Mechanisms of Silique Dehiscence in Rapeseed: A Review of Research Progress
Abstract
1. Introduction
2. The Importance of Mechanized Production to Improve the Yield of Rape
3. Effect of Pod Dehiscence on Rapeseed Production
4. The Cracking Process of Rape Pods
5. Research Progress on Silique Dehiscence Gene
6. Molecular Mechanism of Rapeseed Pod Dehiscence
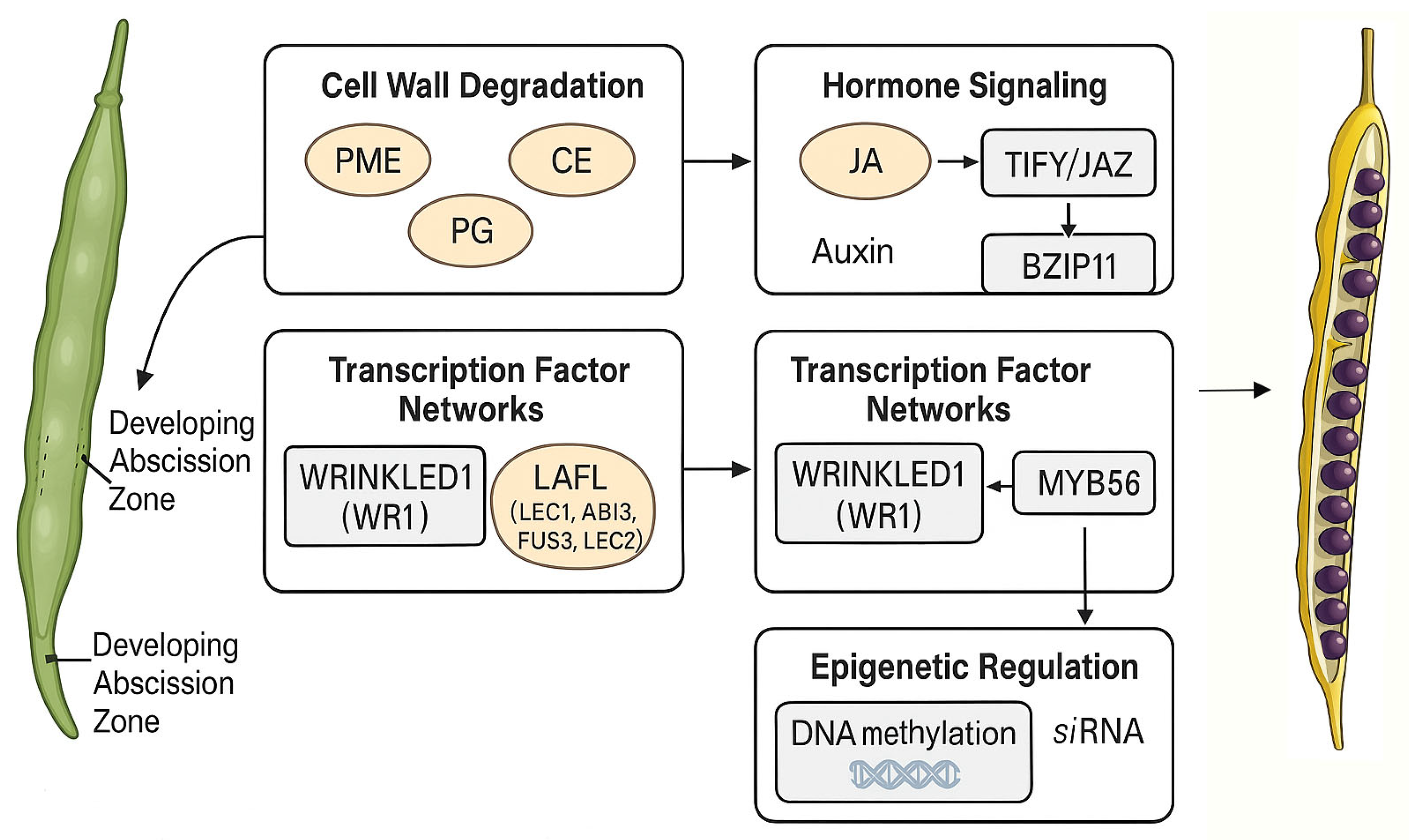
6.1. Regulation Network of Pod Abscission Zone Development in Rapeseed
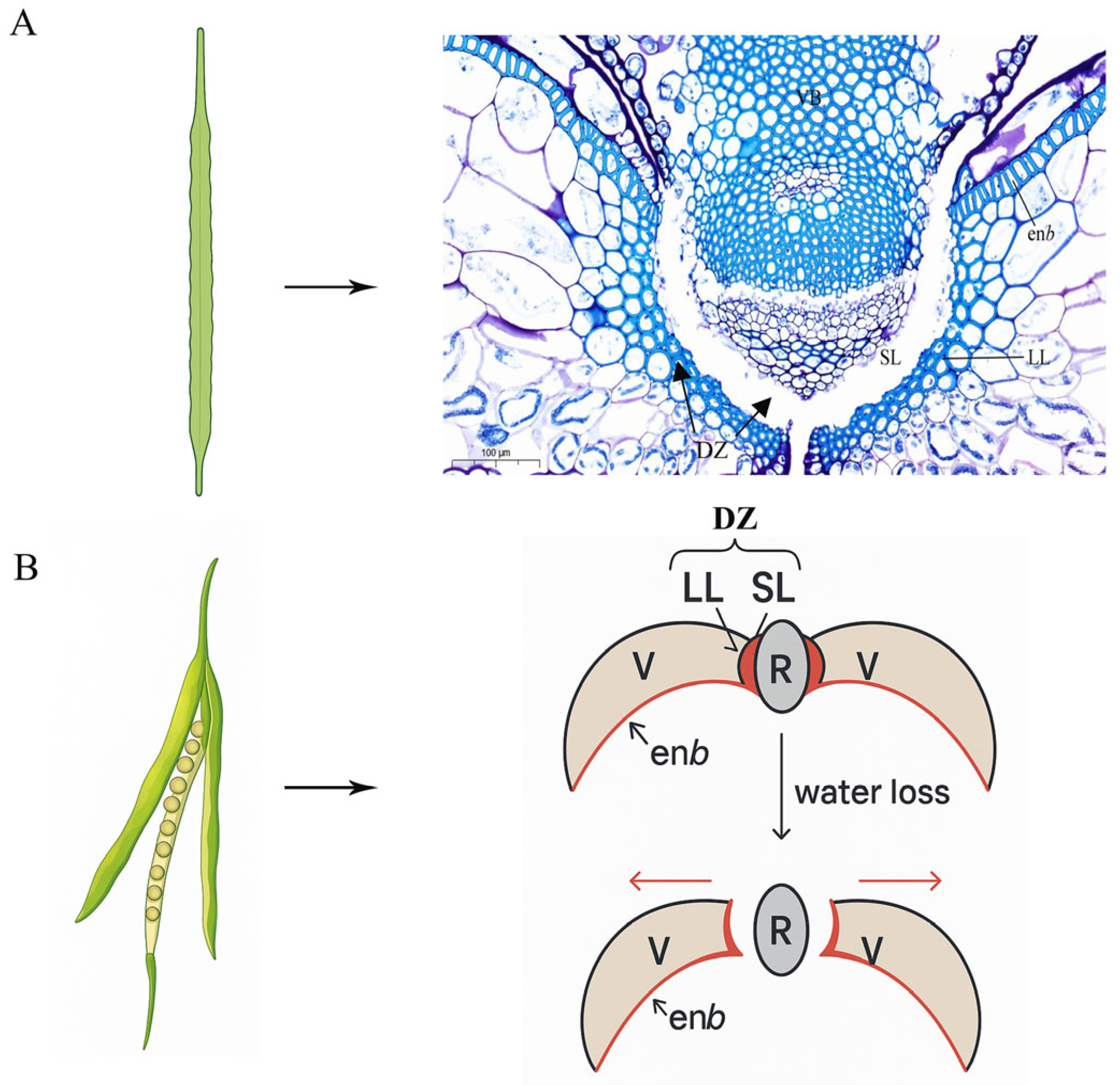
6.2. Development of Lignified Tissue of Rape Silique
6.3. Rape Pod Cell Wall Modification Related Enzymes
6.4. Effect of Hormones on Pod Dehiscence in Rapeseed
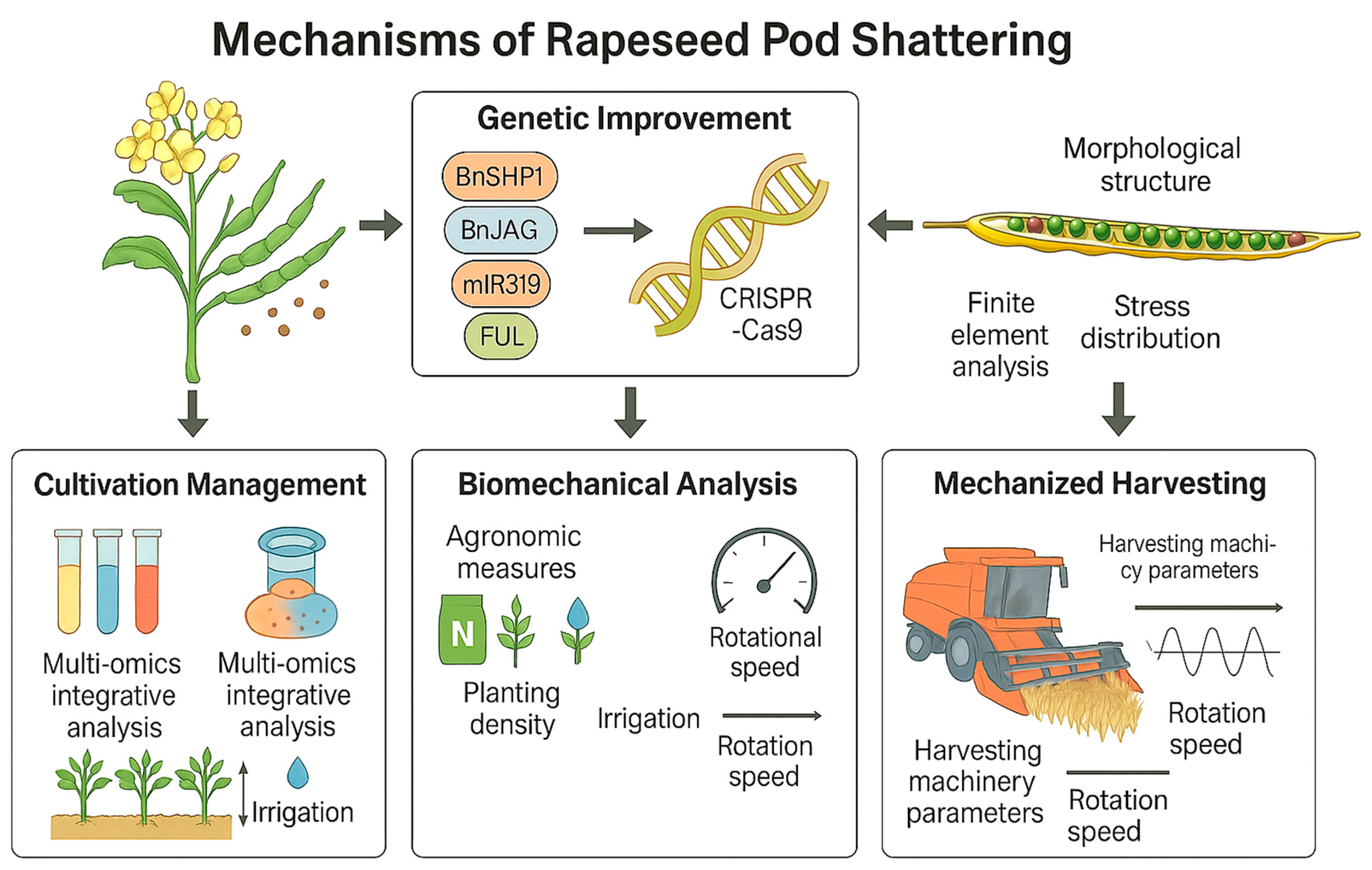
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aquilia, S.; Bello, C.; Pinna, M.; Bianchi, S.; Giurlani, W.; Ciardelli, F.; Rosi, L.; Papini, A.M. Incorporation of Protein Hydrolysate into Rapeseed Meal-Based Materials to Improve Flexibility. Polymers 2025, 17, 1740. [Google Scholar] [CrossRef]
- Quan, X.; Wang, X.; Chen, C.; Ma, T.; Wang, D.; Zhang, Y.; Liu, J. A comparative evaluation of using bio- and petroleum-based rejuvenators in aged asphalt rejuvenation: Rheological performance, microscopic properties, and life cycle assessments. Constr. Build. Mater. 2025, 492, 142866. [Google Scholar] [CrossRef]
- Witaszek, K.; Kupryaniuk, K.; Kupryaniuk, J.; Panasiewicz, J.; Czekala, W. Optimization of Straw Particle Size for Enhanced Biogas Production: A Comparative Study of Wheat and Rapeseed Straw. Energies 2025, 18, 1794. [Google Scholar] [CrossRef]
- Decsi, K.; Hegedus, G.; Kutasy, B.; Virag, E. RNA-seq datasets of field rapeseed (Brassica napus) cultures conditioned by Elice16Indures biostimulator. Data Brief 2022, 45, 108602. [Google Scholar] [CrossRef] [PubMed]
- MacIntosh, S.C.; Shaw, M.; Connelly, M.; Yao, Z.J. Food and Feed Safety of NS-B5oo27-4 Omega-3 Canola (Brassica napus): A New Source of Long-Chain Omega-3 Fatty Acids. Front. Nutr. 2021, 8, 716659. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.D.; Han, X.; Dai, C.; Lu, S.P.; He, H.Z.; Yao, X.; Chen, P.; Yang, C.; Zhao, L.; Yang, Q.Y.; et al. Functional genomics of Brassica napus: Progresses, challenges, and perspectives. J. Integr. Plant Biol. 2024, 66, 484–509. [Google Scholar] [CrossRef]
- Yun, J.; Wang, C.; Zhang, F.; Chen, L.; Sun, Z.; Cai, Y.; Luo, Y.; Liao, J.; Wang, Y.; Cha, Y.; et al. A nitrogen fixing symbiosis-specific pathway required for legume flowering. Sci. Adv. 2023, 9, ade1150. [Google Scholar] [CrossRef]
- Watson, C.A.; Reckling, M.; Preissel, S.; Bachinger, J.; Bergkvist, G.; Kuhlman, T.; Lindstrom, K.; Nemecek, T.; Topp, C.F.E.; Vanhatalo, A.; et al. Grain Legume Production and Use in European Agricultural Systems. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 144, pp. 235–303. [Google Scholar]
- Gao, Z.; Tu, Y.; Liao, C.; Guo, P.; Tian, Y.; Zhou, Y.; Xie, Q.; Chen, G.; Hu, Z. Overexpression of SlALC Increases Drought and Salt Tolerance and Affects Fruit Dehiscence in Tomatoes. Int. J. Mol. Sci. 2024, 25, 9433. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.F.; Tang, Y.; Li, J.G.; Zhao, M.L. RNA-Seq Provides New Insights into the Molecular Events Involved in “Ball-Skin versus Bladder Effect” on Fruit Cracking in Litchi. Int. J. Mol. Sci. 2021, 22, 454. [Google Scholar] [CrossRef]
- Shirokova, A.V.; Volovik, V.T.; Zagoskina, N.V.; Zaitsev, G.P.; Khudyakova, H.K.; Korovina, L.M.; Krutius, O.N.; Nikolaeva, T.N.; Simonova, O.B.; Alekseev, A.A.; et al. From Dimness to Glossiness-Characteristics of the Spring Rapeseed Mutant Form without Glaucous Bloom (Brassica napus L.). Agronomy 2020, 10, 1563. [Google Scholar] [CrossRef]
- Karlsson, I.; Friberg, H.; Kolseth, A.-K.; Steinberg, C.; Persson, P. Agricultural factors affecting Fusarium communities in wheat kernels. Int. J. Food Microbiol. 2017, 252, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Zumajo-Cardona, C.; Ambrose, B.A.; Madrigal, Y.; Pabon-Mora, N. Dehiscent fruits in Brassicaceae and Papaveraceae: Convergent morpho-anatomical features with divergent underlying genetic mechanisms. Ann. Bot. 2025, mcaf079. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, M.; Rabbani, A.M.; Iqbal, S.; Fayyaz, L.; Afzal, M. Genetic diversity in Brassica species AND Eruca sativa for yield associated parameters. Genet 2014, 46, 537–543. [Google Scholar] [CrossRef][Green Version]
- Li, Y.-L.; Yu, Y.-K.; Zhu, K.-M.; Ding, L.-N.; Wang, Z.; Yang, Y.-H.; Cao, J.; Xu, L.-Z.; Li, Y.-M.; Tan, X.-L. Down-regulation of MANNANASE7 gene in Brassica napus L. enhances silique dehiscence-resistance. Plant Cell Rep. 2021, 40, 361–374. [Google Scholar] [CrossRef]
- Marsh, J.I.; Nestor, B.J.; Petereit, J.; Tay Fernandez, C.G.; Bayer, P.E.; Batley, J.; Edwards, D. Legume-wide comparative analysis of pod shatter locus PDH1 reveals phaseoloid specificity, high cowpea expression, and stress responsive genomic context. Plant J. 2023, 115, 68–80. [Google Scholar] [CrossRef]
- Sun, W.; Chen, Y.; Zeng, J.; Li, C.; Yao, M.; Liu, M.; Ma, Z.; Huang, L.; Yan, J.; Zhan, J.; et al. The Tartary buckwheat bHLH gene ALCATRAZ contributes to silique dehiscence in Arabidopsis thaliana. Plant Sci. 2023, 333, 111733. [Google Scholar] [CrossRef]
- Zhang, T.; Hong, Y.; Zhang, X.; Yuan, X.; Chen, S. Relationship between Key Environmental Factors and the Architecture of Fruit Shape and Size in Near-Isogenic Lines of Cucumber (Cucumis sativus L.). Int. J. Mol. Sci. 2022, 23, 14033. [Google Scholar] [CrossRef]
- Pan, Y.; Luo, Y.; Bao, J.; Wu, C.; Wang, J.; Liu, M.; Yan, F. Screening candidate genes for fruit size based on QTL-seq in Chinese jujube. Front. Plant Sci. 2024, 15, 1361771. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, M.; Bai, M.; Xu, Y.; Fan, S.; Yang, G. Effect of calcium on relieving berry cracking in grape (Vitis vinifera L.) ‘Xiangfei’. PeerJ 2020, 8, 9896. [Google Scholar] [CrossRef]
- Ren, H.; Zhao, Q.; Feng, Y.; Tang, P.; Wang, Y.; Jiang, J.; Hu, C.; Wang, Y.; Cui, B.; Xie, X.; et al. Gene-specific silencing of SlPL16, a pectate lyase coding gene, extends the shelf life of tomato fruit. Postharvest Biol. Technol. 2023, 201, 112368. [Google Scholar] [CrossRef]
- D’Aquino, S.; Strano, M.C.; Gentile, A.; Palma, A. Decay Incidence and Quality Changes of Film Packaged ‘Simeto’ Mandarins Treated with Sodium Bicarbonate. Horticulturae 2022, 8, 354. [Google Scholar] [CrossRef]
- Yudina, L.; Sukhova, E.; Mudrilov, M.; Nerush, V.; Pecherina, A.; Smirnov, A.A.; Dorokhov, A.S.; Chilingaryan, N.O.; Vodeneev, V.; Sukhov, V. Ratio of Intensities of Blue and Red Light at Cultivation Influences Photosynthetic Light Reactions, Respiration, Growth, and Reflectance Indices in Lettuce. Biology 2022, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Litt, P.K.; Kelly, A.; Omar, A.; Johnson, G.; Vinyard, B.T.; Kniel, K.E.; Sharma, M. Temporal and Agricultural Factors Influence Escherichia coli Survival in Soil and Transfer to Cucumbers. Appl. Environ. Microbiol. 2021, 87, e02418-20. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Javed, H.H.; Hu, Y.; Luo, Y.-Q.; Peng, X.; Wu, Y.-C. Research progress and mitigation strategies for pod shattering resistance in rapeseed. PeerJ 2024, 12, 18105. [Google Scholar] [CrossRef]
- Qing, Y.; Li, Y.; Xu, L.; Ma, Z. Screen Oilseed Rape (Brassica napus) Suitable for Low-Loss Mechanized Harvesting. Agric 2021, 11, 504. [Google Scholar] [CrossRef]
- Chu, W.; Liu, J.; Cheng, H.; Li, C.; Fu, L.; Wang, W.; Wang, H.; Hao, M.; Mei, D.; Liu, K.; et al. A lignified-layer bridge controlled by a single recessive gene is associated with high pod-shatter resistance in Brassica napus L. Crop J. 2022, 10, 638–646. [Google Scholar] [CrossRef]
- Hussain, Q.; Zhan, J.; Liang, H.; Wang, X.; Liu, G.; Shi, J.; Wang, H. Key genes and mechanisms underlying natural variation of silique length in oilseed rape (Brassica napus L.) germplasm. Crop J. 2022, 10, 617–626. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, R.; Wang, W.; Wang, H.; Qiu, Y.; Raman, R.; Mei, D.; Raman, H.; Hu, Q. A copia-like retrotransposon insertion in the upstream region of the SHATTERPROOF1 gene, BnSHP1. A9, is associated with quantitative variation in pod shattering resistance in oilseed rape. J. Exp. Bot. 2020, 71, 5402–5413. [Google Scholar] [CrossRef]
- Li, H.; Li, F.; Wang, M.; Hou, C.; Jia, F.; Wang, X.; Li, M. Growth and selenium bioaccumulation in rape seedlings promoted by strain Limosilactobacillus sp. LF-17. BMC Plant Biol. 2025, 25, 429. [Google Scholar] [CrossRef]
- Weymann, W.; Boettcher, U.; Sieling, K.; Kage, H. Effects of weather conditions during different growth phases on yield formation of winter oilseed rape. Field Crops Res. 2015, 173, 41–48. [Google Scholar] [CrossRef]
- Zheng, M.; Terzaghi, W.; Wang, H.; Hua, W. Integrated strategies for increasing rapeseed yield. Trends Plant Sci. 2022, 27, 742–745. [Google Scholar] [CrossRef]
- de Oliveira, A.M.R.C.B.; Yu, P. Research progress and future study on physicochemical, nutritional, and structural characteristics of canola and rapeseed feedstocks and co-products from bio-oil processing and nutrient modeling evaluation methods. Crit. Rev. Food Sci. Nutr. 2023, 63, 6484–6490. [Google Scholar] [CrossRef]
- Zhang, M.; Li, G.; Yang, Y.; Jin, M.; Wang, G. Test Trials and Analysis of Pod-Shattering Characteristics of Harvested Rapeseed Silique. Appl. Sci. 2023, 13, 9369. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Wei, C.; Yang, C.; Zhong, S. Effect of Mechanized Ridge Tillage with Rice-Rape Rotation on Paddy Soil Structure. Agric 2022, 12, 2147. [Google Scholar] [CrossRef]
- Hu, Q.; Hua, W.; Yin, Y.; Zhang, X.; Liu, L.; Shi, J.; Zhao, Y.; Qin, L.; Chen, C.; Wang, H. Rapeseed research and production in China. Crop J. 2017, 5, 127–135. [Google Scholar] [CrossRef]
- Qian, L.; Lu, H.; Gao, Q.; Lu, H.L. Household-owned farm machinery vs. outsourced machinery services: The impact of agricultural mechanization on the land leasing behavior of relatively large-scale farmers in China. Land Use Policy 2022, 115, 106008. [Google Scholar] [CrossRef]
- Jiang, T.; Guan, Z.; Li, H.; Zhang, M.; Mu, S.; Wu, C.; Jin, M. Collaborative optimization method of cleaning operational performance and multiparameter online control system for combine harvesters. Comput. Electron. Agric. 2025, 235, 110389. [Google Scholar] [CrossRef]
- Yang, M.; He, J.; Wan, S.; Li, W.; Chen, W.; Wang, Y.; Jiang, X.; Cheng, P.; Chu, P.; Shen, W.; et al. Fine mapping of the BnaC04. BIL1 gene controlling plant height in Brassica napus L. BMC Plant Biol. 2021, 21, 359. [Google Scholar] [CrossRef]
- Ping, X.; Ye, Q.; Yan, M.; Zeng, J.; Yan, X.; Li, H.; Li, J.; Liu, L. Integrated genetic mapping and transcriptome analysis reveal the BnaA03. IAA7 protein regulates plant architecture and gibberellin signaling in Brassica napus L. Theor. Appl. Genet. 2022, 135, 3497–3510. [Google Scholar] [CrossRef]
- Guo, C.Y.; Bai, Z.H.; Wang, X.Z.; Zhang, W.S.; Chen, X.J.; Lakshmanan, P.; Ma, L.; Lu, J.W.; Liu, B.; Shi, X.J.; et al. Spatio-temporal assessment of greenhouse gas emission from rapeseed production in China by coupling nutrient flows model with LCA approach. Food Energy Secur. 2022, 11, e398. [Google Scholar] [CrossRef]
- Kazemi, H.; Bourkheili, S.H.; Kamkar, B.; Soltani, A.; Gharanjic, K.; Nazari, N.M. Estimation of greenhouse gas (GHG) emission and energy use efficiency (EUE) analysis in rainfed canola production (case study: Golestan province, Iran). Energy 2016, 116, 694–700. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Mei, H.C.; Yan, Z.H.; Hu, A.B.; Wang, S.M.; Feng, C.H.; Chen, K.H.; Li, W.; Zhang, X.H.; Ji, P.P.; et al. Year-Round Production of Cotton and Wheat or Rapeseed Regulated by Different Nitrogen Rates with Crop Straw Returning. Agronomy 2023, 13, 1254. [Google Scholar] [CrossRef]
- Jung, J.; Maeda, M.; Chang, A.; Bhandari, M.; Ashapure, A.; Landivar-Bowles, J. The potential of remote sensing and artificial intelligence as tools to improve the resilience of agriculture production systems. Curr. Opin. Biotechnol. 2021, 70, 15–22. [Google Scholar] [CrossRef]
- Karunathilake, E.M.B.M.; Le, A.T.; Heo, S.; Chung, Y.S.; Mansoor, S. The Path to Smart Farming: Innovations and Opportunities in Precision Agriculture. Agric 2023, 13, 1593. [Google Scholar] [CrossRef]
- Zhan, G.C.; Zong, W.Y.; Ma, L.; Wei, J.Y.; Liu, W. Biomechanical properties of ready-to-harvest rapeseed plants: Measurement and analysis. Inf. Process. Agric. 2023, 10, 391–399. [Google Scholar] [CrossRef]
- Steponavicius, D.; Kemzuraite, A.; Bausa, L.; Zaleckas, E. Evaluation of the Effectiveness of Pod Sealants in Increasing Pod Shattering Resistance in Oilseed Rape (Brassica napus L.). Energies 2019, 12, 2256. [Google Scholar] [CrossRef]
- Li, Q.; Luo, T.; Cheng, T.; Yang, S.T.; She, H.J.; Li, J.; Wang, B.; Kuai, J.; Wang, J.; Xu, Z.H.; et al. Evaluation and Screening of Rapeseed Varieties (Brassica napus L.) Suitable for Mechanized Harvesting with High Yield and Quality. Agronomy 2023, 13, 795. [Google Scholar] [CrossRef]
- Kowsalya, K.; Halka, J.; Anand, M.; Sahayarayan, J.J.; Rajkumar, R.; Arun, M. Unraveling the multifaceted role of ethephon in plant physiology: From seed germination to crop maturation and harvesting. J. Plant Biochem. Biotechnol. 2025, 34, 639–664. [Google Scholar] [CrossRef]
- Wang, C.M.; Xu, M.C.; Wang, Y.C.; Batchelor, W.D.; Zhang, J.; Kuai, J.; Ling, L. Long-Term Optimal Management of Rapeseed Cultivation Simulated with the CROPGRO-Canola Model. Agronomy 2022, 12, 1191. [Google Scholar] [CrossRef]
- Zhang, Z.; Cong, R.H.; Ren, T.; Li, H.; Zhu, Y.; Lu, J.W. Optimizing agronomic practices for closing rapeseed yield gaps under intensive cropping systems in China. J. Integr. Agric. 2020, 19, 1241–1249. [Google Scholar] [CrossRef]
- Lou, H.X.; Zhao, B.W.; Peng, Y.; El-Badri, A.M.; Batool, M.; Wang, C.Y.; Wang, Z.K.; Huang, W.; Wang, T.Y.; Li, Z.; et al. Auxin plays a key role in nitrogen and plant density-modulated root growth and yield in different plant types of rapeseed. Field Crops Res. 2023, 302, 109066. [Google Scholar] [CrossRef]
- Raman, H.; Raman, R.; Sharma, N.; Cui, X.B.; McVittie, B.; Qiu, Y.; Zhang, Y.Y.; Hu, Q.; Liu, S.Y.; Gororo, N. Novel quantitative trait loci from an interspecific Brassica rapa derivative improve pod shatter resistance in Brassica napus. Front. Plant Sci. 2023, 14, 1233996. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Qiu, Z.P.; Wang, H. Tipping points in seed dispersal mutualism driven by environmental stochasticity. Siam J. Appl. Math. 2024, 84, 114–138. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Y.Y.; Wu, X.M.; Wang, J.B. The basis of pod dehiscence: Anatomical traits of the dehiscence zone and expression of eight pod shatter-related genes in four species of Brassicaceae. Biol. Plant. 2016, 60, 343–354. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, H.; Zhang, W.; Liu, Y.; Ge, D.; Feng, C.; Chen, W.; Song, C.; Sijun, G.; Zhang, Q.; et al. Biomass-Based Rapeseed (Brassica napus) Pod Morphological Model. Int. J. Agric. Biol. 2018, 20, 1193–1200. [Google Scholar]
- Tang, M.; Tong, C.; Liang, L.; Du, C.; Zhao, J.; Xiao, L.; Tong, J.; Dai, X.; Helal, M.M.U.; Dai, W.; et al. A recessive high-density pod mutant resource of Brassica napus. Plant Sci. 2020, 293, 110411. [Google Scholar] [CrossRef]
- Mahmood, U.; Li, X.; Qian, M.; Fan, Y.; Yu, M.; Li, S.; Shahzad, A.; Qu, C.; Li, J.; Liu, L.; et al. Comparative transcriptome and co-expression network analysis revealed the genes associated with senescence and polygalacturonase activity involved in pod shattering of rapeseed. Biotechnol. Biofuels Bioprod. 2023, 16, 20. [Google Scholar] [CrossRef]
- Zhang, F.G.; Liu, N.; Chen, T.H.; Xu, H.; Li, R.; Wang, L.Y.; Zhou, S.; Cai, Q.A.; Hou, X.Z.; Wang, L.; et al. Genome-wide identification of GH28 family and insight into its contributions to pod shattering resistance in Brassica napus L. BMC Genom. BMC Genom. 2024, 25, 492. [Google Scholar] [CrossRef]
- Wang, D.; Lu, Q.; Jin, S.; Fan, X.; Ling, H. Pectin, Lignin and Disease Resistance in Brassica napus L.: An Update. Horticulturae 2023, 9, 112. [Google Scholar] [CrossRef]
- Liljegren, S.J.; Ditta, G.S.; Eshed, Y.; Savidge, B.; Bowman, J.L.; Yanofsky, M.F. SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 2000, 404, 766–770. [Google Scholar] [CrossRef]
- Zhai, Y.; Cai, S.; Hu, L.; Yang, Y.; Amoo, O.; Fan, C.; Zhou, Y. CRISPR/Cas9-mediated genome editing reveals differences in the contribution of INDEHISCENT homologues to pod shatter resistance in Brassica napus L. Theor. Appl. Genet. 2019, 132, 2111–2123. [Google Scholar] [CrossRef] [PubMed]
- Ohama, N.; Moo, T.L.; Chung, K.; Mitsuda, N.; Boonyaves, K.; Urano, D.; Chua, N.-H. MEDIATOR15 destabilizes DELLA protein to promote gibberellin-mediated plant development. New Phytol. 2025, 245, 2665–2680. [Google Scholar] [CrossRef]
- Lynch, T.J.; Erickson McNally, B.J.; Losic, T.; Lundquist, J.; Finkelstein, R. ABI5 Binding Proteins are substrates of key components in the ABA core signaling pathway. bioRxiv 2024. [Google Scholar] [CrossRef]
- Roberts, J.A.; Elliott, K.A.; Gonzalez-Carranza, Z.H. Abscission, dehiscence, and other cell separation processes. Annu. Rev. Plant Biol. 2002, 53, 131–158. [Google Scholar] [CrossRef] [PubMed]
- Patharkar, O.R.; Walker, J.C. Connections between abscission, dehiscence, pathogen defense, drought tolerance, and senescence. Plant Sci. 2019, 284, 25–29. [Google Scholar] [CrossRef]
- Afridi, M.; Ahmad, K.; Malik, S.S.; Rehman, N.; Yasin, M.; Khan, S.M.; Hussain, A.; Khan, M.R. Genome-wide identification, phylogeny, and expression profiling analysis of shattering genes in rapeseed and mustard plants. J. Genet. Eng. Biotechnol. 2022, 20, 124. [Google Scholar] [CrossRef]
- Stephenson, P.; Stacey, N.; Bruser, M.; Pullen, N.; Ilyas, M.; O’Neill, C.; Wells, R.; Ostergaard, L. The power of model-to-crop translation illustrated by reducing seed loss from pod shatter in oilseed rape. Plant Reprod. 2019, 32, 331–340. [Google Scholar] [CrossRef]
- Braatz, J.; Harloff, H.-J.; Mascher, M.; Stein, N.; Himmelbach, A.; Jung, C. CRISPR-Cas9 Targeted Mutagenesis Leads to Simultaneous Modification of Different Homoeologous Gene Copies in Polyploid Oilseed Rape (Brassica napus). Plant Physiol. 2017, 174, 935–942. [Google Scholar] [CrossRef]
- Cui, Y.; Su, Y.; Bian, J.; Han, X.; Guo, H.; Yang, Z.; Chen, Y.; Li, L.; Li, T.; Deng, X.W.; et al. Single-nucleus RNA and ATAC sequencing analyses provide molecular insights into early pod development of peanut fruit. Plant Commun. 2024, 5, 100979. [Google Scholar] [CrossRef]
- Guo, M.W.; Zhu, L.; Li, H.Y.; Liu, W.P.; Wu, Z.N.; Wang, C.H.; Liu, L.; Li, Z.Y.; Li, J. Mechanism of pod shattering in the forage legume Medicago ruthenica. Plant Physiol. Biochem. 2022, 185, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, W.; Sun, L.; Zhu, H.; Hou, R.; Zhang, H.; Tang, X.; Clark, C.B.; Swarm, S.A.; Nelson, R.L.; et al. Artificial selection of mutations in two nearby genes gave rise to shattering resistance in soybean. Nat. Commun. 2024, 15, 7588. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Liu, Y.; Hassan, M.M.; Abraham, P.E.; Merlet, J.; Townsend, A.; Jacobson, D.; Buell, C.R.; Tuskan, G.A.; Yang, X. Advances in the Application of Single-Cell Transcriptomics in Plant Systems and Synthetic Biology. Biodesign Res. 2024, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Mukhtiar, A.; Ullah, S.; Yang, B.; Jiang, Y.-Q. Unlocking genetic potential: A review of the role of CRISPR/Cas technologies in rapeseed improvement. Stress Biol. 2025, 5, 31. [Google Scholar] [CrossRef]
- Jedlickova, V.; Hejret, V.; Demko, M.; Jedlicka, P.; Stefkova, M.; Robert, H.S. Transcriptome analysis of thermomorphogenesis in ovules and during early seed development in Brassica napus. BMC Genom. 2023, 24, 236. [Google Scholar] [CrossRef]
- Khan, D.; Ziegler, D.J.; Kalichuk, J.L.; Hoi, V.; Huynh, N.; Hajihassani, A.; Parkin, I.A.P.; Robinson, S.J.; Belmonte, M.F. Gene expression profiling reveals transcription factor networks and subgenome bias during Brassica napus seed development. Plant J. 2022, 109, 477–489. [Google Scholar] [CrossRef]
- Han, X.; Peng, Y.; Yin, S.; Zhao, H.; Zong, Z.; Tan, Z.; Zhang, Y.; Ma, W.; Guo, L. Transcriptional regulation of transcription factor genes WRI1 and LAFL during Brassica napus seed development. Plant Physiol. 2024, 197, kiae378. [Google Scholar] [CrossRef]
- Ziegler, D.J.; Khan, D.; Pulgar-Vidal, N.; Parkin, I.A.P.; Robinson, S.J.; Belmonte, M.F. Genomic asymmetry of the Brassica napus seed: Epigenetic contributions of DNA methylation and small RNAs to subgenome bias. Plant J. 2023, 115, 690–708. [Google Scholar] [CrossRef]
- Khan, M.H.U.; Hu, L.; Zhu, M.; Zhai, Y.; Khan, S.U.; Ahmar, S.; Amoo, O.; Zhang, K.; Fan, C.; Zhou, Y. Targeted mutagenesis of EOD3 gene in Brassica napus L. regulates seed production. J. Cell. Physiol. 2021, 236, 1996–2007. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, J.; Qi, X.; Shi, X.; Zhou, J.; Wang, X.; Xiang, X. Correlation analysis of the transcriptome and metabolome reveals the regulatory network for lipid synthesis in developing Brassica napus embryos. Plant Mol. Biol. 2019, 99, 31–44. [Google Scholar] [CrossRef]
- Zhang, C.; Chang, W.; Li, X.; Yang, B.; Zhang, L.; Xiao, Z.; Li, J.; Lu, K. Transcriptome and Small RNA Sequencing Reveal the Mechanisms Regulating Harvest Index in Brassica napus. Front. Plant Sci. 2022, 13, 855486. [Google Scholar] [CrossRef]
- Nichol, J.B.; Samuel, M.A. Characterizing the role of endocarp a and b cells layers during pod (silique) development in Brassicaceae. Plant Signal. Behav. 2024, 19, 2384243. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, J.; Lin, L.; Li, P.; Li, S.; Wang, Y.; Li, J.; Sun, Q.; Liang, J.; Wang, Y. Overexpression of Cinnamoyl-CoA Reductase 2 in Brassica napus Increases Resistance to Sclerotinia sclerotiorum by Affecting Lignin Biosynthesis. Front. Plant Sci. 2021, 12, 732733. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liao, X.; Jin, X.; Tan, L.; Lu, Q.; Yuan, C.; Xue, Y.; Yin, N.; Lin, N.; Chai, Y. MYB43 in Oilseed Rape (Brassica napus) Positively Regulates Vascular Lignification, Plant Morphology and Yield Potential but Negatively Affects Resistance to Sclerotinia sclerotiorum. Genes 2020, 11, 581. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Wang, M.; Jiang, J.; Zhou, Q.; Yin, J.; Li, J.; Lian, J.; Xue, Y.; Chai, Y. Downregulation of Brassica napus MYB69 (BnMYB69) increases biomass growth and disease susceptibility via remodeling phytohormone, chlorophyll, shikimate and lignin levels. Front. Plant Sci. 2023, 14, 1157836. [Google Scholar] [CrossRef]
- Yin, N.; Li, B.; Liu, X.; Liang, Y.; Lian, J.; Xue, Y.; Qu, C.; Lu, K.; Wei, L.; Wang, R.; et al. Two types of cinnamoyl-CoA reductase function divergently in accumulation of lignins, flavonoids and glucosinolates and enhance lodging resistance in Brassica napus. Crop J. 2022, 10, 647–660. [Google Scholar] [CrossRef]
- Hou, J.; Riaz, M.; Yan, L.; Lu, K.; Jiang, C. Effect of exogenous L-aspartate nano-calcium on root growth, calcium forms and cell wall metabolism of Brassica napus L. Nanoimpact 2022, 27, 100415. Nanoimpact 2022, 27, 100415. [Google Scholar] [CrossRef]
- Wang, D.; Jin, S.; Chen, Z.; Shan, Y.; Li, L. Genome-wide identification of the pectin methylesterase inhibitor genes in Brassica napus and expression analysis of selected members. Front. Plant Sci. 2022, 13, 940284. [Google Scholar] [CrossRef]
- Dou, Y.; Yang, Y.; Mund, N.K.; Wei, Y.; Liu, Y.; Wei, L.; Wang, Y.; Du, P.; Zhou, Y.; Liesche, J.; et al. Comparative Analysis of Herbaceous and Woody Cell Wall Digestibility by Pathogenic Fungi. Molecules 2021, 26, 7220. [Google Scholar] [CrossRef]
- Xu, B.; Gong, X.; Chen, S.; Hu, M.; Zhang, J.; Peng, Q. Transcriptome Analysis Reveals the Complex Molecular Mechanisms of Brassica napus-Sclerotinia sclerotiorum Interactions. Front. Plant Sci. 2021, 12, 716935. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Hampton, J.G.; Shaw, M.L.; Rolston, M.P.; Khan, K.M.; Saville, D.J. Oxidative damage in forage rape (Brassica napus L.) seeds following heat stress during seed development. J. Agron. Crop Sci. 2020, 206, 101–117. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Y.; Mao, L.; Qu, C.; Lu, K.; Li, J.; Liu, L. Genome-wide association study and transcriptome analysis dissect the genetic control of silique length in Brassica napus L. Biotechnol. Biofuels 2021, 14, 214. [Google Scholar] [CrossRef]
- Yan, J.; Liao, X.; He, R.; Zhong, M.; Feng, P.; Li, X.; Tang, D.; Liu, X.; Zhao, X. Ectopic expression of GA 2-oxidase 6 from rapeseed (Brassica napus L.) causes dwarfism, late flowering and enhanced chlorophyll accumulation in Arabidopsis thaliana. Plant Physiol. Biochem. 2017, 111, 10–19. [Google Scholar] [CrossRef]
- Zhao, M.; Li, J.; Zhou, S.; Li, K.; Niu, L.; Zhao, L.; Xu, D. Analysis of the effects of sulfamethoxazole on the secondary metabolites and antioxidants in oilseed rape (Brassica napus L.) and the underlying mechanisms. Sci. Total Environ. 2023, 902, 165768. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Lei, W.; Wu, J.; Li, C.; Shi, H.; Meng, L.; Yuan, F.; Zhou, Q.; Cui, C. Transcriptome analysis reveals gene responses to herbicide, tribenuron methyl, in Brassica napus L. during seed germination. BMC Genom. 2021, 22, 299. [Google Scholar] [CrossRef]
- Shahid, M.; Cai, G.; Zu, F.; Zhao, Q.; Qasim, M.U.; Hong, Y.; Fan, C.; Zhou, Y. Comparative Transcriptome Analysis of Developing Seeds and Silique Wall Reveals Dynamic Transcription Networks for Effective Oil Production in Brassica napus L. Int. J. Mol. Sci. 2019, 20, 1982. [Google Scholar] [CrossRef]
- Liu, Y.; Hua, Y.-p.; Chen, H.; Zhou, T.; Yue, C.-p.; Huang, J.-y. Genome-scale identification of plant defensin (PDF) family genes and molecular characterization of their responses to diverse nutrient stresses in allotetraploid rapeseed. PeerJ 2021, 9, 12007. [Google Scholar] [CrossRef]
- Zaman, Q.U.; Chu, W.; Shi, Y.; Hao, M.; Mei, D.; Jacqueline, B.; Zhang, B.; Li, C.; Hu, Q. Characterization of SHATTERPRROOF Homoeologs and CRISPR-Cas9-Mediated Genome Editing Enhances Pod-Shattering Resistance in Brassica napus L. Cris. J. 2021, 4, 360–370. [Google Scholar] [CrossRef]
- Zaman, Q.U.; Chu, W.; Hao, M.; Shi, Y.; Sun, M.; Sang, S.-F.; Mei, D.; Cheng, H.; Liu, J.; Li, C.; et al. CRISPR/Cas9-Mediated Multiplex Genome Editing of JAGGED Gene in Brassica napus L. Biomolecules 2019, 9, 725. Biomolecules 2019, 9, 725. [Google Scholar] [CrossRef]
- Cao, B.; Wang, H.; Bai, J.; Wang, X.; Li, X.; Zhang, Y.; Yang, S.; He, Y.; Yu, X. miR319-Regulated TCP3 Modulates Silique Development Associated with Seed Shattering in Brassicaceae. Cells 2022, 11, 3096. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Qiu, Y.; Coombes, N.; Raman, H. Identification and validation of genomic regions for pod shatter resistance in Brassica rapa using QTL-seq and traditional QTL mapping. Bmc Plant Biol. 2025, 25, 175. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.; Liu, J.; Wang, L.; Yang, G.; Leng, S. Yield, dry matter and N characteristics in canola as affected by fertilizer N rate and split-application ratio under high soil fertility condition. J. Plant Nutr. 2020, 43, 655–666. [Google Scholar] [CrossRef]
- Dogra, P.; Thakur, A.; Kukreja, S.; Alfagham, A.T.; Gupta, R.K.; Ahmad, M.; Dahiya, Y.; Siddiqui, M.H.; Alamri, S. Synergistic Effects of Salicylic Acid, Hydrogel, and Sulphur Sources for Boosting the Yield of Rapeseed under Limited Irrigation. Bioresources 2025, 20, 1883–1899. [Google Scholar] [CrossRef]
- Wang, R.; Wu, W.; Cheng, X.; Peng, W. High plant density increases sunlight interception and yield of direct-seeded winter canola in China. Exp. Agric. 2023, 59, e2. [Google Scholar] [CrossRef]
- Zhang, M.; Li, G.; Yang, Y.; Jin, M.; Jiang, T. Design and Parameter Optimization of Variable Speed Reel for Oilseed Rape Combine Harvester. Agric 2023, 13, 1521. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Deng, W.; Dai, B.; Yu, Q.; Zhou, W.; Zan, X.; Song, X. Mechanisms of Silique Dehiscence in Rapeseed: A Review of Research Progress. Curr. Issues Mol. Biol. 2025, 47, 755. https://doi.org/10.3390/cimb47090755
Zhou M, Deng W, Dai B, Yu Q, Zhou W, Zan X, Song X. Mechanisms of Silique Dehiscence in Rapeseed: A Review of Research Progress. Current Issues in Molecular Biology. 2025; 47(9):755. https://doi.org/10.3390/cimb47090755
Chicago/Turabian StyleZhou, Menglin, Wuming Deng, Bingbing Dai, Qingqing Yu, Wei Zhou, Xiaofei Zan, and Xi Song. 2025. "Mechanisms of Silique Dehiscence in Rapeseed: A Review of Research Progress" Current Issues in Molecular Biology 47, no. 9: 755. https://doi.org/10.3390/cimb47090755
APA StyleZhou, M., Deng, W., Dai, B., Yu, Q., Zhou, W., Zan, X., & Song, X. (2025). Mechanisms of Silique Dehiscence in Rapeseed: A Review of Research Progress. Current Issues in Molecular Biology, 47(9), 755. https://doi.org/10.3390/cimb47090755





