ZmNLR-7-Mediated Synergistic Regulation of ROS, Hormonal Signaling, and Defense Gene Networks Drives Maize Immunity to Southern Corn Leaf Blight
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatics Analysis of ZmNLR-7
2.2. Vector Construction and Subcellular Localization
2.3. Pathogen Cultivation
2.4. Plant Materials and Treatments
2.5. RNA Extraction and RT-PCR Analysis
2.6. EMS Mutant Identification
2.7. EMS Mutant Identification Physiological Parameter Analysis of ZmNLR-7
2.8. RNA Sequencing (RNA-Seq) Analysis
2.9. Statistical Analysis
3. Results
3.1. Bioinformatics Analysis of the ZmNLR-7 Gene
3.2. Analysis of Subcellular Localization of ZmNLR-7
3.3. Analysis of the Expression Pattern of ZmNLR-7
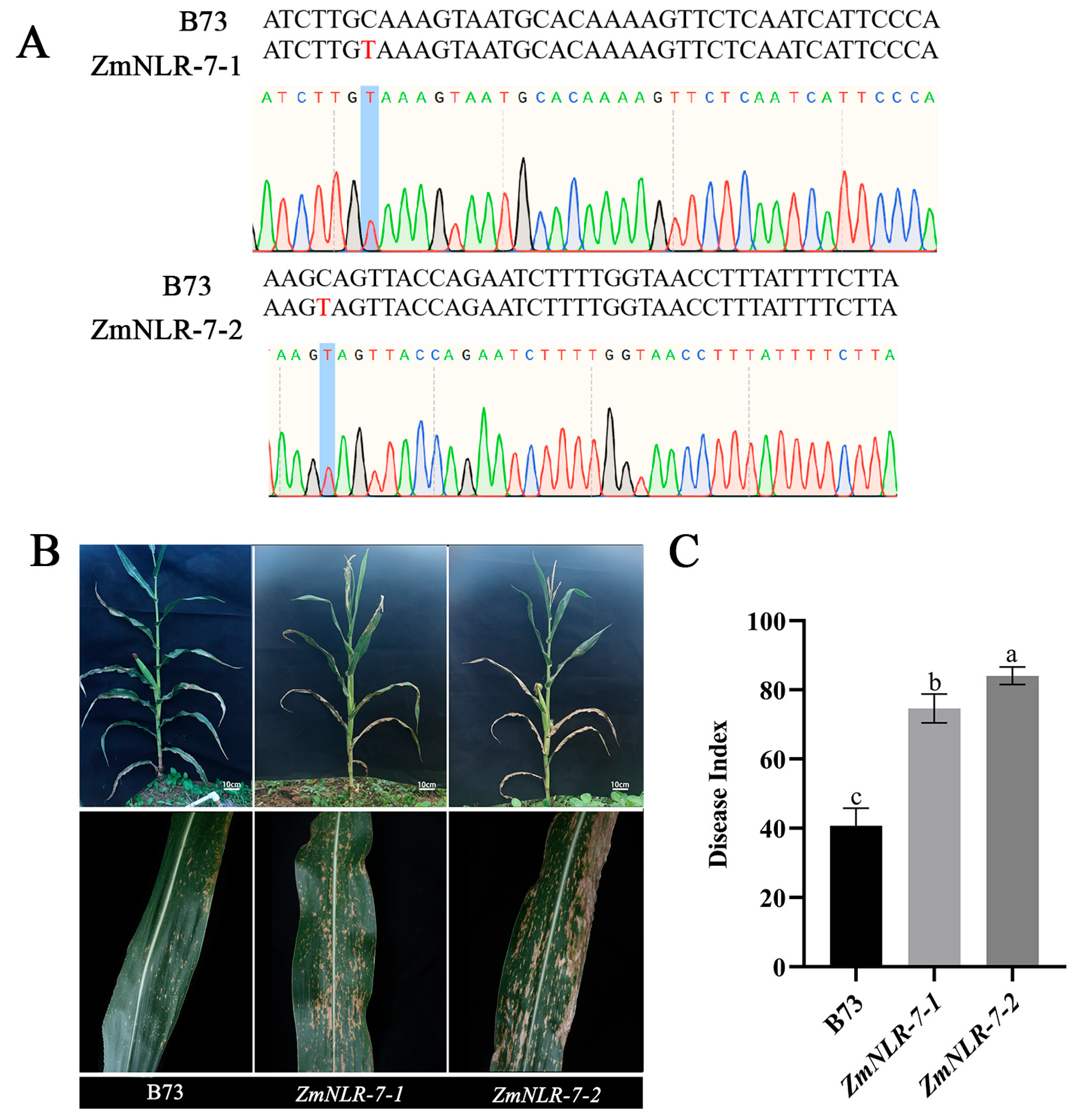
3.4. Obtaining Homozygous Mutants of ZmNLR-7 Maize and Phenotypic Analysis of Response to Infection with Bipolaris Maydis
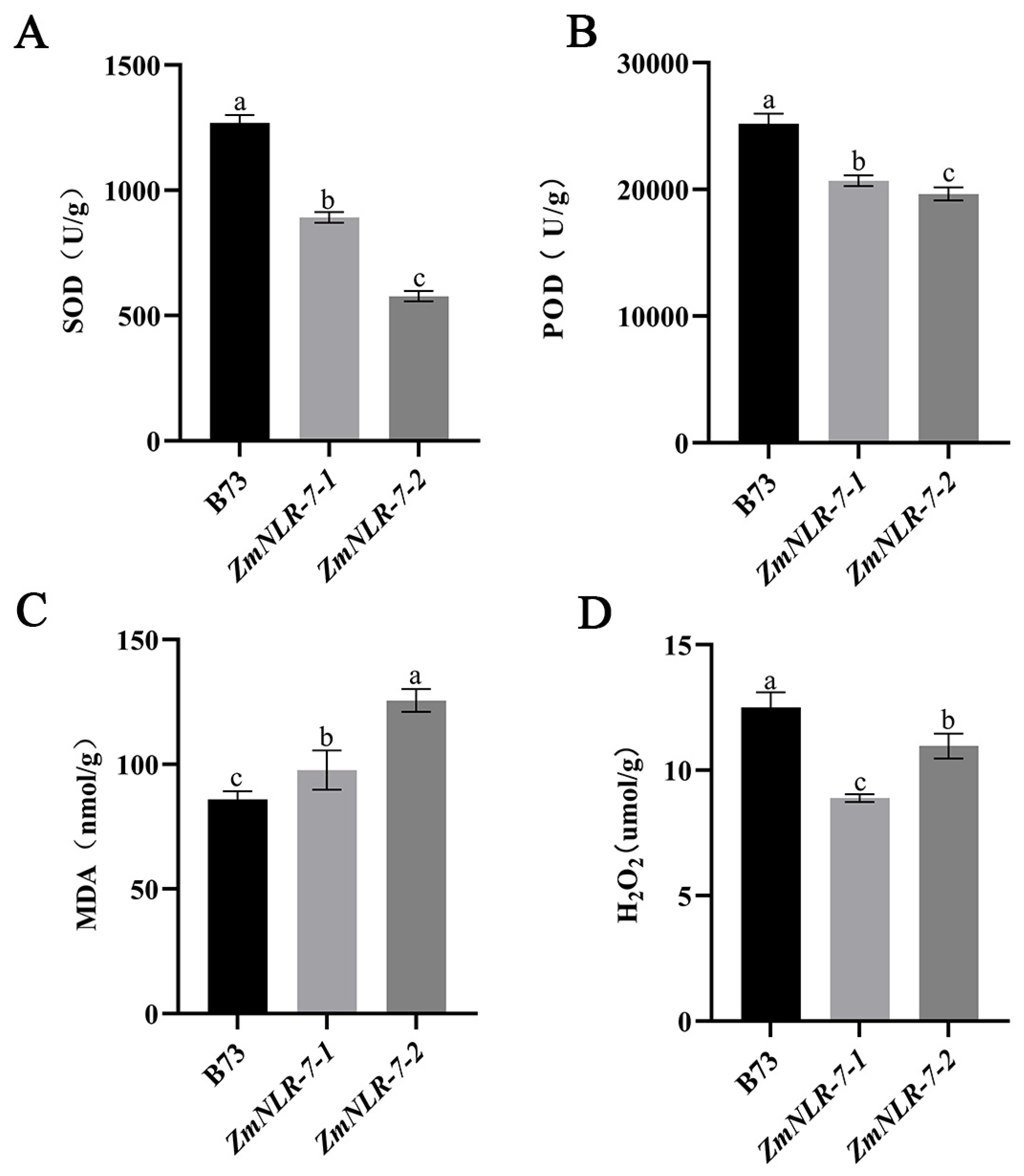
3.5. Determination of Physiological Indicators of ZmNLR-7 Maize Mutants in Response to Infection with Bipolaris Maydis
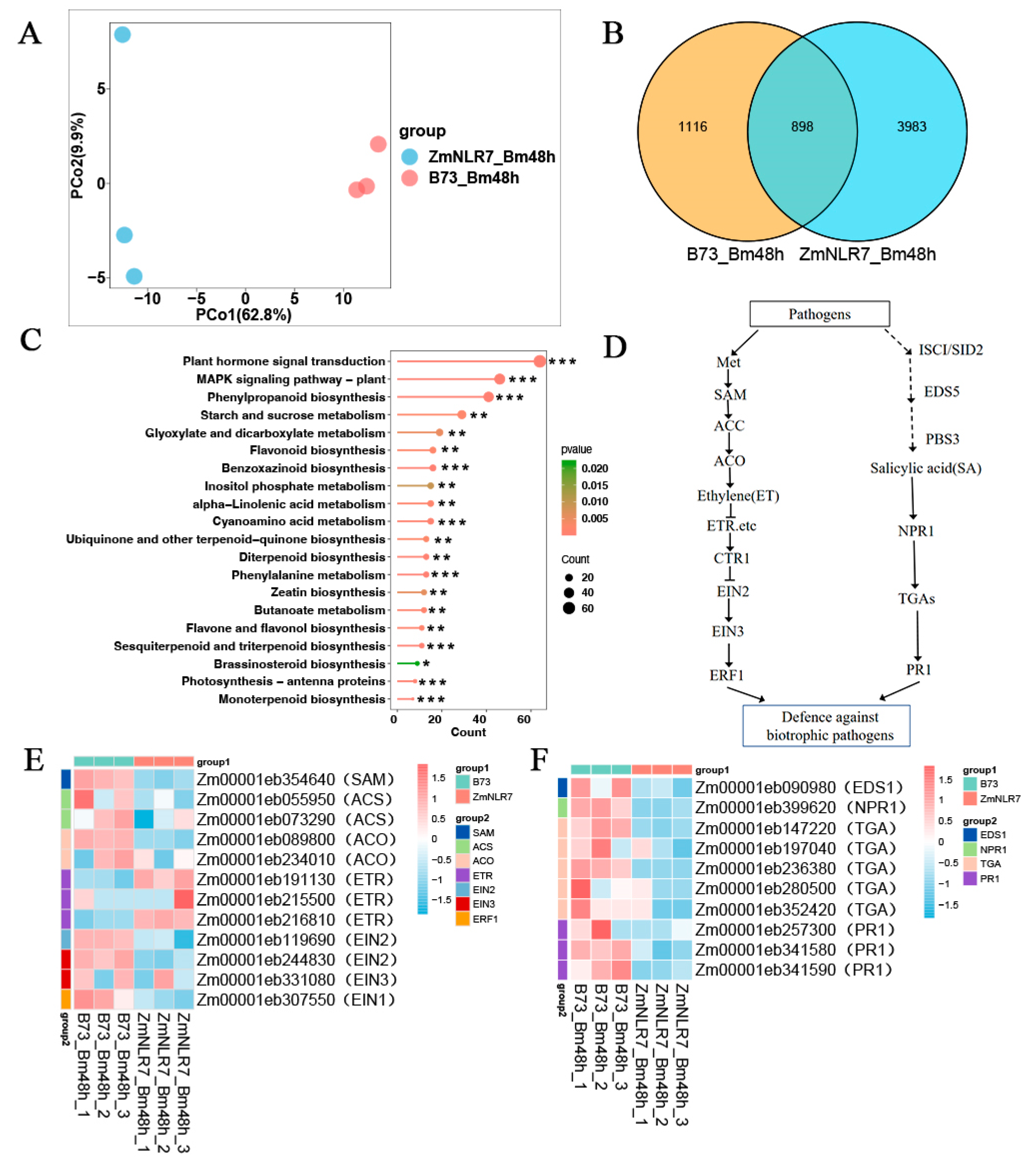
3.6. Transcriptomic Analysis of ZmNLR-7 Maize Mutant in Response to Bipolaris Maydis Infection
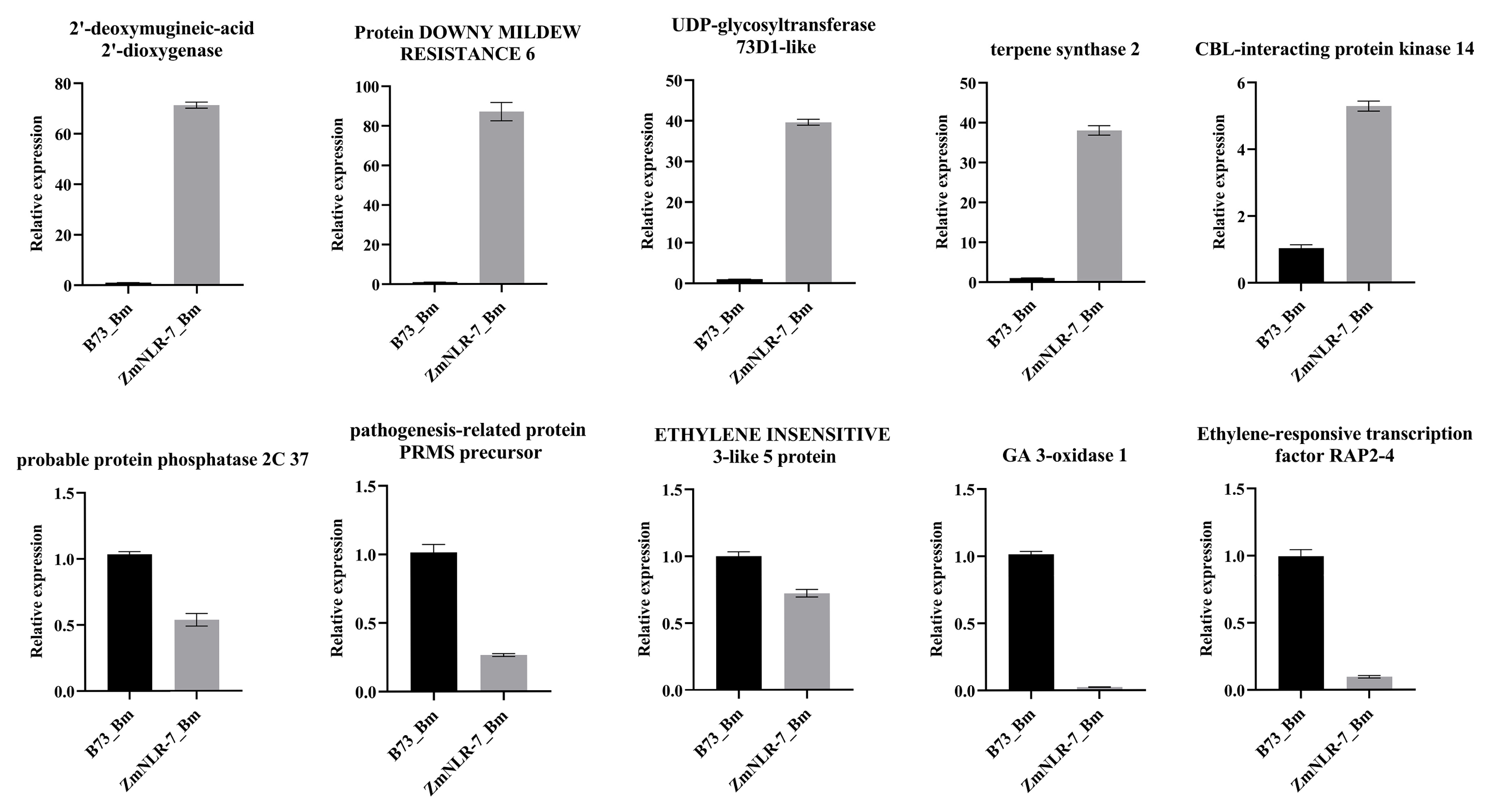
3.7. Fluorescence Quantitative Verification of ZmNLR-7 Transcriptomics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Niu, H.; Liu, C.; Wang, H.; Yin, W.; Xia, X. PTI-ETI synergistic signal mechanisms in plant immunity. Plant Biotechnol. J. 2024, 22, 2113–2128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Menke, F.L.; Yoshioka, K.; Moder, W.; Shirano, Y.; Klessig, D.F. High humidity suppresses ssi4-mediated cell death and disease resistance upstream of MAP kinase activation, H2O2 production and defense gene expression. Plant J. 2004, 39, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; Wang, Y.; Cai, B.; Zhou, J.-M.; He, S.Y.; Xin, X.-F. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Ngou, B.P.M.; Ahn, H.-K.; Ding, P.; Jones, J.D.G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 2021, 592, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-S.; Zhu, Y.-X.; Zhang, Y.-F.; Zhong, X.; Pan, K.-Y.; Jiang, Y.; Wen, C.-K.; Yang, Z.-N.; Yao, X. Ethylene activates the EIN2-EIN3/EIL1 signaling pathway in tapetum and disturbs anther development in Arabidopsis. Cells 2022, 11, 3177. [Google Scholar] [CrossRef] [PubMed]
- Duxbury, Z.; Wu, C.-H.; Ding, P. A comparative overview of the intracellular guardians of plants and animals: NLRs in innate immunity and beyond. Annu. Rev. Plant Biol. 2021, 72, 155–184. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Liu, Y.-S.; Budai-Hadrian, O.; Sela, M.; Carmel-Goren, L.; Zamir, D.; Fluhr, R. Comparative genetics of nucleotide binding site-leucine rich repeat resistance gene homologues in the ge-nomes of two dicotyledons: Tomato and Arabidopsis. Genetics 2000, 155, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Wendel, J.; Fluhr, R. Divergent evolution of plant NBS-LRR resistance gene homologues in dicot and cereal genomes. J. Mol. Evol. 2000, 50, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Tamborski, J.; Krasileva, K.V. Evolution of plant NLRs: From natural history to precise modifications. Annu. Rev. Plant Biol. 2020, 71, 355–378. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A.; Takemoto, D. Plant innate immunity–direct and indirect recognition of general and specific pathogen-associated molecules. Curr. Opin. Immunol. 2004, 16, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.B.; Bogdanove, A.J.; Sessa, G. Understanding the functions of plant disease resistance proteins. Annu. Rev. Plant Biol. 2003, 54, 23–61. [Google Scholar] [CrossRef] [PubMed]
- Danot, O.; Marquenet, E.; Vidal-Ingigliardi, D.; Richet, E. Wheel of life, wheel of death: A mechanistic insight into signaling by STAND proteins. Structure 2009, 17, 172–182. [Google Scholar] [CrossRef] [PubMed]
- van der Biezen, E.A.; Jones, J.D. The NB-ARC domain: A novel signalling motif shared by plant resistance gene products and regulators of cell death in animals. Curr. Biol. 1998, 8, R226–R228. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Xie, L.; Chakraborty, S.; Wang, A.; Matny, O.; Jugovich, M.; Kolmer, J.A.; Richardson, T.; Bhatt, D.; Hoque, M.; et al. A five-transgene cassette confers broad-spectrum resistance to a fungal rust pathogen in wheat. Nat. Biotechnol. 2021, 39, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Hatta, M.A.M.; Arora, S.; Ghosh, S.; Matny, O.; Smedley, M.A.; Yu, G.; Chakraborty, S.; Bhatt, D.; Xia, X.; Steuernagel, B.J. The wheat Sr22, Sr33, Sr35 and Sr45 genes confer resistance against stem rust in barley. Plant Biotechnol. J. 2021, 19, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Dracatos, P.M.; Lu, J.; Sánchez-Martín, J.; Wulff, B.B. Resistance that stacks up: Engineering rust and mildew disease control in the cereal crops wheat and barley. Plant Biotechnol. J. 2023, 21, 1938–1951. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, C.; Zhao, X.; Liu, J.; Cui, T.; Ren, Y.; Niu, Y.; Tang, Z. Research Progress on Pyramiding Breeding of Disease-Resistant Related Genes in Wheat. Shanxi Agric. Sci. 2017, 45, 308–313. [Google Scholar]
- Feng, Y.; Ma, Y.; Feng, F.; Chen, X.; Qi, W.; Ma, Z.; Song, R. Accumulation of 22 kDa α-zein-mediated nonzein protein in protein body of maize endosperm. New Phytol. 2022, 233, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Cao, Q.; Dong, J.; Qiao, M.; Wang, Z.; Zhang, Z.; Sun, H.; Xie, H.; Ge, M.; Zhang, Y.; et al. Transcription factor ZmNLP8 modulates nitrate utilization by transactivating ZmNiR1.2 in maize. Plant J. 2025, 122, e70263. [Google Scholar] [CrossRef] [PubMed]
- Hang, T.; Ling, X.; He, C.; Xie, S.; Jiang, H.; Ding, T. Isolation of the zmers4 gene from maize and its functional analysis in transgenic plants. Front. Microbiol. 2021, 12, 632908. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Ge, L.; Ye, X.; Xu, L.; Si, W.; Ding, T. ZmGLP1, a germin-like protein from maize, plays an important role in the regulation of pathogen resistance. Int. J. Mol. Sci. 2022, 23, 14316. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Jiang, H.; Song, Z.; Liu, W.; Rao, S.; Jiang, H.; Wu, G.; Ding, T. Overexpression of ZmEREB211 confers enhanced susceptibility to Pseudomonas syringae pv. tomato DC3000 in Arabidopsis. Plant Sci. 2025, 356, 112482. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.-C.; Zhang, Y.-R.; Liu, Z.-G.; Li, X.-C.; Yu, Z.; Ping, B.-Y.; Sun, Y.-Q.; Burg, H.v.D.; Ma, F.-W.; Zhao, T.; et al. Deciphering Plant NLR Genomic Evolution: Synteny-Informed Classification Unveils Insights into TNL Gene Loss. Mol. Biol. Evol. 2025, 42, msaf015. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Jia, A.; Ma, S.; Sun, Y.; Chang, X.; Han, Z.; Chai, J. NLR signaling in plants: From resistosomes to second messengers. Trends Biochem. Sci. 2023, 48, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.T.; Anderson, R.G.; Cherkis, K.A.; Law, T.F.; Liu, Q.L.; Machius, M.; Nimchuk, Z.L.; Yang, L.; Chung, E.-H.; El Kasmi, F.; et al. TIR-only protein RBA1 recognizes a pathogen effector to regulate cell death in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, E2053–E2062. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Lapin, D.; Liu, L.; Sun, Y.; Song, W.; Zhang, X.; Logemann, E.; Yu, D.; Wang, J.; Jirschitzka, J.; et al. Direct pathogen-induced assembly of an NLR immune receptor complex to form a holoenzyme. Science 2020, 370, eabe3069. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, F.; Du, X.; Zhang, X.; Huang, X.; Li, Z.; Zhang, Y.; Gan, P.; Li, H.; Li, M.; et al. TaANK-TPR1 enhances wheat resistance against stripe rust via controlling gene expression and protein activity of NLR protein TaRPP13L1. Dev. Cell 2025, 60, p1702–p1718.e6. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Zhang, Y.; Qin, Z.; Wang, S.; Liu, C.; Cui, Z.; Liu, D.; Wang, H. Puccinia triticina effector Pt-1234 modulates wheat immunity by targeting transcription factor TaNAC069 via its C subdomain. Crop J. 2025, 13, 69–78. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, S.; Liu, X.; Wang, G.-F. The maize ZmVPS23-like protein relocates the nucleotide-binding leucine-rich repeat protein Rp1-D21 to endosomes and suppresses the defense response. Plant Cell 2023, 35, 2369–2390. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Li, M.; Dong, L.; Zhang, H.; Zhang, D.; Lu, P.; Wu, Q.; Xie, J.; Chen, Y.; Guo, G.; et al. An atypical NLR pair TdCNL1/TdCNL5 from wild emmer confers powdery mildew resistance in wheat. Nat. Genet. 2025, 57, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, H.; Ding, Z.; Yan, J.; Yu, H.; Pan, R.; Hu, J.; Guan, Y.; Hua, J. Low temperature enhances plant immunity via salicylic acid pathway genes that are repressed by ethylene. Plant Physiol. 2019, 182, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhu, K.; Shen, X.; Meng, J.; Huang, X.; Tan, Y.; Cardinale, F.; Liu, J.; Li, G.; Liu, J. Two interacting ethylene response factors negatively regulate peach resistance to Lasiodiplodia theobromae. Plant Physiol. 2023, 192, 3134–3151. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, C.; Du, J.; Sun, Y.; Hu, R.; Liu, S.; Xu, Q.; He, X.; Tang, C.-X.; Xu, R.; et al. DEAD-box protein SMA1 activates immunity likely through the formation of nuclear condensates with EDS1 in Arabidopsis. Cell Rep. 2025, 44, 115895. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, J.; Lu, W.; Deng, D. Gibberellin in plant height control: Old player, new story. Plant Cell Rep. 2017, 36, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, Y.; Cheng, Z.; Zheng, X.; Cai, C.; Wang, H.; Lu, K.; Zhu, C.; Ding, Y. Important Factors Controlling Gibberellin Homeostasis in Plant Height Regulation. J. Agric. Food Chem. 2023, 71, 15895–15907. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, B.; Yang, X.; Zhang, R.; Dong, S.; Liu, Y.; Jiang, H.; Wu, G.; Ding, T. ZmNLR-7-Mediated Synergistic Regulation of ROS, Hormonal Signaling, and Defense Gene Networks Drives Maize Immunity to Southern Corn Leaf Blight. Curr. Issues Mol. Biol. 2025, 47, 573. https://doi.org/10.3390/cimb47070573
Su B, Yang X, Zhang R, Dong S, Liu Y, Jiang H, Wu G, Ding T. ZmNLR-7-Mediated Synergistic Regulation of ROS, Hormonal Signaling, and Defense Gene Networks Drives Maize Immunity to Southern Corn Leaf Blight. Current Issues in Molecular Biology. 2025; 47(7):573. https://doi.org/10.3390/cimb47070573
Chicago/Turabian StyleSu, Bo, Xiaolan Yang, Rui Zhang, Shijie Dong, Ying Liu, Hubiao Jiang, Guichun Wu, and Ting Ding. 2025. "ZmNLR-7-Mediated Synergistic Regulation of ROS, Hormonal Signaling, and Defense Gene Networks Drives Maize Immunity to Southern Corn Leaf Blight" Current Issues in Molecular Biology 47, no. 7: 573. https://doi.org/10.3390/cimb47070573
APA StyleSu, B., Yang, X., Zhang, R., Dong, S., Liu, Y., Jiang, H., Wu, G., & Ding, T. (2025). ZmNLR-7-Mediated Synergistic Regulation of ROS, Hormonal Signaling, and Defense Gene Networks Drives Maize Immunity to Southern Corn Leaf Blight. Current Issues in Molecular Biology, 47(7), 573. https://doi.org/10.3390/cimb47070573





