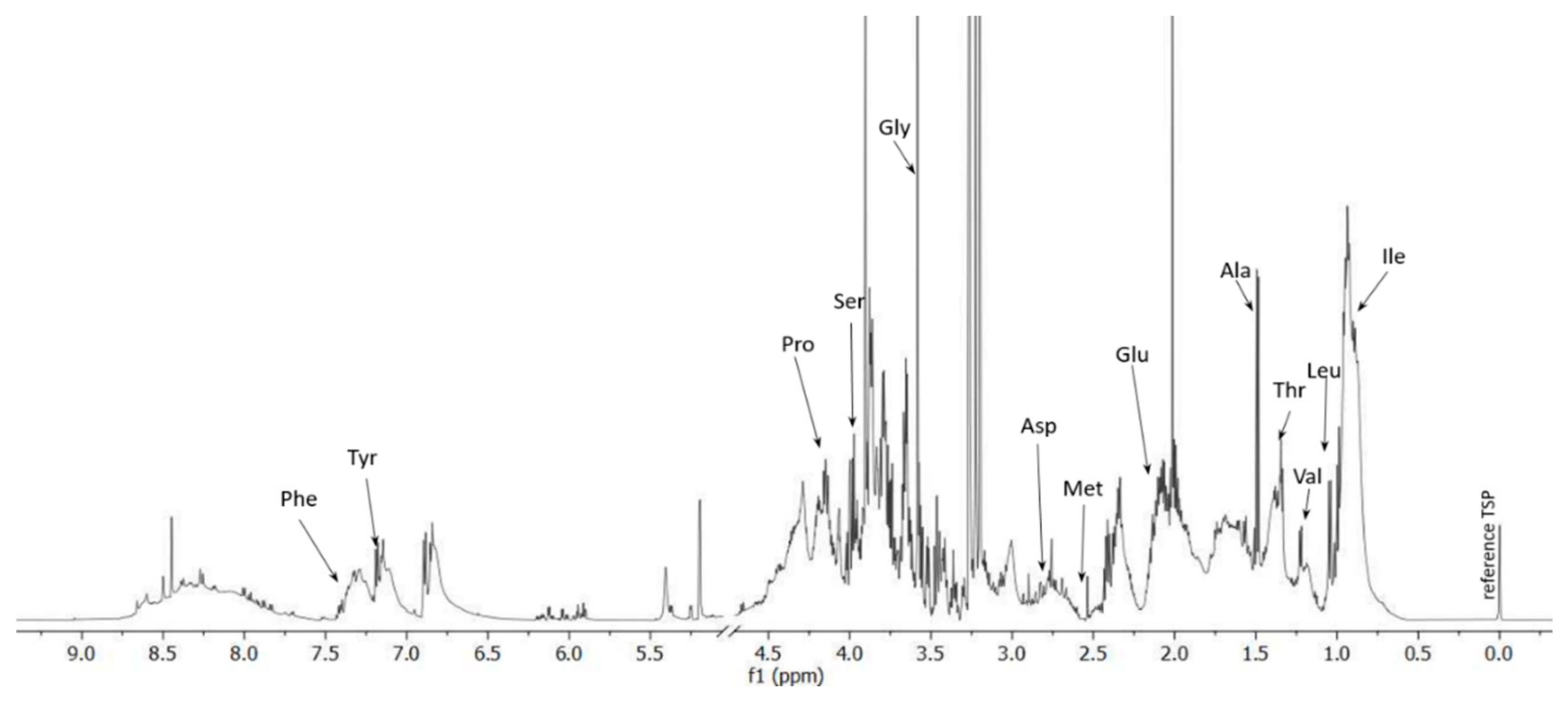
Figure 1.
1D 1H NMR spectrum of the larvae hydrolysate.
Figure 1.
1D 1H NMR spectrum of the larvae hydrolysate.
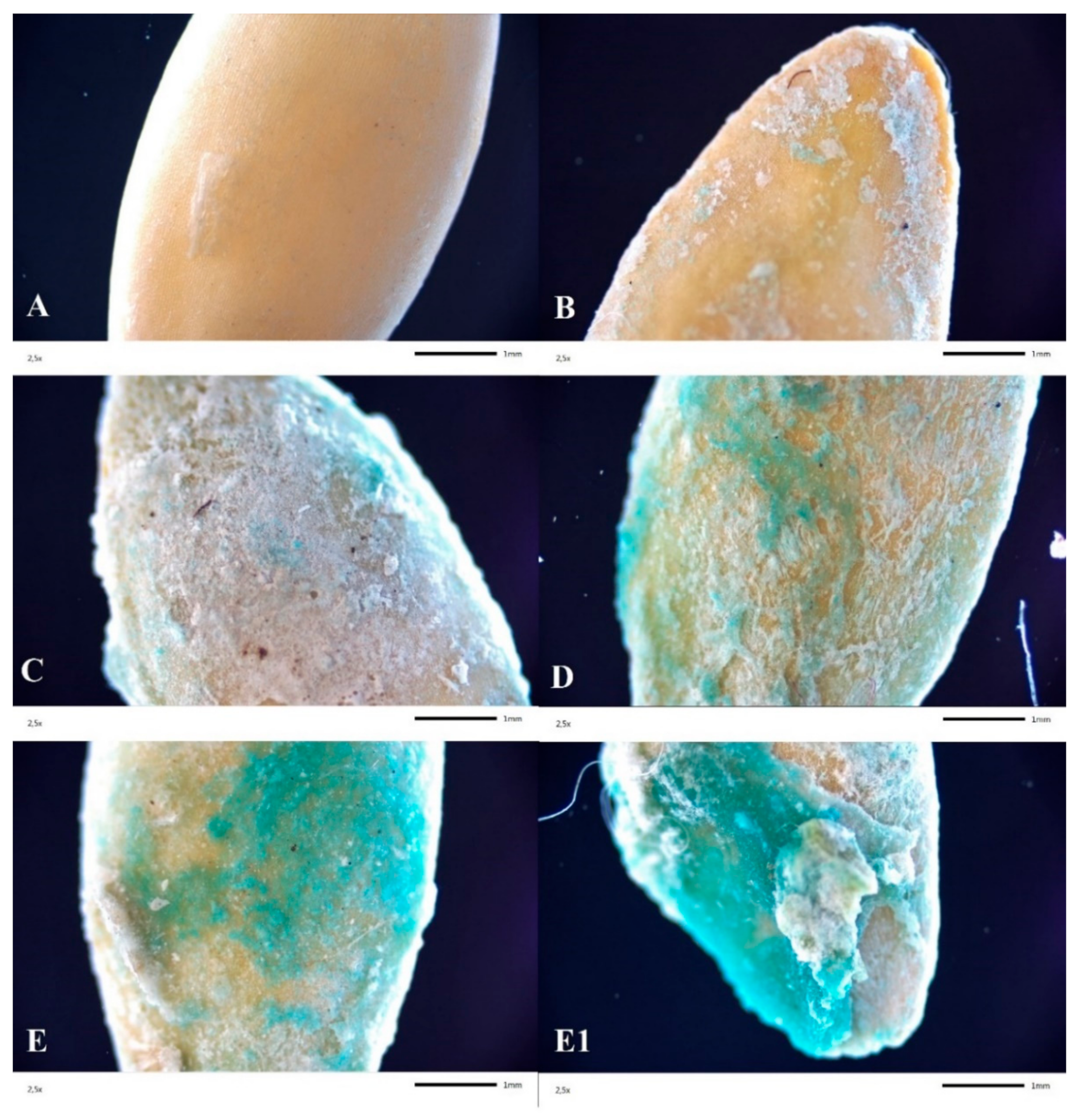
Figure 2.
Optical microscope images of S1 (A), S5.1 (B), S5.2 (C), S5.3 (D) and S5.4 (E,E1), zoom ×2.5 (S1—Uncoated seeds, S5.1—Seed coating containing a solution of 2% alginate with amino acids (37.6 mL/100 g), micronutrients (37.5 mL/100 g), and NPK, S5.2—Seed coating containing a solution of 2% alginate with amino acids (75.1 mL/100 g), micronutrients (75.1 mL/100 g), and NPK, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK, S5.4—Seed coating containing a solution of 2% alginate with amino acids (150 mL/100 g), micronutrients (150 mL/100 g), and NPK).
Figure 2.
Optical microscope images of S1 (A), S5.1 (B), S5.2 (C), S5.3 (D) and S5.4 (E,E1), zoom ×2.5 (S1—Uncoated seeds, S5.1—Seed coating containing a solution of 2% alginate with amino acids (37.6 mL/100 g), micronutrients (37.5 mL/100 g), and NPK, S5.2—Seed coating containing a solution of 2% alginate with amino acids (75.1 mL/100 g), micronutrients (75.1 mL/100 g), and NPK, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK, S5.4—Seed coating containing a solution of 2% alginate with amino acids (150 mL/100 g), micronutrients (150 mL/100 g), and NPK).
Figure 3.
SEM microscopic image of the surface morphology after modification with different concentrations of the AA (a) S1—reference sample, (b) S5.1 sample, (c) S5.2 sample, (d) S5.3 sample, (e) S5.4 sample; InLENS (S1—Uncoated seeds, S5.1—Seed coating containing a solution of 2% alginate with amino acids (37.6 mL/100 g), micronutrients (37.5 mL/100 g), and NPK, S5.2—Seed coating containing a solution of 2% alginate with amino acids (75.1 mL/100 g), micronutrients (75.1 mL/100 g), and NPK, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK, S5.4—Seed coating containing a solution of 2% alginate with amino acids (150 mL/100 g), micronutrients (150 mL/100 g), and NPK).
Figure 3.
SEM microscopic image of the surface morphology after modification with different concentrations of the AA (a) S1—reference sample, (b) S5.1 sample, (c) S5.2 sample, (d) S5.3 sample, (e) S5.4 sample; InLENS (S1—Uncoated seeds, S5.1—Seed coating containing a solution of 2% alginate with amino acids (37.6 mL/100 g), micronutrients (37.5 mL/100 g), and NPK, S5.2—Seed coating containing a solution of 2% alginate with amino acids (75.1 mL/100 g), micronutrients (75.1 mL/100 g), and NPK, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK, S5.4—Seed coating containing a solution of 2% alginate with amino acids (150 mL/100 g), micronutrients (150 mL/100 g), and NPK).
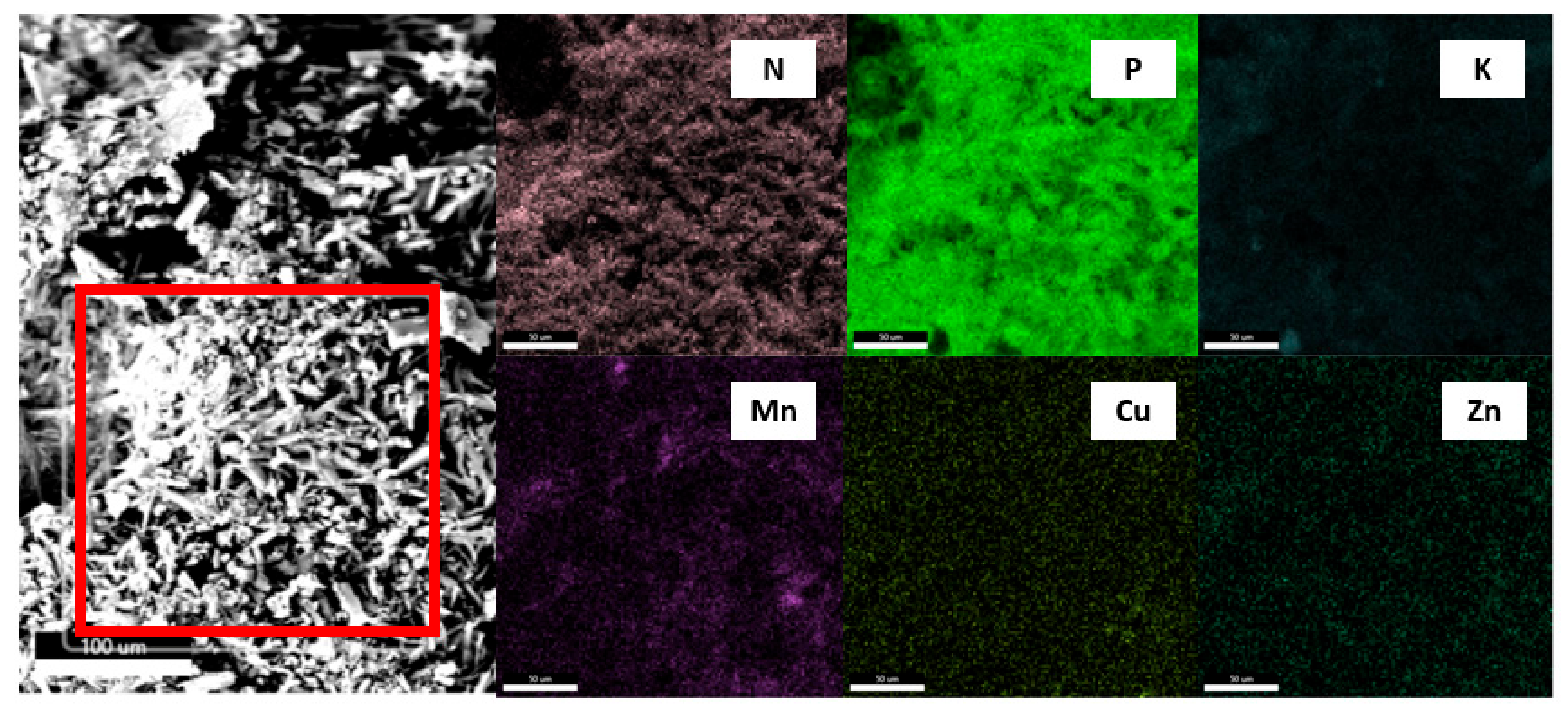
Figure 4.
Sample surface mapping with ROI selected (area marked in red). Element map for ROI.
Figure 4.
Sample surface mapping with ROI selected (area marked in red). Element map for ROI.
Figure 5.
Cucumber sprout growth parameters: fresh mass (A), chlorophyll content (B), steam length (C), root length (D), root volume (E), and root diameter (F) (S1—Uncoated seeds, S5.1—Seed coating containing a solution of 2% alginate with amino acids (37.6 mL/100 g), micronutrients (37.5 mL/100 g), and NPK, S5.2—Seed coating containing a solution of 2% alginate with amino acids (75.1 mL/100 g), micronutrients (75.1 mL/100 g), and NPK, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK, S5.4—Seed coating containing a solution of 2% alginate with amino acids (150 mL/100 g), micronutrients (150 mL/100 g), and NPK).
Figure 5.
Cucumber sprout growth parameters: fresh mass (A), chlorophyll content (B), steam length (C), root length (D), root volume (E), and root diameter (F) (S1—Uncoated seeds, S5.1—Seed coating containing a solution of 2% alginate with amino acids (37.6 mL/100 g), micronutrients (37.5 mL/100 g), and NPK, S5.2—Seed coating containing a solution of 2% alginate with amino acids (75.1 mL/100 g), micronutrients (75.1 mL/100 g), and NPK, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK, S5.4—Seed coating containing a solution of 2% alginate with amino acids (150 mL/100 g), micronutrients (150 mL/100 g), and NPK).
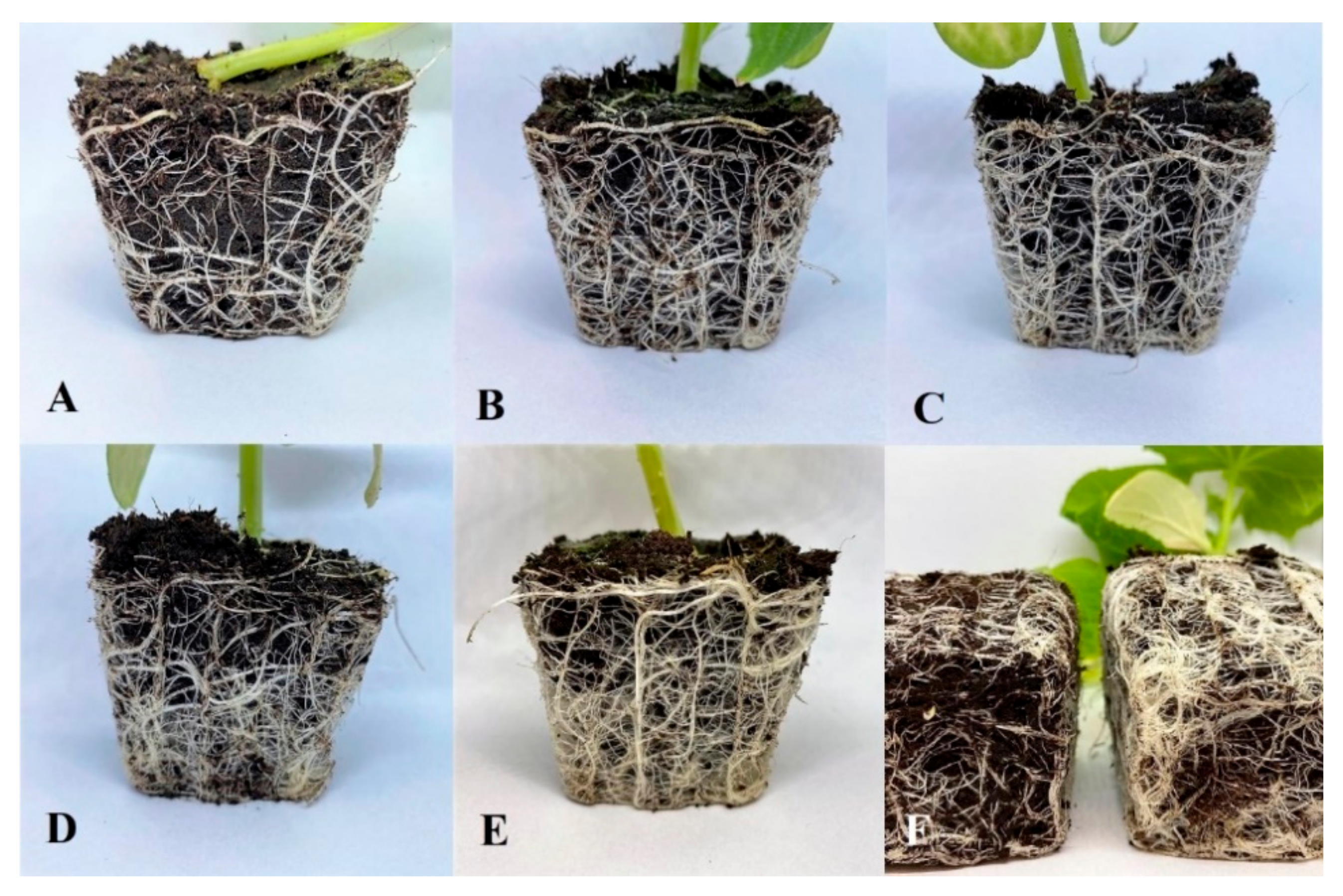
Figure 6.
Root ball arrangement in pots for S1 (A), S5.1 (B), S5.2 (C), S5.3 (D), S5.4 (E), and S1 and S5.4 (F) (S1—Uncoated seeds, S5.1—Seed coating containing a solution of 2% alginate with amino acids (37.6 mL/100 g), micronutrients (37.5 mL/100 g), and NPK, S5.2—Seed coating containing a solution of 2% alginate with amino acids (75.1 mL/100 g), micronutrients (75.1 mL/100 g), and NPK, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK, S5.4—Seed coating containing a solution of 2% alginate with amino acids (150 mL/100 g), micronutrients (150 mL/100 g), and NPK).
Figure 6.
Root ball arrangement in pots for S1 (A), S5.1 (B), S5.2 (C), S5.3 (D), S5.4 (E), and S1 and S5.4 (F) (S1—Uncoated seeds, S5.1—Seed coating containing a solution of 2% alginate with amino acids (37.6 mL/100 g), micronutrients (37.5 mL/100 g), and NPK, S5.2—Seed coating containing a solution of 2% alginate with amino acids (75.1 mL/100 g), micronutrients (75.1 mL/100 g), and NPK, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK, S5.4—Seed coating containing a solution of 2% alginate with amino acids (150 mL/100 g), micronutrients (150 mL/100 g), and NPK).
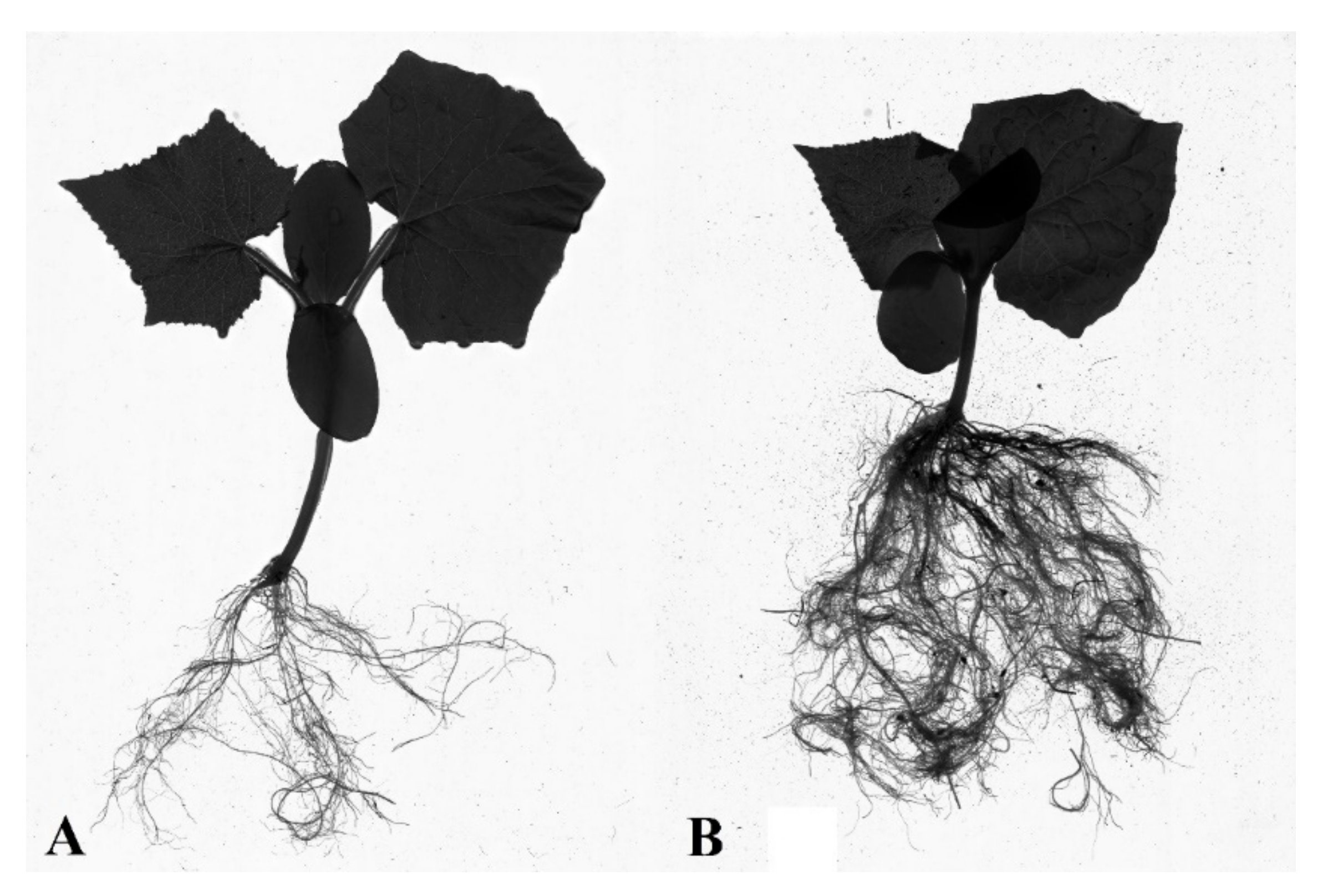
Figure 7.
Overground part and root ball of cucumber in pots for S1 (A) and S5.3 (B). (S1—Uncoated seeds, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK).
Figure 7.
Overground part and root ball of cucumber in pots for S1 (A) and S5.3 (B). (S1—Uncoated seeds, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK).
Table 1.
Composition of seeds coating solutions (ALG—sodium alginate, GA—gum arabic, CMC—carboxymethyl cellulose, AA—amino acid solution).
Table 1.
Composition of seeds coating solutions (ALG—sodium alginate, GA—gum arabic, CMC—carboxymethyl cellulose, AA—amino acid solution).
| Sample | Solution | ALG | GA | CMC | AA |
|---|
| % | % | % | mL/100 g |
|---|
| S1 | - | - | - | - | - |
| S2 | NPK + ALG | 2 | - | - | - |
| S3 | NPK + ALG | 4 | - | - | - |
| S4 | NPK + CMC + ALG | 2 | - | 1 | - |
| S5 | NPK + ALG + AA | 2 | - | - | 37.5 |
| S6 | NPK+ GA | - | 2 | - | - |
| S7 | NPK + GA | - | 4 | - | - |
| S8 | NPK + GA + AA | - | 4 | - | 37.5 |
Table 2.
Compositions of seeds coating solutions (ALG—sodium alginate, M—microelements solution, GA—gum arabic, CMC—carboxymethyl cellulose, AA—amino acid solution).
Table 2.
Compositions of seeds coating solutions (ALG—sodium alginate, M—microelements solution, GA—gum arabic, CMC—carboxymethyl cellulose, AA—amino acid solution).
| Sample | Coating Composition | ALG | M | AA |
|---|
| % | mL/100 g | mL/100 g |
|---|
| S5.1 | NPK + ALG + M + B | 2 | 37.5 | 37.6 |
| S5.2 | NPK + ALG + M + B | 2 | 75.0 | 75.1 |
| S5.3 | NPK + ALG + M + B | 2 | 112 | 112 |
| S5.4 | NPK + ALG + M + B | 2 | 150 | 150 |
Table 3.
The concentration of amino acids in the sample based on three technical replicates.
Table 3.
The concentration of amino acids in the sample based on three technical replicates.
| Amino Acid | Concentration (mM) |
|---|
| Serine | 31.4 ± 1.5 |
| Proline | 48.8 ± 1.5 |
| Glutamate | 55.8 ± 1.3 |
| Methionine | 2.48 ± 0.58 |
| Aspartate | 9.19 ± 0.51 |
| Phenylalanine | 2.12 ± 0.41 |
| Isoleucine | 4.33 ± 0.31 |
| Tyrosine | 9.93 ± 0.29 |
| Threonine | 8.49 ± 0.26 |
| Valine | 8.11 ± 0.24 |
| Alanine | 19.5 ± 0.1 |
| Leucine | 10.1 ± 0.7 |
| Glycine | 71.2 ± 0.6 |
| Total | 281 ± 7 |
Table 4.
Germination power of coated seeds (S1—Uncoated seeds, S2—Seed coating containing a solution of 2% alginate with NPK, S3—Seed coating containing a solution of 4% alginate with NPK, S4—Seed coating containing a solution of 2% alginate, 1% carboxymethyl cellulose with NPK, S5—Seed coating containing a solution of 2% alginate with amino acids (37.5 mL/100 g) and NPK, S6—Seed coating containing a 2% solution of gum arabic with NPK, S7—Seed coating containing a solution of 4% gum arabic with NPK, S8—Seed coating containing a solution of 4% gum arabic with amino acids (37.5 mL/100 g) and NPK).
Table 4.
Germination power of coated seeds (S1—Uncoated seeds, S2—Seed coating containing a solution of 2% alginate with NPK, S3—Seed coating containing a solution of 4% alginate with NPK, S4—Seed coating containing a solution of 2% alginate, 1% carboxymethyl cellulose with NPK, S5—Seed coating containing a solution of 2% alginate with amino acids (37.5 mL/100 g) and NPK, S6—Seed coating containing a 2% solution of gum arabic with NPK, S7—Seed coating containing a solution of 4% gum arabic with NPK, S8—Seed coating containing a solution of 4% gum arabic with amino acids (37.5 mL/100 g) and NPK).
| Day | Germination Power |
|---|
| % |
|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 |
|---|
| 1 | 44 | 48 | 12 | 56 | 8 | 0 | 0 | 0 |
| 2 | 96 | 100 | 76 | 92 | 92 | 16 | 32 | 0 |
| 3 | 100 | 100 | 88 | 96 | 100 | 16 | 32 | 8 |
Table 5.
Effect of thickening agent on growth parameters of cucumber—germination test seeds (S1—Uncoated seeds, S2—Seed coating containing a solution of 2% alginate with NPK, S3—Seed coating containing a solution of 4% alginate with NPK, S4—Seed coating containing a solution of 2% alginate, 1% carboxymethyl cellulose with NPK, S5—Seed coating containing a solution of 2% alginate with amino acids (37.5 mL/100 g) and NPK).
Table 5.
Effect of thickening agent on growth parameters of cucumber—germination test seeds (S1—Uncoated seeds, S2—Seed coating containing a solution of 2% alginate with NPK, S3—Seed coating containing a solution of 4% alginate with NPK, S4—Seed coating containing a solution of 2% alginate, 1% carboxymethyl cellulose with NPK, S5—Seed coating containing a solution of 2% alginate with amino acids (37.5 mL/100 g) and NPK).
| Group | Solution | Chlorophyll | Stem Length | Root Area | Root Length | Root Volume |
|---|
| ALG | GA | CMC | AA |
|---|
| % | % | % | mL/100 g | mg/m2 | mm | cm2 | cm | cm3 |
|---|
| S1 | - | - | - | - | 646 ± 77 a,b | 16.8 ± 2.2 a,b,c | 6.74 ± 1.73 a | 37.6 ± 9.6 | 0.100 ± 0.030 a |
| S2 | 2 | - | - | - | 552 ± 93 a,c | 13.9 ± 1.2 a,d,e | 7.07 ± 2.40 b | 40.9 ± 16.5 | 0.100 ± 0.030 b |
| S3 | 4 | - | - | - | 540 ± 87 b,d | 11.2 ± 1.6 b,d,f,g | 6.46 ± 2.58 c | 47.6 ± 15.8 a | 0.0700 ± 0.0400 c,d |
| S4 | 2 | - | 1 | - | 673 ± 42 c,d,e | 14.6 ± 1.8 c,f,h | 6.51 ± 1.85 d | 33.7 ± 9.8 | 0.100 ± 0.030 c,e |
| S5 | 2 | - | - | 37.5 | 577 ± 57 e | 16.8 ± 1.7 d,g,h | 9.09 ± 2.07 a,b,c,d | 46.6 ± 11.9 a | 0.140 ± 0.040 a,b,d,e |
Table 6.
Chemical composition of the microareas of the surface of the seeds, obtained by the energy dispersive X-ray analysis (S1—Uncoated seeds, S5.1—Seed coating containing a solution of 2% alginate with amino acids (37.6 mL/100 g), micronutrients (37.5 mL/100 g), and NPK, S5.2—Seed coating containing a solution of 2% alginate with amino acids (75.1 mL/100 g), micronutrients (75.1 mL/100 g), and NPK, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK, S5.4—Seed coating containing a solution of 2% alginate with amino acids (150 mL/100 g), micronutrients (150 mL/100 g), and NPK).
Table 6.
Chemical composition of the microareas of the surface of the seeds, obtained by the energy dispersive X-ray analysis (S1—Uncoated seeds, S5.1—Seed coating containing a solution of 2% alginate with amino acids (37.6 mL/100 g), micronutrients (37.5 mL/100 g), and NPK, S5.2—Seed coating containing a solution of 2% alginate with amino acids (75.1 mL/100 g), micronutrients (75.1 mL/100 g), and NPK, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK, S5.4—Seed coating containing a solution of 2% alginate with amino acids (150 mL/100 g), micronutrients (150 mL/100 g), and NPK).
| Sample | N | P | K | Mn | Cu | Zn |
|---|
| % |
|---|
| S1 | 80.9 ± 2.1 a,b,c,d | 8.63 ± 1.24 a,b,c | 7.40 ± 2.33 a,b,c,d | N/A | N/A | N/A |
| S5.1 | 23.2 ± 10.0 a,e,f | 28.87 ± 14.4 | 44.5 ± 6.1 a | 2.43 ± 1.93 | 0.300 ± 0.100 | 0.567 ± 0.503 |
| S5.2 | 2.57 ± 3.09 b,e | 43.3 ± 8.12 a | 45.9 ± 2.5 b | 2.43 ± 0.70 | 4.40 ± 0.60 | 1.50 ± 1.21 |
| S5.3 | 8.06 ± 2.68 c,f | 36.5 ± 4.3 b | 51.0 ± 5.5 c | 1.93 ± 0.70 | 1.36 ± 0.57 | 1.20 ± 0.62 |
| S5.4 | 12.4 ± 2.0 d | 44.0 ± 1.7 c | 38.5 ± 3.8 d | 3.27 ± 2.64 | 1.10 ± 0.66 | 0.833 ± 0.462 |
Table 7.
Macro and micronutrient levels in enrichment media and coated seeds (M—micronutrient solution, ALG—alginate, S1—Uncoated seeds, S5.1—Seed coating containing a solution of 2% alginate with amino acids (37.6 mL/100 g), micronutrients (37.5 mL/100 g), and NPK, S5.2—Seed coating containing a solution of 2% alginate with amino acids (75.1 mL/100 g), micronutrients (75.1 mL/100 g), and NPK, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK, S5.4—Seed coating containing a solution of 2% alginate with amino acids (150 mL/100 g), micronutrients (150 mL/100 g), and NPK).
Table 7.
Macro and micronutrient levels in enrichment media and coated seeds (M—micronutrient solution, ALG—alginate, S1—Uncoated seeds, S5.1—Seed coating containing a solution of 2% alginate with amino acids (37.6 mL/100 g), micronutrients (37.5 mL/100 g), and NPK, S5.2—Seed coating containing a solution of 2% alginate with amino acids (75.1 mL/100 g), micronutrients (75.1 mL/100 g), and NPK, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK, S5.4—Seed coating containing a solution of 2% alginate with amino acids (150 mL/100 g), micronutrients (150 mL/100 g), and NPK).
| Materials | N | P | K | Cu | Mn | Zn |
|---|
| % | % | % | mg/L | mg/L | mg/L |
|---|
| Enrichment solutions | AA | 2.58 ± 0.39 | 3.65 ± 0.55 | 5.73 ± 0.86 | 2.49 ± 0.37 | 1.67 ± 0.25 | 9.66 ± 1.45 |
| ALG+NPK solution | 1.02 ± 0.15 | 1.45 ± 0.22 | 2.14 ± 0.32 | 0.391 ± 0.059 | 14.0 ± 2.1 | 19.0 ± 2.9 |
| M | - | - | - | 6224 ± 934 | 5104 ± 766 | 5072 ± 761 |
| Cucumber seeds | S1 | 4.14 ± 0.62 | 1.08 ± 0.16 | 0.722 ± 0.108 | 21.2 ± 3.2 | 38.6 ± 5.8 | 83.9 ± 12.6 |
| S5.1 | 5.21 ± 0.78 | 2.83 ± 0.42 | 3.52 ± 0.53 | 499 ± 74 | 482 ± 72 | 524 ± 79 |
| S5.2 | 5.29 ± 0.79 | 2.87 ± 0.43 | 3.58 ± 0.54 | 1128 ± 169 | 957 ± 144 | 1003 ± 151 |
| S5.3 | 5.01 ± 0.75 | 2.89 ± 0.43 | 3.53 ± 0.53 | 1579 ± 237 | 1370 ± 206 | 1463 ± 219 |
| S5.4 | 5.01 ± 0.75 | 3.07 ± 0.46 | 3.94 ± 0.59 | 1833 ± 275 | 1421 ± 213 | 1571 ± 236 |
Table 8.
Evaluation of leachability of elements in the coating—water and neutral ammonium citrate extraction (S1—Uncoated seeds, S5.1—Seed coating containing a solution of 2% alginate with amino acids (37.6 mL/100 g), micronutrients (37.5 mL/100 g), and NPK, S5.2—Seed coating containing a solution of 2% alginate with amino acids (75.1 mL/100 g), micronutrients (75.1 mL/100 g), and NPK, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK, S5.4—Seed coating containing a solution of 2% alginate with amino acids (150 mL/100 g), micronutrients (150 mL/100 g), and NPK).
Table 8.
Evaluation of leachability of elements in the coating—water and neutral ammonium citrate extraction (S1—Uncoated seeds, S5.1—Seed coating containing a solution of 2% alginate with amino acids (37.6 mL/100 g), micronutrients (37.5 mL/100 g), and NPK, S5.2—Seed coating containing a solution of 2% alginate with amino acids (75.1 mL/100 g), micronutrients (75.1 mL/100 g), and NPK, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK, S5.4—Seed coating containing a solution of 2% alginate with amino acids (150 mL/100 g), micronutrients (150 mL/100 g), and NPK).
| Extractor | Materials | P | K | Cu | Mn | Zn |
|---|
| % |
|---|
| Water extraction | S1 | 0.00 | 0.685 | 1.96 | 3.25 | 2.16 |
| S5.1 | 5.82 | 7.51 | 14.1 | 12.4 | 5.33 |
| S5.2 | 7.54 | 9.48 | 7.10 | 9.64 | 5.26 |
| S5.3 | 5.50 | 7.07 | 4.48 | 9.01 | 6.89 |
| S5.4 | 7.75 | 9.24 | 5.46 | 9.48 | 6.71 |
| Neutral ammonium citrate extraction | S1 | 7.60 | 9.11 | 56.9 | 32.2 | 19.4 |
| S5.1 | 69.8 | 68.4 | 100 | 100 | 100 |
| S5.2 | 60.5 | 59.6 | 100 | 100 | 92.3 |
| S5.3 | 67.5 | 65.9 | 100 | 100 | 86.0 |
| S5.4 | 66.2 | 57.4 | 100 | 82.5 | 67.1 |
Table 9.
Effect of coating thickness with micronutrients and AAt on growth parameters of cucumber—pot tests) (S1—Uncoated seeds, S5.1—Seed coating containing a solution of 2% alginate with amino acids (37.6 mL/100 g), micronutrients (37.5 mL/100 g), and NPK, S5.2—Seed coating containing a solution of 2% alginate with amino acids (75.1 mL/100 g), micronutrients (75.1 mL/100 g), and NPK, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK, S5.4—Seed coating containing a solution of 2% alginate with amino acids (150 mL/100 g), micronutrients (150 mL/100 g), and NPK).
Table 9.
Effect of coating thickness with micronutrients and AAt on growth parameters of cucumber—pot tests) (S1—Uncoated seeds, S5.1—Seed coating containing a solution of 2% alginate with amino acids (37.6 mL/100 g), micronutrients (37.5 mL/100 g), and NPK, S5.2—Seed coating containing a solution of 2% alginate with amino acids (75.1 mL/100 g), micronutrients (75.1 mL/100 g), and NPK, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK, S5.4—Seed coating containing a solution of 2% alginate with amino acids (150 mL/100 g), micronutrients (150 mL/100 g), and NPK).
| Group | Chlorophyll | SteamLength | Root
Area | Root Diameter | Root
Length | Root
Volume |
|---|
| mg/m2 | cm | cm2 | mm | mm | cm3 |
|---|
| S1 | 398 ± 74 a,b | 7.74 ± 2.21 a,b,c,d | 23.5 ± 15.0 a,b,c,d | 0.440 ± 0.11 | 170 ± 16 a,b,c,d | 0.280 ± 0.210 a,b,c,d |
| S5.1 | 553 ± 6 a,c,d | 5.23 ± 1.08 a,e | 117 ± 77 a | 0.580 ± 0.240 | 679 ± 48 a | 1.74 ± 0.27 a |
| S5.2 | 507 ± 5 b | 3.92 ± 0.71 b | 156 ± 58 b | 0.540 ± 0.100 | 909 ± 60 b,e | 2.18 ± 0.12 b |
| S5.3 | 469 ± 67 c | 3.42 ± 0.95 c,e | 165 ± 50 c | 0.600 ± 0.110 | 865 ± 18 c | 2.58 ± 0.07 c |
| S5.4 | 457 ± 50 d | 4.90 ± 1.46 d | 115 ± 67 d | 1.10 ± 0.14 | 557 ± 29 d,e | 2.45 ± 0.65 d |
Table 10.
Macro- and micronutrient content of plants and effect of coating on bioavailability (TF) of micronutrients (S1—Uncoated seeds, S5.1—Seed coating containing a solution of 2% alginate with amino acids (37.6 mL/100 g), micronutrients (37.5 mL/100 g), and NPK, S5.2—Seed coating containing a solution of 2% alginate with amino acids (75.1 mL/100 g), micronutrients (75.1 mL/100 g), and NPK, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK, S5.4—Seed coating containing a solution of 2% alginate with amino acids (150 mL/100 g), micronutrients (150 mL/100 g), and NPK).
Table 10.
Macro- and micronutrient content of plants and effect of coating on bioavailability (TF) of micronutrients (S1—Uncoated seeds, S5.1—Seed coating containing a solution of 2% alginate with amino acids (37.6 mL/100 g), micronutrients (37.5 mL/100 g), and NPK, S5.2—Seed coating containing a solution of 2% alginate with amino acids (75.1 mL/100 g), micronutrients (75.1 mL/100 g), and NPK, S5.3—Seed coating containing a solution of 2% alginate with amino acids (112 mL/100 g), micronutrients (112 mL/100 g), and NPK, S5.4—Seed coating containing a solution of 2% alginate with amino acids (150 mL/100 g), micronutrients (150 mL/100 g), and NPK).
| Group | Content | TF |
|---|
| mg/kg | % |
|---|
| Cu | Mn | Zn | P | K | Cu | Mn | Zn |
|---|
| S1 | 7.88 ± 1.18 | 44.3 ± 6.7 | 59.1 ± 8.9 | 6835 ± 1025 | 34838 ± 5226 | - | - | - |
| S5.1 | 8.92 ± 1.34 | 46.7 ± 7.0 | 83.7 ± 12.6 | 7860 ± 1179 | 45024 ± 6754 | 10.3 ± 1.6 | 58.3 ± 8.8 | 82.3 ± 12.4 |
| S5.2 | 11.4 ± 1.7 | 47.5 ± 7.1 | 81.8 ± 12.3 | 8939 ± 1341 | 46731 ± 7010 | 6.71 ± 1.01 | 32.2 ± 4.8 | 55.6 ± 8.3 |
| S5.3 | 11.0 ± 1.7 | 56.3 ± 8.5 | 106 ± 16 | 9089 ± 1364 | 47231 ± 7085 | 8.20 ± 1.23 | 27.9 ± 4.2 | 79.4 ± 11.9 |
| S5.4 | 13.9 ± 2.1 | 72.5 ± 10.9 | 154 ± 23 | 8834 ± 1325 | 4589 ± 688 | 5.52 ± 0.83 | 26.5 ± 4.0 | 77.5 ± 11.6 |