Transcriptomic Analysis Reveals Candidate Hub Genes and Putative Pathways in Arabidopsis thaliana Roots Responding to Verticillium longisporum Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Materials and Pathogen Inoculation
2.2. Raw Transcriptome Data Collection
2.3. Data Processing and Integration
2.4. Enrichment Analysis of Differentially Expressed Genes
2.5. PPI Network Construction and Module Analysis
2.6. Hub Gene Identification and Co-Expression Network Construction
2.7. RT-qPCR Analysis
2.8. Prediction of the NAC042 Regulatory Network
3. Results
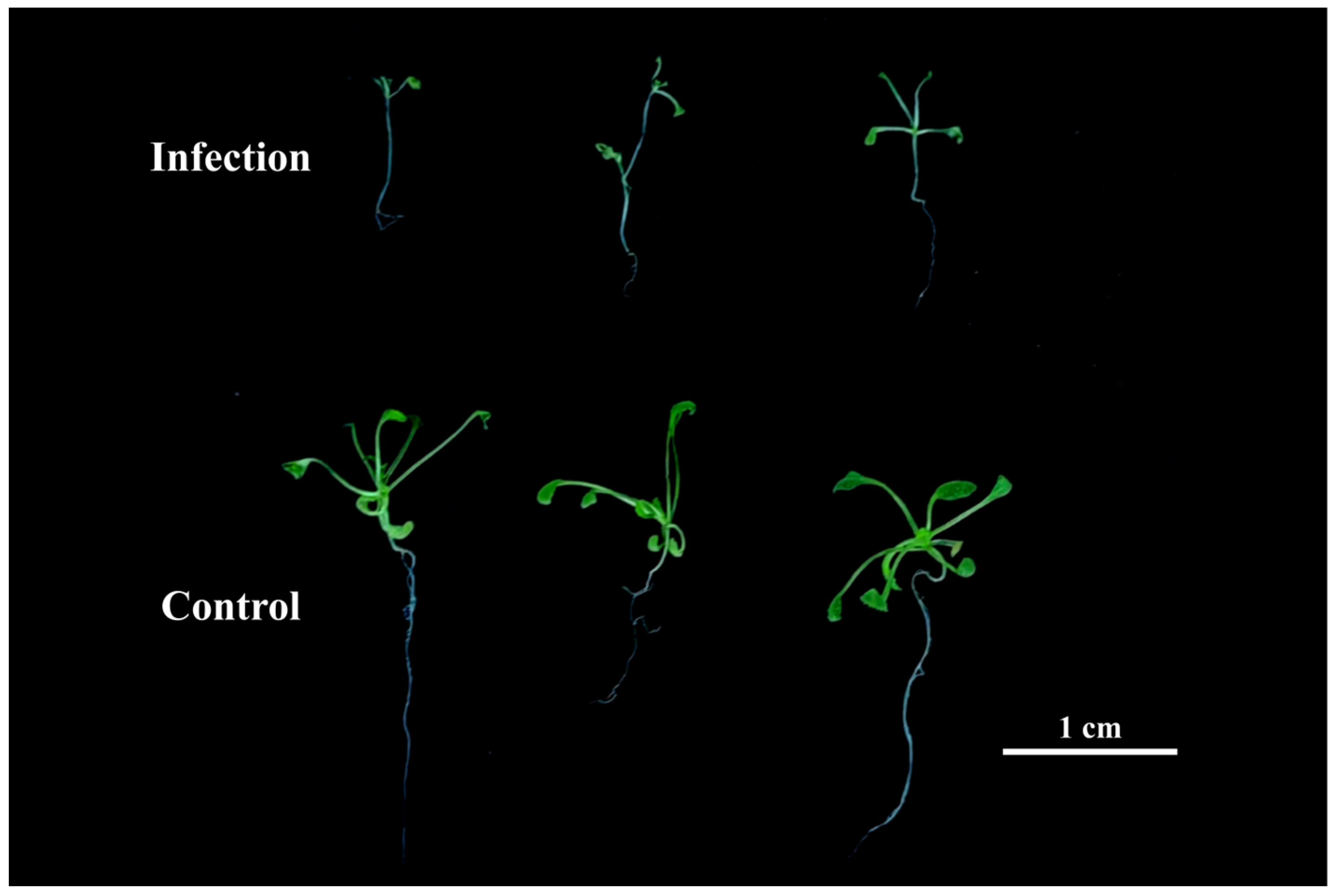
3.1. Phenotypic Evaluation in Response to V. longisporum Infection
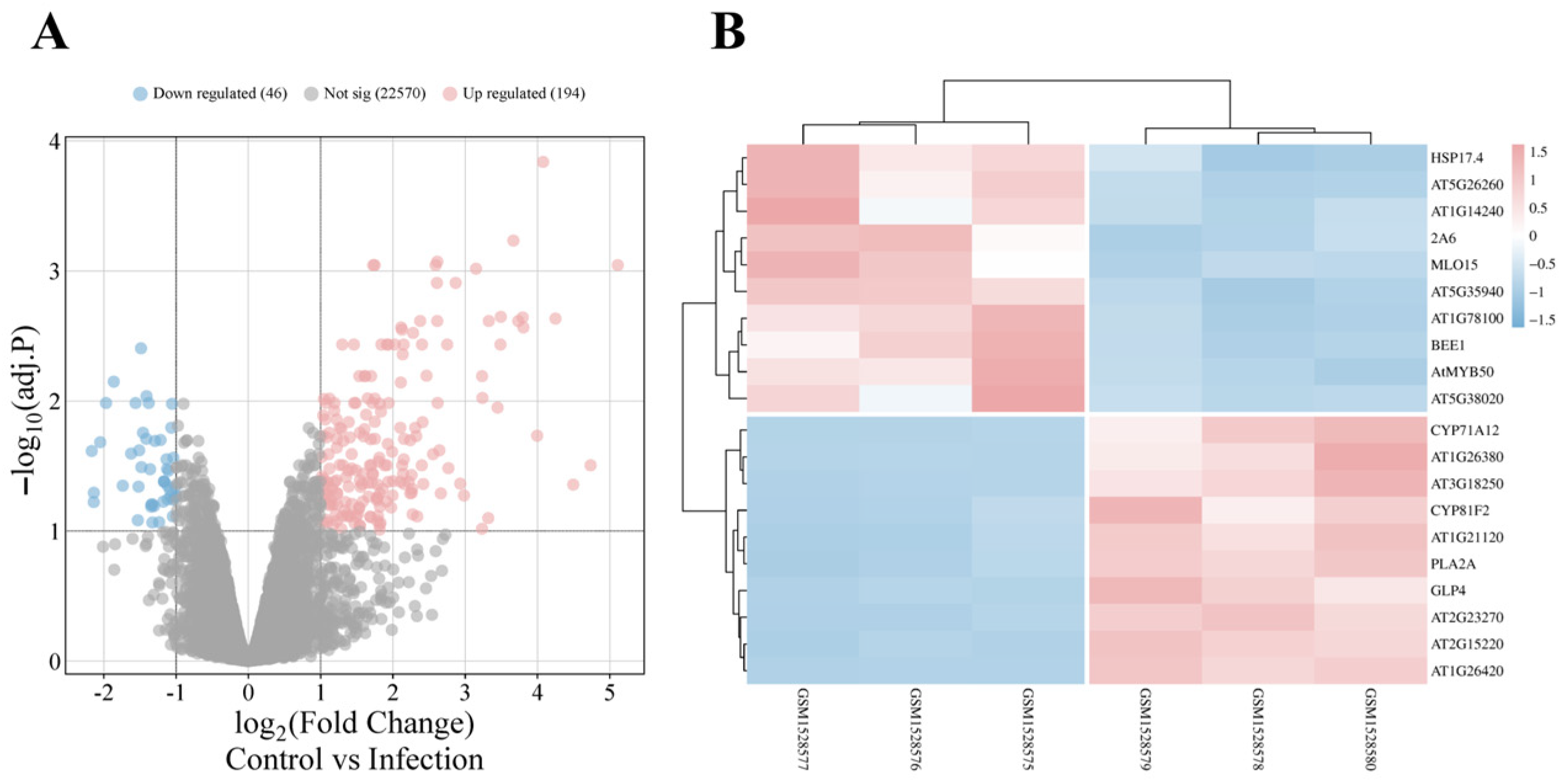
3.2. Identification of DEGs Responsive to V. longisporum Infection
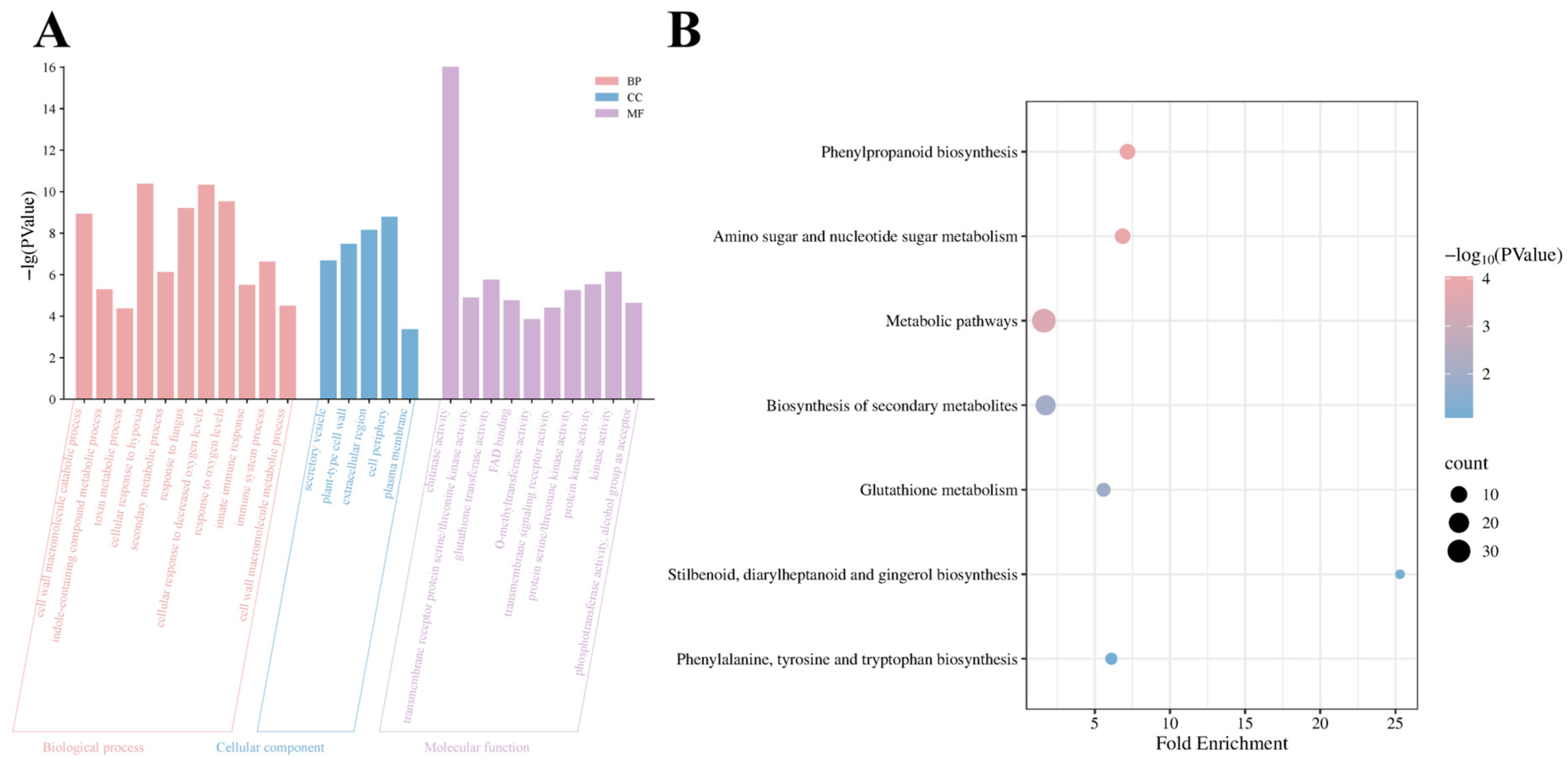
3.3. Functional Characterization of DEGs
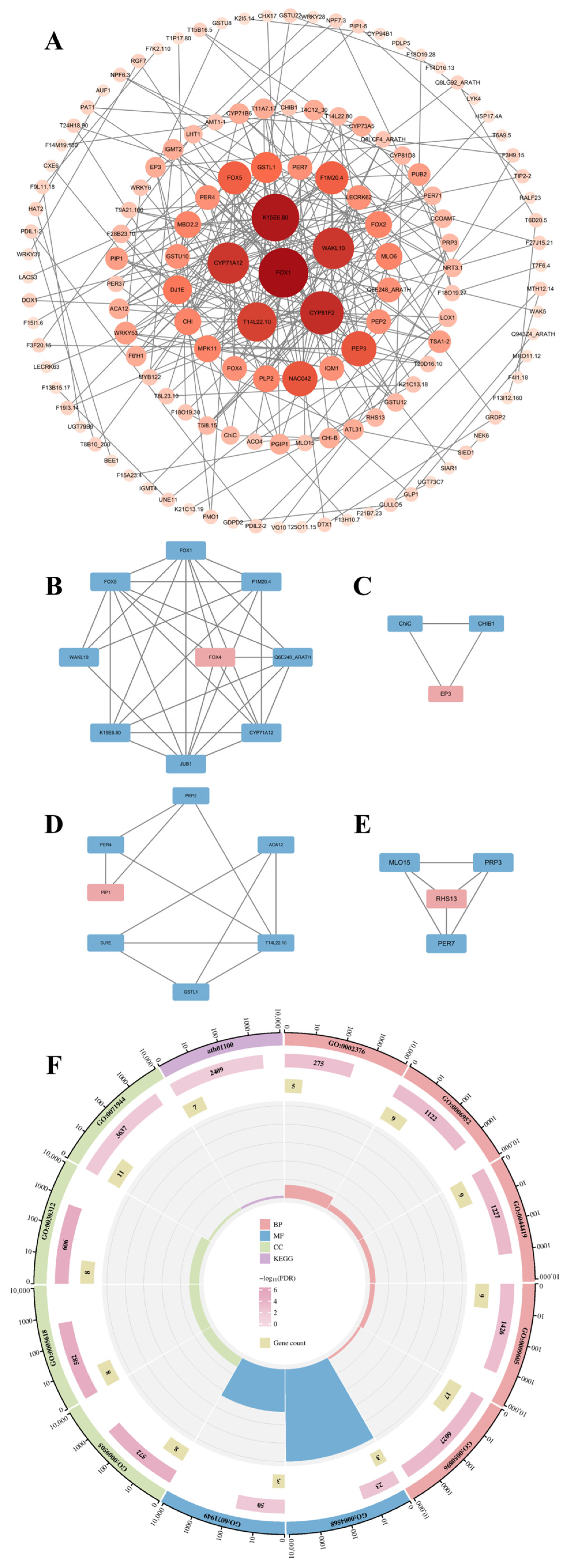
3.4. Construction and Module Analysis of the PPI Network
3.5. Comprehensive Screening and Analysis of Hub Genes
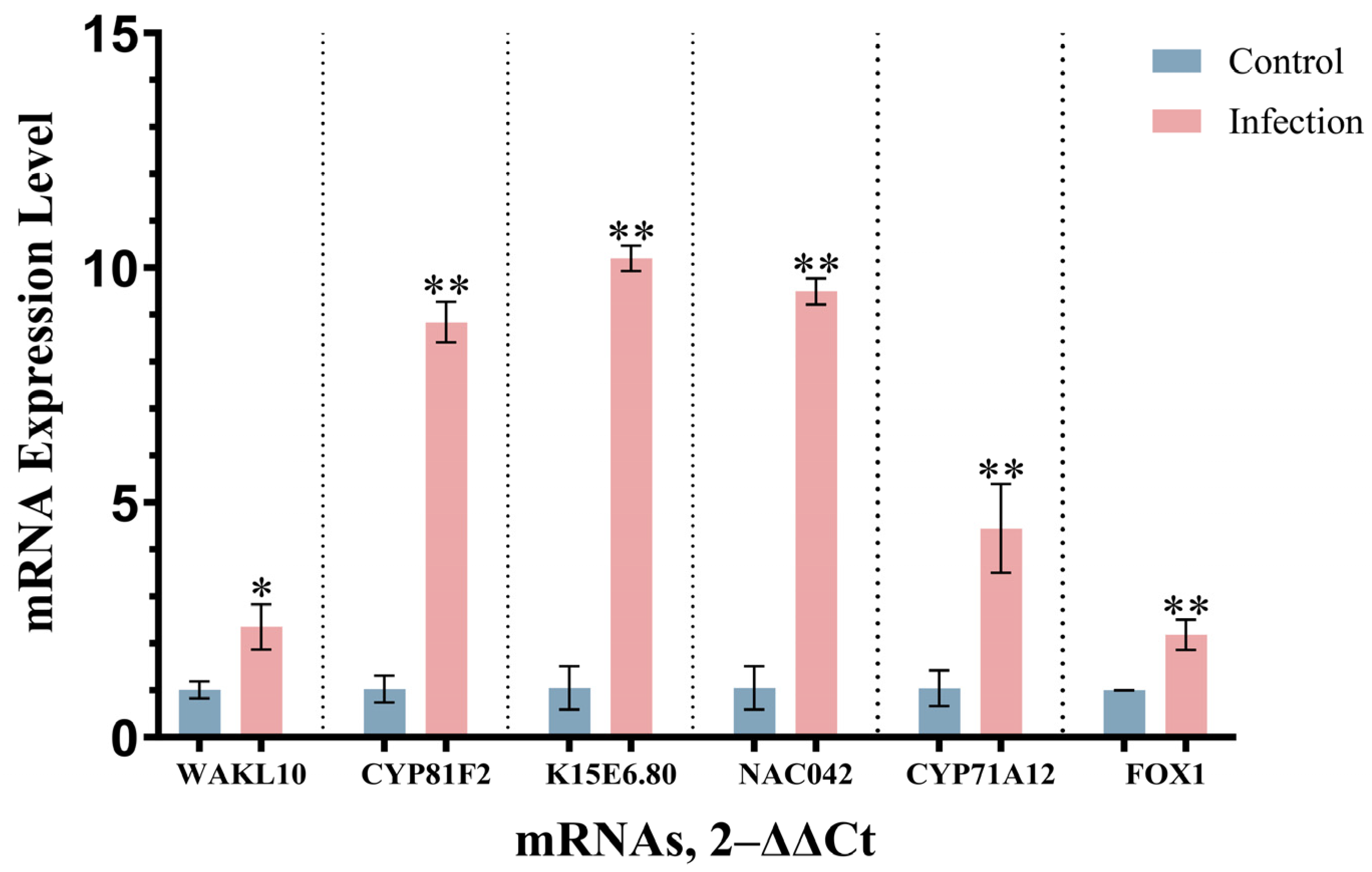
3.6. Analysis of RT-qPCR
3.7. Identification of TFs in DEGs and Prediction of Upstream miRNAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daayf, F. Verticillium wilts in crop plants: Pathogen invasion and host defence responses. Can. J. Plant Pathol. 2014, 37, 8–20. [Google Scholar] [CrossRef]
- Fradin, E.F.; Thomma, B.P.H.J. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Zhao, M.; Li, T.T.; Wang, L.; Liao, C.; Liu, D.; Zhang, H.; Zhao, Y.; Liu, L.; Ge, X.; et al. Interactions between Verticillium dahliae and cotton: Pathogenic mechanism and cotton resistance mechanism to Verticillium wilt. Front. Plant Sci. 2023, 14, 1174281. [Google Scholar] [CrossRef]
- Sayari, M.; Dolatabadian, A.; El-Shetehy, M.; Daayf, F. Genomic insights into Verticillium: A review of progress in the genomics era. Front. Microbiol. 2024, 15, 1463779. [Google Scholar] [CrossRef] [PubMed]
- Klosterman, S.J.; Subbarao, K.V.; Kang, S.; Veronese, P.; Gold, S.E.; Thomma, B.P.H.J.; Chen, Z.; Henrissat, B.; Lee, Y.H.; Park, J.; et al. Comparative genomics yields insights into niche adaptation of plant vascular wilt pathogens. PLoS Pathog. 2011, 7, e1002137. [Google Scholar] [CrossRef]
- Zhang, D.D.; Dai, X.F.; Klosterman, S.J.; Subbarao, K.V.; Chen, J.Y. The secretome of Verticillium dahliae in collusion with plant defence responses modulates Verticillium wilt symptoms. Biol. Rev. Camb. Philos. Soc. 2022, 97, 1810–1822. [Google Scholar] [CrossRef]
- Depotter, J.R.L.; Deketelaere, S.; Inderbitzin, P.; Von Tiedemann, A.; Höfte, M.; Subbarao, K.V.; Wood, T.A.; Thomma, B.P.H.J. Verticillium longisporum, the invisible threat to oilseed rape and other brassicaceous plant hosts. Mol. Plant Pathol. 2016, 17, 1004–1016. [Google Scholar] [CrossRef]
- Ingram, R. Verticillium dahliae var. longisporum, a stable diploid. Trans. Br. Mycol. Soc. 1968, 51, 339–341. [Google Scholar] [CrossRef]
- Karapapa, V.K.; Bainbridge, B.W.; Heale, J.B. Morphological and molecular characterization of Verticillium longisporum comb. nov., pathogenic to oilseed rape. Mycol. Res. 1997, 101, 1281–1294. [Google Scholar] [CrossRef]
- Inderbitzin, P.; Bostock, R.M.; Davis, R.M.; Usami, T.; Platt, H.W.; Subbarao, K.V. Phylogenetics and taxonomy of the fungal vascular wilt pathogen Verticillium, with the descriptions of five new species. PLoS ONE 2011, 6, e28341. [Google Scholar] [CrossRef]
- Inderbitzin, P.; Davis, R.M.; Bostock, R.M.; Subbarao, K.V. Identification and Differentiation of Verticillium Species and V. longisporum Lineages by Simplex and Multiplex PCR Assays. PLoS ONE 2013, 8, e65990. [Google Scholar] [CrossRef] [PubMed]
- Inderbitzin, P.; Davis, R.M.; Bostock, R.M.; Subbarao, K.V. The Ascomycete Verticillium longisporum Is a Hybrid and a Plant Pathogen with an Expanded Host Range. PLoS ONE 2011, 6, e18260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Hu, Q.; Johansson, A.; Dixelius, C. Verticillium longisporum and V. dahliae: Infection and disease in Brassica napus. Plant Pathol. 2006, 55, 137–144. [Google Scholar] [CrossRef]
- Eynck, C.; Koopmann, B.; Grunewaldt-Stoecker, G.; Karlovsky, P.; von Tiedemann, A. Differential interactions of Verticillium longisporum and V. dahliae with Brassica napus detected with molecular and histological techniques. Eur. J. Plant Pathol. 2007, 118, 259–274. [Google Scholar] [CrossRef]
- Kamble, A.; Koopmann, B.; von Tiedemann, A. Induced resistance to Verticillium longisporum in Brassica napus by β-aminobutyric acid. Plant Pathol. 2013, 62, 552–561. [Google Scholar] [CrossRef]
- Heale, J.B.; Karapapa, V.K. The Verticillium threat to Canada’s major oilseed crop: Canola. Can. J. Plant Pathol. 1999, 21, 1–7. [Google Scholar] [CrossRef]
- Fradin, E.F.; Abd-El-Haliem, A.; Masini, L.; van den Berg, G.C.M.; Joosten, M.H.; Thomma, B.P. Interfamily transfer of tomato Ve1 mediates Verticillium resistance in Arabidopsis. Plant Physiol. 2011, 156, 2255–2265. [Google Scholar] [CrossRef]
- Wang, Y.; Fredua-Agyeman, R.; Yu, Z.; Hwang, S.F.; Strelkov, S.E. Genome-wide association study of Verticillium longisporum resistance in Brassica genotypes. Front. Plant Sci. 2024, 15, 1436982. [Google Scholar] [CrossRef]
- Yadeta, K.A.; Valkenburg, D.J.; Hanemian, M.; Marco, Y.; Thomma, B.P.H.J. The Brassicaceae-specific EWR1 gene provides resistance to vascular wilt pathogens. PLoS ONE 2014, 9, e88230. [Google Scholar] [CrossRef]
- Dölfors, F.; Ilbäck, J.; Bejai, S.; Fogelqvist, J.; Dixelius, C. Nitrate transporter protein NPF5.12 and major latex-like protein MLP6 are important defense factors against Verticillium longisporum. J. Exp. Bot. 2024, 75, 4148–4164. [Google Scholar] [CrossRef]
- Pröbsting, M.; Schenke, D.; Hossain, R.; Häder, C.; Thurau, T.; Wighardt, L.; Schuster, A.; Zhou, Z.; Ye, W.; Rietz, S.; et al. Loss of function of CRT1a (calreticulin) reduces plant susceptibility to Verticillium longisporum in both Arabidopsis thaliana and oilseed rape (Brassica napus). Plant Biotechnol. J. 2020, 18, 2328–2344. [Google Scholar] [CrossRef]
- Roos, J.; Bejai, S.; Mozūraitis, R.; Dixelius, C. Susceptibility to Verticillium longisporum is linked to monoterpene production by TPS23/27 in Arabidopsis. Plant J. 2015, 81, 572–585. [Google Scholar] [CrossRef]
- Ulrich, L.; Schmitz, J.; Thurow, C.; Gatz, C. Coronatine Insensitive 1-mediated repression of immunity-related genes in Arabidopsis roots is lifted upon infection with Verticillium longisporum. J. Exp. Bot. 2025, 76, 2356–2372. [Google Scholar] [CrossRef] [PubMed]
- Fröschel, C.; Iven, T.; Walper, E.; Bachmann, V.; Weiste, C.; Dröge-Laser, W. A Gain-of-Function Screen Reveals Redundant ERF Transcription Factors Providing Opportunities for Resistance Breeding Toward the Vascular Fungal Pathogen Verticillium longisporum. Mol. Plant Microbe Interact. 2019, 32, 1095–1109. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Smoot, M.E.; Ono, K.; Ruscheinski, J.; Wang, P.L.; Ideker, T. Cytoscape 2.8: New features for data integration and network visualization. Bioinformatics 2011, 27, 431–432. [Google Scholar] [CrossRef]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010, 38, W214–W220. [Google Scholar] [CrossRef]
- Jin, J.; Tian, F.; Yang, D.C.; Meng, Y.Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017, 45, D1040–D1045. [Google Scholar] [CrossRef]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef]
- Oughtred, R.; Rust, J.; Chang, C.; Breitkreutz, B.J.; Stark, C.; Willems, A.; Boucher, L.; Leung, G.; Kolas, N.; Zhang, F.; et al. The BioGRID database: A comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 2021, 30, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Kemmochi, I.; Kobayashi, I.; Tsuchiya, M.; Sakai, H.; Shimizu, M. Breeding materials for resistance to Verticillium wilt in Japanese cabbage (Brassica oleracea L. var. capitata). J. Jpn. Soc. Hortic Sci. 2000, 69, 483–491. [Google Scholar] [CrossRef][Green Version]
- Rygulla, W.; Snowdon, R.J.; Friedt, W.; Happstadius, I.; Cheung, W.Y.; Chen, D. Identification of quantitative trait loci for resistance against Verticillium longisporum in oilseed rape (Brassica napus). Phytopathology 2008, 98, 215–221. [Google Scholar] [CrossRef]
- Song, R.; Li, J.; Xie, C.; Jian, W.; Yang, X. An Overview of the Molecular Genetics of Plant Resistance to the Verticillium Wilt Pathogen Verticillium dahliae. Int. J. Mol. Sci. 2020, 21, 1120. [Google Scholar] [CrossRef]
- Wittstock, U.; Halkier, B.A. Glucosinolate research in the Arabidopsis era. Trends Plant Sci. 2002, 7, 263–270. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Vallad, G.E.; Park, S.Y.; Kang, S.; Koike, S.T.; Bolda, M.; Burman, P.; Polonik, W.; Subbarao, K.V. Phenological and phytochemical changes correlate with differential interactions of Verticillium dahliae with broccoli and cauliflower. Phytopathology 2011, 101, 523–534. [Google Scholar] [CrossRef]
- Witzel, K.; Hanschen, F.S.; Klopsch, R.; Ruppel, S.; Schreiner, M.; Grosch, R. Verticillium longisporum infection induces organ-specific glucosinolate degradation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 508. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Cytochrome P450 research and The Journal of Biological Chemistry. J. Biol. Chem. 2019, 294, 1671–1680. [Google Scholar] [CrossRef]
- Iven, T.; König, S.; Singh, S.; Braus-Stromeyer, S.A.; Bischoff, M.; Tietze, L.F.; Braus, G.H.; Lipka, V.; Feussner, I.; Dröge-Laser, W. Transcriptional Activation and Production of Tryptophan-Derived Secondary Metabolites in Arabidopsis Roots Contributes to the Defense against the Fungal Vascular Pathogen Verticillium longisporum. Mol. Plant 2012, 5, 1389–1402. [Google Scholar] [CrossRef]
- Yun, H.S.; Sul, W.J.; Chung, H.S.; Lee, J.H.; Kwon, C. Secretory membrane traffic in plant-microbe interactions. New Phytol. 2023, 237, 53–59. [Google Scholar] [CrossRef]
- Xu, J.; Xu, X.; Tian, L.; Wang, G.; Zhang, X.; Wang, X.; Guo, W. Discovery and identification of candidate genes from the chitinase gene family for Verticillium dahliae resistance in cotton. Sci. Rep. 2016, 6, 29022. [Google Scholar] [CrossRef]
- Zhang, M.; Su, J.; Zhang, Y.; Xu, J.; Zhang, S.Q. Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Curr. Opin. Plant Biol. 2018, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.S.; Wang, L. The phenylpropanoid pathway and plant defence-a genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ye, C.; Zhao, Y.; Cheng, X.; Wang, Y.; Jiang, Y.Q.; Yang, B. An oilseed rape WRKY-type transcription factor regulates ROS accumulation and leaf senescence in Nicotiana benthamiana and Arabidopsis through modulating transcription of RbohD and RbohF. Planta 2018, 247, 1323–1338. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wingate, V.P.M.; Lawton, M.A.; Lamb, C.J. Glutathione causes a massive and selective induction of plant defense genes. Plant Physiol. 1988, 87, 206–210. [Google Scholar] [CrossRef]
- Boro, P.; Chattopadhyay, S. Crosstalk between MAPKs and GSH under stress: A critical review. J. Biosci. 2022, 47, 71. [Google Scholar] [CrossRef]
- Liu, Y.; He, C. A review of redox signaling and the control of MAP kinase pathway in plants. Redox Biol. 2017, 11, 192–204. [Google Scholar] [CrossRef]
- Zhao, S.P.; Kamran, M.; Rizwan, M.; Ali, S.; Yan, L.; Alwahibi, M.S.; Elshikh, M.S.; Riaz, M. Regulation of proline metabolism, AsA-GSH cycle, cadmium uptake and subcellular distribution in Brassica napus L. under the effect of nano-silicon. Environ. Pollut. 2023, 335, 122321. [Google Scholar] [CrossRef]
- Ngounou Wetie, A.G.; Sokolowska, I.; Woods, A.G.; Roy, U.; Deinhardt, K.; Darie, C.C. Protein-protein interactions: Switch from classical methods to proteomics and bioinformatics-based approaches. Cell. Mol. Life Sci. 2014, 71, 205–228. [Google Scholar] [CrossRef]
- Müller, T.M.; Böttcher, C.; Morbitzer, R.; Götz, C.C.; Lehmann, J.; Lahaye, T.; Glawischnig, E. TRANSCRIPTION ACTIVATOR-LIKE EFFECTOR NUCLEASE-Mediated Generation and Metabolic Analysis of Camalexin-Deficient cyp71a12 cyp71a13 Double Knockout Lines. Plant Physiol. 2015, 168, 849–858. [Google Scholar] [CrossRef]
- Pastorczyk, M.; Kosaka, A.; Piślewska-Bednarek, M.; López, G.; Frerigmann, H.; Kułak, K.; Glawischnig, E.; Molina, A.; Takano, Y.; Bednarek, P. The role of CYP71A12 monooxygenase in pathogen-triggered tryptophan metabolism and Arabidopsis immunity. New Phytol. 2020, 225, 400–412. [Google Scholar] [CrossRef]
- Meier, S.; Ruzvidzo, O.; Morse, M.; Donaldson, L.; Kwezi, L.; Gehring, C.; Newbigin, E. The Arabidopsis wall associated kinase-like 10 gene encodes a functional guanylyl cyclase and is co-expressed with pathogen defense related genes. PLoS ONE 2010, 5, e8904. [Google Scholar] [CrossRef] [PubMed]
- Marín-de la Rosa, N.; Sotillo, B.; Miskolczi, P.; Gibbs, D.J.; Vicente, J.; Carbonero, P.; Oñate-Sánchez, L.; Holdsworth, M.J.; Bhalerao, R.; Alabadí, D.; et al. Large-scale identification of gibberellin-related transcription factors defines group VII ETHYLENE RESPONSE FACTORS as functional DELLA partners. Plant Physiol. 2014, 166, 1022–1032. [Google Scholar] [CrossRef]
- Shahnejat-Bushehri, S.; Nobmann, B.; Devi Allu, A.; Balazadeh, S. JUB1 suppresses Pseudomonas syringae-induced defense responses through accumulation of DELLA proteins. Plant Signal. Behav. 2016, 11, e1181245. [Google Scholar] [CrossRef] [PubMed]
- Saga, H.; Ogawa, T.; Kai, K.; Suzuki, H.; Ogata, Y.; Sakurai, N.; Shibata, D.; Ohta, D. Identification and characterization of ANAC042, a transcription factor family gene involved in the regulation of camalexin biosynthesis in Arabidopsis. Mol. Plant Microbe Interact. 2012, 25, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, C.; Li, K.; Li, X.; Xu, M.; Guo, Y. CLE14 functions as a “brake signal” to suppress age-dependent and stress-induced leaf senescence by promoting JUB1-mediated ROS scavenging in Arabidopsis. Mol. Plant 2022, 15, 179–188. [Google Scholar] [CrossRef]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef]
- Javed, T.; Gao, S.J. WRKY transcription factors in plant defense. Trends Genet. 2023, 39, 787–801. [Google Scholar] [CrossRef]
- Häffner, E.; Karlovsky, P.; Splivallo, R.; Traczewska, A.; Diederichsen, E. ERECTA, salicylic acid, abscisic acid, and jasmonic acid modulate quantitative disease resistance of Arabidopsis thaliana to Verticillium longisporum. BMC Plant Biol. 2014, 14, 85. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepinie, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Dröge-Laser, W.; Snoek, B.L.; Snel, B.; Weiste, C. The Arabidopsis bZIP transcription factor family-an update. Curr. Opin. Plant Biol. 2018, 45(Pt A), 36–49. [Google Scholar] [CrossRef]
- Sharif, R.; Raza, A.; Chen, P.; Li, Y.; El-Ballat, E.M.; Rauf, A.; Hano, C.; El-Esawi, M.A. HD-ZIP Gene Family: Potential Roles in Improving Plant Growth and Regulating Stress-Responsive Mechanisms in Plants. Genes 2021, 12, 1256. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Hou, X.L.; Xing, G.M.; Liu, J.X.; Duan, A.Q.; Xu, Z.S.; Li, M.Y.; Zhuang, J.; Xiong, A.S. Advances in AP2/ERF super-family transcription factors in plant. Crit. Rev. Biotechnol. 2020, 40, 750–776. [Google Scholar] [CrossRef] [PubMed]
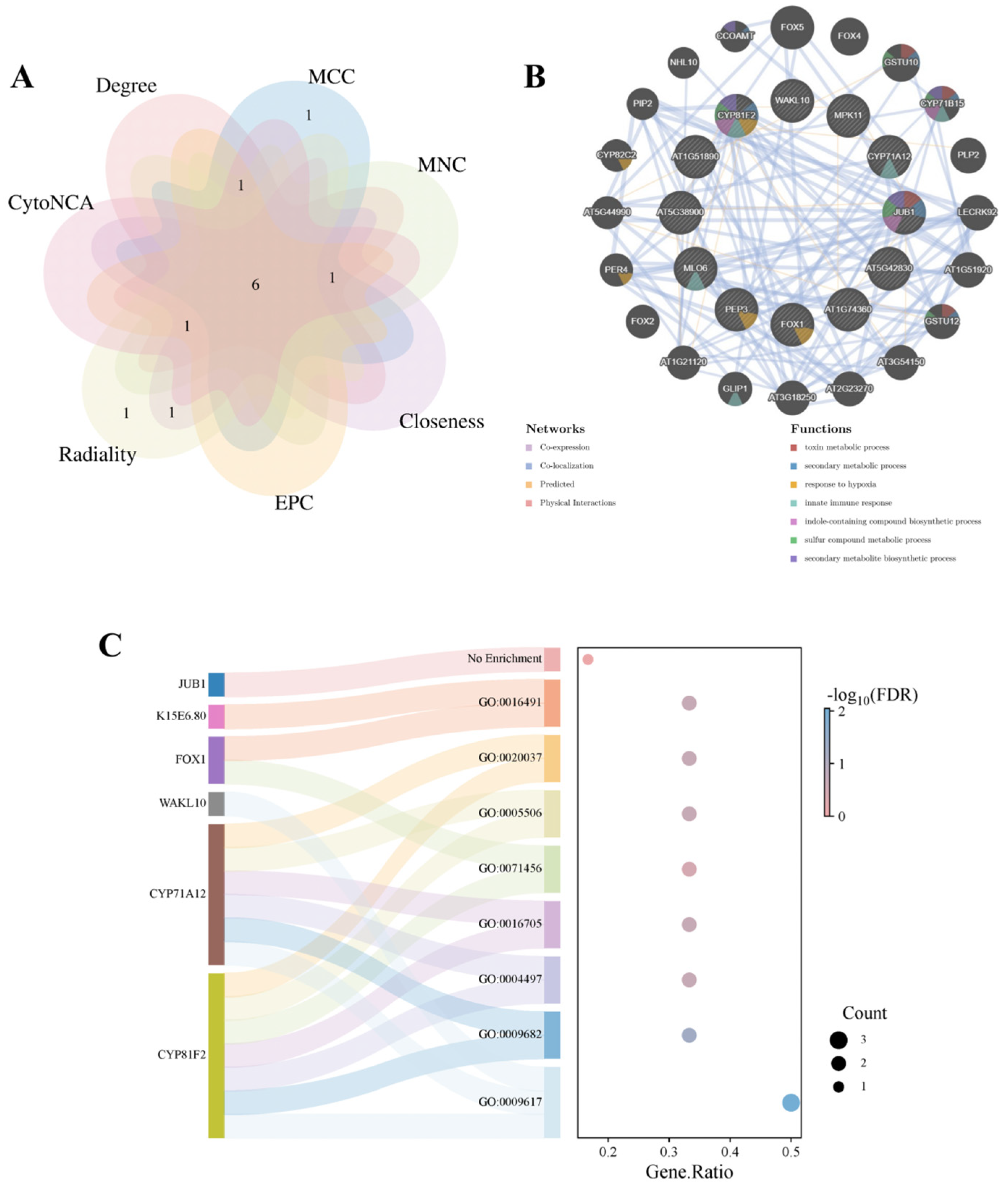

| Degree | MCC | MNC | Closeness | Radiality | EPC | CytoNCA |
|---|---|---|---|---|---|---|
| FOX1 | FOX1 | FOX1 | FOX1 | FOX1 | T14L22.10 | FOX1 |
| FOX5 | K15E6.80 | CYP81F2 | K15E6.80 | F1M20.4 | FOX1 | K15E6.80 |
| F1M20.4 | CYP81F2 | K15E6.80 | CYP81F2 | WAKL10 | K15E6.80 | CYP71A12 |
| NAC042 | CYP71A12 | T14L22.10 | WAKL10 | MLO6 | CYP81F2 | NAC042 |
| CYP71A12 | WAKL10 | WAKL10 | CYP71A12 | CYP71A12 | CYP71A12 | FOX5 |
| WAKL10 | T14L22.10 | CYP71A12 | F1M20.4 | CYP81F2 | WAKL10 | T14L22.10 |
| K15E6.80 | PEP3 | FOX5 | T14L22.10 | K15E6.80 | NAC042 | F1M20.4 |
| CYP81F2 | NAC042 | NAC042 | NAC042 | NAC042 | FOX5 | WAKL10 |
| T14L22.10 | FOX5 | F1M20.4 | MLO6 | MPK11 | F1M20.4 | CYP81F2 |
| TAIR ID | Gene Symbol | Full Name | Function |
|---|---|---|---|
| AT1G26380 | FOX1 | FAD-binding Berberine family protein | Within the biosynthetic pathway of the Arabidopsis cyanogenic phytoalexin 4-hydroxy indole-3-carbonyl nitrile (4-OH-ICN), FOX1 catalyzes the dehydrogenation of indole cyanohydrin, generating indole carbonyl nitrile. |
| AT5G38900 | K15E6.80 | Thioredoxin superfamily protein | This enzyme exhibits protein disulfide oxidoreductase activity and participates in defense responses against fungal pathogens during incompatible interactions. |
| AT5G57220 | CYP81F2 | cytochrome P450, family 81, subfamily F, polypeptide 2 | As a CYP81F subfamily member participating in glucosinolate metabolism, loss-of-function mutants exhibit compromised fungal resistance, while its mRNA demonstrates intercellular mobility. |
| AT2G30750 | CYP71A12 | cytochrome P450 family 71 polypeptide | This putative cytochrome P450 cooperates with CYP71A13 to generate dihydrocamalexic acid (DHCA), the biosynthetic precursor of camalexin. This defense phytoalexin localizes to intercellular spaces and mediates P. syringae resistance in mature Arabidopsis plants via non-antimicrobial pathways. |
| AT1G79680 | WAKL10 | WALL ASSOCIATED KINASE (WAK)-LIKE 10 | The encoded bifunctional kinase/guanylate cyclase protein likely participates in biotic stress response mechanisms through essential cGMP second messenger signaling. |
| AT2G43000 | NAC042 | NAC domain containing protein 42 | This gene encodes an H2O2-inducible NAC transcription factor regulating senescence. Its overexpression significantly postpones senescence and confers enhanced tolerance to diverse abiotic stresses. |
| TAIR ID | Gene Symbol | TF Family | Describe |
|---|---|---|---|
| Upregulated | |||
| AT2G43000 | NAC042 | NAC | See Table 2. |
| AT1G62300 | WRKY6 | WRKY | Encodes a transcription factor WRKY6. Regulates Phosphate1 (Pho1) expression in response to low phosphate stress. Together with WRKY28 and WRKY41 plays a role redundant to WRKY51 in the suppression of RPW8.1. |
| AT4G18170 | WRKY28 | WRKY | This gene encodes WRKY6, a transcription factor that modulates Phosphate1 expression under low-Pi stress. Functionally redundant with WRKY28 and WRKY41, it cooperatively suppresses RPW8.1 alongside WRKY51. |
| AT4G22070 | WRKY31 | WRKY | Classified under Group II-b of the WRKY transcription factor family. |
| AT4G23810 | WRKY53 | WRKY | As a Group III WRKY transcription factor, it participates in antagonistic regulatory networks with WRKY53 and CRK5, collectively controlling chlorophyll metabolism, senescence progression, and stomatal conductance. |
| AT1G74650 | MYB31 | MYB | Member of the R2R3 factor gene family; wax regulator associated with reproductive development. |
| AT1G74080 | MYB122 | MYB | As an R2R3-MYB transcription factor, it modulates cuticular wax biosynthesis during reproductive morphogenesis. |
| AT1G06850 | bZIP52 | bZIP | This bZIP transcription factor mediates heat stress adaptation by relocating exclusively to the nucleus upon thermal challenge. |
| AT1G70920 | HB18 | HD-ZIP | Encodes homeodomain-leucine zipper transcription factor HD-Zip 18. |
| AT3G23230 | TDR1 | ERF | This gene encodes a B-3 subfamily ERF/AP2 transcription factor characterized by a single AP2 domain. The ERF B-3 clade comprises 18 members, with ATERF-1, ATERF-2, and ATERF-5 representing characterized examples. |
| Downregulated | |||
| AT1G18400 | BEE1 | bHLH | Encodes the brassinosteroid signaling component BEE1 (BR-ENHANCED EXPRESSION 1). Positively modulates the shade avoidance syndrome in Arabidopsis seedlings. |
| AT5G47370 | HAT2 | HD-ZIP | Homeobox-leucine zipper genes induced by auxin, but not by other phytohormones. Plays opposite roles in the shoot and root tissues in regulating auxin-mediated morphogenesis. |
| AT3G49930 | AT3G49930 | C2H2 | A zinc finger protein belonging to the C2H2/C2HC superfamily characterized by tandem zinc-binding motifs. |
| AT1G57560 | MYB50 | MYB | As an R2R3-MYB transcription factor, it promotes root cell elongation via transcriptional activation of PECTIN METHYLESTERASE INHIBITOR 8 (PMEI8), while its expression is suppressed by UPB1. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Q.; Zhou, Y.; Ni, S. Transcriptomic Analysis Reveals Candidate Hub Genes and Putative Pathways in Arabidopsis thaliana Roots Responding to Verticillium longisporum Infection. Curr. Issues Mol. Biol. 2025, 47, 536. https://doi.org/10.3390/cimb47070536
Zheng Q, Zhou Y, Ni S. Transcriptomic Analysis Reveals Candidate Hub Genes and Putative Pathways in Arabidopsis thaliana Roots Responding to Verticillium longisporum Infection. Current Issues in Molecular Biology. 2025; 47(7):536. https://doi.org/10.3390/cimb47070536
Chicago/Turabian StyleZheng, Qiwei, Yangpujia Zhou, and Sui Ni. 2025. "Transcriptomic Analysis Reveals Candidate Hub Genes and Putative Pathways in Arabidopsis thaliana Roots Responding to Verticillium longisporum Infection" Current Issues in Molecular Biology 47, no. 7: 536. https://doi.org/10.3390/cimb47070536
APA StyleZheng, Q., Zhou, Y., & Ni, S. (2025). Transcriptomic Analysis Reveals Candidate Hub Genes and Putative Pathways in Arabidopsis thaliana Roots Responding to Verticillium longisporum Infection. Current Issues in Molecular Biology, 47(7), 536. https://doi.org/10.3390/cimb47070536






