TP53 IVS3 16 bp Variant and Breast Cancer Risk in Western Mexican Women: A Case–Control Study
Abstract
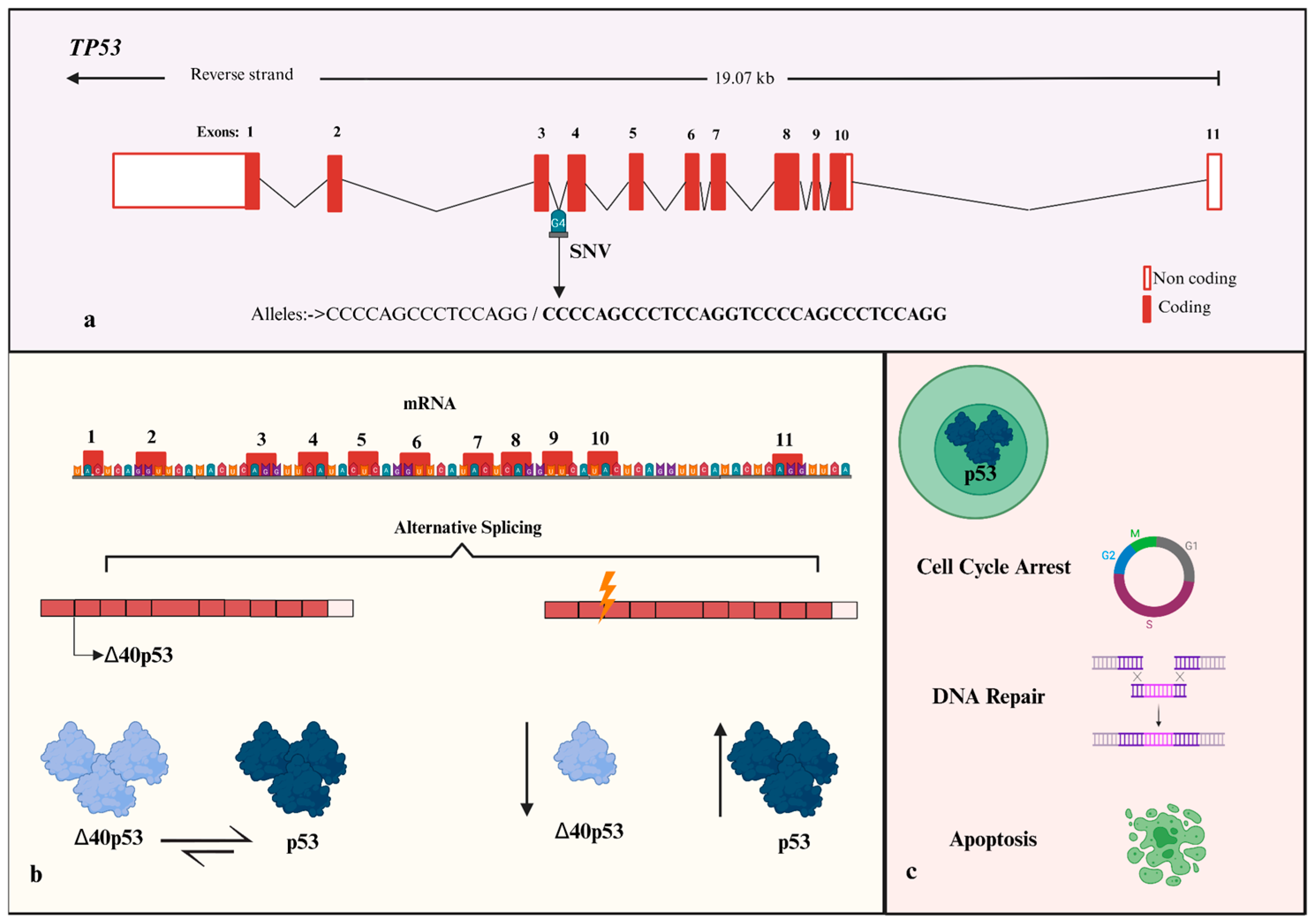
1. Introduction
2. Materials and Methods
2.1. Subjects and Clinical Setting
2.2. Clinical Evaluations
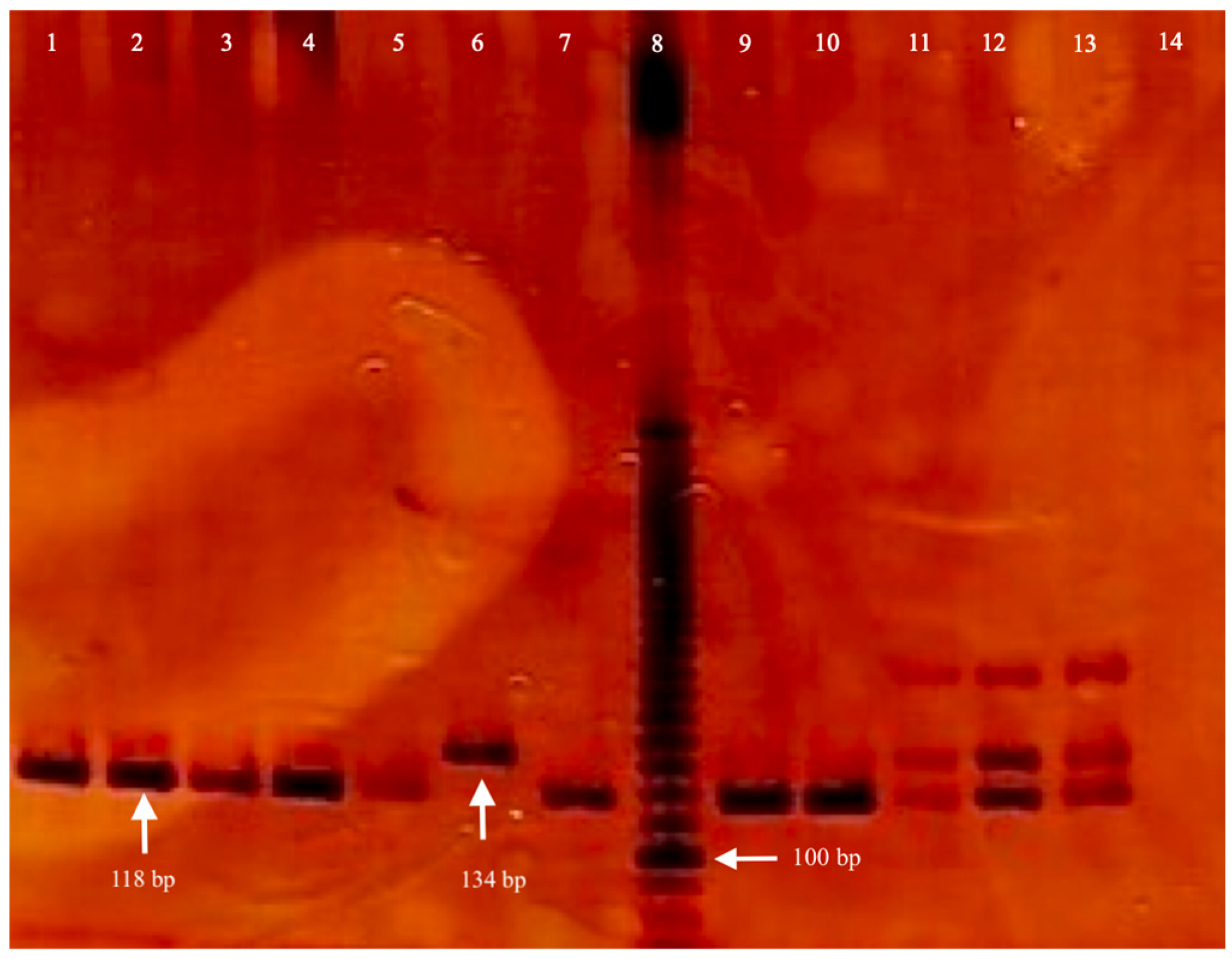
2.3. Genomic DNA Isolation and Genotyping
2.4. Ethics
2.5. Statistical Analysis
3. Results
3.1. Description of Clinical Variables
3.2. Genetic Association
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Feroz, W.; Sheikh, A.M.A. Exploring the multiple roles of guardian of the genome: P53. Egypt. J. Med. Hum. Genet. 2020, 21, 49. [Google Scholar] [CrossRef]
- Bang, S.; Kaur, S.; Kurokawa, M. Regulation of the p53 Family Proteins by the Ubiquitin Proteasomal Pathway. Int. J. Mol. Sci. 2019, 21, 261. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, H.; Wiesmüller, L.; Chen, M. Canonical and non-canonical functions of p53 isoforms: Potentiating the com-plexity of tumor development and therapy resistance. Cell Death Dis. 2024, 15, 412. [Google Scholar] [CrossRef] [PubMed]
- Marcel, V.; Tran, P.L.; Sagne, C.; Martel-Planche, G.; Vaslin, L.; Teulade-Fichou, M.-P.; Hall, J.; Mergny, J.-L.; Hainaut, P.; Van Dyck, E. G-quadruplex structures in TP53 intron 3: Role in alternative splicing and in production of p53 mRNA isoforms. Carcinogenesis 2011, 32, 271–278. [Google Scholar] [CrossRef]
- Halim, F.; Azhar, Y.; Suwarman, S.; Hernowo, B. p53 Mutation as Plausible Predictor for Endocrine Resistance Therapy in Luminal Breast Cancer. F1000Research 2022, 11, 330. [Google Scholar] [CrossRef]
- Marvalim, C.; Datta, A.; Lee, S.C. Role of p53 in breast cancer progression: An insight into p53 targeted therapy. Theranostics 2023, 13, 1421–1442. [Google Scholar] [CrossRef]
- Gallardo-Alvarado, L.N.; Tussié-Luna, M.I.; Díaz-Chávez, J.; Segura, Y.X.; Bargallo-Rocha, E.; Villarreal, C.; Herrera-Montalvo, L.A.; Herrera-Medina, E.M.; Leon, D.F.C.-D. Prevalence of germline mutations in the TP53 gene in patients with early-onset breast cancer in the Mexican population. BMC Cancer 2019, 19, 118. [Google Scholar] [CrossRef]
- Cárdenas-Sánchez, J.; Erazo-Valle-Solís, A.A.; Arce-Salinas, C.; Bargalló-Rocha, E.; Piña, V.B.; Cervantes-Sánchez, M.G.; Flores-Balcázar, C.H.; Lluch-Hernández, A.; Maffuz-Aziz, A.; Pérez-Sánchez, V.M.; et al. Consenso Mexicano sobre diagnóstico y tratamiento del cáncer mamario. Octava revisión. Colima 2019. GAMO 2022, 18, 141–231. [Google Scholar] [CrossRef]
- Schneider, K.; Zelley, K.; Nichols, K.E.; Levine, A.S.; Garber, J. Li-Fraumeni Syndrome. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. Available online: http://www.ncbi.nlm.nih.gov/books/NBK1311/ (accessed on 1 September 2025).
- Sagne, C.; Marcel, V.; Amadou, A.; Hainaut, P.; Olivier, M.; Hall, J. A meta-analysis of cancer risk associated with the TP53 intron 3 duplication polymorphism (rs17878362): Geographic and tumor-specific effects. Cell Death Dis. 2013, 4, e492. [Google Scholar] [CrossRef] [PubMed]
- Morten, B.C.; Chiu, S.; Oldmeadow, C.; Lubinski, J.; Scott, R.J.; Avery-Kiejda, K.A. The intron 3 16 bp duplication poly-morphism of p53 (rs17878362) is not associated with increased risk of developing triple-negative breast cancer. Breast Cancer Res. Treat. 2019, 173, 727–733. [Google Scholar] [CrossRef]
- Costa, S.; Pinto, D.; Pereira, D.; Rodrigues, H.; Cameselle-Teijeiro, J.; Medeiros, R.; Schmitt, F. Importance of TP53 codon 72 and intron 3 duplication 16bppolymorphisms in prediction of susceptibility on breast cancer. BMC Cancer 2008, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Faghani, M.; Ghasemi, F.; Nikhbakht, M.; Salehi, M. TP53 PIN3 polymorphism associated with breast cancer risk in Iranian women. Indian J. Cancer 2011, 48, 298–302. [Google Scholar] [CrossRef]
- Hao, W.; Xu, X.; Shi, H.; Zhang, C.; Chen, X. No association of TP53 codon 72 and intron 3 16-bp duplication polymorphisms with breast cancer risk in Chinese Han women: New evidence from a population-based case–control investigation. Eur. J. Med. Res. 2018, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Floris, M.; Pira, G.; Castiglia, P.; Idda, M.L.; Steri, M.; De Miglio, M.R.; Piana, A.; Cossu, A.; Azara, A.; Arru, C.; et al. Impact on breast cancer susceptibility and clinicopathological traits of common genetic polymorphisms in TP53, MDM2 and ATM genes in Sardinian women. Oncol. Lett. 2022, 24, 331. [Google Scholar] [CrossRef]
- Dyer, S.C.; Austine-Orimoloye, O.; Azov, A.G.; Barba, M.; Barnes, I.; Barrera-Enriquez, V.P.; Becker, A.; Bennett, R.; Beracochea, M.; Berry, A.; et al. Ensembl 2025. Nucleic Acids Res. 2025, 53, D948–D957. [Google Scholar] [CrossRef]
- de Pública, A.D.I. INAH—The Mexican National Institute of Anthropology and History, Mexico City. Available online: https://mexicocity.cdmx.gob.mx/tag/inah/ (accessed on 15 January 2025).
- González-Quezada, B.; Creary, L.; Munguia-Saldaña, A.; Flores-Aguilar, H.; Fernández-Viña, M.; Gorodezky, C. Exploring the ancestry and admixture of Mexican Oaxaca Mestizos from Southeast Mexico using next-generation sequencing of 11 HLA loci. Hum. Immunol. 2019, 80, 157–162. [Google Scholar] [CrossRef]
- Soler-Vila, H.; Galán, I.; Valencia-Martín, J.L.; León-Muñoz, L.M.; Guallar-Castillón, P.; Rodríguez-Artalejo, F. Binge drinking in Spain, 2008–2010. Alcohol. Clin. Exp. Res. 2014, 38, 810–819. [Google Scholar] [CrossRef]
- Tobacco Use—Health, United States. Available online: https://www.cdc.gov/nchs/hus/topics/tobacco-use.htm (accessed on 29 June 2025).
- American Joint Committee on Cancer, ACS. Available online: https://www.facs.org/quality-programs/cancer-programs/american-joint-committee-on-cancer/ (accessed on 15 January 2025).
- Teichgraeber, D.C.; Guirguis, M.S.; Whitman, G.J. Breast Cancer Staging: Updates in the AJCC Cancer Staging Manual, 8th Edition, and Current Challenges for Radiologists, From the AJR Special Series on Cancer Staging. Am. J. Roentgenol. 2021, 217, 278–290. [Google Scholar] [CrossRef]
- Guidelines Detail, NCCN. Available online: https://www.nccn.org/guidelines/guidelines-detail?id=1419 (accessed on 29 June 2025).
- Sullu, Y.; Tomak, L.; Demirag, G.; Kuru, B.; Ozen, N.; Karagoz, F. Evaluation of the relationship between Ki67 expression level and neoadjuvant treatment response and prognosis in breast cancer based on the Neo-Bioscore staging system. Discov. Oncol. 2023, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut Off Points. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK541070/ (accessed on 29 June 2025).
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef] [PubMed]
- Akkiprik, M.; Sonmez, O.; Gulluoglu, B.M.; Caglar, H.B.; Kaya, H.; Demirkalem, P.; Abacioglu, U.; Sengoz, M.; Sav, A.; Ozer, A. Analysis of p53 gene polymorphisms and protein over-expression in patients with breast cancer. Pathol. Oncol. Res. 2009, 15, 359–368. [Google Scholar] [CrossRef]
- Freudenheim, J.L. Alcohol’s Effects on Breast Cancer in Women. Alcohol. Res. Curr. Rev. 2020, 40, 11. [Google Scholar] [CrossRef]
- Fan, D.; Xia, Q.; Lin, D.; Ma, Y.; Rao, J.; Liu, L.; Tang, H.; Xu, T.; Li, P.; Chen, G.; et al. Role of breastfeeding on maternal and childhood cancers: An umbrella review of meta-analyses. J. Glob. Health 2023, 13, 04067. [Google Scholar] [CrossRef]
- Naaman, S.C.; Shen, S.; Zeytinoglu, M.; Iyengar, N.M. Obesity and Breast Cancer Risk: The Oncogenic Implications of Metabolic Dysregulation. J. Clin. Endocrinol. Metab. 2022, 107, 2154–2166. [Google Scholar] [CrossRef]
- Morten, B.C.; Wong-Brown, M.W.; Scott, R.J.; Avery-Kiejda, K.A. The presence of the intron 3 16 bp duplication poly-morphism of p53 (rs17878362) in breast cancer is associated with a low Δ40p53:p53 ratio and better outcome. Carcinogenesis 2016, 37, 81–86. [Google Scholar] [CrossRef]
- Perriaud, L.; Marcel, V.; Sagne, C.; Favaudon, V.; Guédin, A.; De Rache, A.; Guetta, C.; Hamon, F.; Teulade-Fichou, M.-P.; Hainaut, P.; et al. Impact of G-quadruplex structures and intronic polymorphisms rs17878362 and rs1642785 on basal and ionizing radiation-induced expression of alternative p53 transcripts. Carcinogenesis 2014, 35, 2706–2715. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X. Aberrant alternative splicing in cancer: Splicing events and their regulatory mechanisms (Review). Int. J. Oncol. 2024, 65, 90. [Google Scholar] [CrossRef] [PubMed]
| Variable | Case Group n a = 216 | Control Group n = 198 | p b |
|---|---|---|---|
| Age (years), mean | 55.6 ± 10.1 | 56.4 ± 12.8 | 0.5 |
| BC with FIH c, n (%) | 46 (21.3) | 12 (6.1) | <0.001 |
| Alcoholism, n (%) | 72 (33.3) | 60 (30.6) | 0.1 |
| Tobacco smoke, n (%) | 63 (29.4) | 0 | 0.6 |
| Sedentary lifestyle, n (%) | 108 (54.8) | 114 (52.8) | 0.7 |
| Pregnancy, n (%) | 194 (89.8) | - | |
| Lactation (months), mean | 6.6 ± 12.1 | - | |
| Menopause, n (%) | 187 (86.6) | 124 (62.4) | <0.001 |
| Menopause years | 40 ± 16.7 | 35 ± 21 | 0.03 |
| Body mass index | 27.6 ± 4.4 | 28.5 ± 5.06 | 0.08 |
| Underweight, n (%) | 1 (0.5) | 3 (1.6) | 0.2 |
| Normal, (%) | 69 (32.5) | 53 (26.7) | 0.9 |
| Overweight, n (%) | 81 (37.5) | 79 (41.1) | 0.9 |
| Obese, n (%) | 65 (30.7) | 63 (32.8) | 0.4 |
| Variable | n a = 216 |
|---|---|
| Disease duration (years), mean | 3.6 ± 5.3 |
| Clinical stage, n (%) | |
| I | 44 (20.4) |
| II | 99 (45.8) |
| III | 61 (28.2) |
| IV | 12 (5.6) |
| Histology, n (%) | |
| Ductal | 183 (84.7) |
| Lobulillar | 25 (11.6) |
| Other | 8 (3.7) |
| Histological grade, n (%) | |
| Well-differentiated | 24 (11.1) |
| Moderately differentiated | 158 (73.1) |
| Poorly differentiated | 34 (15.8) |
| Variable | n a = 216 |
|---|---|
| Objective of treatment | |
| Neoadjuvant, n (%) | 57 (26.4) |
| Adjuvant, n (%) | 4 (1.9) |
| Palliative, n (%) | 7 (3.2) |
| Chemotherapy | |
| Anthracyclines, n (%) | 99 (45.8) |
| Taxanes, n (%) | 83 (38.4) |
| Carboplatin, n (%) | 3 (1.4) |
| Cyclophosphamide, n (%) | 85 (39.4) |
| Hormone therapy | |
| Tamoxifen, n (%) | 38 (17.6) |
| Aromatase inhibitor, n (%) | 87 (40.3) |
| Tamoxifen and aromatase inhibitor, n (%) | 24 (11.1) |
| Radiotherapy, n (%) | 14 (6.5) |
| Variable | n a = 216 |
|---|---|
| Treatment provided during sampling | |
| Without treatment, n (%) | 19 (8.9) |
| Chemotherapy, n (%) | 30 (13.9) |
| Targeted therapy, n (%) | 31 (14.4) |
| Hormone therapy, n (%) | 137 (63.4) |
| Radiotherapy, n (%) | 6 (2.8) |
| Inheritance Models | Control Group n a (%) | Case Group n (%) | OR b (95%CI c) | p d |
|---|---|---|---|---|
| Allele | ||||
| D f | 405 (0.92) | 349 (0.88) | Reference | |
| I g | 35 (0.08) | 47 (0.12) | 0.64 (0.41–1.02) | 0.07 |
| Co-dominant | ||||
| D/D | 187(0.85) | 151 (0.76) | Reference | |
| D/I | 31 (0.14) | 47 (0.24) | 0.53 (0.32–0.88) | 0.02 |
| I/I | 2 (0.01) | 0 (0.0) | NS e | 0.58 |
| Dominant | ||||
| D/D | 187(0.85) | 151 (0.76) | Reference | |
| D/I + I/I | 33 (0.15) | 47 (0.24) | 0.57 (0.35–0.93) | 0.03 |
| Recessive | ||||
| D/D + D/I | 218 (0.99) | 198 (1.00) | Reference | |
| I/I | 2 (0.01) | 0 (0.0) | NS e | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araiza-Guzmán, M.; Gutiérrez-Zepeda, B.M.; Saldaña-Cruz, A.M.; Montoya-Delgado, I.B.; Rubio-Delgado, D.; Benítez-Villa, P.; Hernández-Corona, D.M.; Daneri-Navarro, A.; del Toro-Arreola, A.; Márquez-Pedroza, J.; et al. TP53 IVS3 16 bp Variant and Breast Cancer Risk in Western Mexican Women: A Case–Control Study. Curr. Issues Mol. Biol. 2025, 47, 744. https://doi.org/10.3390/cimb47090744
Araiza-Guzmán M, Gutiérrez-Zepeda BM, Saldaña-Cruz AM, Montoya-Delgado IB, Rubio-Delgado D, Benítez-Villa P, Hernández-Corona DM, Daneri-Navarro A, del Toro-Arreola A, Márquez-Pedroza J, et al. TP53 IVS3 16 bp Variant and Breast Cancer Risk in Western Mexican Women: A Case–Control Study. Current Issues in Molecular Biology. 2025; 47(9):744. https://doi.org/10.3390/cimb47090744
Chicago/Turabian StyleAraiza-Guzmán, Mariana, Bricia M. Gutiérrez-Zepeda, Ana M. Saldaña-Cruz, Ingrid B. Montoya-Delgado, Diana Rubio-Delgado, Pablo Benítez-Villa, Diana M. Hernández-Corona, Adrian Daneri-Navarro, Alicia del Toro-Arreola, Jazmin Márquez-Pedroza, and et al. 2025. "TP53 IVS3 16 bp Variant and Breast Cancer Risk in Western Mexican Women: A Case–Control Study" Current Issues in Molecular Biology 47, no. 9: 744. https://doi.org/10.3390/cimb47090744
APA StyleAraiza-Guzmán, M., Gutiérrez-Zepeda, B. M., Saldaña-Cruz, A. M., Montoya-Delgado, I. B., Rubio-Delgado, D., Benítez-Villa, P., Hernández-Corona, D. M., Daneri-Navarro, A., del Toro-Arreola, A., Márquez-Pedroza, J., Quintero-Ramos, A., & Contreras-Haro, B. (2025). TP53 IVS3 16 bp Variant and Breast Cancer Risk in Western Mexican Women: A Case–Control Study. Current Issues in Molecular Biology, 47(9), 744. https://doi.org/10.3390/cimb47090744









