The Effects of Engeletin on Insulin Resistance Induced in Human HepG2 Liver Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line Used in Experiments
2.2. Preparation of Drugs Used in Experiments and Determination of Cytotoxicity with MTT Assay
2.3. Induction of Insulin Resistance in Cell Culture
2.4. Experimental Groups
- 1.
- Control (HEALTHY GROUP);
- 2.
- High-dose glucose + high-dose insulin (IR GROUP);
- 3.
- IR + Engeletin at 100 µM (IR + ENG100 GROUP);
- 4.
- IR + Engeletin at 10 µM (IR + ENG10 GROUP);
- 5.
- IR + Metformin (control drug) (IR + MET GROUP);
- 6.
- IR + Metformin + Engeletin at 100 µM (IR + MET+ENG100 GROUP);
- 7.
- IR + Metformin + Engeletin at 10 µM (IR + MET + ENG10 GROUP).
2.5. Molecular Analyses
2.5.1. mRNA Isolation
2.5.2. Reverse Transcriptase Reaction and cDNA Synthesis
2.5.3. Quantitative Determination of mRNA Expression
2.6. Biochemical Analyses
Oxidative Stress Analysis
2.7. Statistical Analyses
3. Results
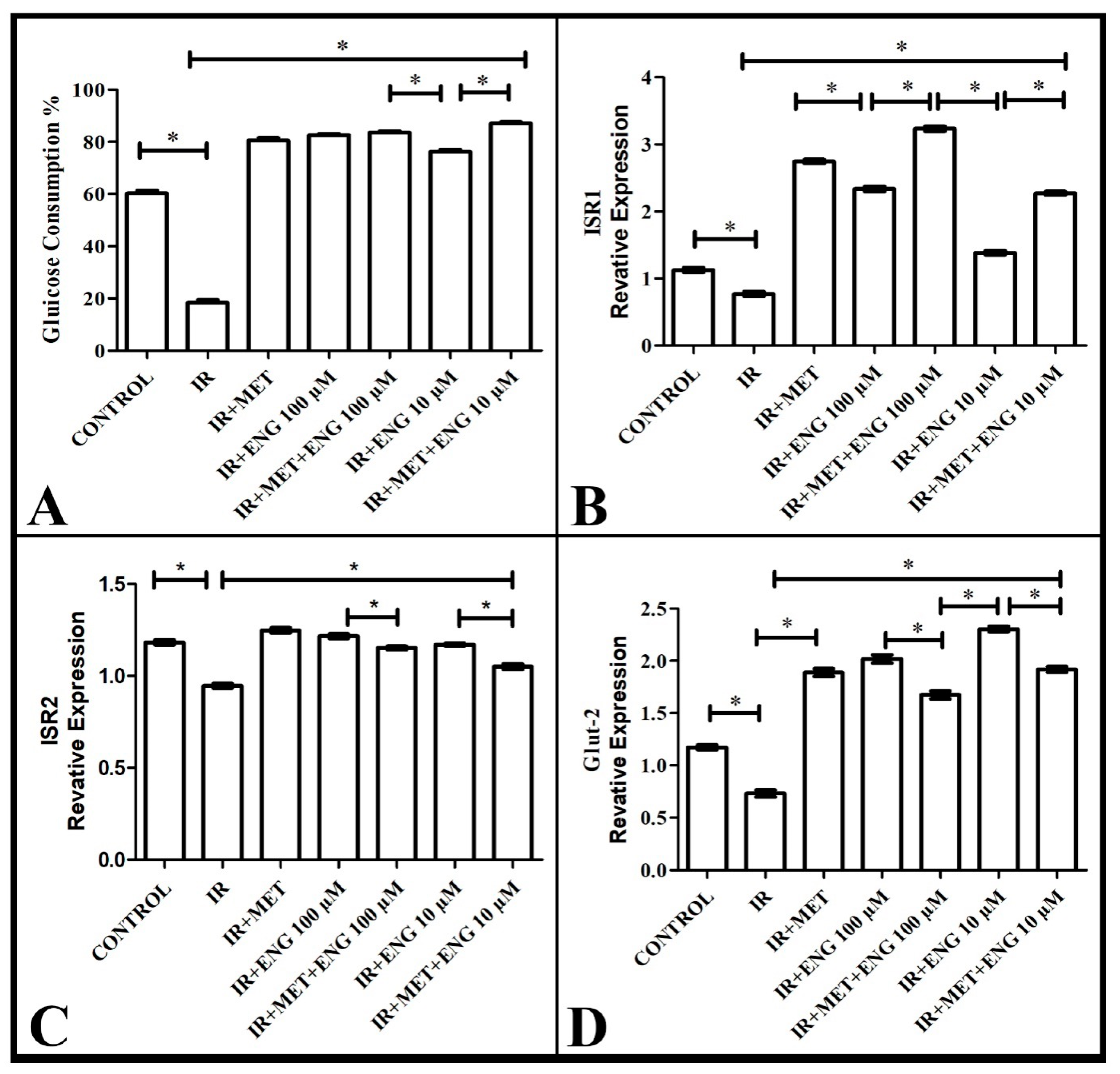
3.1. Findings on Effective Dose of Engeletin and Glucose Consumption
3.2. Real-Time PCR Results
3.2.1. ISR-1 mRNA Expression Results
3.2.2. ISR-2 mRNA Expression Results
3.2.3. GLUT-2 mRNA Expression Results
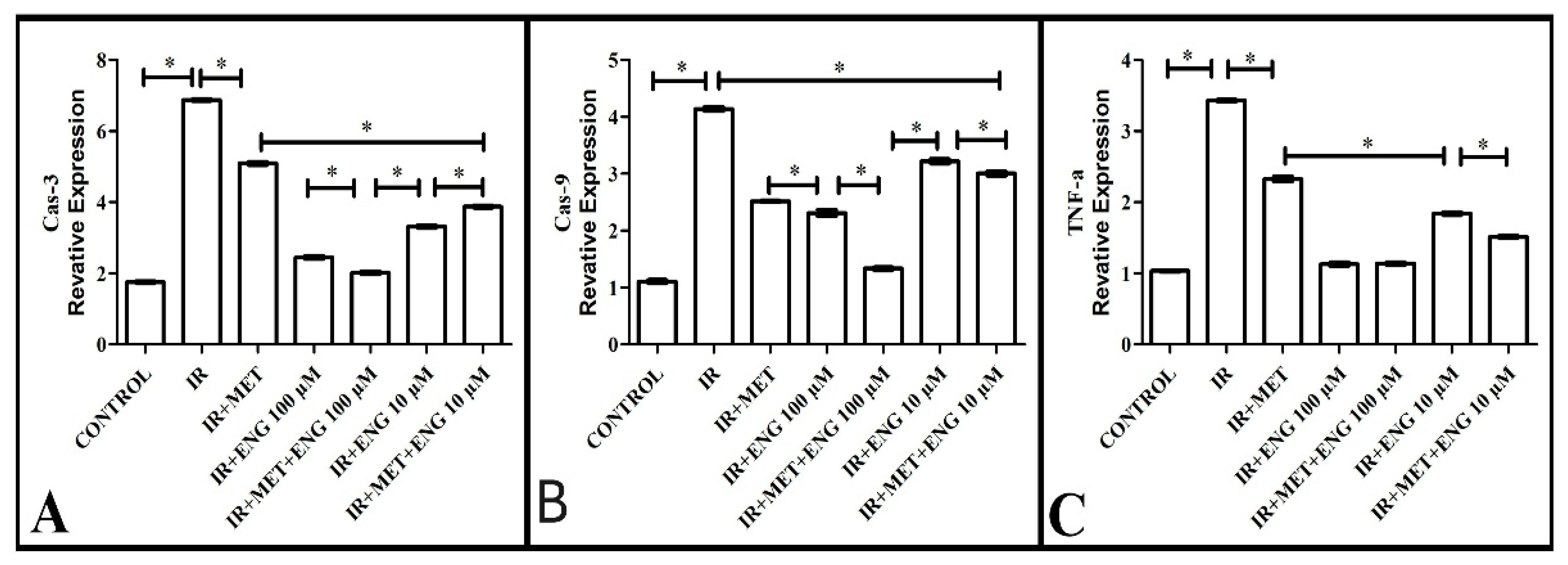
3.2.4. Cas-3 mRNA Expression Results
3.2.5. Cas-9 mRNA Expression Results
3.2.6. TNF-α mRNA Expression Results
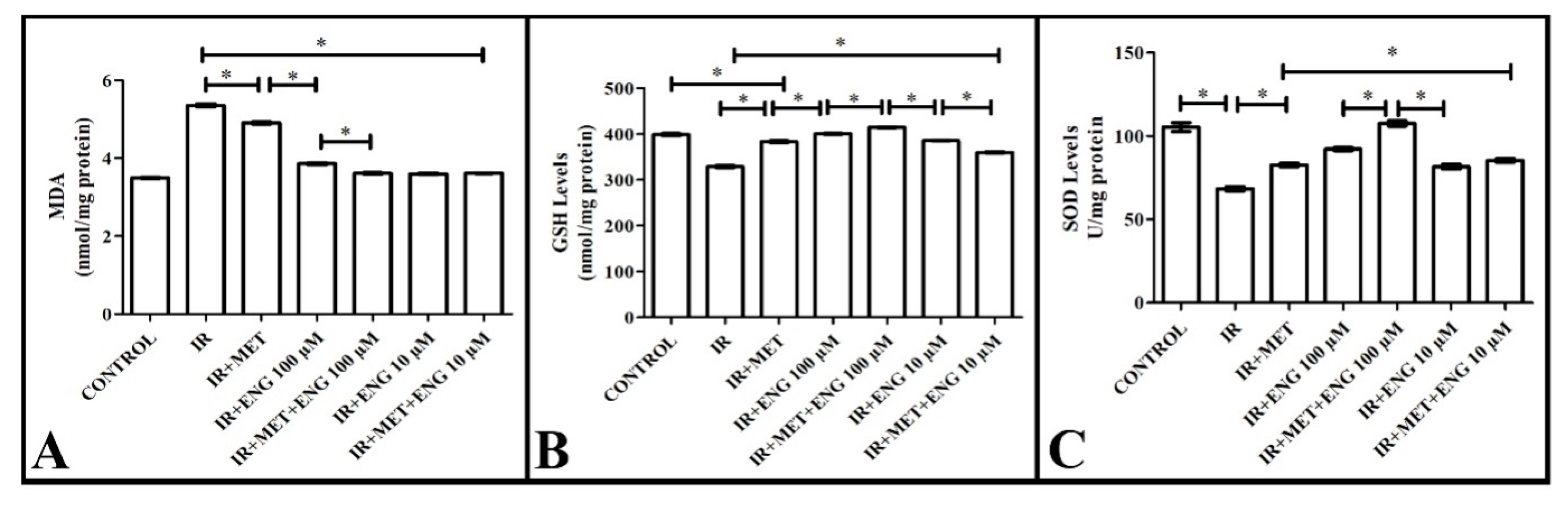
3.3. Biochemical Analyses Results
3.3.1. MDA Analysis Results
3.3.2. GSH Analysis Results
3.3.3. SOD Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Yang, W.; Cintina, I.; Hoerger, T.; Neuwahl, S.J.; Shao, H.; Laxy, M.; Zhang, P. Estimating Costs of Diabetes Complications in People <65 years in the U.S. Using Panel Data. J. Diabetes Complicat. 2020, 34, 107735. [Google Scholar] [CrossRef]
- Zhao, X.; An, X.; Yang, C.; Sun, W.; Ji, H.; Lian, F. The Crucial Role and Mechanism of Insulin Resistance in Metabolic Disease. Front. Endocrinol. 2023, 14, 1149239. [Google Scholar] [CrossRef]
- Zhou, M.; Konigsberg, W.H.; Hao, C.; Pan, Y.; Sun, J.; Wang, X. Bioactivity and Mechanisms of Flavonoids in Decreasing Insulin Resistance. J. Enzym. Inhib. Med. Chem. 2023, 38, 2199168. [Google Scholar] [CrossRef]
- Russo, B.; Picconi, F.; Malandrucco, I.; Frontoni, S. Flavonoids and Insulin-Resistance: From Molecular Evidences to Clinical Trials. Int. J. Mol. Sci. 2019, 20, 2061. [Google Scholar] [CrossRef]
- Zhong, X.; Huang, R.; Chen, X.; Lei, Y. A Review on the Pharmacological Aspects of Engeletin as Natural Compound. Drug Des. Dev. Ther. 2023, 17, 3833–3843. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Wang, G.; Zhang, Y.; Zhang, F.; Yang, L.; Liu, Z.; Shen, Z. Engeletin Inhibits Lipopolysaccharide/d-Galactosamine-Induced Liver Injury in Mice through Activating PPAR-γ. J. Pharmacol. Sci. 2019, 140, 218–222. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, L.; Zhang, Z.; Sun, Y.; Wang, X.; Wei, J. Protective and Therapeutic Effects of Engeletin on LPS-Induced Acute Lung Injury. Inflammation 2018, 41, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Ji, H.; Shi, J.; Zhu, X.; Zhi, Z. Engeletin Attenuates Aβ1–42-Induced Oxidative Stress and Neuroinflammation by Keap1/Nrf2 Pathway. Inflammation 2020, 43, 1759–1771. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Sun, J.; Tian, G.; Shi, Z. Engeletin Suppresses Lung Cancer Progression by Inducing Apoptotic Cell Death through Modulating the XIAP Signaling Pathway: A Molecular Mechanism Involving ER Stress. Biomed. Pharmacother. 2020, 128, 110221. [Google Scholar] [CrossRef]
- Namwong, A.; Thongkrajai, P.; Konsue, A. Effect of a Thai Folk Recipe on Phytochemical Screening, Antioxidant Activities, and Glucosidase Inhibition by Different Solvent Extracts. Pharmacogn. Res. 2020, 12, 225–229. [Google Scholar]
- Gunawan, V.A.; Soetjipto, H.; Mustika, A. Hypoglicemic and Antioxidant Activity of Petiveria Alliacea in Diabetic Rat Models. Biomol. Health Sci. J. 2020, 3, 19–23. [Google Scholar] [CrossRef]
- Wirasathien, L.; Pengsuparp, T.; Suttisri, R.; Ueda, H.; Moriyasu, M.; Kawanishi, K. Inhibitors of Aldose Reductase and Advanced Glycation End-Products Formation from the Leaves of Stelechocarpus Cauliflorus R.E. Fr. Phytomedicine 2007, 14, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Hou, Y.; Wu, J.; Li, G.; Chen, W.; Hu, L.; Gan, D. MiR-93-5p Promotes Insulin Resistance to Regulate Type 2 Diabetes Progression in HepG2 Cells by Targeting HGF. Mol. Med. Rep. 2021, 23, 329. [Google Scholar] [CrossRef] [PubMed]
- Kheirollahzadeh, F.; Eftekhari, E.; Ghollasi, M.; Behzadi, P. Anti-Hyperglycemic Effects of Eryngium Billardierei F. Delaroche Extract on Insulin-Resistance HepG2 Cells in Vitro. Mol. Biol. Rep. 2022, 49, 3401–3411. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Method 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W.; Li, Y. A Simple Method for Clinical Assay of Superoxide Dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of Total, Protein-Bound, and Nonprotein Sulfhydryl Groups in Tissue with Ellman’s Reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohisni, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Barazzoni, R.; Gortan Cappellari, G.; Ragni, M.; Nisoli, E. Insulin Resistance in Obesity: An Overview of Fundamental Alterations. Eat. Weight Disord.—Stud. Anorex. Bulim. Obes. 2018, 23, 149–157. [Google Scholar] [CrossRef]
- Freeman, A.M.; Acevedo, L.A.; Pennings, N. Insulin Resistance. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lebovitz, H. Insulin Resistance: Definition and Consequences. Exp. Clin. Endocrinol. Diabetes 2001, 109 (Suppl. S2), S135–S148. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Nascimbeni, F.; Mantovani, A.; Targher, G. Hypertension, Diabetes, Atherosclerosis and NASH: Cause or Consequence? J. Hepatol. 2018, 68, 335–352. [Google Scholar] [CrossRef]
- Giannarelli, R.; Aragona, M.; Coppelli, A.; Del Prato, S. Reducing Insulin Resistance with Metformin: The Evidence Today. Diabetes Metab. 2003, 29, 6S28–6S35. [Google Scholar] [CrossRef]
- Babu, P.V.A.; Liu, D.; Gilbert, E.R. Recent Advances in Understanding the Anti-Diabetic Actions of Dietary Flavonoids. J. Nutr. Biochem. 2013, 24, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-T.; Chang, F.-R.; Tsai, Y.-H.; Wu, Y.-C.; Hsieh, T.-J. 2-Bromo-4′-Methoxychalcone and 2-Iodo-4′-Methoxychalcone Prevent Progression of Hyperglycemia and Obesity via 5′-Adenosine-Monophosphate-Activated Protein Kinase in Diet-Induced Obese Mice. Int. J. Mol. Sci. 2018, 19, 2763. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Martín, M.Á.; Goya, L.; Ramos, S. Cocoa Flavonoids Attenuate High Glucose-Induced Insulin Signalling Blockade and Modulate Glucose Uptake and Production in Human HepG2 Cells. Food Chem. Toxicol. 2014, 64, 10–19. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Cocoa Flavonoids Improve Insulin Signalling and Modulate Glucose Production via AKT and AMPK in HEpG2 Cells. Mol. Nutr. Food Res. 2013, 57, 974–985. [Google Scholar] [CrossRef]
- Li, D.; Fan, J.; Du, L.; Ren, G. Prenylated Flavonoid Fractions from Glycyrrhiza Glabra Alleviate Insulin Resistance in HepG2 Cells by Regulating the ERK/IRS-1 and PI3K/Akt Signaling Pathways. Arch. Pharm. Res. 2024, 47, 127–145. [Google Scholar] [CrossRef]
- Thorens, B. GLUT2, Glucose Sensing and Glucose Homeostasis. Diabetologia 2015, 58, 221–232. [Google Scholar] [CrossRef]
- Shen, Z.; Hou, Y.; Zhao, G.; Tan, L.; Chen, J.; Dong, Z.; Ni, C.; Pei, L. Physiological Functions of Glucose Transporter-2: From Cell Physiology to Links with Diabetes Mellitus. Heliyon 2024, 10, e25459. [Google Scholar] [CrossRef] [PubMed]
- Hajiaghaalipour, F.; Khalilpourfarshbafi, M.; Arya, A. Modulation of Glucose Transporter Protein by Dietary Flavonoids in Type 2 Diabetes Mellitus. Int. J. Biol. Sci. 2015, 11, 508–524. [Google Scholar] [CrossRef]
- Han, S.; You, L.; Hu, Y.; Wei, S.; Liu, T.; Cho, J.Y.; Hu, W. Ginsenoside F2 Enhances Glucose Metabolism by Modulating Insulin Signal Transduction in Human Hepatocarcinoma Cells. J. Ginseng Res. 2023, 47, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Hurrle, S.; Hsu, W.H. The Etiology of Oxidative Stress in Insulin Resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef]
- Kohen, R.; Nyska, A. Invited Review: Oxidation of Biological Systems: Oxidative Stress Phenomena, Antioxidants, Redox Reactions, and Methods for Their Quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef]
- Huang, L.; Bian, M.; Lu, S.; Wang, J.; Yu, J.; Jiang, L.; Zhang, J. Engeletin Alleviates Erastin-Induced Oxidative Stress and Protects against Ferroptosis via Nrf2/Keap1 Pathway in Bone Marrow Mesenchymal Stem Cells. Tissue Cell 2023, 82, 102040. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, A.; Moradi-Sarabi, M.; Sayahi, A.; Zal, F. Protective Effects of Quercetin against Hyperglycemia-Induced Oxidative Stress in Hepatic HepG2 Cell Line. Avicenna J. Phytomed. 2021, 11, 269–280. [Google Scholar]
- Chen, F.; Xiong, H.; Wang, J.; Ding, X.; Shu, G.; Mei, Z. Antidiabetic Effect of Total Flavonoids from Sanguis Draxonis in Type 2 Diabetic Rats. J. Ethnopharmacol. 2013, 149, 729–736. [Google Scholar] [CrossRef]
- Alipourfard, I.; Bakhtiyari, S.; Gheysarzadeh, A.; Di Renzo, L.; De Lorenzo, A.; Mikeladze, D.; Khamoushi, A. The Key Role of Akt Protein Kinase in Metabolic-Inflammatory Pathways Cross-Talk: TNF-α Down-Regulation and Improving of Insulin Resistance in HepG2 Cell Line. Curr. Mol. Med. 2021, 21, 257–264. [Google Scholar] [CrossRef]
- Bourebaba, N.; Kornicka-Garbowska, K.; Marycz, K.; Bourebaba, L.; Kowalczuk, A. Laurus Nobilis Ethanolic Extract Attenuates Hyperglycemia and Hyperinsulinemia-Induced Insulin Resistance in HepG2 Cell Line through the Reduction of Oxidative Stress and Improvement of Mitochondrial Biogenesis—Possible Implication in Pharmacotherapy. Mitochondrion 2021, 59, 190–213. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Li, J.; Cheng, Y.; Chen, J.; Shen, M.; Zhang, S.; Wei, H. Insulin Resistance Contributes to Multidrug Resistance in HepG2 Cells via Activation of the PERK Signaling Pathway and Upregulation of Bcl-2 and P-Gp. Oncol. Rep. 2016, 35, 3018–3024. [Google Scholar] [CrossRef]
- Li, B.; Yang, X.; Zhang, P.; Guo, J.; Rong, K.; Wang, X.; Cao, X.; Zhou, T.; Zhao, J. Engeletin Alleviates the Inflammation and Apoptosis in Intervertebral Disc Degeneration via Inhibiting the NF-ΚB and MAPK Pathways. J. Inflamm. Res. 2022, 15, 5767–5783. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Z.; Pang, Z.; Qi, G.; Hua, B.; Yan, Z.; Yuan, H. Engeletin Protects Against TNF-α-Induced Apoptosis and Reactive Oxygen Species Generation in Chondrocytes and Alleviates Osteoarthritis in Vivo. J. Inflamm. Res. 2021, 14, 745–760. [Google Scholar] [CrossRef]

| Human Bactin-F | 5′-ATT GCC GAC AGG ATG CAG AAG-3′—21 bp |
| Human Bactin-R | 5′-AGA AGC ATT TGC GGT GGA CG-3′—20 bp |
| Human Cas9-F | 5′-CTT CGT TTC TGC GAA CTA ACA GG-3′—23 bp |
| Human Cas9-R | 5′-GCA CCA CTG GGG TAA GGT TT-3′—20 bp |
| Human TNF-a-F | 5′-CCT CTC TCT AAT CAG CCC TCT G-3′—22 bp |
| Human TNF-a-R | 5′-GAG GAC CTG GGA GTA GAT GAG-3′—21 bp |
| Human Insulin R1-F | 5′-CCC AGG ACC CGC ATT CAA A-3′—19 bp |
| Human Insulin R1-R | 5′-GGC GGT AGA TAC CAA TCA GGT-3′—21 bp |
| Human Insulin R2-F | 5′-ACT TCA CAT TGC AAA CGC CT-3′—20 bp |
| Human Insulin R2-R | 5′-GGA ATT GCT AGC ACG CCT AC-3′—20 bp |
| Human Glut2-F | 5′-GCT GCT CAA CTA ATC ACC ATG C-3′—22 bp |
| Human Glut2-R | 5′-TGG TCC CAA TTT TGA AAA CCC C-3′—22 bp |
| Human Cas3-F | 5′-TGG CGA AAT TCA AAG GAT G-3′—19 bp |
| Human Cas3-R | 5′-TAA CCC GGG TAA GAA TGT GC-3′—20 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toktay, E.; Parlak, S.N.; Kavas, T.; Un, H.; Ugan, R.A.; Yayla, M. The Effects of Engeletin on Insulin Resistance Induced in Human HepG2 Liver Cells. Curr. Issues Mol. Biol. 2025, 47, 535. https://doi.org/10.3390/cimb47070535
Toktay E, Parlak SN, Kavas T, Un H, Ugan RA, Yayla M. The Effects of Engeletin on Insulin Resistance Induced in Human HepG2 Liver Cells. Current Issues in Molecular Biology. 2025; 47(7):535. https://doi.org/10.3390/cimb47070535
Chicago/Turabian StyleToktay, Erdem, Secil Nazife Parlak, Tugba Kavas, Harun Un, Rustem Anıl Ugan, and Muhammed Yayla. 2025. "The Effects of Engeletin on Insulin Resistance Induced in Human HepG2 Liver Cells" Current Issues in Molecular Biology 47, no. 7: 535. https://doi.org/10.3390/cimb47070535
APA StyleToktay, E., Parlak, S. N., Kavas, T., Un, H., Ugan, R. A., & Yayla, M. (2025). The Effects of Engeletin on Insulin Resistance Induced in Human HepG2 Liver Cells. Current Issues in Molecular Biology, 47(7), 535. https://doi.org/10.3390/cimb47070535






