Macrophage Inhibitory Factor in Myocardial Oxidative Stress and Inflammation During Thioacetamide-Induced Liver Fibrosis: Modulation by Betaine
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Preparation of the Myocardial Samples and Biochemical Analysis
2.4. Determination of Oxidative/Nitosative and Antioxidative Parameters in Myocardial Tissue
2.5. Determination of Proinflammatory Cytokines (IL-6 and TNF) and Profibrogenic Mediators (TGF-b1 and PDGF-BB) in Myocardial Tissue
2.6. Preparation of Myocardial Tissue for Pathohistological Analysis
2.7. Statistical Analysis
3. Results
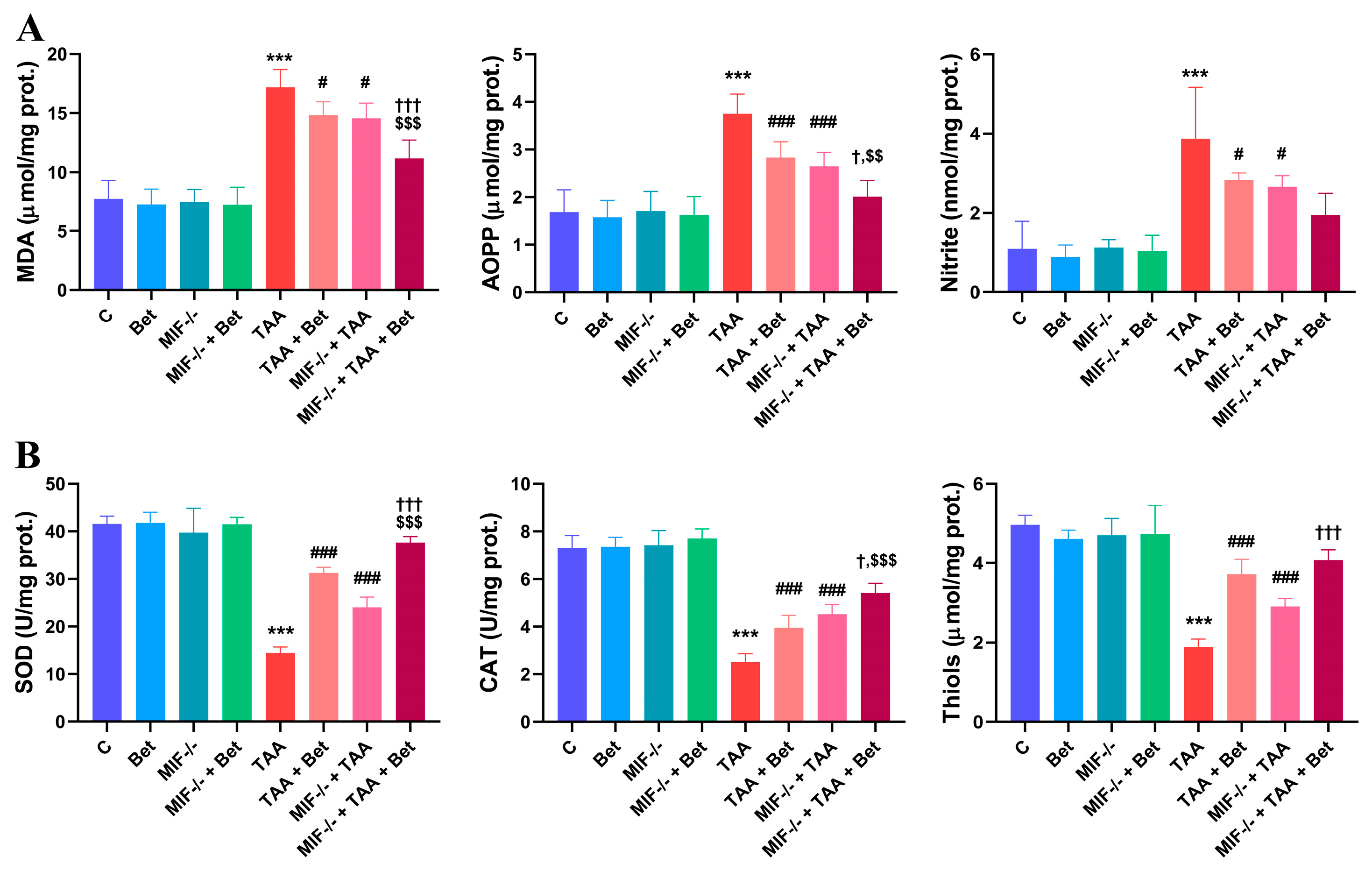
3.1. Oxidative/Nitrosative and Antixidative Parameters in Myocardial Tissue
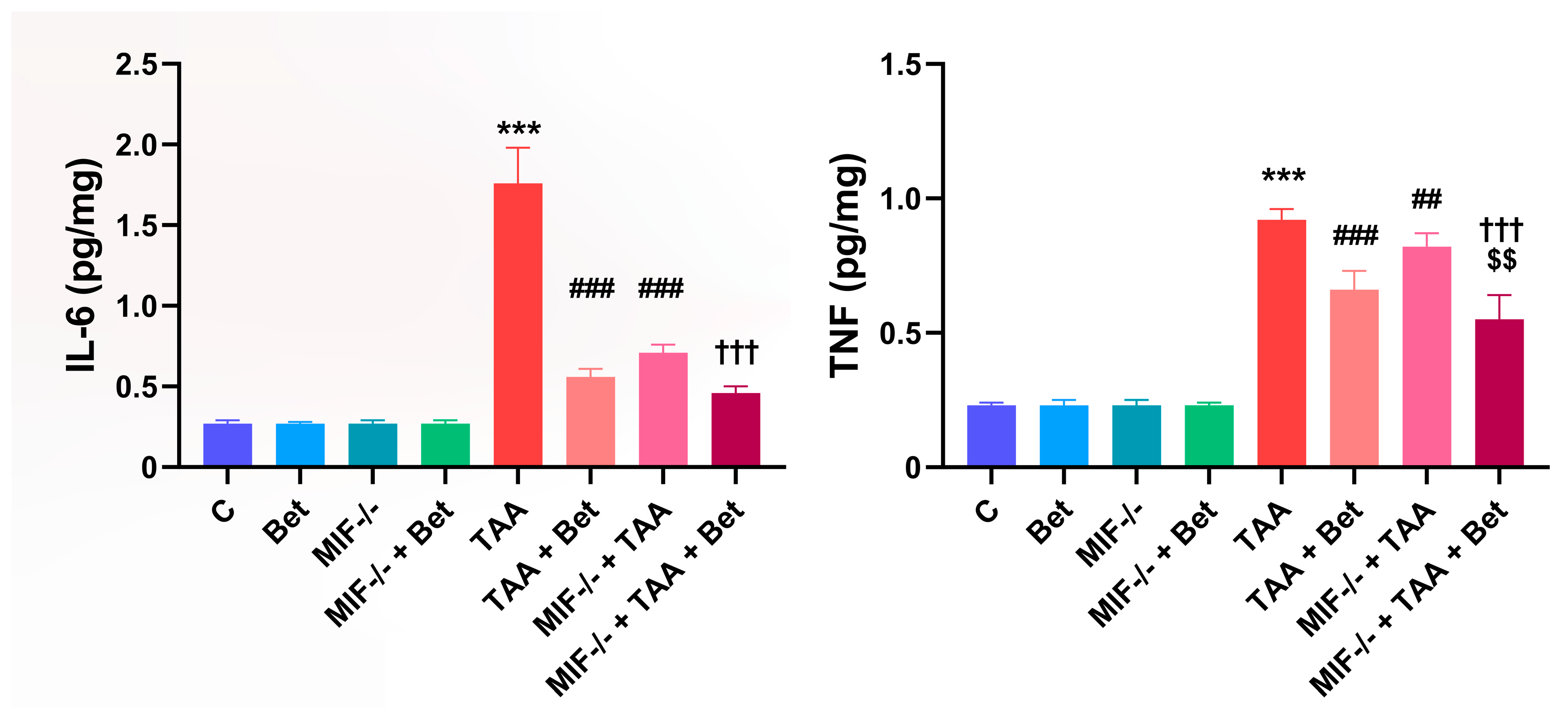
3.2. Determination of Proinflammatory Cytokines (IL-6 and TNF) in Myocardial Tissue
3.3. Profibrogenic Mediators (TGF-β1 and PDGF-BB) in Myocardial Tissue
3.4. Pathohistological Analysis of the Myocardial Tissue
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MIF | Macrophage Inhibitory Factor |
| TAA | Thioacetamide |
| Bet | Betaine |
| IL-6 | Interleukin-6 |
| TNF | Tumor Necrosis Factor |
| TGF-β1 | Transforming Growth Factor-beta1 |
| PDGF-BB | Platelet Derived Growth Factor-BB |
| MDA | Malondialdehyde |
| AOPP | Advanced Oxidation Protein Product |
| SOD | Superoxide Dismutase |
| CLD | Chronic Liver Disease |
| HCC | Hepatocellular carcinoma |
| ECM | Extracellular Matrix |
| EDTA | Ethylenediaminetetraacetic acid |
| DTNB | 2,2-dithiobis nitrobenzoic acid |
| PBS | Phosphate-buffered saline |
| NO2− | Nitrate |
| CAT | Catalase |
| GSH | Reduced Glutathione |
| H&E | Hematoxylin and Eosin |
| MT | Masson-Trichrome |
| ARE | Antioxidant response element |
| DAMPs | Damage-associated molecular patterns |
| PAMPs | Pathogen-associated molecular patterns |
References
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of Liver Diseases in the World. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Gulati, K.; Reshi, M.R.; Rai, N.; Ray, A. Hepatotoxicity: Its Mechanisms, Experimental Evaluation and Protective Strategies. Am. J. Pharmacol. 2018, 1, 1004. [Google Scholar]
- Parolaa, M.; Pinzani, M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Aspects Med. 2019, 65, 37–55. [Google Scholar] [CrossRef]
- Dhar, D.; Baglieri, J.; Kisseleva, T.; Brenner, D.A. Mechanisms of liver fibrosis and its role in liver cancer. Exp. Biol. Med. 2020, 245, 96–108. [Google Scholar] [CrossRef]
- Liu, Y.; Meyer, C.; Xu, C.; Weng, H.; Hellerbrand, C.; ten Dijke, P.; Dooley, S. Animal models of chronic liver diseases. Am. J. Physiol. Gastrointest Liver Physiol. 2013, 304, G449–G468. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.; Hamesch, K.; Lunova, M.; Kim, Y.; Weiskirchen, R.; Strnad, P.; Friedman, S. Standard Operating Procedures in Experimental Liver Research: Thioacetamide model in mice and rats. Lab. Anim. 2015, 49, 21–29. [Google Scholar] [CrossRef] [PubMed]
- International Union of Pure and Applied Chemistry. Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013; The Royal Society of Chemistry: London, UK, 2014; p. 856. [Google Scholar]
- Schyman, P.; Printz, R.L.; Estes, S.K.; Boyd, K.L.; Shiota, M.; Wallqvist, A. Identification of the toxicity pathways associated with thioacetamide-induced injuries in rat liver and kidney. Front. Pharmacol. 2018, 9, 1272. [Google Scholar] [CrossRef] [PubMed]
- Vukićević, D.; Rovčanin, B.; Gopčević, K.; Stanković, S.; Vučević, D.; Jorgačević, B.; Mladenović, D.; Vesković, M.; Samardžić, J.; Ješić, R.; et al. The Role of MIF in Hepatic Function, Oxidative Stress, and Inflammation in Thioacetamide-induced Liver Injury in Mice: Protective Effects of Betaine. Curr. Med. Chem. 2021, 28, 3249–3268. [Google Scholar] [CrossRef] [PubMed]
- Bashandy, S.A.E.; Ebaid, H.; Moussa, S.A.A.; Alhazza, I.M.; Hassan, I.; Alaamer, A.; Tamimi, J.A. Potential effects of the combination of nicotinamide, vitamin B2 and vitamin C on oxidative-mediated hepatotoxicity induced by thioacetamide. Lipids Health Dis. 2018, 17, 29. [Google Scholar] [CrossRef]
- Hajovsky, H.; Hu, G.; Koen, Y.; Sarma, D.; Cui, W.; Moore, D.S.; Staudinger, J.L.; Hanzlik, R.P. Metabolism and toxicity of thioacetamide and thioacetamide S-oxide in rat hepatocytes. Chem. Res. Toxicol. 2012, 25, 1955–1963. [Google Scholar] [CrossRef]
- Jorgačević, B.; Stanković, S.; Filipović, J.; Samardžić, J.; Vučević, D.; Radosavljević, T. Betaine Modulating MIF-Mediated Oxidative Stress, Inflammation and Fibrogenesis in Thioacetamide-Induced Nephrotoxicity. Curr. Med. Chem. 2022, 129, 5254–5267. [Google Scholar] [CrossRef] [PubMed]
- Türkmen, N.B.; Yüce, H.; Taşlidere, A.; Şahin, Y.; Çiftçi, O. The Ameliorate Effects of Nerolidol on Thioasteamide-Induced Oxidative Damage in Heart and Kidney Tissue. Turk. J. Pharm. Sci. 2022, 19, 1–8. [Google Scholar] [CrossRef]
- Nascimento, M.; Piran, R.; Da Costa, R.M.; Giordani, M.A.; Carneiro, F.S.; Aguiar, D.H.; Dias, M.C.; Sugizaki, M.M.; Luvizotto, R.A.; Nascimento, A.F.; et al. Hepatic injury induced by thioacetamide causes aortic endothelial dysfunction by a cyclooxygenase-dependent mechanism. Life Sci. 2018, 212, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Naraki, K.; Roohbakhsh, A.; Hayes, A.G.; Karimi, G. The Protective Effects of Rutin on the Liver, Kidneys, and Heart by Counteracting Organ Toxicity Caused by Synthetic and Natural Compounds. Food. Sci. Nutr. 2022, 11, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Liu, H.; Nam, S.W.; Kunos, G.; Lee, S.S. Mechanisms of TNFalpha-induced cardiac dysfunction in cholestatic bile duct-ligated mice: Interaction between TNFa and endocannabinoids. J. Hepatol. 2010, 53, 298–306. [Google Scholar] [CrossRef]
- Amirtharaj, G.J.; Natarajan, S.K.; Pulimood, A.; Balasubramanian, K.A.; Venkatraman, A.; Ramachandran, A. Role of Oxygen Free Radicals, Nitric Oxide and Mitochondria in Mediating Cardiac Alterations During Liver Cirrhosis Induced by Thioacetamide. Cardiovasc. Toxicol. 2017, 17, 175–184. [Google Scholar] [CrossRef]
- Al-Hamoudi, W.K. Cardiovascular changes in cirrhosis: Pathogenesis and clinical implications. Saudi J. Gastroenterol. 2010, 16, 145–153. [Google Scholar] [CrossRef]
- Fattouh, A.M.; El-Shabrawi, M.H.; Mahmoud, E.H.; Ahmed, W.O. Evaluation of cardiac functions of cirrhotic children using serum brain natriuretic peptide and tissue Doppler imaging. Ann. Pediatr. Cardiol. 2016, 9, 22–28. [Google Scholar] [CrossRef]
- Ostovaneh, M.R.; Ambale-Venkatesh, B.; Fuji, T.; Bakhshi, H.; Shah, R.; Murthy, V.L.; Tracy, R.P.; Guallar, E.; Wu, C.O.; Bluemke, D.A.; et al. Association of Liver Fibrosis with Cardiovascular Diseases in the General Population. Cir. Cardiovasc. Imaging 2018, 11, e007241. [Google Scholar] [CrossRef]
- Roger, T.; David, J.; Glauser, M.P.; Calandra, T. MIF Regulates Innate Immune Responses through Modulation of Toll-like Receptor 4. Nature 2001, 414, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Tanuwidjaya, E.; Schittenhelm, R.B.; Faridi, P. Soluble HLA Peptidome: A New Resource for Cancer Biomarkers. Drug News Perspect. 2010, 23, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Marin, V.; Odena, G.; Poulsen, K.; Tiribelli, C.; Bellentani, S.; Barchetti, A.; Bru, P.S.; Rosso, N.; Bataller, R.; Laura, E.; et al. Role of MIF in Hepatic Inflammatory Diseases and Fibrosis. In MIF Family Cytokines in Innate Immunity and Homeostasis; Bucala, R., Bernhagen, J., Eds.; Progress in Inflammation Research; Springer International: Cham, Switzerland, 2017; pp. 109–134. [Google Scholar]
- Heinrichs, D.; Knauel, M.; Offermanns, C.; Berres, M.L.; Nellen, A.; Leng, L.; Schmitz, P.; Bucala, R.; Trautwein, C.; Weber, C.; et al. Macrophage migration inhibitory factor (MIF) exerts antifibrotic effects in experimental liver fibrosis. Proc. Natl. Acad. Sci. USA 2011, 108, 17444–17449. [Google Scholar] [CrossRef] [PubMed]
- Marin, V.; Poulsen, K.; Odena, G.; McMullen, M.R.; Altamirano, J.; Sancho-Bru, P.; Tiribelli, C.; Caballeria, J.; Rosso, N.; Bataller, R.; et al. Hepatocyte-Derived Macrophage Migration Inhibitory Factor Mediates Alcohol-Induced Liver Injury in Mice and Patients. J. Hepatol. 2017, 67, 1018–1025. [Google Scholar] [CrossRef]
- Calandra, T.; Roger, T. Macrophage migration inhibitory factor: A regulator of innate immunity. Nat. Rev. Immunol. 2003, 3, 791–800. [Google Scholar] [CrossRef]
- Park, M.C.; Kwon, O.C.; Lee, S.W.; Song, J.J.; Park, Y.B. MiR-451 suppresses inflammatory responses in ankylosing spondylitis by targeting macrophage migration inhibitory factor. Clin. Exp. Rheumatol. 2020, 38, 275–281. [Google Scholar] [CrossRef]
- Mizue, Y.; Ghani, S.; Leng, L.; McDonald, C.; Kong, P.; Baugh, J.; Lane, S.J.; Craft, J.; Nishihira, J.; Donnelly, S.C.; et al. Role for macrophage migration inhibitory factor in asthma. Natl. Acad. Sci. 2005, 102, 14410–14415. [Google Scholar] [CrossRef]
- Cavalli, E.; Ciurleo, R.; Petralia, M.C.; Fagone, P.; Bella, R.; Mangano, K.; Nicoletti, F.; Bramanti, P.; Basile, M.S. Emerging Role of the Macrophage Migration Inhibitory Factor Family of Cytokines in Neuroblastoma. Pathogenic Effectors and Novel Therapeutic Targets? Molecules 2020, 25, 1194. [Google Scholar] [CrossRef]
- Bruchfeld, A.; Carrero, J.J.; Qureshi, A.R.; Lindholm, B.; Barany, P.; Heimburger, O.; Hu, M.; Lin, X.; Stenvinkel, P.; Miller, E.J. Macrophage migration inhibitory factor: Critical role in obesity, insulin resistance and associated comorbidities. Mediators Inflamm. 2009, 610479. [Google Scholar] [CrossRef]
- Zernecke, A.; Bernhagen, J.; Weber, C. Macrophage Migration Inhibitory Factor in Cardiovascular Disease. Circulation 2008, 117, 1594–1602. [Google Scholar] [CrossRef]
- Voss, S.; Krüger, S.; Scherschel, K.; Warnke, S.; Schwarzl, M.; Schrage, B.; Girdauskas, E.; Meyer, C.; Blankenberg, S.; Westermann, D.; et al. Macrophage Migration Inhibitory Factor (MIF) Expression Increases during Myocardial Infarction and Supports Pro-Inflammatory Signaling in Cardiac Fibroblasts. Biomolecules 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Tilstam, P.V.; Qi, D.; Leng, L.; Young, L.; Bucala, R. MIF Family Cytokines in Cardiovascular Diseases and Prospects for Precision-Based Therapeutics. Expert. Opin. Ther. Targets 2017, 21, 671–683. [Google Scholar] [CrossRef]
- Luedike, P.; Alatzides, G.; Papathanasiou, M.; Heisler, M.; Pohl, J.; Lehmann, N.; Rassaf, T. Circulating Macrophage Migration Inhibitory Factor (MIF) in Patients with Heart Failure. Cytokine 2018, 110, 104–109. [Google Scholar] [CrossRef]
- Luedike, P.; Alatzides, G.; Papathanasiou, M.; Heisler, M.; Pohl, J.; Lehmann, N.; Rassaf, T. Predictive Potential of Macrophage Migration Inhibitory Factor (MIF) in Patients with Heart Failure with Preserved Ejection Fraction (HFpEF). Eur. J. Med. Res. 2018, 23, 22. [Google Scholar] [CrossRef]
- Ruze, A.; Chen, B.D.; Liu, F.; Chen, X.C.; Gai, M.T.; Li, X.M.; Ma, Y.T.; Du, X.J.; Yang, Y.N.; Gao, X.M. Macrophage migration inhibitory factor plays an essential role in ischemic preconditioning-mediated cardioprotection. Clin. Sci. 2019, 133, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Sinitski, D.; Kontos, C.; Krammer, C.; Asare, Y.; Kapurniotu, A.; Bernhagen, J. Macrophage Migration Inhibitory Factor (MIF)-Based Therapeutic Concepts in Atherosclerosis and Inflammation. Thromb. Haemost. 2019, 119, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.K.; Paal, M.C.; Donohue, T.M., Jr.; Ganesan, M.; Osna, N.A.; Kharbanda, K.K. Beneficial Effects of Betaine: A Comprehensive Review. Biology 2021, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Day, C.R.; Kempson, S.A. Betaine chemistry, Roles, and potential use in liver disease. Biochim. Biophys. Acta 2016, 1860, 1098–1106. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Mar, M.H.; Howe, J.C.; Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307, Erratum in J. Nutr. 2003, 133, 2918. [Google Scholar] [CrossRef]
- Lever, M.; Slow, S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin. Biochem. 2010, 43, 732–744. [Google Scholar] [CrossRef]
- Veskovic, M.; Mladenovic, D.; Milenkovic, M.; Tosic, J.; Borozan, S.; Gopcevic, K.; Labudovic-Borovic, M.; Dragutinovic, V.; Vucevic, D.; Jorgacevic, B.; et al. Betaine modulates oxidative stress, inflammation, apoptosis, autophagy, and Akt/mTOR signaling in methionine-choline deficiency-induced fatty liver disease. Eur. J. Pharmacol. 2019, 848, 39–48. [Google Scholar] [CrossRef]
- Bingűl, I.; Başaran-Kűcűkgergin, C.; Aydin, A.F.; Coban, J.; Doğan-Ekici, I.; Doğru-Abbasoğlu, S.; Uysal, M. Betaine treatment decreased oxidative stress, inflammation, and stellate cell activation in rats with alcoholic liver fibrosis. Environ. Toxicol. Pharmacol. 2016, 45, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-T.; Chen, C.-Y.; Pan, Y.-H.; Wang, S.-H.; Mersmann, H.J.; Ding, S.-T. Alleviation of Carbon-Tetrachloride- Induced Liver Injury and Fibrosis by Betaine Supplementation in Chickens. Evid. Based Complement. Alternat. Med. 2015, 725379. [Google Scholar] [CrossRef]
- El Hadi, H.; Di Vincenzo, A.; Vettor, R.; Rossato, M. Relationship between Heart Disease and Liver Disease: A Two-Way Street. Cells 2020, 9, 567. [Google Scholar] [CrossRef]
- Szkudelska, K.; Chan, M.H.; Okulicz, M.; Jasaszwili, M.; Lukomska, A.; Malek, E.; Shah, M.; Sunder, S.; Szkudelski, T. Betaine supplementation to rats alleviates disturbances induced by high-fat diet: Pleiotropic effects in model of type 2 diabetes. J. Physiol. Pharmacol. 2021, 72, 763–775. [Google Scholar] [CrossRef]
- Schwahn, B.C.; Laryea, M.D.; Chen, Z.; Melnyk, S.; Pogribny, I.; Garrow, T.; James, S.J.; Rozen, R. Betaine rescue of an animal model with methylenetetrahydrofolate reductase deficiency. Biochem. J. 2004, 382(Pt3), 831–840. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin Phenol rea-gent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Girotti, M.J.; Khan, N.; McLellan, B.A. Earlymeasurement of systemic lipid peroxidation products in the plasma of major blunt trauma patients. J. Trauma 1991, 31, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Witko, V.; Nguyen, A.T.; Descamps-Latscha, B. Microtiter plate assay for phagocyte-derived taurine-chloramines. J. Clin. Lab. Anal. 1992, 6, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Selmeci, L.; Seres, L.; Antal, M.; Lukács, J.; Regoly-Mérei, A.; Acsády, G. Advanced Oxidation Protein Products (AOPP) for Monitoring Oxidative Stress in Critically Ill Patients: A Simple, Fast and Inexpensive Automated Technique. Clin. Chem. Lab. Med. 2005, 43, 294–297. [Google Scholar] [CrossRef]
- Granger, D.L.; Taintor, R.R.; Boockvar, K.S.; Hibbs, J.B. Measurement of nitrate and nitrite in biological samples using nitrate reductase and Griess reaction. Methods Enzymol. 1996, 268, 142–151. [Google Scholar] [CrossRef]
- Sun, M.; Zigman, S. An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Anal. Biochem. 1978, 90, 81–89. [Google Scholar] [CrossRef]
- Beers, R.F., Jr.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E. Tissue glutathione. In The DTNB-GSSG Reductase Recycling Assay for Total Glutathione (GSH+1/2GSSG); Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1986; pp. 317–323. [Google Scholar]
- Matyas, C.; Haskó, G.; Liaudet, L.; Trojnar, E.; Pacher, P. Interplay of cardiovascular mediators, oxidative stress and inflammation in liver disease and its complications. Nat. Rev. Cardiol. 2021, 18, 117–135. [Google Scholar]
- Bernardi, M.; Moreau, R.; Angeli, P.; Schnabl, B.; Arroyo, V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J. Hepatol. 2015, 63, 1272–1284. [Google Scholar] [CrossRef]
- Xanthopoulos, A.; Starling, R.C.; Kitai, T.; Triposkiadis, F. Heart Failure and Liver Disease: Cardiohepatic Interactions. JACC Heart Fail. 2019, 7, 87–97. [Google Scholar] [CrossRef]
- Radosavljevic, T.; Vukicevic, D.; Djuretić, J.; Gopcevic, K.; Labudovic Borovic, M.; Stankovic, S.; Samardzic, J.; Radosavljevic, M.; Vucevic, D.; Jakovljevic, V. The Role of Macrophage Inhibitory Factor in TAA-Induced Liver Fibrosis in Mice: Modulatory Effects of Betaine. Biomedicines 2024, 12, 1337. [Google Scholar] [CrossRef]
- Yukitake, H.; Takizawa, M.; Kimura, H. Macrophage Migration Inhibitory Factor as an Emerging Drug Target to Regulate Antioxidant Response Element System. Oxid. Med. Cell. Longev. 2017, 8584930. [Google Scholar] [CrossRef]
- Sumaiya, K.; Langford, D.; Natarajaseenivasan, K.; Shanmughapriya, S. Macrophage Migration Inhibitory Factor (MIF): A Multifaceted Cytokine Regulated by Genetic and Physiological Strategies. Pharmacol. Therap. 2022, 233, 108024. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Wong, D.W.L.; Bucala, R.; Djudjaj, S.; Boor, P. Evolving complexity of MIF signaling. Cell. Signal. 2019, 57, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.J.; Li, J.; Leng, L.; McDonald, C.; Atsumi, T.; Bucala, R.; Young, L.H. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature 2008, 451, 578–582. [Google Scholar] [CrossRef]
- Almohawes, Z.N.; Okail, H.A.; Al-Megrin, W.A.; El-Khadragy, M.F.; Ibrahim, M.A.; Fathalla, A.S.; Soliman, D.; Mohamed, S.R. The cardioprotective effect of whey protein against thioacetamide-induced toxicity through its antioxidant, anti-inflammatory, and anti-apoptotic effects in male albino rats. Front. Vet. Sci. 2025, 12, 1590722. [Google Scholar] [CrossRef]
- Genesca, J.; Gonzalez, A.; Segura, R.; Catalan, R.; Marti, R.; Varela, E.; Cadelina, G.; Martinez, M.; Lopez-Talavera, J.C.; Esteban, R.; et al. Interleukin-6, Nitric Oxide, and the Clinical and Hemodynamic Alterations of Patients with Liver Cirrhosis. Am. J. Gastroenterol. 1999, 94, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zheng, R.; Hu, S.; Ma, Y.; Choudhry, M.A.; Messina, J.L.; Rue, L.W.; Bland, K.I.; Chaudry, I.H. Mechanism of Cardiac Depression after Trauma-Hemorrhage: Increased Cardiomyocyte IL-6 and Effect of Sex Steroids on IL-6 Regulation and Cardiac Function. Am. J. Physiol.-Heart Circul. Physiol. 2004, 287, H2183–H2191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fontes, J.A.; Rose, N.R.; Čiháková, D. The Varying Faces of IL-6: From Cardiac Protection to Cardiac Failure. Cytokine 2015, 74, 62–68. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djuretić, J.; Filipovic, J.; Brankovic, M.; Stankovic, S.; Samardzic, J.; Vucevic, D.; Radosavljevic, T. Macrophage Inhibitory Factor in Myocardial Oxidative Stress and Inflammation During Thioacetamide-Induced Liver Fibrosis: Modulation by Betaine. Curr. Issues Mol. Biol. 2025, 47, 728. https://doi.org/10.3390/cimb47090728
Djuretić J, Filipovic J, Brankovic M, Stankovic S, Samardzic J, Vucevic D, Radosavljevic T. Macrophage Inhibitory Factor in Myocardial Oxidative Stress and Inflammation During Thioacetamide-Induced Liver Fibrosis: Modulation by Betaine. Current Issues in Molecular Biology. 2025; 47(9):728. https://doi.org/10.3390/cimb47090728
Chicago/Turabian StyleDjuretić, Jasmina, Jelena Filipovic, Milica Brankovic, Sanja Stankovic, Janko Samardzic, Danijela Vucevic, and Tatjana Radosavljevic. 2025. "Macrophage Inhibitory Factor in Myocardial Oxidative Stress and Inflammation During Thioacetamide-Induced Liver Fibrosis: Modulation by Betaine" Current Issues in Molecular Biology 47, no. 9: 728. https://doi.org/10.3390/cimb47090728
APA StyleDjuretić, J., Filipovic, J., Brankovic, M., Stankovic, S., Samardzic, J., Vucevic, D., & Radosavljevic, T. (2025). Macrophage Inhibitory Factor in Myocardial Oxidative Stress and Inflammation During Thioacetamide-Induced Liver Fibrosis: Modulation by Betaine. Current Issues in Molecular Biology, 47(9), 728. https://doi.org/10.3390/cimb47090728







