Ground Tire Rubber Modified by Elastomers via Low-Temperature Extrusion Process: Physico-Mechanical Properties and Volatile Organic Emission Assessment
Abstract
:1. Introduction
2. Materials and Methods
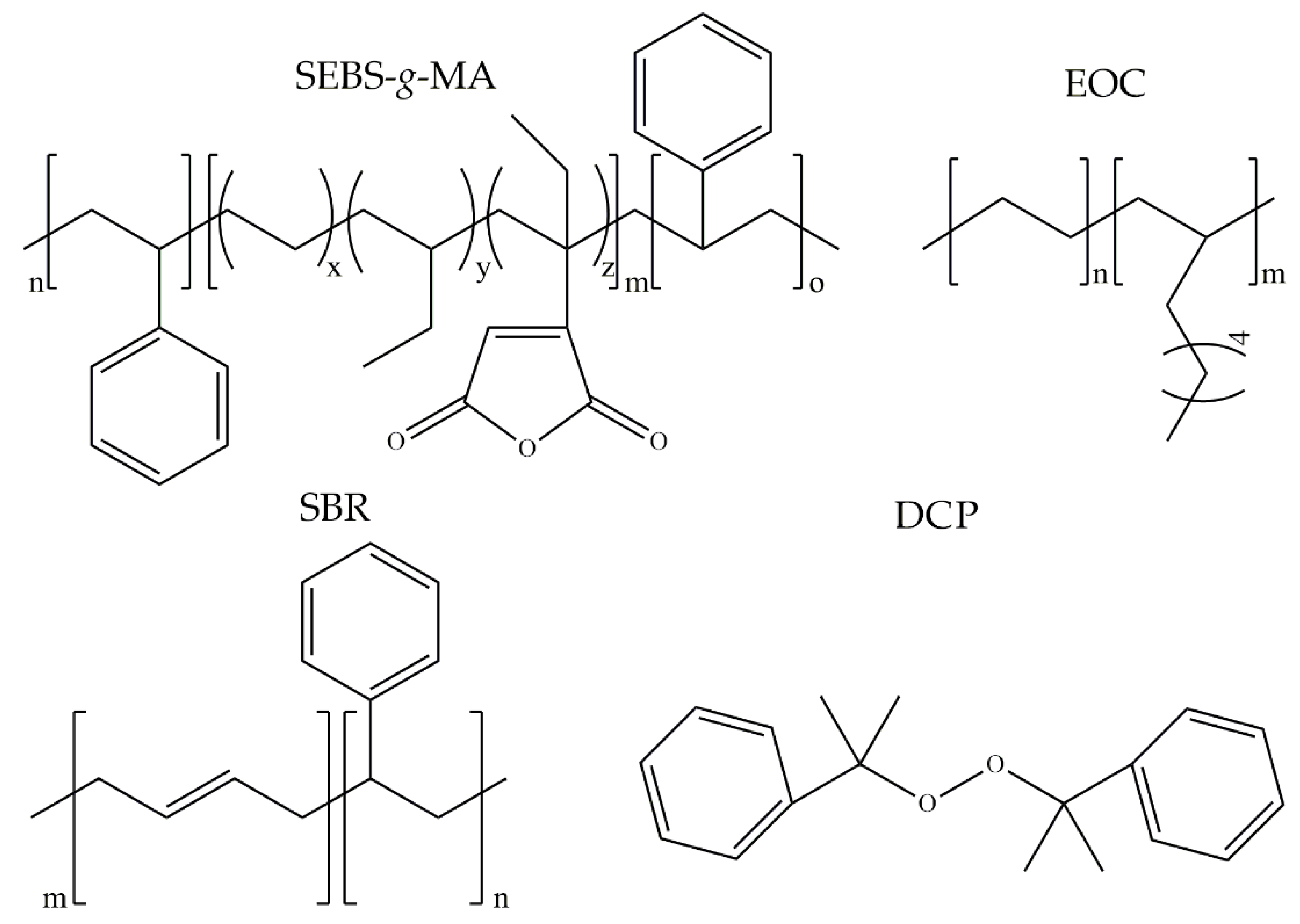
2.1. Materials
- Ground tire rubber (GTR)—obtained from passenger and truck tires, with particle sizes up to 0.6 mm, was received from Grupa Recykl S.A. (Śrem, Poland). GTR composition determined by thermogravimetric analysis showed: rubbers and additives (62.3 wt.%), carbon black (26.9 wt.%), silica and ash content (10.8 wt.%). Two peaks related to the presence of natural rubber and styrene-butadiene rubber were observed on differential thermogravimetry plots, confirming that recycled rubber was prepared from waste tires [37].
- Styrene-butadiene rubber (KER 9001)—is a high styrene resin containing about 83% of styrene bonded in the polymer (SBR), and it is characterized with softening point at 35–40 °C, hardness 65–75 Shore D, and volatile matter maximum of 1 wt.% The rubber was supplied by Synthos Rubbers (Oświęcim, Poland).
- Styrene-ethylene/butylene-styrene grafted with maleic anhydride with tradename TAIPOL SEBS 7126—it is characterized by bond maleic anhydride content 1.2–1.8 wt.%, melt flow index (5 kg at 230 °C) 15–25 g/10 min, and volatile matter maximum 0.5 wt.%. The copolymer was supplied by TSRC Corporation (Kaohsiung, Taiwan).
- Ethylene-octene copolymer (EOC) with tradename Solumer 851L—is characterized with melt flow index (2.16 kg at 190 °C) 1 g/10 min and glass transition temperature at −59 °C. The copolymer was supplied by SK Global Chemical Co., Ltd. (Seoul, Korea).
- Dicumyl peroxide (DCP)—organic peroxide commercially used for the curing of unsaturated polyester resins, natural and synthetic rubbers, as well as polyolefins. It is characterized by a peroxide assay minimum of 98% and an active oxygene assay minimum of 5.8%. The peroxide was supplied by Pergan GmbH (Bocholt, Germany).
2.2. Sample Preparation
2.3. Characterization Methods
3. Results and Discussion
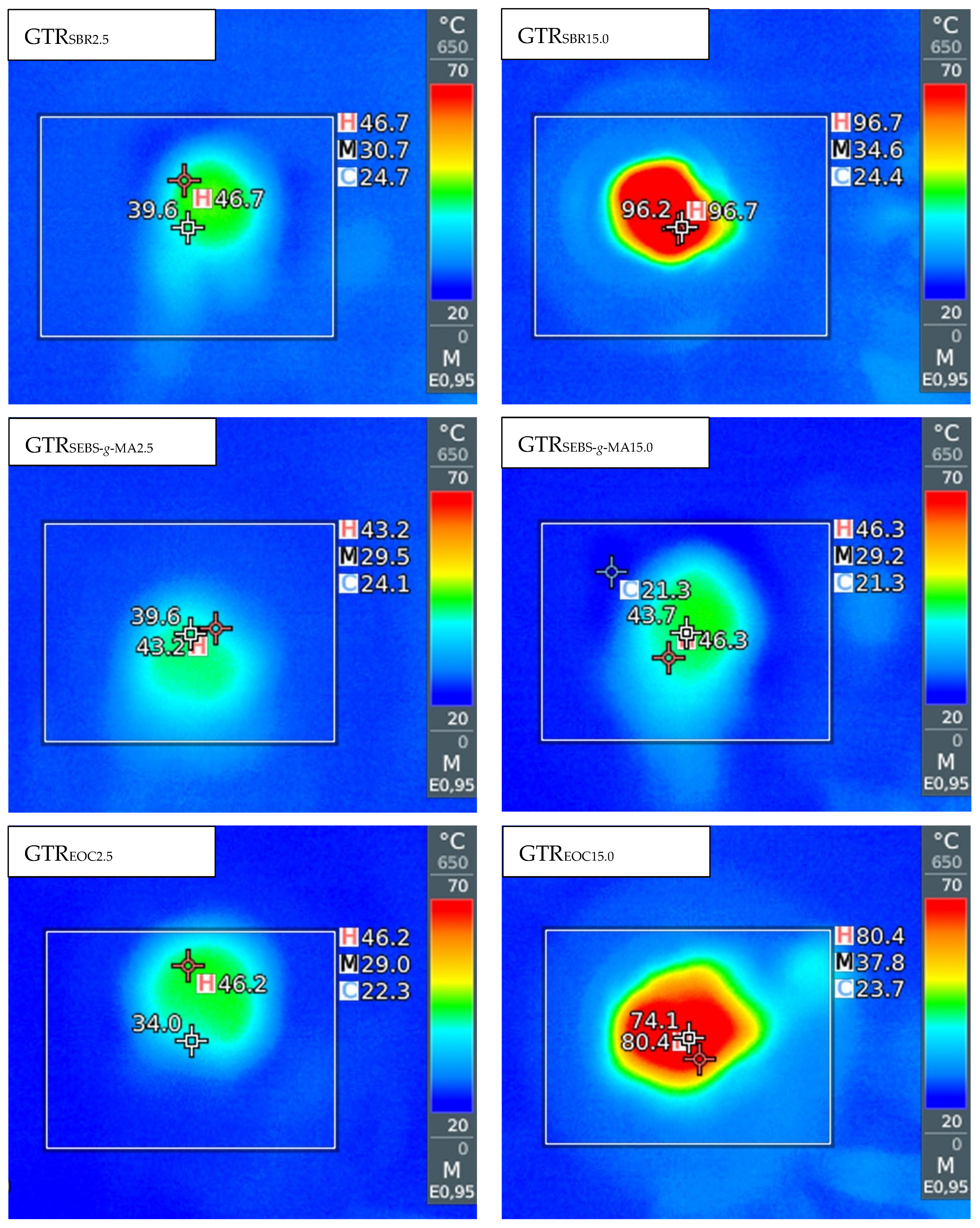
3.1. Temperature and Energy Consumption Measurements
3.2. Curing Characteristics of Modified GTR
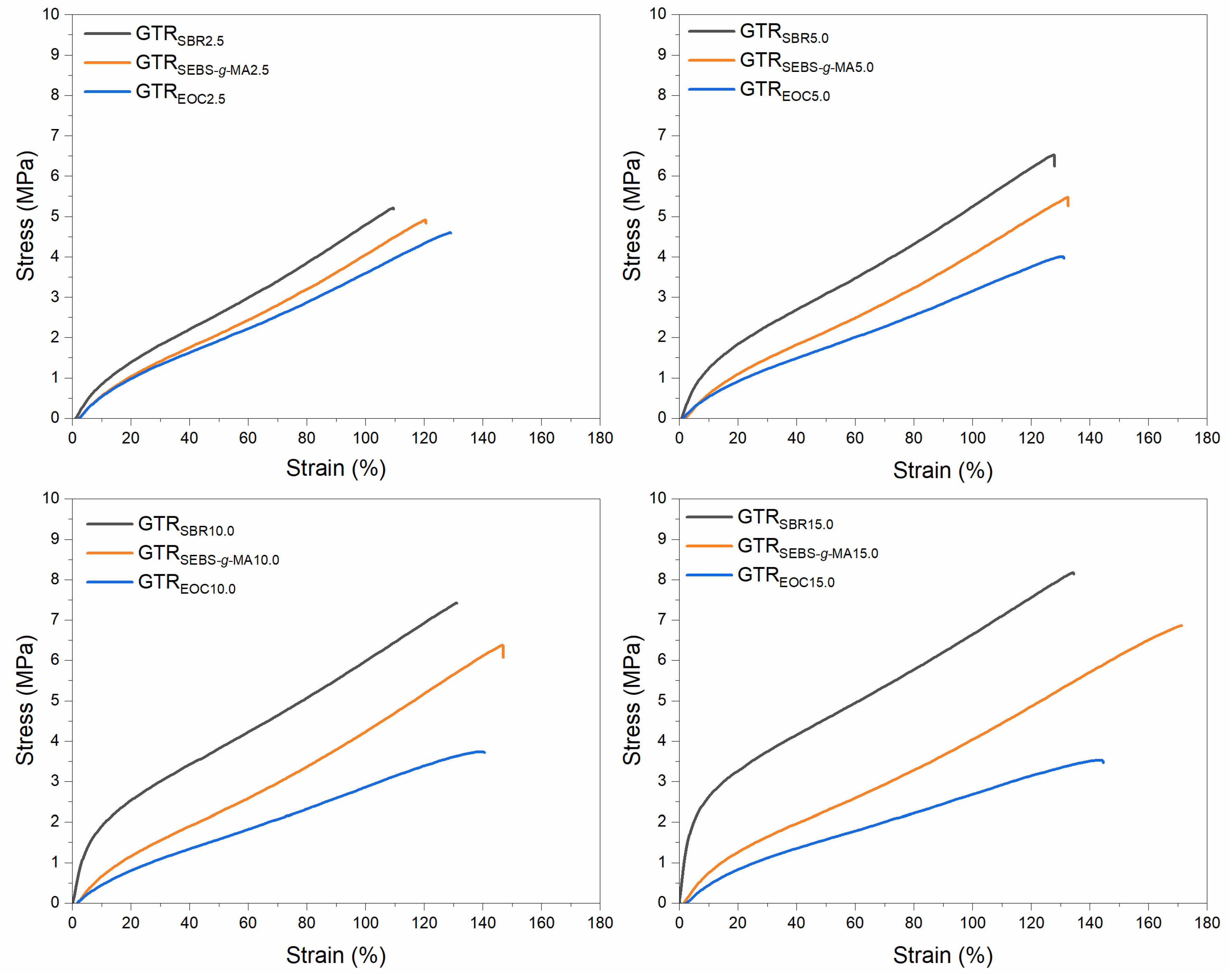
3.3. Physico-Mechanical Properties of Modified GTR
3.4. XRF Analysis of Modified GTR
3.5. Volatile Organic Compound Emission Profile Determined for Modified GTR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodgers, B. Introduction to Tire Engineering. In Tire Engineering: An Introduction, 1st ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 1–24. [Google Scholar]
- Mokhtar, F.N.; Abdel Rehim, I.V.; Mahmoud, E.A. Applicability of using recycled rubber-tire materials for acoustic insulation in barriers of residential areas in Egypt. ARPN J. Eng. Appl. Sci. 2017, 12, 806–820. [Google Scholar]
- Hidalgo-Signes, C.; Garzón-Roca, J.; Grima-Palop, J.M.; Insa-Franco, R. Use of rubber shreds to enhance attenuation of railway sub-ballast layers made of unbound aggregates. Mater. De Construcción 2017, 67, 115. [Google Scholar] [CrossRef] [Green Version]
- Alfayez, S.A.; Suleiman, A.R.; Nehdi, M.L. Recycling tire rubber in asphalt pavements: State of the art. Sustainability 2020, 12, 9076. [Google Scholar] [CrossRef]
- Shu, X.; Huang, B. Recycling of waste tire rubber in asphalt and portland cement concrete: An overview. Constr. Build. Mater. 2014, 67, 217–224. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, D.; Xiao, F. Recent developments in the application of chemical approaches to rubberized asphalt. Constr. Build. Mater. 2017, 131, 101–113. [Google Scholar] [CrossRef]
- Bosscher, P.J.; Edil, T.B.; Kuraoka, S. Design of Highway Embankments Using Tire Chips. J. Geotech. Geoenviron. Eng. 1997, 123, 295–304. [Google Scholar] [CrossRef]
- Baričević, A.; Jelčić Rukavina, M.; Pezer, M.; Štirmer, N. Influence of recycled tire polymer fibers on concrete properties. Cem. Concr. Compos. 2018, 91, 29–41. [Google Scholar] [CrossRef]
- Nakomcic-Smaragdakis, B.; Cepic, Z.; Senk, N.; Doric, J.; Radovanović, L. Use of scrap tires in cement production and their impact on nitrogen and sulfur oxides emissions. Energy Sources Part A Recover. Util. Environ. Eff. 2016, 38, 485–493. [Google Scholar] [CrossRef]
- Nakajima, Y.; Matsuyuki, M. Utilization of waste tires as fuel for cement production. Conserv. Recycl. 1981, 4, 145–152. [Google Scholar] [CrossRef]
- Echterhof, T. Review on the use of alternative carbon sources in EAF steelmaking. Metals 2021, 11, 222. [Google Scholar] [CrossRef]
- Conesa, J.A.; Martín-Gullón, I.; Font, R.; Jauhiainen, J. Complete study of the pyrolysis and gasification of scrap tires in a pilot plant reactor. Environ. Sci. Technol. 2004, 38, 3189–3194. [Google Scholar] [CrossRef]
- Amari, T.; Themelis, N.J.; Wernick, I.K. Resource recovery from used rubber tires. Resour. Policy 1999, 25, 179–188. [Google Scholar] [CrossRef]
- Picado-Santos, L.G.; Capitão, S.D.; Neves, J.M.C. Crumb rubber asphalt mixtures: A literature review. Constr. Build. Mater. 2020, 247, 118577. [Google Scholar] [CrossRef]
- Hejna, A.; Korol, J.; Przybysz-Romatowska, M.; Zedler, Ł.; Chmielnicki, B.; Formela, K. Waste tire rubber as low-cost and environmentally-friendly modifier in thermoset polymers—A review. Waste Manag. 2020, 108, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Formela, K.; Hejna, A.; Zedler, Ł.; Colom, X.; Cañavate, J. Microwave treatment in waste rubber recycling–recent advances and limitations. Express Polym. Lett. 2019, 13, 565–588. [Google Scholar] [CrossRef]
- Roychand, R.; Gravina, R.J.; Zhuge, Y.; Ma, X.; Youssf, O.; Mills, J.E. A comprehensive review on the mechanical properties of waste tire rubber concrete. Constr. Build. Mater. 2020, 237, 117651. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrique, D. Waste rubber recycling: A review on the evolution and properties of thermoplastic elastomers. Materials 2020, 13, 782. [Google Scholar] [CrossRef] [Green Version]
- Formela, K.; Klein, M.; Colom, X.; Saeb, M.R. Investigating the combined impact of plasticizer and shear force on the efficiency of low temperature reclaiming of ground tire rubber (GTR). Polym. Degrad. Stab. 2016, 125, 1–11. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, X.; Liang, M.; Lu, C. Enhancement of processability and foamability of ground tire rubber powder and LDPE blends through solid state shear milling. J. Polym. Res. 2011, 18, 533–539. [Google Scholar] [CrossRef]
- Si, H.; Chen, T.; Zhang, Y. Effects of high shear stress on the devulcanization of ground tire rubber in a twin-screw extruder. J. Appl. Polym. Sci. 2013, 128, 2307–2318. [Google Scholar] [CrossRef]
- Nunes, A.T.; dos Santos, R.E.; Pereira, J.S.; Barbosa, R.; Ambrósio, J.D. Characterization of waste tire rubber devulcanized in twin-screw extruder with thermoplastics. Prog. Rubber Plast. Recycl. Technol. 2018, 34, 143–157. [Google Scholar] [CrossRef]
- Barbosa, R.; Ambrósio, J.D. Devulcanization of natural rubber compounds by extrusion using thermoplastics and characterization of revulcanized compounds. J. Polym. Res. 2019, 26, 160. [Google Scholar] [CrossRef]
- Wang, Y.H.; Chen, Y.K.; Rodrigue, D. Production of thermoplastic elastomers based on recycled PE and ground tire rubber: Morphology, mechanical properties and effect of compatibilizer addition. Int. Polym. Proc. 2018, 33, 525–534. [Google Scholar] [CrossRef]
- Kim, J.I.; Ryu, S.H.; Chang, Y.W. Mechanical and dynamic mechanical properties of waste rubber powder/HDPE composite. J. Appl. Polym. Sci. 2000, 77, 2595–2602. [Google Scholar] [CrossRef]
- Seghar, S.; Asaro, L.; Rolland-Monnet, M.; Aït Hocine, N. Thermo-mechanical devulcanization and recycling of rubber industry waste. Resour. Conserv. Recycl. 2019, 144, 180–186. [Google Scholar] [CrossRef]
- Zedler, Ł.; Klein, M.; Saeb, M.R.; Colom, X.; Cañavate, J.; Formela, K. Synergistic effects of bitumen plasticization and microwave treatment on short-term devulcanization of ground tire rubber. Polymers 2018, 10, 1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zedler, Ł.; Burger, P.; Wang, S.; Formela, K. Ground tire rubber modified by ethylene-vinyl acetate copolymer: Processing, physico-mechanical properties, volatile organic compounds emission and recycling possibility. Materials 2020, 13, 4669. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef] [Green Version]
- Formela, K.; Cysewska, M.; Haponiuk, J.T. Thermomechanical reclaiming of ground tire rubber via extrusion at low temperature: Efficiency and limits. J. Vinyl Addit. Technol. 2016, 22, 213–221. [Google Scholar] [CrossRef]
- OEHHA. Evaluation of Health Effects of Recycled Waste Tires in Playground and Track Products; Office of Environmental Health Hazard Assessment of California Environmental Protection Agency: Sacramento, CA, USA, 2007.
- Mohajerani, A.; Burnett, L.; Smith, J.V.; Markovski, S.; Rodwell, G.; Rahman, M.T.; Kurmus, H.; Mirzababaei, M.; Arulrajah, A.; Horpibulsuk, S.; et al. Recycling waste rubber tyres in construction materials and associated environmental considerations: A review. Resour. Conserv. Recycl. 2020, 155, 104679. [Google Scholar] [CrossRef]
- Janajreh, I.; Hussain, M.; Elagroudy, S.; Moustakas, K. Recycled tire granular for playground in hot regions: Technical assessment. J. Mater. Cycles Waste Manag. 2021, 23, 107–120. [Google Scholar] [CrossRef]
- Birkholz, D.A.; Belton, K.L.; Guidotti, T. Toxicological evaluation for the hazard assessment of tire crumb for use in public playgrounds. J. Air Waste Manag. Assoc. 2003, 53, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.C.; Fiore, S.; Ruffino, B.; Santagata, E.; Lanotte, M. Assessment of gaseous emissions produced on site by bituminous mixtures containing crumb rubber. Constr. Build. Mater. 2014, 67, 291–296. [Google Scholar] [CrossRef]
- Skoczyńska, E.; Leonards, P.E.G.; Llompart, M.; Boer, J. Analysis of recycled rubber: Development of an analytical method and determination of polycyclic aromatic hydrocarbons and heterocyclic aromatic compounds in rubber matrices. Chemosphere 2021, 276, 130076. [Google Scholar] [CrossRef]
- Zedler, Ł.; Kowalkowska-Zedler, D.; Vahabi, H.; Saeb, M.R.; Colom, X.; Cañavate, J.; Wang, S.; Formela, K. Preliminary investigation on auto-thermal extrusion of ground tire rubber. Materials 2019, 12, 2090. [Google Scholar] [CrossRef] [Green Version]
- Menon, A.R.R.; Pillai, C.K.S.; Nando, G.B. Vulcanization of natural rubber modified with cashew nut shell liquid and its phosphorylated derivative—A comparative study. Polymer 1998, 39, 4033–4036. [Google Scholar] [CrossRef]
- Khang, T.H.; Ariff, Z.M. Vulcanization kinetics study of natural rubber compounds having different formulation variables. J. Therm. Anal. Calorim. 2012, 109, 1545–1553. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical mechanics of crosslinked polymer networks I. rubberlike elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Kraus, G.J. Swelling of filler-reinforced vulcanizates. J. Appl. Polym. Sci. 1963, 7, 861–871. [Google Scholar] [CrossRef]
- Król, S.; Zabiegała, B.; Namieśnik, J. Measurement of benzene concentration in urban air using passive sampling. Anal. Bioanal. Chem. 2012, 403, 1067–1082. [Google Scholar] [CrossRef]
- Marć, M.; Zabiegała, B.; Namieśnik, J. Application of passive sampling technique in monitoring research on quality of atmospheric air in the area of Tczew. Poland. Int. J. Environ. Anal. Chem. 2014, 94, 151–167. [Google Scholar] [CrossRef]
- Marć, M.; Formela, K.; Klein, M.; Namieśnik, J.; Zabiegała, B. The emissions of monoaromatic hydrocarbons from small polymeric toys placed in chocolate food products. Sci. Total Environ. 2015, 530–531, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Marć, M.; Namieśnik, J.; Zabiegała, B. The miniaturized emission chamber system and home-made passive flux sampler studies of monoaromatic hydrocarbons emissions from selected commercially available floor coverings. Build. Environ. 2017, 123, 1–13. [Google Scholar] [CrossRef]
- Nohr, M.; Horn, W.; Wiegner, K.; Richter, M.; Lorenz, W. Development of a material with reproducible emission of selected volatile organic compounds—µ-Chamber study. Chemosphere 2014, 107, 224–229. [Google Scholar] [CrossRef]
- Marć, M.; Zabiegała, B. An investigation of selected monoaromatic hydrocarbons released from the surface of polystyrene lids used in coffee-to-go cups. Microchem. J. 2017, 133, 496–505. [Google Scholar] [CrossRef]
- Marć, M. Emissions of selected monoaromatic hydrocarbons as a factor affecting the removal of single-use polymer barbecue and kitchen utensils from everyday use. Sci. Total Environ. 2020, 720, 137485. [Google Scholar] [CrossRef] [PubMed]
- Schripp, T.; Nachtwey, B.; Toelke, J.; Salthammer, T.; Uhde, E.; Wensing, M.; Bahadir, M. A microscale device for measuring emissions from materials for indoor use. Anal. Bioanal. Chem. 2007, 387, 1907–1919. [Google Scholar] [CrossRef]
- Galeja, M.; Wypiór, K.; Wachowicz, J.; Kędzierski, P.; Hejna, A.; Marć, M.; Klewicz, K.; Gabor, J.; Okła, H.; Swinarew, A.S. POM/EVA blends with future utility in fused deposition modeling. Materials 2020, 13, 2912. [Google Scholar] [CrossRef]
- Marć, M.; Namieśnik, J.; Zabiegała, B. BTEX concentration levels in urban air in the area of the Tri-City agglomeration (Gdansk, Gdynia, Sopot), Poland. Air Qual. Atmos. Health 2014, 7, 489–504. [Google Scholar] [CrossRef]
- Zabiegała, B.; Sarbu, C.; Urbanowicz, M.; Namieśnik, J. A comparative study of the performance of passive samplers. J. Air Waste Manag. Assoc. 2011, 61, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Massold, E.; Bahr, C.; Salthammer, T.; Brown, S.K. Determination of VOC and TVOC in air using thermal desorption GC–MS—practical implications for test chamber experiments. Chromatographia 2005, 62, 75–85. [Google Scholar] [CrossRef]
- European Collaborative Action Indoor Air Quality & Its Impact on Man (ECA-IAQ). Report No 19, Total Volatile Organic Compounds (TVOC) in Indoor Air Quality Investigations. In Environment and Quality of Life; Publications Office of the European Union: Luxembourg, 1997. [Google Scholar]
- Marć, M.; Tsakovski, S.; Tobiszewski, M. Emissions and toxic units of solvent, monomer and additive residues released to gaseous phase from latex balloons. Environ. Res. 2021, 195, 110700. [Google Scholar] [CrossRef]
- Kumar, C.R.; Fuhrmann, I.; Karger-Kocsis, J. LDPE-based thermoplastic elastomers containing ground tire rubber with and without dynamic curing. Polym. Degrad. Stab. 2002, 76, 137–144. [Google Scholar] [CrossRef]
- Karabork, F.; Pehlivan, E.; Akdemir, A. Characterization of styrene butadiene rubber and microwave devulcanized ground tire rubber composites. J. Polym. Eng. 2014, 34, 543–554. [Google Scholar] [CrossRef]
- De, D.; De, D. Processing and material characteristics of a reclaimed ground rubber tire reinforced styrene butadiene rubber. Mater. Sci. Appl. 2011, 2, 486–495. [Google Scholar] [CrossRef] [Green Version]
- Yehia, A.; Ismail, M.N.; Hefny, Y.A.; Abdel-Bary, E.M.; Mull, M.A. Mechano-chemical reclamation of waste rubber powder and its effect on the performance of NR and SBR vulcanizates. J. Elastomers Plast. 2004, 36, 109–123. [Google Scholar] [CrossRef]
- Magioli, M.; Sirqueira, A.S.; Soares, B.G. The effect of dynamic vulcanization on the mechanical, dynamic mechanical and fatigue properties of TPV based on polypropylene and ground tire rubber. Polym. Test. 2010, 29, 840–849. [Google Scholar] [CrossRef]
- Linag, H.; Rodrigue, D.; Brisson, J. Characterization of recycled styrene butadiene rubber ground tire rubber: Combining X-ray fluorescence, differential scanning calorimetry, and dynamical thermal analysis for quality control. J. Appl. Polym. Sci. 2015, 132, 42692. [Google Scholar] [CrossRef]
- Gągol, M.; Boczkaj, G.; Haponiuk, J.; Formela, K. Investigation of volatile low molecular weight compounds formed during continuous reclaiming of ground tire rubber. Polym. Degrad. Stab. 2015, 119, 113–120. [Google Scholar] [CrossRef]
- Ginsberg, G.; Toal, B.; Simcox, N.; Bracker, A.; Golembiewski, B.; Kurland, T.; Hedman, C. Human health risk assessment of synthetic turf fields based upon investigation of five fields in Connecticut. J. Toxicol. Environ. Health Part A 2011, 74, 1150–1174. [Google Scholar] [CrossRef]
- Hulse, G.E.; Kersting, R.J.; Warfel, D.R. Chemistry of dicumyl peroxide-induced crosslinking of linear polyethylene. J. Polym. Sci. Polym. Chem. Ed. 1981, 19, 655–667. [Google Scholar] [CrossRef]
- Kamarulzaman, N.H.; Le-Minh, N.; Stuetz, R.M. Identification of VOCs from natural rubber by different headspace techniques coupled using GC-MS. Talanta 2019, 191, 535–544. [Google Scholar] [CrossRef]
- Sato, S.; Honda, Y.; Kuwahara, M.; Watanabe, T. Degradation of vulcanized and nonvulcanized polyisoprene rubbers by lipid peroxidation catalyzed by oxidative enzymes and transition metals. Biomacromolecules 2003, 4, 321–329. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Zedler, Ł.; Formela, K. Processing, performance properties, and storage stability of ground tire rubber modified by dicumyl peroxide and ethylene-vinyl acetate copolymers. Polymers 2021, 13, 4014. [Google Scholar] [CrossRef]
- Thomas, D.K. Crosslinking efficiency of dicumyl peroxide in natural rubber. J. Appl. Polym. Sci. 1962, 6, 613–616. [Google Scholar] [CrossRef]
- Di Somma, I.; Marotta, R.; Andreozzi, R.; Caprio, V. Kinetic and chemical characterization of thermal decomposition of di-cumylperoxide in cumene. J. Hazard. Mater. 2011, 187, 157–163. [Google Scholar] [CrossRef]
- Wewerka, D.; Hummel, K.; Inselsbacher, W. Reactions in crosslinked polymers. 3. A metal mold for collecting volatile products during the crosslinking reactions. Rubber Chem. Technol. 1976, 49, 1142–1144. [Google Scholar] [CrossRef]
- Lv, J.; Chen, L.; Chen, W.; Gao, H.; Peng, M. Kinetic analysis and self-accelerating decomposition temperature (SADT) of dicumyl peroxide. Thermochim. Acta 2013, 571, 60–63. [Google Scholar] [CrossRef]



| Sample Coding | GTRXY | X—Modifier Type: EOC; SBR or SEBS-g-MA | Y—The Amount of Modifier: 2.5; 5; 10 and 15 phr |
|---|---|---|---|
| GTR Modification | Modification was performed using a co-rotating twin screw extruder EHP 2 × 20 Sline with an L/d ratio of 40 and d = 20 mm produced by Zamak Mercator (Skawina, Poland). Rotational screw speed: 150 rpm Barrel temperature (from hopper to extrusion die): 35/60/60/60/60/60/60/25/25/25 °C Prior to extrusion, a premix of GTR and DCP (2 phr) was prepared. GTR/DCP premix and elastomeric modifier were dosed with a total throughput: 3 kg/h. | ||
| Modified GTR Formulation | Modified GTR samples were formed into sheets of about 2 mm using hydraulic press PH-90 manufactured by ZUP Nysa (Nysa, Poland) Temperature: 170 °C, Pressure: 9.8 MPa Samples were compressed according to the optimal vulcanization time determined by ISO 6502 standard. | ||
| Sample Code | Temperature at Die (°C) | SME (kWh/kg) | Extruder Energy Consumption (kWh/kg) |
|---|---|---|---|
| GTRSBR2.5 | 46 ± 1 | 0.131 ± 0.002 | 0.433 ± 0.030 |
| GTRSBR5.0 | 50 ± 2 | 0.136 ± 0.003 | 0.440 ± 0.025 |
| GTRSBR10.0 | 92 ± 4 | 0.250 ± 0.007 | 0.580 ± 0.022 |
| GTRSBR15.0 | 96 ± 1 | 0.262 ± 0.004 | 0.587 ± 0.021 |
| GTRSEBS-g-MA2.5 | 43 ± 1 | 0.090 ± 0.004 | 0.407 ± 0.039 |
| GTRSEBS-g-MA5.0 | 42 ± 1 | 0.111 ± 0.007 | 0.413 ± 0.021 |
| GTRSEBS-g-MA10.0 | 44 ± 1 | 0.123 ± 0.008 | 0.433 ± 0.030 |
| GTRSEBS-g-MA15.0 | 47 ± 2 | 0.145 ± 0.006 | 0.440 ± 0.044 |
| GTREOC2.5 | 44 ± 1 | 0.091 ± 0.005 | 0.420 ± 0.022 |
| GTREOC5.0 | 78 ± 2 | 0.138 ± 0.011 | 0.453 ± 0.021 |
| GTREOC10.0 | 79 ± 2 | 0.176 ± 0.006 | 0.480 ± 0.025 |
| GTREOC15.0 | 81 ± 1 | 0.195 ± 0.002 | 0.487 ± 0.030 |
| Sample Code | Curing Parameters | ||||||
|---|---|---|---|---|---|---|---|
| ML (dNm) | MH (dNm) | ΔM (dNm) | t2 (min.) | t90 (min.) | CRI (min−1) | R300 (%) | |
| GTRSBR2.5 | 10.8 | 19.9 | 9.1 | 0.4 | 5.6 | 19.0 | 0.6 |
| GTRSBR5.0 | 10.2 | 18.2 | 8.0 | 0.5 | 5.7 | 19.4 | 0.5 |
| GTRSBR10.0 | 7.2 | 14.9 | 7.7 | 0.2 | 5.3 | 19.8 | 0.6 |
| GTRSBR15.0 | 6.5 | 13.1 | 6.6 | 0.2 | 5.4 | 19.3 | 0.7 |
| GTRSEBS-g-MA2.5 | 10.9 | 20.3 | 9.4 | 0.4 | 5.7 | 18.8 | 0.4 |
| GTRSEBS-g-MA5.0 | 8.8 | 19.4 | 10.6 | 0.2 | 5.6 | 18.7 | 0.2 |
| GTRSEBS-g-MA10.0 | 9.9 | 16.4 | 6.6 | 0.6 | 6.1 | 18.0 | 0.3 |
| GTRSEBS-g-MA15.0 | 9.3 | 15.4 | 6.2 | 0.6 | 6.3 | 17.5 | 0.3 |
| GTREOC2.5 | 9.6 | 19.9 | 10.3 | 0.1 | 5.4 | 19.0 | 0.6 |
| GTREOC5.0 | 8.3 | 16.9 | 8.7 | 0.3 | 5.1 | 21.0 | 0.9 |
| GTREOC10.0 | 6.4 | 14.8 | 8.4 | 0.3 | 5.4 | 19.6 | 0.6 |
| GTREOC15.0 | 5.2 | 12.7 | 7.4 | 0.4 | 6.2 | 17.2 | 0.4 |
| Sample Code | Tensile Strength (MPa) | Elongation at Break (%) | Hardness (Shore A) | Density (g/cm3) | Swelling Degree (%) | Sol Fraction (%) | Cross-Link Density (mol/cm3 × 10−4) |
|---|---|---|---|---|---|---|---|
| GTRSBR2.5 | 5.2 ± 0.3 | 113 ± 8 | 71 ± 1 | 1.162 ± 0.002 | 122 ± 4 | 9.4 ± 0.2 | 1.30 ± 0.07 |
| GTRSBR5.0 | 6.4 ± 0.3 | 127 ± 7 | 75 ± 1 | 1.158 ± 0.005 | 133 ± 1 | 9.1 ± 0.1 | 1.13 ± 0.01 |
| GTRSBR10.0 | 7.5 ± 0.1 | 133 ± 4 | 81 ± 1 | 1.149 ± 0.002 | 146 ± 1 | 7.9 ± 0.1 | 1.01 ± 0.02 |
| GTRSBR15.0 | 8.1 ± 0.3 | 136 ± 4 | 84 ± 1 | 1.144 ± 0.001 | 157 ± 1 | 7.0 ± 0.2 | 0.92 ± 0.01 |
| GTRSEBS-g-MA2.5 | 5.0 ± 0.1 | 120 ± 6 | 66 ± 1 | 1.155 ± 0.001 | 125 ± 3 | 9.5 ± 0.1 | 1.25 ± 0.04 |
| GTRSEBS-g-MA5.0 | 5.6 ± 0.2 | 134 ± 3 | 66 ± 1 | 1.144 ± 0.004 | 138 ± 0 | 9.4 ± 0.1 | 1.07 ± 0.01 |
| GTRSEBS-g-MA10.0 | 6.3 ± 0.1 | 148 ± 6 | 67 ± 1 | 1.126 ± 0.001 | 151 ± 2 | 9.3 ± 0.2 | 0.94 ± 0.03 |
| GTRSEBS-g-MA15.0 | 6.9 ± 0.1 | 170 ± 5 | 67 ± 1 | 1.112 ± 0.002 | 172 ± 1 | 9.2 ± 0.1 | 0.77 ± 0.01 |
| GTREOC2.5 | 4.5 ± 0.1 | 126 ± 3 | 64 ± 1 | 1.150 ± 0.001 | 131 ± 1 | 10.0 ± 0.2 | 1.13 ± 0.02 |
| GTREOC5.0 | 4.1 ± 0.1 | 130 ± 4 | 63 ± 1 | 1.138 ± 0.003 | 141 ± 1 | 9.7 ± 0.2 | 1.03 ± 0.02 |
| GTREOC10.0 | 3.8 ± 0.2 | 139 ± 5 | 61 ± 1 | 1.121 ± 0.001 | 167 ± 4 | 10.5 ± 0.1 | 0.76 ± 0.03 |
| GTREOC15.0 | 3.5 ± 0.3 | 143 ± 8 | 60 ± 1 | 1.100 ± 0.001 | 187 ± 3 | 10.4 ± 0.3 | 0.64 ± 0.02 |
| Sample Composition | Sample Preparation | Tensile Strength (MPa) | Elongation at Break (%) | Hardness (Sh A) | References |
|---|---|---|---|---|---|
| GTR/SBR + DCP 100/2.5, 100/5, 100/10, 100/15 | Extrusion at 60 °C; compression molding at 170 °C | 5.2 ± 0.3 6.4 ± 0.3 7.5 ± 0.1 8.1 ± 0.3 | 113 ± 8 127 ± 7 133 ± 4 136 ± 4 | 71 ± 1 75 ± 1 81 ± 1 84 ± 1 | This study |
| LDPE/SBR/GTR + DCP 50/25/25 | Two-roll mills at 60 °C (GTR and SBR); internal mixer at 130 °C at a rotor speed of 60 rpm (LDPE, GTR/SBR, and DCP); compression molding at 135 °C | 4.1 | 33 | 82 | [56] |
| SBR/GTR + sulfur system 50/50 | Microwave devulcanization of GTR; two-roll mill at room temperature; compression molding at 170 °C | 4.7–4.9 | 366–445 | 66–67 | [57] |
| SBR/GTR + sulfur system 40/60 | Two-roll mills at room temperature; compression molding at 160 °C | 5.0 | 445 | 60 | [58] |
| SBR/GTR + sulfur system 0/100, 10/90, 20/80 | Mechano-chemical devulcanization of GTR; two-roll mills at 50 °C; compression molding at 142 °C | 3.1 4.8 6.0 | 100 160 200 | - | [59] |
| PP/SBR/GTR + DCP 30/40/30 | Internal mixer at 185 °C at a rotor speed of 60 rpm; injection molding at 240 °C | 10–11 | 175–225 | - | [60] |
| Element (wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Zn | Si | S | Ca | Al | Br | Fe | ||
| Uncured | GTRSBR2.5 | 0.88 | 0.71 | 0.82 | 0.19 | 0.05 | 0.03 | 0.02 |
| GTRSBR15.0 | 0.56 | 0.40 | 0.40 | 0.12 | 0.05 | 0.02 | 0.01 | |
| GTRSEBS-g-MA2.5 | 1.17 | 0.87 | 0.82 | 0.22 | - | 0.04 | 0.03 | |
| GTRSEBS-g-MA15.0 | 0.91 | 0.67 | 0.65 | 0.21 | 0.04 | 0.03 | 0.02 | |
| GTREOC2.5 | 1.01 | 0.76 | 0.72 | 0.22 | 0.05 | 0.04 | 0.02 | |
| GTREOC15.0 | 0.68 | 0.42 | 0.35 | 0.14 | 0.07 | 0.02 | 0.02 | |
| Cured | GTRSBR2.5 | 1.10 | 1.18 | 1.04 | 0.25 | 0.11 | 0.04 | 0.03 |
| GTRSBR15.0 | 1.01 | 0.97 | 0.91 | 0.22 | 0.15 | 0.04 | 0.02 | |
| GTRSEBS-g-MA2.5 | 0.96 | 1.09 | 0.99 | 0.20 | 0.08 | 0.03 | 0.02 | |
| GTRSEBS-g-MA15.0 | 1.07 | 1.15 | 0.89 | 0.24 | 0.08 | 0.04 | 0.02 | |
| GTREOC2.5 | 1.27 | 1.28 | 1.17 | 0.29 | 0.12 | 0.05 | 0.03 | |
| GTREOC15.0 | 1.02 | 0.92 | 0.83 | 0.23 | 0.11 | 0.04 | 0.02 | |
| Retention Time (min) | Identified Compound | Chemical Structure | Molecular Weight (g/mol) | Match Quality (%) | Source | References |
|---|---|---|---|---|---|---|
| 4.02 | benzene | 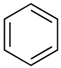 | 78.11 | 91 | styrene-butadiene rubber present in GTR | [62] |
| 5.30 | toluene |  | 92.14 | 94 | styrene-butadiene rubber present in GTR | [62,63] |
| 6.73 | xylene |  | 106.17 | 97 | styrene-butadiene rubber present in GTR | [62,63] |
| 7.04 | styrene |  | 104.15 | 97 | styrene-butadiene rubber present in GTR | [62,63] |
| 8.12 | benzaldehyde |  | 106.12 | 96 | styrene-butadiene rubber present in GTR | [62] |
| 8.78 | α-methylstyrene | 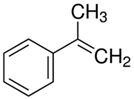 | 118.18 | 96 | dicumyl peroxide decomposition | [64] |
| 9.68 | cymene | 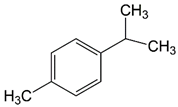 | 134.22 | 94 | styrene-butadiene rubber present in GTR | |
| 9.89 | limonene | 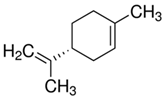 | 136.23 | 94 | natural rubber present in GTR | [28,65,66] |
| 10.18 | acetophenone | 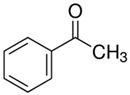 | 120.15 | 94 | dicumyl peroxide decomposition | [64] |
| 12.93 | dodecene |  | 168.32 | 96 | aliphatic thermplastics and natural rubber present in GTR | - |
| 16.01 | tetradecane |  | 198.39 | 98 | aliphatic thermplastics and natural rubber present in GTR | - |
| Sample Code | TVOCs [µg] Measured | TVOCs [µg/g] Measured | |||
|---|---|---|---|---|---|
| During Extrusion (Radiello®) | After Extrusion (Micro-Chamber/ Thermal ExtractorTM) | After Extrusion (SHS-GC-MS) | After curing (Micro-Chamber/ Thermal ExtractorTM) | After Curing (SHS-GC-MS) | |
| GTRSBR2.5 | 9.8 | 4.5 | 82 | 44.8 | 1645 |
| GTRSBR15.0 | 10.1 | 5.7 | 130 | 48.9 | 1965 |
| GTRSEBS-g-MA2.5 | - | 3.3 | 79 | 75.3 | 1608 |
| GTRSEBS-g-MA15.0 | 7.2 | 5.9 | 80 | 67.5 | 316 |
| GTREOC2.5 | 9.3 | 3.0 | 71 | 60.8 | 1854 |
| GTREOC15.0 | 6.4 | 2.8 | 48 | 57.1 | 227 |
| Identified VOC | α-Methylstyrene | Acetophenone | α-Cumyl Alcohol | Methyl Cumyl Ether | Benzothiazole | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | (µg/g) | (% TVOC) | (µg/g) | (% TVOC) | (µg/g) | (% TVOC) | (µg/g) | (% TVOC) | (µg/g) | (% TVOC) | |
| Uncured | GTRSBR2.5 | 0.2 | 5.2 | 0.4 | 8.6 | 0.9 | 20.0 | - | - | 0.3 | 5.8 |
| GTRSBR15.0 | 0.2 | 4.2 | 0.7 | 11.7 | 3.0 | 52.1 | <0.1 | 0.3 | 0.1 | 2.2 | |
| GTRSEBS-g-MA2.5 | 0.1 | 3.0 | 0.2 | 6.4 | 0.4 | 11.2 | - | - | 0.3 | 8.0 | |
| GTRSEBS-g-MA15.0 | 0.2 | 3.9 | 0.5 | 9.1 | 2.1 | 35.7 | <0.1 | 0.1 | 0.2 | 4.2 | |
| GTREOC2.5 | 0.1 | 1.8 | 0.1 | 4.3 | 0.4 | 13.4 | - | - | 0.3 | 8.3 | |
| GTREOC15.0 | 0.1 | 1.5 | 0.1 | 3.0 | 0.3 | 11.8 | <0.1 | 0.2 | 0.2 | 8.4 | |
| Cured | GTRSBR2.5 | 1.1 | 2.6 | 5.4 | 12.2 | 35.0 | 78.3 | 1.4 | 3.2 | 0.1 | 0.2 |
| GTRSBR15.0 | 1.5 | 3.0 | 9.1 | 18.5 | 34.8 | 71.2 | 2.0 | 4.1 | 0.1 | 0.3 | |
| GTRSEBS-g-MA2.5 | 2.1 | 2.8 | 9.6 | 12.7 | 59.3 | 78.8 | 2.0 | 2.6 | 0.2 | 0.2 | |
| GTRSEBS-g-MA15.0 | 1.9 | 2.8 | 11.5 | 17.0 | 49.5 | 73.3 | 2.2 | 3.3 | 0.1 | 0.2 | |
| GTREOC2.5 | 1.6 | 2.6 | 7.8 | 12.9 | 47.7 | 78.5 | 1.6 | 2.7 | 0.2 | 0.3 | |
| GTREOC15.0 | 1.7 | 3.0 | 8.7 | 15.3 | 43.4 | 76.0 | 1.6 | 2.8 | 0.2 | 0.3 | |
| Concentration (µg/g) | ||||||
|---|---|---|---|---|---|---|
| α-Methylstyrene | Acetophenone | α-Cumyl Alcohol | Methyl Cumyl Ether | Benzothiazole | ||
| Uncured | GTRSBR2.5 | 12 | 7 | 26 | 2 | - |
| GTRSBR15.0 | 36 | 14 | 34 | 3 | - | |
| GTRSEBS-g-MA2.5 | 9 | 6 | 15 | - | - | |
| GTRSEBS-g-MA15.0 | 9 | - | 29 | 3 | - | |
| GTREOC2.5 | 4 | 10 | 17 | - | - | |
| GTREOC15.0 | - | - | - | - | - | |
| Cured | GTRSBR2.5 | 7 | 89 | 1515 | 12 | 3 |
| GTRSBR15.0 | 8 | 251 | 1656 | 24 | 5 | |
| GTRSEBS-g-MA2.5 | 6 | 71 | 1498 | 13 | - | |
| GTRSEBS-g-MA15.0 | 7 | 32 | 243 | 10 | - | |
| GTREOC2.5 | 4 | 163 | 1659 | 15 | 4 | |
| GTREOC15.0 | 20 | 24 | 146 | 12 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiśniewska, P.; Zedler, Ł.; Marć, M.; Klein, M.; Haponiuk, J.; Formela, K. Ground Tire Rubber Modified by Elastomers via Low-Temperature Extrusion Process: Physico-Mechanical Properties and Volatile Organic Emission Assessment. Polymers 2022, 14, 546. https://doi.org/10.3390/polym14030546
Wiśniewska P, Zedler Ł, Marć M, Klein M, Haponiuk J, Formela K. Ground Tire Rubber Modified by Elastomers via Low-Temperature Extrusion Process: Physico-Mechanical Properties and Volatile Organic Emission Assessment. Polymers. 2022; 14(3):546. https://doi.org/10.3390/polym14030546
Chicago/Turabian StyleWiśniewska, Paulina, Łukasz Zedler, Mariusz Marć, Marek Klein, Józef Haponiuk, and Krzysztof Formela. 2022. "Ground Tire Rubber Modified by Elastomers via Low-Temperature Extrusion Process: Physico-Mechanical Properties and Volatile Organic Emission Assessment" Polymers 14, no. 3: 546. https://doi.org/10.3390/polym14030546






