TGF-β-Enriched Exosomes from Acute Myeloid Leukemia Activate Smad2/3–MMP2 and ERK1/2 Signaling to Promote Leukemic Cell Proliferation, Migration, and Immune Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
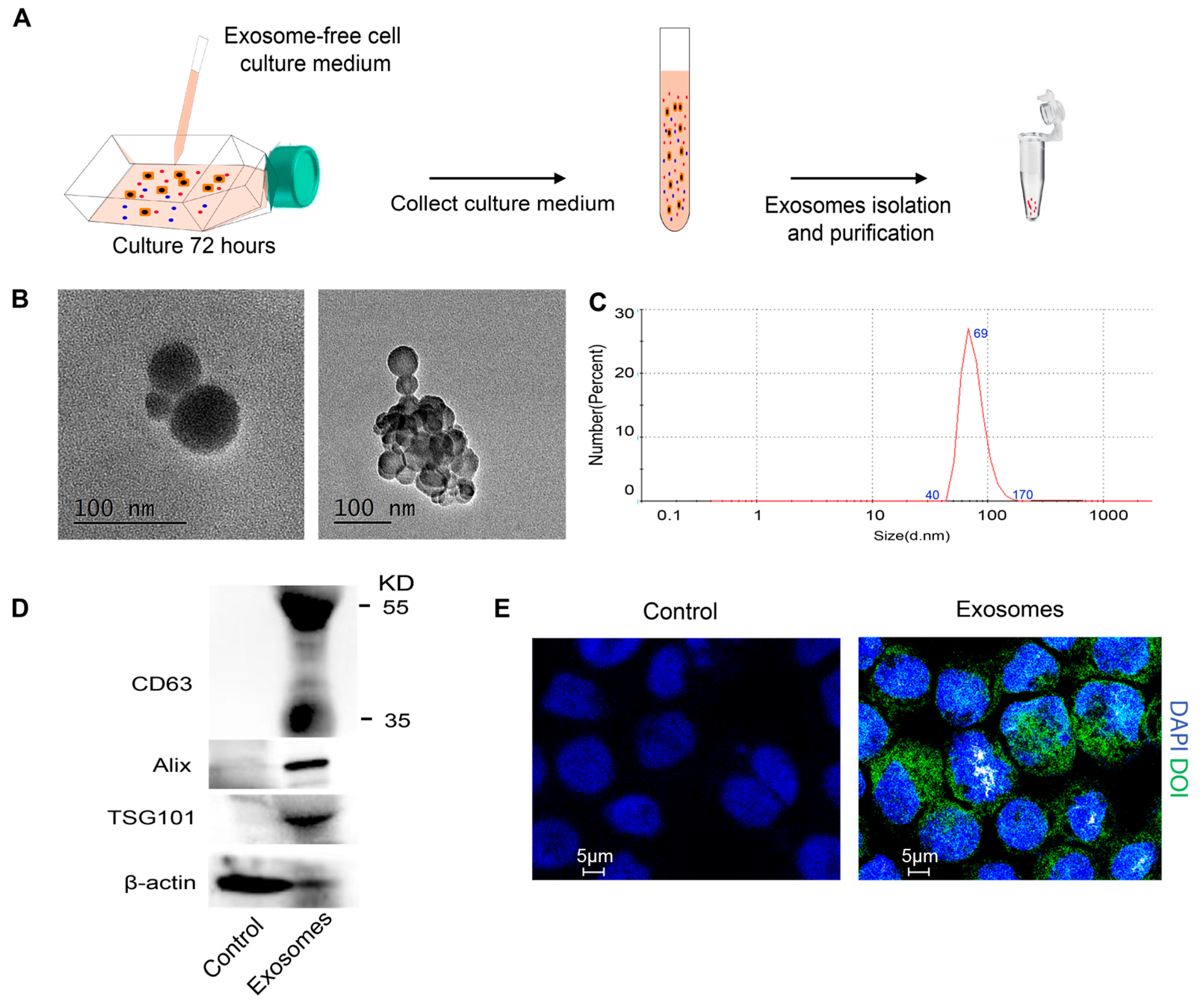
2.2. Isolation of Exosomes
2.3. Transmission Electron Microscopy
2.4. Dynamic Light Scattering Analysis
2.5. Exosome Labeling and Tracing
2.6. Cell Proliferation Test
2.7. Cell Migration Assay
2.8. Western Blotting
2.9. Dataset Information of Transcriptomics Analysis
2.10. Gene Set Enrichment Analysis
2.11. Single-Cell RNA-Seq Processing
2.12. Cell–Cell Interaction Analysis
2.13. Statistical Analysis
3. Results
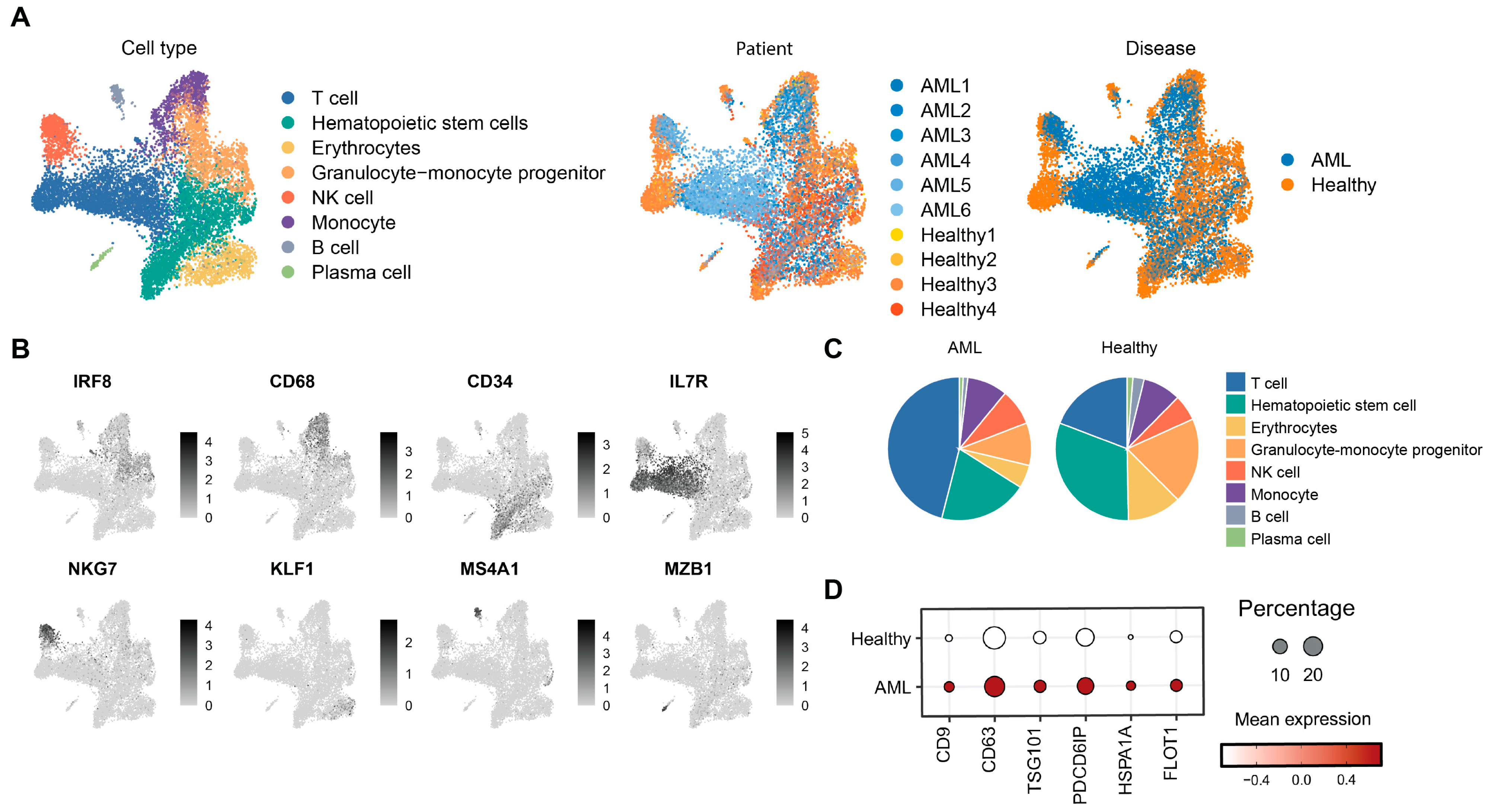
3.1. Single-Cell Transcriptomic Profiling Reveals Disrupted Hematopoiesis and Enhanced Exosome Activity in AML Bone Marrow
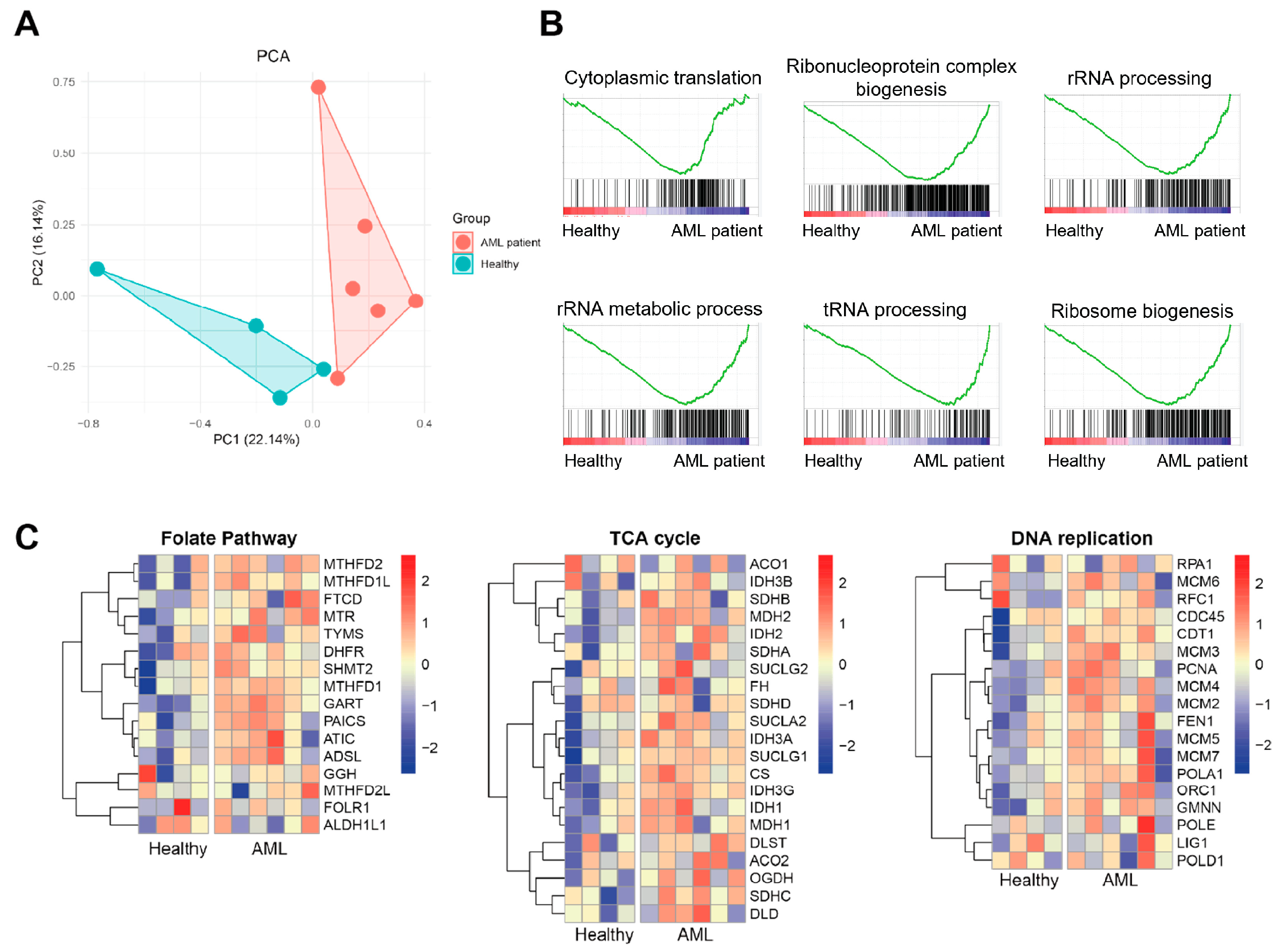
3.2. Exosomal Transcriptomic Signatures Reveal Enhanced Ribosome Biogenesis and Metabolic Reprogramming in AML Patients
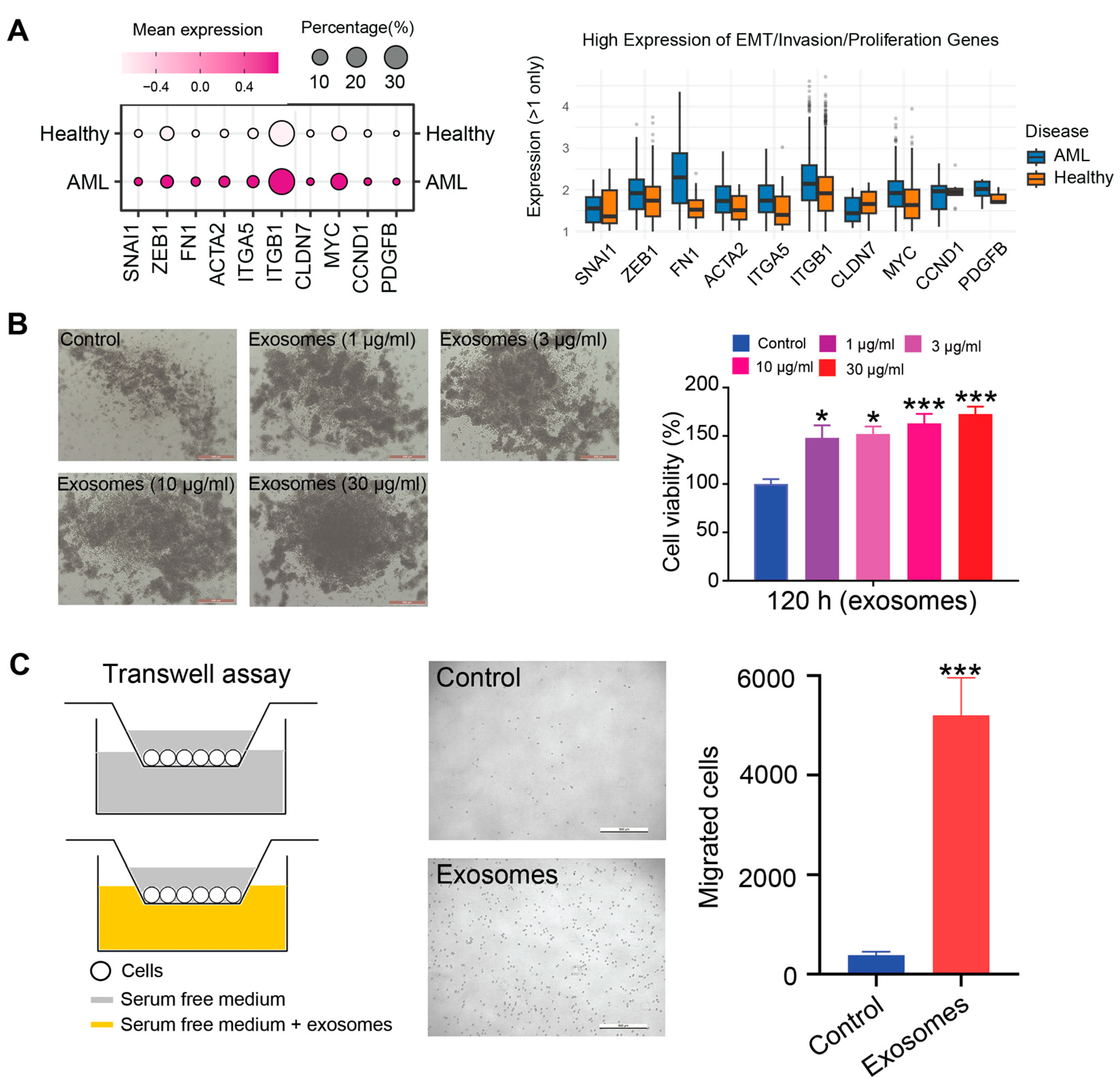
3.3. AML-Exos Affect Thp-1 Cell Proliferation and Migration
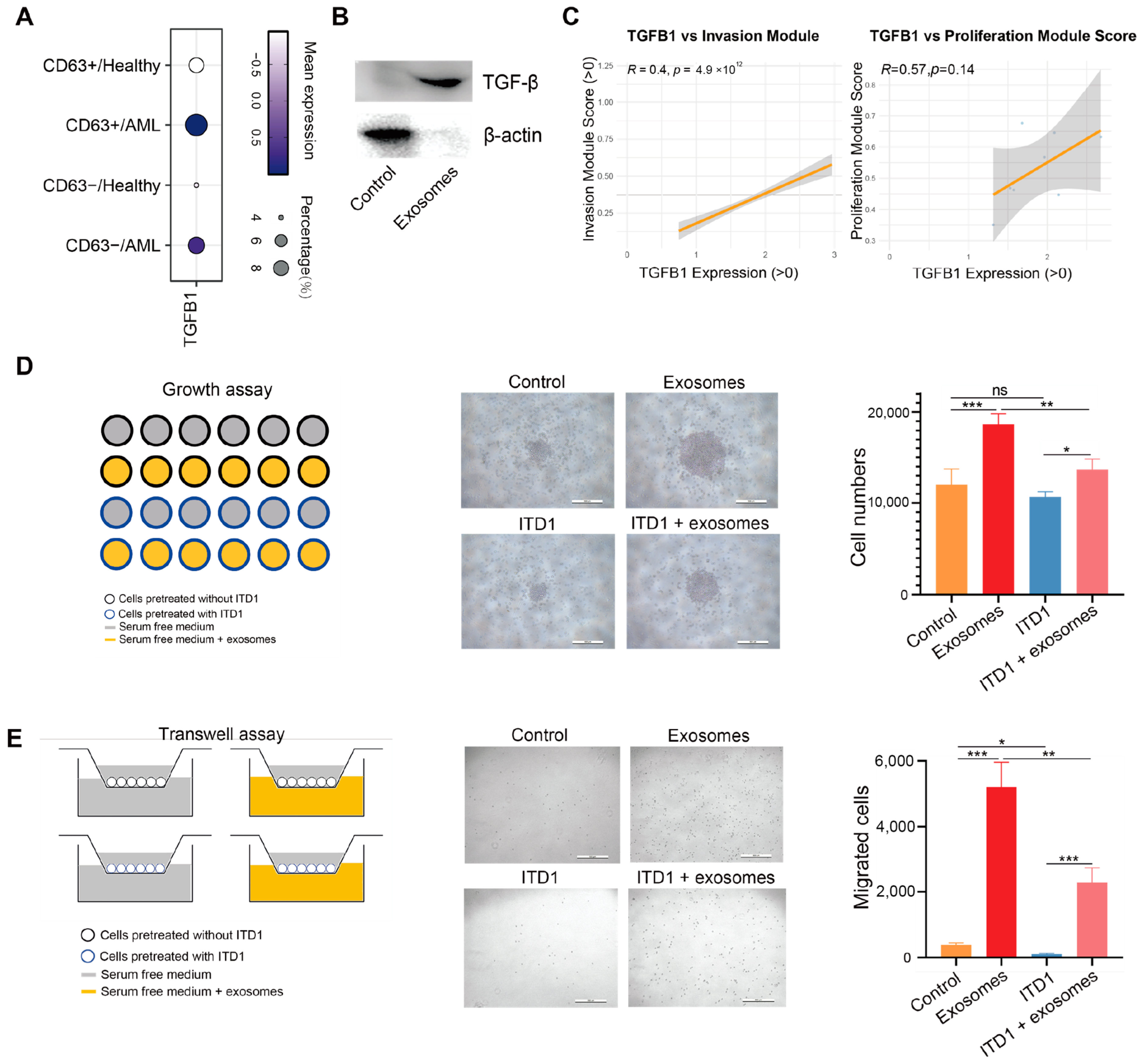
3.4. Exosomal TGF-β Promotes AML Cell Proliferation and Migration
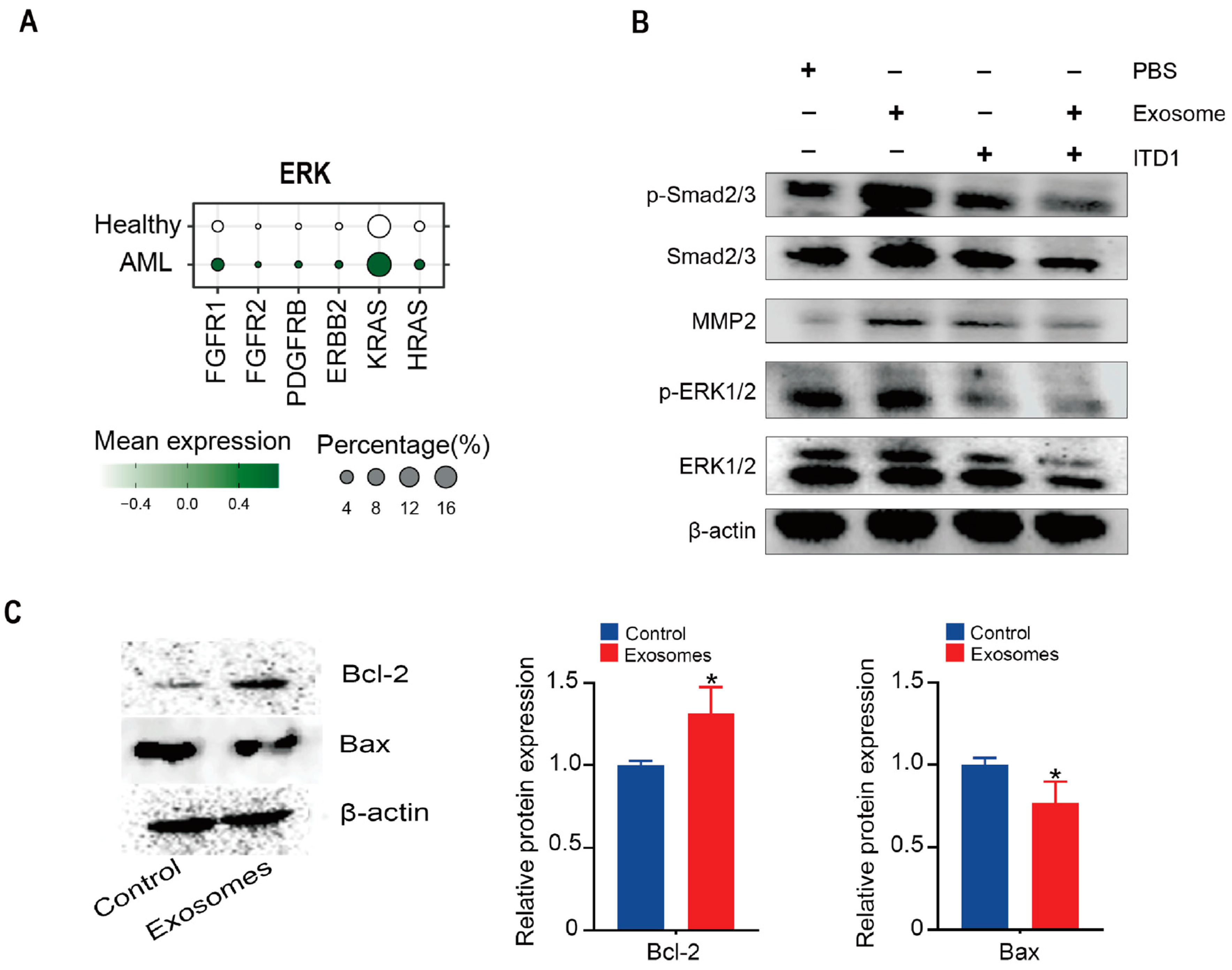
3.5. Exosomal TGF-β Induces ERK1/2 and Smad2/3–MMP2 Signaling and Suppresses Apoptosis
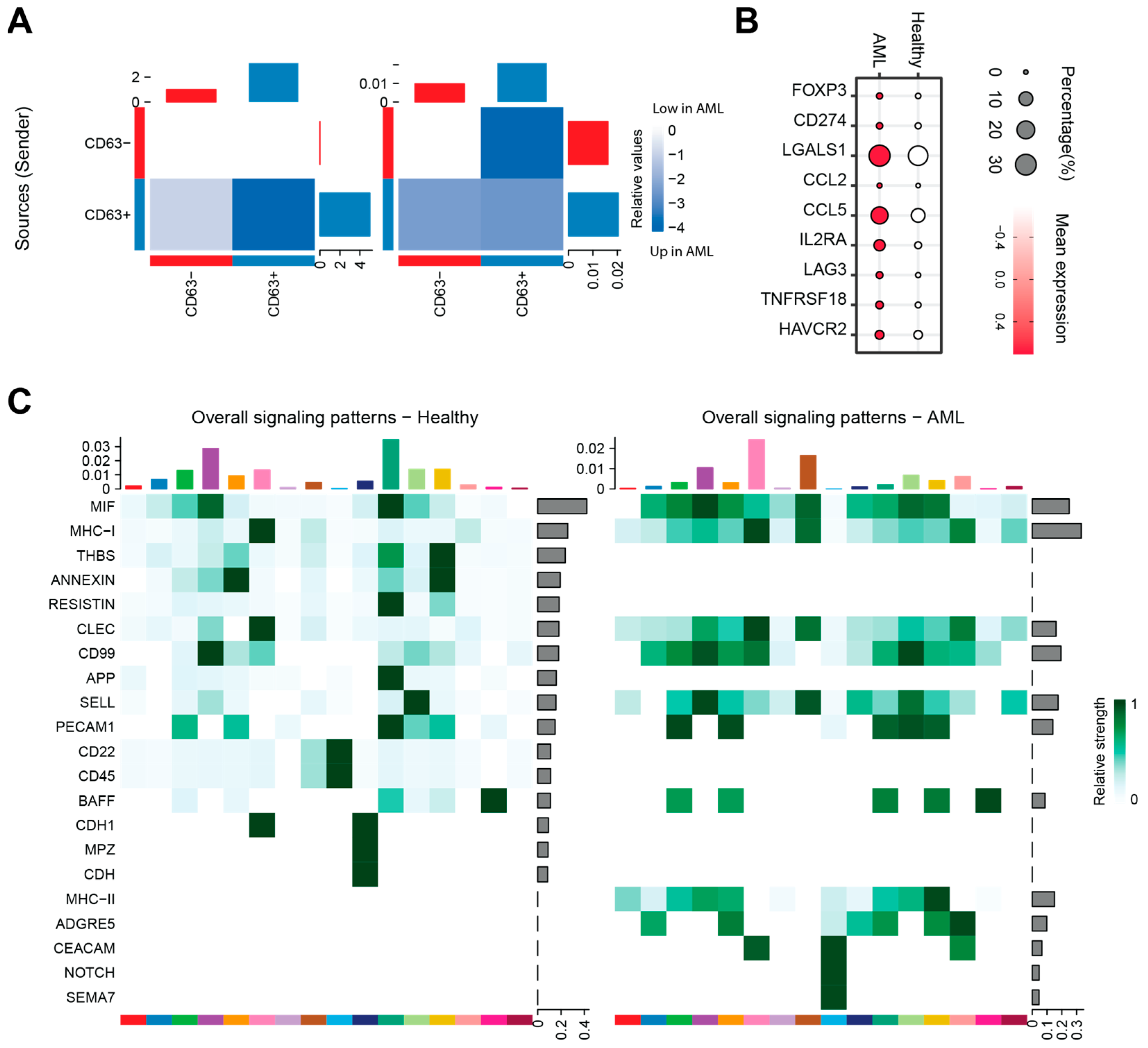
3.6. The Impact of AML-Exos on Immune Modulation
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kantarjian, H.; Borthakur, G.; Daver, N.; DiNardo, C.D.; Issa, G.; Jabbour, E.; Kadia, T.; Sasaki, K.; Short, N.J.; Yilmaz, M.; et al. Current status and research directions in acute myeloid leukemia. Blood Cancer J. 2024, 14, 163. [Google Scholar] [CrossRef]
- Senapati, J.; Kadia, T.M.; Daver, N.G.; DiNardo, C.D.; Borthakur, G.; Ravandi, F.; Kantarjian, H.M. Therapeutic horizon of acute myeloid leukemia: Success, optimism, and challenges. Cancer 2025, 131, e35806. [Google Scholar] [CrossRef]
- Xiong, H.; Huang, Z.; Yang, Z.; Lin, Q.; Yang, B.; Fang, X.; Liu, B.; Chen, H.; Kong, J. Recent Progress in Detection and Profiling of Cancer Cell-Derived Exosomes. Small 2021, 17, e2007971. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, P.; Yin, N.; Xu, Z.; Liu, X.; Wu, A.; Zhang, X.; Li, Z.; Zhang, Z.; Zhong, T.; et al. Glioblastoma stem cell-derived exosomal miR-374b-3p promotes tumor angiogenesis and progression through inducing M2 macrophages polarization. IScience 2024, 27, 109270. [Google Scholar] [CrossRef]
- Lv, Y.; Li, Y.; Zhou, J.; Liu, X.; Wang, D.; Wang, D.; Tong, D.; Wang, S.; An, H.; Kang, X. Exosomal miR-122-5p for regulation of secretory functions of fibroblasts and promotion of breast cancer metastasis by targeting MKP-2: An experimental study. Cancer Biol. Ther. 2025, 26, 2500104. [Google Scholar] [CrossRef]
- Li, X.; Lu, X.; Liu, M.; Chen, J.; Lu, X. Extracellular vesicles: Messengers of cross-talk between gastric cancer cells and the tumor microenvironment. Front. Cell Dev. Biol. 2025, 13, 1561856. [Google Scholar] [CrossRef]
- Trino, S.; Lamorte, D.; Caivano, A.; De Luca, L.; Sgambato, A.; Laurenzana, I. Clinical relevance of extracellular vesicles in hematological neoplasms: From liquid biopsy to cell biopsy. Leukemia 2021, 35, 661–678. [Google Scholar] [CrossRef]
- Zhang, Y.; Alexander, P.B.; Wang, X.F. TGF-beta Family Signaling in the Control of Cell Proliferation and Survival. Cold Spring Harb. Perspect. Biol. 2017, 9, a022145. [Google Scholar] [CrossRef]
- Huynh, L.K.; Hipolito, C.J.; Ten, D.P. A Perspective on the Development of TGF-beta Inhibitors for Cancer Treatment. Biomolecules 2019, 9, 743. [Google Scholar] [CrossRef]
- Ashiru, O.; Boutet, P.; Fernandez-Messina, L.; Aguera-Gonzalez, S.; Skepper, J.N.; Vales-Gomez, M.; Reyburn, H.T. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res. 2010, 70, 481–489. [Google Scholar] [CrossRef]
- Szczepanski, M.J.; Szajnik, M.; Welsh, A.; Whiteside, T.L.; Boyiadzis, M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica 2011, 96, 1302–1309. [Google Scholar] [CrossRef]
- Tohumeken, S.; Baur, R.; Bottcher, M.; Stoll, A.; Loschinski, R.; Panagiotidis, K.; Braun, M.; Saul, D.; Volkl, S.; Baur, A.S.; et al. Palmitoylated Proteins on AML-Derived Extracellular Vesicles Promote Myeloid-Derived Suppressor Cell Differentiation via TLR2/Akt/mTOR Signaling. Cancer Res. 2020, 80, 3663–3676. [Google Scholar] [CrossRef]
- Wojtowicz-Praga, S. Reversal of tumor-induced immunosuppression by TGF-beta inhibitors. Investig. New Drugs 2003, 21, 21–32. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-beta signaling in health, disease, and therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef]
- Allgayer, H.; Mahapatra, S.; Mishra, B.; Swain, B.; Saha, S.; Khanra, S.; Kumari, K.; Panda, V.K.; Malhotra, D.; Patil, N.S.; et al. Epithelial-to-mesenchymal transition (EMT) and cancer metastasis: The status quo of methods and experimental models 2025. Mol. Cancer 2025, 24, 167. [Google Scholar] [CrossRef]
- Khalili-Tanha, G.; Radisky, E.S.; Radisky, D.C.; Shoari, A. Matrix met alloproteinase-driven epithelial-mesenchymal transition: Implications in health and disease. J. Transl. Med. 2025, 23, 436. [Google Scholar] [CrossRef]
- Glaviano, A.; Lau, H.S.; Carter, L.M.; Lee, E.; Lam, H.Y.; Okina, E.; Tan, D.; Tan, W.; Ang, H.L.; Carbone, D.; et al. Harnessing the tumor microenvironment: Targeted cancer therapies through modulation of epithelial-mesenchymal transition. J. Hematol. Oncol. 2025, 18, 6. [Google Scholar] [CrossRef]
- Can, C.; Yang, X.; Jia, H.; Wu, H.; Guo, X.; Wei, Y.; Jia, Z.; Liu, W.; Zhang, A.; He, N.; et al. Exosomal circ_0006896 promotes AML progression via interaction with HDAC1 and restriction of antitumor immunity. Mol. Cancer 2025, 24, 4. [Google Scholar] [CrossRef]
- Cui, C.H.; Uyama, T.; Miyado, K.; Terai, M.; Kyo, S.; Kiyono, T.; Umezawa, A. Menstrual blood-derived cells confer human dystrophin expression in the murine model of Duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation. Mol. Biol. Cell 2007, 18, 1586–1594. [Google Scholar] [CrossRef]
- Jia, J.; Liu, B.; Wang, D.; Wang, X.; Song, L.; Ren, Y.; Guo, Z.; Ma, K.; Cui, C. CD93 promotes acute myeloid leukemia development and is a potential therapeutic target. Exp. Cell Res. 2022, 420, 113361. [Google Scholar] [CrossRef]
- Van Galen, P.; Hovestadt, V.; Wadsworth, I.M.; Hughes, T.K.; Griffin, G.K.; Battaglia, S.; Verga, J.A.; Stephansky, J.; Pastika, T.J.; Lombardi, S.J.; et al. Single-Cell RNA-Seq Reveals AML Hierarchies Relevant to Disease Progression and Immunity. Cell 2019, 176, 1265–1281. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdottir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Hao, Y.; Stuart, T.; Kowalski, M.H.; Choudhary, S.; Hoffman, P.; Hartman, A.; Srivastava, A.; Molla, G.; Madad, S.; Fernandez-Granda, C.; et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 2024, 42, 293–304. [Google Scholar] [CrossRef]
- Jin, S.; Plikus, M.V.; Nie, Q. CellChat for systematic analysis of cell-cell communication from single-cell transcriptomics. Nat. Protoc. 2025, 20, 180–219. [Google Scholar] [CrossRef]
- Leisch, M.; Weiss, L.; Lindlbauer, N.; Jungbauer, C.; Egle, A.; Rohde, E.; Greil, R.; Grabmer, C.; Pleyer, L. Red blood cell alloimmunization in 184 patients with myeloid neoplasms treated with azacitidine—A retrospective single center experience. Leuk. Res. 2017, 59, 12–19. [Google Scholar] [CrossRef]
- Lieu, Y.K.; Reddy, E.P. Impaired adult myeloid progenitor CMP and GMP cell function in conditional c-myb-knockout mice. Cell Cycle 2012, 11, 3504–3512. [Google Scholar] [CrossRef]
- Sharma, N.D.; Keewan, E.; Matlawska-Wasowska, K. Metabolic Reprogramming and Cell Adhesion in Acute Leukemia Adaptation to the CNS Niche. Front. Cell Dev. Biol. 2021, 9, 767510. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Li, G.; Wang, L.; Zhao, Y.; Zheng, B.; Lin, F.; Xie, L. Exosomal miR-320d promotes angiogenesis and colorectal cancer metastasis via targeting GNAI1 to affect the JAK2/STAT3 signaling pathway. Cell Death Dis. 2024, 15, 913. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, Z.; Li, R.; Wang, H. Tumor-Derived Exosomal circ_0020095 Promotes Colon Cancer Cell Proliferation and Metastasis by Inhibiting M1 Macrophage Polarization. J. Biochem. Mol. Toxicol. 2025, 39, e70225. [Google Scholar] [CrossRef]
- Ludwig, N.; Yerneni, S.S.; Azambuja, J.H.; Pietrowska, M.; Widlak, P.; Hinck, C.S.; Gluszko, A.; Szczepanski, M.J.; Karmer, T.; Kallinger, I.; et al. TGFbeta(+) small extracellular vesicles from head and neck squamous cell carcinoma cells reprogram macrophages towards a pro-angiogenic phenotype. J. Extracell. Vesicles 2022, 11, e12294. [Google Scholar] [CrossRef]
- Pimenta, D.B.; Varela, V.A.; Datoguia, T.S.; Caraciolo, V.B.; Lopes, G.H.; Pereira, W.O. The Bone Marrow Microenvironment Mechanisms in Acute Myeloid Leukemia. Front. Cell Dev. Biol. 2021, 9, 764698. [Google Scholar] [CrossRef]
- Danielpour, D. Advances and Challenges in Targeting TGF-beta Isoforms for Therapeutic Intervention of Cancer: A Mechanism-Based Perspective. Pharmaceuticals 2024, 17, 533. [Google Scholar] [CrossRef]
- Syed, V. TGF-beta Signaling in Cancer. J. Cell Biochem. 2016, 117, 1279–1287. [Google Scholar] [CrossRef]
- Raimondo, S.; Saieva, L.; Corrado, C.; Fontana, S.; Flugy, A.; Rizzo, A.; De Leo, G.; Alessandro, R. Chronic myeloid leukemia-derived exosomes promote tumor growth through an autocrine mechanism. Cell Commun. Signal. 2015, 13, 8. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, J.; Chen, X.; Zhang, Y.; Xu, M.; Yang, Z. The regulation of cancer cell migration by lung cancer cell-derived exosomes through TGF-beta and IL-10. Oncol. Lett. 2016, 11, 1527–1530. [Google Scholar] [CrossRef]
- Naji, N.S.; Sathish, M.; Karantanos, T. Inflammation and Related Signaling Pathways in Acute Myeloid Leukemia. Cancers 2024, 16, 3974. [Google Scholar] [CrossRef]
- Shi, X.; Yang, J.; Deng, S.; Xu, H.; Wu, D.; Zeng, Q.; Wang, S.; Hu, T.; Wu, F.; Zhou, H. TGF-beta signaling in the tumor metabolic microenvironment and targeted therapies. J. Hematol. Oncol. 2022, 15, 135. [Google Scholar] [CrossRef]
- Lian, G.Y.; Wang, Q.M.; Mak, T.S.; Huang, X.R.; Yu, X.Q.; Lan, H.Y. Inhibition of tumor invasion and metastasis by targeting TGF-beta-Smad-MMP2 pathway with Asiatic acid and Naringenin. Mol. Ther. Oncolytics 2021, 20, 277–289. [Google Scholar] [CrossRef]
- Ma, Y.; Kuang, Y.; Bo, W.; Liang, Q.; Zhu, W.; Cai, M.; Tian, Z. Exercise Training Alleviates Cardiac Fibrosis through Increasing Fibroblast Growth Factor 21 and Regulating TGF-beta1-Smad2/3-MMP2/9 Signaling in Mice with Myocardial Infarction. Int. J. Mol. Sci. 2021, 22, 12341. [Google Scholar] [CrossRef]
- Khushman, M.; Bhardwaj, A.; Patel, G.K.; Laurini, J.A.; Roveda, K.; Tan, M.C.; Patton, M.C.; Singh, S.; Taylor, W.; Singh, A.P. Exosomal Markers (CD63 and CD9) Expression Pattern Using Immunohistochemistry in Resected Malignant and Nonmalignant Pancreatic Specimens. Pancreas 2017, 46, 782–788. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gogenur, I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Manara, M.C.; Fiori, V.; Sparti, A.; Scotlandi, K. CD99: A Key Regulator in Immune Response and Tumor Microenvironment. Biomolecules 2025, 15, 632. [Google Scholar] [CrossRef]
- Takamatsu, H.; Kumanogoh, A. Diverse roles for semaphorin-plexin signaling in the immune system. Trends Immunol. 2012, 33, 127–135. [Google Scholar] [CrossRef]
- Shang, Y.; Smith, S.; Hu, X. Role of Notch signaling in regulating innate immunity and inflammation in health and disease. Protein Cell 2016, 7, 159–174. [Google Scholar] [CrossRef]
- Zhao, L.; Li, T.; Zhou, Y.; Wang, P.; Luo, L. Monoclonal antibody targeting CEACAM1 enhanced the response to anti-PD1 immunotherapy in non-small cell lung cancer. Int. Immunopharmacol. 2024, 143 Pt. 2, 113395. [Google Scholar] [CrossRef]
- Sergazy, S.; Seydahmetova, R.; Gulyayev, A.; Shulgau, Z.; Aljofan, M. The Role of Exosomes in Cancer Progression and Therapy. Biology 2025, 14, 27. [Google Scholar] [CrossRef]
- Mir, R.; Baba, S.K.; Elfaki, I.; Algehainy, N.; Alanazi, M.A.; Altemani, F.H.; Tayeb, F.J.; Barnawi, J.; Husain, E.; Bedaiwi, R.I.; et al. Unlocking the Secrets of Extracellular Vesicles: Orchestrating Tumor Microenvironment Dynamics in Metastasis, Drug Resistance, and Immune Evasion. J. Cancer 2024, 15, 6383–6415. [Google Scholar] [CrossRef]
- Boyiadzis, M.; Whiteside, T.L. Exosomes in acute myeloid leukemia inhibit hematopoiesis. Curr. Opin. Hematol. 2018, 25, 279–284. [Google Scholar] [CrossRef]
- Boyiadzis, M.; Whiteside, T.L. Plasma-Deriv. Exosomes Acute Myeloid Leuk. Detect. Minimal Residual Dis.: Are we ready? Expert. Rev. Mol. Diagn. 2016, 16, 623–629. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, J.Q.; Liu, T.J.; Chen, Y.L.; Ma, Z.K.; Fan, Y.Z.; Wang, Z.X.; Xu, S.; Wang, K.; Wang, X.Y.; et al. Knocking down AR promotes osteoblasts to recruit prostate cancer cells by altering exosomal circ-DHPS/miR-214-3p/CCL5 pathway. Asian J. Androl. 2024, 26, 195–204. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, W.; Li, B.; Zou, J.; Li, Y.; Xiao, Y.; He, Y.; Yoshida, S.; Zhou, Y. Exosomal non-coding RNAs in angiogenesis: Functions, mechanisms and potential clinical applications. Heliyon 2023, 9, e18626. [Google Scholar] [CrossRef]
- Lu, Y.; Zheng, J.; Lin, P.; Lin, Y.; Zheng, Y.; Mai, Z.; Chen, X.; Xia, T.; Zhao, X.; Cui, L. Tumor Microenvironment-Derived Exosomes: A Double-Edged Sword for Advanced T Cell-Based Immunotherapy. ACS Nano 2024, 18, 27230–27260. [Google Scholar] [CrossRef]
- Shehata, M.; Schwarzmeier, J.D.; Hilgarth, M.; Hubmann, R.; Duechler, M.; Gisslinger, H. TGF-beta1 induces bone marrow reticulin fibrosis in hairy cell leukemia. J. Clin. Investig. 2004, 113, 676–685. [Google Scholar] [CrossRef]
- Ganesan, S.; Awan-Toor, S.; Guidez, F.; Maslah, N.; Rahimy, R.; Aoun, C.; Gou, P.; Guiguen, C.; Soret, J.; Ravdan, O.; et al. Comprehensive analysis of mesenchymal cells reveals a dysregulated TGF-beta/WNT/HOXB7 axis in patients with myelofibrosis. JCI Insight 2024, 9, e173665. [Google Scholar] [CrossRef]
- Naka, K.; Hoshii, T.; Muraguchi, T.; Tadokoro, Y.; Ooshio, T.; Kondo, Y.; Nakao, S.; Motoyama, N.; Hirao, A. TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature 2010, 463, 676–680. [Google Scholar] [CrossRef]
- Shingai, Y.; Yokota, T.; Okuzaki, D.; Sudo, T.; Ishibashi, T.; Doi, Y.; Ueda, T.; Ozawa, T.; Nakai, R.; Tanimura, A.; et al. Autonomous TGFbeta signaling induces phenotypic variation in human acute myeloid leukemia. Stem Cells 2021, 39, 723–736. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, L.; Zhang, B.; Li, J.; Dou, X.; Zhao, R.C. TGF-beta1-induced PI3K/Akt/NF-kappaB/MMP9 signalling pathway is activated in Philadelphia chromosome-positive chronic myeloid leukaemia hemangioblasts. J. Biochem. 2011, 149, 405–414. [Google Scholar] [CrossRef]
- Militi, S.; Nibhani, R.; Pook, M.; Pauklin, S. SMAD2/3-SMYD2 and developmental transcription factors cooperate with cell-cycle inhibitors to guide tissue formation. Protein Cell 2025, 16, 260–285. [Google Scholar] [CrossRef]
- Mishra, S.K.; Millman, S.E.; Zhang, L. Metabolism in acute myeloid leukemia: Mechanistic insights and therapeutic targets. Blood 2023, 141, 1119–1135. [Google Scholar] [CrossRef]
- Nistico, N.; Maisano, D.; Iaccino, E.; Vecchio, E.; Fiume, G.; Rotundo, S.; Quinto, I.; Mimmi, S. Role of Chronic Lymphocytic Leukemia (CLL)-Derived Exosomes in Tumor Progression and Survival. Pharmaceuticals 2020, 13, 244. [Google Scholar] [CrossRef]
- Wang, L.; Shi, M.; Sung, A.Y.; Yin, C.C.; Bai, Y.; Chen, M. Role of the bone marrow microenvironment in multiple myeloma: Impact of niches on drug resistance mechanisms. Semin. Diagn. Pathol. 2025, 42, 150916. [Google Scholar] [CrossRef]
- Tai, Y.L.; Chen, K.C.; Hsieh, J.T.; Shen, T.L. Exosomes in cancer development and clinical applications. Cancer Sci. 2018, 109, 2364–2374. [Google Scholar] [CrossRef]
- Krylova, S.V.; Feng, D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef]
- Taylor, A.W. Review of the activation of TGF-beta in immunity. J. Leukoc. Biol. 2009, 85, 29–33. [Google Scholar] [CrossRef]
- Teixeira, A.F.; Wang, Y.; Iaria, J.; Ten, D.P.; Zhu, H.J. Simultaneously targeting extracellular vesicle trafficking and TGF-beta receptor kinase activity blocks signaling hyperactivation and metastasis. Signal Transduct. Target. Ther. 2023, 8, 456. [Google Scholar] [CrossRef]
- Yang, C.; Robbins, P.D. The roles of tumor-derived exosomes in cancer pathogenesis. Clin. Dev. Immunol. 2011, 2011, 842849. [Google Scholar] [CrossRef]
- Li, C.; Wen, Y.; Wang, J.; Li, L.; He, Y.; Cheng, Y.; Chen, J.; Huang, J.; Ouyang, C.; Liu, Y.; et al. Human Mesenchymal Stem Cell-Derived Exosomes as Engineering Vehicles of Daunorubicin for Targeted c-Mpl+ AML Therapy. Int. J. Nanomed. 2025, 20, 5267–5289. [Google Scholar] [CrossRef]
- Matsuzaka, Y.; Tanihata, J.; Komaki, H.; Ishiyama, A.; Oya, Y.; Ruegg, U.; Takeda, S.I.; Hashido, K. Characterization and Functional Analysis of Extracellular Vesicles and Muscle-Abundant miRNAs (miR-1, miR-133a, and miR-206) in C2C12 Myocytes and mdx Mice. PLoS ONE 2016, 11, e0167811. [Google Scholar] [CrossRef]
- Aggarwal, R.; Shao, A.; Potel, K.N.; So, S.W.; Swingen, C.M.; Wright, C.A.; Hocum, S.L.; McFalls, E.O.; Butterick, T.A.; Kelly, R.F. Stem cell-derived exosome patch with coronary artery bypass graft restores cardiac function in chronically ischemic porcine myocardium. J. Thorac. Cardiovasc. Surg. 2023, 166, e512–e530. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Q.; Zan, J.; Tian, J.; Zhang, Y.; Wu, F.; Zhao, H.; Peng, Q.; Liu, S.; Chen, Q.; et al. Proteomic Analysis of Human Follicular Fluid-Derived Exosomes Reveals That Insufficient Folliculogenesis in Aging Women is Associated With Infertility. Mol. Cell. Proteom. 2025, 24, 100930. [Google Scholar] [CrossRef]
- Lundy, D.J.; Liao, C.T. Extracellular Vesicles in Aging and Age-Related Diseases. How Important are they? Adv. Biol. 2025, 9, e2400656. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, J. TGF-β-Enriched Exosomes from Acute Myeloid Leukemia Activate Smad2/3–MMP2 and ERK1/2 Signaling to Promote Leukemic Cell Proliferation, Migration, and Immune Modulation. Curr. Issues Mol. Biol. 2025, 47, 690. https://doi.org/10.3390/cimb47090690
Jia J. TGF-β-Enriched Exosomes from Acute Myeloid Leukemia Activate Smad2/3–MMP2 and ERK1/2 Signaling to Promote Leukemic Cell Proliferation, Migration, and Immune Modulation. Current Issues in Molecular Biology. 2025; 47(9):690. https://doi.org/10.3390/cimb47090690
Chicago/Turabian StyleJia, Jie. 2025. "TGF-β-Enriched Exosomes from Acute Myeloid Leukemia Activate Smad2/3–MMP2 and ERK1/2 Signaling to Promote Leukemic Cell Proliferation, Migration, and Immune Modulation" Current Issues in Molecular Biology 47, no. 9: 690. https://doi.org/10.3390/cimb47090690
APA StyleJia, J. (2025). TGF-β-Enriched Exosomes from Acute Myeloid Leukemia Activate Smad2/3–MMP2 and ERK1/2 Signaling to Promote Leukemic Cell Proliferation, Migration, and Immune Modulation. Current Issues in Molecular Biology, 47(9), 690. https://doi.org/10.3390/cimb47090690





