The Impact of Arsenic, Cadmium, Lead, Mercury, and Thallium Exposure on the Cardiovascular System and Oxidative Mechanisms in Children
Abstract
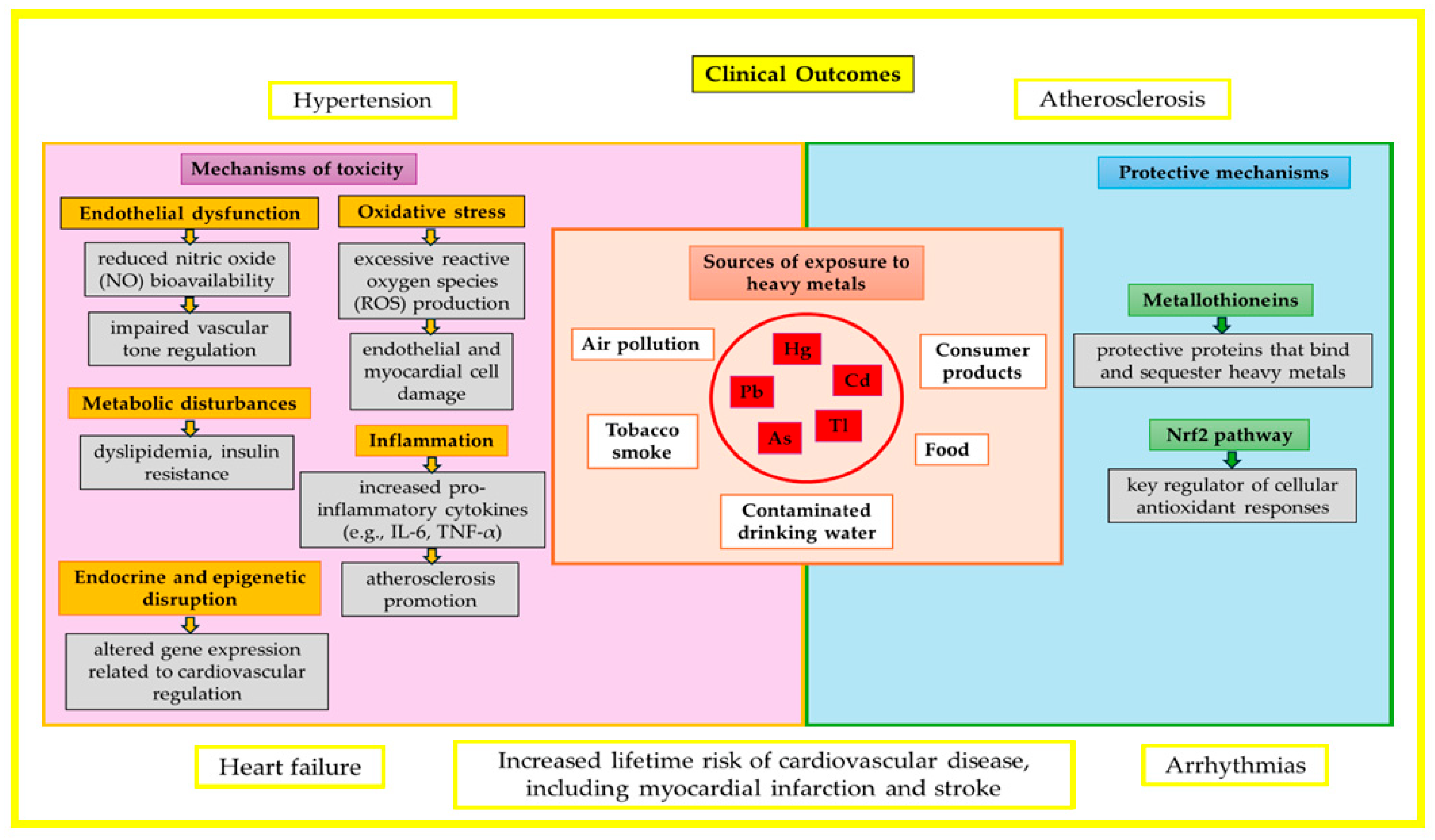
1. Introduction
2. Mercury
3. Lead
4. Cadmium
5. Arsenic
6. Thallium
7. Sources of Heavy Metal Exposure and Diagnostic Guidelines
8. Prenatal Exposure to Heavy Metals and the Risk of Congenital Heart Defects
9. Preventive Strategies and Public Health Implications
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADMA | Asymmetric dimethylarginine |
| BP | Blood pressure |
| BMP | Signaling pathway |
| cIMT | Carotid intima-media thickness |
| CAT | Catalase |
| CHDs | Congenital heart defects |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| DBP | Diastolic blood pressure |
| EF | Ejection fraction |
| GA | Gestational age |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| HR | Heart rate |
| HDL | High-density lipoprotein |
| IL-6 | Interleukin-6 |
| iAs | Inorganic arsenic |
| LAD | Left atrial diameter |
| LDL | Low-density lipoprotein |
| LVM | Left ventricular mass |
| MAP | Mean arterial pressure |
| •NO | Nitric oxide radical |
| Notch | Signaling pathway |
| PWV | Pulse wave velocity |
| ROS | Reactive oxygen species |
| RHR | Resting heart rate |
| SBP | Systolic blood pressure |
| SF | Shortening fraction |
| SOD | Superoxide dismutase |
| TC | Total cholesterol |
| TC/HDL | Total cholesterol to high-density lipoprotein ratio |
| TNF-α | Tumor necrosis factor alpha |
| Wnt | Signaling pathway |
References
- Viraraghavan, T.; Srinivasan, A. Thallium: Environmental Pollution and Health Effects. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2011; pp. 325–333. [Google Scholar]
- Hasani, M.; Khazdouz, M.; Sobhani, S.; Mardi, P.; Riahi, S.; Agh, F.; Mahdavi-Gorabi, A.; Mohammadipournami, S.; Gomnam, F.; Qorbani, M. Association of heavy metals and bio-elements blood level with metabolic syndrome: A systematic review and meta-analysis of observational studies. J. Diabetes Metab. Disord. 2024, 23, 1719–1752. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Ramond, A.; O’Keeffe, L.M.; Shahzad, S.; Kunutsor, S.K.; Muka, T.; Gregson, J.; Willeit, P.; Warnakula, S.; Khan, H.; et al. Environmental toxic metal contaminants and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2018, 362, k3310. [Google Scholar] [CrossRef]
- Al Osman, M.; Yang, F.; Massey, I.Y. Exposure routes and health effects of heavy metals on children. BioMetals 2019, 32, 563–573. [Google Scholar] [CrossRef]
- Akimzhanova, Z.; Guney, M.; Kismelyeva, S.; Zhakiyenova, A.; Yagofarova, A. Contamination by eleven harmful elements in children’s jewelry and toys from Central Asian market. Environ. Sci. Pollut. Res. 2020, 27, 21071–21083. [Google Scholar] [CrossRef] [PubMed]
- Gul, A.; Gul, D.-S.; Mohiuddin, S. Metals as toxicants in event-based expedited production of children’s jewelry. Environ. Sci. Pollut. Res. 2023, 30, 73964–73973. [Google Scholar] [CrossRef] [PubMed]
- Gul, D.-S.; Gul, A.; Tanoli, A.K.; Ahmed, T.; Mirza, M.A. Contamination by hazardous elements in low-priced children’s plastic toys bought on the local markets of Karachi, Pakistan. Environ. Sci. Pollut. Res. 2022, 29, 51964–51975. [Google Scholar] [CrossRef]
- Tepanosyan, G.; Sahakyan, L.; Belyaeva, O.; Asmaryan, S.; Saghatelyan, A. Continuous impact of mining activities on soil heavy metals levels and human health. Sci. Total Environ. 2018, 639, 900–909. [Google Scholar] [CrossRef]
- Osorio-Yáñez, C.; Ayllon-Vergara, J.C.; Aguilar-Madrid, G.; Arreola-Mendoza, L.; Hernández-Castellanos, E.; Barrera-Hernández, A.; De Vizcaya-Ruiz, A.; Del Razo, L.M. Carotid Intima-Media Thickness and Plasma Asymmetric Dimethylarginine in Mexican Children Exposed to Inorganic Arsenic. Environ. Health Perspect. 2013, 121, 1090–1096. [Google Scholar] [CrossRef]
- Osorio-Yáñez, C.; Ayllon-Vergara, J.C.; Arreola-Mendoza, L.; Aguilar-Madrid, G.; Hernández-Castellanos, E.; Sánchez-Peña, L.C.; Del Razo, L.M. Blood Pressure, Left Ventricular Geometry, and Systolic Function in Children Exposed to Inorganic Arsenic. Environ. Health Perspect. 2015, 123, 629–635. [Google Scholar] [CrossRef]
- Farzan, S.F.; Howe, C.G.; Chen, Y.; Gilbert-Diamond, D.; Korrick, S.; Jackson, B.P.; Weinstein, A.R.; Karagas, M.R. Prenatal and postnatal mercury exposure and blood pressure in childhood. Environ. Int. 2021, 146, 106201. [Google Scholar] [CrossRef]
- Shi, H.; Su, M.; Shen, P.; Ma, J.; Zhou, Q.; Yang, Z.; Chai, P.; Sun, S.; Lin, H.; Shui, L.; et al. Associations Between Metals and Nonmetals in Drinking Water, Cardiovascular Events, and Diet. JACC Adv. 2025, 4, 101669. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Lelieveld, J.; Steven, S.; Oelze, M.; Kröller-Schön, S.; Sørensen, M.; Münzel, T. The “exposome” concept—how environmental risk factors influence cardiovascular health. Acta Biochim. Pol. 2019, 66, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, J.P.; Jone, P.-N.; Agbaje, A.O.; Ryan, H.H.; Trasande, L.; Perng, W.; Farzan, S.F. Environmental Exposures and Pediatric Cardiology: A Scientific Statement From the American Heart Association. Circulation 2024, 149, E1165–E1175. [Google Scholar] [CrossRef] [PubMed]
- Świątkiewicz, I.; Wróblewski, M.; Nuszkiewicz, J.; Sutkowy, P.; Wróblewska, J.; Woźniak, A. The Role of Oxidative Stress Enhanced by Adiposity in Cardiometabolic Diseases. Int. J. Mol. Sci. 2023, 24, 6382. [Google Scholar] [CrossRef]
- Yim, G.; Margetaki, K.; Romano, M.E.; Kippler, M.; Vafeiadi, M.; Roumeliotaki, T.; Bempi, V.; Farzan, S.F.; Chatzi, L.; Howe, C.G. Metal mixture exposures and serum lipid levels in childhood: The Rhea mother-child cohort in Greece. J. Expo. Sci. Environ. Epidemiol. 2024, 34, 688–698. [Google Scholar] [CrossRef]
- Abarikwu, S.O. Lead, Arsenic, Cadmium, Mercury: Occurrence, Toxicity and Diseases. In Pollutant Diseases, Remediation and Recycling; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer International Publishing: Cham, Switzerland, 2013; pp. 351–386. ISBN 978-3-319-02387-8. [Google Scholar]
- Yao, X.; Steven Xu, X.; Yang, Y.; Zhu, Z.; Zhu, Z.; Tao, F.; Yuan, M. Stratification of population in NHANES 2009–2014 based on exposure pattern of lead, cadmium, mercury, and arsenic and their association with cardiovascular, renal and respiratory outcomes. Environ. Int. 2021, 149, 106410. [Google Scholar] [CrossRef]
- Horton, L.M.; Mortensen, M.E.; Iossifova, Y.; Wald, M.M.; Burgess, P. What Do We Know of Childhood Exposures to Metals (Arsenic, Cadmium, Lead, and Mercury) in Emerging Market Countries? Int. J. Pediatr. 2013, 2013, 872596. [Google Scholar] [CrossRef]
- Lameijer, W.; van Zwieten, P.A. Acute cardiovascular toxicity of thallium (I) ions. Arch. Toxicol. 1976, 35, 49–61. [Google Scholar] [CrossRef]
- Marjanović Čermak, A.M.; Mustać, S.; Cvjetko, P.; Pavičić, I.; Kifer, D.; Bešić, E.; Domijan, A.-M. Thallium Toxicity and its Interference with Potassium Pathways Tested on Various Cell Lines. Biol. Trace Elem. Res. 2024, 202, 5025–5035. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.; Fu, Z.; Shu, Y.; Zhang, J.; Lu, C.; Mo, X. Associations between total mercury and methyl mercury exposure and cardiovascular risk factors in US adolescents. Environ. Sci. Pollut. Res. 2018, 25, 6265–6272. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.; Carocci, A.; Lauria, G.; Catalano, A. Mercury Exposure and Heart Diseases. Int. J. Environ. Res. Public Health 2017, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, R.A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Viñas, G.; Ballester, F.; Llop, S. Chronic mercury exposure and blood pressure in children and adolescents: A systematic review. Environ. Sci. Pollut. Res. 2019, 26, 2238–2252. [Google Scholar] [CrossRef]
- Arik, E.; Gungor, O.; Temiz, F.; Kurutas, E.; Dilber, C. Evaluation of Blood Nitrotyrosine and Nitric Oxide Levels in Acute Mercury Intoxication in Children. Ann. Med. Res. 2024, 31, 486. [Google Scholar] [CrossRef]
- Magnuson, J.T.; Sandheinrich, M.B. Relation among Mercury, Selenium, and Biomarkers of Oxidative Stress in Northern Pike (Esox lucius). Toxics 2023, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, M.; Wang, R.; Gao, M.; Hu, L.; Liu, S.; Xu, M. Roles of glutathione peroxidase 4 on the mercury-triggered ferroptosis in renal cells: Implications for the antagonism between selenium and mercury. Metallomics 2023, 15, 3–5. [Google Scholar] [CrossRef]
- Bose-O’Reilly, S.; McCarty, K.M.; Steckling, N.; Lettmeier, B. Mercury Exposure and Children’s Health. Curr. Probl. Pediatr. Adolesc. Health Care 2010, 40, 186–215. [Google Scholar] [CrossRef]
- Shrivastav, A.; Swetanshu; Singh, P. The Impact of Environmental Toxins on Cardiovascular Diseases. Curr. Probl. Cardiol. 2024, 49, 102120. [Google Scholar] [CrossRef]
- Costet, N.; Doyen, M.; Rouget, F.; Michineau, L.; Monfort, C.; Cirtiu, C.M.; Kadhel, P.; Multigner, L.; Pladys, P.; Cordier, S. Early exposure to mercury and cardiovascular function of seven-year old children in Guadeloupe (French West Indies). Environ. Res. 2024, 246, 117955. [Google Scholar]
- Hill, D.T.; Petroni, M.; Larsen, D.A.; Bendinskas, K.; Heffernan, K.; Atallah-Yunes, N.; Parsons, P.J.; Palmer, C.D.; MacKenzie, J.A.; Collins, M.B.; et al. Linking metal (Pb, Hg, Cd) industrial air pollution risk to blood metal levels and cardiovascular functioning and structure among children in Syracuse, NY. Environ. Res. 2021, 193, 110557. [Google Scholar] [CrossRef]
- Liu, J.; Portnoy, J.; Um, P.; Cui, N.; Rudo-Hutt, A.; Yan, C.; Raine, A.; Chen, A. Blood lead and mercury levels are associated with low resting heart rate in community adolescent boys. Int. J. Hyg. Environ. Health 2021, 233, 113685. [Google Scholar] [CrossRef]
- Niemeier, R.T.; Maier, A.; Reichard, J.F. Rapid Review of Dermal Penetration and Absorption of Inorganic Lead Compounds for Occupational Risk Assessment. Ann. Work Expo. Health 2022, 66, 291–311. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, P. Probabilistic Integrated Human Mixture Risk Assessment of Multiple Metals Through Seafood Consumption. Risk Anal. 2019, 39, 426–438. [Google Scholar] [CrossRef]
- Petit, D.; Véron, A.; Flament, P.; Deboudt, K.; Poirier, A. Review of pollutant lead decline in urban air and human blood: A case study from northwestern Europe. Comptes Rendus Géoscience 2015, 347, 247–256. [Google Scholar] [CrossRef]
- Karakis, I.; Landau, D.; Gat, R.; Shemesh, N.; Tirosh, O.; Yitshak-Sade, M.; Sarov, B.; Novack, L. Maternal metal concentration during gestation and pediatric morbidity in children: An exploratory analysis. Environ. Health Prev. Med. 2021, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Monir, A.U.; Gundberg, C.M.; Yagerman, S.E.; van der Meulen, M.C.H.; Budell, W.C.; Boskey, A.L.; Dowd, T.L. The effect of lead on bone mineral properties from female adult C57/BL6 mice. Bone 2010, 47, 888–894. [Google Scholar] [CrossRef]
- Zeng, X.; Huo, X.; Xu, X.; Liu, D.; Wu, W. E-waste lead exposure and children’s health in China. Sci. Total Environ. 2020, 734, 139286. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Shu, Y.; Fu, Z.; Hu, Y.; Mo, X. Associations between lead concentrations and cardiovascular risk factors in U.S. adolescents. Sci. Rep. 2017, 7, 9121. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, J.P.; Wang, Y.; Penny, D.J.; Baranowski, T. Relation Between Lead Exposure and Trends in Blood Pressure in Children. Am. J. Cardiol. 2018, 122, 1890–1895. [Google Scholar] [CrossRef]
- Govarts, E.; Gilles, L.; Rodriguez Martin, L.; Santonen, T.; Apel, P.; Alvito, P.; Anastasi, E.; Andersen, H.R.; Andersson, A.-M.; Andryskova, L.; et al. Harmonized human biomonitoring in European children, teenagers and adults: EU-wide exposure data of 11 chemical substance groups from the HBM4EU Aligned Studies (2014–2021). Int. J. Hyg. Environ. Health 2023, 249, 114119. [Google Scholar] [CrossRef]
- Zheng, X.; Huo, X.; Zhang, Y.; Wang, Q.; Zhang, Y.; Xu, X. Cardiovascular endothelial inflammation by chronic coexposure to lead (Pb) and polycyclic aromatic hydrocarbons from preschool children in an e-waste recycling area. Environ. Pollut. 2019, 246, 587–596. [Google Scholar] [CrossRef]
- Lee, C.-K.; Wu, C.; Lin, C.-Y.; Huang, P.-C.; Sung, F.-C.; Su, T.-C. Positive Association between Endothelium–Platelet Microparticles and Urinary Concentration of Lead and Cadmium in Adolescents and Young Adults. Nutrients 2021, 13, 2913. [Google Scholar] [CrossRef]
- Ferreira de Mattos, G.; Costa, C.; Savio, F.; Alonso, M.; Nicolson, G.L. Lead poisoning: Acute exposure of the heart to lead ions promotes changes in cardiac function and Cav1.2 ion channels. Biophys. Rev. 2017, 9, 807–825. [Google Scholar] [CrossRef] [PubMed]
- Halabicky, O.M.; Téllez-Rojo, M.M.; Miller, A.L.; Goodrich, J.M.; Dolinoy, D.C.; Hu, H.; Peterson, K.E. Associations of prenatal and childhood Pb exposure with allostatic load in adolescence: Findings from the element cohort study. Environ. Res. 2023, 235, 116647. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.P.; Svensson, K.; Gennings, C.; Burris, H.H.; Oken, E.; Amarasiriwardena, C.; Basnet, P.; Pizano-Zarate, M.L.; Schnaas, L.; Tamayo-Ortiz, M.; et al. Prenatal lead exposure modifies the effect of shorter gestation on increased blood pressure in children. Environ. Int. 2018, 120, 464–471. [Google Scholar] [CrossRef]
- Chen, Z.; Huo, X.; Zhang, S.; Cheng, Z.; Huang, Y.; Xu, X. Relations of blood lead levels to echocardiographic left ventricular structure and function in preschool children. Chemosphere 2021, 268, 128793. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, Y.; Mao, W.; Sui, H.; Yong, L.; Yang, D.; Jiang, D.; Zhang, L.; Gong, Y. Dietary cadmium exposure assessment among the Chinese population. PLoS ONE 2017, 12, e0177978. [Google Scholar] [CrossRef]
- Huang, N.; Wang, B.; Liu, S.; Wang, K.; Wang, R.; Liu, F.; Chen, C. Cadmium exposure in infants and children: Toxicity, health effects, dietary risk assessment and mitigation strategies. Crit. Rev. Food Sci. Nutr. 2024, 1–23. [Google Scholar] [CrossRef]
- Berglund, M.; Larsson, K.; Grandér, M.; Casteleyn, L.; Kolossa-Gehring, M.; Schwedler, G.; Castaño, A.; Esteban, M.; Angerer, J.; Koch, H.M.; et al. Exposure determinants of cadmium in European mothers and their children. Environ. Res. 2015, 141, 69–76. [Google Scholar] [CrossRef]
- Pirard, C.; Koppen, G.; De Cremer, K.; Van Overmeire, I.; Govarts, E.; Dewolf, M.-C.; Van De Mieroop, E.; Aerts, D.; Biot, P.; Casteleyn, L.; et al. Hair mercury and urinary cadmium levels in Belgian children and their mothers within the framework of the COPHES/DEMOCOPHES projects. Sci. Total Environ. 2014, 472, 730–740. [Google Scholar] [CrossRef]
- Riederer, A.M.; Belova, A.; George, B.J.; Anastas, P.T. Urinary cadmium in the 1999–2008 U.S. National health and nutrition examination survey (NHANES). Environ. Sci. Technol. 2013, 47, 1137–1147. [Google Scholar] [CrossRef]
- Chandravanshi, L.; Shiv, K.; Kumar, S. Developmental toxicity of cadmium in infants and children: A review. Environ. Anal. Health Toxicol. 2021, 36, e2021003. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Tao, C.; Li, Z.; Huang, Y.; Yan, W.; Zhao, S.; Gao, B.; Xu, Q.; Qin, Y.; Wang, X.; et al. Association of Endocrine-Disrupting Chemicals with All-Cause and Cause-Specific Mortality in the U.S.: A Prospective Cohort Study. Environ. Sci. Technol. 2023, 57, 2877–2886. [Google Scholar] [CrossRef]
- Akhtar, E.; Roy, A.K.; Haq, M.A.; von Ehrenstein, O.S.; Ahmed, S.; Vahter, M.; Ekstrom, E.-C.; Kippler, M.; Wagatsuma, Y.; Raqib, R. A longitudinal study of rural Bangladeshi children with long-term arsenic and cadmium exposures and biomarkers of cardiometabolic diseases. Environ. Pollut. 2021, 271, 116333. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liang, C.; Wang, Z.; Wang, X.; Xie, L.; Tao, S.; Yan, S.; Wu, X.; Wei, Z.; Tong, J.; et al. Association between prenatal metals exposure and blood pressure in 5–6 years children: A birth cohort study. Environ. Res. 2023, 219, 114974. [Google Scholar] [CrossRef]
- Xu, Z.; Weng, Z.; Liang, J.; Liu, Q.; Zhang, X.; Xu, J.; Xu, C.; Gu, A. Association between urinary cadmium concentrations and liver function in adolescents. Environ. Sci. Pollut. Res. 2022, 29, 39768–39776. [Google Scholar] [CrossRef]
- Abu Bakar, N.; Wan Ibrahim, W.N.; Mohd Faudzi, S.M. Arsenic contamination in rice and drinking water: An insight on human cognitive function. J. Hazard. Mater. Adv. 2025, 17, 100543. [Google Scholar] [CrossRef]
- Gump, B.B.; Heffernan, K.; Brann, L.S.; Hill, D.T.; Labrie-Cleary, C.; Jandev, V.; MacKenzie, J.A.; Atallah-Yunes, N.H.; Parsons, P.J.; Palmer, C.D.; et al. Exposure to Arsenic and Subclinical Cardiovascular Disease in 9- to 11-Year-Old Children, Syracuse, New York. JAMA Netw. Open 2023, 6, e2321379. [Google Scholar] [CrossRef]
- Farzan, S.F.; Karagas, M.R.; Chen, Y. In utero and early life arsenic exposure in relation to long-term health and disease. Toxicol. Appl. Pharmacol. 2013, 272, 384–390. [Google Scholar] [CrossRef]
- Bommarito, P.A.; Fry, R.C. Developmental windows of susceptibility to inorganic arsenic: A survey of current toxicologic and epidemiologic data. Toxicol. Res. 2016, 5, 1503–1511. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Su, P.-H.; Sun, C.-W.; Liu, H.-J.; Chang, C.-L.; Wang, S.-L. Early-life arsenic exposure promotes atherogenic lipid metabolism in adolescence: A 15-year birth cohort follow-up study in central Taiwan. Environ. Int. 2018, 118, 97–105. [Google Scholar] [CrossRef]
- Fujihara, J.; Nishimoto, N. Thallium-poisoner’s poison: An overview and review of current knowledge on the toxicological effects and mechanisms. Curr. Res. Toxicol. 2024, 6, 100157. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Wang, Y.; Li, Z.; Fu, G.; Mao, L.; Song, Y.; Qu, Y.; Ye, L.; Zhou, Q.; Yang, F.; et al. Thallium exposure at low concentration leads to early damage on multiple organs in children: A case study followed-up for four years. Environ. Pollut. 2020, 258, 113319. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, Y.; Wang, K.; Ding, Y.; Li, Z.; Lu, M.; Xu, D. Association of urinary thallium with hypertension in children and adolescents aged 8–17 years: NHANES 2005–2018. Environ. Sci. Pollut. Res. 2023, 30, 102927–102935. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Dapul, H.; Laraque, D. Lead Poisoning in Children. Adv. Pediatr. 2014, 61, 313–333. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Backstrand, J.R. Lead Toxicity and Pollution in Poland. Int. J. Environ. Res. Public Health 2020, 17, 4385. [Google Scholar] [CrossRef]
- Food & Drugs Administration Lead in Food and Foodwares. Available online: https://www.fda.gov/food/environmental-contaminants-food/lead-food-and-foodwares (accessed on 19 June 2025).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for vitamin C. EFSA J. 2013, 11. [Google Scholar] [CrossRef]
- Hore, P.; Alex-Oni, K.; Sedlar, S.; Bardhi, N.; Ehrlich, J. Elevated Blood Lead Levels in a Pregnant Woman and her Family from Traditional Kansa (Bronze) and Pital (Brass) Metalware—New York City, 2024. MMWR-Morbidity Mortal. Wkly. Rep. 2025, 74, 298–301. [Google Scholar] [CrossRef]
- Bellinger, D.C. Prenatal Exposures to Environmental Chemicals and Children’s Neurodevelopment: An Update. Saf. Health Work. 2013, 4, 1–11. [Google Scholar] [CrossRef]
- Podgórska, A.; Puścion-Jakubik, A.; Grodzka, A.; Naliwajko, S.K.; Markiewicz-Żukowska, R.; Socha, K. Natural and Conventional Cosmetics—Mercury Exposure Assessment. Molecules 2021, 26, 4088. [Google Scholar] [CrossRef]
- Li, Z.; Chen, B.; Li, Y.; Le, X.C. Reduction of mercury emissions from anthropogenic sources including coal combustion. J. Environ. Sci. 2021, 100, 363–368. [Google Scholar] [CrossRef]
- Kazantzis, G. Mercury exposure and early effects: An overview. Med. Lav. 2002, 93, 139–147. [Google Scholar]
- Berlin, M. Mercury in dental amalgam: A risk analysis. NeuroToxicology 2020, 81, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-D.; Zheng, W. Human Exposure and Health Effects of Inorganic and Elemental Mercury. J. Prev. Med. Public Health 2012, 45, 344–352. [Google Scholar] [CrossRef]
- World Health Organization. Mercury. Available online: https://www.who.int/news-room/fact-sheets/detail/mercury-and-health (accessed on 19 June 2025).
- World Health Organization. Nations Unite to Eliminate Mercury-Containing Medical Devices. Available online: https://www.who.int/news/item/15-05-2024-nations-unite-to-eliminate-mercury-containing-medical-devices (accessed on 19 June 2025).
- Hu, X.F.; Lowe, M.; Chan, H.M. Mercury exposure, cardiovascular disease, and mortality: A systematic review and dose-response meta-analysis. Environ. Res. 2021, 193, 110538. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Omeljaniuk, W.J.; Garley, M.; Nikliński, J. Mercury Exposure and Health Effects: What Do We Really Know? Int. J. Mol. Sci. 2025, 26, 2326. [Google Scholar] [CrossRef] [PubMed]
- Ciosek, Ż.; Kot, K.; Rotter, I. Iron, Zinc, Copper, Cadmium, Mercury, and Bone Tissue. Int. J. Environ. Res. Public Health 2023, 20, 2197. [Google Scholar] [CrossRef] [PubMed]
- Willers, S.; Gerhardsson, L.; Lundh, T. Environmental tobacco smoke (ETS) exposure in children with asthma—relation between lead and cadmium, and cotinine concentrations in urine. Respir. Med. 2005, 99, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.-R.; Chen, W.-Y.; Liao, C.-M. Assessing human exposure risk to cadmium through inhalation and seafood consumption. J. Hazard. Mater. 2012, 227-228, 353–361. [Google Scholar] [CrossRef]
- Rahman, M.S.; Miah, M.A.M.; Khaled, H.M.; Islam, A.; Panaullah, G.M. Arsenic Concentrations in Groundwater, Soils, and Irrigated Rice in Southwestern Bangladesh. Commun. Soil Sci. Plant Anal. 2010, 41, 1889–1895. [Google Scholar] [CrossRef]
- Samal, A.C.; Bhattacharya, P.; Biswas, P.; Maity, J.P.; Bundschuh, J.; Santra, S.C. Variety-specific arsenic accumulation in 44 different rice cultivars (O. sativa L.) and human health risks due to co-exposure of arsenic-contaminated rice and drinking water. J. Hazard. Mater. 2021, 407, 124804. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.D.; Sahni, S. Mitigation of Arsenic Contamination through Biotechnological Approaches in Rice. J. Exp. Agric. Int. 2023, 45, 180–185. [Google Scholar] [CrossRef]
- Nuvolone, D.; Petri, D.; Aprea, M.C.; Bertelloni, S.; Voller, F.; Aragona, I. Thallium Contamination of Drinking Water: Health Implications in a Residential Cohort Study in Tuscany (Italy). Int. J. Environ. Res. Public Health 2021, 18, 4058. [Google Scholar] [CrossRef]
- Zou, H.; Zou, S. Advanced thallium toxicity. Pr. Neurol. 2022, 23, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Salles, F.J.; Paniz, F.P.; Batista, B.L.; Nardocci, A.C.; Olympio, K.P.K. Potentially Toxic Elements in Costume Cosmetics Used by Children and Adults Are Associated with Cancer Risk. Int. J. Environ. Res. Public Health 2022, 20, 531. [Google Scholar] [CrossRef]
- Rajkumar, V.; Lee, V.R.; Gupta, V. Heavy Metal Toxicity. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560920/ (accessed on 10 June 2025).
- Al-Saleh, I.; Shinwari, N.; Mashhour, A.; Mohamed, G.E.D.; Rabah, A. Heavy metals (lead, cadmium and mercury) in maternal, cord blood and placenta of healthy women. Int. J. Hyg. Environ. Health 2011, 214, 79–101. [Google Scholar] [CrossRef]
- The Pediatric Environmental Health Specialty Units Heavy Metals in Baby Foods and Fruit Juices. Available online: https://nyscheck.org/wp-content/uploads/2022/04/NYSCEHC_HeavyMetal_Food_Updated_March2022_LZ.pdf (accessed on 10 June 2025).
- Lv, H.; Jiang, Y.; Ye, K.; Wang, J.; Wang, W.; Du, J.; Hu, L.; Guo, W.; Qin, R.; Xu, X.; et al. Prenatal Parental Exposure to Metals and Birth Defects: A Prospective Birth Cohort Study. Environ. Sci. Technol. 2024, 58, 14110–14120. [Google Scholar] [CrossRef]
- Gorini, F.; Tonacci, A. Ambient Air Pollution and Congenital Heart Disease: Updated Evidence and Future Challenges. Antioxidants 2025, 14, 48. [Google Scholar] [CrossRef]
- Zubrzycki, M.; Schramm, R.; Costard-Jäckle, A.; Grohmann, J.; Gummert, J.F.; Zubrzycka, M. Cardiac Development and Factors Influencing the Development of Congenital Heart Defects (CHDs): Part I. Int. J. Mol. Sci. 2024, 25, 7117. [Google Scholar] [CrossRef]
- Sharma, V. Congenital Heart Defects. Insights Biomed. 2019, 4, 4–5. [Google Scholar] [CrossRef]
- Dattilo, G.; Tulino, V.; Tulino, D.; Lamari, A.; Marte, F.; Patanè, S. Interatrial defect, ventricular septal defect and patent ductus arteriosus in a 2-day-old newborn infant. Int. J. Cardiol. 2009, 134, e82–e83. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, Y.; Sun, J.; Jiao, X.; Wu, Z.; Wang, J.; Qiu, J.; Mao, B.; Liu, Q. Relationship between pregnant women’s combined exposure to heavy metals and their offspring’s congenital heart defects in Lanzhou, China. Front. Pediatr. 2025, 12, 1291076. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, J.; Deng, S.; Liu, B.; Jing, X.; Yan, Y.; Liu, Y.; Wang, J.; Zhou, X.; She, Q. The molecular mechanisms of cardiac development and related diseases. Signal Transduct. Target. Ther. 2024, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Greene, S.B.; Martin, J.F. BMP signaling in congenital heart disease: New developments and future directions. Birth Defects Res. Part A Clin. Mol. Teratol. 2011, 91, 441–448. [Google Scholar] [CrossRef]
- Luxán, G.; D’Amato, G.; MacGrogan, D.; de la Pompa, J.L. Endocardial Notch Signaling in Cardiac Development and Disease. Circ. Res. 2016, 118, e1–e18. [Google Scholar] [CrossRef]
- Wang, C.; Pi, X.; Yin, S.; Liu, M.; Tian, T.; Jin, L.; Liu, J.; Li, Z.; Wang, L.; Yuan, Z.; et al. Maternal exposure to heavy metals and risk for severe congenital heart defects in offspring. Environ. Res. 2022, 212, 113432. [Google Scholar] [CrossRef]
- Pan, Z.; Gong, T.; Liang, P. Heavy Metal Exposure and Cardiovascular Disease. Circ. Res. 2024, 134, 1160–1178. [Google Scholar] [CrossRef]
- Gorini, F.; Tonacci, A. Toxic metals in pregnancy and congenital heart defects. Insights and new perspectives for a technology-driven reduction in food sources. Explor. Cardiol. 2023, 1, 114–140. [Google Scholar] [CrossRef]
- Dang, Y.; Sun, J.; Wu, Z.; Mao, B.; Hang, Q.; Huang, J.; Zhao, X.; Xia, J.; Chen, C.; Yao, W.; et al. Prenatal exposure to barium and arsenic and the odds of congenital heart defects in offspring: A nested case–control study within a birth cohort in Lanzhou, China. Front. Public Health 2025, 13, 1597178. [Google Scholar] [CrossRef]
- Ou, Y.; Bloom, M.S.; Nie, Z.; Han, F.; Mai, J.; Chen, J.; Lin, S.; Liu, X.; Zhuang, J. Associations between toxic and essential trace elements in maternal blood and fetal congenital heart defects. Environ. Int. 2017, 106, 127–134. [Google Scholar] [CrossRef]
| Metal | Clinical Outcomes | Proposed Mechanism | Study Population | Ref. |
|---|---|---|---|---|
| Mercury | ↓ HR in boys; no effect in girls | Autonomic nervous system dysfunction affecting HR, partly due to oxidative stress Combined myocardial and autonomic disruption via oxidative stress (observed in co-exposure to Hg and Pb) | 532 adolescents; mean age 12 years | [33] |
| ↑ cIMT, and PWV; no effect on HR or BP | Endothelial dysfunction, partly due to oxidative stress | 291 children, aged 9–11 years | [32] | |
| ↑ DBP, MAP; no effect on SBP | 395 children, aged 5–6 years | [11] | ||
| ↑ DBP, MAP at age 5–6, esp. 3rd trimester | 2534 children | [57] | ||
| ↑ TC (notably in girls); no effect on SBP or DBP | Possible lipid metabolism disruption indicates potential early CVD risk | 1129 adolescents, aged 12–19 years | [22] | |
| Lead | Slight ↑ HR in boys; no effect in girls | Autonomic nervous system dysfunction affecting HR, partly due to oxidative stress | 532 adolescents, mean age 12 years | [33] |
| ↑ SBP in preterm children (<37 GA weeks), with potential long-term cardiovascular risk | Combined effects of impaired nephrogenesis, oxidative stress, and endothelial dysfunction may contribute to long-term cardiovascular risk | 565 children, aged 4–6 years | [47] | |
| ↓ LVPW, LVM, EF, SF | Oxidative stress-induced myocardial remodeling and inflammation | 486 children, ages 2–6 years | [48] | |
| ↑ LDL; no effect on SBP or DBP | Possible lipid metabolism disruption indicates potential early CVD risk | 11,662 adolescents | [40] | |
| None confirmed in either study | No cardiovascular effects observed | 291 children, aged 9–11 years | [32] | |
| 2534 children | [57] | |||
| Cadmium | ↓ SBP, DBP, HR; ↑ LVM; ↓ PWV | Vascular and autonomic dysfunction | 291 children, aged 9–11 years | [32] |
| ↓ TC, HDL, LDL; ↑ SBP, DBP | Altered lipid metabolism with unclear impact on cardiovascular risk | 540 children, ages 4.5 and 9 years | [56] | |
| No cardiovascular effects observed | None confirmed in either study | 2534 children | [57] | |
| Arsenic | ↓ TC, HDL | Altered lipid metabolism with unclear impact on cardiovascular risk | 540 children, ages 4.5 and 9 years | [56] |
| ↑ DBP, SBP, MAP at age 5–6, esp. 3rd trimester | Endothelial dysfunction, partly due to oxidative stress | 2534 children | [57] | |
| ↑ SBP, DBP, LVM, LAD (marginal); ↓ EF, SF | Endothelial dysfunction mediated by oxidative stress and vascular remodeling | 161 children, ages 3–8 years | [10] | |
| ↑ cIMT; no effect on lipids | Endothelial dysfunction mediated by ADMA | 199 children, ages 3–14 years | [9] | |
| ↑ cIMT, concentric cardiac hypertrophy | Early cardiovascular alterations due to endothelial dysfunction | 245 children, ages 9–11 years | [60] | |
| ↑ LDL, TC, non-HDL, TC/HDL ratio | Widespread dyslipidemia is associated with early cardiovascular risk | 237 children, aged approximately 2, 5, 8, 11, and 14 years | [63] | |
| Thallium | ↓ LDL, TC; ↑ phosphocreatine kinase, creatine kinase isoenzyme; ↓ ischemia-modified albumin | Altered lipid metabolism with unclear impact on cardiovascular risk Subclinical myocardial injury contributes to increased cardiovascular risk | 6 children | [65] |
| ↓ prevalence of hypertension with higher urinary Tl | No clear mechanism | 2295 children, ages 8–17 years | [66] |
| Category | Types of CHDs | Heavy Metal | Potential Effects on the Developing Heart | Ref. |
|---|---|---|---|---|
| Septal defects | Atrial septal defect | Mercury | Oxidative stress, epigenetic disruption | [104] |
| Arsenic | [107] | |||
| Ventricular septal defect | Mercury | [104] | ||
| Atrioventricular septal defect | Mercury | [104] | ||
| Arsenic | [107] | |||
| Conotruncal defects | Tetralogy of Fallot | Mercury | [104] | |
| d-transposition of the great arteries | Mercury | [104] | ||
| Truncus arteriosus | Mercury | [104] | ||
| Isolated defects | Patent ductus arteriosus | Arsenic | [107] | |
| CHDs (general) | - | Lead | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróblewski, M.; Miłek, J.; Godlewski, A.; Wróblewska, J. The Impact of Arsenic, Cadmium, Lead, Mercury, and Thallium Exposure on the Cardiovascular System and Oxidative Mechanisms in Children. Curr. Issues Mol. Biol. 2025, 47, 483. https://doi.org/10.3390/cimb47070483
Wróblewski M, Miłek J, Godlewski A, Wróblewska J. The Impact of Arsenic, Cadmium, Lead, Mercury, and Thallium Exposure on the Cardiovascular System and Oxidative Mechanisms in Children. Current Issues in Molecular Biology. 2025; 47(7):483. https://doi.org/10.3390/cimb47070483
Chicago/Turabian StyleWróblewski, Marcin, Justyna Miłek, Antoni Godlewski, and Joanna Wróblewska. 2025. "The Impact of Arsenic, Cadmium, Lead, Mercury, and Thallium Exposure on the Cardiovascular System and Oxidative Mechanisms in Children" Current Issues in Molecular Biology 47, no. 7: 483. https://doi.org/10.3390/cimb47070483
APA StyleWróblewski, M., Miłek, J., Godlewski, A., & Wróblewska, J. (2025). The Impact of Arsenic, Cadmium, Lead, Mercury, and Thallium Exposure on the Cardiovascular System and Oxidative Mechanisms in Children. Current Issues in Molecular Biology, 47(7), 483. https://doi.org/10.3390/cimb47070483





