Restoration of Hair Luster via Novel Biomarker COL7A1 by Minoxidil, Caffeine, and Biotin
Abstract
1. Introduction
2. Materials and Methods
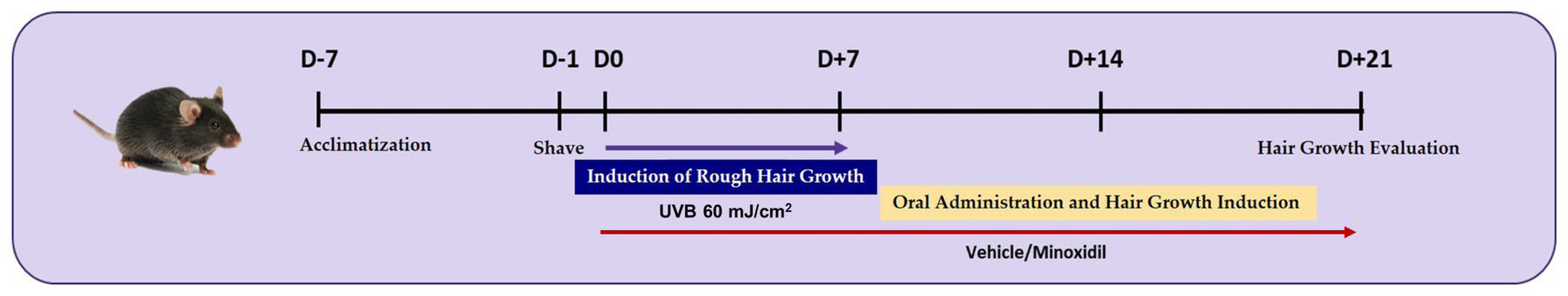
2.1. In Vivo UVB-Induced Hair Luster Loss Mouse Model
2.2. In Vitro and Ex Vivo Experiments
2.2.1. In Vitro Human Hair Follicle Dermal Papilla Cell Culture
2.2.2. Ex Vivo Human Hair Follicles Culture
2.2.3. UVB Irradiation and Treatment
2.3. RNA Extraction and qRT-PCR
2.4. Ethics
2.5. Statistical Analysis
3. Results
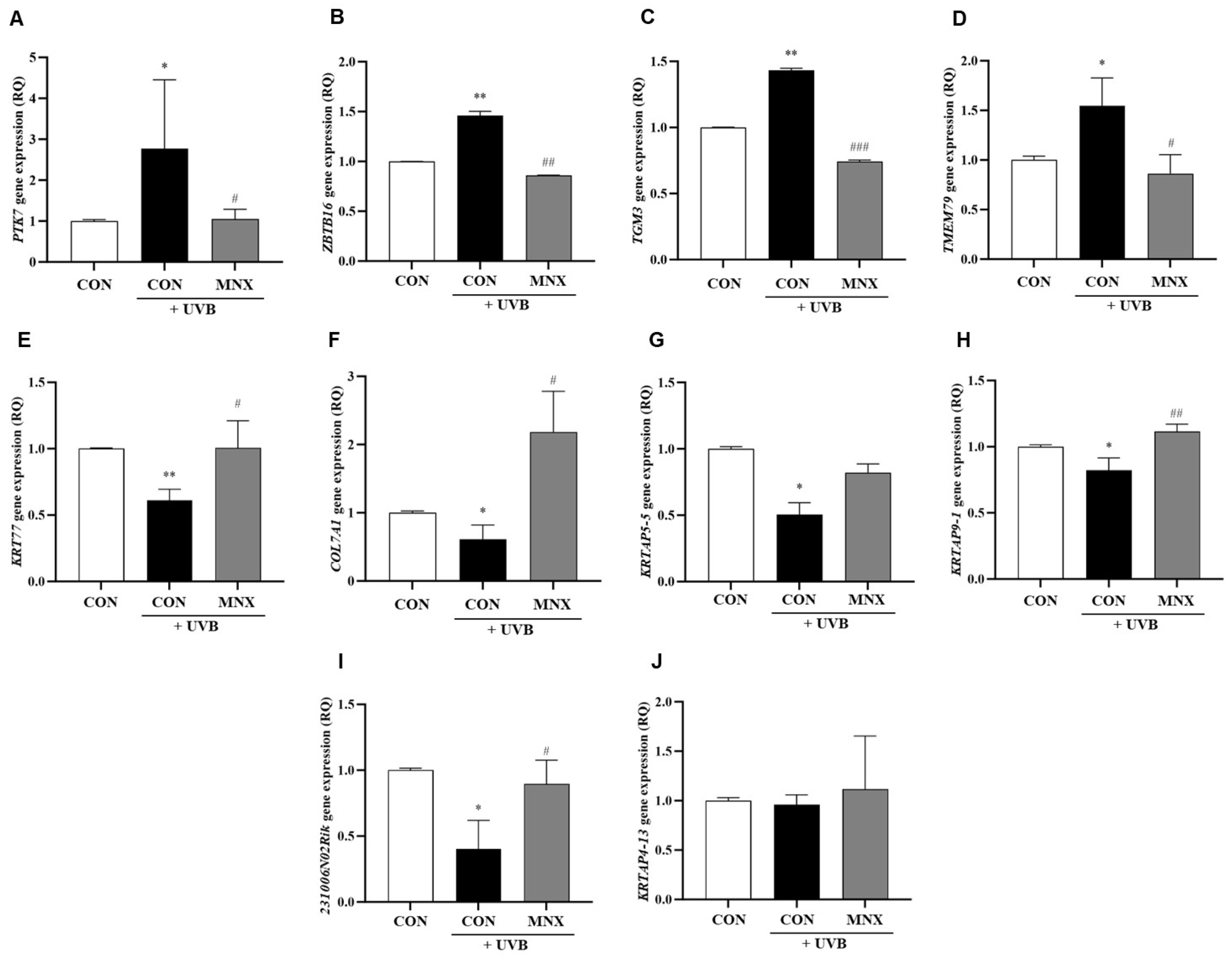
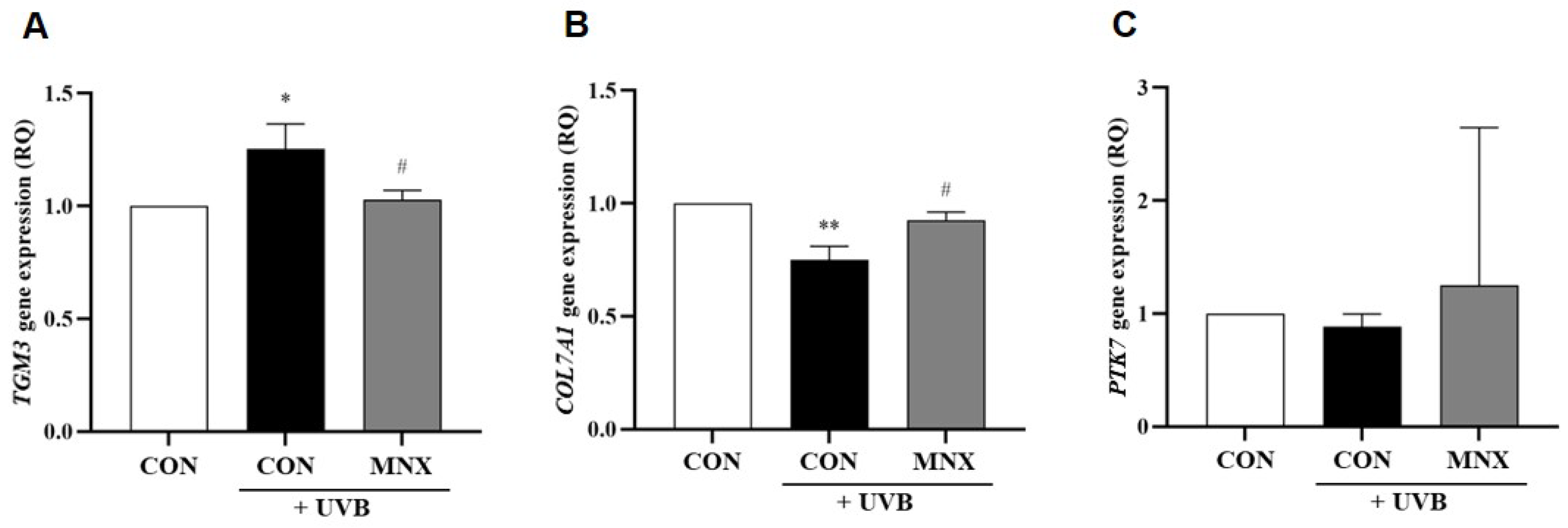
3.1. Gene Expression Analysis in Skin Tissue of the UVB-Induced Hair Luster Mouse Model
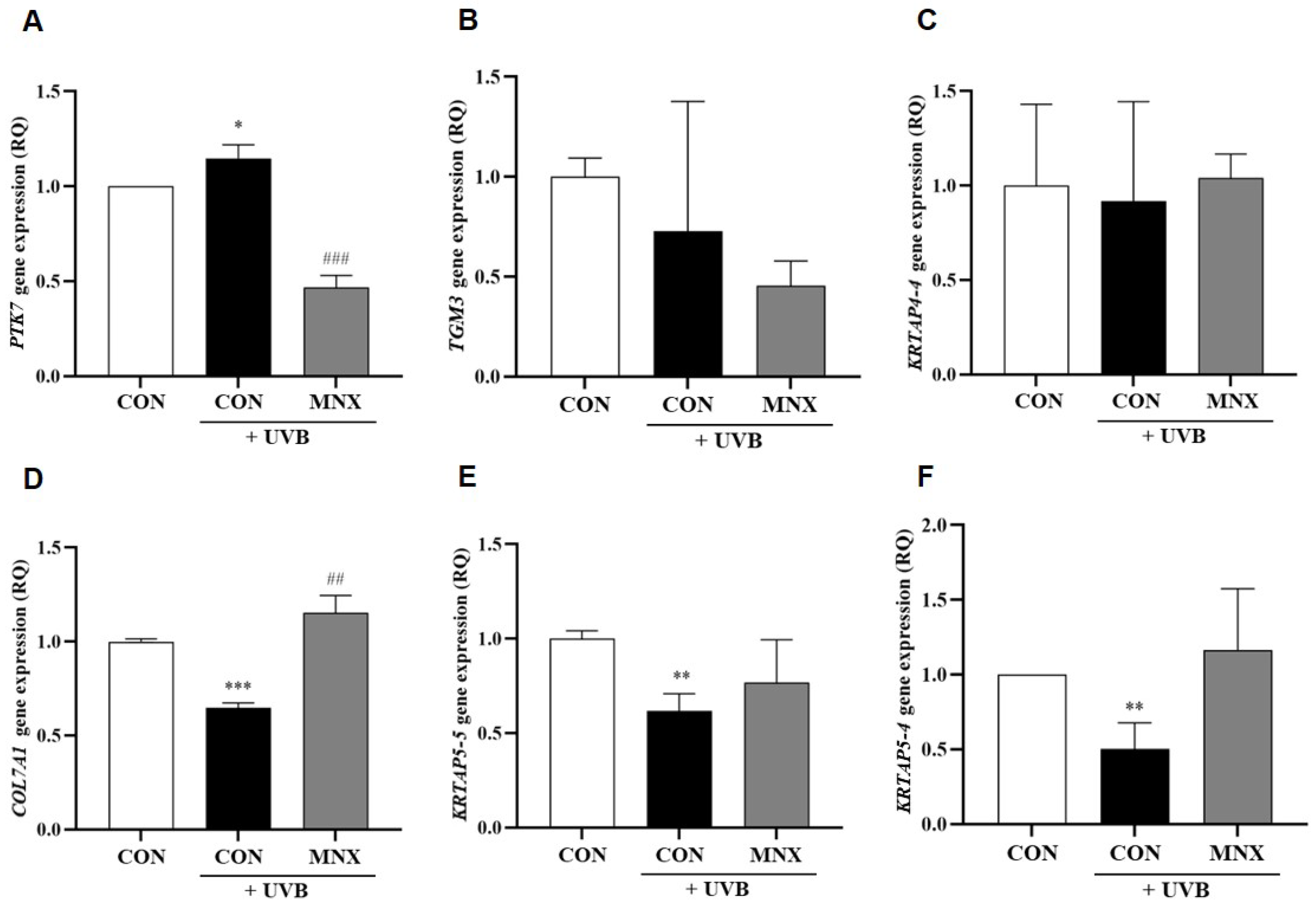
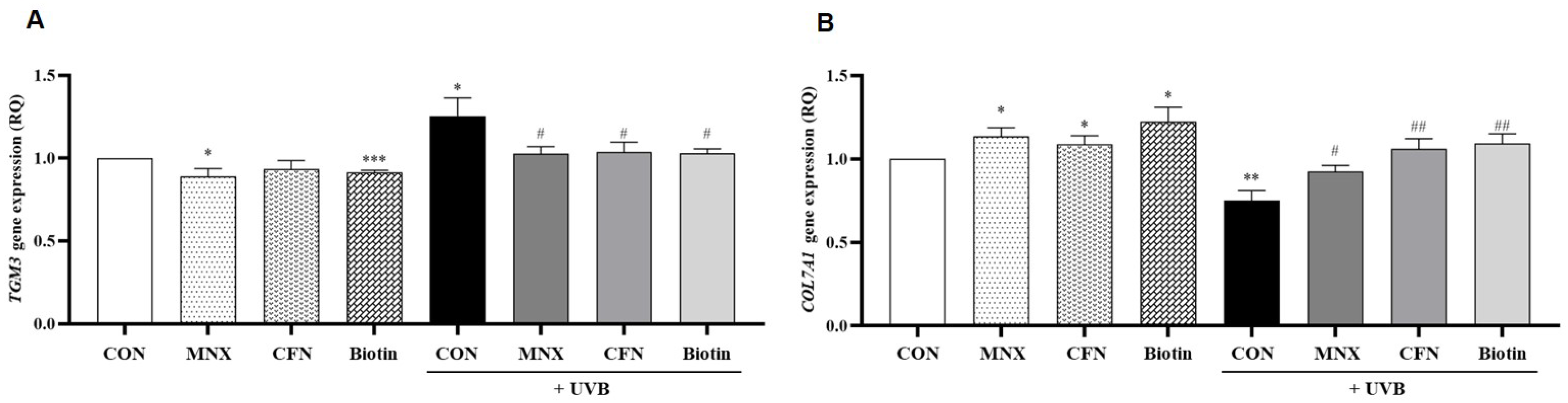
3.2. Gene Expression Analysis in Human Hair Follicle Dermal Papilla Cells
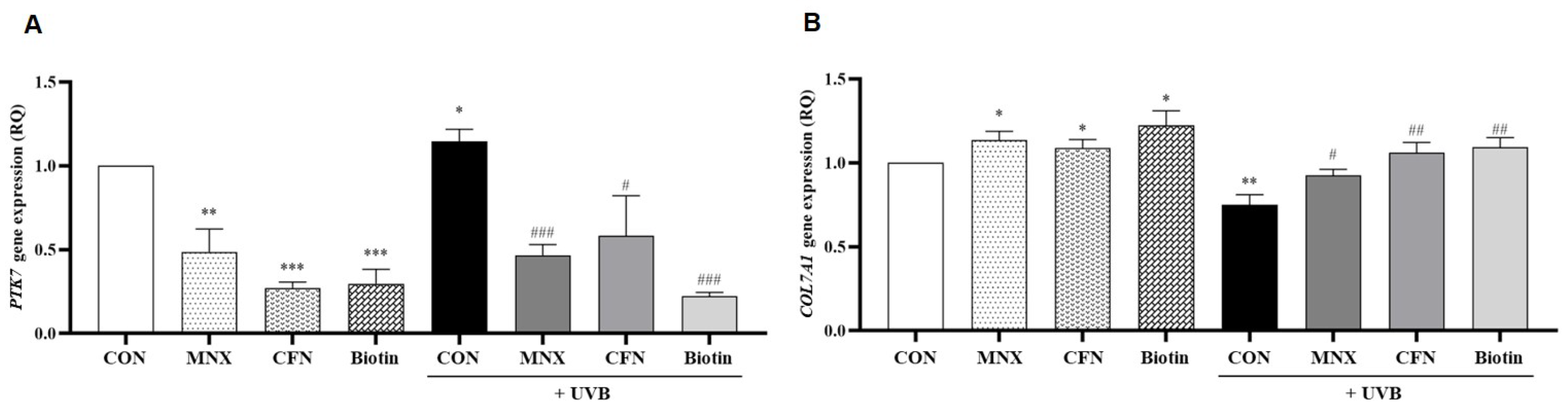
3.3. Gene Expression Analysis in Human Hair Follicle Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMZ | Basement Membrane Zone |
| COL7A1 | Collagen Type VII Alpha 1 |
| cDNA | Complementary DNA |
| DNA | Deoxyribonucleic Acid |
| HFDPCs | Human Hair Follicle Dermal Papilla Cells |
| IACUC | Institutional Animal Care and Use Committee |
| IRB | Institutional Review Board |
| KRTAP | Keratin-Associated Protein |
| NGS | Next-Generation Sequencing |
| PTK7 | Protein Tyrosine Kinase 7 |
| qRT-PCR | Quantitative Real-Time Reverse Transcriptase Polymerase Chain Reaction |
| RNA | Ribonucleic Acid |
| TGM3 | Transglutaminase 3 |
| TMEM79 | Transmembrane Protein 79 |
| UV | Ultraviolet |
| UVB | Ultraviolet B |
References
- Lee, Y.I.; Kim, J.; Park, S.R.; Ham, S.; Lee, H.J.; Park, C.R.; Kim, H.N.; Kang, B.H.; Jung, I.; Suk, J.M.; et al. Age-related changes in scalp biophysical parameters: A comparative analysis of the 20s and 50s age groups. Skin Res. Technol. 2023, 29, e13433. [Google Scholar] [CrossRef] [PubMed]
- Thor, D.; Pagani, A.; Bukowiecki, J.; Houschyar, K.S.; Kolle, S.T.; Wyles, S.P.; Duscher, D. A Novel Hair Restoration Technology Counteracts Androgenic Hair Loss and Promotes Hair Growth in A Blinded Clinical Trial. J. Clin. Med. 2023, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Ko, E.J.; Seok, J.; Han, H.S.; Yoo, K.H.; Song, M.; Song, K.; Kim, B.J.J.F.I.N. Efficacy and safety of Latilactobacillus curvatus LB-P9 on hair health: A randomized, double-blind, placebo-controlled clinical trial. Front. Nutr. 2024, 11, 1447863. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Choi, A.R.; Baek, J.H.; Kim, H.O.; Shin, M.K.; Koh, J.S. Twelve-Point scale grading system of scanning electron microscopic examination to investigate subtle changes in damaged hair surface. Skin Res. Technol. 2016, 22, 406–411. [Google Scholar] [CrossRef]
- Fellows, A.P.; Casford, M.T.L.; Davies, P.B. Nanoscale Molecular Characterization of Hair Cuticle Cells Using Integrated Atomic Force Microscopy-Infrared Laser Spectroscopy. Appl. Spectrosc. 2020, 74, 1540–1550. [Google Scholar] [CrossRef]
- Chung, K.B.; Lee, Y.I.; Kim, Y.J.; Do, H.A.; Suk, J.; Jung, I.; Kim, D.Y.; Lee, J.H. Quantitative Analysis of Hair Luster in a Novel Ultraviolet-Irradiated Mouse Model. Mol. Sci. 2024, 25, 1885. [Google Scholar] [CrossRef]
- Soma, T.; Iino, M.; Tajima, M.; Kishimoto, J. Expression of novel keratin associated protein 5 genes in the cuticle layer of human hair follicles. Dermatol. Sci. 2005, 38, 110–112. [Google Scholar] [CrossRef]
- Wang, Q.; Phang, J.M.; Chakraborty, S.; Zhang, L.; Klahn, M.; Nalaparaju, A.; Lim, F.C.H. Atomistic Characterization of Healthy and Damaged Hair Surfaces: A Molecular Dynamics Simulation Study of Fatty Acids on Protein Layer. Chembiochem 2024, 25, e202400128. [Google Scholar] [CrossRef]
- Fernandes, C.; Medronho, B.; Alves, L.; Rasteiro, M.G. On Hair Care Physicochemistry: From Structure and Degradation to Novel Biobased Conditioning Agents. Polymers 2023, 15, 608. [Google Scholar] [CrossRef]
- Dario, M.F.; Baby, A.R.; Velasco, M.V. Effects of solar radiation on hair and photoprotection. Photochem. Photobiol. B 2015, 153, 240–246. [Google Scholar] [CrossRef]
- Jeon, S.-Y.; Pi, L.Q.; Lee, W.S. Comparison of hair shaft damage after UVA and UVB irradiation. Cosmetic. Sci. 2008, 59, 151–156. [Google Scholar]
- Lee, W.S. Photoaggravation of hair aging. Trichology 2009, 1, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Won, H.J.; Kim, T.M.; An, I.S.; Bae, H.J.; Park, S.Y. Protection and Restoration of Damaged Hair via a Polyphenol Complex by Promoting Mechanical Strength, Antistatic, and Ultraviolet Protection Properties. Biomim. 2023, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Yamazaki, J.; Okita, N.; Shimotori, M.; Igarashi, K.; Sano, T. Mechanism of cuticle hole development in human hair due to UV-Radiation exposure. Cosmet 2018, 5, 24. [Google Scholar] [CrossRef]
- Trüeb, R.; Hoffmann, R. Aging of hair. In Textbook of Men’s Health and Aging; CRC Press: Boca Raton, FL, USA, 2007; pp. 715–728. [Google Scholar]
- Wertheim-Tysarowska, K.; Sobczynska-Tomaszewska, A.; Kowalewski, C.; Skronski, M.; Swieckowski, G.; Kutkowska-Kazmierczak, A.; Wozniak, K.; Bal, J. The COL7A1 mutation database. Hum. Mutat. 2012, 33, 327–331. [Google Scholar] [CrossRef]
- Nystrom, A.; Velati, D.; Mittapalli, V.R.; Fritsch, A.; Kern, J.S.; Bruckner-Tuderman, L. Collagen VII plays a dual role in wound healing. Clin. Investig. 2013, 123, 3498–3509. [Google Scholar] [CrossRef]
- Joubeh, S.; Mori, O.; Owaribe, K.; Hashimoto, T. Immunofluorescence analysis of the basement membrane zone components in human anagen hair follicles. Exp. Dermatol. 2003, 12, 365–370. [Google Scholar] [CrossRef]
- Loing, E.; Lachance, R.; Ollier, V.; Hocquaux, M. A new strategy to modulate alopecia using a combination of two specific and unique ingredients. Cosmet. Sci. 2013, 64, 45–58. [Google Scholar]
- Brown, T.M.; Krishnamurthy, K. Histology, Hair and Follicle; StatPearls: Tampa, FL, USA, 2025. [Google Scholar]
- Shinkuma, S. Dystrophic epidermolysis bullosa: A review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 275–284. [Google Scholar] [CrossRef]
- Xiao, A.; Sathe, N.C.; Tsuchiya, A. Epidermolysis Bullosa Acquisita; StatPearls: Tampa, FL, USA, 2025. [Google Scholar]
- Xie, D.; Bilgic-Temel, A.; Abu Alrub, N.; Murrell, D.F. Pathogenesis and clinical features of alopecia in epidermolysis bullosa: A systematic review. Pediatr. Dermatol. 2019, 36, 430–436. [Google Scholar] [CrossRef]
- Trehan, A.; Anand, R.; Chaudhary, G.; Garg, H.; Verma, M.K. Efficacy and Safety of Skin Radiance Collagen on Skin and Hair Matrix: A Placebo-Controlled Clinical Trial in Healthy Human Subjects. Clin. Cosmet. Investig. Dermatol. 2024, 17, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Q.; Wang, L.; Xu, W.; He, Y.; Li, Y.; He, S.; Ma, H. Improvement of skin condition by oral administration of collagen hydrolysates in chronologically aged mice. Sci. Food. Agric. 2017, 97, 2721–2726. [Google Scholar] [CrossRef] [PubMed]
- Yagoda, M.; Gans, E. A nutritional supplement formulated with peptides, lipids, collagen and hyaluronic acid optimizes key aspects of physical appearance in nails, hair and skin. J. Nutr. Food. Sci. 2014, 5, 1–6. [Google Scholar] [CrossRef]
- Shimomura, Y.; Ito, M. Human hair keratin-associated proteins. J. Investig. Dermatol. Symp. Proc. 2005, 10, 230–233. [Google Scholar] [CrossRef]
- Fujikawa, H.; Fujimoto, A.; Farooq, M.; Ito, M.; Shimomura, Y. Characterization of the human hair keratin-associated protein 2 (KRTAP2) gene family. Investig. Dermatol. 2012, 132, 1806–1813. [Google Scholar] [CrossRef]
- Basmanav, F.B.; Cesarato, N.; Kumar, S.; Borisov, O.; Kokordelis, P.; Ralser, D.J.; Wehner, M.; Axt, D.; Xiong, X.; Thiele, H.; et al. Assessment of the Genetic Spectrum of Uncombable Hair Syndrome in a Cohort of 107 Individuals. JAMA Dermatol. 2022, 158, 1245–1253. [Google Scholar] [CrossRef]
- Lee, J.; Andreeva, A.; Sipe, C.W.; Liu, L.; Cheng, A.; Lu, X. PTK7 regulates myosin II activity to orient planar polarity in the mammalian auditory epithelium. Curr. Biol. 2012, 22, 956–966. [Google Scholar] [CrossRef][Green Version]
- Goren, A.; Naccarato, T.; Situm, M.; Kovacevic, M.; Lotti, T.; McCoy, J. Mechanism of action of minoxidil in the treatment of androgenetic alopecia is likely mediated by mitochondrial adenosine triphosphate synthase-induced stem cell differentiation. Biol. Regul. Homeost. Agents 2017, 31, 1049–1053. [Google Scholar]
- Patel, D.P.; Swink, S.M.; Castelo-Soccio, L. A Review of the Use of Biotin for Hair Loss. Ski. Appendage Disord. 2017, 3, 166–169. [Google Scholar] [CrossRef]
- Szendzielorz, E.; Spiewak, R. Caffeine as an Active Molecule in Cosmetic Products for Hair Loss: Its Mechanisms of Action in the Context of Hair Physiology and Pathology. Molecules 2025, 30, 167. [Google Scholar] [CrossRef]
- Ji, J.; Qian, Q.; Cheng, W.; Ye, X.; Jing, A.; Ma, S.; Ding, Y.; Ma, X.; Wang, Y.; Sun, Q.; et al. FOXP4-mediated induction of PTK7 activates the Wnt/beta-catenin pathway and promotes ovarian cancer development. Cell Death Dis. 2024, 15, 332. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Guo, D.; Yan, T.; Sun, S.; Wang, Y.; Zheng, H.; Wang, G.; Du, J. ZBTB16 inhibits DNA replication and induces cell cycle arrest by targeting WDHD1 transcription in lung adenocarcinoma. Oncogene 2024, 43, 1796–1810. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.D.; Irwin, D.M.; Zhang, Y.P. Molecular evolution of the keratin associated protein gene family in mammals, role in the evolution of mammalian hair. BMC Evol. Biol. 2008, 8, 241. [Google Scholar] [CrossRef] [PubMed]
- Chermnykh, E.S.; Alpeeva, E.V.; Vorotelyak, E.A. Transglutaminase 3: The Involvement in Epithelial Differentiation and Cancer. Cells 2020, 9, 1996. [Google Scholar] [CrossRef]
- Sasaki, T.; Shiohama, A.; Kubo, A.; Kawasaki, H.; Ishida-Yamamoto, A.; Yamada, T.; Hachiya, T.; Shimizu, A.; Okano, H.; Kudoh, J.; et al. A homozygous nonsense mutation in the gene for Tmem79, a component for the lamellar granule secretory system, produces spontaneous eczema in an experimental model of atopic dermatitis. J. Allergy Clin. Immunol. 2013, 132, 1111–1120.e4. [Google Scholar] [CrossRef]
- Hinbest, A.J.; Eldirany, S.A.; Ho, M.; Bunick, C.G. Molecular Modeling of Pathogenic Mutations in the Keratin 1B Domain. Int. J. Mol. Sci. 2020, 21, 6641. [Google Scholar] [CrossRef]
- Rouanet, S.; Warrick, E.; Gache, Y.; Scarzello, S.; Avril, M.F.; Bernerd, F.; Magnaldo, T. Genetic correction of stem cells in the treatment of inherited diseases and focus on xeroderma pigmentosum. Int. J. Mol. Sci. 2013, 14, 20019–20036. [Google Scholar] [CrossRef]
- Hainzl, S.; Peking, P.; Kocher, T.; Murauer, E.M.; Larcher, F.; Del Rio, M.; Duarte, B.; Steiner, M.; Klausegger, A.; Bauer, J.W.; et al. COL7A1 Editing via CRISPR/Cas9 in Recessive Dystrophic Epidermolysis Bullosa. Mol. Ther. 2017, 25, 2573–2584. [Google Scholar] [CrossRef]
- Suzuki, H.; Fukunishi, Y.; Kagawa, I.; Saito, R.; Oda, H.; Endo, T.; Kondo, S.; Bono, H.; Okazaki, Y.; Hayashizaki, Y. Protein-protein interaction panel using mouse full-length cDNAs. Genome Res. 2001, 11, 1758–1765. [Google Scholar] [CrossRef]
| Species | Primer Name | Cat. No |
|---|---|---|
| Human | PTK7 | Hs00897151_m1 |
| TGM3 | Hs00897151_m1 | |
| KRTAP4-4 | Hs00540375_s1 | |
| COL7A1 | Hs00164310_m1 | |
| KRTAP5-5 | Hs03037438_s1 | |
| KRTAP5-4 | Hs04195613_s1 | |
| GAPDH | Hs02786624_g1 | |
| Mouse | PTK7 | Mm00613362_m1 |
| ZTBT16 | Mm01176868_m1 | |
| TGM3 | Mm01268669_m1 | |
| TMEM79 | Mm00470361_m1 | |
| KRTAP4-13 | Mm00471879_s1 | |
| KRT77 | Mm02343482_m1 | |
| COL7A1 | Mm01227938_m1 | |
| KRTAP5-5 | Mm03015615_s1 | |
| KRTAP9-1 | Mm00497172_s1 | |
| 231006N02Rik | Mm03991538_s1 | |
| GAPDH | Mm99999915_g1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.H.; Lee, Y.I.; Do, H.-A.; Jung, I.; Park, J.H.; Lee, S.J.; Lee, J.H. Restoration of Hair Luster via Novel Biomarker COL7A1 by Minoxidil, Caffeine, and Biotin. Curr. Issues Mol. Biol. 2025, 47, 468. https://doi.org/10.3390/cimb47060468
Nguyen NH, Lee YI, Do H-A, Jung I, Park JH, Lee SJ, Lee JH. Restoration of Hair Luster via Novel Biomarker COL7A1 by Minoxidil, Caffeine, and Biotin. Current Issues in Molecular Biology. 2025; 47(6):468. https://doi.org/10.3390/cimb47060468
Chicago/Turabian StyleNguyen, Ngoc Ha, Young In Lee, Hyeon-Ah Do, Inhee Jung, Jae Hyun Park, Sung Jun Lee, and Ju Hee Lee. 2025. "Restoration of Hair Luster via Novel Biomarker COL7A1 by Minoxidil, Caffeine, and Biotin" Current Issues in Molecular Biology 47, no. 6: 468. https://doi.org/10.3390/cimb47060468
APA StyleNguyen, N. H., Lee, Y. I., Do, H.-A., Jung, I., Park, J. H., Lee, S. J., & Lee, J. H. (2025). Restoration of Hair Luster via Novel Biomarker COL7A1 by Minoxidil, Caffeine, and Biotin. Current Issues in Molecular Biology, 47(6), 468. https://doi.org/10.3390/cimb47060468






