Erythroblasts Promote the Development of a Suppressive Lymphocyte Phenotype via Treg Induction and PD1 Upregulation on the Surfaces of B-Cells: A Study on the Subpopulation-Specific Features of Erythroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Experimental Models
2.2. Erythroblast Cells Isolation
2.3. Cell Culturing and Harvesting the Conditioned Media of Erythroid Cells
2.4. Phenotypic Analysis of Lymphoid Cells Exposed to Erythroblast-Derived Soluble Factors
2.5. Phenotypic Characterization of Erythroblasts
2.6. Data Analysis
3. Results
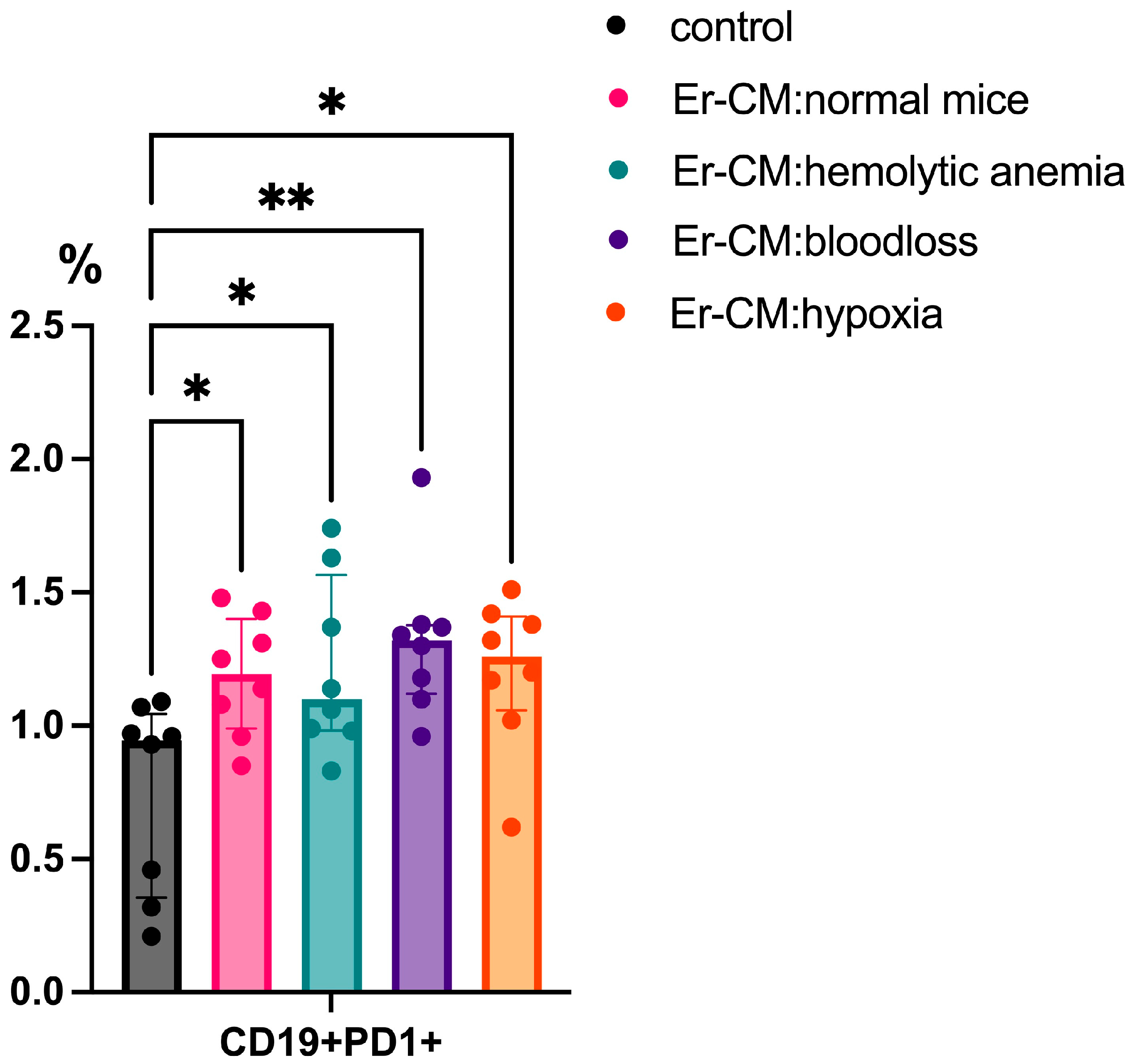
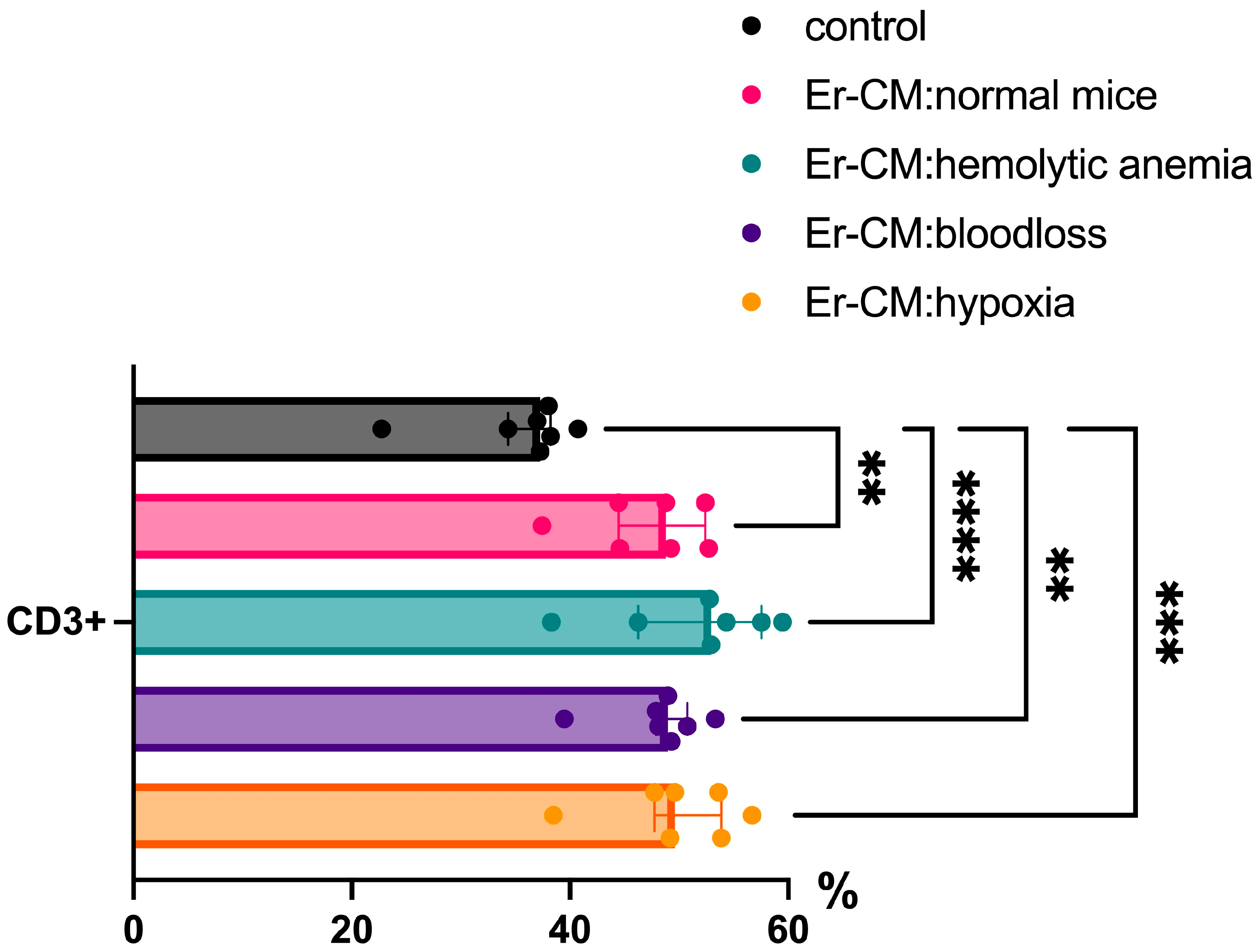
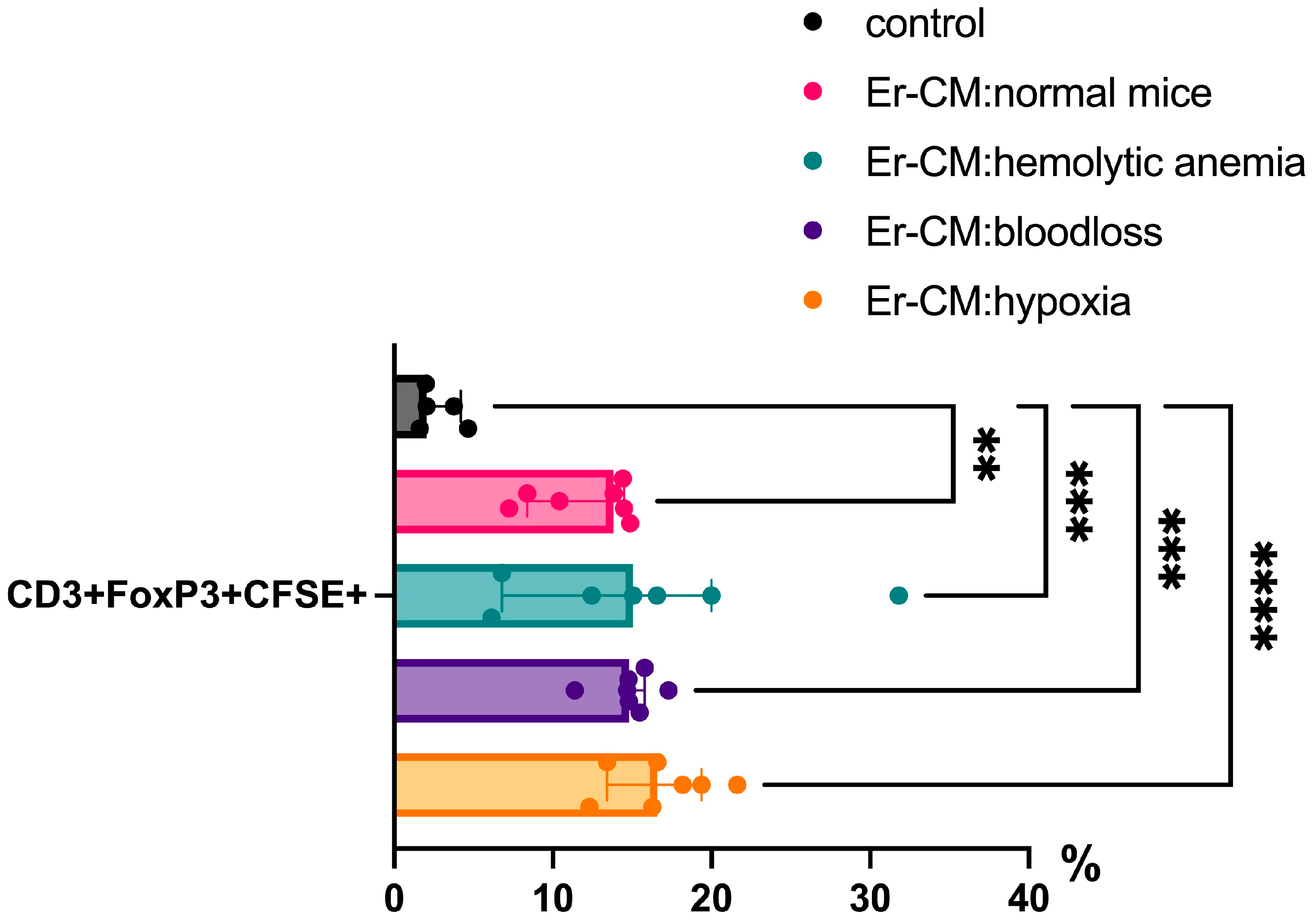
3.1. Effect of Erythroblast-Conditioned Media on Lymphocyte Phenotypes In Vitro
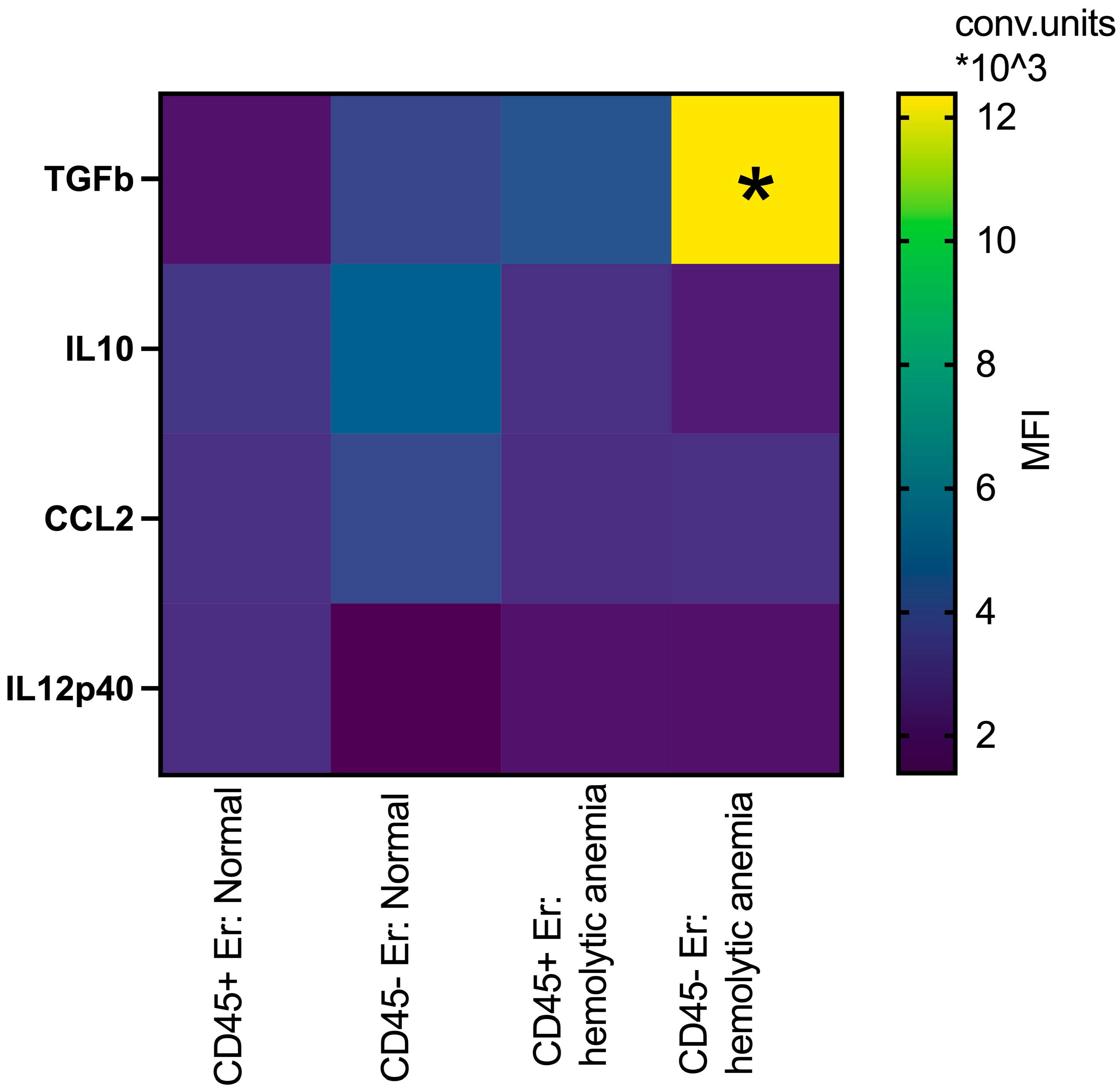
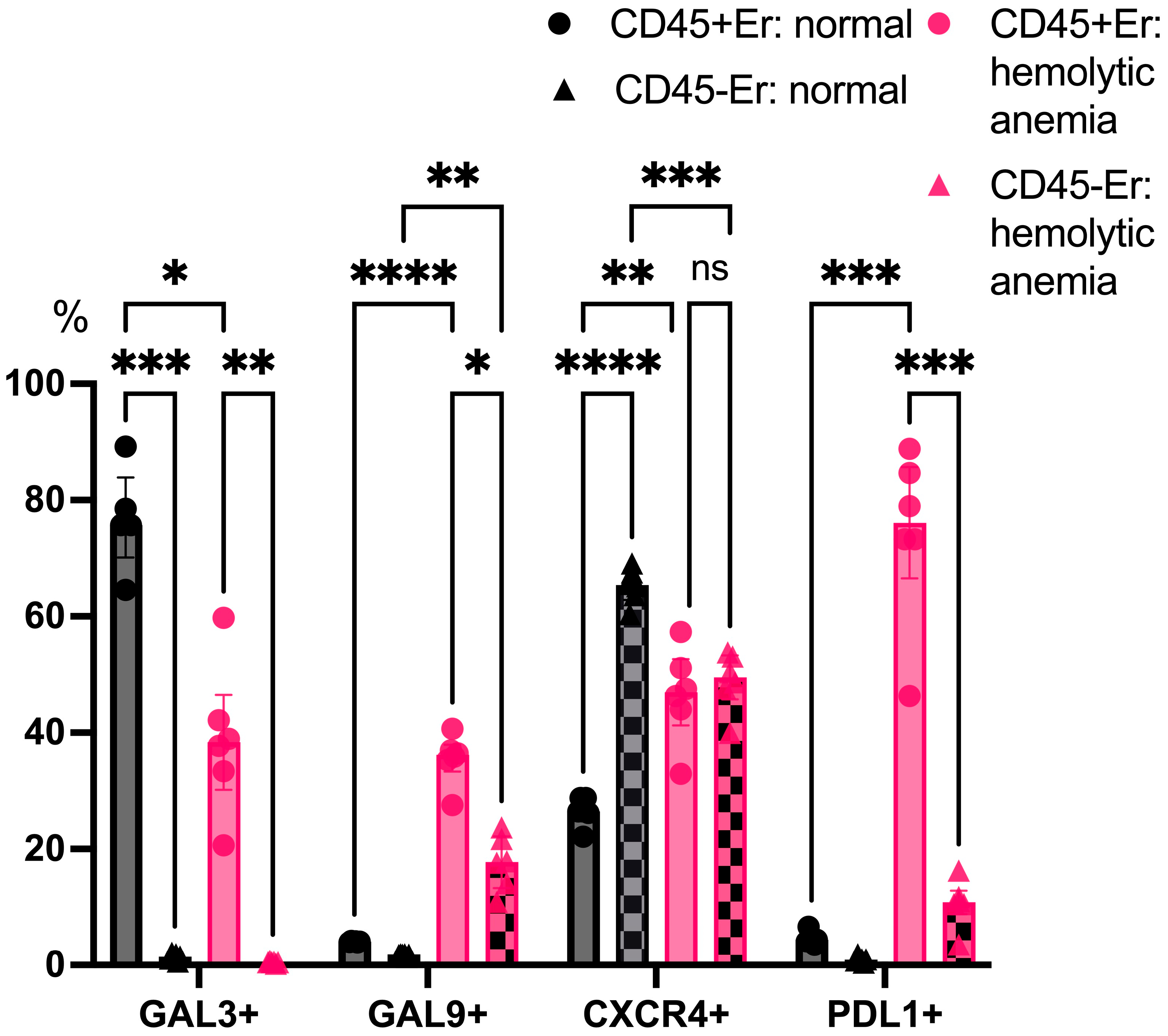
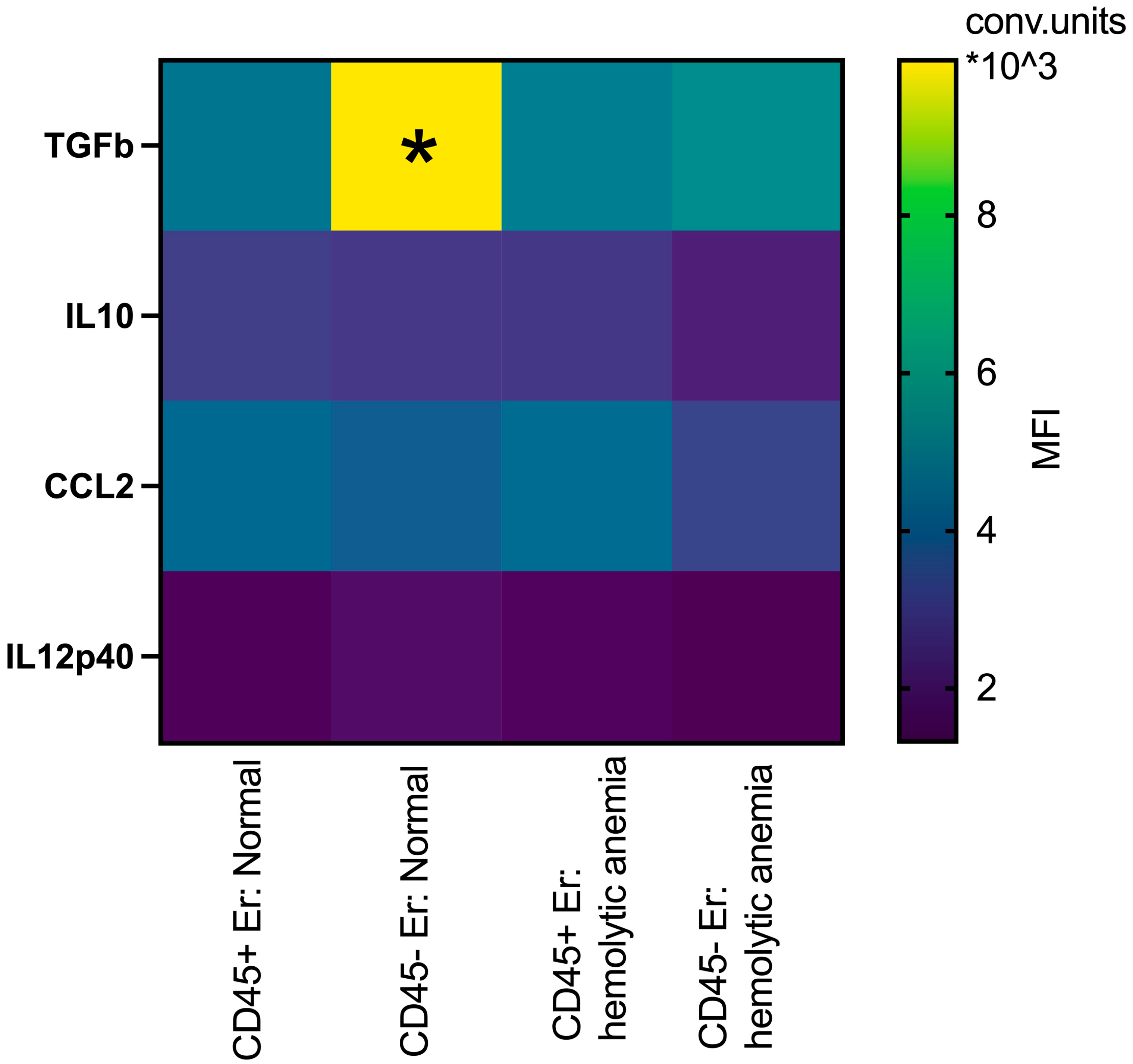
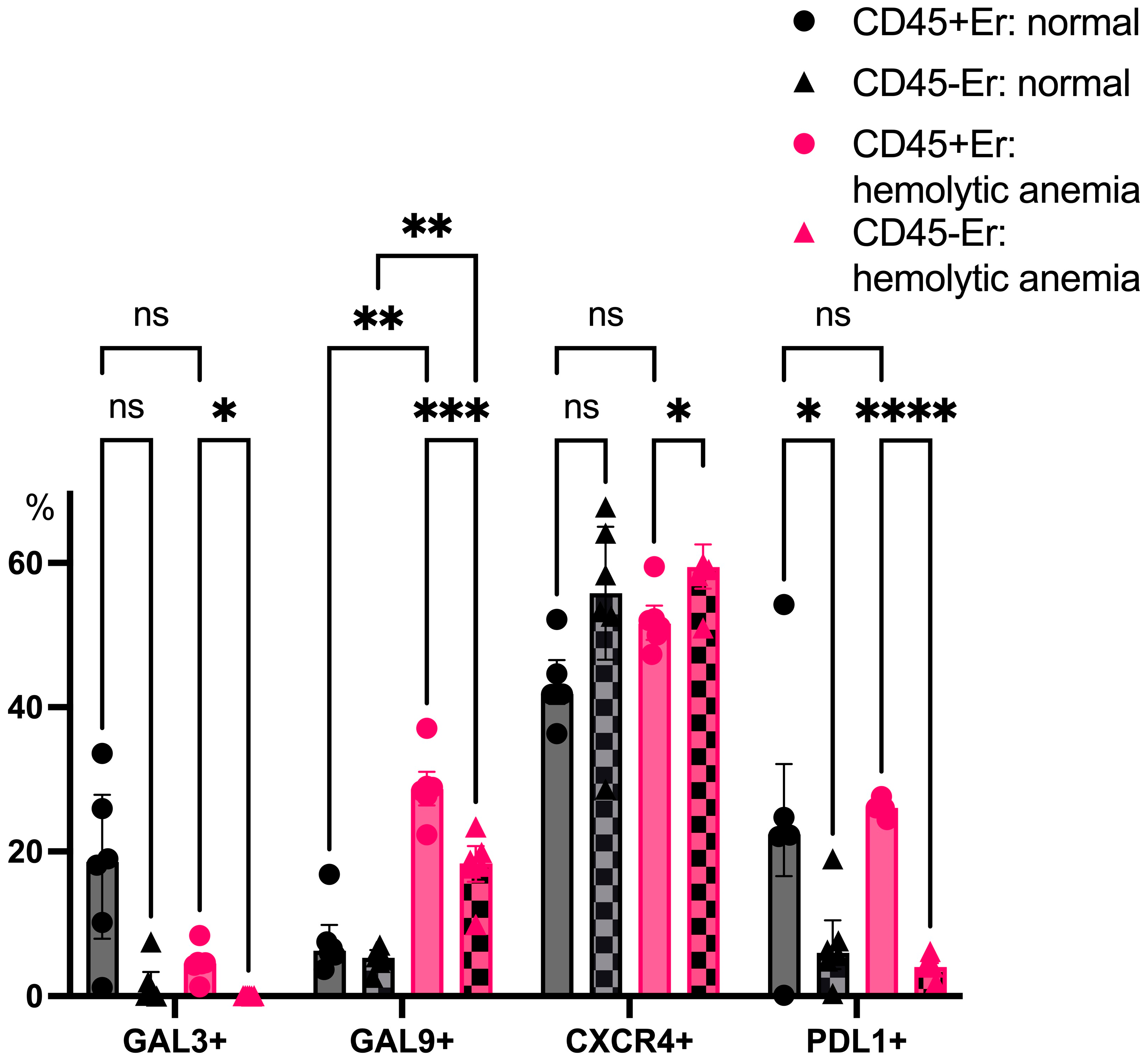
3.2. Phenotypic Features of CD45+ and CD45- Erythroblasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
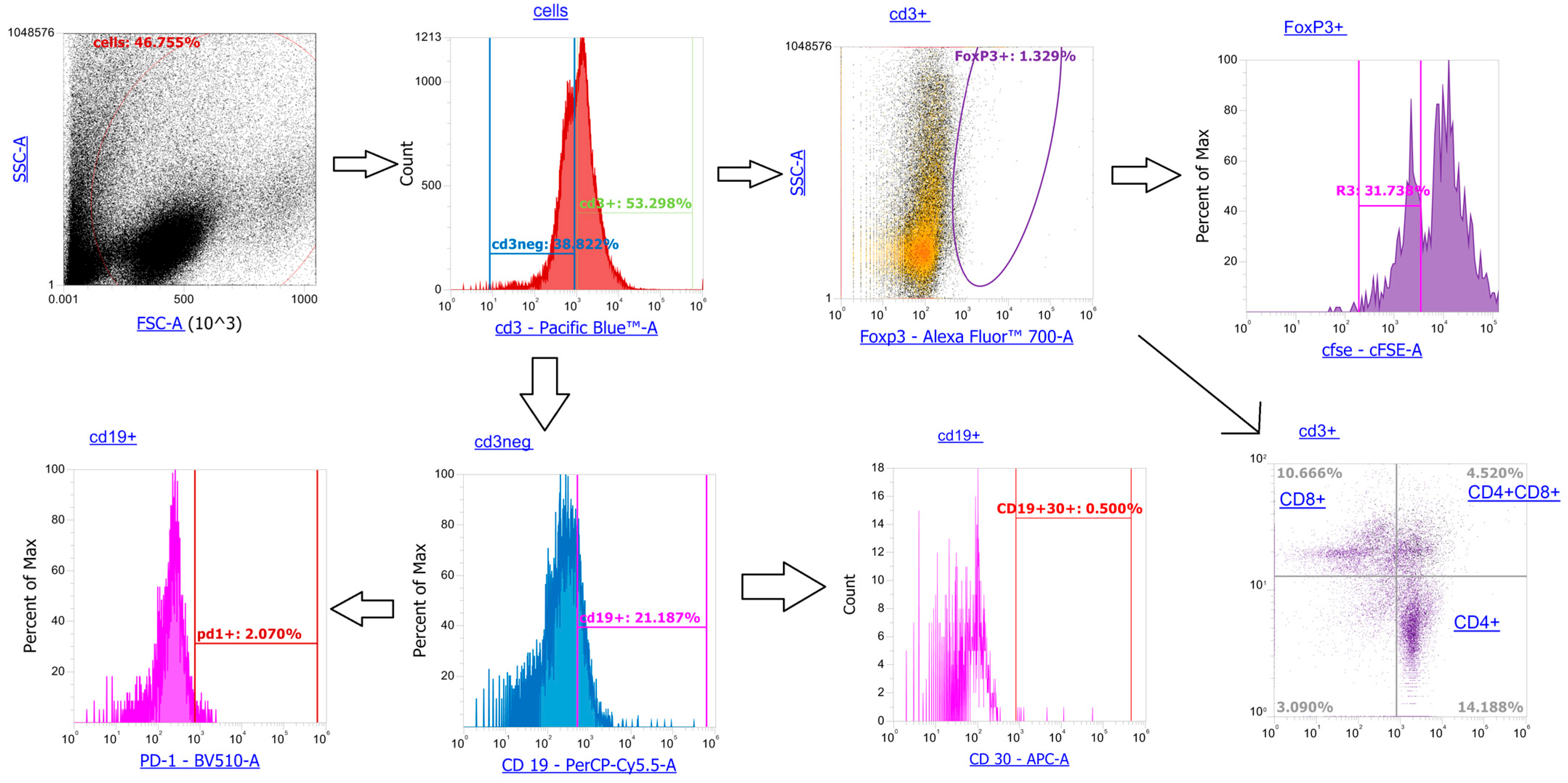
Appendix B
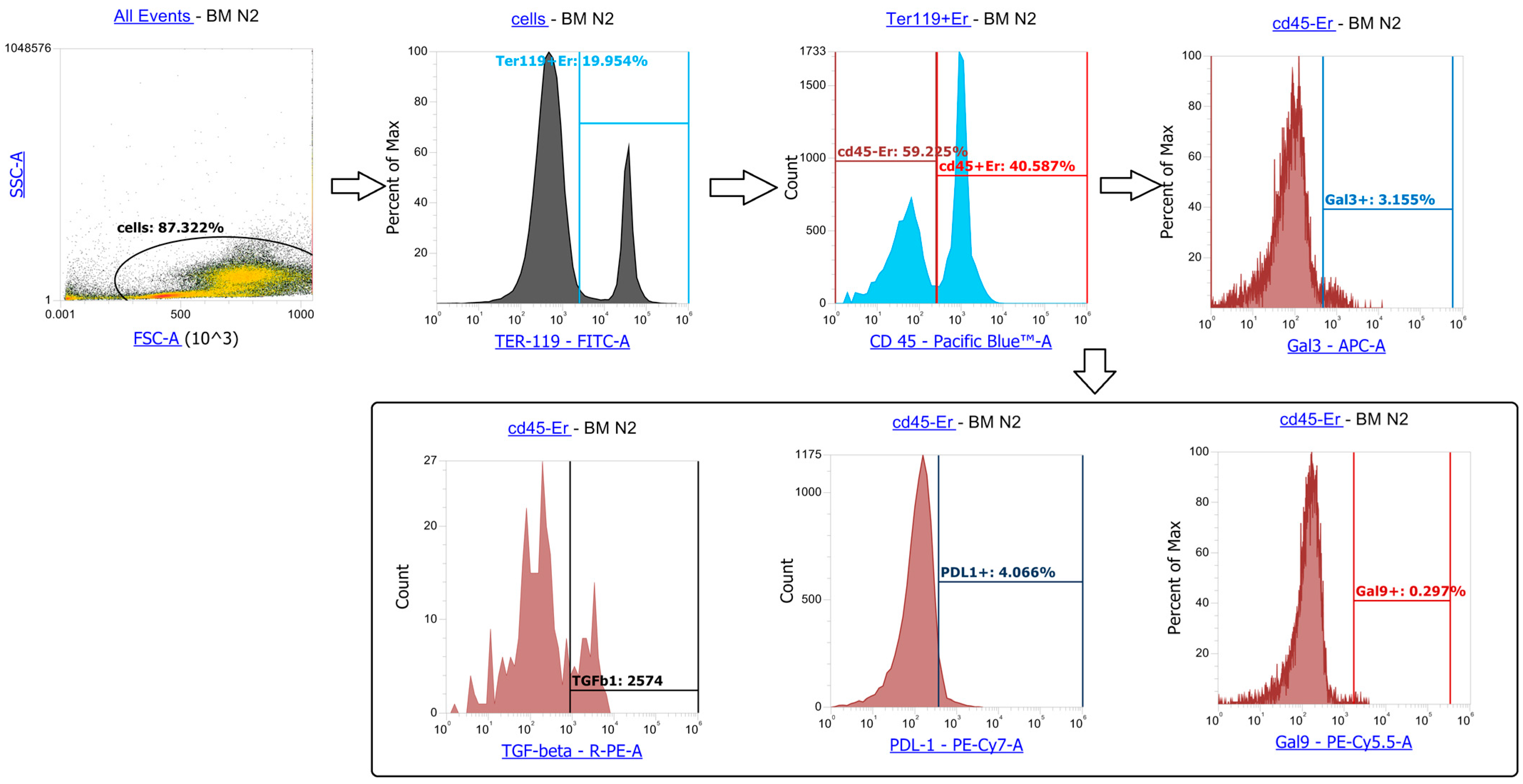
References
- Meerson, F.Z.; Sukhikh, G.T. Influence of adaptation to periodic hypoxia on certain parameters of immunological reactivity. Immunology 1981, 3, 34–38. (In Russian) [Google Scholar]
- Macario, A.J.; Conway de Macario, E.; Dugan, C.B. Erythroblasts can generate immunosuppression in vivo. Medicina 1981, 41, 83–90. [Google Scholar] [PubMed]
- Kozlov, V.A.; Tsyrlova, I.G.; Cheglyakova, V.V. Immunoregulatory Cells of A Non-Lymphoid Nature: ER Suppressors. Dokl. Biol. Sci. 1984, 275, 268–270. [Google Scholar]
- Alamo, I.G.; Kannan, K.B.; Loftus, T.J.; Ramos, H.; Efron, P.A.; Mohr, A.M. Severe trauma and chronic stress activates extramedullary erythropoiesis. J. Trauma. Acute Care Surg. 2017, 83, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Paulson, R.F.; Ruan, B.; Hao, S.; Chen, Y. Stress Erythropoiesis is a Key Inflammatory Response. Cells 2020, 9, 634. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; He, R.; Long, H.; Guo, B.; Jia, Q.; Qin, D.; Liu, S.-Q.; Wang, Z.; Xiang, T.; Zhang, J.; et al. Late-stage tumors induce anemia and immunosuppressive extramedullary erythroid progenitor cells. Nat. Med. 2018, 24, 1536–1544. [Google Scholar] [CrossRef]
- Bozorgmehr, N.; Okoye, I.; Mashhouri, S.; Lu, J.; Koleva, P.; Walker, J.; Elahi, S. CD71+ erythroid cells suppress T-cell effector functions and predict immunotherapy outcomes in patients with virus-associated solid tumors. J. Immunother. Cancer 2023, 11, e006595. [Google Scholar] [CrossRef]
- Lines, J.L.; Pantazi, E.; Mak, J.; Sempere, L.F.; Wang, L.; O’Connell, S.; Ceeraz, S.; Suriawinata, A.A.; Yan, S.; Ernstoff, M.S.; et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014, 74, 1924–1932, Erratum in Cancer Res. 2014, 74, 3195. [Google Scholar] [CrossRef]
- Grzywa, T.M.; Nowis, D.; Golab, J. The role of CD71+ erythroid cells in the regulation of the immune response. Pharmacol. Ther. 2021, 228, 107927, Erratum in Pharmacol. Ther. 2022, 234, 108034. [Google Scholar] [CrossRef]
- Majka, M.; Janowska-Wieczorek, A.; Ratajczak, J.; Ehrenman, K.; Pietrzkowski, Z.; Kowalska, M.A.; Gewirtz, A.M.; Emerson, S.G.; Ratajczak, M.Z. Numerous growth factors, cytokines, and chemokines are secreted by human CD34+ cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood 2001, 97, 3075–3085. [Google Scholar] [CrossRef]
- Han, Y.M.; Liu, Q.Y.; Hou, J.; Gu, Y.; Zhang, Y.; Chen, Z.B.; Fan, J.; Zhou, W.P.; Qiu, S.J.; Zhang, Y.H.; et al. Tumor-Induced Generation of Splenic Erythroblast-like Ter-Cells Promotes Tumor Progression. Cell 2018, 173, 634–648.e12, Erratum in Cell 2021, 184, 1392. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qiao, Y.-D.; Li, X.; Xu, J.-L.; Ye, Q.-J.; Jiang, N.; Zhang, H.; Wu, X.-Y. Intratumoral CD45+CD71+ erythroid cells induce immune tolerance and predict tumor recurrence in hepatocellular carcinoma. Cancer Lett. 2021, 499, 85–98. [Google Scholar] [CrossRef]
- Seledtsov, V.I.; Seledtsova, G.V.; Samarin, D.M.; Taraban, V.Y.; Sennikov, S.V.; Kozlov, V.A. Characterization of erythroid cell-derived natural suppressor activity. Immunobiology 1998, 198, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Perik-Zavodskaia, O.; Perik-Zavodskii, R.; Nazarov, K.; Volynets, M.; Alrhmoun, S.; Shevchenko, J.; Sennikov, S. Murine bone marrow erythroid cells have two branches of differentiation defined by the presence of CD45 and a different immune transcriptome than fetal liver erythroid cells. Int. J. Mol. Sci. 2023, 24, 15752. [Google Scholar] [CrossRef]
- Nazarov, K.; Perik-Zavodskii, R.; Perik-Zavodskaia, O.; Alrhmoun, S.; Volynets, M.; Shevchenko, J.; Sennikov, S. Phenotypic alterations in erythroid nucleated cells of spleen and bone marrow in acute hypoxia. Cells 2023, 12, 2810. [Google Scholar] [CrossRef]
- Nazarov, K.; Perik-Zavodskii, R.; Perik-Zavodskaia, O.; Alrhmoun, S.; Volynets, M.; Shevchenko, J.; Sennikov, S. Acute blood loss in mice forces differentiation of both CD45-positive and CD45-negative erythroid cells and leads to a decreased CCL3 chemokine production by bone marrow erythroid cells. PLoS ONE 2024, 19, e0309455. [Google Scholar] [CrossRef] [PubMed]
- Delyea, C.; Bozorgmehr, N.; Koleva, P.; Dunsmore, G.; Shahbaz, S.; Huang, V.; Elahi, S. CD71+ Erythroid suppressor cells promote fetomaternal tolerance through arginase-2 and PDL-1. J. Immunol. 2018, 200, 4044–4058. [Google Scholar] [CrossRef]
- Epeldegui, M.; Conti, D.V.; Guo, Y.; Cozen, W.; Penichet, M.L.; Martínez-Maza, O. Elevated numbers of PD-L1 expressing B cells are associated with the development of AIDS-NHL. Sci. Rep. 2019, 9, 9371, Erratum in Sci. Rep. 2020, 10, 748. [Google Scholar] [CrossRef]
- Shahbaz, S.; Bozorgmehr, N.; Koleva, P.; Namdar, A.; Jovel, J.; Fava, R.A.; Elahi, S.; Marrack, P. CD71+VISTA+ erythroid cells promote the development and function of regulatory T cells through TGF-β. PLoS Biol. 2018, 16, e2006649. [Google Scholar] [CrossRef]
- Elahi, S.; Ertelt, J.M.; Kinder, J.M.; Jiang, T.T.; Zhang, X.; Xin, L.; Chaturvedi, V.; Strong, B.S.; Qualls, J.E.; Steinbrecher, K.A.; et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 2013, 504, 158–162. [Google Scholar] [CrossRef]
- Shevach, E.M.; Tran, D.Q.; Davidson, T.S.; Andersson, J. The critical contribution of TGF-beta to the induction of Foxp3 expression and regulatory T cell function. Eur. J. Immunol. 2008, 38, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Seledtsova, G.V.; Seledtsov, V.I.; Samarin, D.M.; Senyukov, V.V.; Ivanova, I.P.; Akimenko, Z.A.; Tsyrlova, I.G.; Wolpe, S.S.; Kozlov, V.A. Erythroid cells in immunoregulation: Characterization of a novel suppressor factor. Immunol. Lett. 2004, 93, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Gorman, J.V.; Colgan, J.D. Regulation of T cell responses by the receptor molecule Tim-3. Immunol. Res. 2014, 59, 56–65. [Google Scholar] [CrossRef]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 2020, 20, 173–185. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarov, K.; Perik-Zavodskii, R.; Shevchenko, J.; Sennikov, S. Erythroblasts Promote the Development of a Suppressive Lymphocyte Phenotype via Treg Induction and PD1 Upregulation on the Surfaces of B-Cells: A Study on the Subpopulation-Specific Features of Erythroblasts. Curr. Issues Mol. Biol. 2025, 47, 550. https://doi.org/10.3390/cimb47070550
Nazarov K, Perik-Zavodskii R, Shevchenko J, Sennikov S. Erythroblasts Promote the Development of a Suppressive Lymphocyte Phenotype via Treg Induction and PD1 Upregulation on the Surfaces of B-Cells: A Study on the Subpopulation-Specific Features of Erythroblasts. Current Issues in Molecular Biology. 2025; 47(7):550. https://doi.org/10.3390/cimb47070550
Chicago/Turabian StyleNazarov, Kirill, Roman Perik-Zavodskii, Julia Shevchenko, and Sergey Sennikov. 2025. "Erythroblasts Promote the Development of a Suppressive Lymphocyte Phenotype via Treg Induction and PD1 Upregulation on the Surfaces of B-Cells: A Study on the Subpopulation-Specific Features of Erythroblasts" Current Issues in Molecular Biology 47, no. 7: 550. https://doi.org/10.3390/cimb47070550
APA StyleNazarov, K., Perik-Zavodskii, R., Shevchenko, J., & Sennikov, S. (2025). Erythroblasts Promote the Development of a Suppressive Lymphocyte Phenotype via Treg Induction and PD1 Upregulation on the Surfaces of B-Cells: A Study on the Subpopulation-Specific Features of Erythroblasts. Current Issues in Molecular Biology, 47(7), 550. https://doi.org/10.3390/cimb47070550





