Correlation Between In Silico Docking/Simulation Results and In Vitro MAGL Inhibition Potency of Selected Triterpenes
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Molecular Docking and Molecular Dynamics Simulations
2.3. Correlation of Affinity, Free Energy of Binding, and Docking Scores and IC50 of MAGL Inhibition Data from In Vitro Studies
2.4. In Silico Prediction of the Pharmacokinetic Parameters
3. Results
3.1. Articles That Reported the Evaluation of the Inhibition of MAGL Activity by Triterpenes, Including Euphol and/or Pristimerin
3.2. Molecular Docking and Molecular Dynamic Simulations of Triterpenes on MAGL
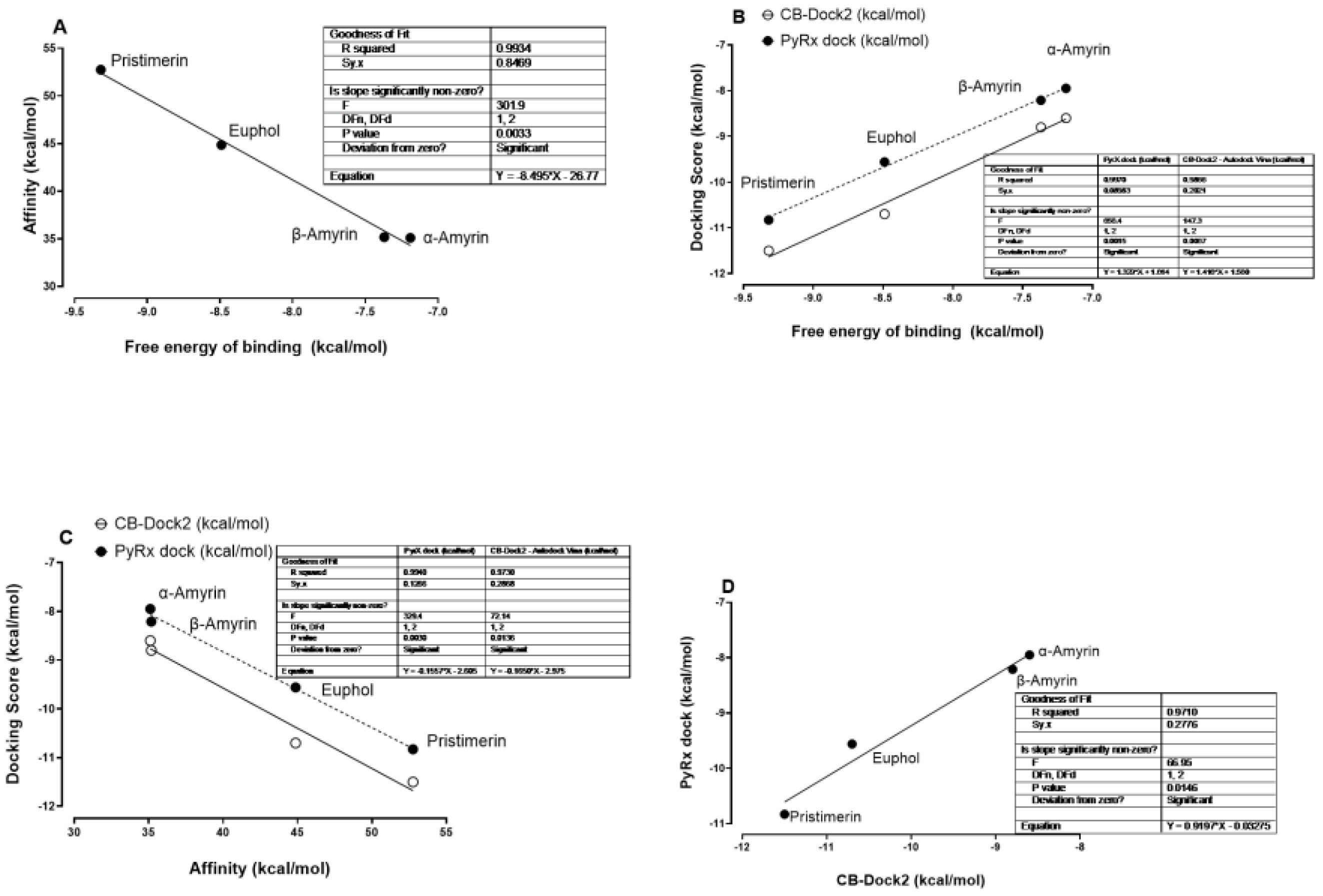
3.3. Comparison of Affinity, Free Energy of Binding and Docking Scores by Both PyRx-0.8, and CB-Dock2 and IC50 of MAGL Inhibition Data from In Vitro Studies
3.4. Results of In Silico Prediction of Pharmacokinetic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
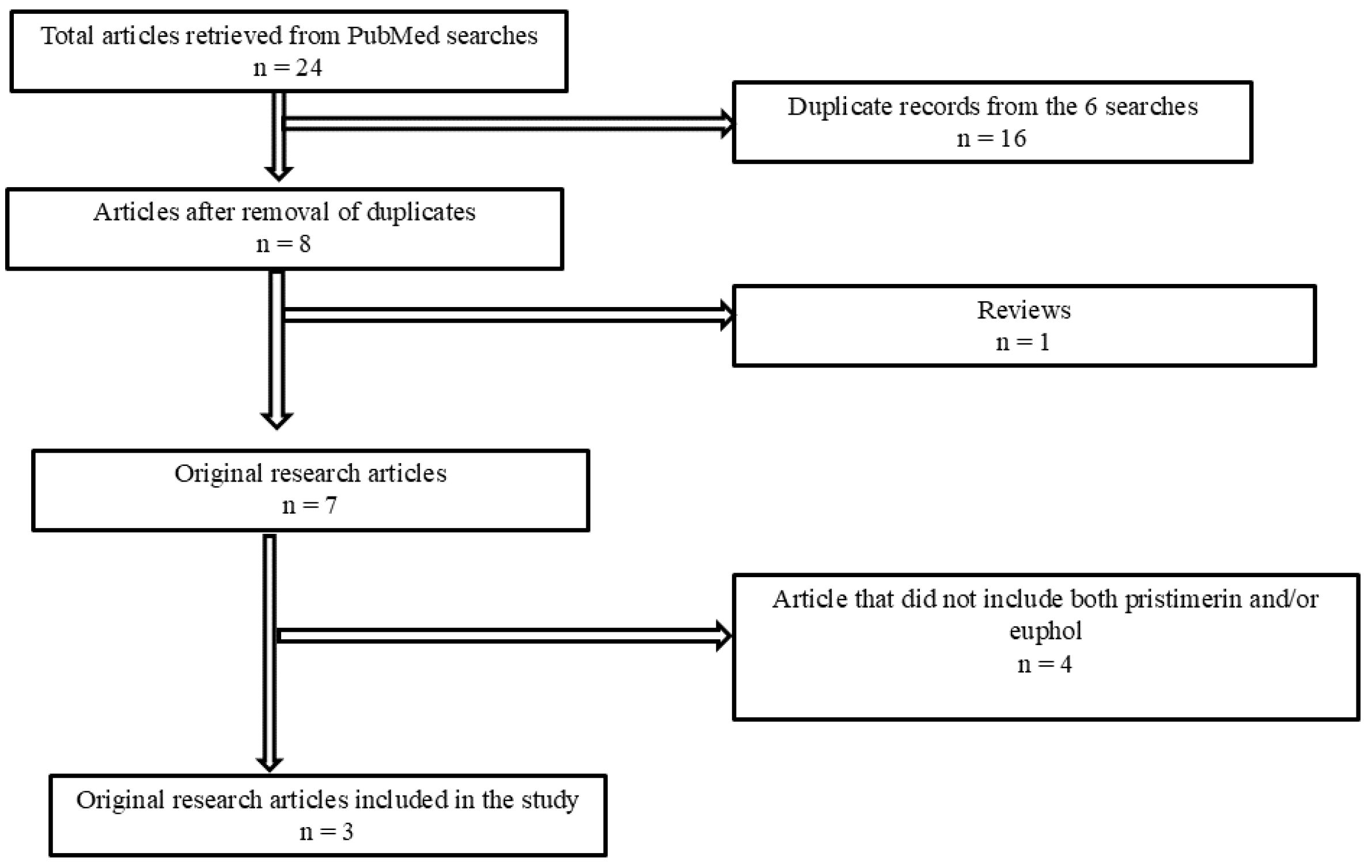
Appendix B
| Article Number | Primary Reason for Exclusion | Article Type | Reference |
|---|---|---|---|
| 1. | Review article | Review | [25] |
| 2. | Did not evaluate MAGL inhibition by either pristimerin or euphol | Original research | [40] |
| 3. | Did not evaluate MAGL inhibition by either pristimerin or euphol | Original research | [41] |
| 4. | Did not evaluate MAGL inhibition by either pristimerin or euphol | Original research | [42] |
| 5. | Did not evaluate MAGL inhibition by either pristimerin or euphol | Original research | [43] |
Appendix C
| Model Name | α-Amyrin | β-Amyrin | Euphol | Pristimerin | |
|---|---|---|---|---|---|
| Absorption | Water solubility (log mol/L) | −6.804 | −6.84 | −7.53 | −5.902 |
| Caco2 permeability (log Papp in 10–6 cm/s) | 1.288 | 1.287 | 1.218 | 1.077 | |
| Intestinal absorption (human) (% Absorbed) | 97.574 | 97.246 | 93.056 | 100 | |
| Skin Permeability (log Kp) | −2.898 | −2.894 | −2.933 | −2.649 | |
| P-glycoprotein substrate (Yes/No) | No | No | No | No | |
| P-glycoprotein I inhibitor (Yes/No) | Yes | Yes | Yes | Yes | |
| P-glycoprotein II inhibitor (Yes/No) | Yes | Yes | Yes | Yes | |
| Distribution | VDss (human) (log L/kg) | 0.443 | 0.446 | 0.692 | −0.353 |
| Fraction unbound (human) | 0 | 0 | 0 | 0 | |
| BBB permeability (log BB) | 0.735 | 0.728 | 0.702 | −0.344 | |
| CNS permeability (log PS) | −1.971 | −1.971 | −2.247 | −1.102 | |
| Metabolism | CYP2D6 substrate (Yes/No) | No | No | No | No |
| CYP3A4 substrate (Yes/No) | Yes | Yes | Yes | Yes | |
| CYP1A2 inhibitor (Yes/No) | No | No | No | No | |
| CYP2C19 inhibitor (Yes/No) | No | No | No | No | |
| CYP2C9 inhibitor (Yes/No) | No | No | No | No | |
| CYP2D6 inhibitor (Yes/No) | No | No | No | No | |
| CYP3A4 inhibitor (Yes/No) | No | No | No | No | |
| Execretion | Total Clearance (log mL/min/kg) | 0.119 | −0.044 | 0.403 | −0.034 |
| Renal OCT2 substrate (Yes/No) | No | No | No | No | |
| Toxicity | Max. tolerated dose (human) (log mg/kg/day) | 0.203 | 0.212 | −0.35 | 0.324 |
| Oral Rat Acute Toxicity (LD50) (mol/kg) | 2.156 | 2.169 | 1.867 | 3.234 | |
| Oral Rat Chronic Toxicity (LOAEL) (log mg/kg_bw/day) | 0.908 | 0.926 | 0.775 | 1.757 | |
| Hepatotoxicity (Yes/No) | No | No | No | No | |
| Skin Sensitisation (Yes/No) | No | No | No | No |
References
- Dinh, T.P.; Carpenter, D.; Leslie, F.M.; Freund, T.F.; Katona, I.; Sensi, S.L.; Kathuria, S.; Piomelli, D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl. Acad. Sci. USA 2002, 99, 10819–10824. [Google Scholar] [CrossRef]
- Saario, S.M.; Savinainen, J.R.; Laitinen, J.T.; Jarvinen, T.; Niemi, R. Monoglyceride lipase-like enzymatic activity is responsible for hydrolysis of 2-arachidonoylglycerol in rat cerebellar membranes. Biochem. Pharmacol. 2004, 67, 1381–1387. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Inhibiting degradation of 2-arachidonoylglycerol as a therapeutic strategy for neurodegenerative diseases. Pharmacol. Ther. 2023, 244, 108394. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kishimoto, S.; Oka, S.; Gokoh, M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog. Lipid Res. 2006, 45, 405–446. [Google Scholar] [CrossRef] [PubMed]
- Hashimotodani, Y.; Ohno-Shosaku, T.; Tanimura, A.; Kita, Y.; Sano, Y.; Shimizu, T.; Di Marzo, V.; Kano, M. Acute inhibition of diacylglycerol lipase blocks endocannabinoid-mediated retrograde signalling: Evidence for on-demand biosynthesis of 2-arachidonoylglycerol. J. Physiol. 2013, 591, 4765–4776. [Google Scholar] [CrossRef]
- Thomas, A.; Okine, B.N.; Finn, D.P.; Masocha, W. Peripheral deficiency and antiallodynic effects of 2-arachidonoyl glycerol in a mouse model of paclitaxel-induced neuropathic pain. Biomed. Pharmacother. 2020, 129, 110456. [Google Scholar] [CrossRef]
- Al-Romaiyan, A.; Masocha, W. Pristimerin, a triterpene that inhibits monoacylglycerol lipase activity, prevents the development of paclitaxel-induced allodynia in mice. Front. Pharmacol. 2022, 13, 944502. [Google Scholar] [CrossRef]
- Curry, Z.A.; Wilkerson, J.L.; Bagdas, D.; Kyte, S.L.; Patel, N.; Donvito, G.; Mustafa, M.A.; Poklis, J.L.; Niphakis, M.J.; Hsu, K.L.; et al. Monoacylglycerol Lipase Inhibitors Reverse Paclitaxel-Induced Nociceptive Behavior and Proinflammatory Markers in a Mouse Model of Chemotherapy-Induced Neuropathy. J. Pharmacol. Exp. Ther. 2018, 366, 169–183. [Google Scholar] [CrossRef]
- King, A.R.; Dotsey, E.Y.; Lodola, A.; Jung, K.M.; Ghomian, A.; Qiu, Y.; Fu, J.; Mor, M.; Piomelli, D. Discovery of potent and reversible monoacylglycerol lipase inhibitors. Chem. Biol. 2009, 16, 1045–1052. [Google Scholar] [CrossRef]
- Chicca, A.; Marazzi, J.; Gertsch, J. The antinociceptive triterpene beta-amyrin inhibits 2-arachidonoylglycerol (2-AG) hydrolysis without directly targeting cannabinoid receptors. Br. J. Pharmacol. 2012, 167, 1596–1608. [Google Scholar] [CrossRef]
- Shen, J.; Kai, J.; Tang, Y.; Zhang, L.; Su, S.; Duan, J.A. The Chemical and Biological Properties of Euphorbia kansui. Am. J. Chin. Med. 2016, 44, 253–273. [Google Scholar] [CrossRef]
- Yuan, X.; Yan, Q.; Hu, H.; He, X.-P.; Wang, L.-X.; Liu, Y.-C.; Liu, Y.; Guo, K.; Li, S.-H. Bioactive triterpenoids from the traditional Chinese medicine Swertia mileensis. Phytochem. Lett. 2023, 55, 1–5. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z.J.; Feng, Y.J.; Wang, D.Y. Chemical constituents of roots of Celastrus orbiculatus. Zhong Yao Cai 2013, 36, 569–572. [Google Scholar] [PubMed]
- Zhang, Q.; Zhou, Q.R.; Lou, J.W.; Chen, P.D.; Yao, W.F.; Tao, W.W.; Tang, Y.P.; Dai, G.C.; Wang, K.; Zhang, L. Chemical Constituents from Euphorbia kansui. Molecules 2017, 22, 2176. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Li, M.; Jiang, Q.; Ke, H.; Liang, Q.; Zeng, L.F.; Fang, J. Evolving Roles of Natural Terpenoids From Traditional Chinese Medicine in the Treatment of Osteoporosis. Front. Endocrinol. 2022, 13, 901545. [Google Scholar] [CrossRef]
- Dutra, R.C.; Simao da Silva, K.A.; Bento, A.F.; Marcon, R.; Paszcuk, A.F.; Meotti, F.C.; Pianowski, L.F.; Calixto, J.B. Euphol, a tetracyclic triterpene produces antinociceptive effects in inflammatory and neuropathic pain: The involvement of cannabinoid system. Neuropharmacology 2012, 63, 593–605. [Google Scholar] [CrossRef]
- Maia, J.L.; Lima-Junior, R.C.; Melo, C.M.; David, J.P.; David, J.M.; Campos, A.R.; Santos, F.A.; Rao, V.S. Oleanolic acid, a pentacyclic triterpene attenuates capsaicin-induced nociception in mice: Possible mechanisms. Pharmacol. Res. 2006, 54, 282–286. [Google Scholar] [CrossRef]
- Dato, F.M.; Neudorfl, J.M.; Gutschow, M.; Goldfuss, B.; Pietsch, M. omega-Quinazolinonylalkyl aryl ureas as reversible inhibitors of monoacylglycerol lipase. Bioorg. Chem. 2020, 94, 103352. [Google Scholar] [CrossRef]
- Di Stefano, M.; Masoni, S.; Bononi, G.; Poli, G.; Galati, S.; Gado, F.; Manzi, S.; Vagaggini, C.; Brai, A.; Caligiuri, I.; et al. Design, synthesis, ADME and biological evaluation of benzylpiperidine and benzylpiperazine derivatives as novel reversible monoacylglycerol lipase (MAGL) inhibitors. Eur. J. Med. Chem. 2024, 263, 115916. [Google Scholar] [CrossRef]
- Hernandez-Torres, G.; Cipriano, M.; Heden, E.; Bjorklund, E.; Canales, A.; Zian, D.; Feliu, A.; Mecha, M.; Guaza, C.; Fowler, C.J.; et al. A reversible and selective inhibitor of monoacylglycerol lipase ameliorates multiple sclerosis. Angew. Chem. Int. Ed. Engl. 2014, 53, 13765–13770. [Google Scholar] [CrossRef]
- Kochnev, Y.; Ahmed, M.; Maldonado, A.M.; Durrant, J.D. MolModa: Accessible and secure molecular docking in a web browser. Nucleic Acids Res. 2024, 52, W498–W506. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y. Protein-Ligand Blind Docking Using CB-Dock2. Methods Mol. Biol. 2024, 2714, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Chaput, L.; Villoutreix, B.O. Virtual screening web servers: Designing chemical probes and drug candidates in the cyberspace. Brief. Bioinform. 2021, 22, 1790–1818. [Google Scholar] [CrossRef] [PubMed]
- Masocha, W.; Aly, E.; Albaloushi, A.; Al-Romaiyan, A. Licofelone, a Dual COX/LOX Inhibitor, Ameliorates Paclitaxel-Induced Mechanical Allodynia in Rats in a Cannabinoid Receptor-Dependent Manner. Biomedicines 2024, 12, 1545. [Google Scholar] [CrossRef] [PubMed]
- Bugnon, M.; Rohrig, U.F.; Goullieux, M.; Perez, M.A.S.; Daina, A.; Michielin, O.; Zoete, V. SwissDock 2024: Major enhancements for small-molecule docking with Attracting Cavities and AutoDock Vina. Nucleic Acids Res. 2024, 52, W324–W332. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [CrossRef]
- Soliman, A.M.; Ghorab, M.M.; Higgins, M.; Dinkova-Kostova, A.T.; Korany, M.; Amin, M.A.; Khedr, M.A.; Sakr, T.M. NQO1 induction and radiation-based biodistribution study of a new quinoline derivative identified in a screen of 6,8-diiodoquinazolinone sulfonamide conjugates. Eur. J. Med. Chem. 2025, 296, 117855. [Google Scholar] [CrossRef]
- Chen, R.Z.; Yang, F.; Zhang, M.; Sun, Z.G.; Zhang, N. Cellular and Molecular Mechanisms of Pristimerin in Cancer Therapy: Recent Advances. Front. Oncol. 2021, 11, 671548. [Google Scholar] [CrossRef]
- Sun, J.; Tian, Z.; Wu, J.; Li, J.; Wang, Q.; Huang, S.; Wang, M. Pristimerin Exerts Pharmacological Effects Through Multiple Signaling Pathways: A Comprehensive Review. Drug Des. Devel. Ther. 2024, 18, 1673–1694. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef]
- Munawar, N.; Oriowo, M.A.; Masocha, W. Antihyperalgesic Activities of Endocannabinoids in a Mouse Model of Antiretroviral-Induced Neuropathic Pain. Front. Pharmacol. 2017, 8, 136. [Google Scholar] [CrossRef]
- Guindon, J.; Lai, Y.; Takacs, S.M.; Bradshaw, H.B.; Hohmann, A.G. Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: Effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacol. Res. 2013, 67, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Scalvini, L.; Piomelli, D.; Mor, M. Monoglyceride lipase: Structure and inhibitors. Chem. Phys. Lipids 2016, 197, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Contreras, J.A.; Hellman, U.; Tornqvist, H.; Holm, C. cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. J. Biol. Chem. 1997, 272, 27218–27223. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guan, S.; E, J.; Yang, Z.; Zhang, X.; Ju, J.; Wang, S.; Zhang, H. Insight into the Inhibitory Mechanism of Aryl Formyl Piperidine Derivatives on Monoacylglycerol Lipase through Molecular Dynamics Simulations. Molecules 2022, 27, 7512. [Google Scholar] [CrossRef]
- Afzal, O.; Kumar, S.; Kumar, R.; Firoz, A.; Jaggi, M.; Bawa, S. Docking based virtual screening and molecular dynamics study to identify potential monoacylglycerol lipase inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 3986–3996. [Google Scholar] [CrossRef]
- Jha, V.; Galati, S.; Volpi, V.; Ciccone, L.; Minutolo, F.; Rizzolio, F.; Granchi, C.; Poli, G.; Tuccinardi, T. Discovery of a new ATP-citrate lyase (ACLY) inhibitor identified by a pharmacophore-based virtual screening study. J. Biomol. Struct. Dyn. 2021, 39, 3996–4004. [Google Scholar] [CrossRef]
- Parkkari, T.; Haavikko, R.; Laitinen, T.; Navia-Paldanius, D.; Rytilahti, R.; Vaara, M.; Lehtonen, M.; Alakurtti, S.; Yli-Kauhaluoma, J.; Nevalainen, T.; et al. Discovery of triterpenoids as reversible inhibitors of alpha/beta-hydrolase domain containing 12 (ABHD12). PLoS ONE 2014, 9, e98286. [Google Scholar] [CrossRef]
- Benabdelaziz, I.; Gomez-Ruiz, S.; Benkhaled, M.; Carralero, S.; Schenker, P.; Salm, A.; Gertsch, J.; Haba, H. New cycloartane-type ester triterpenes from Euphorbia pterococca and biological evaluation. Fitoterapia 2018, 127, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Veloso, C.C.; Ferreira, R.C.M.; Rodrigues, V.G.; Duarte, L.P.; Klein, A.; Duarte, I.D.; Romero, T.R.L.; Perez, A.C. Tingenone, a pentacyclic triterpene, induces peripheral antinociception due to cannabinoid receptors activation in mice. Inflammopharmacology 2018, 26, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Merali, Z.; Cayer, C.; Kent, P.; Liu, R.; Cal, V.; Harris, C.S.; Arnason, J.T. Sacred Maya incense, copal (Protium copal-Burseraceae), has antianxiety effects in animal models. J. Ethnopharmacol. 2018, 216, 63–70. [Google Scholar] [CrossRef] [PubMed]
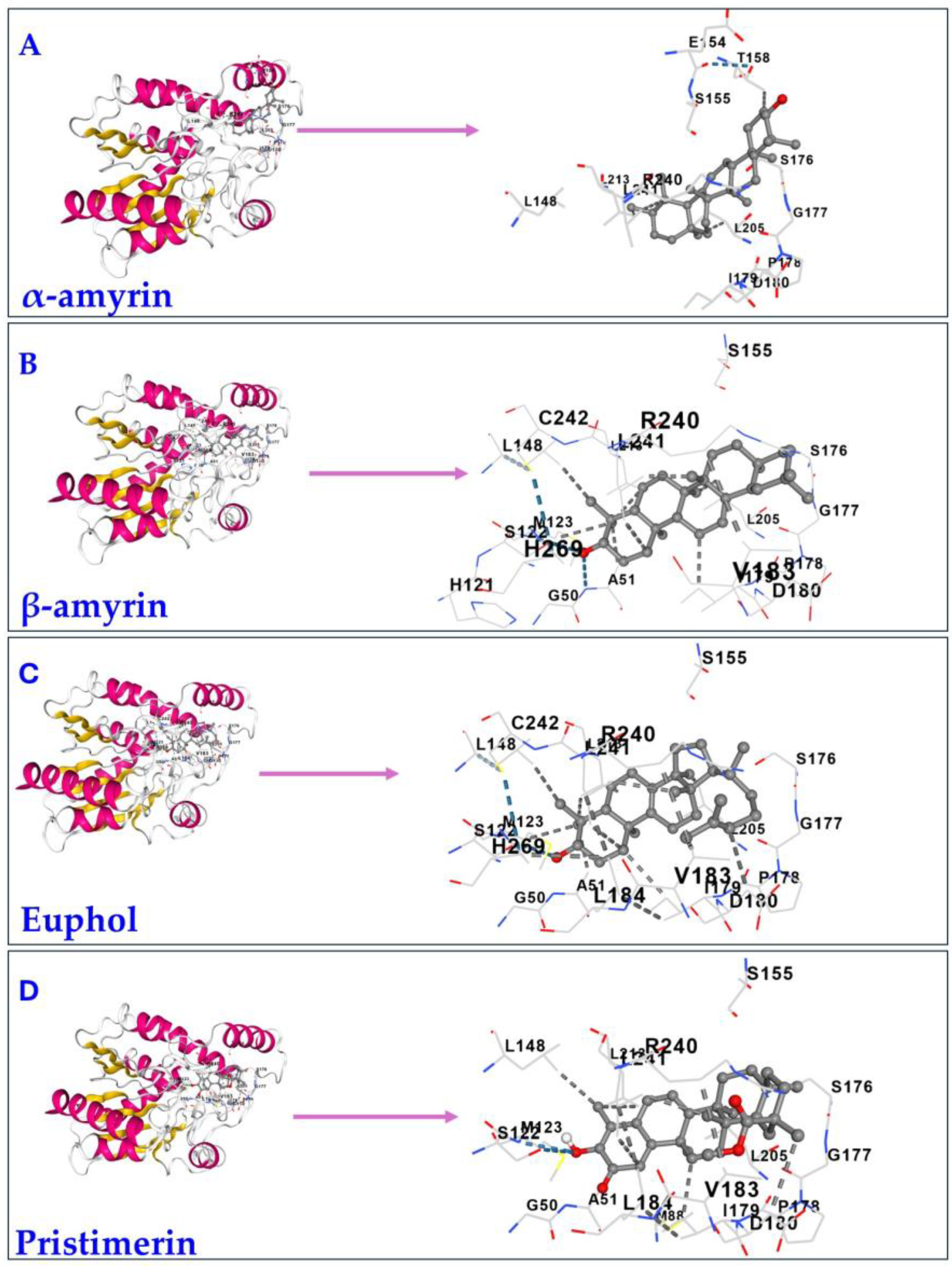
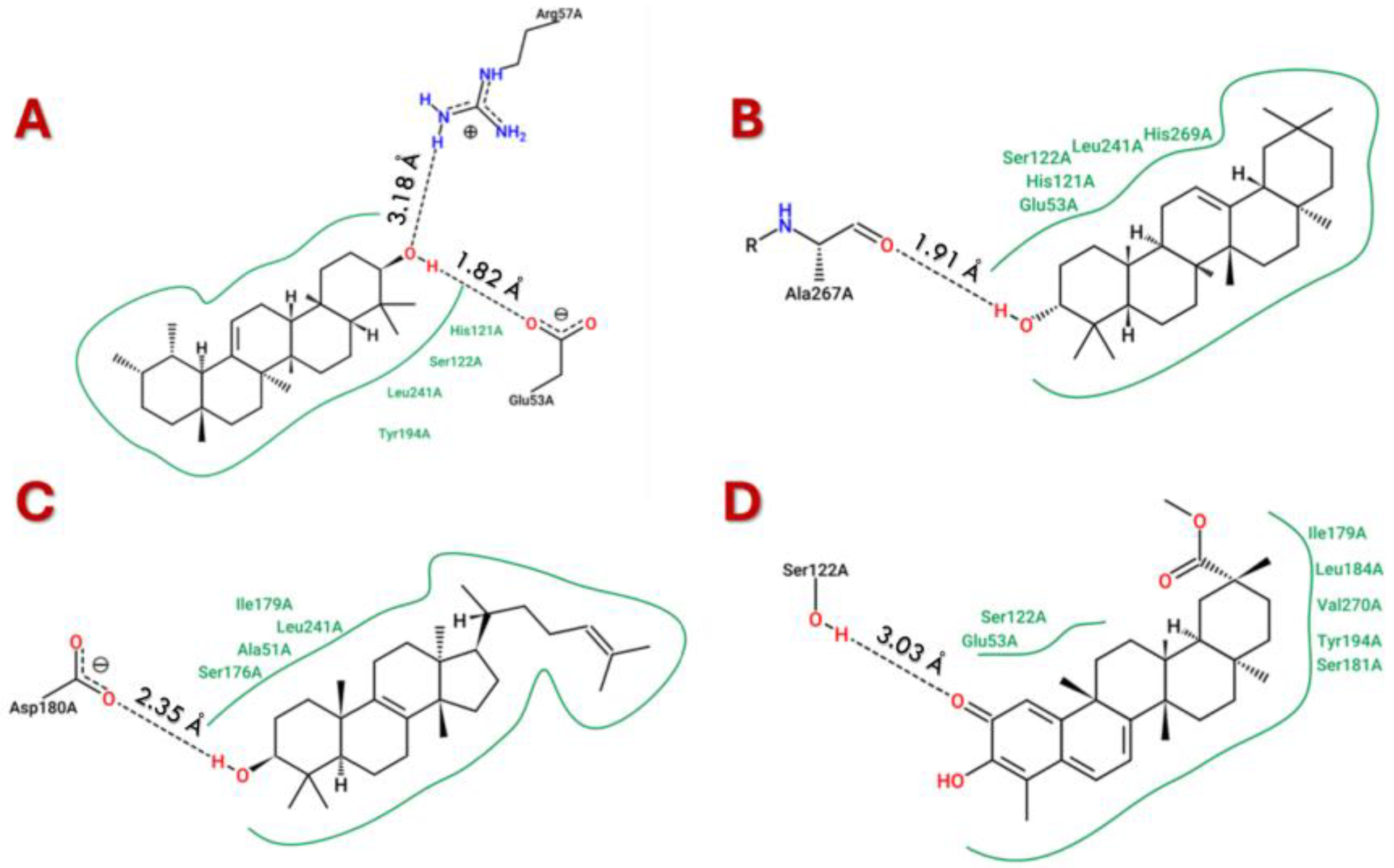
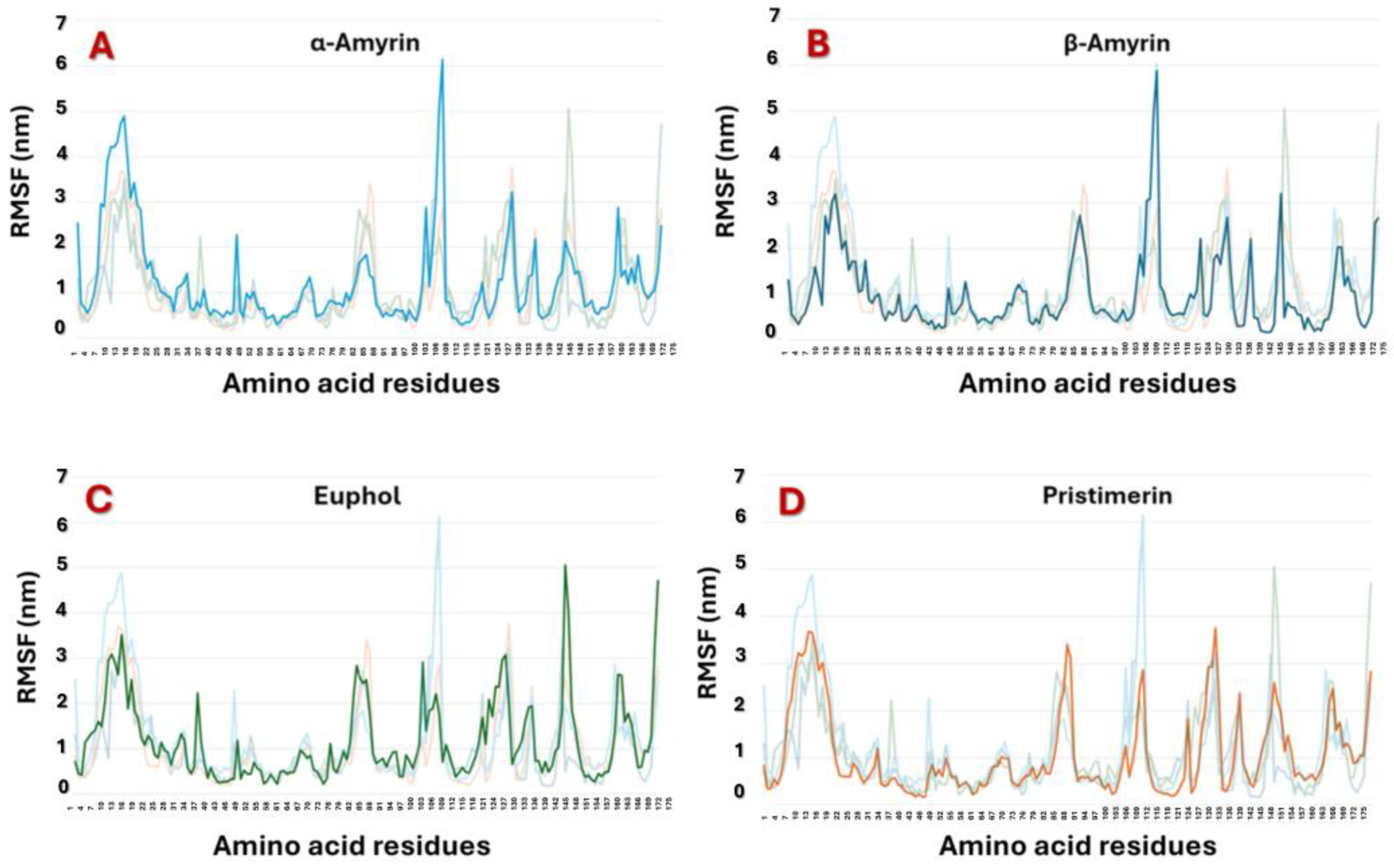

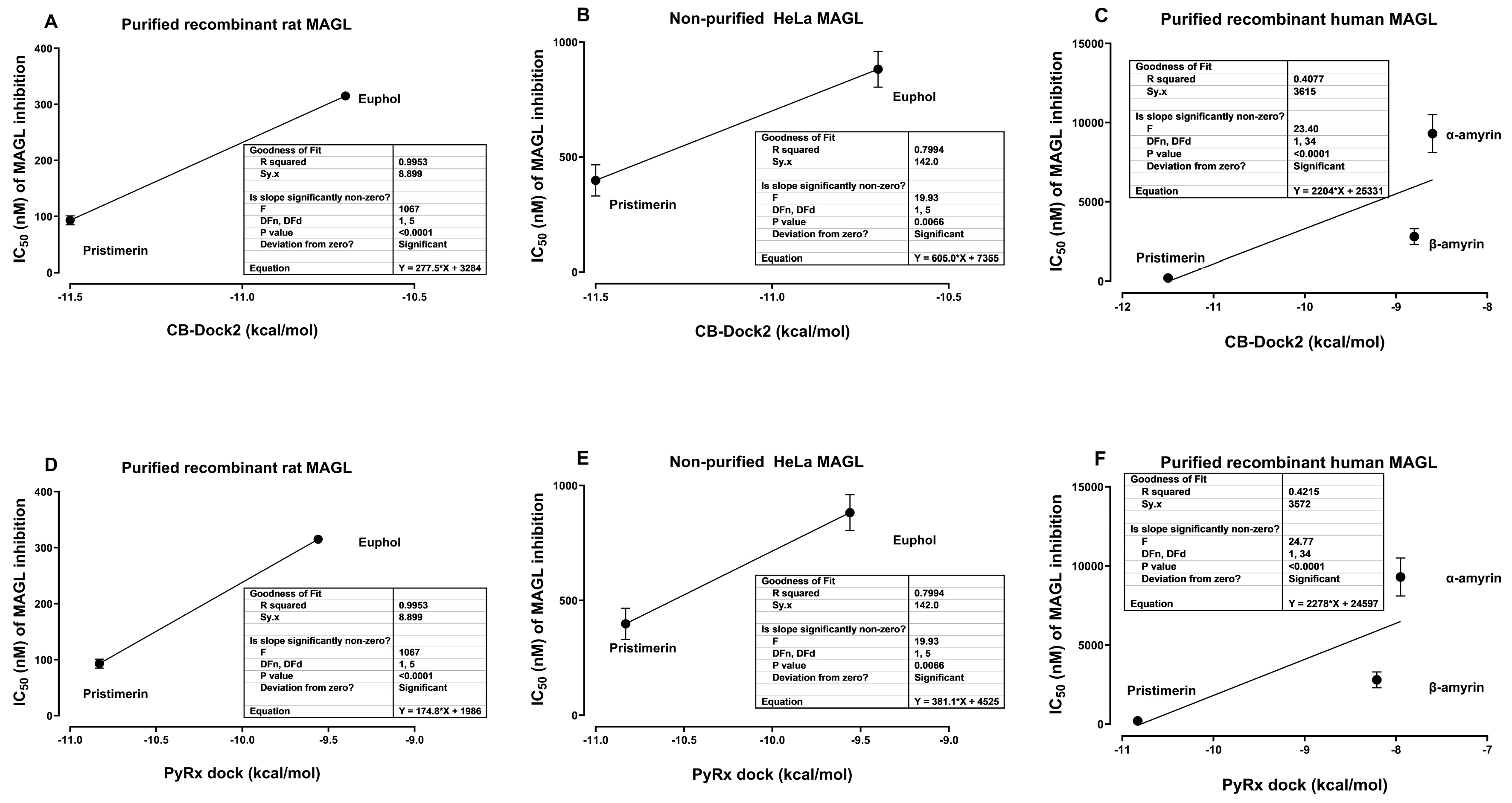
| Triterpene | IC50 * | Source/Type of MAGL | Reference |
|---|---|---|---|
| α-Amyrin | 9300 ± 1200 nM | Recombinant human MAGL | [11] |
| β-Amyrin | 2800 ± 500 nM | Recombinant human MAGL | [11] |
| Euphol | 315 ± 1 nM | Purified recombinant rat MAGL | [10] |
| 882 ± 78 nM | Non-purified (cell lysates of MAGL-transfected HeLa cells) | [10] | |
| Pristimerin | 93 ± 8 nM | Purified recombinant rat MAGL | [10] |
| 398 ± 68 nM | Non-purified (cell lysates of MGL-transfected HeLa cells | [10] | |
| 204 ± 16.2 nM | Recombinant human MAGL | [11] | |
| 130 nM | Recombinant human MAGL | [8] | |
| 1600 nM | Non-purified mouse brain | [8] | |
| 596 nM | Non-purified Mouse paw skin | [8] |
| Triterpene | Vina Score (kcal/mol) | Contact Residues |
|---|---|---|
| α-Amyrin | −8.6 | GLY50 ALA51 MET88 SER122 MET123 LEU148 ALA151 ASN152 GLU154 SER155 ALA156 THR158 PHE159 SER176 GLY177 PRO178 ILE179 ASP180 VAL183 LEU184 LEU205 PHE209 GLY210 LEU213 LEU214 ARG240 LEU241 HIS269 |
| β-Amyrin | −8.8 | GLY50 ALA51 GLY52 HIS121 SER122 MET123 LEU148 ALA151 ASN152 GLU154 SER155 ALA156 THR158 PHE159 SER176 GLY177 PRO178 ILE179 ASP180 SER181 VAL183 LEU184 LEU205 PHE209 GLY210 LEU213 LEU214 VAL217 ARG240 LEU241 CYS242 HIS269 |
| Euphol | −10.7 | GLY50 ALA51 MET88 SER122 MET123 LEU148 LEU150 ALA151 ASN152 GLU154 SER155 ALA156 THR158 PHE159 LYS160 SER176 GLY177 PRO178 ILE179 ASP180 VAL183 LEU184 LEU205 PHE209 GLY210 LEU213 LEU214 VAL217 ARG240 LEU241 CYS242 HIS269 |
| Pristimerin | −11.5 | GLY50 ALA51 MET88 SER122 MET123 LEU148 ALA151 SER155 ALA156 THR158 PHE159 SER176 GLY177 PRO178 ILE179 ASP180 VAL183 LEU184 LEU205 GLY210 LEU213 LEU214 VAL217 ARG240 LEU241 |
| Scores | α-Amyrin | β-Amyrin | Euphol | Pristimerin |
|---|---|---|---|---|
| PubChem CID | 73,170 | 73,145 | 441,678 | 159,516 |
| Free energy of binding (kcal/mol) | −7.19 | −7.37 | −8.49 | −9.32 |
| Affinity (kcal/mol) | 35.10 | 35.17 | 44.86 | 52.75 |
| PyRx-0.8 dock (kcal/mol) | −7.95 | −8.21 | −9.56 | −10.83 |
| CB-Dock2 (kcal/mol) | −8.6 | −8.8 | −10.7 | −11.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masocha, W.; Khedr, M.A. Correlation Between In Silico Docking/Simulation Results and In Vitro MAGL Inhibition Potency of Selected Triterpenes. Curr. Issues Mol. Biol. 2025, 47, 691. https://doi.org/10.3390/cimb47090691
Masocha W, Khedr MA. Correlation Between In Silico Docking/Simulation Results and In Vitro MAGL Inhibition Potency of Selected Triterpenes. Current Issues in Molecular Biology. 2025; 47(9):691. https://doi.org/10.3390/cimb47090691
Chicago/Turabian StyleMasocha, Willias, and Mohammed A. Khedr. 2025. "Correlation Between In Silico Docking/Simulation Results and In Vitro MAGL Inhibition Potency of Selected Triterpenes" Current Issues in Molecular Biology 47, no. 9: 691. https://doi.org/10.3390/cimb47090691
APA StyleMasocha, W., & Khedr, M. A. (2025). Correlation Between In Silico Docking/Simulation Results and In Vitro MAGL Inhibition Potency of Selected Triterpenes. Current Issues in Molecular Biology, 47(9), 691. https://doi.org/10.3390/cimb47090691





