bead-free morphology that mimics ECM, making these wound dressings appropriate for providing an environment for cell proliferation and adhesion to accelerate the diabetic wound healing process. |
| Films and Membranes | These wound dressings are transparent, showing that the wound healing process can be observed without removing them. They also display good mechanical performance. | They are not suitable for exuding wounds due to their inability to absorb a large volume of biological fluids. | The mechanical properties of films and membranes were like those of human skin, making them skin compatible and easily handled during diabetic wound management. |
| Hydrogels | They are used as potential drug delivery systems in wound dressing applications and display other interesting properties such as high porosity, high swelling capacity, excellent biocompatibility, etc. | The biopolymer hydrogel dressings demonstrate poor mechanical performance that makes them not compatible with the human skin. | The drug release profiles were controlled release of bioactive agents from the polymeric hydrogels, resulting in an improved wound healing process. The high porosity of the hydrogels led to good swelling capability. |
| Foams and Wafers | These wound dressings exhibit high porosity that could provide cell growth and adhesion to accelerate the wound healing process. | They are not suitable for dry wounds. | The WVTR experiments of foams and wafers loaded with drugs exhibited moderate WTVR that can provide appropriate moisture to accelerate the healing of diabetic wounds. |
| Sponges and Bandages | These wound dressings are also displayed high porosity that could offer suitable gaseous permeation, superior cell proliferation, migration, and attachment for the accelerated wound healing process. | The very high porosity of the polymeric sponges or bandages can result in high uptake of wound exudate and high WVTR that may cause wound dehydration. | Polymeric sponges and bandages were mostly loaded with antibacterial agents for diabetic wound treatment, and they exhibited excellent antibacterial activity, demonstrating that these dressings are potential candidates for the management of infected diabetic wounds |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alven, S.; Peter, S.; Mbese, Z.; Aderibigbe, B.A. Polymer-Based Wound Dressing Materials Loaded with Bioactive Agents: Potential Materials for the Treatment of Diabetic Wounds. Polymers 2022, 14, 724. https://doi.org/10.3390/polym14040724
Alven S, Peter S, Mbese Z, Aderibigbe BA. Polymer-Based Wound Dressing Materials Loaded with Bioactive Agents: Potential Materials for the Treatment of Diabetic Wounds. Polymers. 2022; 14(4):724. https://doi.org/10.3390/polym14040724
Chicago/Turabian StyleAlven, Sibusiso, Sijongesonke Peter, Zintle Mbese, and Blessing A. Aderibigbe. 2022. "Polymer-Based Wound Dressing Materials Loaded with Bioactive Agents: Potential Materials for the Treatment of Diabetic Wounds" Polymers 14, no. 4: 724. https://doi.org/10.3390/polym14040724
APA StyleAlven, S., Peter, S., Mbese, Z., & Aderibigbe, B. A. (2022). Polymer-Based Wound Dressing Materials Loaded with Bioactive Agents: Potential Materials for the Treatment of Diabetic Wounds. Polymers, 14(4), 724. https://doi.org/10.3390/polym14040724









