Sensitisation of HeLa Cell Cultures to Xanthone Treatment by RNAi-Mediated Silencing of NANOG and STAT3
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
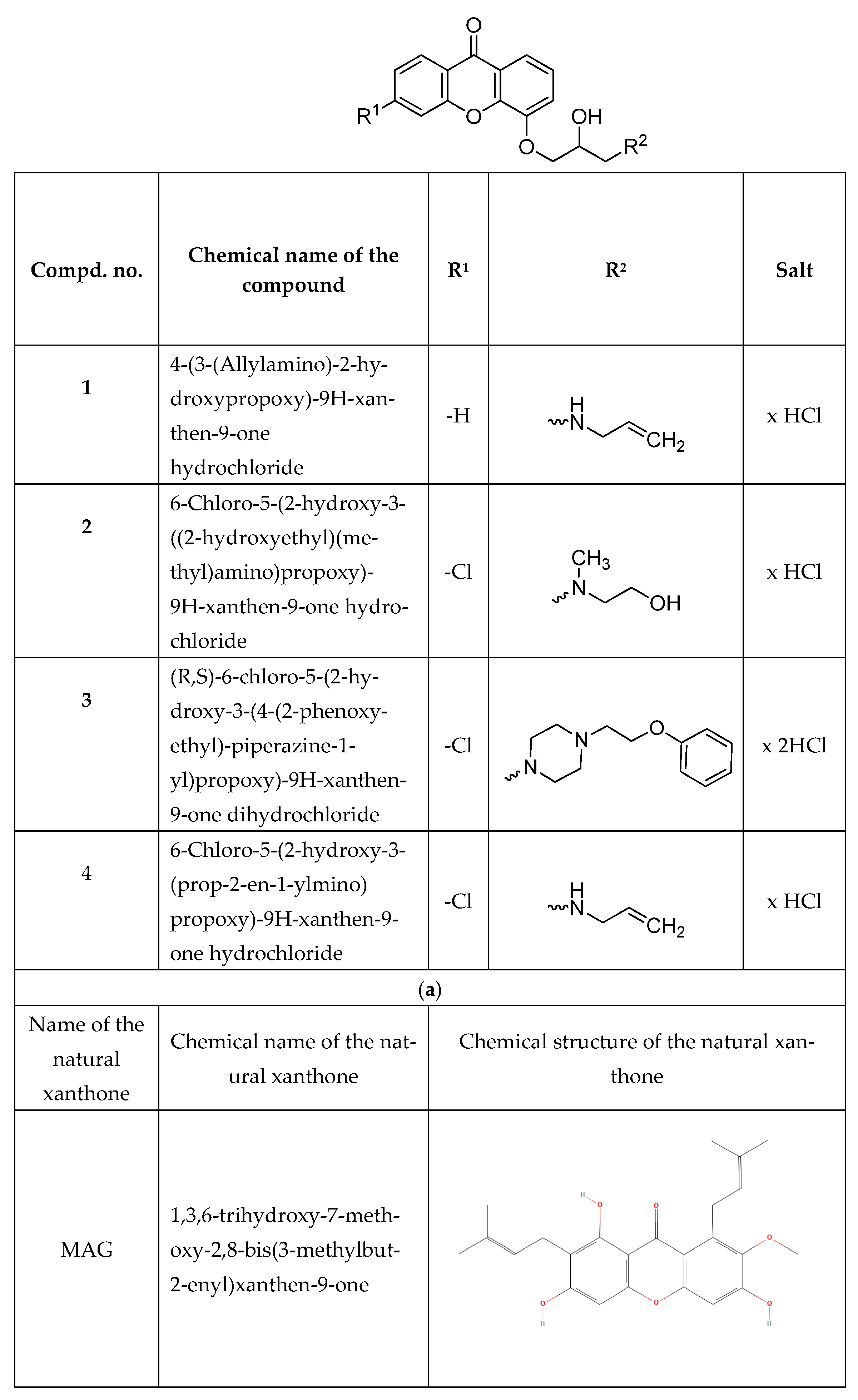
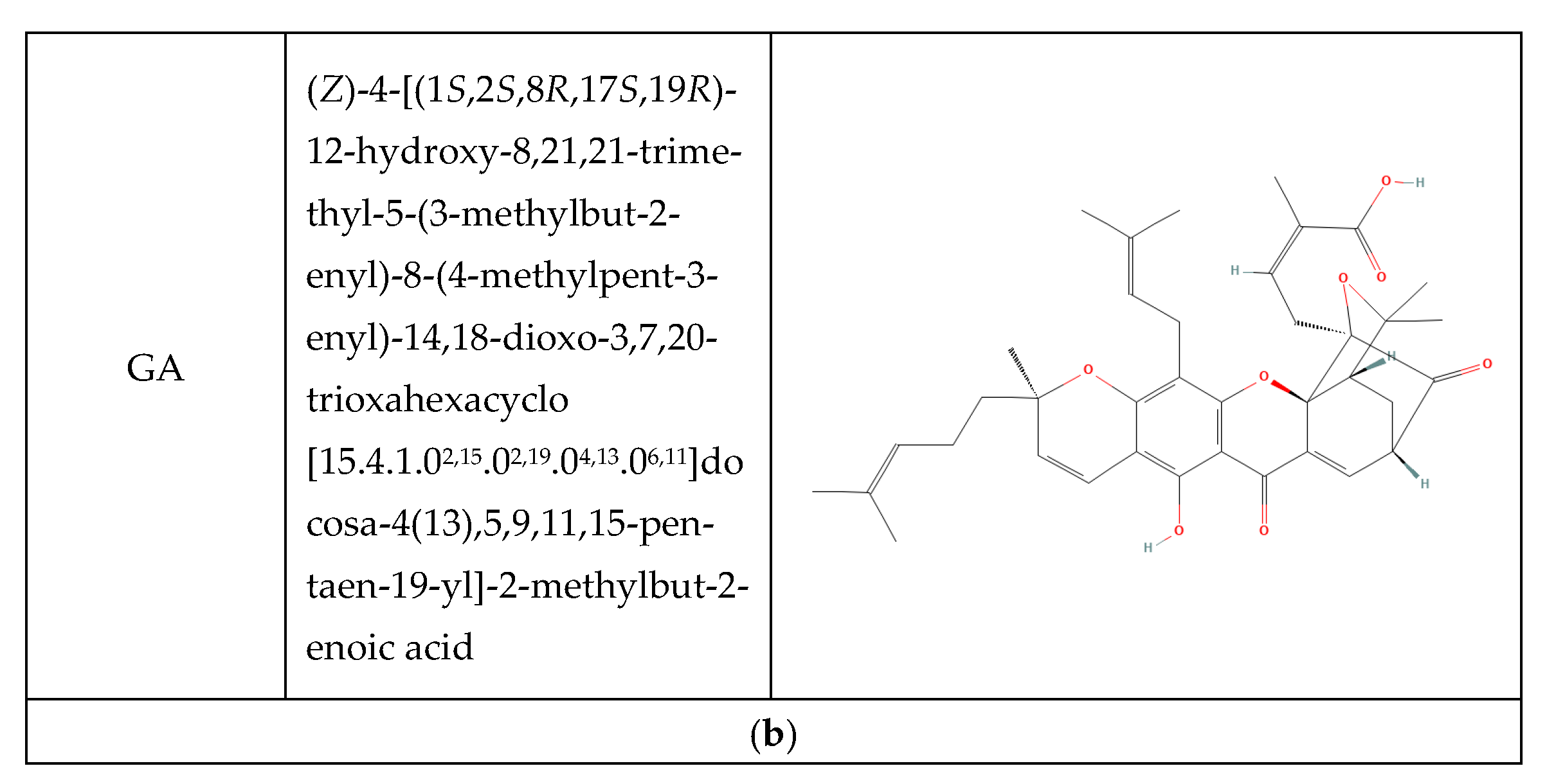
2.2. Synthesis and Purification of Synthetic Xanthone Derivatives
2.3. Cell Culture and Treatment Conditions
2.4. Detection of Reactive Oxygen Species (ROS)
2.5. Plasmids and Transfections
2.6. RNA Extraction
2.7. Real-Time™ RT-PCR
2.8. ELISA
2.9. Cell Proliferation
2.10. Cytotoxicity and Apoptosis Determination
2.11. Statistical Analysis
3. Results
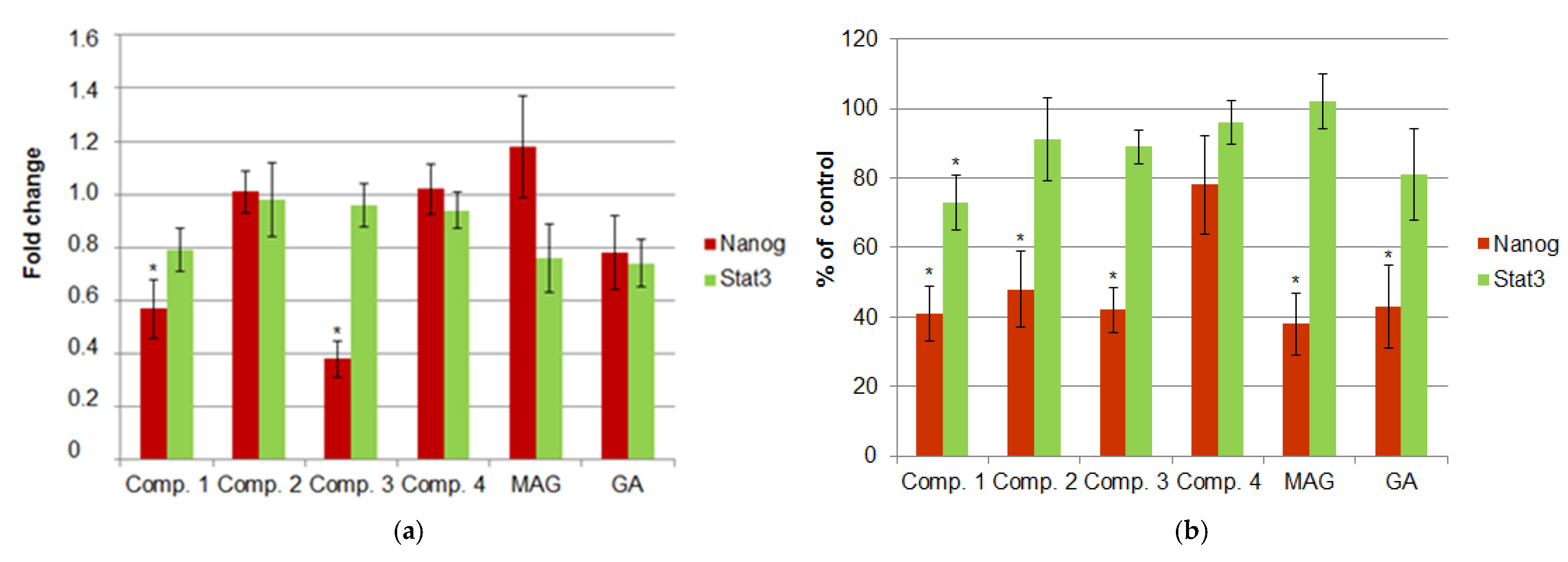
3.1. Expression of NANOG and STAT3 Under Xanthone Treatment
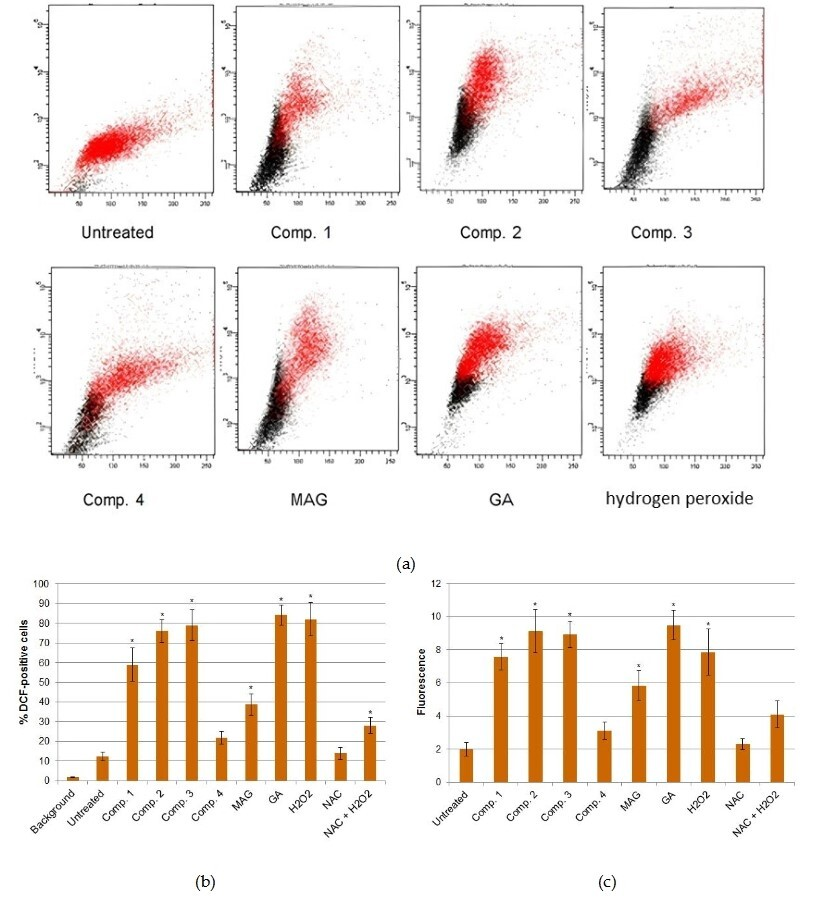
3.2. Induction of ROS in Xanthone-Treated Cells
3.3. Knockdown of NANOG and STAT3 by shRNA-Mediated Gene Silencing
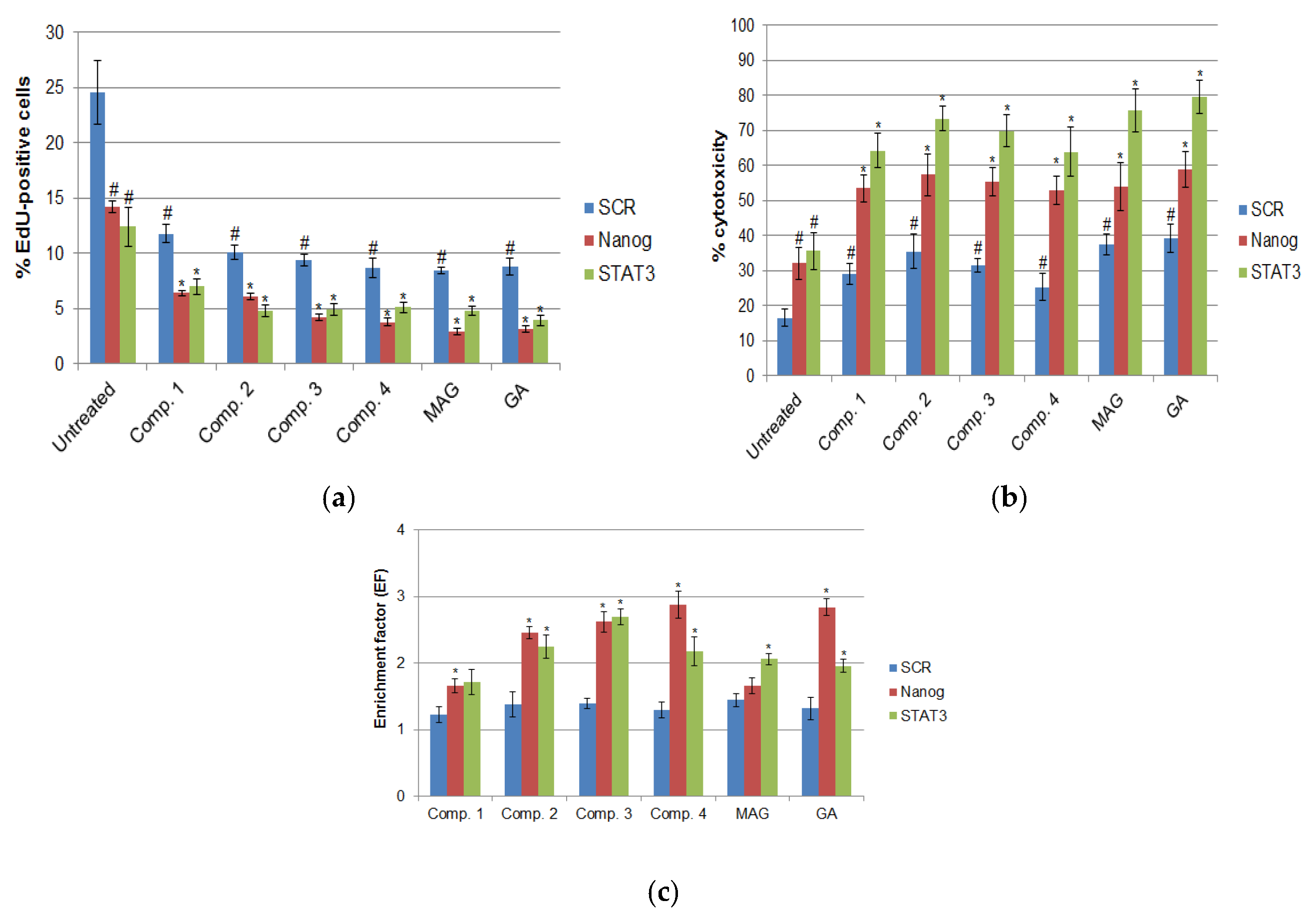
3.4. Cell Viability, Proliferation, and Apoptosis After RNAi-Mediated Gene Silencing and Xanthone Treatments
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSC | Cancer stem cells |
| DAPI | 4′,6-diamidino-2-phenylindole dihydrochloride |
| DCF | 2′,7′-dichlorofluorescein diacetate |
| DMSO | Dimethyl sulfoxide |
| D-PBS | Dulbecco’s Phosphate Buffered Saline |
| EdU | 5-ethynyl-2′-deoxyuridine |
| EF | Enrichment factor |
| FBS | Foetal bovine serum |
| GA | Gambogic acid |
| GFP | Green fluorescent protein |
| H2DCFDA | 2′,7′-dichlorodihydrofluorescein diacetate |
| HPV | Human Papillomavirus |
| LDH | Lactate dehydrogenase |
| MAG | α-mangostin |
| NAC | N-acetyl-L-cysteine |
| RNAi | RNA interference |
| ROR | HOX transcript antisense intergenic RNA |
| ROS | Reactive oxygen species |
| SCR | Scrambled non-targeting sequence |
| shRNA | Short hairpin RNA |
| siRNA | Small interfering RNA |
| STATs | Signal transducers and activators of transcription |
References
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, Y.S.; Priyangga, K.T.A.; Jumina; Pranowo, H.D.; Sholikhah, E.N.; Zulkarnain, A.K.; Fatimi, H.A.; Julianus, J. An Update on the Anticancer Activity of Xanthone Derivatives: A Review. Pharmaceuticals 2021, 14, 1144. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Rashid, S.; Fatima, K.; Adnan, M.; Shafie, A.; Akhtar, M.S.; Ganie, A.H.; Eldin, S.M.; Islam, A.; Khan, I.; et al. Biochemical features and therapeutic potential of α-Mangostin: Mechanism of action, medicinal values, and health benefits. Biomed. Pharmacother. 2023, 163, 114710. [Google Scholar] [CrossRef]
- Omolo, J.J.; Johnson, M.M.; van Vuuren, S.F.; de Koning, C.B. The synthesis of xanthones, xanthenediones, and spirobenzofurans: Their antibacterial and antifungal activity. Bioorg Med. Chem. Lett. 2011, 21, 7085–7088. [Google Scholar] [CrossRef] [PubMed]
- Klein-Júnior, L.C.; Campos, A.; Niero, R.; Corrêa, R.; Vander Heyden, Y.; Filho, V.C. Xanthones and Cancer: From Natural Sources to Mechanisms of Action. Chem. Biodivers. 2020, 17, e1900499. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Lin, L.; Li, H. Gambogic Acid as a Candidate for Cancer Therapy: A Review. Int. J. Nanomed. 2020, 15, 10385–10399. [Google Scholar] [CrossRef]
- Yi, T.; Yi, Z.; Cho, S.-G.; Luo, J.; Pandey, M.K.; Aggarwal, B.B.; Liu, M. Gambogic acid inhibits angiogenesis and prostate tumor growth by suppressing vascular endothelial growth factor receptor 2 signaling. Cancer Res. 2008, 68, 1843–1850. [Google Scholar] [CrossRef]
- Ibrahim, M.Y.; Hashim, N.M.; Mariod, A.A.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Arbab, I.A. α-mangostin from Garcinia mangostana Linn: An updated review of its pharmacological properties. Arab. J. Chem. 2014, 9, 317–329. [Google Scholar] [CrossRef]
- Meylina, L.; Muchtaridi, M.; Joni, I.M.; Mohammed, A.F.A.; Wathoni, N. Nanoformulations of α-Mangostin for Cancer Drug Delivery System. Pharmaceutics 2021, 13, 1993. [Google Scholar] [CrossRef]
- Vasefifar, P.; Motafakkerazad, R.; Maleki, L.A.; Najafi, S.; Ghrobaninezhad, F.; Najafzadeh, B.; Alemohammad, H.; Amini, M.; Baghbanzadeh, A.; Baradaran, B. Nanog, as a key cancer stem cell marker in tumor progression. Gene 2022, 827, 146448. [Google Scholar] [CrossRef]
- Najafzadeh, B.; Asadzadeh, Z.; Motafakker Azad, R.; Mokhtarzadeh, A.; Baghbanzadeh, A.; Alemohammad, H.; Shadbad, M.A.; Vasefifar, P.; Najafi, S.; Baradaran, B. The oncogenic potential of NANOG: An important cancer induction mediator. J. Cell Physiol. 2021, 236, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Li, Q.; Jeter, C.R.; Fan, Q.; Tang, D.G.; Liu, B. Regulation of NANOG in cancer cells. Mol. Carcinog. 2015, 54, 679–687. [Google Scholar] [CrossRef]
- Zhang, J.; Espinoza, L.A.; Kinders, R.J.; Lawrence, S.M.; Pfister, T.D.; Zhou, M.; Veenstra, T.D.; Thorgeirsson, S.S.; Jessup, J.M. NANOG modulates stemness in human colorectal cancer. Oncogene 2013, 32, 4397–4405. [Google Scholar] [CrossRef]
- Watanabe, M.; Ohnishi, Y.; Inoue, H.; Wato, M.; Tanaka, A.; Kakudo, K.; Nozaki, M. NANOG expression correlates with differentiation, mestatsis and resistance to properative adjuvant therapy in oral squamous cell carcinoma. Oncol. Lett. 2014, 7, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Habu, N.; Imanishi, Y.; Kameyama, K.; Shimoda, M.; Tokumaru, Y.; Sakamoto, K.; Fujii, R.; Shigetomi, S.; Otsuka, K.; Sato, Y.; et al. Expression of Oct3/4 and Nanog in the head and neck squamous carcinoma cells and its clinical implications for delayed neck metastasis in stage I/II oral tongue squamous cell carcinoma. BMC Cancer 2015, 15, 730. [Google Scholar] [CrossRef]
- Alemohammad, H.; Asadzadeh, Z.; Motafakker Azad, R.; Hemmat, N.; Najafzadeh, B.; Vasefifar, P.; Najafi, S.; Baradaran, B. Signaling pathways and microRNAs, the orchestrators of NANOG activity during cancer induction. Life Sci. 2020, 260, 118337. [Google Scholar] [CrossRef] [PubMed]
- Afroze, S.N.; Rao, G.V.; Suri, S. Elucidating the immunohistochemistry of Nanog: A transcription marker in the oral squamous cell carcinoma with emphasis on its origin as embryonic stem cell. J. Oral. Maxillofac. Pathol. 2022, 26, 476–482. [Google Scholar] [CrossRef]
- Kenda Suster, N.; Virant-Klun, I. Presence and role of stem cells in ovarian cancer. World J. Stem Cells 2019, 11, 383–397. [Google Scholar] [CrossRef]
- Oh, S.J.; Lee, J.; Kim, Y.; Song, K.-H.; Cho, E.; Kim, M.; Jung, H.; Kim, T.W. Far Beyond Cancer Immunotherapy: Reversion of Multi-Malignant Phenotypes of Immunotherapeutic-Resistant Cancer by Targeting the NANOG Signaling Axis. Immune Netw. 2020, 20, e7. [Google Scholar] [CrossRef]
- Wang, M.-L.; Chiou, S.-H.; Wu, C.-W. Targeting cancer stem cells: Emerging role of Nanog transcription factor. Onco Targets Ther. 2013, 6, 1207–1220. [Google Scholar]
- Bourguignon, L.Y.; Peyrollier, K.; Xia, W.; Gilad, E. Hyaluronian-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J. Biol. Chem. 2008, 283, 17635–17651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, Y.; Yang, Y.; Zhong, C.; Ji, T.; Duan, J.; Wang, Y. RNAi mediated silencing of Nanog expression suppresses the growth of human colorectal cancer stem cells. Biochem. Biophys. Res. Commun. 2021, 534, 254–260. [Google Scholar] [CrossRef]
- Alemohammad, H.; Motafakkerazad, R.; Asadzadeh, Z.; Farsad, N.; Hemmat, N.; Najafzadeh, B.; Vasefifar, P.; Baradaran, B. siRNA-mediated silencing of Nanog reduces stemness properties and increases the sensitivity of HepG2 cells to cisplatin. Gene 2022, 821, 146333. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Zhang, C.; Martincuks, A.; Herrmann, A.; Yu, H. STAT proteins in cancer: Orchestration of metabolism. Nat. Rev. Cancer. 2023, 23, 115–134. [Google Scholar] [CrossRef]
- Halim, C.E.; Deng, S.; Ong, M.S.; Yap, C.T. Involvement of STAT5 in Oncogenesis. Biomedicines 2020, 8, 316. [Google Scholar] [CrossRef] [PubMed]
- Xiong, A.; Yang, Z.; Shen, Y.; Zhou, J.; Shen, Q. Transcriptional factor STAT3 as a novel molecular target for cancer prevention. Cancers 2014, 6, 926–957. [Google Scholar] [CrossRef]
- Pham, T.H.; Park, H.M.; Kim, J.; Hong, J.T.; Yoon, D.Y. STAT3 and p53, Dual Target for Cancer Therapy. Biomedicines 2020, 8, 637. [Google Scholar] [CrossRef]
- Siveen, K.S.; Sikka, S.; Surana, R.; Dai, X.; Zhang, J.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors. Biochim. Biophys. Acta 2014, 1845, 136–154. [Google Scholar] [CrossRef]
- Verhoeven, Y.; Tilborghs, S.; Jacobs, J.; De Waele, J.; Quatannens, D.; Deben, C.; Prenen, H.; Pauwels, P.; Trinh, X.B.; Wouters, A.; et al. The potential and controversy of targeting STAT family members in cancer. Semin. Cancer Biol. 2020, 60, 41–56. [Google Scholar] [CrossRef]
- Strobel, T.D.; Weber, M.; Heber, N.; Holzer, A.; Hoppe-Seyler, K.; Hoppe-Seyler, F. Revisiting the role of endogenous STAT3 in HPV-positive cervical cancer cells. J. Med. Virol. 2023, 95, e29230. [Google Scholar] [CrossRef]
- Szkaradek, N.; Sypniewski, D.; Waszkielewicz, A.M.; Gunia-Krzyżak, A.; Galilejczyk, A.; Gałka, S.; Ga|Ka, S.; Marona, H.; Bednarek, I. Synthesis and in vitro evaluation of the anticancer potential of new aminoalkanol derivatives of xanthone. Anti-Cancer Agents Med. Chem. 2016, 16, 1587–1604. [Google Scholar] [CrossRef]
- α-Mangostin; Gambogic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 25 June 2025).
- Gawlik-Rzemieniewska, N.; Galilejczyk, A.; Krawczyk, M.; Bednarek, I. Silencing expression of the NANOG gene and changes in migration and metastasis of urinary bladder cancer cells. Arch. Med. Sci. 2016, 12, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, I.; Sypniewski, D.; Gawlik, N.; Galilejczyk, A.; Goraus, K. The efficiency of silencing expression of the gene coding STAT3 transcriptional factor and susceptibility of bladder cancer cells to apoptosis. Contemp. Oncol. 2012, 16, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lao, Y.; Zhao, Y.; Qin, J.; Fu, W.; Zhang, Y.; Xu, H. Screening active compounds from Garcinia species native to China reveals novel compounds targeting the STAT/JAK signaling pathway. BioMed Res. Int. 2015, 2015, 910453. [Google Scholar] [CrossRef]
- Lee, C.H.; Ying, T.H.; Chiou, H.L.; Hsieh, S.C.; Wen, S.H.; Chou, R.H.; Hsieh, Y.H. Alpha-mangostin induces apoptosis through activation of reactive oxygen species and ASK1/p38 signaling pathway in cervical cancer cells. Oncotarget 2017, 8, 47425–47439. [Google Scholar] [CrossRef] [PubMed]
- Krajarng, A.; Imoto, M.; Tashiro, E.; Fujimaki, T.; Shinjo, S.; Watanapokasin, R. Apoptosis induction associated with the ER stress response through up-regulation of JNK in HeLa cells by gambogic acid. BMC Complement. Altern. Med. 2015, 15, 26. [Google Scholar] [CrossRef]
- Wong, O.G.; Cheung, A.N. Stem cell transcription factor NANOG in cancers—Is eternal youth a curse ? Expert. Opin. Ther. Targets 2016, 20, 407–417. [Google Scholar] [CrossRef]
- Han, M.K.; Song, E.K.; Guo, Y.; Ou, X.; Mantel, C.; Broxmeyer, H.E. SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell 2008, 2, 241–251. [Google Scholar] [CrossRef]
- Ali Azouaou, S.; Emhemmed, F.; Idris-Khodja, N.; Lobstein, A.; Schini-Kerth, V.; Muller, C.D.; Fuhrmann, G. Selective ROS-dependent p53-associated anticancer effects of the hypoxoside derivative rooperol on human teratocarcinomal cancer stem-like cells. Investig. New Drugs 2015, 33, 64–74. [Google Scholar] [CrossRef]
- Ren, T.; Bai, Y.Y.; Yang, M.Z.; Xu, N.; Guo, X.Z.; Qin, L.J.; Huang, Z.L.; Zhong, Q.Y.; Huang, Y.J.; Lin, W.Z.; et al. Gambogic acid suppresses nasopharyngeal carcinoma via rewiring molecular network of cancer malignancy and immunosurveillance. Biomed Pharmacother. 2022, 150, 113012. [Google Scholar] [CrossRef]
- Shan, T.; Cui, X.J.; Li, W.; Lin, W.R.; Lu, H.W.; Li, Y.M.; Chen, X.; Wu, T. α-mangostin suppresses human gastric adenocarcinoma cells in vitro via blockade of Stat3 signaling pathway. Acta Pharmacol. Sin. 2014, 35, 1065–1073. [Google Scholar] [CrossRef]
- Titze-de-Almeida, R.; David, C.; Titze-de-Almeida, S.S. The race of 10 synthetic RNAi-based drugs to the pharmaceutical market. Pharm. Res. 2017, 34, 1339–1363. [Google Scholar] [CrossRef] [PubMed]
- Falamarzian, A.; Aliabadi, H.M.; Molavi, O.; Seubert, J.M.; Lai, R.; Uludag, H.; Lavasanifar, A. Effective down-regulation of signal transducer and activator of transcription 3 (STAT3) by polyplexes of siRNA and lipid-substituted polyethyleneimine for sensitization of breast tumor cells to conventional chemotherapy. J. Biomed. Mater. Res. Part A 2014, 102, 3216–3228. [Google Scholar] [CrossRef] [PubMed]
- Xi, S.; Gooding, W.E.; Grandis, J.R. In vivo antitumor efficacy of STAT3 blockade using a transcription factor decoy approach: Implications for cancer therapy. Oncogene 2005, 24, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Crowe, P.J.; Goldstein, D.; Yang, J.-L. STAT3 inhibition, a novel approach to enhancing targeted therapy in human cancers (review). Int. J. Oncol. 2012, 41, 1181–1191. [Google Scholar] [CrossRef]
- Munoz, J.; Dhillon, N.; Janku, F.; Watowich, S.S.; Hong, D.S. STAT3 inhibitors: Finding a home in lymphoma and leukemia. Oncologist 2014, 19, 536–544. [Google Scholar] [CrossRef]
- Odate, S.; Veschi, V.; Yan, S.; Lam, N.; Woessner, R.; Thiele, C.J. Inhibition of STAT3 with the generation 2.5 antisense oligonucleotide, AZD9150, decreases neuroblastoma tumorigenicity and increases chemosensitivity. Clin. Cancer Res. 2017, 23, 1771–1784. [Google Scholar] [CrossRef]
- Hong, D.; Kurzrock, R.; Kim, Y.; Woessner, R.; Younes, A.; Nemunaitis, J.; Fowler, N.; Zhou, T.; Schmidt, J.; Jo, M.; et al. AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer. Sci. Transl. Med. 2015, 7, 314ra185. [Google Scholar] [CrossRef]
- Liu, T.; Peng, H.; Zhang, M.; Deng, Y.; Wu, Z. Cucurbitacin B, a small molecule inhibitor of the Stat3 signaling pathway, enhances the chemosensitivity of laryngeal squamous cell carcinoma cells to cisplatin. Eur. J. Pharmacol. 2010, 641, 15–22. [Google Scholar] [CrossRef]
- Ji, W.; Jiang, Z. Effect of shRNA-mediated inhibition of Nanog gene expression on the behavior of human gastric cells. Oncol. Lett. 2013, 6, 367–374. [Google Scholar] [CrossRef][Green Version]
- Zhou, J.-J.; Deng, X.G.; He, X.Y.; Zhou, Y.; Yu, M.; Gao, W.C.; Zeng, B.; Zhou, Q.-B.; Li, Z.-H.; Chen, R.-F. Knockdown of NANOG enhances chemosensitivity of liver cancer cells to doxorubicin by reducing MDR1 expression. Int. J. Oncol. 2014, 44, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Shi, L.; Wang, T.; Liu, Z.; Wang, Z. Nanog siRNA plus cisplatin may enhance the sensitivity of chemotherapy in esophageal cancer. J. Cancer Res. Clin. Oncol. 2012, 138, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, P.; Hua, Y.; Xi, H.; Meng, Z.; Liu, T.; Zeng, B.; Zhou, Q.-B.; Li, Z.-H.; Chen, R.-F. ROR functions as a ceRNA to regulate Nanog expression by sponging miR-145 and predicts poor prognosis in pancreatic cancer. Oncotarget 2016, 7, 1608–1618. [Google Scholar] [CrossRef]
- Zhou, J.-J.; Cheng, D.; He, X.-Y.; Meng, Z.; Ye, H.-L.; Chen, R.-F. Knockdown of long non-coding RNA HOTAIR sensitizes hepatocellular carcinoma cell to cisplatin by suppressing the STAT3/ABCB1 signaling pathway. Oncol. Lett. 2017, 14, 7986–7992. [Google Scholar] [CrossRef] [PubMed]
- Zsoldos, B.; Nagy, N.; Donkó-Tóth, V.; Keglevich, P.; Weber, M.; Dékány, M.; Nehr-Majoros, A.; Szőke, É.; Helyes, Z.; Hazai, L. Novel Piperazine Derivatives of Vindoline as Anticancer Agents. Int. J. Mol. Sci. 2024, 25, 7929. [Google Scholar] [CrossRef]
- Wang, H.; Deng, J.; Ren, H.Y.; Jia, P.; Zhang, W.; Li, M.-Q.; Li, S.-W.; Zhou, Q.-H. STAT3 influences the characteristics of stem cells in cervical carcinoma. Oncol. Lett. 2017, 14, 2131–2136. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruszka, O.; Żelaszczyk, D.; Marona, H.; Bednarek, I.A. Sensitisation of HeLa Cell Cultures to Xanthone Treatment by RNAi-Mediated Silencing of NANOG and STAT3. Curr. Issues Mol. Biol. 2025, 47, 529. https://doi.org/10.3390/cimb47070529
Gruszka O, Żelaszczyk D, Marona H, Bednarek IA. Sensitisation of HeLa Cell Cultures to Xanthone Treatment by RNAi-Mediated Silencing of NANOG and STAT3. Current Issues in Molecular Biology. 2025; 47(7):529. https://doi.org/10.3390/cimb47070529
Chicago/Turabian StyleGruszka, Oliwia, Dorota Żelaszczyk, Henryk Marona, and Ilona Anna Bednarek. 2025. "Sensitisation of HeLa Cell Cultures to Xanthone Treatment by RNAi-Mediated Silencing of NANOG and STAT3" Current Issues in Molecular Biology 47, no. 7: 529. https://doi.org/10.3390/cimb47070529
APA StyleGruszka, O., Żelaszczyk, D., Marona, H., & Bednarek, I. A. (2025). Sensitisation of HeLa Cell Cultures to Xanthone Treatment by RNAi-Mediated Silencing of NANOG and STAT3. Current Issues in Molecular Biology, 47(7), 529. https://doi.org/10.3390/cimb47070529






