Photoinduced Inhibition of Neutrophil Extracellular Traps Formation by Dichromatic Light Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Isolation of Primary Human Neutrophils
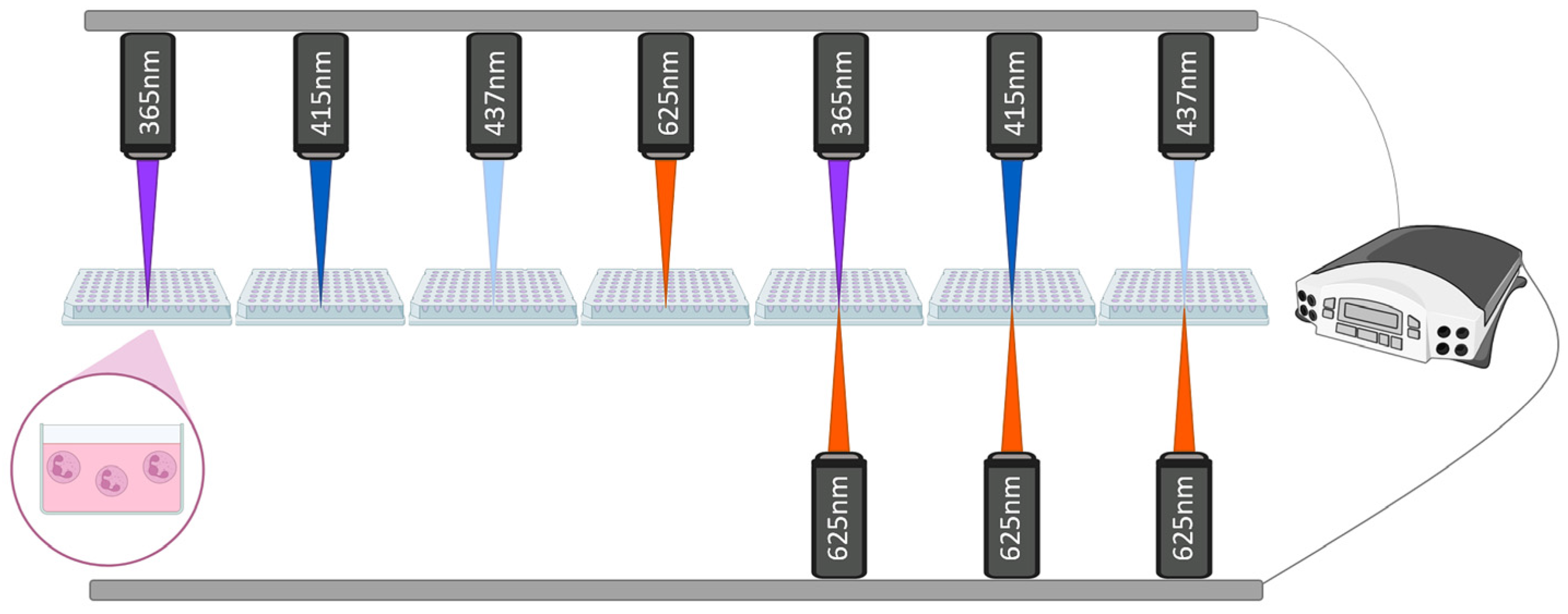
2.3. Experimental Design and Light Irradiation Protocol
2.4. Assessment of NETosis with Fluorescence Microscopy
2.5. Luminol-Enhanced Chemiluminescence Assay
2.6. Statistical Analysis
3. Results
3.1. Effects of Monochromatic and Dichromatic Irradiation on NETs Formation
3.2. Differential Modulation of NETosis Induced by Monochromatic Light with ROS-Targeting Inhibitors
4. Discussion
Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Takei, H.; Araki, A.; Watanabe, H.; Ichinose, A.; Sendo, F. Rapid Killing of Human Neutrophils by the Potent Activator Phorbol 12-Myristate 13-Acetate (PMA) Accompanied by Changes Different from Typical Apoptosis or Necrosis. J. Leukoc. Biol. 1996, 59, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, D.; Khan, M.A.; Palaniyar, N. ROS Induces NETosis by Oxidizing DNA and Initiating DNA Repair. Cell Death Discov. 2021, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef]
- Klein, R.M. Effects of green light on biological systems. Biol. Rev. 1992, 67, 199–284. [Google Scholar] [CrossRef]
- Hillenkamp, F. Interaction between Laser Radiation and Biological Systems. In Lasers in Biology and Medicine; Springer: New York, NY, USA, 1980. [Google Scholar]
- Johnson, C.B. Blue Light Effects in Biological Systems. Phytochemistry 1986, 25, 1785. [Google Scholar] [CrossRef]
- Sundström, V. Light in Elementary Biological Reactions. Prog. Quantum Electron. 2000, 24, 187–238. [Google Scholar] [CrossRef]
- Prindeze, N.J.; Moffatt, L.T.; Shupp, J.W. Mechanisms of Action for Light Therapy: A Review of Molecular Interactions. Exp. Biol. Med. 2012, 237, 1241–1248. [Google Scholar] [CrossRef]
- Golovynska, I.; Golovynskyi, S.; Qu, J. Comparing the Impact of NIR, Visible and UV Light on ROS Upregulation via Photoacceptors of Mitochondrial Complexes in Normal, Immune and Cancer Cells. Photochem. Photobiol. 2023, 99, 106–119. [Google Scholar] [CrossRef]
- Gao, X.; Xing, D. Molecular Mechanisms of Cell Proliferation Induced by Low Power Laser Irradiation. J. Biomed. Sci. 2009, 16, 4. [Google Scholar] [CrossRef]
- Gonzalez-Lima, F.; Rojas, J.C. Low-Level Light Therapy of the Eye and Brain. Eye Brain 2011, 2011, 49–67. [Google Scholar] [CrossRef]
- Karu, T.I. Mitochondrial Signaling in Mammalian Cells Activated by Red and Near-IR Radiation. Photochem. Photobiol. 2008, 84, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Streeter, J.; De Taboada, L.; Oron, U. Mechanisms of Action of Light Therapy for Stroke and Acute Myocardial Infarction. Mitochondrion 2004, 4, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Hollis, V.S.; Palacios-Callender, M.; Springett, R.J.; Delpy, D.T.; Moncada, S. Monitoring Cytochrome Redox Changes in the Mitochondria of Intact Cells Using Multi-Wavelength Visible Light Spectroscopy. Biochim. Biophys. Acta Bioenerg. 2003, 1607, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I.; Pyatibrat, L.V.; Kolyakov, S.F.; Afanasyeva, N.I. Absorption Measurements of a Cell Monolayer Relevant to Phototherapy: Reduction of Cytochrome c Oxidase under near IR Radiation. J. Photochem. Photobiol. B 2005, 81, 98–106. [Google Scholar] [CrossRef]
- Chen, A.C.-H.; Huang, Y.-Y.; Arany, P.R.; Hamblin, M.R. Role of Reactive Oxygen Species in Low Level Light Therapy. In Proceedings of the Mechanisms for Low-Light Therapy IV; SPIE: Bellingham, WA, USA, 2009; Volume 7165. [Google Scholar]
- Passarella, S.; Karu, T. Absorption of Monochromatic and Narrow Band Radiation in the Visible and near IR by Both Mitochondrial and Non-Mitochondrial Photoacceptors Results in Photobiomodulation This Paper Is Devoted to the Memory of Prof. Lorenzo Bolognani Who Was One of the Pioneers in the Field of Photobiomodulation. J. Photochem. Photobiol. B 2014, 140, 344–358. [Google Scholar]
- Rajendran, N.K.; George, B.P.; Chandran, R.; Tynga, I.M.; Houreld, N.; Abrahamse, H. The Influence of Light on Reactive Oxygen Species and NF-KB in Disease Progression. Antioxidants 2019, 8, 640. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef]
- George, S.; Hamblin, M.R.; Abrahamse, H. Effect of Red Light and near Infrared Laser on the Generation of Reactive Oxygen Species in Primary Dermal Fibroblasts. J. Photochem. Photobiol. B 2018, 188, 60–68. [Google Scholar] [CrossRef]
- Arzumanyan, G.; Mamatkulov, K.; Arynbek, Y.; Zakrytnaya, D.; Jevremović, A.; Vorobjeva, N. Radiation from UV-A to Red Light Induces ROS-Dependent Release of Neutrophil Extracellular Traps. Int. J. Mol. Sci. 2023, 24, 5770. [Google Scholar] [CrossRef]
- Mailloux, R.J. Teaching the Fundamentals of Electron Transfer Reactions in Mitochondria and the Production and Detection of Reactive Oxygen Species. Redox Biol. 2015, 4, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P. Disruption of Mitochondrial Redox Circuitry in Oxidative Stress. Chem. Biol. Interact. 2006, 163, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, P.; Singh, B.K. Mitochondria: A Hub of Redox Activities and Cellular Distress Control. Mol. Cell. Biochem. 2007, 305, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Brillo, V.; Chieregato, L.; Leanza, L.; Muccioli, S.; Costa, R. Mitochondrial Dynamics, Ros, and Cell Signaling: A Blended Overview. Life 2021, 11, 332. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive Oxygen Species Generating Systems Meeting Challenges of Photodynamic Cancer Therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef]
- Borbély, P.; Gasperl, A.; Pálmai, T.; Ahres, M.; Asghar, M.A.; Galiba, G.; Müller, M.; Kocsy, G. Light Intensity-and Spectrum-Dependent Redox Regulation of Plant Metabolism. Antioxidants 2022, 11, 1311. [Google Scholar] [CrossRef]
- Sommer, A.P. Mitochondrial Cytochrome c Oxidase Is Not the Primary Acceptor for near Infrared Light—It Is Mitochondrial Bound Water: The Principles of Low-Level Light Therapy. Ann. Transl. Med. 2019, 7, S13. [Google Scholar] [CrossRef]
- Salet, C.; Moreno, G. New Trends in Photobiology Photosensitization of Mitochondria. Molecular and Cellular Aspects. J. Photochem. Photobiol. B 1990, 5, 133–150. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Liu, S.; Huang, B.; Cao, J.; Wang, Y.; Xiao, H.; Zhu, Y.; Zhang, H. ROS Fine-Tunes the Function and Fate of Immune Cells. Int. Immunopharmacol. 2023, 119, 110069. [Google Scholar] [CrossRef]
- Manoharan, R.R.; Prasad, A.; Pospíšil, P.; Kzhyshkowska, J. ROS Signaling in Innate Immunity via Oxidative Protein Modifications. Front. Immunol. 2024, 15, 1359600. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, P.; Guo, S.; Schrodi, S.J.; He, D. Apoptosis, Autophagy, NETosis, Necroptosis, and Pyroptosis Mediated Programmed Cell Death as Targets for Innovative Therapy in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 809806. [Google Scholar] [CrossRef]
- Juha, M.; Molnár, A.; Jakus, Z.; Ledó, N. NETosis: An Emerging Therapeutic Target in Renal Diseases. Front. Immunol. 2023, 14, 1253667. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Chelombitko, M.A.; Sud’ina, G.F.; Zinovkin, R.A.; Chernyak, B.V. Role of Mitochondria in the Regulation of Effector Functions of Granulocytes. Cells 2023, 12, 2210. [Google Scholar] [CrossRef] [PubMed]
- Neubert, E.; Bach, K.M.; Busse, J.; Bogeski, I.; Schön, M.P.; Kruss, S.; Erpenbeck, L. Blue and Long-Wave Ultraviolet Light Induce in Vitro Neutrophil Extracellular Trap (NET) Formation. Front. Immunol. 2019, 10, 2428. [Google Scholar] [CrossRef] [PubMed]
- Zawrotniak, M.; Bartnicka, D.; Rapala-Kozik, M. UVA and UVB Radiation Induce the Formation of Neutrophil Extracellular Traps by Human Polymorphonuclear Cells. J. Photochem. Photobiol. B 2019, 196, 111511. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, D.; Palaniyar, N. ApoNETosis: Discovery of a Novel Form of Neutrophil Death with Concomitant Apoptosis and NETosis. Cell Death Dis. 2018, 9, 839. [Google Scholar] [CrossRef]
- Azzouz, D.; Khan, M.A.; Sweezey, N.; Palaniyar, N. Two-in-One: UV Radiation Simultaneously Induces Apoptosis and NETosis. Cell Death Discov. 2018, 4, 51. [Google Scholar] [CrossRef]
- Migliario, M.; Tonello, S.; Rocchetti, V.; Rizzi, M.; Renò, F. Near Infrared Laser Irradiation Induces NETosis via Oxidative Stress and Autophagy. Lasers Med. Sci. 2018, 33, 1919–1924. [Google Scholar] [CrossRef]
- Azzouz, D.; Palaniyar, N. How Do ROS Induce NETosis? Oxidative DNA Damage, DNA Repair, and Chromatin Decondensation. Biomolecules 2024, 14, 1307. [Google Scholar] [CrossRef]
- Karu, T. Primary and Secondary Mechanisms of Action of Visible to Near-IR Radiation on Cells. J. Photochem. Photobiol. B 1999, 49, 1–17. [Google Scholar] [CrossRef]
- Masuda, H.; Kimura, M.; Nishioka, A.; Kato, H.; Morita, A. Dual Wavelength 5-Aminolevulinic Acid Photodynamic Therapy Using a Novel Flexible Light-Emitting Diode Unit. J. Dermatol. Sci. 2019, 93, 109–115. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Benedicenti, S.; Signore, A.; Arshad, M.; Chiniforush, N. Simultaneous Dual-Wavelength Laser Irradiation against Implant-Adherent Biofilms of Staphylococcus Aureus, Escherichia Coli, and Candida Albicans for Improved Antimicrobial Photodynamic Therapy. Bioengineering 2024, 11, 48. [Google Scholar] [CrossRef]
- Brinkmann, V.; Laube, B.; Abed, U.A.; Goosmann, C.; Zychlinsky, A. Neutrophil Extracellular Traps: How to Generate and Visualize Them. J. Vis. Exp. 2010, 36, e1724. [Google Scholar] [CrossRef]
- Zang, C.; Stevens, J.A.; Link, J.J.; Guo, L.; Wang, L.; Zhong, D. Ultrafast Proteinquake Dynamics in Cytochrome c. J. Am. Chem. Soc. 2009, 131, 2846–2852. [Google Scholar] [CrossRef]
- Shiro, Y.; Isogai, Y.; Nakamura, H.; Iizuka, T. Physiological Functions and Molecular Structures of New Types of Hemoproteins. In Progress in Biotechnology; Elsevier: Amsterdam, The Netherlands, 2002; Volume 22. [Google Scholar]
- Berg, J.M.; Tymoczko, J.L.; Gatto, G.J.; Stryer, L. Biochemistry, 8th ed.; W.H. Freeman & Co. Ltd.: New York, NY, USA, 2015. [Google Scholar]
- Mehaney, D.A.; Darwish, H.A.; Hegazy, R.A.; Nooh, M.M.; Tawdy, A.M.; Gawdat, H.I.; El-Sawalhi, M.M. Analysis of Oxidative Stress Status, Catalase and Catechol-O- Methyltransferase Polymorphisms in Egyptian Vitiligo Patients. PLoS ONE 2014, 9, e99286. [Google Scholar] [CrossRef][Green Version]
- Park, S.H.; Baek, K.H.; Shin, I.; Shin, I. Subcellular Hsp70 Inhibitors Promote Cancer Cell Death via Different Mechanisms. Cell Chem. Biol. 2018, 25, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, H.; Schwappacher, R.; Joshua, J.; Zhuang, S.; Scott, B.T.; Klos, M.; Casteel, D.E.; Frangos, J.A.; Dillmann, W.; Boss, G.R.; et al. Nongenomic Thyroid Hormone Signaling Occurs through a Plasma Membrane -Localized Receptor. Sci. Signal. 2014, 7, ra48. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic Accumulation of Succinate Controls Reperfusion Injury through Mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, M.A.; Jones, R.D. Photoinhibition of Marine Nitrifying Bacteria. II. Dark Recovery after Monochromatic or Polychromatic Irradiation. Mar. Ecol. Prog. Ser. 1996, 141, 193–198. [Google Scholar] [CrossRef]
- Bode, K.; Hauri-Hohl, M.; Jaquet, V.; Weyd, H. Unlocking the Power of NOX2: A Comprehensive Review on Its Role in Immune Regulation. Redox Biol. 2023, 64, 102795. [Google Scholar] [CrossRef]
 ) indicates the mean value from each of the five experiments. The colors are conventionally characterized by their wavelengths, and the slanted pattern refers to PMA. ns., not significant; ***, p < 0.001; ****, p < 0.0001.
) indicates the mean value from each of the five experiments. The colors are conventionally characterized by their wavelengths, and the slanted pattern refers to PMA. ns., not significant; ***, p < 0.001; ****, p < 0.0001.
 ) indicates the mean value from each of the five experiments. The colors are conventionally characterized by their wavelengths, and the slanted pattern refers to PMA. ns., not significant; ***, p < 0.001; ****, p < 0.0001.
) indicates the mean value from each of the five experiments. The colors are conventionally characterized by their wavelengths, and the slanted pattern refers to PMA. ns., not significant; ***, p < 0.001; ****, p < 0.0001.
 ) indicates the mean value from each of the five experiments. The colors are conventionally characterized by their wavelengths. Abbreviations: CL, chemiluminescence; a.u., arbitrary units; *, p < 0.05; ****, p < 0.0001.
) indicates the mean value from each of the five experiments. The colors are conventionally characterized by their wavelengths. Abbreviations: CL, chemiluminescence; a.u., arbitrary units; *, p < 0.05; ****, p < 0.0001.
 ) indicates the mean value from each of the five experiments. The colors are conventionally characterized by their wavelengths. Abbreviations: CL, chemiluminescence; a.u., arbitrary units; *, p < 0.05; ****, p < 0.0001.
) indicates the mean value from each of the five experiments. The colors are conventionally characterized by their wavelengths. Abbreviations: CL, chemiluminescence; a.u., arbitrary units; *, p < 0.05; ****, p < 0.0001.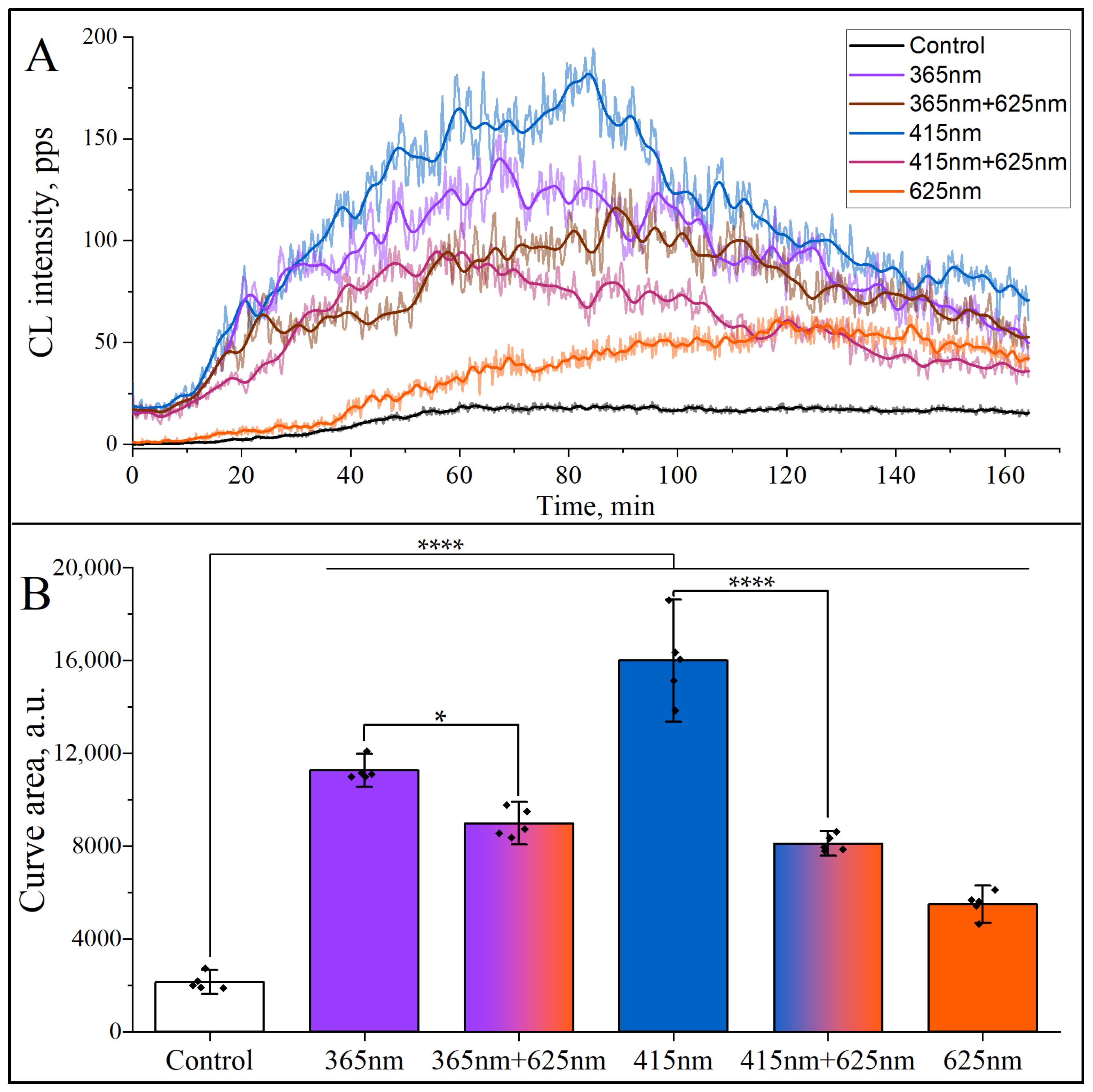
 ) indicates the mean value from each of the five experiments. Colors are conventionally characterized by their wavelengths. The checkered and dotted patterns correspond to Apocynin and MitoTEMPO, respectively. ns, not significant; *, p < 0.05; ***, p < 0.001; ****, p < 0.0001.
) indicates the mean value from each of the five experiments. Colors are conventionally characterized by their wavelengths. The checkered and dotted patterns correspond to Apocynin and MitoTEMPO, respectively. ns, not significant; *, p < 0.05; ***, p < 0.001; ****, p < 0.0001.
 ) indicates the mean value from each of the five experiments. Colors are conventionally characterized by their wavelengths. The checkered and dotted patterns correspond to Apocynin and MitoTEMPO, respectively. ns, not significant; *, p < 0.05; ***, p < 0.001; ****, p < 0.0001.
) indicates the mean value from each of the five experiments. Colors are conventionally characterized by their wavelengths. The checkered and dotted patterns correspond to Apocynin and MitoTEMPO, respectively. ns, not significant; *, p < 0.05; ***, p < 0.001; ****, p < 0.0001.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamatkulov, K.; Arynbek, Y.; Le, H.D.; Vorobjeva, N.; Arzumanyan, G. Photoinduced Inhibition of Neutrophil Extracellular Traps Formation by Dichromatic Light Irradiation. Curr. Issues Mol. Biol. 2025, 47, 729. https://doi.org/10.3390/cimb47090729
Mamatkulov K, Arynbek Y, Le HD, Vorobjeva N, Arzumanyan G. Photoinduced Inhibition of Neutrophil Extracellular Traps Formation by Dichromatic Light Irradiation. Current Issues in Molecular Biology. 2025; 47(9):729. https://doi.org/10.3390/cimb47090729
Chicago/Turabian StyleMamatkulov, Kahramon, Yersultan Arynbek, Huy Duc Le, Nina Vorobjeva, and Grigory Arzumanyan. 2025. "Photoinduced Inhibition of Neutrophil Extracellular Traps Formation by Dichromatic Light Irradiation" Current Issues in Molecular Biology 47, no. 9: 729. https://doi.org/10.3390/cimb47090729
APA StyleMamatkulov, K., Arynbek, Y., Le, H. D., Vorobjeva, N., & Arzumanyan, G. (2025). Photoinduced Inhibition of Neutrophil Extracellular Traps Formation by Dichromatic Light Irradiation. Current Issues in Molecular Biology, 47(9), 729. https://doi.org/10.3390/cimb47090729







