Physiological Mechanisms of and Therapeutic Approaches to the Gut Microbiome and Low-Grade Inflammation in Obesity
Abstract
1. Introduction
2. Materials and Methods
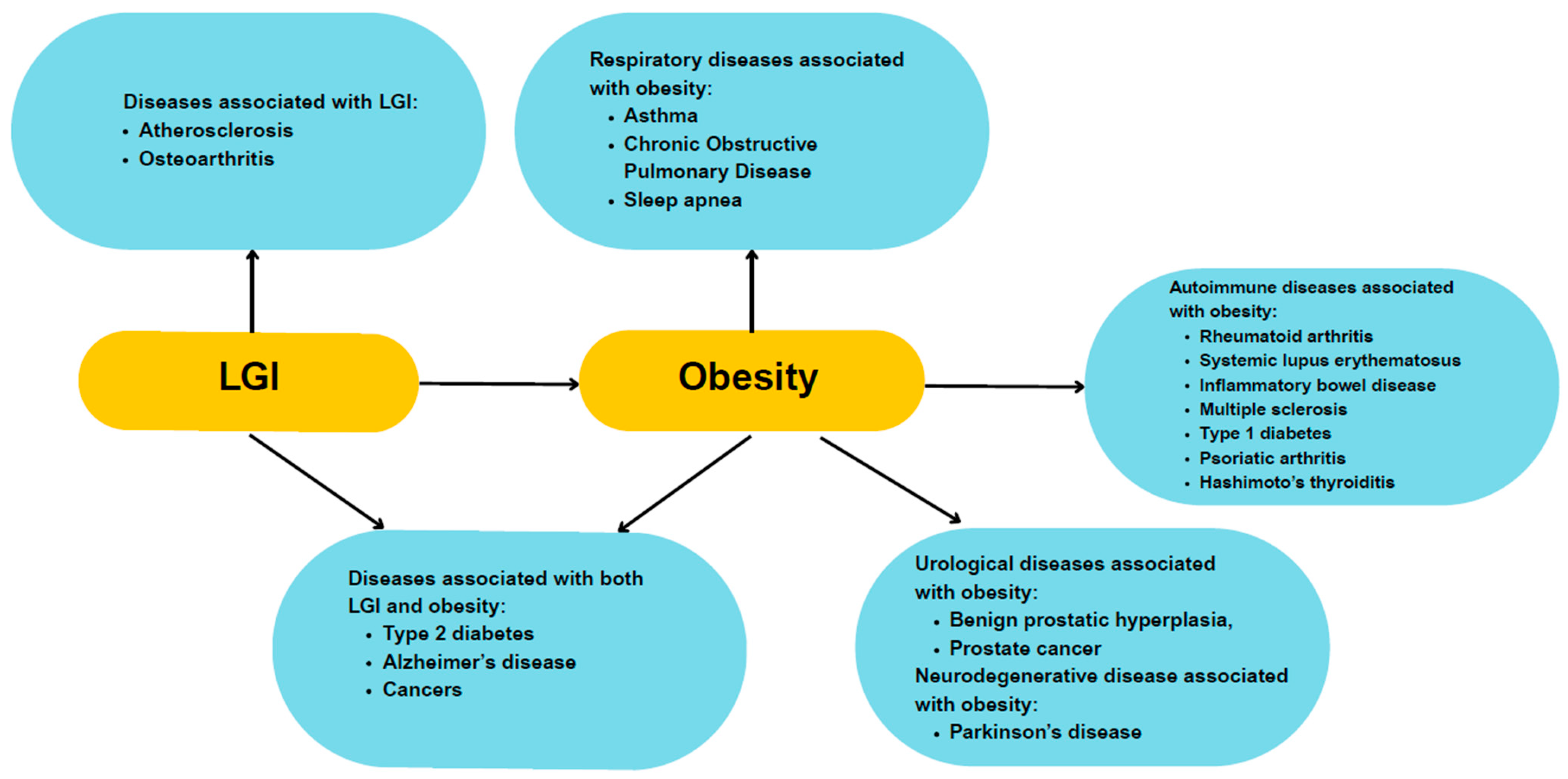
3. Low-Grade Inflammation and Its Link to Obesity
4. The Role of the Gut Microbiome in Obesity: Dysbiosis and Bacterial Metabolites
4.1. Dysbiosis in Obesity
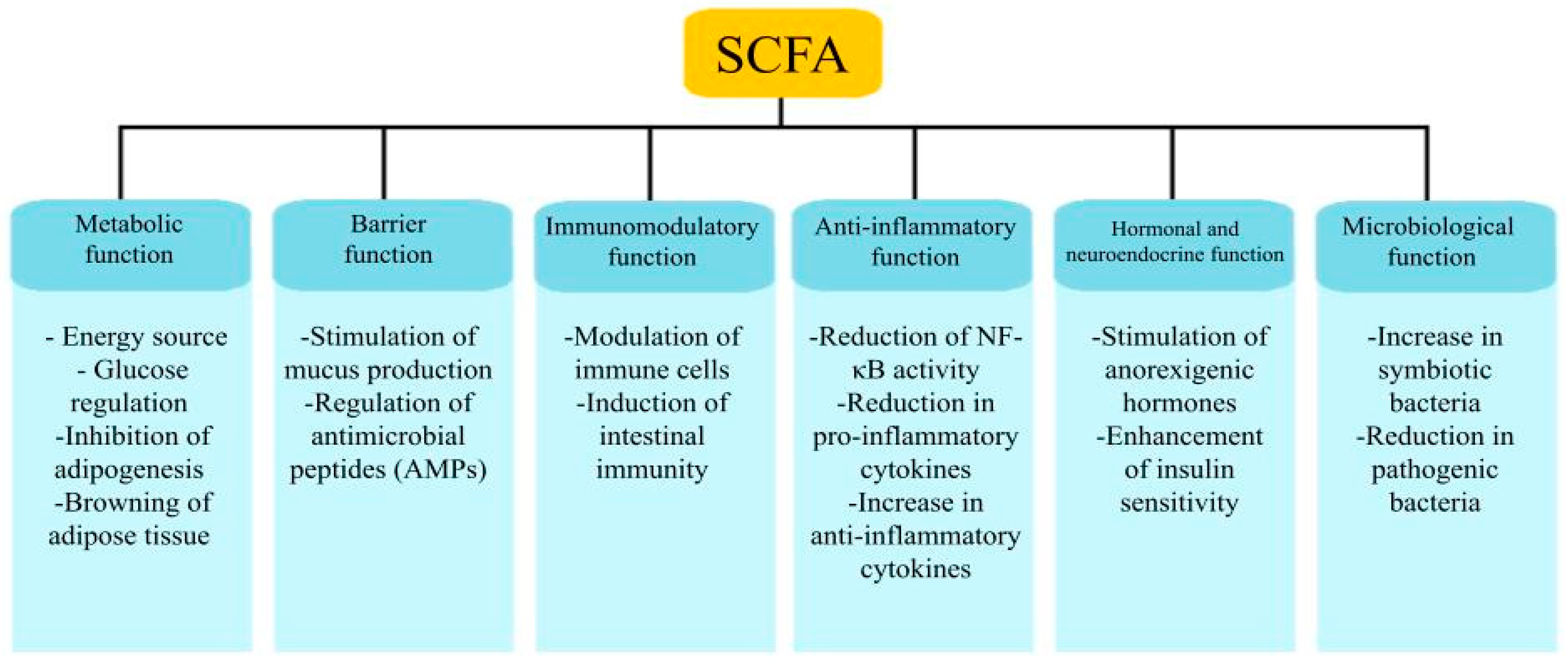
4.2. Bacterial Metabolism Products and Their Impact on Obesity
4.2.1. Carbohydrate Metabolism and Its Products
4.2.2. Lipid Metabolism
4.2.3. Protein Metabolism
5. Implications of the Gut–Microbiome–Inflammation Axis in Obesity
5.1. The Microbiome–Inflammation–Obesity Connection
5.2. Metabolic and Clinical Effects of Dysbiosis and Low-Grade Inflammation in Obesity
5.2.1. Cardiovascular Disease
5.2.2. Type 2 Diabetes and Insulin Resistance
5.2.3. Other Comorbidities
6. Therapeutic Approaches to Modulating the Gut Microbiome
6.1. Probiotics and Prebiotics
6.2. Dietary Interventions
6.3. Pharmacological Approaches
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CKD | Chronic Kidney Disease |
| CNS | Central Nervous System |
| COPD | Chronic Obstructive Pulmonary Disease |
| CRP | C-Reactive Protein |
| DCA | Deoxycholic Acid |
| GALT | Gut-Associated Lymphoid Tissue |
| GPR41 | G Protein-Coupled Receptor 41 |
| GPR43 | G Protein-Coupled Receptor 43 |
| HFD | High Fat Diet |
| IFN-γ | Interferon gamma |
| IL-1 | Interleukin-1 |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-10 | Interleukin-10 |
| IL-12 | Interleukin-12 |
| IL-22 | Interleukin-22 |
| LCA | Lithocholic Acid |
| LGI | Low-Grade Inflammation |
| LPS | Lipopolysaccharides |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NOD | Nucleotide-binding oligomerization domain |
| OS | Oxidative Stress |
| PRRs | Pattern Recognition Receptors |
| SCFAs | Short-Chain Fatty Acids |
| TGF-β | Transforming growth factor-beta |
| Th17 | T helper 17 cells |
| TLRs | Toll-Like Receptors |
| TNF-α | Tumor Necrosis Factor-alpha |
| WHO | World Health Organization |
References
- WHO Consultation on Obesity. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129 (Suppl. 2), S102–S138. [Google Scholar] [CrossRef] [PubMed]
- World Obesity Federation. World Obesity Atlas 2025. Available online: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2025 (accessed on 20 March 2025).
- Kipinoinen, T.; Toppala, S.; Rinne, J.O.; Viitanen, M.H.; Jula, A.M.; Ekblad, L.L. Association of Midlife Inflammatory Markers with Cognitive Performance at 10-Year Follow-Up. Neurology 2022, 99, e2294–e2302. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; Garrett, M.D.; Campbell, K.J.; Schumm, K.; Perkins, N.D. Regulation of NF-kappaB and P53 through Activation of ATR and Chk1 by the ARF Tumour Suppressor. EMBO J. 2005, 24, 1157–1169. [Google Scholar] [CrossRef]
- Wooten, M.W.; Geetha, T.; Seibenhener, M.L.; Babu, J.R.; Diaz-Meco, M.T.; Moscat, J. The P62 Scaffold Regulates Nerve Growth Factor-Induced NF-kappaB Activation by Influencing TRAF6 Polyubiquitination. J. Biol. Chem. 2005, 280, 35625–35629. [Google Scholar] [CrossRef] [PubMed]
- Solanki, R.; Karande, A.; Ranganathan, P. Emerging Role of Gut Microbiota Dysbiosis in Neuroinflammation and Neurodegeneration. Front. Neurol. 2023, 14, 1149618. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory Mechanisms Linking Obesity and Metabolic Disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a Link between Obesity, Metabolic Syndrome and Type 2 Diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Sánchez-Cabo, F.; Fuster, V.; Silla-Castro, J.C.; González, G.; Lorenzo-Vivas, E.; Alvarez, R.; Callejas, S.; Benguría, A.; Gil, E.; Núñez, E.; et al. Subclinical Atherosclerosis and Accelerated Epigenetic Age Mediated by Inflammation: A Multi-Omics Study. Eur. Heart J. 2023, 44, 2698–2709. [Google Scholar] [CrossRef]
- Weijie, Z.; Meng, Z.; Chunxiao, W.; Lingjie, M.; Anguo, Z.; Yan, Z.; Xinran, C.; Yanjiao, X.; Li, S. Obesity-Induced Chronic Low-Grade Inflammation in Adipose Tissue: A Pathway to Alzheimer’s Disease. Ageing Res. Rev. 2024, 99, 102402. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-Grade Inflammation as a Key Mediator of the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Zhong, P.; Tan, S.; Zhu, Z.; Zhu, Z.; Liang, Y.; Huang, W.; Wang, W. Normal-Weight Central Obesity and Risk of Cardiovascular and Microvascular Events in Adults with Prediabetes or Diabetes: Chinese and British Cohorts. Diabetes Metab. Res. Rev. 2023, 39, e3707. [Google Scholar] [CrossRef] [PubMed]
- Qiao, T.; Luo, T.; Pei, H.; Yimingniyazi, B.; Aili, D.; Aimudula, A.; Zhao, H.; Zhang, H.; Dai, J.; Wang, D. Association between Abdominal Obesity Indices and Risk of Cardiovascular Events in Chinese Populations with Type 2 Diabetes: A Prospective Cohort Study. Cardiovasc. Diabetol. 2022, 21, 225. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Z.; Joseph, P.; Hu, B.; Yin, L.; Tse, L.A.; Rangarajan, S.; Wang, C.; Wang, Y.; Islam, S.; et al. Modifiable Risk Factors Associated with Cardiovascular Disease and Mortality in China: A PURE Substudy. Eur. Heart J. 2022, 43, 2852–2863. [Google Scholar] [CrossRef]
- Yang, W.; Yang, Y.; Guo, Y.; Guo, J.; Ma, M.; Han, B. Obesity and Risk for Respiratory Diseases: A Mendelian Randomization Study. Front. Endocrinol. 2023, 14, 1197730. [Google Scholar] [CrossRef]
- Parikesit, D.; Mochtar, C.A.; Umbas, R.; Hamid, A.R.A.H. The Impact of Obesity towards Prostate Diseases. Prostate Int. 2016, 4, 1–6. [Google Scholar] [CrossRef]
- Mazon, J.N.; de Mello, A.H.; Ferreira, G.K.; Rezin, G.T. The Impact of Obesity on Neurodegenerative Diseases. Life Sci. 2017, 182, 22–28. [Google Scholar] [CrossRef]
- Moroni, L.; Farina, N.; Dagna, L. Obesity and Its Role in the Management of Rheumatoid and Psoriatic Arthritis. Clin. Rheumatol. 2020, 39, 1039–1047. [Google Scholar] [CrossRef]
- Carvalho, L.M.; Carvalho, B.G.; Souza, L.L.; da Mota, J.C.; Ribeiro, A.A.; Nicoletti, C.F. Obesity as an Aggravating Factor of Systemic Lupus Erythematosus Disease: What We Already Know and What We Must Explore. A Rapid Scoping Review. Nutr. Burbank Los Angel. Cty. Calif 2024, 128, 112559. [Google Scholar] [CrossRef]
- Khakoo, N.S.; Ioannou, S.; Khakoo, N.S.; Vedantam, S.; Pearlman, M. Impact of Obesity on Inflammatory Bowel Disease. Curr. Gastroenterol. Rep. 2022, 24, 26–36. [Google Scholar] [CrossRef]
- Schreiner, T.-G.; Genes, T.-M. Obesity and Multiple Sclerosis-A Multifaceted Association. J. Clin. Med. 2021, 10, 2689. [Google Scholar] [CrossRef]
- Kueh, M.T.W.; Chew, N.W.S.; Al-Ozairi, E.; le Roux, C.W. The Emergence of Obesity in Type 1 Diabetes. Int. J. Obes. 2024, 48, 289–301. [Google Scholar] [CrossRef]
- Kumthekar, A.; Ogdie, A. Obesity and Psoriatic Arthritis: A Narrative Review. Rheumatol. Ther. 2020, 7, 447–456. [Google Scholar] [CrossRef]
- Ostrowska, L.; Gier, D.; Zyśk, B. The Influence of Reducing Diets on Changes in Thyroid Parameters in Women Suffering from Obesity and Hashimoto’s Disease. Nutrients 2021, 13, 862. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 4277–4283. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The Gut Microbiota and Host Health: A New Clinical Frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial Dysbiosis-Induced Obesity: Role of Gut Microbiota in Homoeostasis of Energy Metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Sears, C.L.; Maruthur, N. Gut Microbiome and Its Role in Obesity and Insulin Resistance. Ann. N. Y. Acad. Sci. 2020, 1461, 37–52. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clément, K. Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated With Obesity, Lipid Metabolism, and Metabolic Health-Pathophysiology and Therapeutic Strategies. Gastroenterology 2021, 160, 573–599. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Wu, C.-Y. The Gut Microbiome in Obesity. J. Formos. Med. Assoc. Taiwan Yi Zhi 2019, 118 (Suppl. 1), S3–S9. [Google Scholar] [CrossRef]
- Castro, A.M.; Macedo-de la Concha, L.E.; Pantoja-Meléndez, C.A. Low-Grade Inflammation and Its Relation to Obesity and Chronic Degenerative Diseases. Rev. Médica Hosp. Gen. México 2017, 80, 101–105. [Google Scholar] [CrossRef]
- Gummlich, L. Obesity-induced neutrophil reprogramming. Nat. Rev. Cancer 2021, 21, 412. [Google Scholar] [CrossRef]
- Singh, V.; Kaur, R.; Kumari, P.; Pasricha, C.; Singh, R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta 2023, 548, 117487. [Google Scholar] [CrossRef]
- Al-Mansoori, L.; Al-Jaber, H.; Prince, M.S.; Elrayess, M.A. Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation 2022, 45, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int. J. Mol. Sci. 2015, 16, 378–400. [Google Scholar] [CrossRef]
- Hildebrandt, X.; Ibrahim, M.; Peltzer, N. Cell Death and Inflammation during Obesity: “Know My Methods, WAT(Son)”. Cell Death Differ. 2023, 30, 279–292. [Google Scholar] [CrossRef]
- Marfella, R.; Esposito, K.; Siniscalchi, M.; Cacciapuoti, F.; Giugliano, F.; Labriola, D.; Ciotola, M.; Di Palo, C.; Misso, L.; Giugliano, D. Effect of Weight Loss on Cardiac Synchronization and Proinflammatory Cytokines in Premenopausal Obese Women. Diabetes Care 2004, 27, 47–52. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Boland, M. Human Digestion—A Processing Perspective. J. Sci. Food Agric. 2016, 96, 2275–2283. [Google Scholar] [CrossRef]
- Wiertsema, S.P.; van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M.J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef] [PubMed]
- Lazar, V.; Ditu, L.-M.; Pircalabioru, G.G.; Gheorghe, I.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front. Immunol. 2018, 9, 1830. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, C.R.; Bäumler, A.J. Dysbiosis: From Fiction to Function. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G602–G608. [Google Scholar] [CrossRef]
- Walker, W.A. Dysbiosis. In The Microbiota in Gastrointestinal Pathophysiology: Implications for Human Health, Prebiotics, Probiotics, and Dysbiosis; Academic Press: Cambridge, MA, USA, 2017; pp. 227–232. [Google Scholar] [CrossRef]
- Duan, M.; Wang, Y.; Zhang, Q.; Zou, R.; Guo, M.; Zheng, H. Characteristics of Gut Microbiota in People with Obesity. PLoS ONE 2021, 16, e0255446. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Muñoz-Garach, A.; Clemente-Postigo, M.; Tinahones, F.J. Importance of gut microbiota in obesity. Eur. J. Clin. Nutr. 2019, 72 (Suppl. 1), 26–37. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Hou, Y.-P.; He, Q.-Q.; Ouyang, H.-M.; Peng, H.-S.; Wang, Q.; Li, J.; Lv, X.-F.; Zheng, Y.-N.; Li, S.-C.; Liu, H.-L.; et al. Human Gut Microbiota Associated with Obesity in Chinese Children and Adolescents. BioMed Res. Int. 2017, 2017, 7585989. [Google Scholar] [CrossRef]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. Bethesda Md 2019, 10 (Suppl. 1), S17–S30. [Google Scholar] [CrossRef]
- Thingholm, L.B.; Rühlemann, M.C.; Koch, M.; Fuqua, B.; Laucke, G.; Boehm, R.; Bang, C.; Franzosa, E.A.; Hübenthal, M.; Rahnavard, A.; et al. Obese Individuals with and without Type 2 Diabetes Show Different Gut Microbial Functional Capacity and Composition. Cell Host Microbe 2019, 26, 252–264.e10. [Google Scholar] [CrossRef]
- Peters, B.A.; Shapiro, J.A.; Church, T.R.; Miller, G.; Trinh-Shevrin, C.; Yuen, E.; Friedlander, C.; Hayes, R.B.; Ahn, J. A Taxonomic Signature of Obesity in a Large Study of American Adults. Sci. Rep. 2018, 8, 9749. [Google Scholar] [CrossRef]
- Andoh, A.; Nishida, A.; Takahashi, K.; Inatomi, O.; Imaeda, H.; Bamba, S.; Kito, K.; Sugimoto, M.; Kobayashi, T. Comparison of the Gut Microbial Community between Obese and Lean Peoples Using 16S Gene Sequencing in a Japanese Population. J. Clin. Biochem. Nutr. 2016, 59, 65–70. [Google Scholar] [CrossRef]
- Meijnikman, A.S.; Aydin, O.; Prodan, A.; Tremaroli, V.; Herrema, H.; Levin, E.; Acherman, Y.; Bruin, S.; Gerdes, V.E.; Backhed, F.; et al. Distinct Differences in Gut Microbial Composition and Functional Potential from Lean to Morbidly Obese Subjects. J. Intern. Med. 2020, 288, 699–710. [Google Scholar] [CrossRef]
- Hu, H.-J.; Park, S.-G.; Jang, H.B.; Choi, M.-K.; Park, K.-H.; Kang, J.H.; Park, S.I.; Lee, H.-J.; Cho, S.-H. Obesity Alters the Microbial Community Profile in Korean Adolescents. PLoS ONE 2015, 10, e0134333. [Google Scholar] [CrossRef]
- Ettehad Marvasti, F.; Moshiri, A.; Sadat Taghavi, M.; Riazi, S.; Taati, M.; Sadati, S.F.; Ghaheri, A.; Masoomi, M.; Vaziri, F.; Fateh, A.; et al. The First Report of Differences in Gut Microbiota Composition between Obese and Normal Weight Iranian Subjects. Iran. Biomed. J. 2020, 24, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Guo, P.; Mao, R.; Ren, Z.; Wen, J.; Yang, Q.; Yan, T.; Yu, J.; Zhang, T.; Liu, Y. Gut Microbiota Signature of Obese Adults Across Different Classifications. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 3933–3947. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Immune Regulation by Microbiome Metabolites. Immunology 2018, 154, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Zhou, P.; Zhang, R. Intestinal Microbiota-Derived Short Chain Fatty Acids in Host Health and Disease. Nutrients 2022, 14, 1977. [Google Scholar] [CrossRef]
- Lange, O.; Proczko-Stepaniak, M.; Mika, A. Short-Chain Fatty Acids-A Product of the Microbiome and Its Participation in Two-Way Communication on the Microbiome-Host Mammal Line. Curr. Obes. Rep. 2023, 12, 108–126. [Google Scholar] [CrossRef]
- Xiong, R.-G.; Zhou, D.-D.; Wu, S.-X.; Huang, S.-Y.; Saimaiti, A.; Yang, Z.-J.; Shang, A.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Anachad, O.; Taouil, A.; Taha, W.; Bennis, F.; Chegdani, F. The Implication of Short-Chain Fatty Acids in Obesity and Diabetes. Microbiol. Insights 2023, 16, 11786361231162720. [Google Scholar] [CrossRef] [PubMed]
- May, K.S.; den Hartigh, L.J. Modulation of Adipocyte Metabolism by Microbial Short-Chain Fatty Acids. Nutrients 2021, 13, 3666. [Google Scholar] [CrossRef] [PubMed]
- Neis, E.P.J.G.; Dejong, C.H.C.; Rensen, S.S. The Role of Microbial Amino Acid Metabolism in Host Metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short Chain Fatty Acids Prevent High-Fat-Diet-Induced Obesity in Mice by Regulating G Protein-Coupled Receptors and Gut Microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef]
- Lamichhane, S.; Sen, P.; Alves, M.A.; Ribeiro, H.C.; Raunioniemi, P.; Hyötyläinen, T.; Orešič, M. Linking Gut Microbiome and Lipid Metabolism: Moving beyond Associations. Metabolites 2021, 11, 55. [Google Scholar] [CrossRef]
- Fujisaka, S.; Watanabe, Y.; Tobe, K. The Gut Microbiome: A Core Regulator of Metabolism. J. Endocrinol. 2023, 256, e220111. [Google Scholar] [CrossRef]
- Godlewska, U.; Bulanda, E.; Wypych, T.P. Bile Acids in Immunity: Bidirectional Mediators between the Host and the Microbiota. Front. Immunol. 2022, 13, 949033. [Google Scholar] [CrossRef]
- Calzadilla, N.; Comiskey, S.M.; Dudeja, P.K.; Saksena, S.; Gill, R.K.; Alrefai, W.A. Bile Acids as Inflammatory Mediators and Modulators of Intestinal Permeability. Front. Immunol. 2022, 13, 1021924. [Google Scholar] [CrossRef]
- Ma, N.; Ma, X. Dietary Amino Acids and the Gut-Microbiome-Immune Axis: Physiological Metabolism and Therapeutic Prospects. Compr. Rev. Food Sci. Food Saf. 2018, 18, 221–242. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Do, J.-S.; Velankanni, P.; Lee, C.-G.; Kwon, H.-K. Gut Microbial Metabolites on Host Immune Responses in Health and Disease. Immune Netw. 2023, 23, e6. [Google Scholar] [CrossRef]
- Hendrikx, T.; Schnabl, B. Indoles: Metabolites Produced by Intestinal Bacteria Capable of Controlling Liver Disease Manifestation. J. Intern. Med. 2019, 286, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Mujawdiya, P.K.; Pivina, L.; Doşa, A.; Semenova, Y.; Benahmed, A.G.; Bjørklund, G. Relationship between Gut Microbiota, Gut Hyperpermeability and Obesity. Curr. Med. Chem. 2021, 28, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Vetrani, C.; Di Nisio, A.; Paschou, S.A.; Barrea, L.; Muscogiuri, G.; Graziadio, C.; Savastano, S.; Colao, A.; On Behalf Of The Obesity Programs Of Nutrition Education Research And Assessment Opera Group, null. From Gut Microbiota through Low-Grade Inflammation to Obesity: Key Players and Potential Targets. Nutrients 2022, 14, 2103. [Google Scholar] [CrossRef]
- Zhou, P.; She, Y.; Dong, N.; Li, P.; He, H.; Borio, A.; Wu, Q.; Lu, S.; Ding, X.; Cao, Y.; et al. Alpha-Kinase 1 Is a Cytosolic Innate Immune Receptor for Bacterial ADP-Heptose. Nature 2018, 561, 122–126. [Google Scholar] [CrossRef]
- Rolland, A.; Douard, V.; Lapaque, N. Role of Pattern Recognition Receptors and Microbiota-Derived Ligands in Obesity. Front. Microbiomes 2024, 3, 1324476. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Castellanos-Juárez, F.X.; Salas-Pacheco, J.M.; Morales-Gurrola, F.G.; Salas-Leal, A.C.; Simental-Mendía, L.E. Association between the Expression of TLR4, TLR2, and MyD88 with Low-Grade Chronic Inflammation in Individuals with Metabolically Healthy Obesity. Mol. Biol. Rep. 2023, 50, 4723–4728. [Google Scholar] [CrossRef]
- Himes, R.W.; Smith, C.W. Tlr2 Is Critical for Diet-Induced Metabolic Syndrome in a Murine Model. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 731–739. [Google Scholar] [CrossRef]
- Davis, J.E.; Braucher, D.R.; Walker-Daniels, J.; Spurlock, M.E. Absence of Tlr2 Protects against High-Fat Diet-Induced Inflammation and Results in Greater Insulin-Stimulated Glucose Transport in Cultured Adipocytes. J. Nutr. Biochem. 2011, 22, 136–141. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y.; Liu, C.; Youn, J.Y.; Cai, H. Toll-Like Receptor 2 (TLR2) Knockout Abrogates Diabetic and Obese Phenotypes While Restoring Endothelial Function via Inhibition of NOX1. Diabetes 2021, 70, 2107–2119. [Google Scholar] [CrossRef] [PubMed]
- Orr, J.S.; Puglisi, M.J.; Ellacott, K.L.J.; Lumeng, C.N.; Wasserman, D.H.; Hasty, A.H. Toll-like Receptor 4 Deficiency Promotes the Alternative Activation of Adipose Tissue Macrophages. Diabetes 2012, 61, 2718–2727. [Google Scholar] [CrossRef]
- Garay-Malpartida, H.M.; Mourão, R.F.; Mantovani, M.; Santos, I.A.; Sogayar, M.C.; Goldberg, A.C. Toll-like Receptor 4 (TLR4) Expression in Human and Murine Pancreatic Beta-Cells Affects Cell Viability and Insulin Homeostasis. BMC Immunol. 2011, 12, 18. [Google Scholar] [CrossRef]
- Gewirtz, A.T.; Navas, T.A.; Lyons, S.; Godowski, P.J.; Madara, J.L. Cutting Edge: Bacterial Flagellin Activates Basolaterally Expressed TLR5 to Induce Epithelial Proinflammatory Gene Expression. J. Immunol. 2001, 167, 1882–1885. [Google Scholar] [CrossRef]
- Tran, H.Q.; Ley, R.E.; Gewirtz, A.T.; Chassaing, B. Flagellin-Elicited Adaptive Immunity Suppresses Flagellated Microbiota and Vaccinates against Chronic Inflammatory Diseases. Nat. Commun. 2019, 10, 5650. [Google Scholar] [CrossRef] [PubMed]
- Vijay-Kumar, M.; Aitken, J.D.; Carvalho, F.A.; Cullender, T.C.; Mwangi, S.; Srinivasan, S.; Sitaraman, S.V.; Knight, R.; Ley, R.E.; Gewirtz, A.T. Metabolic Syndrome and Altered Gut Microbiota in Mice Lacking Toll-like Receptor 5. Science 2010, 328, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, T.P.M.; Herrema, H.; Yu, H.; Bakker, G.J.; Winkelmeijer, M.; Soukhatcheva, G.; Dai, D.; Ma, C.; Havik, S.R.; Balvers, M.; et al. Gut-Derived Bacterial Flagellin Induces Beta-Cell Inflammation and Dysfunction. Gut Microbes 2022, 14, 2111951. [Google Scholar] [CrossRef] [PubMed]
- Thomalla, M.; Schmid, A.; Neumann, E.; Pfefferle, P.I.; Müller-Ladner, U.; Schäffler, A.; Karrasch, T. Evidence of an Anti-Inflammatory Toll-like Receptor 9 (TLR 9) Pathway in Adipocytes. J. Endocrinol. 2019, 240, 325–343. [Google Scholar] [CrossRef]
- Nishimoto, S.; Fukuda, D.; Higashikuni, Y.; Tanaka, K.; Hirata, Y.; Murata, C.; Kim-Kaneyama, J.-R.; Sato, F.; Bando, M.; Yagi, S.; et al. Obesity-Induced DNA Released from Adipocytes Stimulates Chronic Adipose Tissue Inflammation and Insulin Resistance. Sci. Adv. 2016, 2, e1501332. [Google Scholar] [CrossRef]
- Revelo, X.S.; Ghazarian, M.; Chng, M.H.Y.; Luck, H.; Kim, J.H.; Zeng, K.; Shi, S.Y.; Tsai, S.; Lei, H.; Kenkel, J.; et al. Nucleic Acid-Targeting Pathways Promote Inflammation in Obesity-Related Insulin Resistance. Cell Rep. 2016, 16, 717–730. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, P.; Zhou, Y.; Purohit, J.; Hwang, D. NOD1 Activation Induces Proinflammatory Gene Expression and Insulin Resistance in 3T3-L1 Adipocytes. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E587–E598. [Google Scholar] [CrossRef]
- Chan, K.L.; Tam, T.H.; Boroumand, P.; Prescott, D.; Costford, S.R.; Escalante, N.K.; Fine, N.; Tu, Y.; Robertson, S.J.; Prabaharan, D.; et al. Circulating NOD1 Activators and Hematopoietic NOD1 Contribute to Metabolic Inflammation and Insulin Resistance. Cell Rep. 2017, 18, 2415–2426. [Google Scholar] [CrossRef]
- Negroni, A.; Pierdomenico, M.; Cucchiara, S.; Stronati, L. NOD2 and Inflammation: Current Insights. J. Inflamm. Res. 2018, 11, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef]
- Nagpal, R.; Newman, T.M.; Wang, S.; Jain, S.; Lovato, J.F.; Yadav, H. Obesity-Linked Gut Microbiome Dysbiosis Associated with Derangements in Gut Permeability and Intestinal Cellular Homeostasis Independent of Diet. J. Diabetes Res. 2018, 2018, 3462092. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut Microbiota, Intestinal Permeability, and Systemic Inflammation: A Narrative Review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Chassaing, B.; Gewirtz, A.T. Gut Microbiota, Low-Grade Inflammation, and Metabolic Syndrome. Toxicol. Pathol. 2014, 42, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-Grade Inflammation, Diet Composition and Health: Current Research Evidence and Its Translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Chan, J.Y.H.; Wu, K.L.H.; Yu, H.-R.; Lee, W.-C.; Hou, C.-Y.; Tain, Y.-L. Altered Gut Microbiota and Its Metabolites in Hypertension of Developmental Origins: Exploring Differences between Fructose and Antibiotics Exposure. Int. J. Mol. Sci. 2021, 22, 2674. [Google Scholar] [CrossRef]
- O’Donnell, J.A.; Zheng, T.; Meric, G.; Marques, F.Z. The gut microbiome and hypertension. Nat. Rev. Nephrol. 2023, 19, 153–167. [Google Scholar] [CrossRef]
- Jing, Y.; Zhou, H.; Lu, H.; Chen, X.; Zhou, L.; Zhang, J.; Wu, J.; Dong, C. Associations between peripheral blood microbiome and the risk of hypertension. Am. J. Hypertens. 2021, 34, 1064–1070. [Google Scholar] [CrossRef]
- Mizoguchi, R.; Karashima, S.; Miyajima, Y.; Ogura, K.; Kometani, M.; Aono, D.; Konishi, S.; Demura, M.; Tsujiguchi, H.; Hara, A.; et al. Impact of gut microbiome on the renin-aldosterone system: Shika-machi Super Preventive Health Examination results. Hypertens. Res. 2023, 46, 2280–2292. [Google Scholar] [CrossRef]
- Yoshida, N.; Yamashita, T.; Hirata, K. Gut Microbiome and Cardiovascular Diseases. Diseases 2018, 6, 56. [Google Scholar] [CrossRef]
- Bai, S.; Xie, J.; Bai, H.; Tian, T.; Zou, T.; Chen, J.-J. Gut microbiota-derived inflammation-related serum metabolites as potential biomarkers for major depressive disorder. J. Inflamm. Res. 2021, 14, 3755–3766. [Google Scholar] [CrossRef] [PubMed]
- Komaroff, A.L. The Microbiome and Risk for Obesity and Diabetes. JAMA 2017, 317, 355–356. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.L.; Tran, T.; Rhee, C.M.; Kalantar-Zadeh, K.; Vaziri, N.D. Diabetes and the Gut Microbiome. Semin. Nephrol. 2021, 41, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Barlow, G.M.; Yu, A.; Mathur, R. Role of the Gut Microbiome in Obesity and Diabetes Mellitus. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2015, 30, 787–797. [Google Scholar] [CrossRef]
- Caricilli, A.M.; Saad, M.J.A. The Role of Gut Microbiota on Insulin Resistance. Nutrients 2013, 5, 829–851. [Google Scholar] [CrossRef]
- Zhang, S.; Cai, Y.; Meng, C.; Ding, X.; Huang, J.; Luo, X.; Cao, Y.; Gao, F.; Zou, M. The Role of the Microbiome in Diabetes Mellitus. Diabetes Res. Clin. Pract. 2021, 172, 108645. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the Gut Microbiota in Disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, X. The Role of Gut-Liver Axis in Gut Microbiome Dysbiosis Associated NAFLD and NAFLD-HCC. Biomedicines 2022, 10, 524. [Google Scholar] [CrossRef]
- Cao, C.; Zhu, H.; Yao, Y.; Zeng, R. Gut Dysbiosis and Kidney Diseases. Front. Med. 2022, 9, 829349. [Google Scholar] [CrossRef]
- Zou, S.; Fang, L.; Lee, M.-H. Dysbiosis of Gut Microbiota in Promoting the Development of Colorectal Cancer. Gastroenterol. Rep. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Biragyn, A.; Ferrucci, L. Gut Dysbiosis: A Potential Link between Increased Cancer Risk in Ageing and Inflammaging. Lancet Oncol. 2018, 19, e295–e304. [Google Scholar] [CrossRef]
- Bull, M.J.; Plummer, N.T. Part 2: Treatments for Chronic Gastrointestinal Disease and Gut Dysbiosis. Integr. Med. Clin. J. 2015, 14, 25–33. [Google Scholar]
- Borgeraas, H.; Johnson, L.K.; Skattebu, J.; Hertel, J.K.; Hjelmesaeth, J. Effects of Probiotics on Body Weight, Body Mass Index, Fat Mass and Fat Percentage in Subjects with Overweight or Obesity: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Obes. Rev. 2017, 19, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef] [PubMed]
- Torres, B.; Sánchez, M.C.; Virto, L.; Llama-Palacios, A.; Ciudad, M.J.; Collado, L. Use of Probiotics in Preventing and Treating Excess Weight and Obesity. A Systematic Review. Obes. Sci. Pract. 2024, 10, e759. [Google Scholar] [CrossRef]
- Wiciński, M.; Gębalski, J.; Gołębiewski, J.; Malinowski, B. Probiotics for the Treatment of Overweight and Obesity in Humans—A Review of Clinical Trials. Microorganisms 2020, 8, 1148. [Google Scholar] [CrossRef]
- Portune, K.J.; Benítez-Páez, A.; Del Pulgar, E.M.G.; Cerrudo, V.; Sanz, Y. Gut Microbiota, Diet, and Obesity-Related Disorders-The Good, the Bad, and the Future Challenges. Mol. Nutr. Food Res. 2017, 61, 1600252. [Google Scholar] [CrossRef]
- Fic, W.; Polak-Szczybyło, E. Dietary Factors Influencing the Intensity of Low-Grade Inflammation in Obesity. Obesities 2025, 5, 12. [Google Scholar] [CrossRef]
- Wolters, M.; Ahrens, J.; Romaní-Pérez, M.; Watkins, C.; Sanz, Y.; Benítez-Páez, A.; Stanton, C.; Günther, K. Dietary Fat, the Gut Microbiota, and Metabolic Health—A Systematic Review Conducted within the MyNewGut Project. Clin. Nutr. Edinb. Scotl. 2019, 38, 2504–2520. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, X.-J. Effects of a High Fat Diet on Intestinal Microbiota and Gastrointestinal Diseases. World J. Gastroenterol. 2016, 22, 8905–8909. [Google Scholar] [CrossRef]
- Noble, E.E.; Hsu, T.M.; Kanoski, S.E. Gut to Brain Dysbiosis: Mechanisms Linking Western Diet Consumption, the Microbiome, and Cognitive Impairment. Front. Behav. Neurosci. 2017, 11, 9. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Li, P.; Qu, R.; Li, M.; Sheng, P.; Jin, L.; Huang, X.; Xu, Z.Z. Impacts of Food Additives on Gut Microbiota and Host Health. Food Res. Int. Ott. Ont 2024, 196, 114998. [Google Scholar] [CrossRef] [PubMed]
- De Siena, M.; Raoul, P.; Costantini, L.; Scarpellini, E.; Cintoni, M.; Gasbarrini, A.; Rinninella, E.; Mele, M.C. Food Emulsifiers and Metabolic Syndrome: The Role of the Gut Microbiota. Foods 2022, 11, 2205. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Ellerbroek, A.; Silver, T.; Orris, S.; Scheiner, M.; Gonzalez, A.; Peacock, C.A. A High Protein Diet (3.4 g/Kg/d) Combined with a Heavy Resistance Training Program Improves Body Composition in Healthy Trained Men and Women--a Follow-up Investigation. J. Int. Soc. Sports Nutr. 2015, 12, 39. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, X.; Zhao, F.; Shi, X.; Li, H.; Li, Y.; Zhu, W.; Xu, X.; Li, C.; Zhou, G. Meat, Dairy and Plant Proteins Alter Bacterial Composition of Rat Gut Bacteria. Sci. Rep. 2015, 5, 15220. [Google Scholar] [CrossRef]
- Graf, D.; Di Cagno, R.; Fåk, F.; Flint, H.J.; Nyman, M.; Saarela, M.; Watzl, B. Contribution of Diet to the Composition of the Human Gut Microbiota. Microb. Ecol. Health Dis. 2015, 26, 26164. [Google Scholar] [CrossRef]
- Choi, S.-W. A Journey to Explore the Health Properties of Traditional Korean Diet: A Commentary. J. Ethn. Foods 2023, 10, 9. [Google Scholar] [CrossRef]
- Won, S.-M.; Chen, S.; Lee, S.Y.; Lee, K.E.; Park, K.W.; Yoon, J.-H. Lactobacillus sakei ADM14 Induces Anti-Obesity Effects and Changes in Gut Microbiome in High-Fat Diet-Induced Obese Mice. Nutrients 2020, 12, 3703. [Google Scholar] [CrossRef]
- Shin, J.-H.; Jung, S.; Kim, S.-A.; Kang, M.-S.; Kim, M.-S.; Joung, H.; Hwang, G.-S.; Shin, D.-M. Differential Effects of Typical Korean Versus American-Style Diets on Gut Microbial Composition and Metabolic Profile in Healthy Overweight Koreans: A Randomized Crossover Trial. Nutrients 2019, 11, 2450. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gao, J.; Ke, W.; Wang, J.; Li, D.; Liu, R.; Jia, Y.; Wang, X.; Chen, X.; Chen, F.; et al. Resveratrol Reduces Obesity in High-Fat Diet-Fed Mice via Modulating the Composition and Metabolic Function of the Gut Microbiota. Free Radic. Biol. Med. 2020, 156, 83–98. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 8, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef]
- Pisanu, S.; Palmas, V.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Boi, F.; Loviselli, A.; et al. Impact of a Moderately Hypocaloric Mediterranean Diet on the Gut Microbiota Composition of Italian Obese Patients. Nutrients 2020, 12, 2707. [Google Scholar] [CrossRef]
- Myhrstad, M.C.W.; Tunsjø, H.; Charnock, C.; Telle-Hansen, V.H. Dietary Fiber, Gut Microbiota, and Metabolic Regulation—Current Status in Human Randomized Trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Sadagopan, A.; Mahmoud, A.; Begg, M.; Tarhuni, M.; Fotso, M.; Gonzalez, N.A.; Sanivarapu, R.R.; Osman, U.; Latha Kumar, A.; Mohammed, L. Understanding the Role of the Gut Microbiome in Diabetes and Therapeutics Targeting Leaky Gut: A Systematic Review. Cureus 2023, 15, e41559. [Google Scholar] [CrossRef]
- Yanovski, S.Z.; Yanovski, J.A. Long-Term Drug Treatment for Obesity: A Systematic and Clinical Review. JAMA J. Am. Med. Assoc. 2014, 311, 74–86. [Google Scholar] [CrossRef]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin Alters the Gut Microbiome of Individuals with Treatment-Naive Type 2 Diabetes, Contributing to the Therapeutic Effects of the Drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Rosell-Díaz, M.; Fernández-Real, J.M. Metformin, Cognitive Function, and Changes in the Gut Microbiome. Endocr. Rev. 2024, 45, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.B.; Chae, S.U.; Jo, S.J.; Jerng, U.M.; Bae, S.K. The Relationship between the Gut Microbiome and Metformin as a Key for Treating Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 3566. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Sato, T.; Fujita, H.; Kawatani, M.; Yamada, Y. Effects of GLP-1 Receptor Agonist on Changes in the Gut Bacterium and the Underlying Mechanisms. Sci. Rep. 2021, 11, 9167. [Google Scholar] [CrossRef]
- Abdalqadir, N.; Adeli, K. GLP-1 and GLP-2 Orchestrate Intestine Integrity, Gut Microbiota, and Immune System Crosstalk. Microorganisms 2022, 10, 2061. [Google Scholar] [CrossRef]
- Michaelis, L.; Berg, L.; Maier, L. Confounder or Confederate? The Interactions Between Drugs and the Gut Microbiome in Psychiatric and Neurological Diseases. Biol. Psychiatry 2024, 95, 361–369. [Google Scholar] [CrossRef]
- Song, E.-J.; Shin, N.R.; Jeon, S.; Nam, Y.-D.; Kim, H. Lorcaserin and Phentermine Exert Anti-Obesity Effects with Modulation of the Gut Microbiota. Front. Microbiol. 2023, 13, 1109651. [Google Scholar] [CrossRef]
- Uehira, Y.; Ueno, H.; Miyamoto, J.; Kimura, I.; Ishizawa, Y.; Iijima, H.; Muroga, S.; Fujita, T.; Sakai, S.; Samukawa, Y.; et al. Impact of the Lipase Inhibitor Orlistat on the Human Gut Microbiota. Obes. Res. Clin. Pract. 2023, 17, 411–420. [Google Scholar] [CrossRef]
| Ref. | n | Type of Study | Microbiome Composition in People with Obesity | Microbiome Composition in People with Normal Body Weight |
|---|---|---|---|---|
| Andoh et al. [54] | 20 (10 obese, 10 normal weight) | Observational | ↑ Alistipes ↑ Anaerococcus ↑ Corpococcus ↑ Fusobacterium ↑ Parvimona | ↑ Bacteroides ↑ Desulfovibrio ↑ Faecalibacterium ↑ Lachnoanaerobaculum ↑ Olsenella |
| Meijnikman et al. [55] | 177 (95 obese, 82 normal weight) | Observational | ↑ Actinomyces odontolyticus ↑ Collinsella aerofaciens ↑ Ruminococcus torques ↑ Streptococcus australis ↑ Streptococcus thermophilus | ↑ Alistipes senegalensis ↑ Alistipes shahii ↑ Butyrivibrio crossotus ↑ Coprococcus eutactus ↑ Oxalobacter formigenes |
| Hu et al. [56] | 134 (67 obese, 67 normal weight) | Observational | ↑ Alistipes ↑ Sutterellaceae ↑ Veillonellaceae ↑ Prevotella | ↑ Faecalibacterium ↑ Oscillibacter ↑ Rikenellaceae ↑ Ruminococcaceae ↑ Bacteroides |
| Ettehad Marvasti et al. [57] | 100 (50 obese, 50 normal weight) | Observational | ↑ Ratio Firmicutes: Bacteroidetes ↑ Faecalibacterium prausnitzii ↑ Roseburia | ↑ Akkermansia muciniphila ↑ Bifidobacterium ↑ Prevotella |
| Hu et al. [58] | 92 (56 obese, 36 normal weight) | Observational | ↑ Erysipelatoclostridiaceae ↑ Lactobacillales ↑ Bacilli ↑ Negativicutes ↑ Bacteroides ovatus ↑ Bacteroides uniformis ↑ Blautia wexlerae ↑ Bacteroides vulgatus ↑ Citrobacter europaeus ↑ Eubacterium coprostanoligenes ↑ Prevotella copri | ↑ Akkermansia ↑ Eubacterium coprostanoligenes ↑ Lachnospiraceae NK4A136 ↑ Parabacteroides ↑ Eubacterium coprostanoligenes ↑ Tannerellaceae ↑ Prevotella copri |
| Drug Group/ Active Drug Substance | Mechanism of Stimulating Weight Loss | Treatment Side Effects Resulting from Changes in the Microbiome | Changes in the Microbiome | A Possible Way of Influencing the Microbiome |
|---|---|---|---|---|
| Metformin | Reduces glucose absorption in the intestines. | Increased glucose levels, diarrhea. | Increase in the relative abundance of species such as Akkermansia muciniphila and Escherichia coli [144,145]. | Via side effects (bloating, diarrhea, nausea), slowing down glucose absorption in the gut, increase in production for short-chain fatty acids, improving intestinal tightness, immune system modulation, inhibition of fatty acid reabsorption [146]. |
| Glucagon-like peptide-1 receptor agonists: Exanatide, Liraglutide, Lixisenatide, Semaglutide | Signaling satiety, slowing down stomach motility. | Risk of pancreatitis, slow motor function, diarrhea, vomiting. | Decrease in the number of Bacteroidetes bacteria, increase in Actinobacteria. No change in quantity of Akkermansia. Increase in the relative number of Ruminococcus and Actinobacteria [147]. | Suppressing hunger at the CNS level, slowing down of gastrointestinal motility, and via side effects (bloating, diarrhea, nausea, inflammation and pancreatic cancer) [147,148]. Improving intestinal tightness [149]. |
| Naltrekson/Bupropion | Central inhibition of hunger. | Constipation, nausea. | Bupropion increases the amount of conjugation in intestinal bacteria, most likely increasing, among others, the number of antibiotic-resistant E. coli bacteria [149]. | Inhibition of hunger at the CNS level (the mechanisms of action on the CNS have not been thoroughly investigated) [149]. |
| Phentermine | Central inhibition of hunger. | Constipation, nausea. | Change in the quantity of Firmicutes and Bacteroides [150]. | Appetite suppression at the CNS level, sympathomimetic effect (which intensifies fat loss) [150]. |
| Orlistat | Inhibition of lipases in the digestive tract and thus reducing the absorption of fats into the bloodstream. | Increased fat content in the digestive tract and fatty diarrhea, flatulence. | Increase in the number of Lactobacillus genus and Lactobacillus gasseri bacteria [151]. | Inactivation of trihedral lipase and thus a change in the pool of enzymes to which the food content is exposed, decreased absorption of ADEK vitamins, adverse reactions (liver damage, fatty diarrhea, flatulence) [151]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelc, A.; Fic, W.; Typrowicz, T.; Polak-Szczybyło, E. Physiological Mechanisms of and Therapeutic Approaches to the Gut Microbiome and Low-Grade Inflammation in Obesity. Curr. Issues Mol. Biol. 2025, 47, 637. https://doi.org/10.3390/cimb47080637
Pelc A, Fic W, Typrowicz T, Polak-Szczybyło E. Physiological Mechanisms of and Therapeutic Approaches to the Gut Microbiome and Low-Grade Inflammation in Obesity. Current Issues in Molecular Biology. 2025; 47(8):637. https://doi.org/10.3390/cimb47080637
Chicago/Turabian StylePelc, Agnieszka, Weronika Fic, Tymoteusz Typrowicz, and Ewelina Polak-Szczybyło. 2025. "Physiological Mechanisms of and Therapeutic Approaches to the Gut Microbiome and Low-Grade Inflammation in Obesity" Current Issues in Molecular Biology 47, no. 8: 637. https://doi.org/10.3390/cimb47080637
APA StylePelc, A., Fic, W., Typrowicz, T., & Polak-Szczybyło, E. (2025). Physiological Mechanisms of and Therapeutic Approaches to the Gut Microbiome and Low-Grade Inflammation in Obesity. Current Issues in Molecular Biology, 47(8), 637. https://doi.org/10.3390/cimb47080637







