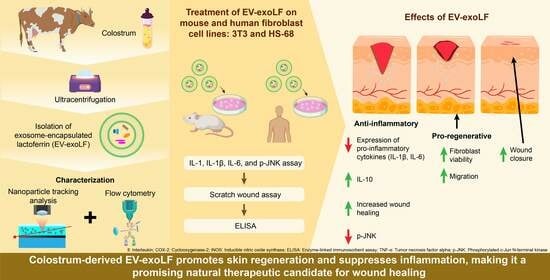
Colostrum-Derived Exosomal Lactoferrin Promotes Skin Fibroblast Regeneration by Suppressing Inflammatory Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. EV-exoLF Extract Isolated from Colostrum
2.3. Cell Line Culture
2.4. CCK-8 Assay
2.5. Nanoparticle Tracking Analysis Measurements
2.6. Flow Cytometry Analysis of EV-exoLF
2.7. Wound Healing Assay
2.8. Enzyme-Linked Immunosorbent Assays
2.9. Western Blotting
3. Results
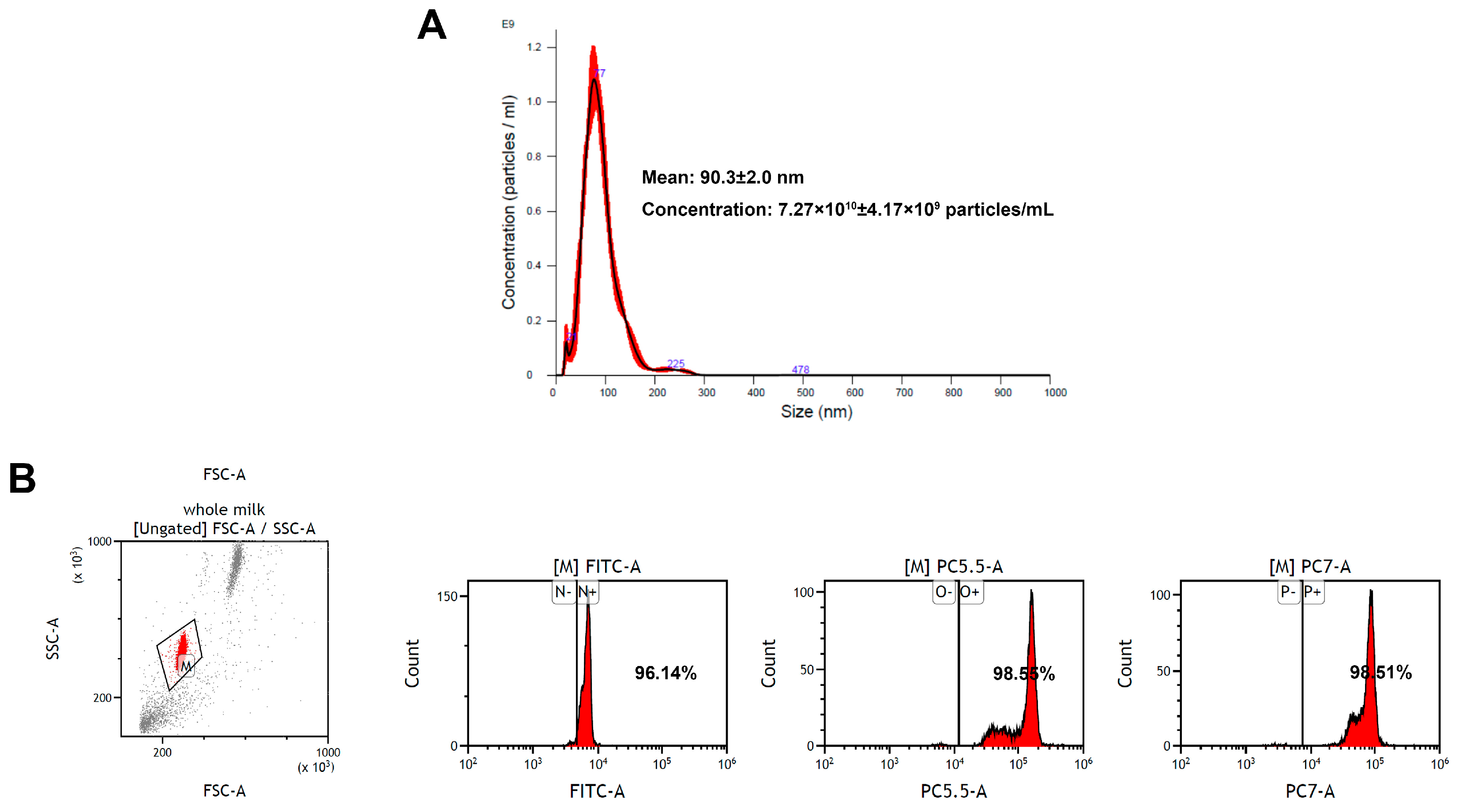
3.1. Characterization of EV-exoLF Derived from Colostrum
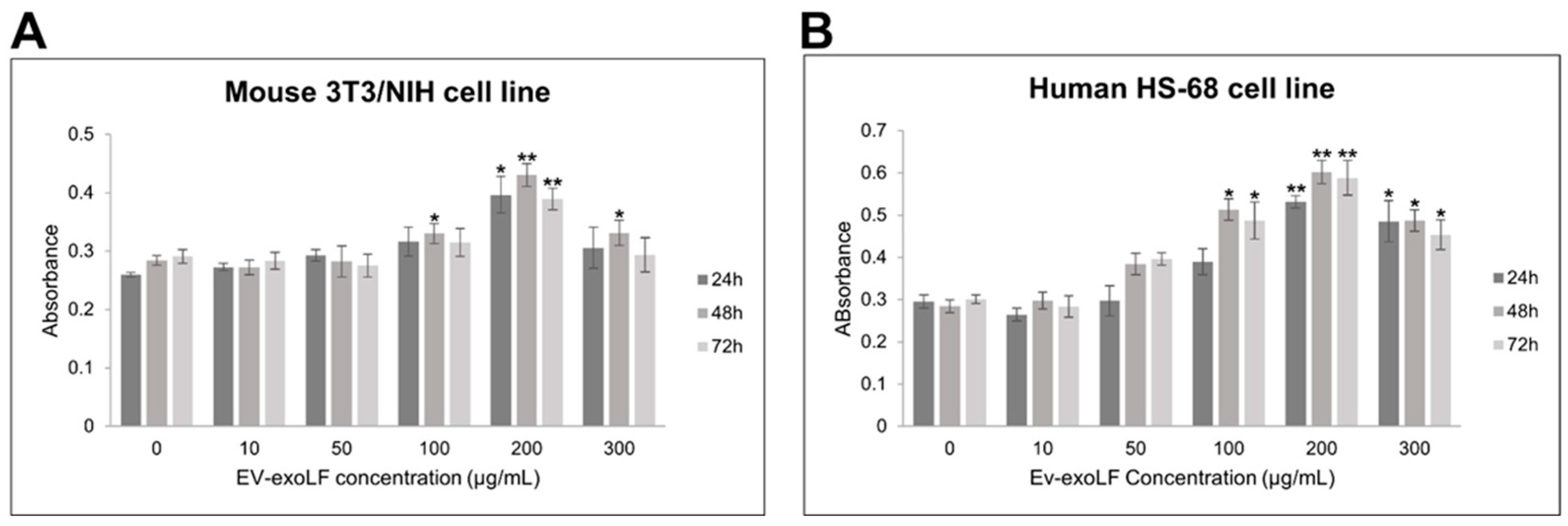
3.2. EV-exoLF Exhibits Dose- and Time-Dependent Effects on Cell Viability
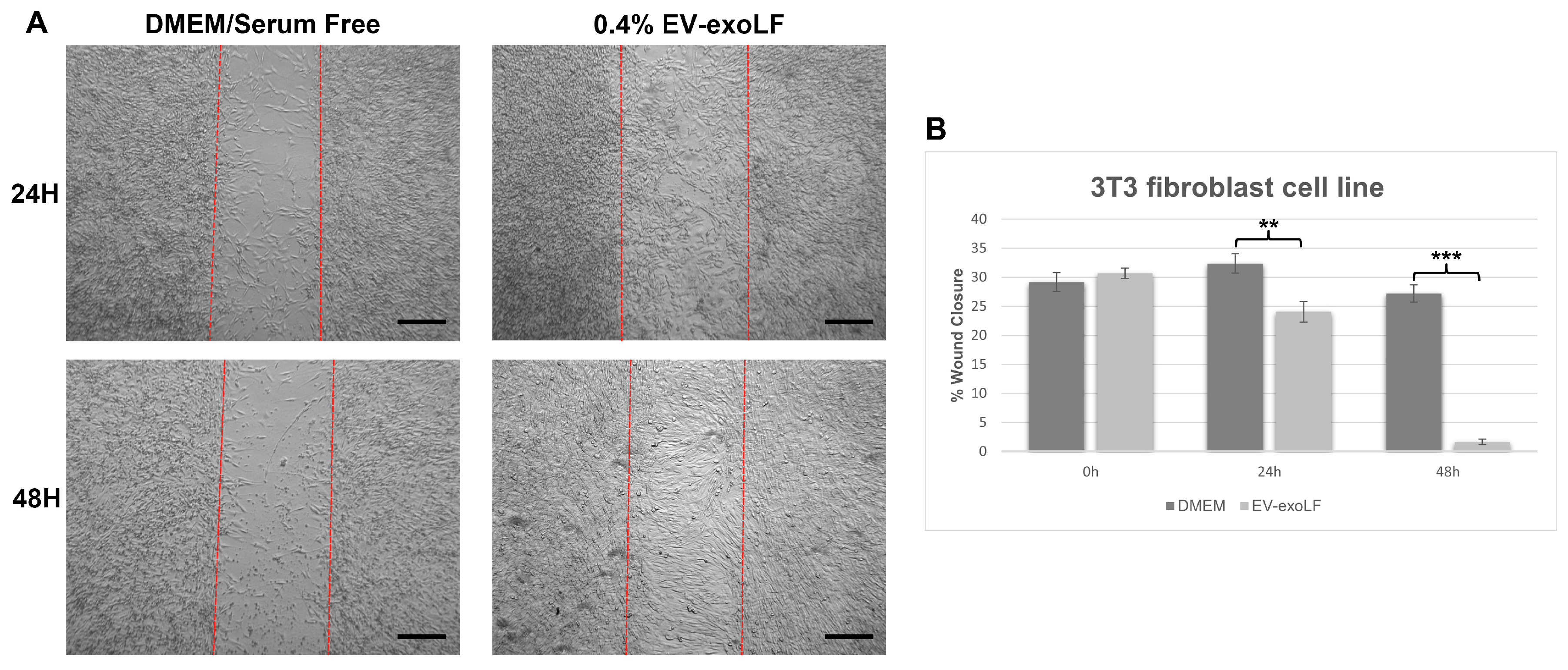
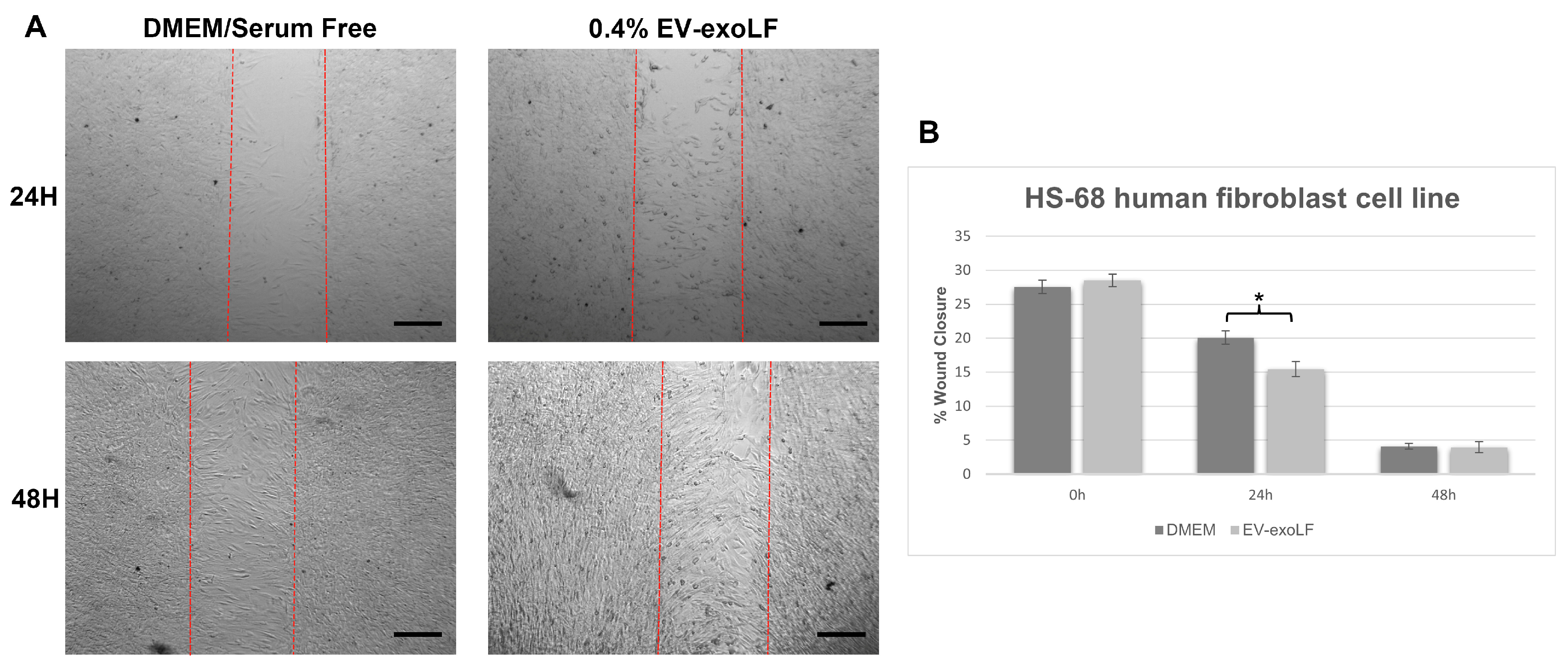
3.3. Capacity of EV-exoLF to Induce Cell Proliferation During the In Vitro Scratch Assay
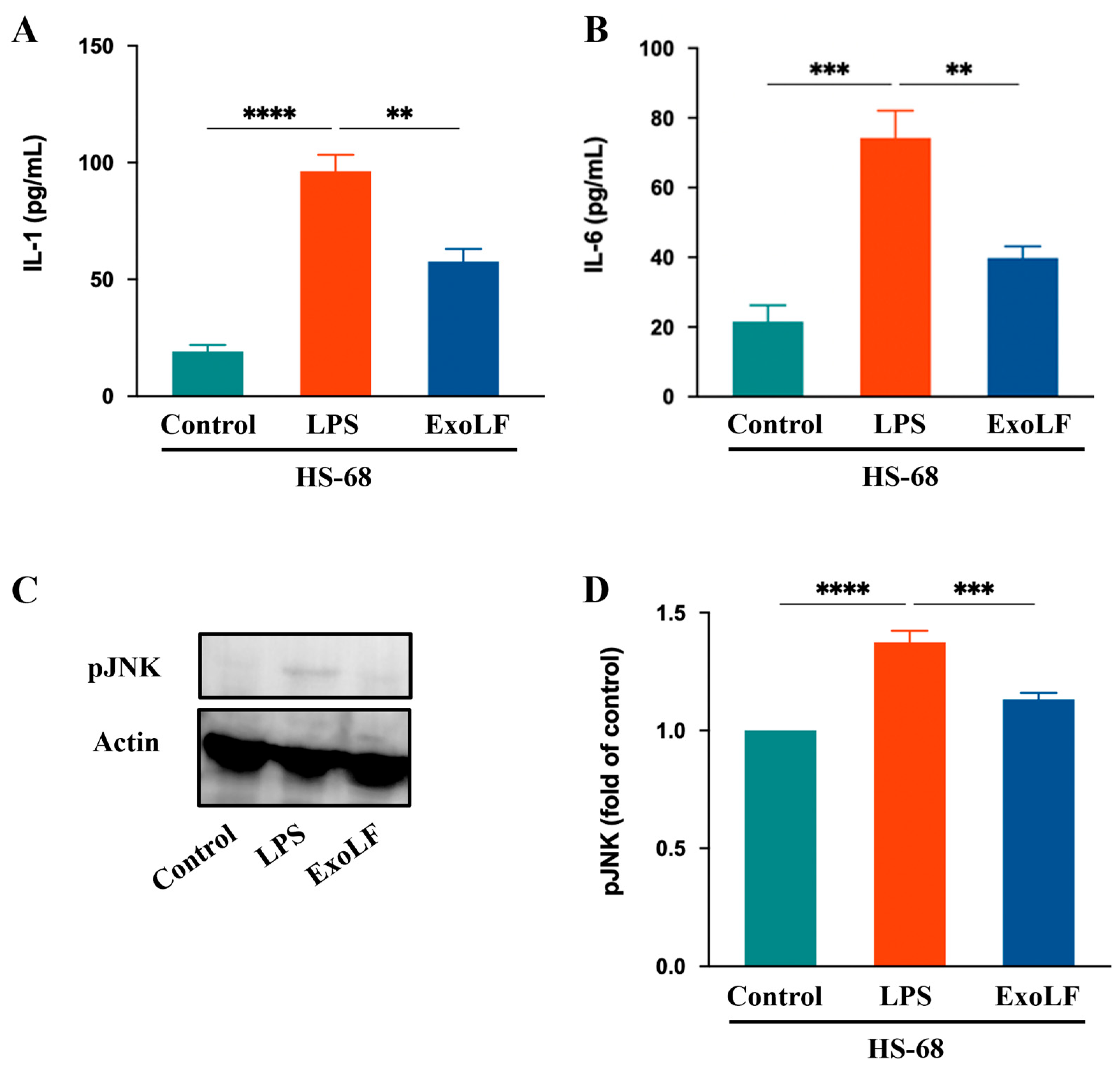
3.4. EV-exoLF Inhibited the Inflammatory Response by Modulating Key Factors in Pro-Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMA | α-Smooth muscle actin |
| ADSC | Adipose-derived mesenchymal stem cell |
| Col1 | Collagen I |
| Col3 | Collagen III |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| ECM | Extracellular matrix |
| ELISA | Enzyme-linked immunosorbent assay |
| EV | Extracellular vesicle |
| EV-exoLF | Exosome-encapsulated lactoferrin |
| HRP | Horseradish peroxidase |
| IL | Interleukin |
| LF | Lactoferrin |
| MSC | Mesenchymal stem cell |
| PBS | Phosphate-buffered saline |
| pJNK | Phosphorylated c-Jun N-terminal kinase |
References
- Fore, J. A review of skin and the effects of aging on skin structure and function. Ostomy Wound Manag. 2006, 52, 24–35; quiz 36-27. [Google Scholar]
- Lai-Cheong, J.E.; McGrath, J.A. Structure and function of skin, hair and nails. Medicine 2009, 37, 223–226. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Baker, P.; Huang, C.; Radi, R.; Moll, S.B.; Jules, E.; Arbiser, J.L. Skin Barrier Function: The Interplay of Physical, Chemical, and Immunologic Properties. Cells 2023, 12, 2745. [Google Scholar] [CrossRef]
- Pena, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Sorg, H.; Sorg, C.G.G. Skin Wound Healing: Of Players, Patterns, and Processes. Eur. Surg. Res. 2023, 64, 141–157. [Google Scholar] [CrossRef]
- Potekaev, N.N.; Borzykh, O.B.; Medvedev, G.V.; Pushkin, D.V.; Petrova, M.M.; Petrov, A.V.; Dmitrenko, D.V.; Karpova, E.I.; Demina, O.M.; Shnayder, N.A. The Role of Extracellular Matrix in Skin Wound Healing. J. Clin. Med. 2021, 10, 5947. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Richardson, R.; Slanchev, K.; Kraus, C.; Knyphausen, P.; Eming, S.; Hammerschmidt, M. Adult zebrafish as a model system for cutaneous wound-healing research. J. Investig. Dermatol. 2013, 133, 1655–1665. [Google Scholar] [CrossRef]
- Seifert, A.W.; Monaghan, J.R.; Voss, S.R.; Maden, M. Skin regeneration in adult axolotls: A blueprint for scar-free healing in vertebrates. PLoS ONE 2012, 7, e32875. [Google Scholar] [CrossRef]
- Takeo, M.; Lee, W.; Ito, M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef] [PubMed]
- Van Loey, N.E.E. Psychological Impact of Living with Scars Following Burn Injury. In Textbook on Scar Management: State of the Art Management and Emerging Technologies; Teot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G., Eds.; Springer: Cham, Switzerland, 2020; pp. 429–434. [Google Scholar]
- Xie, J.; Yao, B.; Han, Y.; Huang, S.; Fu, X. Skin appendage-derived stem cells: Cell biology and potential for wound repair. Burn. Trauma 2016, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rutlin, M.; Abraira, V.E.; Cassidy, C.; Kus, L.; Gong, S.; Jankowski, M.P.; Luo, W.; Heintz, N.; Koerber, H.R.; et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 2011, 147, 1615–1627. [Google Scholar] [CrossRef]
- Dunkin, C.S.J.; Pleat, J.M.; Gillespie, P.H.; Tyler, M.P.H.; Roberts, A.H.N.; McGrouther, D.A. Scarring Occurs at a Critical Depth of Skin Injury: Precise Measurement in a Graduated Dermal Scratch in Human Volunteers. Plast. Reconstr. Surg. 2007, 119, 1722–1732. [Google Scholar] [CrossRef]
- Lumelsky, N.L. Commentary: Engineering of tissue healing and regeneration. Tissue Eng. 2007, 13, 1393–1398. [Google Scholar] [CrossRef]
- Jonidi Shariatzadeh, F.; Currie, S.; Logsetty, S.; Spiwak, R.; Liu, S. Enhancing wound healing and minimizing scarring: A comprehensive review of nanofiber technology in wound dressings. Prog. Mater. Sci. 2025, 147, 101350. [Google Scholar] [CrossRef]
- Lin, X.; Lai, Y. Scarring Skin: Mechanisms and Therapies. Int. J. Mol. Sci. 2024, 25, 1458. [Google Scholar] [CrossRef]
- Hinz, B. Myofibroblasts. Exp. Eye Res. 2016, 142, 56–70. [Google Scholar] [CrossRef]
- Penn, J.W.; Grobbelaar, A.O.; Rolfe, K.J. The role of the TGF-β family in wound healing, burns and scarring: A review. Int. J. Burn. Trauma 2012, 2, 18–28. [Google Scholar]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Sun, X.; Lee, J.H.; Kim, H.W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, S.; Hayashida, K. Advances in surgical applications of growth factors for wound healing. Burn. Trauma 2019, 7, 10. [Google Scholar] [CrossRef]
- Park, J.W.; Hwang, S.R.; Yoon, I.S. Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration. Molecules 2017, 22, 1259. [Google Scholar] [CrossRef]
- Pang, C.; Ibrahim, A.; Bulstrode, N.W.; Ferretti, P. An overview of the therapeutic potential of regenerative medicine in cutaneous wound healing. Int. Wound J. 2017, 14, 450–459. [Google Scholar] [CrossRef]
- Downer, M.; Berry, C.E.; Parker, J.B.; Kameni, L.; Griffin, M. Current Biomaterials for Wound Healing. Bioengineering 2023, 10, 1378. [Google Scholar] [CrossRef]
- Ansari, M.; Darvishi, A. A review of the current state of natural biomaterials in wound healing applications. Front. Bioeng. Biotechnol. 2024, 12, 1309541. [Google Scholar] [CrossRef]
- Kaur, G.; Narayanan, G.; Garg, D.; Sachdev, A.; Matai, I. Biomaterials-Based Regenerative Strategies for Skin Tissue Wound Healing. ACS Appl. Bio Mater. 2022, 5, 2069–2106. [Google Scholar] [CrossRef]
- Prasai, A.; Jay, J.W.; Jupiter, D.; Wolf, S.E.; El Ayadi, A. Role of Exosomes in Dermal Wound Healing: A Systematic Review. J. Investig. Dermatol. 2022, 142 Pt A, 662–678.e8. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, B.; Yang, Y.; Jiang, Q.; Li, T.; Gong, J.; Tang, H.; Zhang, Q. Stem cell-derived exosomes: Emerging therapeutic opportunities for wound healing. Stem Cell Res. Ther. 2023, 14, 107. [Google Scholar] [CrossRef]
- Li, D.; Wu, N. Mechanism and application of exosomes in the wound healing process in diabetes mellitus. Diabetes Res. Clin. Pract. 2022, 187, 109882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- De Toro, J.; Herschlik, L.; Waldner, C.; Mongini, C. Emerging roles of exosomes in normal and pathological conditions: New insights for diagnosis and therapeutic applications. Front. Immunol. 2015, 6, 203. [Google Scholar] [CrossRef] [PubMed]
- Adriano, B.; Cotto, N.M.; Chauhan, N.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. Milk exosomes: Nature’s abundant nanoplatform for theranostic applications. Bioact. Mater. 2021, 6, 2479–2490. [Google Scholar] [CrossRef]
- Rashidi, M.; Bijari, S.; Khazaei, A.H.; Shojaei-Ghahrizjani, F.; Rezakhani, L. The role of milk-derived exosomes in the treatment of diseases. Front. Genet. 2022, 13, 1009338. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Paramonik, A.P.; Sedykh, S.S.; Nevinsky, G.A. Milk Exosomes: Next-Generation Agents for Delivery of Anticancer Drugs and Therapeutic Nucleic Acids. Int. J. Mol. Sci. 2023, 24, 10194. [Google Scholar] [CrossRef]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filen, J.J.; Lahesmaa, R.; Norman, M.; Neve, E.P.; Scheynius, A.; Gabrielsson, S. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007, 179, 1969–1978. [Google Scholar] [CrossRef]
- El-Kattawy, A.M.; Algezawy, O.; Alfaifi, M.Y.; Noseer, E.A.; Hawsawi, Y.M.; Alzahrani, O.R.; Algarni, A.; Kahilo, K.A.; El-Magd, M.A. Therapeutic potential of camel milk exosomes against HepaRG cells with potent apoptotic, anti-inflammatory, and anti-angiogenesis effects for colostrum exosomes. Biomed. Pharmacother. 2021, 143, 112220. [Google Scholar] [CrossRef]
- Giansanti, F.; Panella, G.; Leboffe, L.; Antonini, G. Lactoferrin from Milk: Nutraceutical and Pharmacological Properties. Pharmaceuticals 2016, 9, 61. [Google Scholar] [CrossRef]
- Cui, S.; Lv, X.; Sun, G.; Wu, W.; Xu, H.; Li, Y.; Liu, Y.; Li, J.; Du, G.; Wang, M.; et al. Recent advances and prospects in purification and heterologous expression of lactoferrin. Food Bioeng. 2022, 1, 58–67. [Google Scholar] [CrossRef]
- Rosa, L.; Ianiro, G.; Conte, A.L.; Conte, M.P.; Ottolenghi, L.; Valenti, P.; Cutone, A. Antibacterial, anti-invasive, and anti-inflammatory activity of bovine lactoferrin extracted from milk or colostrum versus whole colostrum. Biochem. Cell Biol. 2024, 102, 331–341. [Google Scholar] [CrossRef] [PubMed]
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, 301.e301–301.e308. [Google Scholar] [CrossRef]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Lonnerdal, B.; Iyer, S. Lactoferrin: Molecular structure and biological function. Annu. Rev. Nutr. 1995, 15, 93–110. [Google Scholar] [CrossRef]
- Comfort, N.; Cai, K.; Bloomquist, T.R.; Strait, M.D.; Ferrante, A.W., Jr.; Baccarelli, A.A. Nanoparticle Tracking Analysis for the Quantification and Size Determination of Extracellular Vesicles. J. Vis. Exp. 2021. [Google Scholar] [CrossRef]
- Bachurski, D.; Schuldner, M.; Nguyen, P.H.; Malz, A.; Reiners, K.S.; Grenzi, P.C.; Babatz, F.; Schauss, A.C.; Hansen, H.P.; Hallek, M.; et al. Extracellular vesicle measurements with nanoparticle tracking analysis—An accuracy and repeatability comparison between NanoSight NS300 and ZetaView. J. Extracell. Vesicles 2019, 8, 1596016. [Google Scholar] [CrossRef]
- Zhang, D.; Li, B.; Zhao, M. Therapeutic Strategies by Regulating Interleukin Family to Suppress Inflammation in Hypertrophic Scar and Keloid. Front. Pharmacol. 2021, 12, 667763. [Google Scholar] [CrossRef]
- Nikoloudaki, G.; Brooks, S.; Peidl, A.P.; Tinney, D.; Hamilton, D.W. JNK Signaling as a Key Modulator of Soft Connective Tissue Physiology, Pathology, and Healing. Int. J. Mol. Sci. 2020, 21, 1015. [Google Scholar] [CrossRef]
- Shaw, T.J.; Martin, P. Wound repair at a glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef]
- Greaves, N.S.; Ashcroft, K.J.; Baguneid, M.; Bayat, A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J. Dermatol. Sci. 2013, 72, 206–217. [Google Scholar] [CrossRef]
- Saleem, M.; Shahzad, K.A.; Marryum, M.; Singh, S.; Zhou, Q.; Du, S.; Wang, S.; Shao, C.; Shaikh, I.I. Exosome-based therapies for inflammatory disorders: A review of recent advances. Stem Cell Res. Ther. 2024, 15, 477. [Google Scholar] [CrossRef] [PubMed]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, M.; Sun, Y.; Yang, D.; Xu, W.; Qian, H. Exosomes: Emerging Cell-Free Based Therapeutics in Dermatologic Diseases. Front. Cell Dev. Biol. 2021, 9, 736022. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R. Keloid and Hypertrophic Scars Are the Result of Chronic Inflammation in the Reticular Dermis. Int. J. Mol. Sci. 2017, 18, 606. [Google Scholar] [CrossRef]
- Ogawa, R.; Akaishi, S. Endothelial dysfunction may play a key role in keloid and hypertrophic scar pathogenesis—Keloids and hypertrophic scars may be vascular disorders. Med. Hypotheses 2016, 96, 51–60. [Google Scholar] [CrossRef]
- Wang, Z.C.; Zhao, W.Y.; Cao, Y.; Liu, Y.Q.; Sun, Q.; Shi, P.; Cai, J.Q.; Shen, X.Z.; Tan, W.Q. The Roles of Inflammation in Keloid and Hypertrophic Scars. Front. Immunol. 2020, 11, 603187. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Wang, S.; Bi, J.; Huo, R. Exosomes from human adipose-derived mesenchymal stem cells inhibit production of extracellular matrix in keloid fibroblasts via downregulating transforming growth factor-beta2 and Notch-1 expression. Bioengineered 2022, 13, 8515–8525. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Shi, J.; Liu, K.; Wang, X.; Jia, Y.; He, T.; Shen, K.; Wang, Y.; Liu, J.; et al. Correction to: Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res. Ther. 2021, 12, 221, Erratum in Stem Cell Res. Ther. 2021, 12, 490. https://doi.org/10.1186/s13287-021-02568-3. [Google Scholar] [CrossRef]
- Somiya, M.; Yoshioka, Y.; Ochiya, T. Biocompatibility of highly purified bovine milk-derived extracellular vesicles. J. Extracell. Vesicles 2018, 7, 1440132. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Shimizu, K.; Rahman, M.; Ishikawa, H.; Takase, H.; Ugawa, S.; Okada, A.; Inoshima, Y. Efficient method for isolation of exosomes from raw bovine milk. Drug Dev. Ind. Pharm. 2019, 45, 359–364. [Google Scholar] [CrossRef]
- Samuel, M.; Chisanga, D.; Liem, M.; Keerthikumar, S.; Anand, S.; Ang, C.S.; Adda, C.G.; Versteegen, E.; Jois, M.; Mathivanan, S. Bovine milk-derived exosomes from colostrum are enriched with proteins implicated in immune response and growth. Sci. Rep. 2017, 7, 5933. [Google Scholar] [CrossRef]
- Stremmel, W.; Weiskirchen, R.; Melnik, B.C. Milk Exosomes Prevent Intestinal Inflammation in a Genetic Mouse Model of Ulcerative Colitis: A Pilot Experiment. Inflamm. Intest. Dis. 2020, 5, 117–123. [Google Scholar] [CrossRef]
- Cui, Z.; Amevor, F.K.; Zhao, X.; Mou, C.; Pang, J.; Peng, X.; Liu, A.; Lan, X.; Liu, L. Potential therapeutic effects of milk-derived exosomes on intestinal diseases. J. Nanobiotechnol. 2023, 21, 496. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Kim, H.; Kim, D.E.; Ahn, Y.; Kim, J.; Jang, Y.J.; Kim, K.; Yang, Y.; Kim, S.H. The Potential of Bovine Colostrum-Derived Exosomes to Repair Aged and Damaged Skin Cells. Pharmaceutics 2022, 14, 307. [Google Scholar] [CrossRef]
- Lu, L.; Bai, W.; Wang, M.; Han, C.; Du, H.; Wang, N.; Gao, M.; Li, D.; Dong, F.; Ge, X. Novel roles of bovine milk-derived exosomes in skin antiaging. J. Cosmet. Dermatol. 2024, 23, 1374–1385. [Google Scholar] [CrossRef]
- Ahn, G.; Kim, Y.H.; Ahn, J.Y. Multifaceted effects of milk-exosomes (Mi-Exo) as a modulator of scar-free wound healing. Nanoscale Adv. 2021, 3, 528–537. [Google Scholar] [CrossRef]
- Johnson, B.Z.; Stevenson, A.W.; Prele, C.M.; Fear, M.W.; Wood, F.M. The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef]
- Moretti, L.; Stalfort, J.; Barker, T.H.; Abebayehu, D. The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation. J. Biol. Chem. 2022, 298, 101530. [Google Scholar] [CrossRef]
- Kong, J.; Grando, S.A.; Li, Y.C. Regulation of IL-1 family cytokines IL-1alpha, IL-1 receptor antagonist, and IL-18 by 1,25-dihydroxyvitamin D3 in primary keratinocytes. J. Immunol. 2006, 176, 3780–3787. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.C.; Tan, M.J.; Philippe, V.; Tan, S.H.; Tan, C.K.; Ku, C.W.; Goh, Y.Y.; Wahli, W.; Michalik, L.; Tan, N.S. Regulation of epithelial–mesenchymal IL-1 signaling by PPARβ/δ is essential for skin homeostasis and wound healing. J. Cell Biol. 2009, 184, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.Y.; Guan, D.W.; Yang, M.; Zhao, R.; Zheng, J.L.; Wang, L.; Yu, T.S.; Cheng, Z.H.; Hu, G.Y.; Zhu, B.L. The time-dependent changes of phospho-JNK expression during the skin incised wound healing in mice. Fa Yi Xue Za Zhi 2008, 24, 241–244. [Google Scholar] [PubMed]
- Xiong, C.Y.; Guan, D.W.; Liu, Z.H.; Zhen, B.; Zhao, R.; Zhu, B.L. Changes of phospho-JNK expression during the skin burned wound healing. Fa Yi Xue Za Zhi 2008, 24, 325–326, 335. [Google Scholar]
- Reich, N.; Tomcik, M.; Zerr, P.; Lang, V.; Dees, C.; Avouac, J.; Palumbo, K.; Horn, A.; Akhmetshina, A.; Beyer, C.; et al. Jun N-terminal kinase as a potential molecular target for prevention and treatment of dermal fibrosis. Ann. Rheum. Dis. 2012, 71, 737–745. [Google Scholar] [CrossRef]
- Takayama, Y.; Takezawa, T. Lactoferrin promotes collagen gel contractile activity of fibroblasts mediated by lipoprotein receptors. Biochem. Cell Biol. 2006, 84, 268–274. [Google Scholar] [CrossRef]
- Takayama, Y.; Aoki, R. Roles of lactoferrin on skin wound healing. Biochem. Cell Biol. 2012, 90, 497–503. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; Campos-Rodríguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin: Balancing Ups and Downs of Inflammation Due to Microbial Infections. Int. J. Mol. Sci. 2017, 18, 501. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Hammouda, M.B.; Ford, A.E.; Liu, Y.; Zhang, J.Y. The JNK Signaling Pathway in Inflammatory Skin Disorders and Cancer. Cells 2020, 9, 857. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Zhu, K.; He, W.; Guo, X.; Wang, T.; Gong, S.; Zhu, Z. Exosomes from mesenchymal stem cells: Potential applications in wound healing. Life Sci. 2024, 357, 123066. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, D.E.; Han, G.; Lim, N.R.; Kim, E.H.; Jang, Y.; Cho, H.; Jang, H.; Kim, K.H.; Kim, S.H.; et al. Harnessing the Natural Healing Power of Colostrum: Bovine Milk-Derived Extracellular Vesicles from Colostrum Facilitating the Transition from Inflammation to Tissue Regeneration for Accelerating Cutaneous Wound Healing. Adv. Healthc. Mater. 2022, 11, e2102027. [Google Scholar] [CrossRef]
- Janik-Karpinska, E.; Ceremuga, M.; Niemcewicz, M.; Synowiec, E.; Sliwinski, T.; Stela, M.; Bijak, M. DNA Damage Induced by T-2 Mycotoxin in Human Skin Fibroblast Cell Line-Hs68. Int. J. Mol. Sci. 2023, 24, 14458. [Google Scholar] [CrossRef]
- Sanadgol, N.; Abedi, M.; Hashemzaei, M.; Kamran, Z.; Khalseh, R.; Beyer, C.; Voelz, C. Exosomes as nanocarriers for brain-targeted delivery of therapeutic nucleic acids: Advances and challenges. J. Nanobiotechnology 2025, 23, 453. [Google Scholar] [CrossRef]
- Ohradanova-Repic, A.; Prazenicova, R.; Gebetsberger, L.; Moskalets, T.; Skrabana, R.; Cehlar, O.; Tajti, G.; Stockinger, H.; Leksa, V. Time to Kill and Time to Heal: The Multifaceted Role of Lactoferrin and Lactoferricin in Host Defense. Pharmaceutics 2023, 15, 1056. [Google Scholar] [CrossRef]
- Isaac, R.; Reis, F.C.G.; Ying, W.; Olefsky, J.M. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021, 33, 1744–1762. [Google Scholar] [CrossRef]
- Valluru, M.; Staton, C.A.; Reed, M.W.; Brown, N.J. Transforming Growth Factor-beta and Endoglin Signaling Orchestrate Wound Healing. Front. Physiol. 2011, 2, 89. [Google Scholar] [CrossRef]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care New Rochelle 2014, 3, 647–661. [Google Scholar] [CrossRef]
- Tran, N.; Garcia, T.; Aniqa, M.; Ali, S.; Ally, A.; Nauli, S.M. Endothelial Nitric Oxide Synthase (eNOS) and the Cardiovascular System: In Physiology and in Disease States. Am. J. Biomed. Sci. Res. 2022, 15, 153–177. [Google Scholar]
- Singh, D.; Rai, V.; Agrawal, D.K. Regulation of Collagen I and Collagen III in Tissue Injury and Regeneration. Cardiol. Cardiovasc. Med. 2023, 7, 5–16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.-H.; Hong, W.-J.; Li, C.-N.; Huang, Y.-H.; Tsai, J.-H.; Huang, C.-Y.; Wu, J.-C.; Kuo, C.-Y.; Kuo, W.-C. Colostrum-Derived Exosomal Lactoferrin Promotes Skin Fibroblast Regeneration by Suppressing Inflammatory Responses. Curr. Issues Mol. Biol. 2025, 47, 549. https://doi.org/10.3390/cimb47070549
Cheng C-H, Hong W-J, Li C-N, Huang Y-H, Tsai J-H, Huang C-Y, Wu J-C, Kuo C-Y, Kuo W-C. Colostrum-Derived Exosomal Lactoferrin Promotes Skin Fibroblast Regeneration by Suppressing Inflammatory Responses. Current Issues in Molecular Biology. 2025; 47(7):549. https://doi.org/10.3390/cimb47070549
Chicago/Turabian StyleCheng, Chu-Hsun, Wei-Jer Hong, Chien-Nien Li, Yung-Hsueh Huang, Jeng-Haw Tsai, Chih-Yuan Huang, Jen-Chin Wu, Chan-Yen Kuo, and Wen-Chun Kuo. 2025. "Colostrum-Derived Exosomal Lactoferrin Promotes Skin Fibroblast Regeneration by Suppressing Inflammatory Responses" Current Issues in Molecular Biology 47, no. 7: 549. https://doi.org/10.3390/cimb47070549
APA StyleCheng, C.-H., Hong, W.-J., Li, C.-N., Huang, Y.-H., Tsai, J.-H., Huang, C.-Y., Wu, J.-C., Kuo, C.-Y., & Kuo, W.-C. (2025). Colostrum-Derived Exosomal Lactoferrin Promotes Skin Fibroblast Regeneration by Suppressing Inflammatory Responses. Current Issues in Molecular Biology, 47(7), 549. https://doi.org/10.3390/cimb47070549