Myo-Inositol Oxygenase (MIOX): A Pivotal Regulator and Therapeutic Target in Multiple Diseases
Abstract
1. Introduction
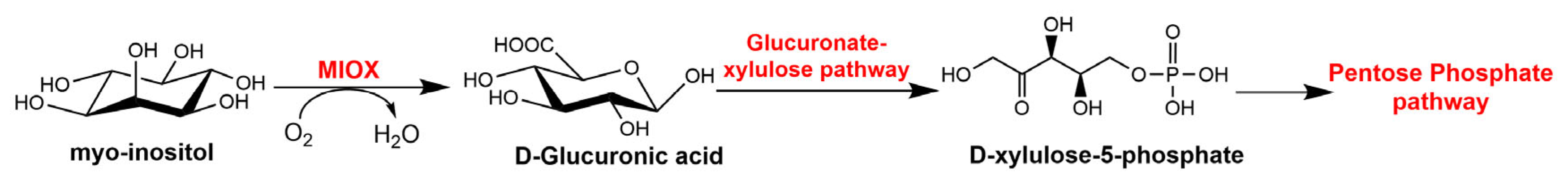
2. Characterization and Biological Function of MIOX
2.1. Characterization of MIOX
2.2. The Function of MIOX
2.2.1. Redox Equilibrium
2.2.2. Endoplasmic Reticulum Stress
2.2.3. Ferroptosis
2.2.4. Cellular Signaling Transduction
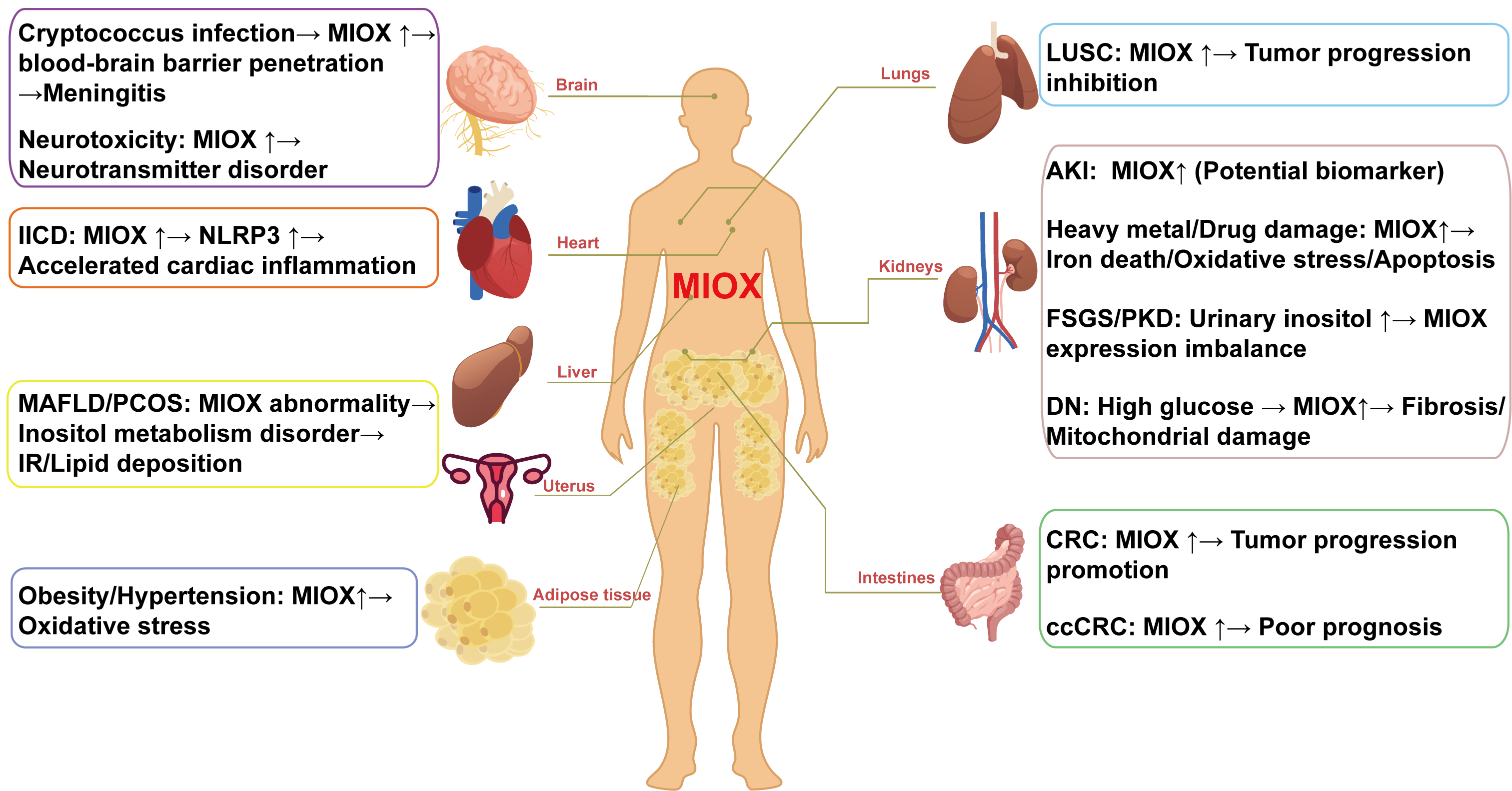
3. Application of MIOX in Disease Diagnosis and Prediction
3.1. Renal Diseases
3.1.1. Acute Kidney Injury
3.1.2. Heavy-Metal- and Drug-Induced Kidney Injury
3.1.3. DN
3.1.4. Other Kidney Diseases
3.2. Metabolic Disease
3.3. Cancer Progression
3.4. Nervous System Disease
3.5. Reproduction and Development
3.6. Other
4. Intervention Strategies Targeting MIOX
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MIOX | Myo-inositol oxygenase |
| MI | Myo-inositol |
| PIP2 | Phosphatidylinositol biphosphate |
| IP3 | Inositol triphosphate |
| DG | D-glucuronic acid |
| PPP | Pentose phosphate pathway |
| ROS | Reactive oxygen species |
| GSH | Glutathione |
| ERS | Endoplasmic reticulum stress |
| UPR | Unfolded protein response |
| GPX4 | Glutathione peroxidase 4 |
| PTC | Papillary thyroid carcinoma |
| HCC | Hepatocellular carcinoma |
| ccRCC | Clear cell renal cell carcinoma |
| NLRP3 | NLR family pyrindomain containing 3 |
| AMPK | AMP-activated protein kinase |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| YY-1 | Yin Yang 1 |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| SREBP1 | Sterol regulatory element-binding protein 1 |
| AGE | Advanced glycation end products |
| RAGE | Receptor of advanced glycation end products |
| PI3K | Phosphoinositide 3-kinase |
| AKT | Protein kinase B |
| NF-κB | Nuclear factor kappa B |
| TGF-β | Transforming growth factor beta |
| STAT3 | Signal transducer and activator of transcription 3 |
| c-Myc | Cellular oncogene |
| CKD | Chronic kidney disease |
| AKI | Acute kidney injury |
| DN | Diabetic nephropathy |
| RSOR | Renal-specific oxidoreductase |
| PKD | Polycystic kidney disease |
| FSGS | Focal segmental glomerulosclerosis |
| eGFR | estimated glomerular filtration rate |
| MAFLD | Metabolic-dysfunction-associated fatty liver disease |
| IR | Insulin resistance |
| PCOS | Polycystic ovary syndrome |
| CRC | Colorectal cancer |
| PRAD | Prostate adenocarcinoma |
| MAPK | Mitogen-activated protein kinase |
| PKC-α | Protein kinase C alpha |
| GNAS | Guanine nucleotide binding protein, alpha-stimulating |
| PI4KB | Phosphatidylinositol 4 kinase III-β |
| APP | Amyloid precursor protein |
| CLU | Clusterin |
References
- Arner, R.J.; Prabhu, K.S.; Thompson, J.T.; Hildenbrandt, G.R.; Liken, A.D.; Reddy, C.C. myo-Inositol oxygenase: Molecular cloning and expression of a unique enzyme that oxidizes myo-inositol and D-chiro-inositol. Biochem. J. 2001, 360, 313–320. [Google Scholar] [CrossRef]
- Facchinetti, F.; Unfer, V.; Dewailly, D.; Kamenov, Z.A.; Diamanti-Kandarakis, E.; Laganà, A.S.; Nestler, J.E.; Soulage, C.O. Inositols in Polycystic Ovary Syndrome: An Overview on the Advances. Trends Endocrinol. Metab. 2020, 31, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Decrock, E.; De Bock, M.; Wang, N.; Gadicherla, A.K.; Bol, M.; Delvaeye, T.; Vandenabeele, P.; Vinken, M.; Bultynck, G.; Krysko, D.V.; et al. IP3, a small molecule with a powerful message. Biochim. Biophys. Acta 2013, 1833, 1772–1786. [Google Scholar] [CrossRef] [PubMed]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef]
- Naber, N.I.; Swan, J.S.; Hamilton, G.A. L-myo-inosose-1 as a probable intermediate in the reaction catalyzed by myo-inositol oxygenase. Biochemistry 1986, 25, 7201–7207. [Google Scholar] [CrossRef]
- Hankes, L.V.; Politzer, W.M.; Touster, O.; Anderson, L. Myo-inositol catabolism in human pentosurics: The predominant role of the glucuronate-xylulose-pentose phosphate pathway. Ann. N. Y. Acad. Sci. 1969, 165, 564–576. [Google Scholar] [PubMed]
- Zhan, M.; Usman, I.M.; Sun, L.; Kanwar, Y.S. Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J. Am. Soc. Nephrol. 2015, 26, 1304–1321. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, C.; Long, Y. Myo-inositol oxygenase (MIOX) accelerated inflammation in the model of infection-induced cardiac dysfunction by NLRP3 inflammasome. Immun. Inflamm. Dis. 2023, 11, e829. [Google Scholar] [CrossRef]
- Deng, F.; Sharma, I.; Dai, Y.; Yang, M.; Kanwar, Y.S. Myo-inositol oxygenase expression profile modulates pathogenic ferroptosis in the renal proximal tubule. J. Clin. Investig. 2019, 129, 5033–5049. [Google Scholar] [CrossRef]
- Cao, L.; Chen, E.; Zhang, H.; Ba, Y.; Yan, B.; Li, T.; Yang, J. Construction of a novel methylation-related prognostic model for colorectal cancer based on microsatellite status. J. Cell Biochem. 2021, 122, 1781–1790. [Google Scholar] [CrossRef]
- Yang, Q.; Dixit, B.; Wada, J.; Tian, Y.; Wallner, E.I.; Srivastva, S.K.; Kanwar, Y.S. Identification of a renal-specific oxido-reductase in newborn diabetic mice. Proc. Natl. Acad. Sci. USA 2000, 97, 9896–9901, in press. [Google Scholar] [CrossRef]
- Yang, B.; Hodgkinson, A.; Millward, B.A.; Demaine, A.G. Polymorphisms of myo-inositol oxygenase gene are associated with Type 1 diabetes mellitus. J. Diabetes Complicat. 2010, 24, 404–408. [Google Scholar] [CrossRef]
- Nayak, B.; Xie, P.; Akagi, S.; Yang, Q.; Sun, L.; Wada, J.; Thakur, A.; Danesh, F.R.; Chugh, S.S.; Kanwar, Y.S. Modulation of renal-specific oxidoreductase/myo-inositol oxygenase by high-glucose ambience. Proc. Natl. Acad. Sci. USA 2005, 102, 17952–17957. [Google Scholar] [CrossRef]
- Bollinger, J.M., Jr.; Diao, Y.; Matthews, M.L.; Xing, G.; Krebs, C. myo-Inositol oxygenase: A radical new pathway for O2 and C-H activation at a nonheme diiron cluster. Dalton Trans. 2009, 6, 905–914. [Google Scholar] [CrossRef]
- Xing, G.; Barr, E.W.; Diao, Y.; Hoffart, L.M.; Prabhu, K.S.; Arner, R.J.; Reddy, C.C.; Krebs, C.; Bollinger, J.M., Jr. Oxygen activation by a mixed-valent, diiron(II/III) cluster in the glycol cleavage reaction catalyzed by myo-inositol oxygenase. Biochemistry 2006, 45, 5402–5412. [Google Scholar] [CrossRef]
- Brown, P.M.; Caradoc-Davies, T.T.; Dickson, J.M.; Cooper, G.J.; Loomes, K.M.; Baker, E.N. Purification, crystallization and preliminary crystallographic analysis of mouse myo-inositol oxygenase. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2006, 62, 811–813. [Google Scholar] [CrossRef]
- González-Álvarez, R.; Pérez-Ibave, D.C.; Garza-Rodríguez, M.L.; Lugo-Trampe, Á.; Delgado-Enciso, I.; Tejero-Barrera, M.E.; Martínez-De-Villarreal, L.E.; Garza-Guajardo, R.; Sánchez-Chaparro, M.M.; Ruiz-Ayma, G.; et al. Molecular cloning of the myo-inositol oxygenase gene from the kidney of baboons. Biomed. Rep. 2017, 7, 301–305. [Google Scholar] [CrossRef]
- Koller, F.; Koller, E. myo-inositol oxygenase from rat kidneys. Substrate-dependent oligomerization. Eur. J. Biochem. 1990, 193, 421–427. [Google Scholar] [CrossRef]
- Gardell, A.M.; Yang, J.; Sacchi, R.; Fangue, N.A.; Hammock, B.D.; Kültz, D. Tilapia (Oreochromis mossambicus) brain cells respond to hyperosmotic challenge by inducing myo-inositol biosynthesis. J. Exp. Biol. 2013, 216, 4615–4625. [Google Scholar] [CrossRef]
- Croze, M.L.; Géloën, A.; Soulage, C.O. Abnormalities in myo-inositol metabolism associated with type 2 diabetes in mice fed a high-fat diet: Benefits of a dietary myo-inositol supplementation. Br. J. Nutr. 2015, 113, 1862–1875. [Google Scholar] [CrossRef]
- Hsu, C.C.; Zhang, X.; Wang, G.; Zhang, W.; Cai, Z.; Pan, B.S.; Gu, H.; Xu, C.; Jin, G.; Xu, X.; et al. Inositol serves as a natural inhibitor of mitochondrial fission by directly targeting AMPK. Mol. Cell 2021, 81, 3803–3819.e3807. [Google Scholar] [CrossRef]
- Sharma, I.; Deng, F.; Liao, Y.; Kanwar, Y.S. Myo-inositol Oxygenase (MIOX) Overexpression Drives the Progression of Renal Tubulointerstitial Injury in Diabetes. Diabetes 2020, 69, 1248–1263. [Google Scholar] [CrossRef]
- Nayak, B.; Kondeti, V.K.; Xie, P.; Lin, S.; Viswakarma, N.; Raparia, K.; Kanwar, Y.S. Transcriptional and post-translational modulation of myo-inositol oxygenase by high glucose and related pathobiological stresses. J. Biol. Chem. 2011, 286, 27594–27611. [Google Scholar] [CrossRef]
- Brown, P.M.; Caradoc-Davies, T.T.; Dickson, J.M.; Cooper, G.J.; Loomes, K.M.; Baker, E.N. Crystal structure of a substrate complex of myo-inositol oxygenase, a di-iron oxygenase with a key role in inositol metabolism. Proc. Natl. Acad. Sci. USA 2006, 103, 15032–15037. [Google Scholar] [CrossRef]
- Xie, P.; Sun, L.; Oates, P.J.; Srivastava, S.K.; Kanwar, Y.S. Pathobiology of renal-specific oxidoreductase/myo-inositol oxygenase in diabetic nephropathy: Its implications in tubulointerstitial fibrosis. Am. J. Physiol. Renal Physiol. 2010, 298, F1393–F1404. [Google Scholar] [CrossRef][Green Version]
- Zheng, X.; Deng, F.; Sharma, I.; Kanwar, Y.S. Myo-inositol oxygenase overexpression exacerbates cadmium-induced kidney injury via oxidant stress and necroptosis. Am. J. Physiol. Renal Physiol. 2022, 322, F344–F359. [Google Scholar] [CrossRef]
- Lu, Y.; Agarwal, A. Myo-inositol oxygenase in cadmium-induced kidney injury. Am. J. Physiol. Renal Physiol. 2022, 322, F470–F472. [Google Scholar] [CrossRef]
- Pallio, G.; Micali, A.; Benvenga, S.; Antonelli, A.; Marini, H.R.; Puzzolo, D.; Macaione, V.; Trichilo, V.; Santoro, G.; Irrera, N.; et al. Myo-inositol in the protection from cadmium-induced toxicity in mice kidney: An emerging nutraceutical challenge. Food Chem. Toxicol. 2019, 132, 110675. [Google Scholar] [CrossRef]
- Sharma, I.; Dutta, R.K.; Singh, N.K.; Kanwar, Y.S. High Glucose-Induced Hypomethylation Promotes Binding of Sp-1 to Myo-Inositol Oxygenase: Implication in the Pathobiology of Diabetic Tubulopathy. Am. J. Pathol. 2017, 187, 724–739. [Google Scholar] [CrossRef]
- Sharma, I.; Liao, Y.; Zheng, X.; Kanwar, Y.S. Modulation of gentamicin-induced acute kidney injury by myo-inositol oxygenase via the ROS/ALOX-12/12-HETE/GPR31 signaling pathway. JCI Insight 2022, 7, e155487. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, Y.; Zhao, J.; Zhou, L.; Li, M. Endoplasmic reticulum stress: Bridging inflammation and obesity-associated adipose tissue. Front. Immunol. 2024, 15, 1381227. [Google Scholar] [CrossRef]
- Tominaga, T.; Sharma, I.; Fujita, Y.; Doi, T.; Wallner, A.K.; Kanwar, Y.S. Myo-inositol oxygenase accentuates renal tubular injury initiated by endoplasmic reticulum stress. Am. J. Physiol. Renal Physiol. 2019, 316, F301–F315. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Geng, J.; Wang, R.; Ma, G.; Liu, P. Identification of biomarkers and mechanism exploration of ferroptosis related genes regulated by m6A in type 2 diabetes mellitus. Hereditas 2025, 162, 24. [Google Scholar] [CrossRef]
- Yang, D.; Wang, J.; Li, C.; Shi, L.; Zhang, M. Ferroptosis-related gene model to predict overall survival of papillary thyroid carcinoma. Am. J. Otolaryngol. 2021, 42, 103163. [Google Scholar] [CrossRef]
- Da, Q.; Ren, M.; Huang, L.; Qu, J.; Yang, Q.; Xu, J.; Ma, Q.; Mao, X.; Cai, Y.; Zhao, D.; et al. Identification and Validation of a Ferroptosis-Related Signature for Predicting Prognosis and Immune Microenvironment in Papillary Renal Cell Carcinoma. Int. J. Gen. Med. 2022, 15, 2963–2977. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, M.; Cui, X.; O’Connell, D.; Yang, Y. Long noncoding RNA NEAT1 promotes ferroptosis by modulating the miR-362-3p/MIOX axis as a ceRNA. Cell Death Differ. 2022, 29, 1850–1863. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, Y.; Xiao, J.; Cheng, C.; Ma, G.; Wang, Y.; Zhang, Y.; Chen, M. Ferroptosis-Related Genes in IgA Nephropathy: Screening for Potential Targets of the Mechanism. Int. J. Genom. 2024, 2024, 8851124. [Google Scholar] [CrossRef]
- Luo, G.; Wang, L.; Zheng, Z.; Gao, B.; Lei, C. Cuproptosis-Related Ferroptosis genes for Predicting Prognosis in kidney renal clear cell carcinoma. Eur. J. Med. Res. 2023, 28, 176. [Google Scholar] [CrossRef]
- Sharma, I.; Deng, F.; Kanwar, Y.S. Modulation of Renal Injury by Variable Expression of Myo-Inositol Oxygenase (MIOX) via Perturbation in Metabolic Sensors. Biomedicines 2020, 8, 217. [Google Scholar] [CrossRef]
- Tominaga, T.; Dutta, R.K.; Joladarashi, D.; Doi, T.; Reddy, J.K.; Kanwar, Y.S. Transcriptional and Translational Modulation of myo-Inositol Oxygenase (Miox) by Fatty Acids: IMPLICATIONS IN RENAL TUBULAR INJURY INDUCED IN OBESITY AND DIABETES. J. Biol. Chem. 2016, 291, 1348–1367. [Google Scholar] [CrossRef]
- Sharma, I.; Tupe, R.S.; Wallner, A.K.; Kanwar, Y.S. Contribution of myo-inositol oxygenase in AGE:RAGE-mediated renal tubulointerstitial injury in the context of diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2018, 314, F107–F121. [Google Scholar] [CrossRef]
- Meng, L.; Gao, J.; Mo, W.; Wang, B.; Shen, H.; Cao, W.; Ding, M.; Diao, W.; Chen, W.; Zhang, Q.; et al. MIOX inhibits autophagy to regulate the ROS -driven inhibition of STAT3/c-Myc-mediated epithelial-mesenchymal transition in clear cell renal cell carcinoma. Redox Biol. 2023, 68, 102956. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Mertoglu, C.; Gunay, M.; Gurel, A.; Gungor, M. Myo-inositol Oxygenase as a Novel Marker in the Diagnosis of Acute Kidney Injury. J. Med. Biochem. 2018, 37, 1–6. [Google Scholar] [CrossRef]
- Konvalinka, A. myo-Inositol oxygenase: A novel kidney-specific biomarker of acute kidney injury? Clin. Chem. 2014, 60, 708–710. [Google Scholar] [CrossRef][Green Version]
- Gaut, J.P.; Crimmins, D.L.; Ohlendorf, M.F.; Lockwood, C.M.; Griest, T.A.; Brada, N.A.; Hoshi, M.; Sato, B.; Hotchkiss, R.S.; Jain, S.; et al. Development of an immunoassay for the kidney-specific protein myo-inositol oxygenase, a potential biomarker of acute kidney injury. Clin. Chem. 2014, 60, 747–757. [Google Scholar] [CrossRef]
- Gilquin, B.; Louwagie, M.; Jaquinod, M.; Cez, A.; Picard, G.; El Kholy, L.; Surin, B.; Garin, J.; Ferro, M.; Kofman, T.; et al. Multiplex and accurate quantification of acute kidney injury biomarker candidates in urine using Protein Standard Absolute Quantification (PSAQ) and targeted proteomics. Talanta 2017, 164, 77–84. [Google Scholar] [CrossRef]
- Mertoglu, C.; Ozmen, Z.C.; Firat, F.; Arici, A.; Unsal, V.; Erdemir, F. Effectiveness of myo-inositol oxygenase in the early diagnosis of experimental acute kidney injury. Bratisl. Lek. Listy 2020, 121, 565–570. [Google Scholar] [CrossRef]
- Omosule, C.L.; Blair, C.J.; Herries, E.; Zaydman, M.A.; Farnsworth, C.; Ladenson, J.; Dietzen, D.J.; Gaut, J.P. Clinical Utility of LC-MS/MS for Blood Myo-Inositol in Patients with Acute Kidney Injury and Chronic Kidney Disease. Clin. Chem. 2024, 70, 1172–1181. [Google Scholar] [CrossRef]
- Altun, A.; Bozkurt, A.; Erdogan, A.; Mertoğlu, C.; Hirik, E.; Keskin, E.; Turan, A. Comparison of serum Kim-1 and Miox levels in patients that underwent percutaneous nephrolithotomy and flexible ureterorenoscopy. Urologia 2023, 90, 335–341. [Google Scholar] [CrossRef]
- Mertoglu, C.; Bozkurt, A.; Keskin, E.; Gunay, M. Evaluation of the effect of Retrograde Intrarenal Surgery with Myo-Inositol Oxygenase. Pak. J. Med. Sci. 2018, 34, 170–174. [Google Scholar] [CrossRef]
- Turan, A.; Hirik, E.; Erdoğan, A.; Altun, A.; Mertoğlu, C.; Şam, E.; Atar, M. Evaluation of the effect of 9.5/11.5-fr ureteral access sheath use on acute kidney injury with the myo-inositol oxygenase biomarker in patients undergoing retrograde intrarenal surgery: A prospective, randomized, and controlled study. Minim. Invasive Ther. Allied Technol. 2025, 34, 1–7. [Google Scholar] [CrossRef]
- Kakkanattu, T.J.; Kaur, J.; Nagesh, V.; Kundu, M.; Kamboj, K.; Kaur, P.; Sethi, J.; Kohli, H.S.; Gupta, K.L.; Ghosh, A.; et al. Serum myo-inositol oxygenase levels at hospital discharge predict progression to chronic kidney disease in community-acquired acute kidney injury. Sci. Rep. 2022, 12, 13225. [Google Scholar] [CrossRef]
- Dutta, R.K.; Kondeti, V.K.; Sharma, I.; Chandel, N.S.; Quaggin, S.E.; Kanwar, Y.S. Beneficial Effects of Myo-Inositol Oxygenase Deficiency in Cisplatin-Induced AKI. J. Am. Soc. Nephrol. 2017, 28, 1421–1436. [Google Scholar] [CrossRef]
- Xie, Y.H.; Wang, L.; Li, M.L.; Gong, Z.C.; Du, J. Role of myo-inositol in acute kidney injury induced by cisplatin. Toxicology 2023, 499, 153653. [Google Scholar] [CrossRef]
- Chiou, Y.Y.; Jiang, S.T.; Ding, Y.S.; Cheng, Y.H. Kidney-based in vivo model for drug-induced nephrotoxicity testing. Sci. Rep. 2020, 10, 13640. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, C.; Miao, X.; Xu, K.; Wu, X.; Liu, C. Increased expression of myo-inositol oxygenase is involved in the tubulointerstitial injury of diabetic nephropathy. Exp. Clin. Endocrinol. Diabetes 2009, 117, 257–265. [Google Scholar] [CrossRef]
- Gao, P.; Xu, B.; Song, P.; Zhu, X.; Yuan, S.; Kanwar, Y.S.; Sun, L. The Kidney Specific Protein myo-Inositol Oxygenase, a Potential Biomarker for Diabetic Nephropathy. Kidney Blood Press. Res. 2018, 43, 1772–1785. [Google Scholar] [CrossRef]
- Lu, L.; Liu, Q.; Zhi, L.; Che, X.; Xiao, B.; Cui, M.; Yu, M.; Yang, B.; Zhang, J.; Zhang, B. Establishment of a Ciliogenesis-Associated Signaling Model for Polycystic Kidney Disease. Kidney Blood Press. Res. 2021, 46, 693–701. [Google Scholar] [CrossRef]
- Altintas, M.M.; Agarwal, S.; Sudhini, Y.; Zhu, K.; Wei, C.; Reiser, J. Pathogenesis of Focal Segmental Glomerulosclerosis and Related Disorders. Annu. Rev. Pathol. 2025, 20, 329–353. [Google Scholar] [CrossRef]
- An, J.N.; Hyeon, J.S.; Jung, Y.; Choi, Y.W.; Kim, J.H.; Yang, S.H.; Oh, S.; Kwon, S.; Lee, S.H.; Cho, J.H.; et al. Urinary myo-inositol is associated with the clinical outcome in focal segmental glomerulosclerosis. Sci. Rep. 2019, 9, 14707. [Google Scholar] [CrossRef]
- Chang, H.H.; Chao, H.N.; Walker, C.S.; Choong, S.Y.; Phillips, A.; Loomes, K.M. Renal depletion of myo-inositol is associated with its increased degradation in animal models of metabolic disease. Am. J. Physiol. Renal Physiol. 2015, 309, F755–F763. [Google Scholar] [CrossRef]
- Dinicola, S.; Minini, M.; Unfer, V.; Verna, R.; Cucina, A.; Bizzarri, M. Nutritional and Acquired Deficiencies in Inositol Bioavailability. Correlations with Metabolic Disorders. Int. J. Mol. Sci. 2017, 18, 2187. [Google Scholar] [CrossRef]
- Arefhosseini, S.; Roshanravan, N.; Tutunchi, H.; Rostami, S.; Khoshbaten, M.; Ebrahimi-Mameghani, M. Myo-inositol supplementation improves cardiometabolic factors, anthropometric measures, and liver function in obese patients with non-alcoholic fatty liver disease. Front. Nutr. 2023, 10, 1092544. [Google Scholar] [CrossRef]
- Tutunchi, H.; Arefhosseini, S.; Ebrahimi-Mameghani, M. Clinical effectiveness of α-lipoic acid, myo-inositol and propolis supplementation on metabolic profiles and liver function in obese patients with NAFLD: A randomized controlled clinical trial. Clin. Nutr. ESPEN 2023, 54, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Rostami, S.; Arefhosseini, S.; Tutunchi, H.; Khoshbaten, M.; Ebrahimi-Mameghani, M. Does myo-inositol supplementation influence oxidative stress biomarkers in patients with non-alcoholic fatty liver disease? Food Sci. Nutr. 2024, 12, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lin, H.; Kong, L.; Ma, J.; Long, Z.; Qin, H.; Huang, Z.; Lin, Y.; Liu, L.; Li, Z. Effects of Mulberry Leaf Extract on the Liver Function of Juvenile Spotted Sea Bass (Lateolabrax maculatus). Aquac. Nutr. 2023, 2023, 2892463. [Google Scholar] [CrossRef]
- Merviel, P.; James, P.; Bouée, S.; Le Guillou, M.; Rince, C.; Nachtergaele, C.; Kerlan, V. Impact of myo-inositol treatment in women with polycystic ovary syndrome in assisted reproductive technologies. Reprod. Health 2021, 18, 13. [Google Scholar] [CrossRef]
- Bremer, A.A. Polycystic ovary syndrome in the pediatric population. Metab. Syndr. Relat. Disord. 2010, 8, 375–394. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, S.; Nian, F.; Xu, S. Identification of a glycolysis-related gene signature associated with clinical outcome for patients with lung squamous cell carcinoma. Cancer Med. 2021, 10, 4017–4029. [Google Scholar] [CrossRef]
- Wu, G.; Li, T.; Chen, Y.; Ye, S.; Zhou, S.; Tian, X.; Anwaier, A.; Zhu, S.; Xu, W.; Hao, X.; et al. Deciphering glutamine metabolism patterns for malignancy and tumor microenvironment in clear cell renal cell carcinoma. Clin. Exp. Med. 2024, 24, 152. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xiang, J.; Wu, X.; Wei, S.; Huang, H.; Xiao, Y.; Zhai, B.; Wang, T. Transcriptome Profiles Reveal a 12-Signature Metabolic Prediction Model and a Novel Role of Myo-Inositol Oxygenase in the Progression of Prostate Cancer. Front. Oncol. 2022, 12, 899861. [Google Scholar] [CrossRef]
- Wang, Y.; Wear, M.; Kohli, G.; Vij, R.; Giamberardino, C.; Shah, A.; Toffaletti, D.L.; Yu, C.A.; Perfect, J.R.; Casadevall, A.; et al. Inositol Metabolism Regulates Capsule Structure and Virulence in the Human Pathogen Cryptococcus neoformans. mBio 2021, 12, e0279021. [Google Scholar] [CrossRef]
- Mackenzie, E.A.; Klig, L.S. Computational modeling and in silico analysis of differential regulation of myo-inositol catabolic enzymes in Cryptococcus neoformans. BMC Mol. Biol. 2008, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, J.T.; Cryder, Z.; Andrzejczyk, N.E.; Harraka, G.; Wolf, D.C.; Gan, J.; Schlenk, D. Metabolomic Profiles in the Brains of Juvenile Steelhead (Oncorhynchus mykiss) Following Bifenthrin Treatment. Environ. Sci. Technol. 2020, 54, 12245–12253. [Google Scholar] [CrossRef]
- Pagé-Larivière, F.; Campagna, C.; Sirard, M.A. Mechanisms Involved in Porcine Early Embryo Survival following Ethanol Exposure. Toxicol. Sci. 2017, 156, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Kısa, B.; Sert, U.Y.; Celik, H.K.; Candar, T.; Erol Koç, E.M.; Taşcı, Y.; Çağlar, G.S. Myo-inositol oxygenese activity in second trimester of pregnancy: Altered myoinositol catabolism in gestational diabetes mellitus. Arch. Physiol. Biochem. 2022, 128, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Jones, M.K.; Eldon, E.D.; Klig, L.S. Inositol in Disease and Development: Roles of Catabolism via myo-Inositol Oxygenase in Drosophila melanogaster. Int. J. Mol. Sci. 2023, 24, 4185. [Google Scholar] [CrossRef]
- Ghaffari, M.H.; Sadri, H.; Trakooljul, N.; Koch, C.; Sauerwein, H. Liver transcriptome profiles of dairy cows with different serum metabotypes. J. Dairy. Sci. 2024, 107, 1751–1765. [Google Scholar] [CrossRef]
- Gonzalez-Uarquin, F.; Sommerfeld, V.; Rodehutscord, M.; Huber, K. Interrelationship of myo-inositol pathways with systemic metabolic conditions in two strains of high-performance laying hens during their productive life span. Sci. Rep. 2021, 11, 4641. [Google Scholar] [CrossRef]
- Chu, S.H.; Geyer, R.P. Tissue content and metabolism of myo-inositol in normal and lipodystrophic gerbils. J. Nutr. 1983, 113, 293–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, T.; Zhang, Y.; Song, Q.; Meng, Q.; Zhou, S.; Wei, L.; Qi, Y.; Guo, Y.; Cong, J. Accumulation and depuration of tire wear particles in zebrafish (Danio rerio) and toxic effects on gill, liver, and gut. Sci. Total Environ. 2024, 951, 175625. [Google Scholar] [CrossRef]
- Arner, R.J.; Prabhu, K.S.; Krishnan, V.; Johnson, M.C.; Reddy, C.C. Expression of myo-inositol oxygenase in tissues susceptible to diabetic complications. Biochem. Biophys. Res. Commun. 2006, 339, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yu, Q.; Huang, Y.; Luo, Y.; Qin, J.; Chen, L.; Li, E.; Wang, X. Study on the osmotic response and function of myo-inositol oxygenase in euryhaline fish nile tilapia (Oreochromis niloticus). Am. J. Physiol. Cell Physiol. 2024, 326, C1054–C1066. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Uarquin, F.; Molano, E.; Heinrich, F.; Sommerfeld, V.; Rodehutscord, M.; Huber, K. Research Note: Jejunum phosphatases and systemic myo-inositol in broiler chickens fed without or with supplemented phytase. Poult. Sci. 2020, 99, 5972–5976. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.A.; Suh, H.J.; Yun, J.W.; Choi, J.W. Differential gene expression in pancreatic tissues of streptozocin-induced diabetic rats and genetically-diabetic mice in response to hypoglycemic dipeptide cyclo (His-Pro) treatment. Mol. Biol. Rep. 2012, 39, 8821–8835. [Google Scholar] [CrossRef]
- Shirakawa, K.; Sano, M. Sodium-Glucose Co-Transporter 2 Inhibitors Correct Metabolic Maladaptation of Proximal Tubular Epithelial Cells in High-Glucose Conditions. Int. J. Mol. Sci. 2020, 21, 7676. [Google Scholar] [CrossRef]
- Thorsell, A.G.; Persson, C.; Voevodskaya, N.; Busam, R.D.; Hammarström, M.; Gräslund, S.; Gräslund, A.; Hallberg, B.M. Structural and biophysical characterization of human myo-inositol oxygenase. J. Biol. Chem. 2008, 283, 15209–15216. [Google Scholar] [CrossRef]

| Molecule or Formulation | Biological Effect |
|---|---|
| Mulberry leaf extract | Upregulates MIOX expression, enhances liver carbohydrate metabolism, and optimizes energy supply [67]. |
| Cyclo (His-Pro) | Inhibits MIOX expression, improves glucose homeostasis, enhances insulin sensitivity, and indirectly alleviates inflammatory responses [86]. |
| Canagliflozin | Inhibits MIOX expression and abnormal glycolysis, blocks glucose uptake, reduces mitochondrial ROS production, and suppresses fibrosis generation [87]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Zhang, M.; Yang, H.; Yang, H.; Tang, Y.; Li, W.; Li, L.; Yu, J.; Yang, X. Myo-Inositol Oxygenase (MIOX): A Pivotal Regulator and Therapeutic Target in Multiple Diseases. Curr. Issues Mol. Biol. 2025, 47, 745. https://doi.org/10.3390/cimb47090745
Han S, Zhang M, Yang H, Yang H, Tang Y, Li W, Li L, Yu J, Yang X. Myo-Inositol Oxygenase (MIOX): A Pivotal Regulator and Therapeutic Target in Multiple Diseases. Current Issues in Molecular Biology. 2025; 47(9):745. https://doi.org/10.3390/cimb47090745
Chicago/Turabian StyleHan, Shaocong, Min Zhang, Huan Yang, Huiqiong Yang, Yanmei Tang, Weixi Li, Li Li, Jie Yu, and Xingxin Yang. 2025. "Myo-Inositol Oxygenase (MIOX): A Pivotal Regulator and Therapeutic Target in Multiple Diseases" Current Issues in Molecular Biology 47, no. 9: 745. https://doi.org/10.3390/cimb47090745
APA StyleHan, S., Zhang, M., Yang, H., Yang, H., Tang, Y., Li, W., Li, L., Yu, J., & Yang, X. (2025). Myo-Inositol Oxygenase (MIOX): A Pivotal Regulator and Therapeutic Target in Multiple Diseases. Current Issues in Molecular Biology, 47(9), 745. https://doi.org/10.3390/cimb47090745






