Serum TNF -α, IL-10 and IL-2 Trajectories and Outcomes in NSCLC and Melanoma Under Anti-PD-1 Therapy: Longitudinal Real-World Evidence from a Single Center
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Methods and Statistical Analysis
2.4. Clinical Endpoints
2.5. Study Purpose
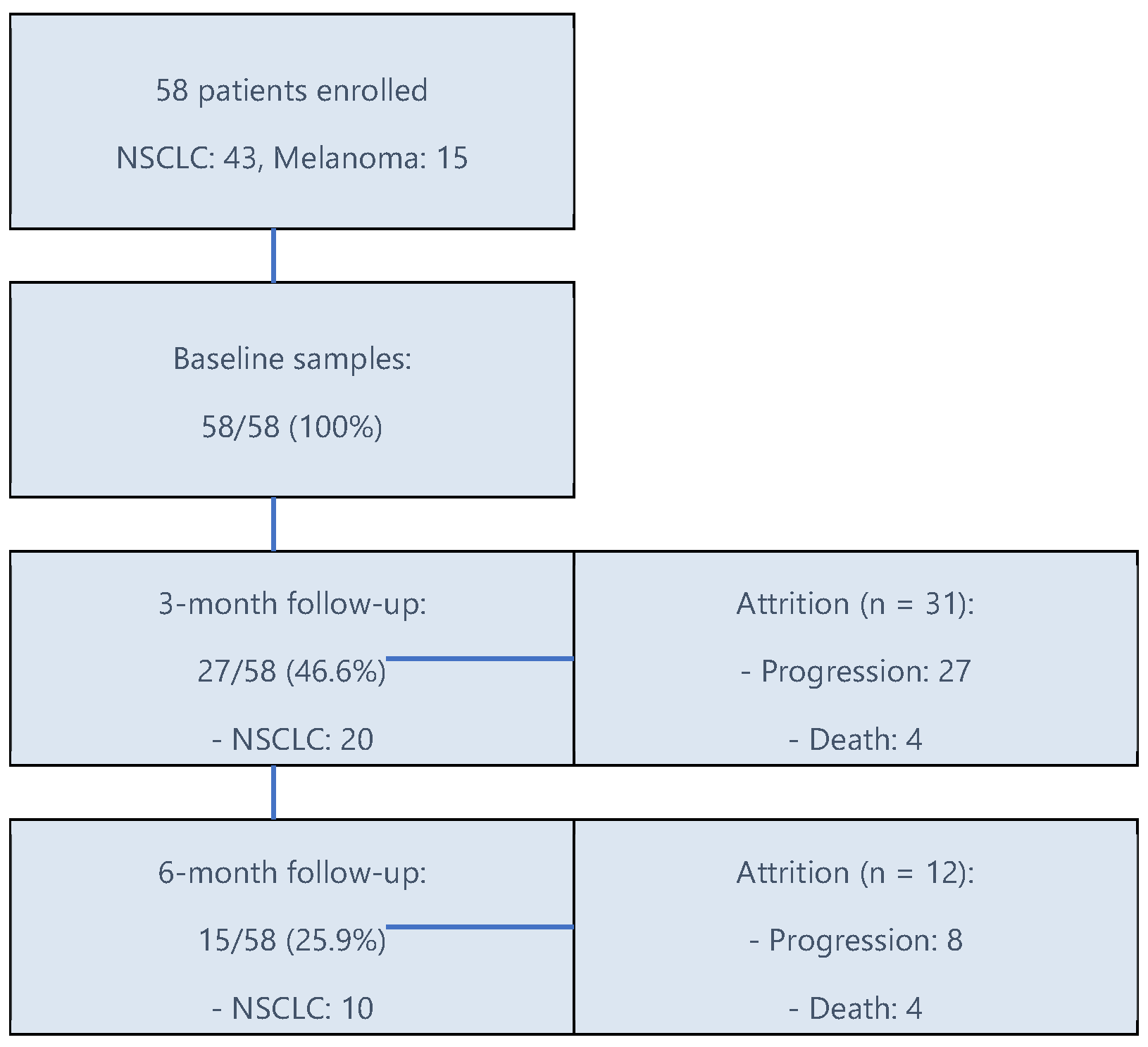
3. Results
3.1. Patients’ Characteristics
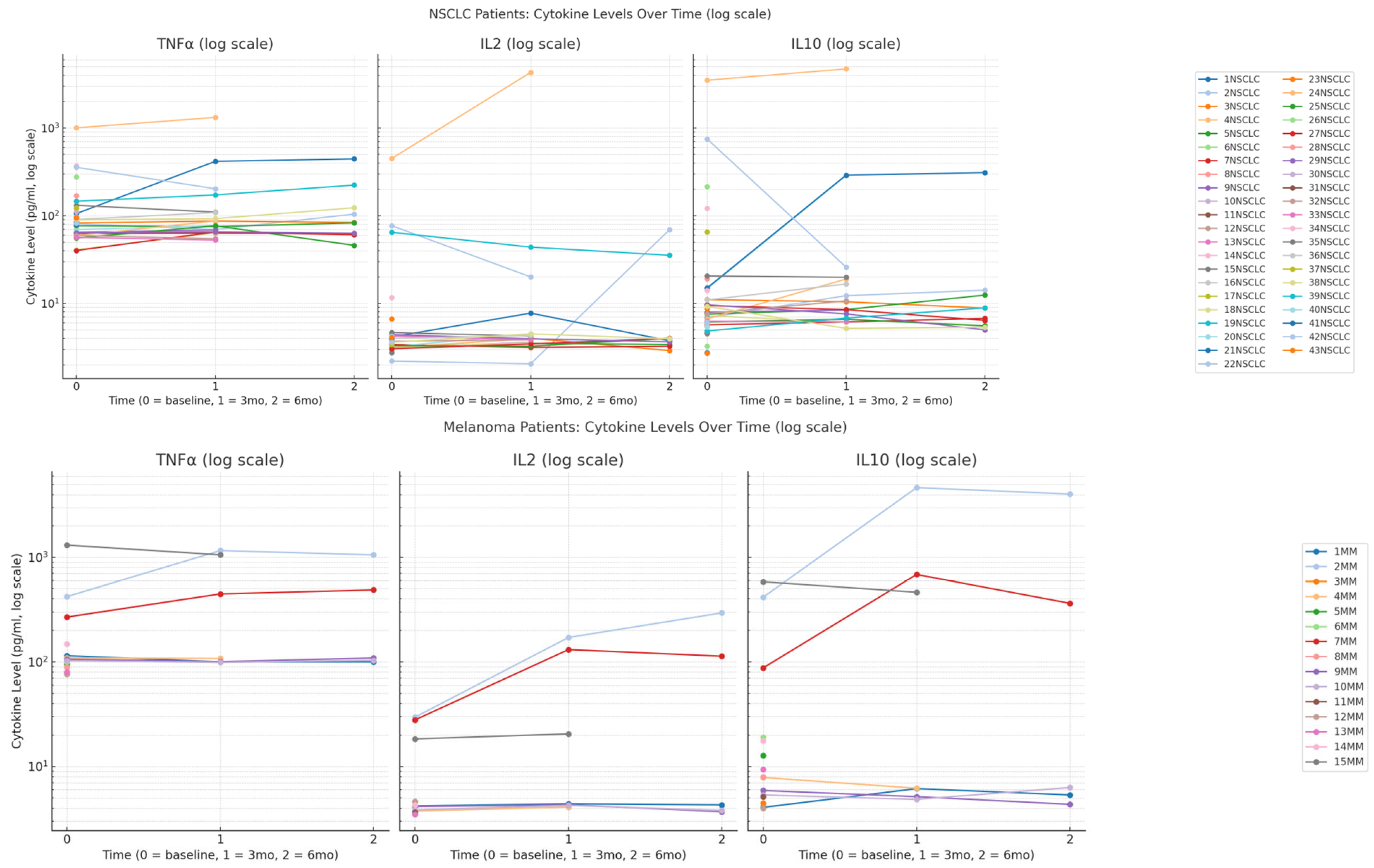
3.2. Cytokines Timeline Trends in Both Cohorts
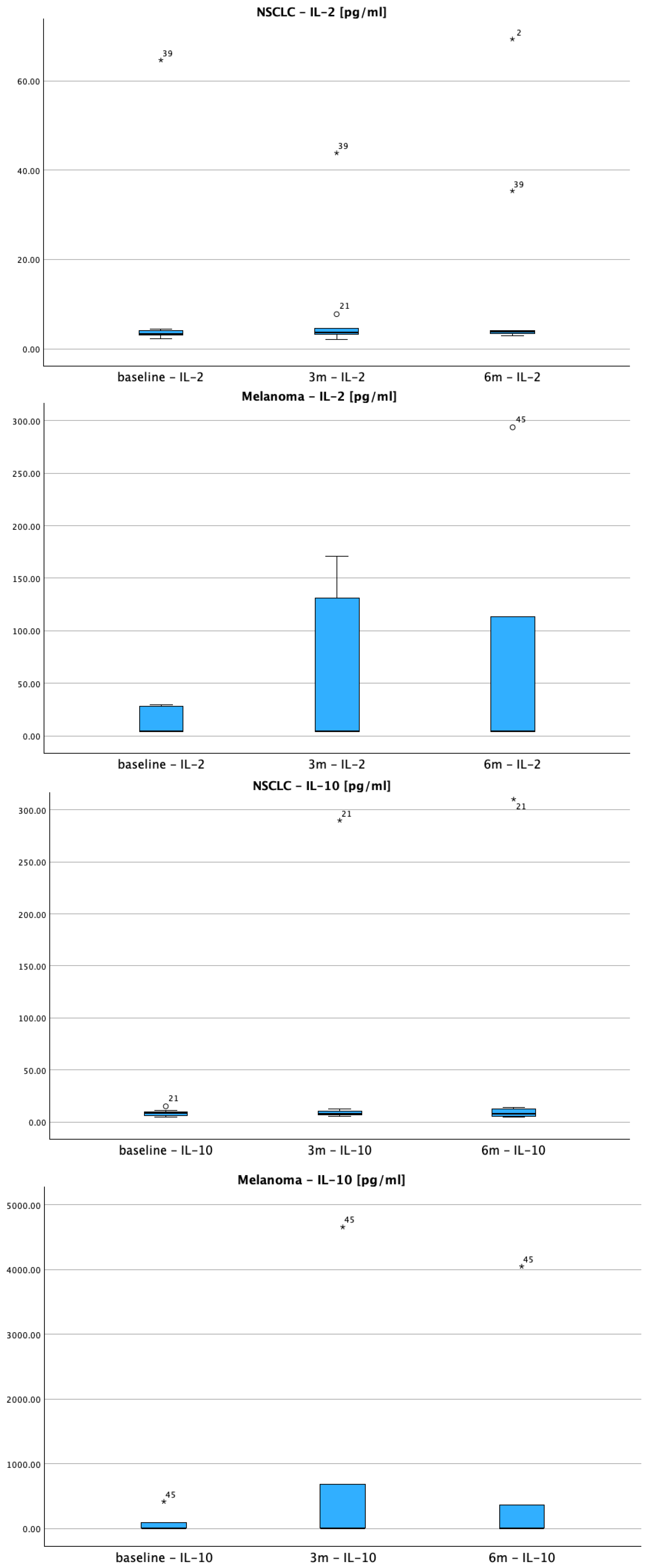
3.2.1. IL-2 Response Patterns
3.2.2. IL-10 Dynamics
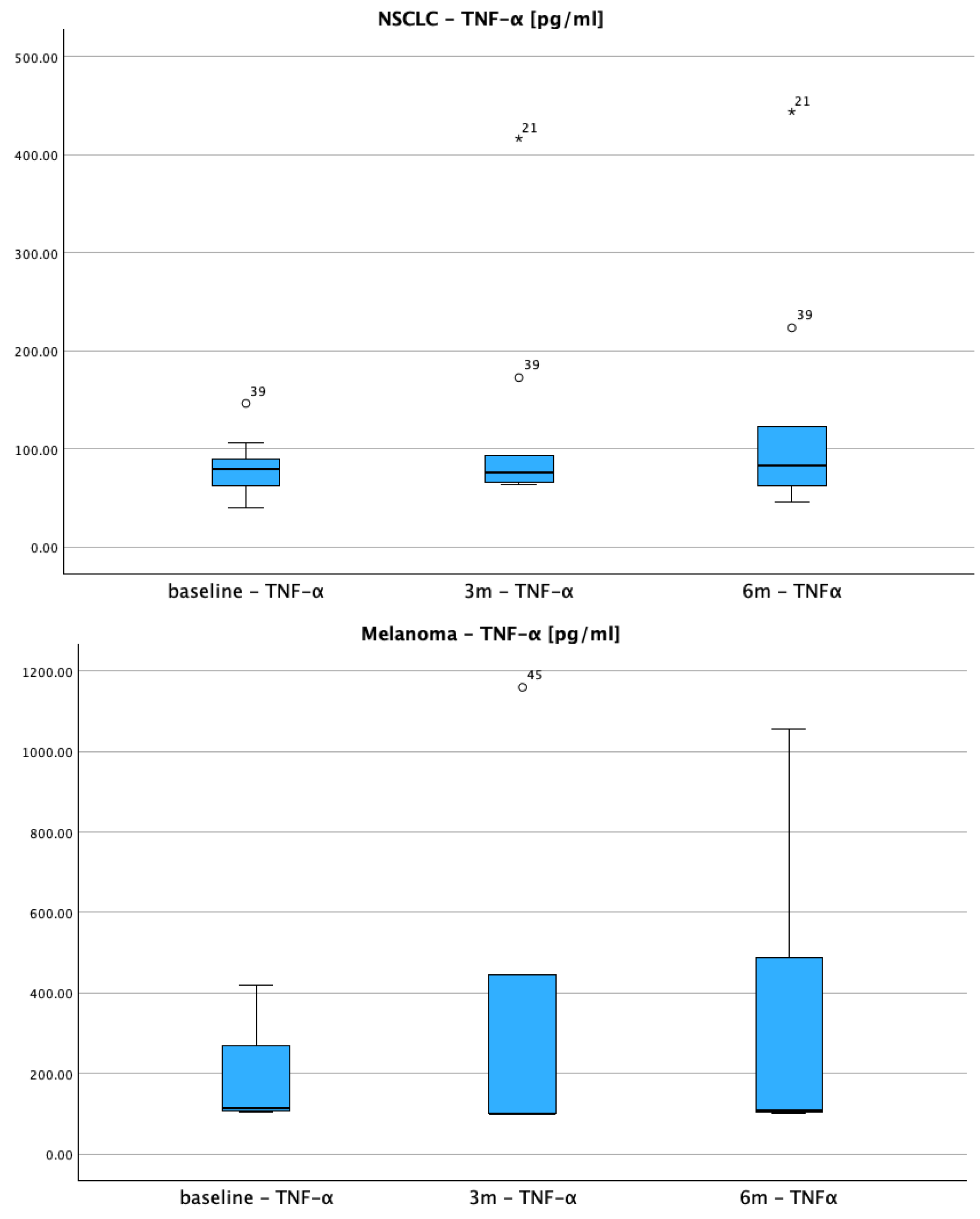
3.2.3. TNF-α Inflammatory Response
3.3. Baseline Cytokine Levels as Predictors of Treatment Response and Survival
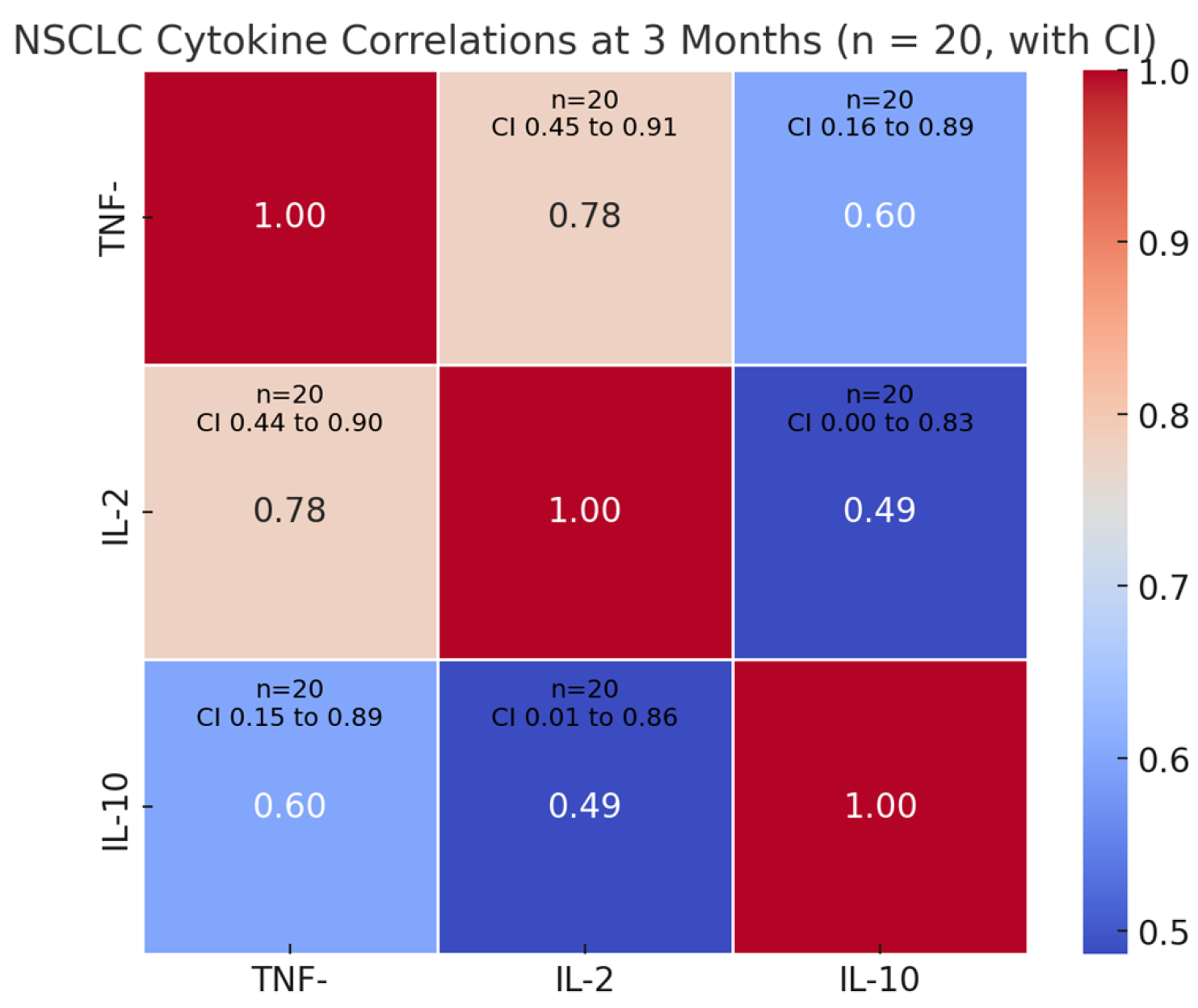
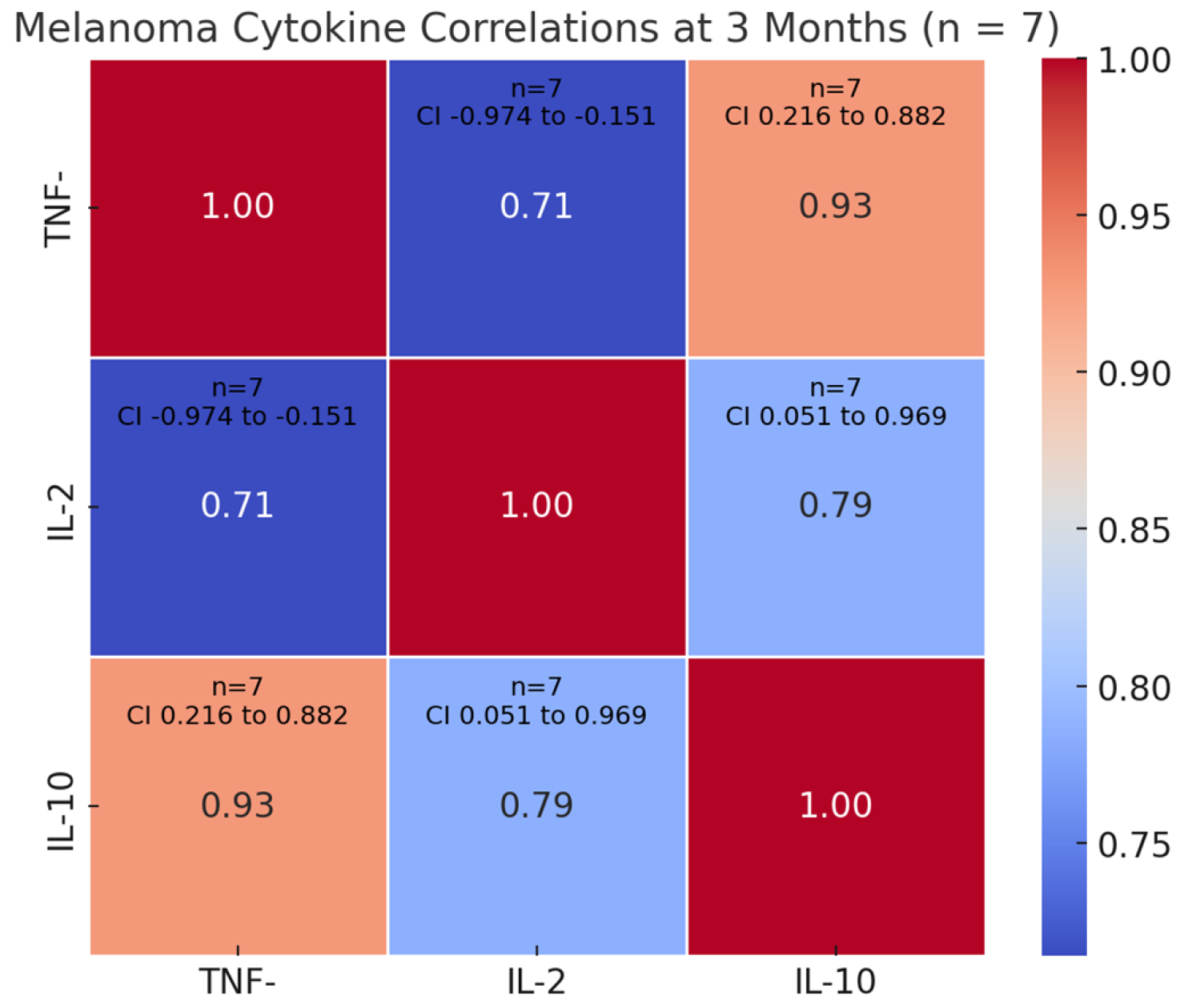
3.4. Biomarker Dynamics at 3 Months Timepoint—Correlations with Clinical Outcomes and Treatment Efficacy
4. Discussion
4.1. Cytokine Dynamics
4.2. Timeline Dynamics of Biomarkers: Associations with Clinical Endpoints and Therapeutic Efficacy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, Y.; Huo, M.; Liu, X.; Li, S.C. Biomarkers and computational models for predicting efficacy to tumor ICI immunotherapy. Front. Immunol. 2024, 15, 1368749. [Google Scholar] [CrossRef]
- Wang, M.; Zhai, X.; Li, J.; Guan, J.; Xu, S.; Li, Y.; Zhu, H. The Role of Cytokines in Predicting the Response and Adverse Events Related to Immune Checkpoint Inhibitors. Front. Immunol. 2021, 12, 670391. [Google Scholar] [CrossRef] [PubMed]
- Manzano, S.; Caffarel, M.M. Cytokine-centered strategies to boost cancer immunotherapy. Mol. Oncol. 2025, 19, 579–583. [Google Scholar] [CrossRef]
- Farooqi, S.J.; Zhao, Z.; Öjlert, Å.K.; Thunold, S.; Juul, H.V.; Bjaanæs, M.M.; Horndalsveen, H.; Nymoen, H.M.G.; Helland, Å.; Haakensen, V.D. Serum cytokines as a biomarker for immune checkpoint inhibitor toxicity in patients with pleural mesothelioma. Front. Immunol. 2024, 15, 1480183. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Song, Y.; Deng, W.; Blake, N.; Luo, X.; Meng, J. Potential predictive biomarkers in antitumor immunotherapy: Navigating the future of antitumor treatment and immune checkpoint inhibitor efficacy. Front. Oncol. 2024, 14, 1483454. [Google Scholar] [CrossRef]
- Vogrig, A.; Dentoni, M.; Florean, I.; Cellante, G.; Domenis, R.; Iacono, D.; Pelizzari, G.; Rossi, S.; Damato, V.; Fabris, M.; et al. Prediction, prevention, and precision treatment of immune checkpoint inhibitor neurological toxicity using autoantibodies, cytokines, and microbiota. Front. Immunol. 2025, 16, 1548897. [Google Scholar] [CrossRef]
- Wei, F.; Sasada, T. Circulating cytokine signatures as a soluble biomarker of immune checkpoint inhibitor therapy in non-small-cell lung cancer. Genes Immun. 2024, 25, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.-C.; Yang, C.-C.; Yang, Y.-F.; Yan, L.-J.; Ding, Z.-N.; Liu, H.; Yan, Y.-C.; Dong, Z.-R.; Wang, D.-X.; Li, T. Peripheral cytokine levels as novel predictors of survival in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 884592. [Google Scholar] [CrossRef]
- Hou, Y.; Li, X.; Yang, Y.; Shi, H.; Wang, S.; Gao, M. Serum cytokines and neutrophil-to-lymphocyte ratio as predictive biomarkers of benefit from PD-1 inhibitors in gastric cancer. Front. Immunol. 2023, 14, 1274431. [Google Scholar] [CrossRef]
- Kao, C.J.; Charmsaz, S.; Alden, S.L.; Brancati, M.; Li, H.L.; Balaji, A.; Munjal, K.; Howe, K.; Mitchell, S.; Leatherman, J.; et al. Immune-related events in individuals with solid tumors on immunotherapy associate with Th17 and Th2 signatures. J. Clin. Investig. 2025, 135, e192014. [Google Scholar] [CrossRef]
- Wang, X.; Montoyo-Pujol, Y.G.; Bermudez, S.; Corpas, G.; Martin, A.; Almazan, F.; Cabrera, T.; López-Nevot, M.A.; Berardi, R. Serum Cytokine Profiles of Melanoma Patients and Their Association with Tumor Progression and Metastasis. J. Oncol. 2021, 2021, 6610769. [Google Scholar] [CrossRef]
- Versluis, J.M.; Blankenstein, S.A.; Dimitriadis, P.; Wilmott, J.S.; Elens, R.; Blokx, W.A.M.; van Houdt, W.; Menzies, A.M.; Schrage, Y.M.; Wouters, M.W.J.M.; et al. Interferon-gamma signature as prognostic and predictive marker in macroscopic stage III melanoma. J. Immunother. Cancer 2024, 12, e008125. [Google Scholar] [CrossRef]
- Yang, F.; Shaibu, Z.; Liu, Q.; Zhu, W. Cytokine profiles as predictive biomarkers for treatment outcomes in advanced gastric cancer patients undergoing PD-1 blockade immunochemotherapy: A meta-analysis. Clin. Exp. Med. 2025, 25, 136. [Google Scholar] [CrossRef]
- Saleem, M.; Watson, A.E.; Anwaar, A.; Jasser, A.O.; Yusuf, N. Optimizing Immunotherapy: The Synergy of Immune Checkpoint Inhibitors with Artificial Intelligence in Melanoma Treatment. Biomolecules 2025, 15, 589. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Liu, J.; Yu, Y.V.; Jin, Y.N. Machine learning-based identification of an immunotherapy-related signature to enhance outcomes and immunotherapy responses in melanoma. Front. Immunol. 2024, 15, 1451103. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M. Biomarkers for Immunotherapy Toxicity: Are Cytokines the Answer? Clin. Cancer Res. 2019, 25, 1452–1454. [Google Scholar] [CrossRef]
- Fu, X.-Y.; Yin, H.; Chen, X.-T.; Yao, J.-F.; Ma, Y.-N.; Song, M.; Xu, H.; Yu, Q.-Y.; Du, S.-S.; Qi, Y.-K.; et al. Three Rounds of Stability-Guided Optimization and Systematical Evaluation of Oncolytic Peptide LTX-315. J. Med. Chem. 2024, 67, 3885–3908. [Google Scholar] [CrossRef]
- Yin, H.; Fu, X.Y.; Gao, H.Y.; Ma, Y.N.; Yao, J.F.; Du, S.S.; Qi, Y.K.; Wang, K.W. Design, synthesis and anticancer evaluation of novel oncolytic peptide-chlorambucil conjugates. Bioorg. Chem. 2023, 138, 106674. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Chen, X.T.; Chi, Q.N.; Ma, Y.N.; Fu, X.Y.; Du, S.S.; Qi, Y.K.; Wang, K.W. The hybrid oncolytic peptide NTP-385 potently inhibits adherent cancer cells by targeting the nucleus. Acta Pharmacol. Sin. 2023, 44, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Bogatu, A.; Wysocka, M.; Wysocki, O.; Butterworth, H.; Landers, D.; Kilgour, E.; Freitas, A. Metareview-informed Explainable Cytokine Storm Detection during CAR-T cell Therapy. arXiv 2022, arXiv:2206.10612. Available online: http://arxiv.org/abs/2206.10612 (accessed on 19 July 2025).
- Jain, K.; Goel, A.; Mehra, D.; Rathore, D.K.; Binayke, A.; Aggarwal, S.; Ganguly, S.; Awasthi, A.; Madan, E.; Ganguly, N.K. Cytokine profiling identifies circulating IL-2, IL23 and sPD-L1 as prognostic biomarkers for treatment outcomes in non-small cell lung cancer patients undergoing anti-PD1 therapy. Front. Oncol. 2025, 15, 1628379. [Google Scholar] [CrossRef]
- Rosner, B. Fundamentals of Biostatistics, 8th ed.; Cengage Learning: Boston, MA, USA, 2016. [Google Scholar]
- Xu, Y.; Fu, Y.; Zhu, B.; Wang, J.; Zhang, B. Predictive Biomarkers of Immune Checkpoint Inhibitors-Related Toxicities. Front. Immunol. 2020, 11, 2023. [Google Scholar] [CrossRef]
- Hussain, F.H.N.; Shafique, K.; Raza, A. Soluble biomarkers as predictors of response to immunotherapy in Non-Small Cell Lung cancer (NSCLC)-insights from the tumor microenvironment perspective. Cancer Immunol. Connect 2025, 1, 1. [Google Scholar] [CrossRef]
- Les, I.; Martínez, M.; Pérez-Francisco, I.; Cabero, M.; Teijeira, L.; Arrazubi, V.; Torrego, N.; Campillo-Calatayud, A.; Elejalde, I.; Kochan, G.; et al. Predictive Biomarkers for Checkpoint Inhibitor Immune-Related Adverse Events. Cancers 2023, 15, 1629. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.U.; Yoon, H.K. Potential predictive value of change in inflammatory cytokines levels subsequent to initiation of immune checkpoint inhibitor in patients with advanced non-small cell lung cancer. Cytokine 2021, 138, 155363. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Fisher, D.E.; Flaherty, K.T. Cell-state dynamics and therapeutic resistance in melanoma from the perspective of MITF and IFNγ pathways. Nat. Rev. Clin. Oncol. 2019, 16, 549–562. [Google Scholar] [CrossRef]
- Spiliopoulou, P.; Vornicova, O.; Genta, S.; Spreafico, A. Shaping the Future of Immunotherapy Targets and Biomarkers in Melanoma and Non-Melanoma Cutaneous Cancers. Int. J. Mol. Sci. 2023, 24, 1294. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, F.; Montfort, A.; Marcheteau, E.; Imbert, C.; Gilhodes, J.; Filleron, T.; Rochaix, P.; Andrieu-Abadie, N.; Levade, T.; Meyer, N.; et al. TNFα blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat. Commun. 2017, 8, 2256. [Google Scholar] [CrossRef]
- Ohri, C.M.; Shikotra, A.; Green, R.H.; Waller, D.A.; Bradding, P. Tumour necrosis factor-alpha expression in tumour islets confers a survival advantage in non-small cell lung cancer. BMC Cancer 2010, 10, 323. [Google Scholar] [CrossRef]
- Lin, A.; Zhang, H.; Meng, H.; Deng, Z.; Gu, T.; Luo, P.; Zhang, J. TNF-Alpha Pathway Alternation Predicts Survival of Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer. Front. Immunol. 2021, 12, 667875. [Google Scholar] [CrossRef]
- Benoot, T.; Piccioni, E.; De Ridder, K.; Goyvaerts, C. TNFα and Immune Checkpoint Inhibition: Friend or Foe for Lung Cancer? Int. J. Mol. Sci. 2021, 22, 8691. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Nie, X.; Desai, S.S.; Villaroel-Espindola, F.; Badri, T.; Zhao, D.; Kim, A.W.; Ji, L.; Zhang, T.; Quinlan, E.; et al. A Burned-Out CD8+ T-cell Subset Expands in the Tumor Microenvironment and Curbs Cancer Immunotherapy. Cancer Discov. 2021, 11, 1700–1715. [Google Scholar] [CrossRef]
- Luo, Y.H.; Shen, C.I.; Chiang, C.L.; Huang, H.C.; Chen, Y.M. Dynamic immune signatures of patients with advanced non–small-cell lung cancer for infection prediction after immunotherapy. Front. Immunol. 2024, 15, 1269253. [Google Scholar] [CrossRef]
- Raeber, M.E.; Sahin, D.; Karakus, U.; Boyman, O. A systematic review of interleukin-2-based immunotherapies in clinical trials for cancer and autoimmune diseases. eBioMedicine 2023, 90, 104539. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, C.; Ren, S. Role of IL-2 in cancer immunotherapy. OncoImmunology 2016, 5, e1163462. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Xiang, B.; Yang, Z.; Liu, J.; Xu, D.; Geng, L.; Zhan, M.; Xu, Y.; Zhang, B. Down-regulation of interleukin-2 predicts poor prognosis and associated with immune escape in lung adenocarcinoma. Int. J. Immunopathol. Pharmacol. 2023, 37, 03946320231202748. [Google Scholar] [CrossRef] [PubMed]
- Pasello, G.; Fabricio, A.S.C.; Del Bianco, P.; Favaretto, A.; Salizzato, V.; Piccin, L.; Zustovich, F.; De Rossi, C.; Pigozzo, J.; Fabozzi, A.; et al. Circulating cytokines as predictors of response to immune checkpoint inhibitors (ICIs) in patients (pts) with melanoma (Mel) and non–small cell lung cancer (NSCLC). J. Clin. Oncol. 2022, 40, 2549. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, S.; Zhang, K.; Zhang, L. Serum cytokine levels and other associated factors as possible immunotherapeutic targets and prognostic indicators for lung cancer. Front. Oncol. 2023, 13, 1064616. [Google Scholar] [CrossRef]
- Rafei-Shamsabadi, D.; Lehr, S.; Behrens, M.; Meiss, F. Additive Intralesional Interleukin-2 Improves Progression-Free Survival in a Distinct Subgroup of Melanoma Patients with Prior Progression under Immunotherapy. Cancers 2022, 14, 540. [Google Scholar] [CrossRef] [PubMed]
- Gellrich, F.; Schmitz, M.; Beissert, S.; Meier, F. Anti-PD-1 and Novel Combinations in the Treatment of Melanoma—An Update. J. Clin. Med. 2020, 9, 223. [Google Scholar] [CrossRef]
- Jacquelot, N.; Seillet, C.; Wang, M.; Pizzolla, A.; Liao, Y.; Hediyeh-Zadeh, S.; Grisaru-Tal, S.; Louis, C.; Huang, Q.; Schreuder, J.; et al. Blockade of the co-inhibitory molecule PD-1 unleashes ILC2-dependent antitumor immunity in melanoma. Nat. Immunol. 2021, 22, 851–864. [Google Scholar] [CrossRef]
- Im, S.J.; Lee, K.; Ha, S.J. Harnessing IL-2 for immunotherapy against cancer and chronic infection: A historical perspective and emerging trends. Exp. Mol. Med. 2024, 56, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Rokade, S.; Damani, A.M.; Oft, M.; Emmerich, J. IL-2 based cancer immunotherapies: An evolving paradigm. Front. Immunol. 2024, 15, 1433989. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lu, M.; Chen, H.; Chen, S.; Luo, X.; Qin, Y.; Zhang, J. Increased IL-10 mRNA expression in tumor-associated macrophage correlated with late stage of lung cancer. J. Exp. Clin. Cancer Res. 2011, 30, 62. [Google Scholar] [CrossRef]
- Vahl, J.M.; Friedrich, J.; Mittler, S.; Trump, S.; Heim, L.; Kachler, K.; Balabko, L.; Fuhrich, N.; Geppert, C.-I.; Trufa, D.I.; et al. Interleukin-10-regulated tumour tolerance in non-small cell lung cancer. Br. J. Cancer 2017, 117, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Tucci, M.; Passarelli, A.; Mannavola, F.; Felici, C.; Stucci, L.S.; Cives, M.; Silvestris, F. Immune System Evasion as Hallmark of Melanoma Progression: The Role of Dendritic Cells. Front. Oncol. 2019, 9, 1148. [Google Scholar] [CrossRef]
- Itakura, E.; Huang, R.R.; Wen, D.R.; Paul, E.; Wünsch, P.H.; Cochran, A.J. IL-10 expression by primary tumor cells correlates with melanoma progression from radial to vertical growth phase and development of metastatic competence. Mod. Pathol. 2011, 24, 801–809. [Google Scholar] [CrossRef]
- Nour, M.A.; Rajabivahid, M.; Mehdi, M.S.S.; Tahmasebi, S.; Dashtgol, S.N.; Dehghani-Ghorbi, M.; Vanan, A.G.; Ghorbaninezhad, F. A new era in melanoma immunotherapy: Focus on DCs metabolic reprogramming. Cancer Cell Int. 2025, 25, 149. [Google Scholar] [CrossRef]
- Sun, Z.; Fourcade, J.; Pagliano, O.; Chauvin, J.M.; Sander, C.; Kirkwood, J.M.; Zarour, H.M. IL10 and PD-1 Cooperate to Limit the Activity of Tumor-Specific CD8+ T Cells. Cancer Res. 2015, 75, 1635–1644. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and hot tumors: From molecular mechanisms to targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 274. [Google Scholar] [CrossRef]
- Salkeni, M.A.; Naing, A. Interleukin-10 in cancer immunotherapy: From bench to bedside. Trends Cancer 2023, 9, 716–725. [Google Scholar] [CrossRef]
- Wu, W.K.; Llewellyn, O.P.C.; Bates, D.O.; Nicholson, L.B.; Dick, A.D. IL-10 regulation of macrophage VEGF production is dependent on macrophage polarisation and hypoxia. Immunobiology 2010, 215, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Carlini, V.; Noonan, D.M.; Abdalalem, E.; Goletti, D.; Sansone, C.; Calabrone, L.; Albini, A. The multifaceted nature of IL-10: Regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front. Immunol. 2023, 14, 1161067. [Google Scholar] [CrossRef]
- Xu-Monette, Z.Y.; Zhang, M.; Li, J.; Young, K.H. PD-1/PD-L1 Blockade: Have We Found the Key to Unleash the Antitumor Immune Response? Front. Immunol. 2017, 8, 1597. [Google Scholar] [CrossRef]
- Karki, R.; Kanneganti, T.D. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat. Rev. Cancer 2019, 19, 197–214. [Google Scholar] [CrossRef]
- Sun, Z.; Ren, Z.; Yang, K.; Liu, Z.; Cao, S.; Deng, S.; Xu, L.; Liang, Y.; Guo, J.; Bian, Y.; et al. A next-generation tumor-targeting IL-2 preferentially promotes tumor-infiltrating CD8+ T-cell response and effective tumor control. Nat. Commun. 2019, 10, 3874. [Google Scholar] [CrossRef]
- Rollings, C.M.; Sinclair, L.V.; Brady, H.J.M.; Cantrell, D.A.; Ross, S.H. Interleukin-2 shapes the cytotoxic T cell proteome and immune environment–sensing programs. Sci. Signal. 2018, 11, eaap8112. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Qin, L.; Sun, J.; Li, K.; Zang, C.; Wang, Q.; Qiao, W.; Liu, B.; Zhao, Y.; Zhang, Y. Dynamic Changes of Cytokine Profiles and Their Correlation With Tumor Recurrence Following Thermal Ablation in Hepatocellular Carcinoma. Technol. Cancer Res. Treat. 2023, 22, 15330338231190644. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Yang, H.; Lee, W.S.; Cheon, J.; Sang, Y.B.; Kang, B.; Chon, H.J.; Kim, C. High levels of baseline serum IL-10 are associated with reduced clinical benefit from first-line immune checkpoint inhibitor therapy in advanced renal cell carcinoma. J. Cancer 2023, 14, 935–942. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, F.; Zhao, C.; Cheng, L.; Zhou, C.; Qiao, M.; Li, X.; Chen, X. Interleukin-10 Is a Promising Marker for Immune-Related Adverse Events in Patients With Non-Small Cell Lung Cancer Receiving Immunotherapy. Front. Immunol. 2022, 13, 840313. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.; Orlowski, R.J.; Xu, X.; Mick, R.; George, S.M.; Yan, P.K.; Manne, S.; Kraya, A.A.; Wubbenhorst, B.; Dorfman, L.; et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat. Med. 2019, 25, 454–461. [Google Scholar] [CrossRef]
- Vathiotis, I.A.; Salichos, L.; Martinez-Morilla, S.; Gavrielatou, N.; Aung, T.N.; Shafi, S.; Wong, P.F.; Jessel, S.; Kluger, H.M.; Syrigos, K.N.; et al. Baseline gene expression profiling determines long-term benefit to programmed cell death protein 1 axis blockade. NPJ Precis. Oncol. 2022, 6, 92. [Google Scholar] [CrossRef]
- Lamichhane, P.; Karyampudi, L.; Shreeder, B.; Krempski, J.; Bahr, D.; Daum, J.; Kalli, K.R.; Goode, E.L.; Block, M.S.; Cannon, M.J.; et al. IL10 Release upon PD-1 Blockade Sustains Immunosuppression in Ovarian Cancer. Cancer Res. 2017, 77, 6667–6678. [Google Scholar] [CrossRef]
- Ng, T.H.S.; Britton, G.J.; Hill, E.V.; Verhagen, J.; Burton, B.R.; Wraith, D.C. Regulation of Adaptive Immunity; The Role of Interleukin-10. Front Immunol. Front. Immunol. 2013, 4, 129. Available online: http://journal.frontiersin.org/article/10.3389/fimmu.2013.00129/abstract (accessed on 19 June 2025). [CrossRef] [PubMed]
- Torisu-Itakura, H.; Lee, J.H.; Huynh, Y.; Ye, X.; Essner, R.; Morton, D.L. Monocyte-derived IL-10 expression predicts prognosis of stage IV melanoma patients. J. Immunother. 2007, 30, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, S.; He, X.; Yuan, Y.; Wei, X. Targeting inflammation as cancer therapy. J. Hematol. Oncol. 2024, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Warner, A.B.; Postow, M.A. Bigger Is Not Always Better: Tumor Size and Prognosis in Advanced Melanoma. Clin. Cancer Res. 2018, 24, 4915–4917. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Yi, M.; Li, T.; Niu, M.; Zhang, H.; Wu, Y.; Wu, K.; Dai, Z. Targeting cytokine and chemokine signaling pathways for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 176. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Muhammad, S.; Fan, T.; Hai, Y.; Gao, Y.; He, J. Reigniting hope in cancer treatment: The promise and pitfalls of IL-2 and IL-2R targeting strategies. Mol. Cancer 2023, 22, 121. [Google Scholar] [CrossRef] [PubMed]
- Whyte, C.E.; Singh, K.; Burton, O.T.; Aloulou, M.; Kouser, L.; Veiga, R.V.; Dashwood, A.; Okkenhaug, H.; Benadda, S.; Moudra, A.; et al. Context-dependent effects of IL-2 rewire immunity into distinct cellular circuits. J. Exp. Med. 2022, 219, e20212391, Erratum in J. Exp. Med. 2022, 219, jem.2021149805312022c. [Google Scholar] [CrossRef] [PubMed]
- Morello:, S.; Capone, M.; Sorrentino, C.; Giannarelli, D.; Madonna, G.; Mallardo, D.; Grimaldi, A.M.; Pinto, A.; Ascierto, P.A. Soluble CD73 as biomarker in patients with metastatic melanoma patients treated with nivolumab. J. Transl. Med. 2017, 15, 244. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Value/Distribution |
|---|---|
| Number of patients | 58 |
| Median age (years) | 62.5 (range: 35–83) |
| Cancer type | Lung: 43 (77.6%) |
| Melanoma: 15 (22.4%) | |
| Sex | Male: 39 (67.2%) |
| Female: 19 (32.8%) | |
| Smokers | Yes: 41 (70.7%) |
| No: 17 (29.3%) | |
| Use of corticosteroids at baseline | Yes: 21 (36.2%) |
| No: 37 (63.8%) | |
| Type of NSCLC | Adenocarcinoma 25 (58.1%) |
| Squamous cell carcinoma 18 (41.9%) | |
| PD-L1 | Positive >1% 22 (50%) |
| Negative <1% 22 (50%) | |
| ECOG | 0–31 (53.45%) |
| 1–20 (34.48%) | |
| 2–7 (12.07%) | |
| Median overall survival (months) | 21.1 |
| Best response | Progression 48.3% (28), Stable disease 43.1% (25), |
| Complete response 6.9% (4), Partial response 1.7% (1) | |
| Median number of Nivolumab cycles | 17 |
| Average line of Nivolumab | 2 |
| Line of Nivolumab | 1–13 (22.4%) |
| 2–32 (58.6%) | |
| 3–7 (12.1%) | |
| 4–4 (6.9%) | |
| Subsequent lines of therapy | 50.0% (29/58) |
| Most frequent: Docetaxel, Carboplatin + Paclitaxel, Gemcitabine, Dabrafenib + Trametinib | |
| Previous lines of treatment | Carboplatin + Pemetrexed 19 (32.76%) |
| Carboplatin +Gemcitabine 11 (18.97%) | |
| Carboplatin + Paclitaxel 5 (8.62%) | |
| Gemcitabine 3 (5.17%) | |
| Carboplatin + Navelbine 2 (3.45%) | |
| Carboplatin 2 (3.45%) | |
| Dacarbazine 2 (3.45%) | |
| Dabrafenib + Trametinib 2 (3.45%) | |
| No previous therapy: 20.69% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grecea-Balaj, A.M.; Soritau, O.; Brie, I.; Perde-Schrepler, M.; Virag, P.; Fischer-Fodor, E.; Todor, N.; Cenariu, M.; Nedelea, I.; Ciuleanu, T.E. Serum TNF -α, IL-10 and IL-2 Trajectories and Outcomes in NSCLC and Melanoma Under Anti-PD-1 Therapy: Longitudinal Real-World Evidence from a Single Center. Curr. Issues Mol. Biol. 2025, 47, 746. https://doi.org/10.3390/cimb47090746
Grecea-Balaj AM, Soritau O, Brie I, Perde-Schrepler M, Virag P, Fischer-Fodor E, Todor N, Cenariu M, Nedelea I, Ciuleanu TE. Serum TNF -α, IL-10 and IL-2 Trajectories and Outcomes in NSCLC and Melanoma Under Anti-PD-1 Therapy: Longitudinal Real-World Evidence from a Single Center. Current Issues in Molecular Biology. 2025; 47(9):746. https://doi.org/10.3390/cimb47090746
Chicago/Turabian StyleGrecea-Balaj, Alina Miruna, Olga Soritau, Ioana Brie, Maria Perde-Schrepler, Piroska Virag, Eva Fischer-Fodor, Nicolae Todor, Mihai Cenariu, Ioana Nedelea, and Tudor Eliade Ciuleanu. 2025. "Serum TNF -α, IL-10 and IL-2 Trajectories and Outcomes in NSCLC and Melanoma Under Anti-PD-1 Therapy: Longitudinal Real-World Evidence from a Single Center" Current Issues in Molecular Biology 47, no. 9: 746. https://doi.org/10.3390/cimb47090746
APA StyleGrecea-Balaj, A. M., Soritau, O., Brie, I., Perde-Schrepler, M., Virag, P., Fischer-Fodor, E., Todor, N., Cenariu, M., Nedelea, I., & Ciuleanu, T. E. (2025). Serum TNF -α, IL-10 and IL-2 Trajectories and Outcomes in NSCLC and Melanoma Under Anti-PD-1 Therapy: Longitudinal Real-World Evidence from a Single Center. Current Issues in Molecular Biology, 47(9), 746. https://doi.org/10.3390/cimb47090746







