Krill Oil Mitigates Cisplatin-Induced Ovarian Toxicity via Attenuation of Oxidative Stress and Inflammatory Pathways
Abstract
1. Introduction
2. Material and Method
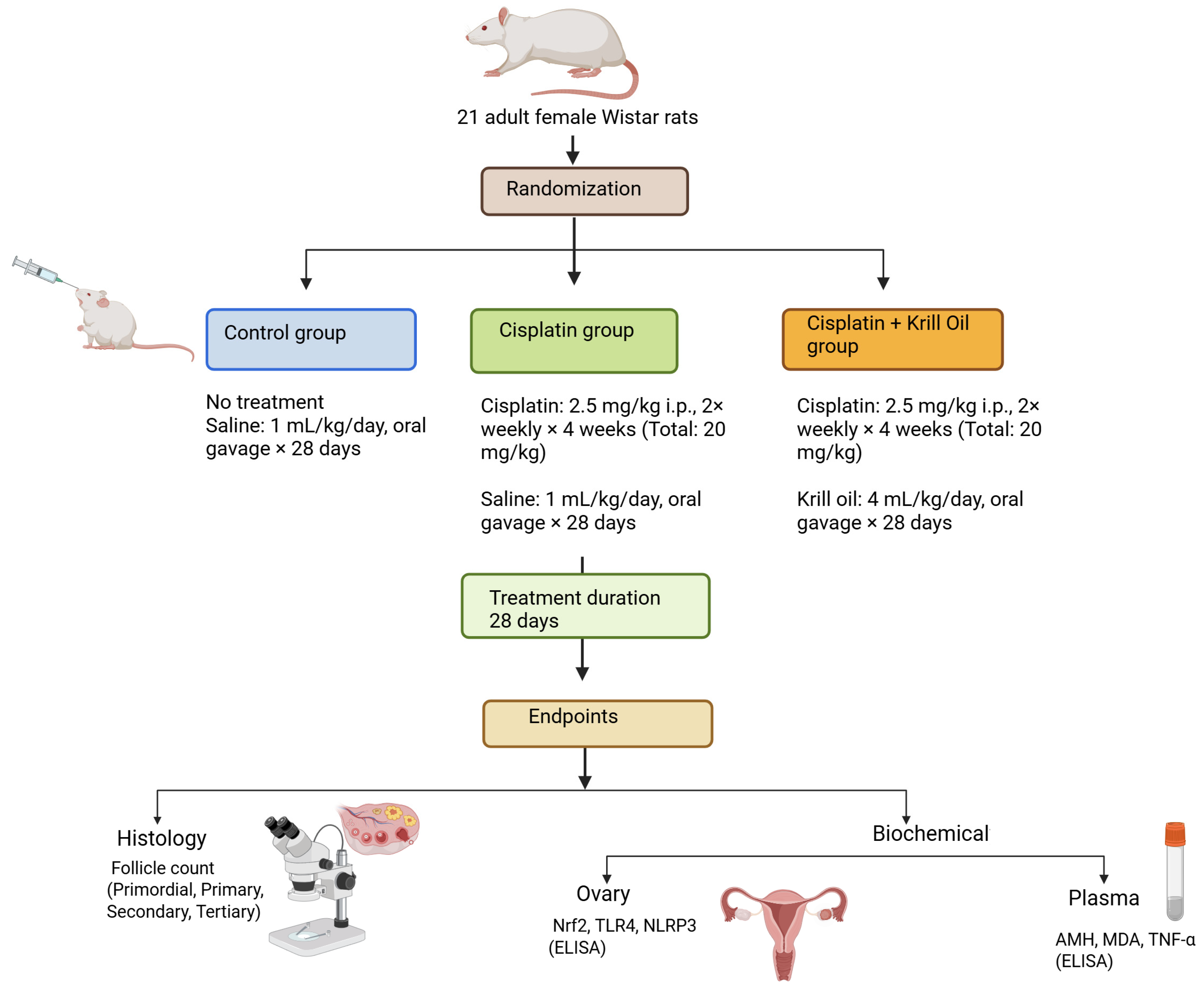
2.1. Animals
2.2. Experimental Protocol
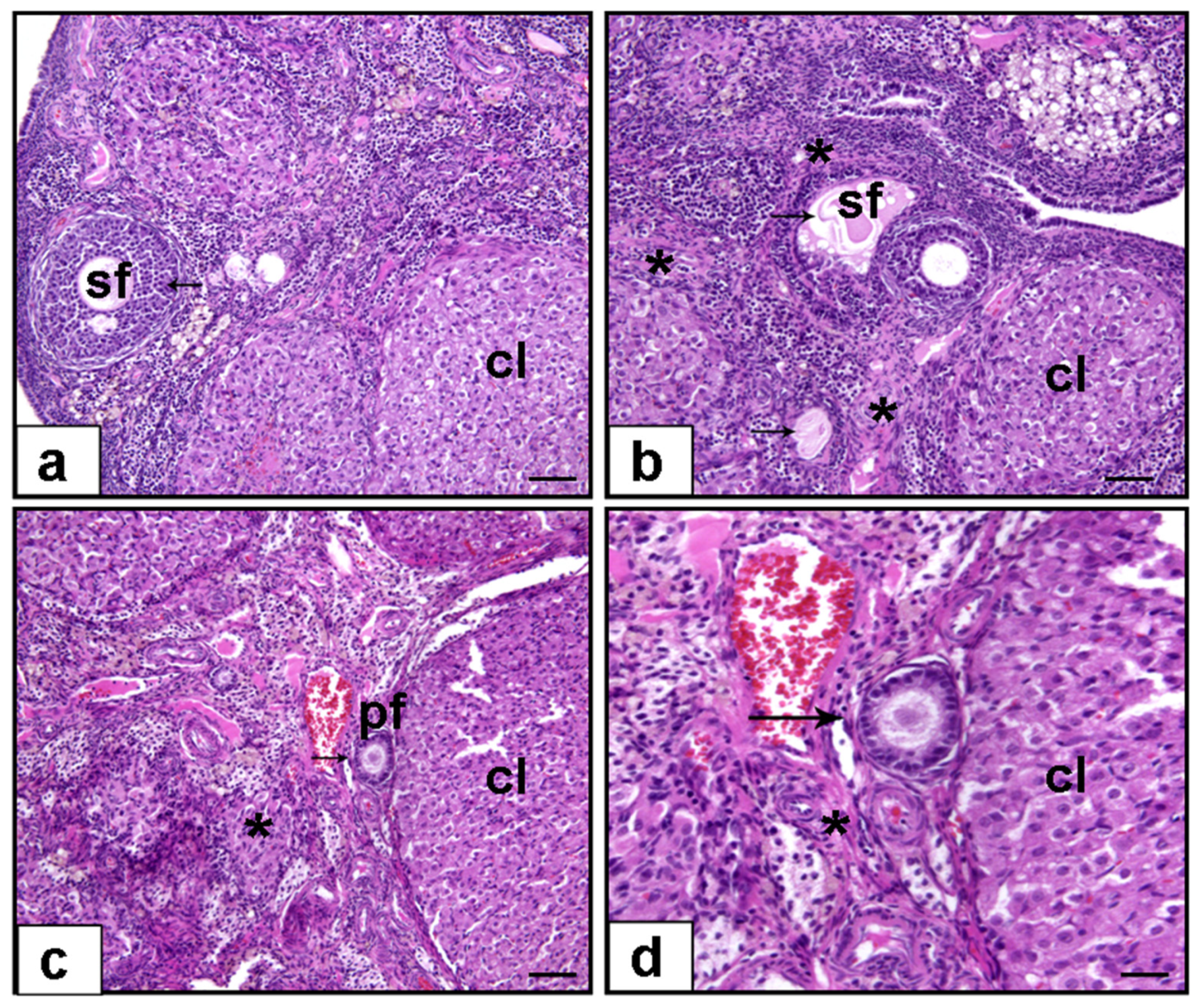
2.3. Histological Examination
2.4. Calculation of Percentage Restoration in Follicle Count
2.5. Biochemical Analyses
2.5.1. Analysis of Plasma Samples
2.5.2. Measurement of AMH Levels
2.5.3. Determination of Lipid Peroxidation
2.5.4. Measurement of TNF-α Levels
2.5.5. Measurement of IL-1β Levels
2.6. Analysis of Ovarian Tissue Samples
2.6.1. Tissue Collection and Storage
2.6.2. Tissue Homogenization
2.6.3. Total Protein Determination
2.6.4. Measurement of Oxidative Stress and Inflammatory Markers
2.6.5. ELISA Data Acquisition and Analysis
2.7. Statistical Analysis
3. Results
3.1. Histopathological Evaluation
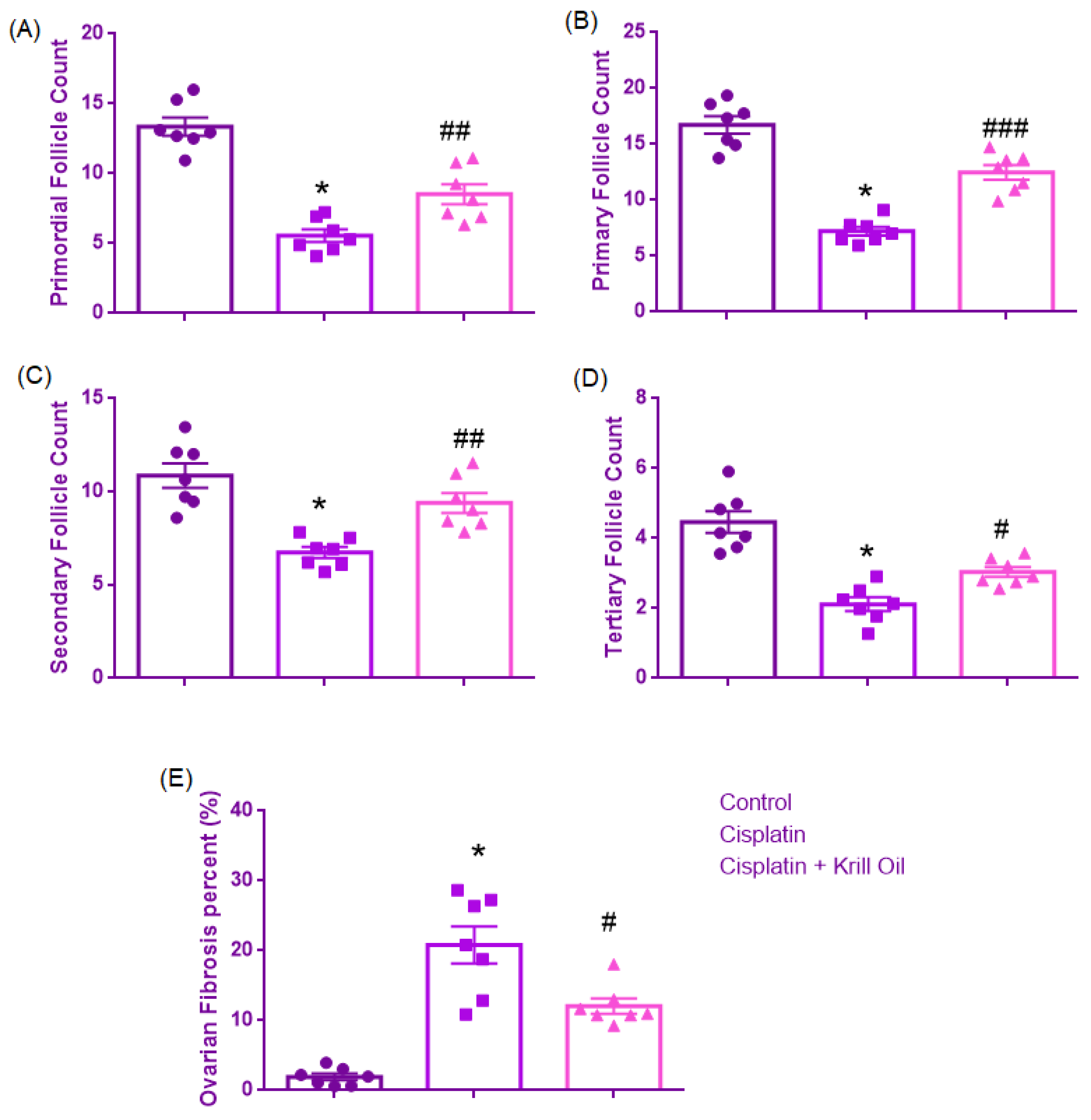
3.1.1. Primordial Follicle Count
3.1.2. Primary Follicle Count
3.1.3. Secondary Follicle Count
3.1.4. Tertiary Follicle Count
3.1.5. Ovarian Fibrosis (%)
3.2. Assessment of Biochemical Parameters
3.2.1. Plasma AMH (ng/mL) Level
3.2.2. Plasma MDA and Nrf2 (Oxidative Stress)
Plasma MDA Level (nmol/g)
Ovarian NrF2 Level (pg/mg)
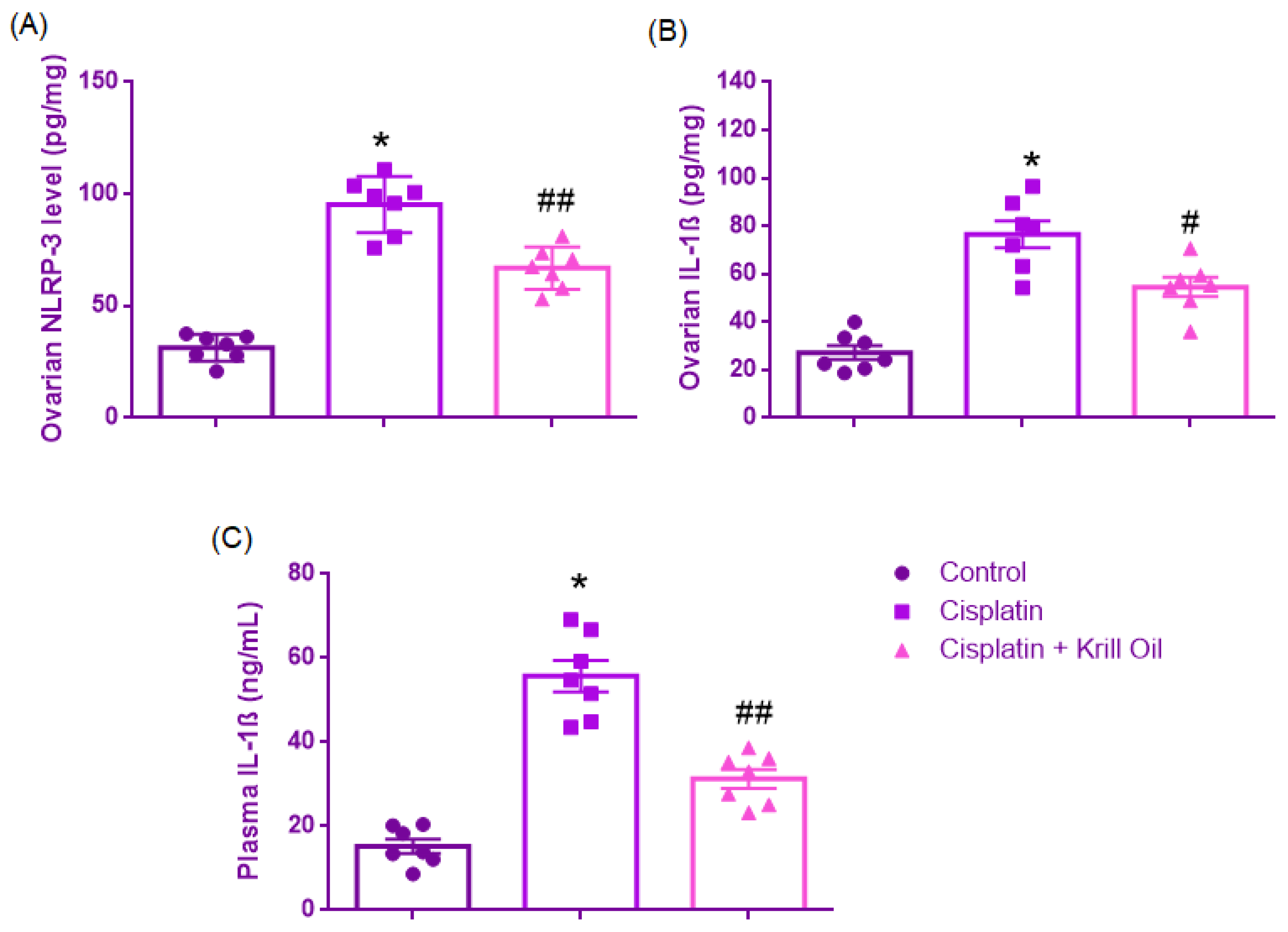
3.2.3. Ovarian NLRP3 and IL-1β + Plasma IL-1β
Ovarian NLRP-3 Level (pg/mg)
Ovarian IL-1β Level (pg/mg)
Plasma IL-1β Level (ng/mL)
3.2.4. Ovarian TLR4 and TNF-α + Plasma TNF-α
Ovarian TLR4 Level (ng/mg)
Ovarian TNF-α Levels (pg/mg)
Plasma TNF-α (pg/mL)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.; Liang, H.; Wang, W.; Liu, J.; Liu, X.; Lao, S.; Liang, W.; He, J. Global cancer statistics for adolescents and young adults: Population based study. J. Hematol. Oncol. 2024, 17, 99. [Google Scholar] [CrossRef]
- Wallace, W.H.B.; Thomson, A.B.; Saran, F.; Kelsey, T.W. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 738–744. [Google Scholar] [CrossRef]
- Morgan, S.; Anderson, R.; Gourley, C.; Wallace, W.; Spears, N. How do chemotherapeutic agents damage the ovary? Hum. Reprod. Update 2012, 18, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef]
- Xing, F.; Wang, M.; Ding, Z.; Zhang, J.; Ding, S.; Shi, L.; Xie, Q.; Ahmad, M.J.; Wei, Z.; Tang, L.; et al. Protective effect and mechanism of melatonin on cisplatin-induced ovarian damage in mice. J. Clin. Med. 2022, 11, 7383. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zheng, Y.; Yang, Y.-H.; Huang, Y.-J.; Hao, Y.-M.; Chen, C.; Wang, B.-Z.; Guo, X.; Wu, H.; Su, G.-H. Krill oil prevents lipopolysaccharide-evoked acute liver injury in mice through inhibition of oxidative stress and inflammation. Food Funct. 2022, 13, 3853–3864. [Google Scholar] [CrossRef]
- Babolmorad, G.; Latif, A.; Domingo, I.K.; Pollock, N.M.; Delyea, C.; Rieger, A.M.; Allison, W.T.; Bhavsar, A.P. Toll-like receptor 4 is activated by platinum and contributes to cisplatin-induced ototoxicity. EMBO Rep. 2021, 22, e51280. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Mentese, A.; Kucuk, H.; Yulug, E.; Alemdar, N.T.; Demir, E.A.; Aliyazicioglu, Y. Ethyl pyruvate attenuates cisplatin-induced ovarian injury in rats via activating Nrf2 pathway. Drug Chem. Toxicol. 2024, 47, 218–226. [Google Scholar] [CrossRef]
- Eid, B.G.; Binmahfouz, L.S.; Shaik, R.A.; Bagher, A.M.; Sirwi, A.; Abdel-Naim, A.B. Icariin inhibits cisplatin-induced ovarian toxicity via modulating NF-κB and PTEN/AKT/mTOR/AMPK axis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Khallaf, W.A.; Sharata, E.E.; Attya, M.E.; Abo-Youssef, A.M.; Hemeida, R.A. LCZ696 (sacubitril/valsartan) mitigates cyclophosphamide-induced premature ovarian failure in rats; the role of TLR4/NF-κB/NLRP3/Caspase-1 signaling pathway. Life Sci. 2023, 326, 121789. [Google Scholar] [CrossRef]
- Li, Z.; Qi, H.; Li, Z.; Bao, Y.; Yang, K.; Min, Q. Research progress on the premature ovarian failure caused by cisplatin therapy. Front. Oncol. 2023, 13, 1276310. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, Q.; Shao, X.; Mou, S.; Gu, L.; Wang, L.; Zhang, Z.; Shen, J.; Zhou, Y.; Qi, C.; et al. NLRP3 inflammasome inhibition attenuates cisplatin-induced renal fibrosis by decreasing oxidative stress and inflammation. Exp. Cell Res. 2019, 383, 111488. [Google Scholar] [CrossRef]
- Huang, J.; Shan, W.; Li, N.; Zhou, B.; Guo, E.; Xia, M.; Lu, H.; Wu, Y.; Chen, J.; Wang, B.; et al. Melatonin provides protection against cisplatin-induced ovarian damage and loss of fertility in mice. Reprod. Biomed. Online 2021, 42, 505–519. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Albahlol, I.A.; Wani, F.A.; Tammam, A.A.-E.; Kelleni, M.T.; Sayeed, M.U.; El-Fadeal, N.M.A.; Mohamed, A.A. Resveratrol protects against cisplatin-induced ovarian and uterine toxicity in female rats by attenuating oxidative stress, inflammation and apoptosis. Chem. Biol. Interact. 2021, 338, 109402. [Google Scholar] [CrossRef]
- Almeida, L.; Vaz-da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.L.; Fernandes-Lopes, C.; Rocha, J.F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53 (Suppl. 1), S7–S15. [Google Scholar] [CrossRef]
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Sarıyer, E.T.; Baş, M.; Yüksel, M. Comparative analysis of the antioxidant and anti-inflammatory effects of krill and fish oil. Int. J. Mol. Sci. 2025, 26, 7360. [Google Scholar] [CrossRef]
- Alijani, S.; Hahn, A.; Harris, W.S.; Schuchardt, J.P. Bioavailability of EPA and DHA in Humans—A Comprehensive Review. Prog. Lipid Res. 2025, 97, 101318. [Google Scholar] [CrossRef]
- Tou, J.C.; Jaczynski, J.; Chen, Y.-C. Krill for Human Consumption: Nutritional Value and Potential Health Benefits. Nutr. Rev. 2007, 65, 63–77. [Google Scholar] [CrossRef]
- Xie, D.; Gong, M.; Wei, W.; Jin, J.; Wang, X.; Wang, X.; Jin, Q. Antarctic Krill (Euphausia Superba) Oil: A Comprehensive Review of Chemical Composition, Extraction Technologies, Health Benefits, and Current Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 514–534. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Lim, S.J.; Ryu, Y.M.; Park, H.R.; Suh, H.J.; Han, S.H. Absorption Rate of Krill Oil and Fish Oil in Blood and Brain of Rats. Lipids Health Dis. 2018, 17, 162. [Google Scholar] [CrossRef] [PubMed]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Shafat, I.; Jones, P.J.H. Supplementation of Krill Oil with High Phospholipid Content Increases Sum of EPA and DHA in Erythrocytes Compared with Low Phospholipid Krill Oil. Lipids Health Dis. 2015, 14, 142. [Google Scholar] [CrossRef]
- Helal, M.G.; El-Kashef, D.H. Krill Oil Alleviates Oxidative Stress, Iron Accumulation and Fibrosis in the Liver and Spleen of Iron-Overload Rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 3950–3961. [Google Scholar] [CrossRef]
- Zadeh-Ardabili, P.M.; Rad, S.K. Anti-Pain and Anti-Inflammation like Effects of Neptune Krill Oil and Fish Oil against Carrageenan Induced Inflammation in Mice Models: Current Statues and Pilot Study. Biotechnol. Rep. 2019, 22, e00341, Erratum in Biotechnol. Rep. 2020, 27, e00510. [Google Scholar] [CrossRef]
- Kükürt, A.; Karapehlivan, M. Protective effect of astaxanthin on experimental ovarian damage in rats. J. Biochem. Mol. Toxicol. 2022, 36, e22966. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sun, X.; Meng, H.; Wu, J.; Guo, X.; Du, L.; Wu, H. Krill oil inhibits NLRP3 inflammasome activation in the prevention of the pathological injuries of diabetic cardiomyopathy. Nutrients 2022, 14, 368. [Google Scholar] [CrossRef] [PubMed]
- Yeral, I.; Sayan, C.D.; Karaca, G.; Simsek, Y.; Sagsoz, N.; Ozkan, Z.S.; Atasoy, P.; Sahin, Y.; Neselioglu, S.; Erel, O. What is the protective effect of krill oil on rat ovary against ischemia–reperfusion injury? J. Obstet. Gynaecol. Res. 2019, 45, 592–599. [Google Scholar] [CrossRef]
- Hirshfield, A.N. Development of follicles in the mammalian ovary. Int. Rev. Cytol. 1991, 124, 43–101. [Google Scholar]
- Myers, M.; Britt, K.L.; Wreford, N.G.M.; Ebling, F.J.P.; Kerr, J.B. Methods for quantifying follicular numbers within the mouse ovary. Reproduction 2004, 127, 569–580. [Google Scholar] [CrossRef]
- Pedersen, T.; Peters, H. Proposal for a classification of oocytes and follicles in the mouse ovary. Reproduction 1968, 17, 555–557. [Google Scholar] [CrossRef]
- Wilbur, K.; Bernheim, F.; Shapiro, O. The thiobarbituric acid reagent as a test for the oxidation of unsaturated fatty acids by various agents. Arch. Biochem. 1949, 24, 305–313. [Google Scholar]
- Meirow, D.; Nugent, D. The effects of radiotherapy and chemotherapy on female reproduction. Hum. Reprod. Update 2001, 7, 535–543. [Google Scholar] [CrossRef]
- Yeh, J.; Kim, B.S.; Peresie, J. Protection against cisplatin-induced ovarian damage by the antioxidant sodium 2-mercaptoethanesulfonate (mesna) in female rats. Am. J. Obs. Gynecol. 2008, 198, 463.e1–463.e7. [Google Scholar] [CrossRef]
- Taskin, M.I.; Yay, A.; Adali, E.; Balcioglu, E.; Inceboz, U. Protective effects of sildenafil citrate administration on cisplatin-induced ovarian damage in rats. Gynecol. Endocrinol. 2015, 31, 272–277. [Google Scholar] [CrossRef]
- Erbaş, O.; Akman, L.; Yavaşoğlu, A.; Terek, M.C.; Akman, T.; Taskiran, D. Oxytocin Improves Follicular Reserve in a Cisplatin-Induced Gonadotoxicity Model in Rats. BioMed Res. Int. 2014, 2014, 703691. [Google Scholar] [CrossRef] [PubMed]
- Said, R.S.; Mantawy, E.M.; El-Demerdash, E. Mechanistic perspective of protective effects of resveratrol against cisplatin-induced ovarian injury in rats: Emphasis on anti-inflammatory and anti-apoptotic effects. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Fanchin, R.; Schonäuer, L.M.; Righini, C.; Guibourdenche, J.; Frydman, R.; Taieb, J. Serum anti-Müllerian hormone is more strongly related to ovarian follicular status than seruminhibin B, estradiol, FSH and LH on day 3. Hum. Reprod. 2003, 18, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Gruijters, M.J.G.; Visser, J.A.; Durlinger, A.L.L.; Themmen, A.P.N. Anti- Müllerian hormone and its role in ovarian function. Mol. Cell Endocrinol. 2003, 211, 85–90. [Google Scholar] [CrossRef]
- Visser, J.A.; Themmen, A.P. Anti-Müllerian hormone and folliculogenesis. Mol. Cell. Endocrinol. 2005, 234, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, M.; Andersen, C.Y.; la Cour Freiesleben, N.; Juul, A.; Løssl, K.; Andersen, A.N. Dynamics and mechanisms of chemotherapyinduced ovarian follicular depletion in women of fertile age. Fertil. Steril. 2010, 94, 156–166. [Google Scholar] [CrossRef]
- Al-Shahat, A.; Hulail, M.A.E.; Soliman, N.M.M.; Khamis, T.; Fericean, L.M.; Arisha, A.H.; Moawad, R.S. Melatonin mitigates cisplatin-induced ovarian dysfunction via altering steroidogenesis, inflammation, apoptosis, oxidative stress, and PTEN/PI3K/Akt/mTOR/AMPK signaling pathway in female rats. Pharmaceutics 2022, 14, 2769. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wen, C.; Hu, B.; Wu, H. Investigating the potential role of α-SNAP in preventing chemotherapy-induced ovarian dysfunction: Insights from cellular and animal models. Heliyon 2024, 10, e32802. [Google Scholar] [CrossRef]
- Barberino, R.S.; Menezes, V.G.; Ribeiro, A.E.; Palheta Jr, R.C.; Jiang, X.; Smitz, J.E.; Matos, M.H.T. Melatonin protects against cisplatin-induced ovarian damage in mice via the MT1 receptor and antioxidant activity. Biol. Reprod. 2017, 96, 1244–1255. [Google Scholar] [CrossRef]
- Lins, T.L.B.; Gouveia, B.B.; Barberino, R.S.; Silva, R.L.; Monte, A.P.; Pinto, J.G.; Campinho, D.S.; Palheta, R.C., Jr.; Matos, M.H. Rutin prevents cisplatin-induced ovarian damage via antioxidant activity and regulation of PTEN and FOXO3a phosphorylation in mouse model. Reprod. Toxicol. 2020, 98, 209–217. [Google Scholar] [CrossRef]
- Mentese, A.; Alemdar, N.T.; Livaoglu, A.; Demir, E.A.; Aliyazicioglu, Y.; Demir, S. Suppression of cisplatin-induced ovarian injury in rats by chrysin: An experimental study. J. Obstet. Gynaecol. 2022, 42, 3584–3590. [Google Scholar] [CrossRef]
- Gui, H.; Jin, Y.; Lin, A.; Wang, P.; Wang, Y.; Zhu, H. Rosmarinic acid relieves cisplatin-induced ovary toxicity in female mice via suppression of oxidative stress and inflammation. J. Biochem. Mol. Toxicol. 2021, 35, e22839. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhu, F.; Wang, S.; Hu, H.; Zhang, D.; He, Z.; Chen, J.; Li, X.; Cheng, L.; Zhong, F. Icariin alleviates cisplatin-induced premature ovarian failure by inhibiting ferroptosis through activation of the Nrf2/ARE pathway. Sci. Rep. 2024, 14, 17318. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, D.; Gao, L.; Wang, Y.; Wang, Y.; Jia, R.; Bai, Y.; Shi, D.; Lu, F. Effects of Astaxanthin on the Physiological State of Porcine Ovarian Granulose Cells Cultured In Vitro. Antioxidants 2024, 13, 1185. [Google Scholar] [CrossRef]
- Nair, D.V.; Rani, M.U.; Reddy, A.G.; Kumar, B.K.; Reddy, M.A.; Lakshman, M.; Rajkumar, U. Protective effect of alpha-lipoic acid and omega-3 fatty acids against cyclophosphamide-induced ovarian toxicity in rats. Vet. World 2020, 13, 188. [Google Scholar] [CrossRef]
- Zhang, B.; Ramesh, G.; Uematsu, S.; Akira, S.; Reeves, W.B. TLR4 Signaling Mediates Inflammation and Tissue Injury in Nephrotoxicity. J. Am. Soc. Nephrol. 2008, 19, 923–932. [Google Scholar] [CrossRef]
- Zhou, J.; An, C.; Jin, X.; Hu, Z.; Safirstein, R.L.; Wang, Y. TAK1 deficiency attenuates cisplatin-induced acute kidney injury. Am. J. Physiol. Ren. Physiol. 2020, 318, F209–F215. [Google Scholar] [CrossRef] [PubMed]
- Domingo, I.K.; Latif, A.; Bhavsar, A.P. Pro-inflammatory signalling PRRopels cisplatin-induced toxicity. Int. J. Mol. Sci. 2022, 23, 7227. [Google Scholar] [CrossRef]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting Edge: NF-κB Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Ekinio, A.; Esaleh, M. Functions of NOD-Like Receptors in Human Diseases. Front. Immunol. 2013, 4, 333. [Google Scholar] [CrossRef]
- Bonaterra, G.A.; Driscoll, D.; Schwarzbach, H.; Kinscherf, R. Krill Oil-In-Water Emulsion Protects against Lipopolysaccharide-Induced Proinflammatory Activation of Macrophages In Vitro. Mar. Drugs 2017, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Smith, A.D.; Solano-Aguilar, G.; Wang, T.T.Y.; Pham, Q.; Beshah, E.; Tang, Q.; Urban, J.F.; Xue, C.; Li, R.W. Mechanistic Insights into the Attenuation of Intestinal Inflammation and Modulation of the Gut Microbiome by Krill Oil Using in Vitro and in Vivo Models. Microbiome 2020, 8, 83. [Google Scholar] [CrossRef]
- Du, Y.; Song, L.; Dong, X.; Li, H.; Xie, W.; Wang, Y.; Che, H. Long-Term Krill Oil Administration Alleviated Early Mild Cognitive Impairment in APP/PS1 Mice. Mol. Nutr. Food Res. 2024, 68, 2200652. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Fletcher, S.; Xin, W.; Claycombe, K.J.; Quignard-Boulange, A.; Zhao, L.; Saxton, A.M.; Moustaid-Moussa, N. N-3 and n-6 Polyunsaturated Fatty Acids Differentially Regulate Adipose Angiotensinogen and Other Inflammatory Adipokines in Part via NF-ΚB-Dependent Mechanisms. J. Nutr. Biochem. 2012, 23, 1661–1667. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes: From Molecules to Man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Mao, L.; Yu, S.; Huang, J.; Xie, Q.; Hu, M.; Mao, L. DHA and EPA Improve Liver IR in HFD-Induced IR Mice through Modulating the Gut Microbiotas-LPS-Liver Axis. J. Funct. Foods 2024, 112, 105917. [Google Scholar] [CrossRef]
- Lee, S.J.; Bai, S.K.; Lee, K.S.; Namkoong, S.; Na, H.J.; Ha, K.S.; Han, J.A.; Yim, S.V.; Chang, K.; Kwon, Y.G.; et al. Astaxanthin Inhibits Nitric Oxide Production and Inflammatory Gene Expression by Suppressing IκB Kinase-Dependent NF-ΚB Activation. Mol. Cells 2003, 16, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, T.; Queiroz, I.; de Mesquita, C.F.; Ruelas, M.G.; Leandro, G.N.; Monteiro, A.R.; Pimentel, F.N. Krill oil supplementation for knee pain: A systematic review and meta-analysis with trial sequential analysis of randomized controlled trials. Inflammopharmacology 2024, 32, 3109–3118. [Google Scholar] [CrossRef]
- Moore, H.C.; Unger, J.M.; Phillips, K.-A.; Boyle, F.; Hitre, E.; Porter, D.; Francis, P.A.; Goldstein, L.J.; Gomez, H.L.; Vallejos, C.S.; et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N. Engl. J. Med. 2015, 372, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Sampalis, F.; Bunea, R.; Pelland, M.F.; Kowalski, O.; Duguet, N.; Dupuis, S. Evaluation of the effects of Neptune Krill Oil™ on the management of premenstrual syndrome and dysmenorrhea. Altern. Med. Rev. 2003, 8, 171–179. [Google Scholar]
- Attri, N.; Arora, D.; Saini, R.; Chandel, M.; Suthar, P.; Dhiman, A. Health promoting benefits of krill oil: Mechanisms, bioactive combinations, and advanced encapsulation technologies. Food Sci. Biotechnol. 2025, 34, 1285–1308. [Google Scholar] [CrossRef]
- Deutsch, L. Evaluation of the effect of Neptune Krill Oil on chronic inflammation and arthritic symptoms. J. Am. Coll. Nutr. 2007, 26, 39–48. [Google Scholar] [CrossRef]
- Costanzo, M.; Cesi, V.; Prete, E.; Negroni, A.; Palone, F.; Cucchiara, S.; Oliva, S.; Leter, B.; Stronati, L. Krill oil reduces intestinal inflammation by improving epithelial integrity and impairing adherent-invasive Escherichia coli pathogenicity. Dig. Liver Dis. 2016, 48, 34–42. [Google Scholar] [CrossRef]
- Rundblad, A.; Holven, K.B.; Bruheim, I.; Myhrstad, M.C.; Ulven, S.M. Effects of krill oil and lean and fatty fish on cardiovascular risk markers: A randomised controlled trial. J. Nutr. Sci. 2018, 7, e3. [Google Scholar] [CrossRef]
- Laslett, L.L.; Scheepers, L.E.; Antony, B.; Wluka, A.E.; Cai, G.; Hill, C.L.; Jones, G.; March, L.; Keen, H.I.; Otahal, P.; et al. Krill oil for knee osteoarthritis: A randomized clinical trial. JAMA 2024, 331, 1997–2006. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aksu, E.; Erbas, O. Krill Oil Mitigates Cisplatin-Induced Ovarian Toxicity via Attenuation of Oxidative Stress and Inflammatory Pathways. Curr. Issues Mol. Biol. 2025, 47, 708. https://doi.org/10.3390/cimb47090708
Aksu E, Erbas O. Krill Oil Mitigates Cisplatin-Induced Ovarian Toxicity via Attenuation of Oxidative Stress and Inflammatory Pathways. Current Issues in Molecular Biology. 2025; 47(9):708. https://doi.org/10.3390/cimb47090708
Chicago/Turabian StyleAksu, Erson, and Oytun Erbas. 2025. "Krill Oil Mitigates Cisplatin-Induced Ovarian Toxicity via Attenuation of Oxidative Stress and Inflammatory Pathways" Current Issues in Molecular Biology 47, no. 9: 708. https://doi.org/10.3390/cimb47090708
APA StyleAksu, E., & Erbas, O. (2025). Krill Oil Mitigates Cisplatin-Induced Ovarian Toxicity via Attenuation of Oxidative Stress and Inflammatory Pathways. Current Issues in Molecular Biology, 47(9), 708. https://doi.org/10.3390/cimb47090708





