Decoding the Tumor Microenvironment: Insights and New Targets from Single-Cell Sequencing and Spatial Transcriptomics
Abstract
1. Introduction
Diagnosis by Single-Cell Sequencing (SCS) and Spatial Transcriptomics (ST)
2. Therapeutic Targets in the TME
2.1. Components of the TME
2.2. Pathways Involved with the TME
2.2.1. VEGF
2.2.2. Hypoxia-Inducible Factor 1-Alpha (HIF-1α)
2.2.3. TGF-β
2.2.4. PD-1/PD-L1
2.2.5. CTLA-4
2.2.6. SRC and FAK
2.2.7. NF-κB Pathway

2.3. Protein Targeting
3. Diagnostics
3.1. Single Cell Sequencing
3.2. Spatial Transcriptomics
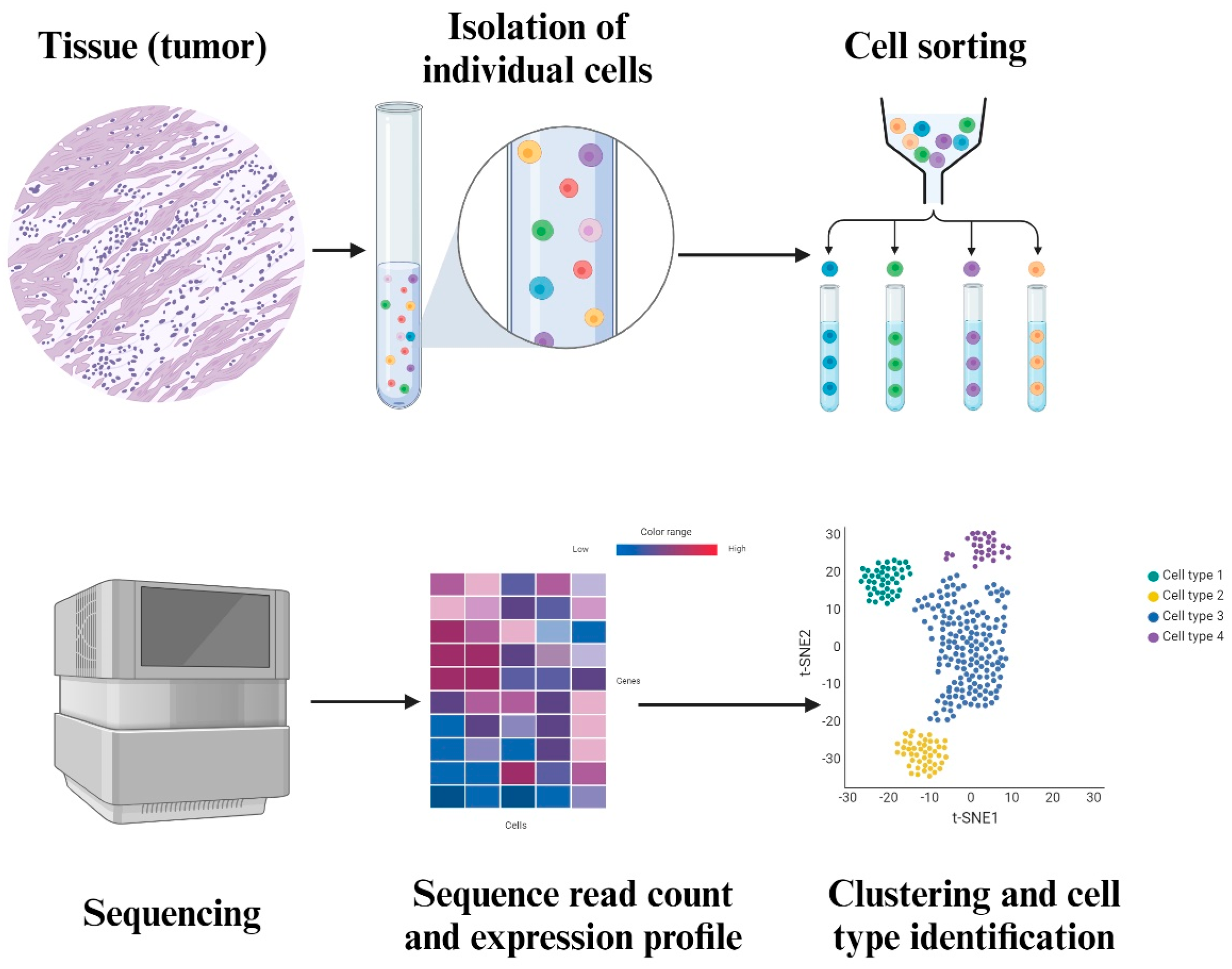
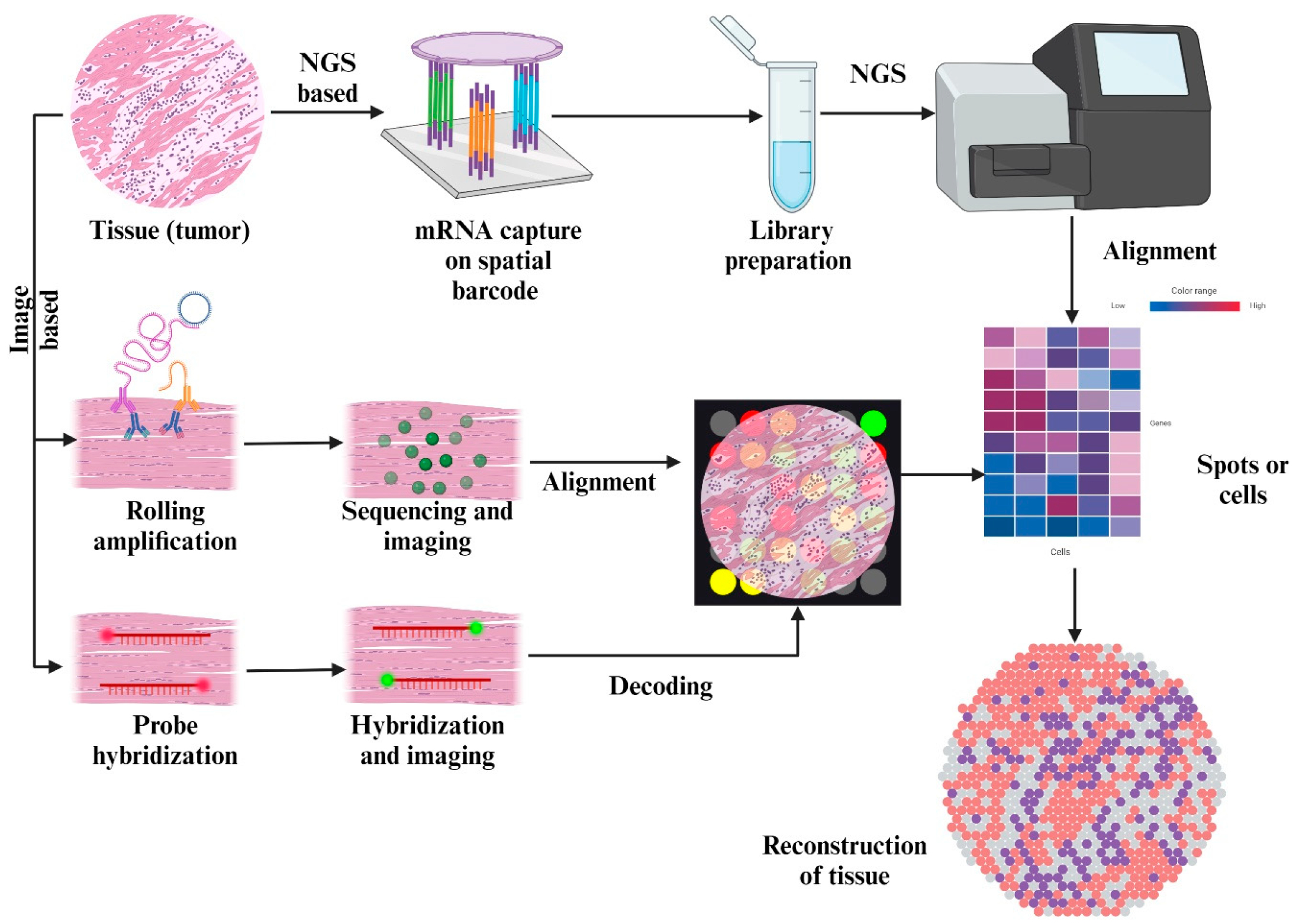
4. Future Directions
| Study and Scientists | Targeted on | Results |
|---|---|---|
| Review of the TME in basal and squamous cell carcinoma; Elizabeth Chiang et al. [132]. | TME in basal and squamous cell carcinoma. | TME interactions promote tumor growth and progression. Immunotherapeutic agents like vismodegib and cemiplimab treat BCC and SCC. |
| Integrated analysis of TME using reconfigurable microfluidic platform; Nan Sethakorn et al. [133]. | Elucidating the pathways underlying the roles of the TME and developing new TME-directed therapies for cancer treatment. | Stacks platform supports multicellular culture and integrated cellular analysis. Enhances TME research; potential for clinical translation and novel insights. |
| Immune modulation in TME; Zimu Deng et al. [134]. | Immune escape mechanisms in the TME to improve cancer immunotherapy. Targeted approaches include CAR T-cell therapy, immune checkpoint blockers, TCR-based cell therapy, cancer vaccines, and oncolytic viruses. | Combines information from 16 original research studies and 2 reviews to highlight the role of the immunosuppressive TME in the initiation, spread, and resistance to treatment of cancer. |
| Targeting the TME for improving therapeutic effectiveness; I-Tsu Chyuan et al. [135]. | Exploring molecular and cellular factors in the TME to uncover resistance mechanisms and develop new combination strategies for cancer immunotherapy. | Reviews advancements in improving therapeutic efficacy in cancer immunotherapy. Highlights the contribution of targeting the TME in cancer immunotherapy. |
| Development of immunotherapy strategies targeting TME; Rilan Bai et al. [136]. | Developing immunotherapy approaches that target the TME across different cancer types. | Precision immunotherapy remodels TME into a positive immune microenvironment. Challenges include a lack of research models and spatiotemporal heterogeneity. |
| Cancer: a mirrored room between tumor bulk and TME; Pablo Hernández-Camarer et al. [137]. | TME and its role in the maintenance of a cancer stem-like phenotype via tumor metastasis, progression, invasiveness, and drug resistance. The paper discusses the importance of understanding the interaction between the TME and metastasis to improve clinical management of cancer. | Demonstrates the feasibility of reprogramming TAMs to an antitumorigenic state, showing potential for improving therapeutic outcomes. |
| Role of TME in cancer progression and therapeutic strategy; Qingjing Wang et al. [138]. | Interaction between PD-1 and the TME, as well as ensuring cancer immunotherapy therapeutics. The paper mentions the inhibition of PD-1, PD-L1, and PD-L2, the construction of CAR-T, and tumor vaccines as popular therapeutic approaches. | TME plays a crucial role in cancer progression and therapeutic strategies. Cancer immunotherapy shows promising therapeutic effects in various cancers. |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, L.; Jing, X.; Ahn, B.-C. Turning tumor microenvironmental foes to friends: A new opportunity for thyroid cancer therapy and redifferentiation. Oral Oncol. 2025, 168, 107513. [Google Scholar] [CrossRef]
- Goenka, A.; Khan, F.; Verma, B.; Sinha, P.; Dmello, C.C.; Jogalekar, M.P.; Gangadaran, P.; Ahn, B. Tumor microenvironment signaling and therapeutics in cancer progression. Cancer Commun. 2023, 43, 525–561. [Google Scholar] [CrossRef]
- Zhu, L.; Li, X.J.; Gangadaran, P.; Jing, X.; Ahn, B.-C. Tumor-associated macrophages as a potential therapeutic target in thyroid cancers. Cancer Immunol. Immunother. 2023, 72, 3895–3917. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Siminzar, P.; Tohidkia, M.R.; Eppard, E.; Vahidfar, N.; Tarighatnia, A.; Aghanejad, A. Recent Trends in Diagnostic Biomarkers of Tumor Microenvironment. Mol. Imaging Biol. 2022, 25, 464–482. [Google Scholar] [CrossRef]
- Tao, S.-C.; Guo, S.-C. Role of extracellular vesicles in tumour microenvironment. Cell Commun. Signal. 2020, 18, 163. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Huang, J.; Tsang, W.-Y.; Li, Z.-H.; Guan, X.-Y. The Origin, Differentiation, and Functions of Cancer-Associated Fibroblasts in Gastrointestinal Cancer. Cell. Mol. Gastroenterol. Hepatol. 2023, 16, 503–511. [Google Scholar] [CrossRef]
- Raaijmakers, K.T.P.M.; Adema, G.J.; Bussink, J.; Ansems, M. Cancer-associated fibroblasts, tumor and radiotherapy: Interactions in the tumor micro-environment. J. Exp. Clin. Cancer Res. 2024, 43, 323. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Walczyk, A.; Kopczyński, J.; Gąsior-Perczak, D.; Pałyga, I.; Kowalik, A.; Chrapek, M.; Hejnold, M.; Góźdź, S.; Kowalska, A.; Hsieh, J.C.-H. Histopathology and immunohistochemistry as prognostic factors for poorly differentiated thyroid cancer in a series of Polish patients. PLoS ONE 2020, 15, e0229264. [Google Scholar] [CrossRef]
- Tang, X.; Huang, Y.; Lei, J.; Luo, H.; Zhu, X. The single-cell sequencing: New developments and medical applications. Cell Biosci. 2019, 9, 53. [Google Scholar] [CrossRef]
- Williams, C.G.; Lee, H.J.; Asatsuma, T.; Vento-Tormo, R.; Haque, A. An introduction to spatial transcriptomics for biomedical research. Genome Med. 2022, 14, 68. [Google Scholar] [CrossRef]
- Choe, K.; Pak, U.; Pang, Y.; Hao, W.; Yang, X. Advances and Challenges in Spatial Transcriptomics for Developmental Biology. Biomolecules 2023, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Misra, P.; Jadhav, A.R.; Bapat, S.A. Single-cell sequencing: A cutting edge tool in molecular medical research. Med. J. Armed Forces India 2022, 78, S7–S13. [Google Scholar] [CrossRef]
- Hadrup, S.; Donia, M.; Straten, P.T. Effector CD4 and CD8 T Cells and Their Role in the Tumor Microenvironment. Cancer Microenviron. 2012, 6, 123–133. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed]
- Chiaruttini, G.; Mele, S.; Opzoomer, J.; Crescioli, S.; Ilieva, K.M.; Lacy, K.E.; Karagiannis, S.N. B cells and the humoral response in melanoma: The overlooked players of the tumor microenvironment. OncoImmunology 2017, 6, e1294296. [Google Scholar] [CrossRef]
- Lewis, C.E.; Pollard, J.W. Distinct Role of Macrophages in Different Tumor Microenvironments. Cancer Res. 2006, 66, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Kim, K.-D.; Lee, H.K. The role of dendritic cells in tumor microenvironments and their uses as therapeutic targets. BMB Rep. 2021, 54, 31–43. [Google Scholar] [CrossRef]
- Yao, H.; He, S. Multi-faceted role of cancer-associated adipocytes in the tumor microenvironment. Mol. Med. Rep. 2021, 24, 866. [Google Scholar] [CrossRef]
- Simon, T.; Salhia, B. Cancer-Associated Fibroblast Subpopulations with Diverse and Dynamic Roles in the Tumor Microenvironment. Mol. Cancer Res. 2022, 20, 183–192. [Google Scholar] [CrossRef]
- Xing, F.; Saidou, J.; Watabe, K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front. Biosci. Landmark Ed. 2010, 15, 166–179. [Google Scholar] [CrossRef]
- Gil Kim, B.; An, H.J.; Kang, S.; Choi, Y.P.; Gao, M.-Q.; Park, H.; Cho, N.H. Laminin-332-Rich Tumor Microenvironment for Tumor Invasion in the Interface Zone of Breast Cancer. Am. J. Pathol. 2011, 178, 373–381. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Brassart-Pasco, S.; Brézillon, S.; Brassart, B.; Ramont, L.; Oudart, J.-B.; Monboisse, J.C. Tumor Microenvironment: Extracellular Matrix Alterations Influence Tumor Progression. Front. Oncol. 2020, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, E.C.; Resnick, M.B. Elastin in the tumor microenvironment. In Tumor Microenvironment; Springer: Cham, Switzerland, 2020; pp. 1–16. [Google Scholar]
- Paskeh, M.D.A.; Entezari, M.; Mirzaei, S.; Zabolian, A.; Saleki, H.; Naghdi, M.J.; Sabet, S.; Khoshbakht, M.A.; Hashemi, M.; Hushmandi, K.; et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J. Hematol. Oncol. 2022, 15, 83. [Google Scholar] [CrossRef]
- Kahlert, C.; Kalluri, R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J. Mol. Med. 2013, 91, 431–437. [Google Scholar] [CrossRef]
- Hou, P.P.; Chen, H.Z. Extracellular vesicles in the tumor immune microenvironment. Cancer Lett. 2021, 516, 48–56. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.M.; Cheresh, D.A. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat. Med. 2011, 17, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.M.; Bouchier-Hayes, D.J.; Harmey, J.H. Vascular Endothelial Growth Factor (VEGF) and Its Role in Non-Endothelial Cells: Autocrine Signalling by VEGF. In VEGF and Cancer; Springer: Boston, MA, USA, 2004; pp. 133–144. [Google Scholar] [CrossRef]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef]
- Pufe, T.; Harde, V.; Petersen, W.; Goldring, M.B.; Tillmann, B.; Mentlein, R. Vascular endothelial growth factor (VEGF) induces matrix metalloproteinase expression in immortalized chondrocytes. J. Pathol. 2004, 202, 367–374. [Google Scholar] [CrossRef]
- Gonzalez-Moreno, O.; Lecanda, J.; Green, J.E.; Segura, V.; Catena, R.; Serrano, D.; Calvo, A. VEGF elicits epithelial-mesenchymal transition (EMT) in prostate intraepithelial neoplasia (PIN)-like cells via an autocrine loop. Exp. Cell Res. 2010, 316, 554–567. [Google Scholar] [CrossRef]
- Ciardiello, F.; Bianco, R.; Damiano, V.; Fontanini, G.; Caputo, R.; Pomatico, G.; De Placido, S.; Bianco, A.R.; Mendelsohn, J.; Tortora, G. Antiangiogenic and antitumor activity of anti-epidermal growth factor receptor C225 monoclonal antibody in combination with vascular endothelial growth factor antisense oligonucleotide in human GEO colon cancer cells. Clin. Cancer Res. 2000, 6, 3739–3747. [Google Scholar]
- Bates, D.; Harper, S. Regulation of vascular permeability by vascular endothelial growth factors. Vasc. Pharmacol. 2002, 39, 225–237. [Google Scholar] [CrossRef]
- Infantino, V.; Santarsiero, A.; Convertini, P.; Todisco, S.; Iacobazzi, V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 5703. [Google Scholar] [CrossRef]
- Nardinocchi, L.; Puca, R.; Guidolin, D.; Belloni, A.S.; Bossi, G.; Michiels, C.; Sacchi, A.; Onisto, M.; D’Orazi, G. Transcriptional regulation of hypoxia-inducible factor 1α by HIPK2 suggests a novel mechanism to restrain tumor growth. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2009, 1793, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Crotti, S.; D’aNgelo, E.; Bedin, C.; Fassan, M.; Pucciarelli, S.; Nitti, D.; Bertazzo, A.; Agostini, M. Tryptophan metabolism along the kynurenine and serotonin pathways reveals substantial differences in colon and rectal cancer. Metabolomics 2017, 13, 148. [Google Scholar] [CrossRef]
- Strickaert, A.; Saiselet, M.; Dom, G.; De Deken, X.; Dumont, J.E.; Feron, O.; Sonveaux, P.; Maenhaut, C. Cancer heterogeneity is not compatible with one unique cancer cell metabolic map. Oncogene 2017, 36, 2637–2642. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Multhoff, G. Hypoxia-/HIF-1α-Driven Factors of the Tumor Microenvironment Impeding Antitumor Immune Responses and Promoting Malignant Progression. Adv. Exp. Med. Biol. 2018, 1072, 171–175. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, H.; Xiang, L.; Bullen, J.W.; Zhang, C.; Samanta, D.; Gilkes, D.M.; He, J.; Semenza, G.L. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc. Natl. Acad. Sci. USA 2015, 112, E6215–E6223, Correction in Proc. Natl. Acad. Sci. USA 2024, 121, e2418194121. [Google Scholar] [CrossRef]
- Mole, D.R.; Maxwell, P.H.; Pugh, C.W.; Ratcliffe, P.J. Regulation of HIF by the von Hippel-Lindau Tumour Suppressor: Implications for Cellular Oxygen Sensing. IUBMB Life 2001, 52, 43–47. [Google Scholar] [CrossRef]
- Maxwell, P.H.; Ratcliffe, P.J. Oxygen sensors and angiogenesis. Semin. Cell Dev. Biol. 2002, 13, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Cockman, M.E.; Masson, N.; Mole, D.R.; Jaakkola, P.; Chang, G.-W.; Clifford, S.C.; Maher, E.R.; Pugh, C.W.; Ratcliffe, P.J.; Maxwell, P.H. Hypoxia Inducible Factor-α Binding and Ubiquitylation by the von Hippel-Lindau Tumor Suppressor Protein. J. Biol. Chem. 2000, 275, 25733–25741. [Google Scholar] [CrossRef] [PubMed]
- Ema, M.; Hirota, K.; Mimura, J.; Abe, H.; Yodoi, J.; Sogawa, K.; Poellinger, L.; Fujii-Kuriyama, Y. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: Their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 1999, 18, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wei, J.; Sun, J. Roles of TGF-β signaling pathway in tumor microenvironment and cancer therapy. Int. Immunopharmacol. 2020, 89, 107101. [Google Scholar] [CrossRef]
- Chan, M.K.-K.; Chung, J.Y.-F.; Tang, P.C.-T.; Chan, A.S.-W.; Ho, J.Y.-Y.; Lin, T.P.-T.; Chen, J.; Leung, K.-T.; To, K.-F.; Lan, H.-Y.; et al. TGF-β signaling networks in the tumor microenvironment. Cancer Lett. 2022, 550, 215925. [Google Scholar] [CrossRef]
- Caja, F.; Vannucci, L. TGFβ: A player on multiple fronts in the tumor microenvironment. J. Immunotoxicol. 2014, 12, 300–307. [Google Scholar] [CrossRef]
- Ali, S.; Rehman, M.U.; Yatoo, A.M.; Arafah, A.; Khan, A.; Rashid, S.; Majid, S.; Ali, A.; Ali, N. TGF-β signaling pathway: Therapeutic targeting and potential for anti-cancer immunity. Eur. J. Pharmacol. 2023, 947, 175678. [Google Scholar] [CrossRef]
- Deckers, M.; van Dinther, M.; Buijs, J.; Que, I.; LöwIk, C.; van der Pluijm, G.; Dijke, P.T. The Tumor Suppressor Smad4 Is Required for Transforming Growth Factor β–Induced Epithelial to Mesenchymal Transition and Bone Metastasis of Breast Cancer Cells. Cancer Res. 2006, 66, 2202–2209. [Google Scholar] [CrossRef]
- Park, B.V.; Freeman, Z.T.; Ghasemzadeh, A.; Chattergoon, M.A.; Rutebemberwa, A.; Steigner, J.; Winter, M.E.; Huynh, T.V.; Sebald, S.M.; Lee, S.J.; et al. TGFβ1-Mediated SMAD3 Enhances PD-1 Expression on Antigen-Specific T Cells in Cancer. Cancer Discov. 2016, 6, 1366–1381. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Chen, X.; Li, L.; Li, Y.; Ping, Y.; Huang, L.; Yue, D.; Zhang, Z.; Wang, F.; et al. CD39/CD73 upregulation on myeloid-derived suppressor cells via TGF-β-mTOR-HIF-1 signaling in patients with non-small cell lung cancer. OncoImmunology 2017, 6, e1320011. [Google Scholar] [CrossRef]
- Shi, X.; Yang, J.; Deng, S.; Xu, H.; Wu, D.; Zeng, Q.; Wang, S.; Hu, T.; Wu, F.; Zhou, H. TGF-β signaling in the tumor metabolic microenvironment and targeted therapies. J. Hematol. Oncol. 2022, 15, 135. [Google Scholar] [CrossRef]
- Tie, Y.; Tang, F.; Peng, D.; Zhang, Y.; Shi, H. TGF-beta signal transduction: Biology, function and therapy for diseases. Mol. Biomed. 2022, 3, 45. [Google Scholar] [CrossRef]
- Fanelli, G.; Romano, M.; Nova-Lamperti, E.; Sunderland, M.W.; Nerviani, A.; Scottà, C.; Bombardieri, M.; Quezada, S.A.; Sacks, S.H.; Noelle, R.J.; et al. PD-L1 signaling on human memory CD4+ T cells induces a regulatory phenotype. PLoS Biol. 2021, 19, e3001199. [Google Scholar] [CrossRef]
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer 2018, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Salmaninejad, A.; Valilou, S.F.; Shabgah, A.G.; Aslani, S.; Alimardani, M.; Pasdar, A.; Sahebkar, A. PD-1/PD-L1 pathway: Basic biology and role in cancer immunotherapy. J. Cell. Physiol. 2019, 234, 16824–16837. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and Its Ligands in Tolerance and Immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef]
- Dosset, M.; Vargas, T.R.; Lagrange, A.; Boidot, R.; Végran, F.; Roussey, A.; Chalmin, F.; Dondaine, L.; Paul, C.; Marie-Joseph, E.L.; et al. PD-1/PD-L1 pathway: An adaptive immune resistance mechanism to immunogenic chemotherapy in colorectal cancer. OncoImmunology 2018, 7, e1433981. [Google Scholar] [CrossRef] [PubMed]
- Oyewole-Said, D.; Konduri, V.; Vazquez-Perez, J.; Weldon, S.A.; Levitt, J.M.; Decker, W.K. Beyond T-Cells: Functional Characterization of CTLA-4 Expression in Immune and Non-Immune Cell Types. Front. Immunol. 2020, 11, 608024. [Google Scholar] [CrossRef]
- Valk, E.; Rudd, C.E.; Schneider, H. CTLA-4 trafficking and surface expression. Trends Immunol. 2008, 29, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.; Song, Q.; Sun, X.; Xue, M.; Yang, Z.; Shang, J. Comprehensive analysis of CTLA-4 in the tumor immune microenvironment of 33 cancer types. Int. Immunopharmacol. 2020, 85, 106633. [Google Scholar] [CrossRef]
- Bour-Jordan, H.; Esensten, J.H.; Martinez-Llordella, M.; Penaranda, C.; Stumpf, M.; Bluestone, J.A. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/ B7 family. Immunol. Rev. 2011, 241, 180–205. [Google Scholar] [CrossRef]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef]
- Li, P.; Chen, D.; Cui, Y.; Zhang, W.; Weng, J.; Yu, L.; Chen, L.; Chen, Z.; Su, H.; Yu, S.; et al. Src Plays an Important Role in AGE-Induced Endothelial Cell Proliferation, Migration, and Tubulogenesis. Front. Physiol. 2018, 9, 765. [Google Scholar] [CrossRef] [PubMed]
- Ikema, K.; Matsumoto, K.; Inomata, Y.; Komohara, Y.; Miyajima, S.; Takeya, M.; Tanihara, H. Induction of matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs correlates with outcome of acute experimental pseudomonal keratitis. Exp. Eye Res. 2006, 83, 1396–1404. [Google Scholar] [CrossRef]
- Zhao, X.; Guan, J.-L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 2011, 63, 610–615. [Google Scholar] [CrossRef]
- Westhoff, M.A.; Serrels, B.; Fincham, V.J.; Frame, M.C.; Carragher, N.O. Src-Mediated Phosphorylation of Focal Adhesion Kinase Couples Actin and Adhesion Dynamics to Survival Signaling. Mol. Cell. Biol. 2004, 24, 8113–8133. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-κB signaling in inflammation and cancer. MedComm 2021, 2, 618–653. [Google Scholar] [CrossRef] [PubMed]
- Cornice, J.; Verzella, D.; Arboretto, P.; Vecchiotti, D.; Capece, D.; Zazzeroni, F.; Franzoso, G. NF-κB: Governing Macrophages in Cancer. Genes 2024, 15, 197. [Google Scholar] [CrossRef]
- Bapat, A.S.; O’cOnnor, C.H.; Schwertfeger, K.L. Targeting the NF-κB pathway enhances responsiveness of mammary tumors to JAK inhibitors. Sci. Rep. 2023, 13, 5349, Correction in Sci. Rep. 2023, 13, 16689. [Google Scholar] [CrossRef]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef]
- Efthymiou, G.; Saint, A.; Ruff, M.; Rekad, Z.; Ciais, D.; Van Obberghen-Schilling, E. Shaping Up the Tumor Microenvironment With Cellular Fibronectin. Front. Oncol. 2020, 10, 641. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Li, J.Q.; Zhang, L.L.; Wang, H.; Wang, F.H.; Tao, Y.W.; Wang, Y.Q.; Guo, Q.R.; Li, J.J.; Liu, Y.; et al. The Biological Functions and Clinical Applications of Integrins in Cancers. Front. Pharmacol. 2020, 11, 579068. [Google Scholar] [CrossRef]
- Kaszak, I.; Witkowska-Piłaszewicz, O.; Niewiadomska, Z.; Dworecka-Kaszak, B.; Ngosa Toka, F.; Jurka, P. Role of Cadherins in Cancer—A Review. Int. J. Mol. Sci. 2020, 21, 7624. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.B.; Rah, B.; Bhat, G.R.; Mushtaq, I.; Parveen, S.; Hassan, R.; Zargar, M.H.; Afroze, D. Transforming Growth Factor-Beta (TGF-β) Signaling in Cancer-A Betrayal Within. Front. Pharmacol. 2022, 13, 791272. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69 (Suppl. 3), 4–10. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, Y.; Li, X.; Long, S.; Shi, Y.; Yu, Y.; Wu, W.; Han, L.; Wang, S. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front. Immunol. 2022, 13, 964442. [Google Scholar] [CrossRef]
- Brunet, J.F.; Denizot, F.; Luciani, M.F.; Roux-Dosseto, M.; Suzan, M.; Mattei, M.G.; Golstein, P. A new member of the immunoglobulin superfamily—CTLA-4. Nature 1987, 328, 267–270. [Google Scholar] [CrossRef]
- Tickner, J.A.; Urquhart, A.J.; Stephenson, S.-A.; Richard, D.J.; O’Byrne, K.J. Functions and Therapeutic Roles of Exosomes in Cancer. Front. Oncol. 2014, 4, 127. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xing, C.; Deng, Y.; Ye, C.; Peng, H. HIF-1α signaling: Essential roles in tumorigenesis and implications in targeted therapies. Genes Dis. 2024, 11, 234–251. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, X. Roles of nucleolin. Saudi Med. J. 2016, 37, 1312–1318. [Google Scholar] [CrossRef]
- Tirosh, I.; Izar, B.; Prakadan, S.M.; Wadsworth, M.H.; Treacy, D.; Trombetta, J.J.; Rotem, A.; Rodman, C.; Lian, C.; Murphy, G.; et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science (1979) 2016, 352, 189–196. [Google Scholar] [CrossRef]
- Chung, W.; Eum, H.H.; Lee, H.-O.; Lee, K.-M.; Lee, H.-B.; Kim, K.-T.; Ryu, H.S.; Kim, S.; Lee, J.E.; Park, Y.H.; et al. Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nat. Commun. 2017, 8, 15081. [Google Scholar] [CrossRef]
- Cui, X.; Liu, S.; Song, H.; Xu, J.; Sun, Y. Single-cell and spatial transcriptomic analyses revealing tumor microenvironment remodeling after neoadjuvant chemoimmunotherapy in non-small cell lung cancer. Mol. Cancer 2025, 24, 111. [Google Scholar] [CrossRef]
- Arora, R.; Cao, C.; Kumar, M.; Sinha, S.; Chanda, A.; McNeil, R.; Samuel, D.; Arora, R.K.; Matthews, T.W.; Chandarana, S.; et al. Spatial transcriptomics reveals distinct and conserved tumor core and edge architectures that predict survival and targeted therapy response. Nat. Commun. 2023, 14, 5029. [Google Scholar] [CrossRef] [PubMed]
- Navin, N.; Kendall, J.; Troge, J.; Andrews, P.; Rodgers, L.; McIndoo, J.; Cook, K.; Stepansky, A.; Levy, D.; Esposito, D.; et al. Tumour evolution inferred by single-cell sequencing. Nature 2011, 472, 90–94. [Google Scholar] [CrossRef]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Marjani, S.L.; Hu, Z.; Weissman, S.M.; Pan, X.; Wu, S. Single-Cell Sequencing for Precise Cancer Research: Progress and Prospects. Cancer Res. 2016, 76, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, B.; Zhao, G.; Lee, Y.; Buzdin, A.; Mu, X.; Zhao, J.; Chen, H.; Li, X. Spatial transcriptomics: Technologies, applications and experimental considerations. Genomics 2023, 115, 110671. [Google Scholar] [CrossRef]
- Chen, A.; Liao, S.; Cheng, M.; Ma, K.; Wu, L.; Lai, Y.; Qiu, X.; Yang, J.; Xu, J.; Hao, S.; et al. Spatiotemporal transcriptomic atlas of mouse organogenesis using DNA nanoball-patterned arrays. Cell 2022, 185, 1777–1792.e21. [Google Scholar] [CrossRef] [PubMed]
- Lyubetskaya, A.; Rabe, B.; Fisher, A.; Lewin, A.; Neuhaus, I.; Brett, C.; Brett, T.; Pereira, E.; Golhar, R.; Kebede, S.; et al. Assessment of spatial transcriptomics for oncology discovery. Cell Rep. Methods 2022, 2, 100340. [Google Scholar] [CrossRef]
- Tapia, C.; Savic, S.; Wagner, U.; Schönegg, R.; Novotny, H.; Grilli, B.; Herzog, M.; Barascud, A.D.; Zlobec, I.; Cathomas, G.; et al. HER2gene status in primary breast cancers and matched distant metastases. Breast Cancer Res. 2007, 9, R31. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Mahmood, U. Molecular Imaging. Radiology 2001, 219, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Anagnostou, V.; Lytle, K.; Parpart-Li, S.; Nesselbush, M.; Riley, D.R.; Shukla, M.; Chesnick, B.; Kadan, M.; Papp, E.; et al. Personalized genomic analyses for cancer mutation discovery and interpretation. Sci. Transl. Med. 2015, 7, 283ra53. [Google Scholar] [CrossRef]
- Mardis, E.R. A decade’s perspective on DNA sequencing technology. Nature 2011, 470, 198–203. [Google Scholar] [CrossRef]
- Meldrum, C.; A Doyle, M.; Tothill, R.W. Next-generation sequencing for cancer diagnostics: A practical perspective. Clin. Biochem. Rev. 2011, 32, 177–195. [Google Scholar]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Teng, F.; Yang, Q.; Wang, J.; Sun, B.; Liu, J.; Zhang, J.; Sun, X.; Zhao, H.; et al. Research progress and the prospect of using single-cell sequencing technology to explore the characteristics of the tumor microenvironment. Genes Dis. 2025, 12, 101239. [Google Scholar] [CrossRef]
- Sun, H.J.; Chen, J.; Ni, B.; Yang, X.; Wu, Y.Z. Recent advances and current issues in single-cell sequencing of tumors. Cancer Lett. 2015, 365, 1–10. [Google Scholar] [CrossRef]
- Song, C.; Park, H.-J.; Kim, M.S. Spatial Transcriptomics in Thyroid Cancer: Applications, Limitations, and Future Perspectives. Cells 2025, 14, 936. [Google Scholar] [CrossRef]
- Cilento, M.A.; Sweeney, C.J.; Butler, L.M. Spatial transcriptomics in cancer research and potential clinical impact: A narrative review. J. Cancer Res. Clin. Oncol. 2024, 150, 296. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Kim, J.K.; Svensson, V.; Marioni, J.C.; Teichmann, S.A. The Technology and Biology of Single-Cell RNA Sequencing. Mol. Cell 2015, 58, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Buenrostro, J.D.; Wu, B.; Chang, H.Y.; Greenleaf, W.J. ATAC-seq: A Method for Assaying Chromatin Accessibility Genome-Wide. Curr. Protoc. Mol. Biol. 2015, 109, 21–29. [Google Scholar] [CrossRef]
- Morton, C.; Sarker, D.; Ross, P. Next-generation sequencing and molecular therapy. Clin. Med. 2023, 23, 65–69. [Google Scholar] [CrossRef]
- Creighton, C.J. Clinical proteomics towards multiomics in cancer. Mass Spectrom. Rev. 2022, 43, 1255–1269. [Google Scholar] [CrossRef]
- Gupta, A.; Pathak, A.; Ranjan, R.; Bandyopadhyay, A.; Rathore, A. Next-generation Sequencing: For the Present Generation Oncologist. Indian Cancer Aware. J. 2022, 1, 2–7. [Google Scholar] [CrossRef]
- Schwaederle, M.; Zhao, M.; Lee, J.J.; Lazar, V.; Leyland-Jones, B.; Schilsky, R.L.; Mendelsohn, J.; Kurzrock, R. Association of Biomarker-Based Treatment Strategies with Response Rates and Progression-Free Survival in Refractory Malignant Neoplasms. JAMA Oncol. 2016, 2, 1452–1459. [Google Scholar] [CrossRef]
- Baron, M.; Chursov, A.; Funkhouser, B.; Kaffey, J.; Kumar, S.; Komatsoulis, G.A.; Kuperwaser, F.; Ramchandran, M.; Sherman, J.; Vucic, E. Use of a systems-biology informed machine learning model to predict drug response using clinically available NGS data. J. Clin. Oncol. 2023, 41, 3136. [Google Scholar] [CrossRef]
- Demirel, H.C.; Arici, M.K.; Tuncbag, N. Computational approaches leveraging integrated connections of multi-omic data toward clinical applications. Mol. Omics 2021, 18, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Park, P.; Shin, S.-Y.; Park, S.Y.; Yun, J.; Shin, C.; Jung, J.; Choi, K.S.; Cha, H.S. Next-Generation Sequencing–Based Cancer Panel Data Conversion Using International Standards to Implement a Clinical Next-Generation Sequencing Research System: Single-Institution Study. JMIR Public Health Surveill. 2020, 8, e14710. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Seigneuret, N.; Sanz, F.; Goldman, M. Data Standards are Needed to Move Translational Medicine Forward. Transl. Med. 2013, 3, 1000119. [Google Scholar]
- Samsom, K.G.; Bosch, L.J.W.; Schipper, L.J.; Roepman, P.; de Bruijn, E.; Hoes, L.R.; Riethorst, I.; Schoenmaker, L.; van der Kolk, L.E.; Retèl, V.P.; et al. Study protocol: Whole genome sequencing Implementation in standard Diagnostics for Every cancer patient (WIDE). BMC Med. Genom. 2020, 13, 169. [Google Scholar] [CrossRef]
- Park, W.; Heo, Y.-J.; Han, D.K. New opportunities for nanoparticles in cancer immunotherapy. Biomater. Res. 2018, 22, 24. [Google Scholar] [CrossRef]
- Saeed, R.F.; Awan, U.A.; Saeed, S.; Mumtaz, S.; Akhtar, N.; Aslam, S. Targeted Therapy and Personalized Medicine. In Therapeutic Approaches in Cancer Treatment; Springer: Cham, Switzerland, 2023; pp. 177–205. [Google Scholar]
- Doyle-Lindrud, S. Personalized cancer care. J. Am. Assoc. Nurse Pract. 2022, 34, 1184–1186. [Google Scholar] [CrossRef]
- Li, Y.-R.; Wilson, M.; Yang, L. Target tumor microenvironment by innate T cells. Front. Immunol. 2022, 13, 999549. [Google Scholar] [CrossRef]
- Russo, M.; Nastasi, C. Targeting the Tumor Microenvironment: A Close Up of Tumor-Associated Macrophages and Neutrophils. Front. Oncol. 2022, 12, 871513. [Google Scholar] [CrossRef]
- Tauriello, D.V. Targeting CAFs to Improve Anti-PD-1 Checkpoint Immunotherapy. Cancer Res. 2023, 83, 655–656. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Fan, Y.; Xiong, Y.; Wang, W.; Chen, J.; Xia, Y.; Lei, J.; Gong, L.; Sun, S.; Jiang, T. Delineating the dynamic evolution from preneoplasia to invasive lung adenocarcinoma by integrating single-cell RNA sequencing and spatial transcriptomics. Exp. Mol. Med. 2022, 54, 2060–2076. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, K.; Sun, Z.; Wang, H.; Xie, J.; Zhang, T.; Sang, S.; Islam, T.; Wang, J.-Y.; Chen, C.; et al. Non-invasive tumor microenvironment evaluation and treatment response prediction in gastric cancer using deep learning radiomics. Cell Rep. Med. 2023, 4, 101146. [Google Scholar] [CrossRef] [PubMed]
- Yuki, K.; Cheng, N.; Nakano, M.; Kuo, C.J. Organoid Models of Tumor Immunology. Trends Immunol. 2020, 41, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Peng, L.; Luo, Y.; Zhang, S.; Pu, Y.; Chen, Y.; Guo, W.; Yao, J.; Shao, M.; Fan, W.; et al. Dissecting esophageal squamous-cell carcinoma ecosystem by single-cell transcriptomic analysis. Nat. Commun. 2021, 12, 5291. [Google Scholar] [CrossRef]
- Chiang, E.; Stafford, H.; Buell, J.; Ramesh, U.; Amit, M.; Nagarajan, P.; Migden, M.; Yaniv, D. Review of the Tumor Microenvironment in Basal and Squamous Cell Carcinoma. Cancers 2023, 15, 2453. [Google Scholar] [CrossRef] [PubMed]
- Sethakorn, N.; Heninger, E.; Breneman, M.T.; Recchia, E.; Ding, A.B.; Jarrard, D.F.; Hematti, P.; Beebe, D.J.; Kosoff, D. Integrated analysis of the tumor microenvironment using a reconfigurable microfluidic cell culture platform. FASEB J. 2022, 36, e22540. [Google Scholar] [CrossRef]
- Deng, Z.; Sun, X.; Cao, J.; Xiao, Q. Editorial: Immune modulation in tumor microenvironment: New perspectives for cancer immunotherapy. Front. Cell Dev. Biol. 2023, 10, 1103705. [Google Scholar] [CrossRef]
- Chyuan, I.-T.; Chu, C.-L.; Hsu, P.-N. Targeting the Tumor Microenvironment for Improving Therapeutic Effectiveness in Cancer Immunotherapy: Focusing on Immune Checkpoint Inhibitors and Combination Therapies. Cancers 2021, 13, 1188. [Google Scholar] [CrossRef]
- Bai, R.; Cui, J. Development of Immunotherapy Strategies Targeting Tumor Microenvironment Is Fiercely Ongoing. Front. Immunol. 2022, 13, 890166. [Google Scholar] [CrossRef]
- Hernández-Camarero, P.; López-Ruiz, E.; Marchal, J.A.; Perán, M. Cancer: A mirrored room between tumor bulk and tumor microenvironment. J. Exp. Clin. Cancer Res. 2021, 40, 217. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, X.; Zhang, Y.; Zhu, M.; Wang, F.X.C.; Mu, J.; Li, J.; Yao, H.; Chen, K. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023, 12, 11149–11165. [Google Scholar] [CrossRef] [PubMed]
| S. No | Proteins in TME | Function | Pathways |
|---|---|---|---|
| 01 | Collagen | Influences prognosis, recurrence, and resistance in cancer [80]. | p53 pathway, tumor necrosis factor (TNF) receptor 2/p38 MAPK signaling pathway |
| 02 | Fibronectin | Serves as a scaffold for matrix proteins and binding sites for the TME to function [81]. | α5β1 pathway |
| 03 | Integrins | Mediate interactions between cell–cell and cell–ECM [82]. | RAS/PI3K/AKT and RAF/MEK/ERK/mTOR pathway |
| 04 | Cadherins | Tissue homeostasis and cell–cell adhesions [83]. | MAPK/ERK, MEK/ERK, Ras/MAPK, PI3K/AKT and ERK/MAPK pathway |
| 05 | TGF-β | It plays a role as a tumor suppressor and clears malignant cells by reducing growth and development [84]. | JAK/STAT, PI3K/Akt, NF-κB, pathway |
| 06 | VEGF | It plays a key role in angiogenesis [85]. | PI3-K/Akt, PI3K/mTOR, PI3K pathways |
| 07 | PD-1 and PD-L1 | These frame the mechanism by which tumor cells achieve immune escape [86]. | |
| 08 | CTLA-4 | Inhibitor of T cell functions [87]. | RAS pathway |
| 09 | Exosomes | Potentially affects the TME, remodels the ECM, and promotes vasculogenesis [88]. | RAB, MAPK, P13-K/Akt, RAS, ESCRT-independent and ESCRT-dependent pathways |
| 10 | HIF-1α | Promotes the malignancy of the progression of tumors [89]. | pVHL, FIH-1, Ras/Raf/MEK: Rat sarcoma/rapidly accelerated fibrosarcoma/MAPK/ERK kinase, Mdm2-p53-mediated ubiquitination and proteasomal degradation, Hsp90 pathways |
| 11 | Nucleolin (NCL) | NCL helps in chromatin remodeling, processes pre-RNA, drives rDNA transcription, and assembles ribosomes [90]. |
| Study (Year) | Modality | Genes Involved | Diagnostic Contributions | Therapeutic Contributions |
|---|---|---|---|---|
| Navin et al., 2011 [95] | SCS | ERBB2, MYC, TP53 | Preliminary single-cell DNA sequencing of breast cancer revealed subclonal copy number variation architecture, differentiating between punctuated and clonal evolution models. | Identification of CNV-driven oncogenes facilitates therapeutic targeting options for HER2+ breast cancer. |
| Patel et al., 2014 [96] | SCS (scRNA-seq) | EGFR, PDGFRA, NF1, TP53 | Intratumoral heterogeneity was shown by single-cell transcriptomics, revealing different expression profiles. Distinguished between stem-like and differentiated states. | Resistance mechanism-related heterogeneity guides combination treatments aimed at several pathways. |
| Tirosh et al., 2016 [91] | SCS (scRNA-seq, melanoma) | MITF, AXL, CD8A, PDCD1 | Detected immune evasion and two transcriptional states—MITF-high (proliferative) and AXL-high (invasive). | Revealed the reasoning for adaptive resistance to BRAF/MEK inhibitors; this data is used to guide patient classification and immunotherapy. |
| Zhang et al., 2016 [97] | SCS | BCR-ABL1, JAK2, TET2, DNMT3A | Monitored the clonal progression of leukemia, differentiating between driver and passenger mutations. | Enhanced precision therapeutics in CML/AML by facilitating the prognosis of therapeutic response and recurrence. |
| Chung et al., 2017 [92] | SCS + multi-omics | PIK3CA, ESR1, TP53 | The incorporation of SCS with protein data was used to delineate genotype–phenotype heterogeneity. | The relationship between genotype and functional protein expression for therapeutic guidance. |
| Wang et al., 2023 [98] | ST (review) | Multiple datasets (methodological) | Condensed computational frameworks for the delineation of spatial heterogeneity in diagnostics. | Formulated a therapeutic stratification methodology that incorporates ST. |
| Chen et al., 2022 [99] | ST | SOX2, OLIG2, GFAP | Emphasized the geographic variability of stemness genes and their interactions with the surroundings. | Therapeutic insights into the targeting of stem cell-like populations inside the tumor microenvironment. |
| Lyubetskaya et al., 2022 [100] | ST | PD-L1, CD274, CXCL13, IGHG1 | In situ characterization of B cell microenvironments and immunological checkpoint expression. | Contributed to the advancement of spatially informed immunotherapies and checkpoint inhibitors. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattabiram, S.; Gangadaran, P.; Dhayalan, S.; Chatterjee, G.; Reyaz, D.; Prakash, K.; Arun, R.; Rajendran, R.L.; Ahn, B.-C.; Aruljothi, K.N. Decoding the Tumor Microenvironment: Insights and New Targets from Single-Cell Sequencing and Spatial Transcriptomics. Curr. Issues Mol. Biol. 2025, 47, 730. https://doi.org/10.3390/cimb47090730
Pattabiram S, Gangadaran P, Dhayalan S, Chatterjee G, Reyaz D, Prakash K, Arun R, Rajendran RL, Ahn B-C, Aruljothi KN. Decoding the Tumor Microenvironment: Insights and New Targets from Single-Cell Sequencing and Spatial Transcriptomics. Current Issues in Molecular Biology. 2025; 47(9):730. https://doi.org/10.3390/cimb47090730
Chicago/Turabian StylePattabiram, Shriya, Prakash Gangadaran, Sanjana Dhayalan, Gargii Chatterjee, Danyal Reyaz, Kruthika Prakash, Raksa Arun, Ramya Lakshmi Rajendran, Byeong-Cheol Ahn, and Kandasamy Nagarajan Aruljothi. 2025. "Decoding the Tumor Microenvironment: Insights and New Targets from Single-Cell Sequencing and Spatial Transcriptomics" Current Issues in Molecular Biology 47, no. 9: 730. https://doi.org/10.3390/cimb47090730
APA StylePattabiram, S., Gangadaran, P., Dhayalan, S., Chatterjee, G., Reyaz, D., Prakash, K., Arun, R., Rajendran, R. L., Ahn, B.-C., & Aruljothi, K. N. (2025). Decoding the Tumor Microenvironment: Insights and New Targets from Single-Cell Sequencing and Spatial Transcriptomics. Current Issues in Molecular Biology, 47(9), 730. https://doi.org/10.3390/cimb47090730










