Design of New Primer Sets for the Development of a Loop-Mediated Isothermal Amplification for Rapid Detection of Neisseria meningitidis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Primer Design
2.3. PCR Amplification of Target Gene
2.4. LAMP Reaction and Optimization
2.5. Visual Detection of LAMP Product Using pH-Sensitive Dye
2.6. Sensitivity and Specificity of the LAMP Reaction
2.7. Statistical Analysis
3. Results
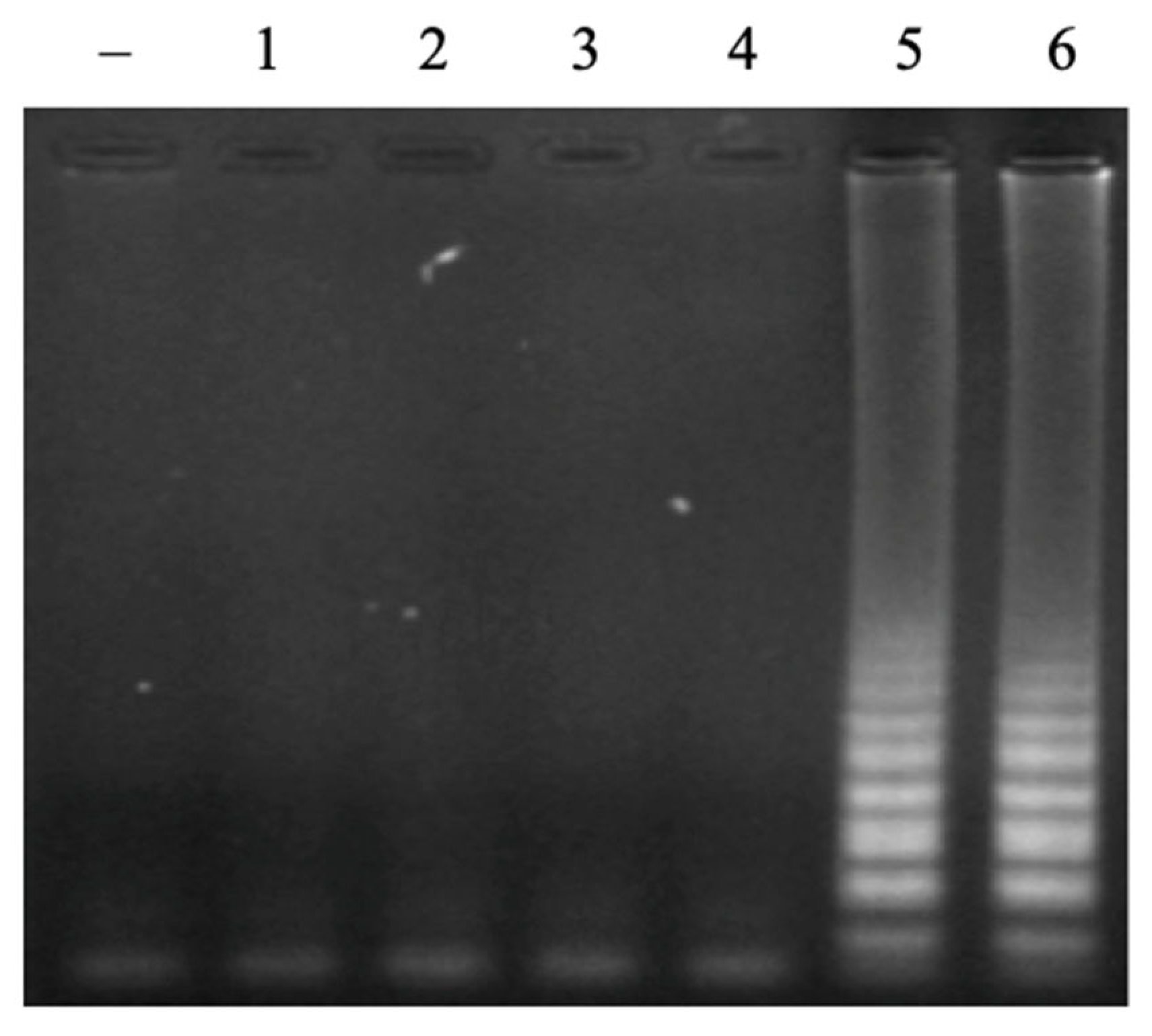
3.1. Designing of New LAMP Primers for Detecting N. meningitidis
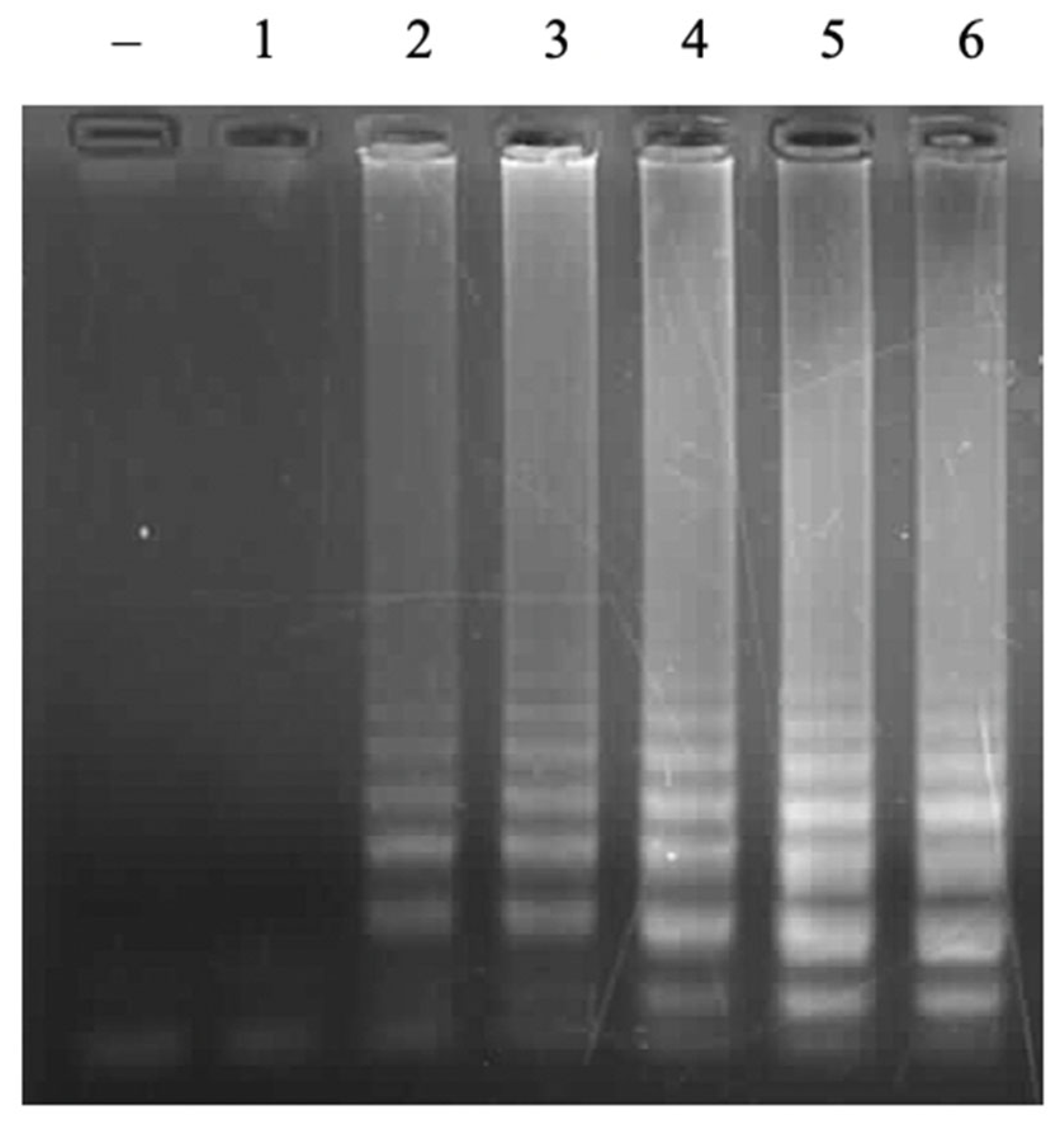
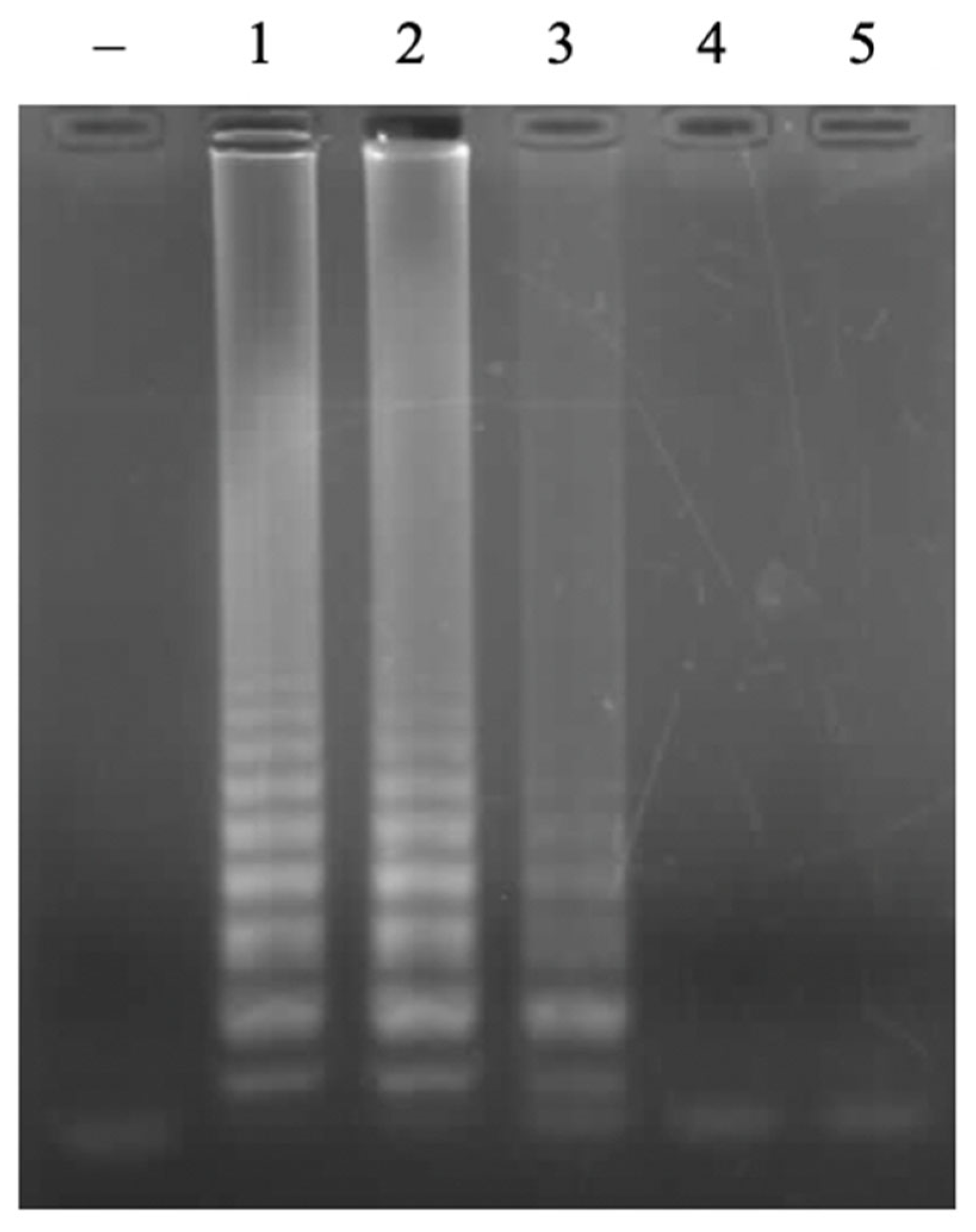
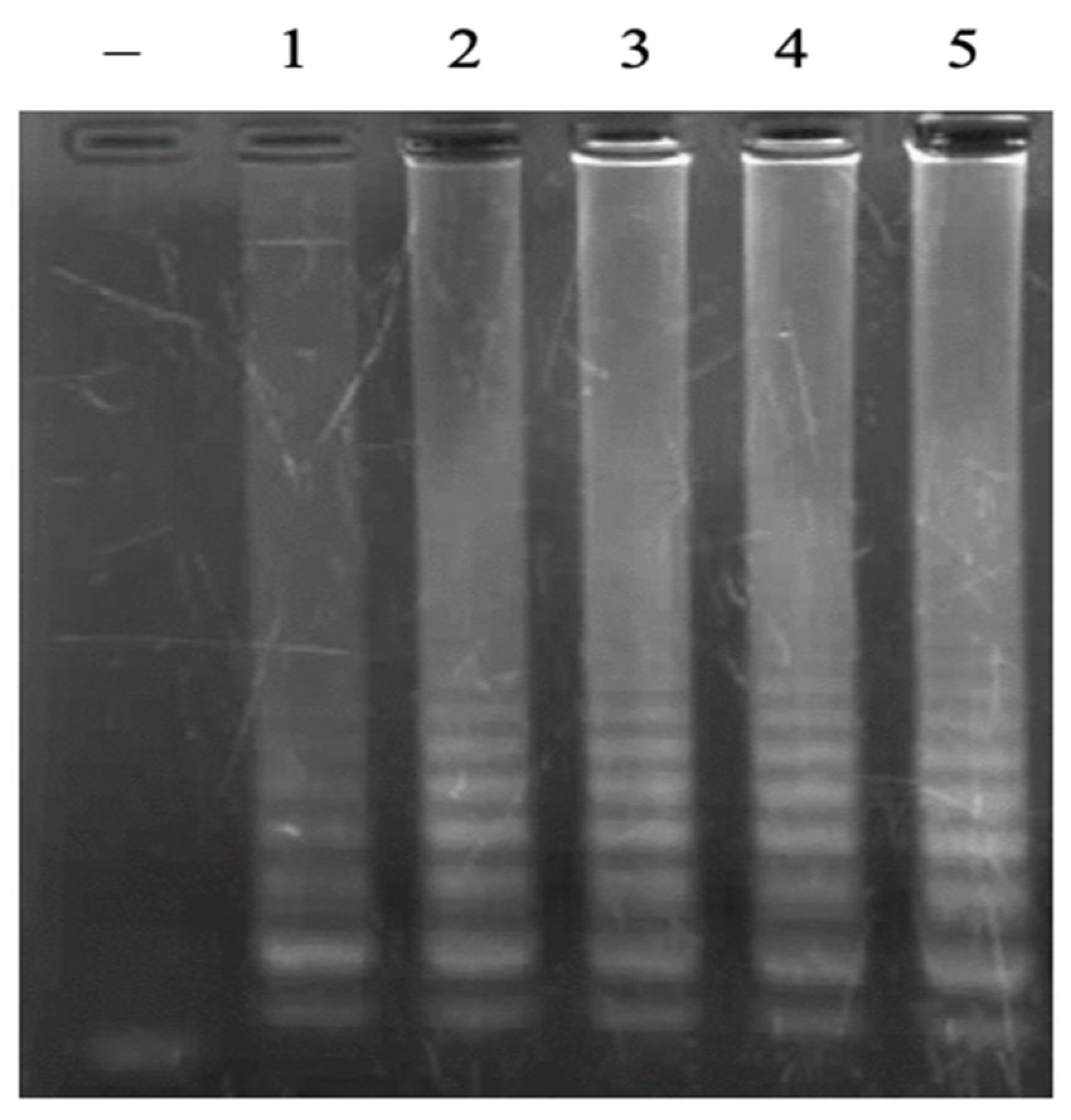
3.2. Optmization of LAMP Reactions
3.2.1. Concentration of FIP/BIP Primers
3.2.2. Concentration of FL/BL Primers
3.2.3. Concentration of dNTPs
3.2.4. Concentration of MgSO4
3.2.5. Concentration of Betaine
3.2.6. Amount of Bst DNA Polymerase
3.2.7. Reaction Temperature
3.2.8. Reaction Time
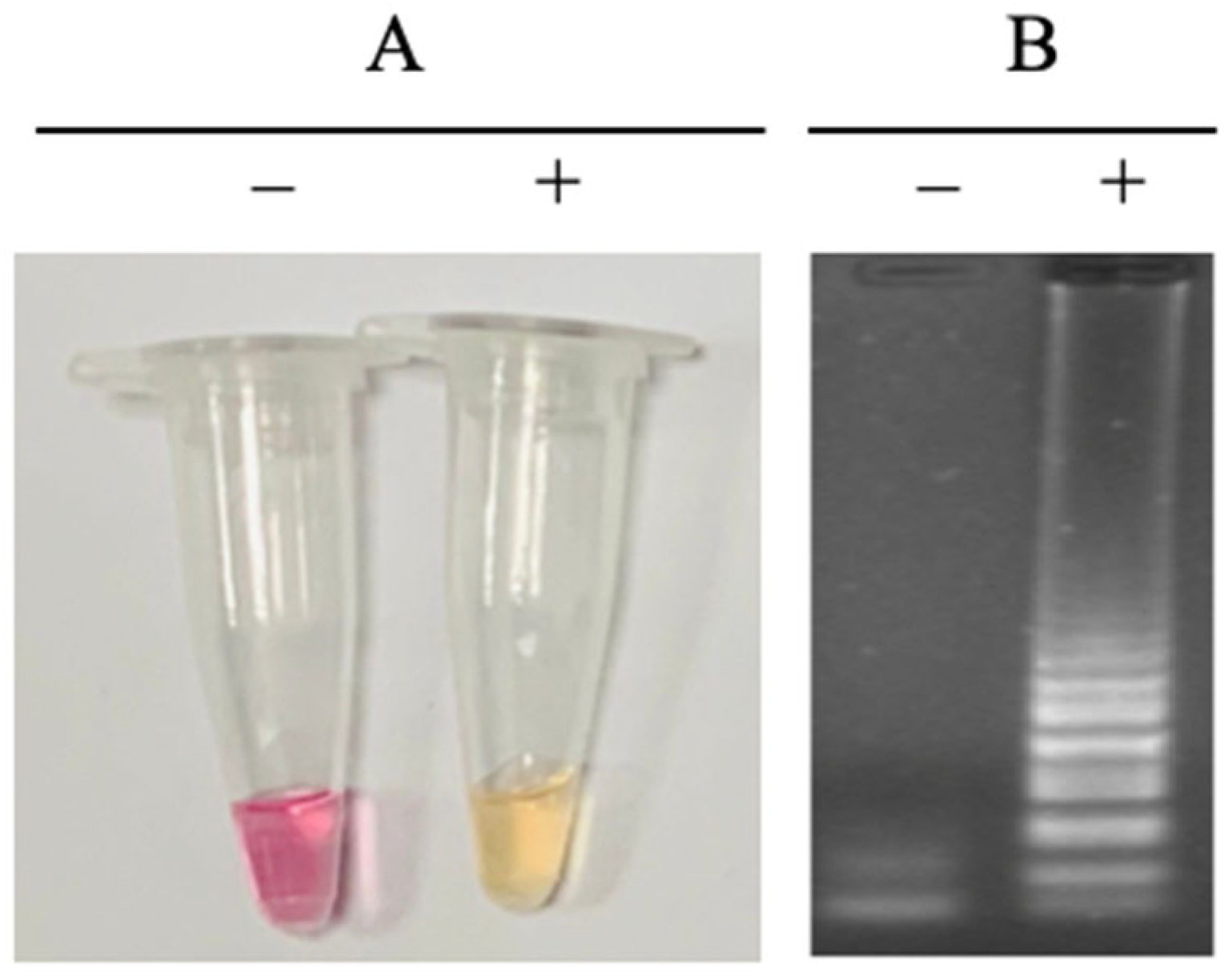
3.3. Detection Method
3.4. Properties of the LAMP Reaction
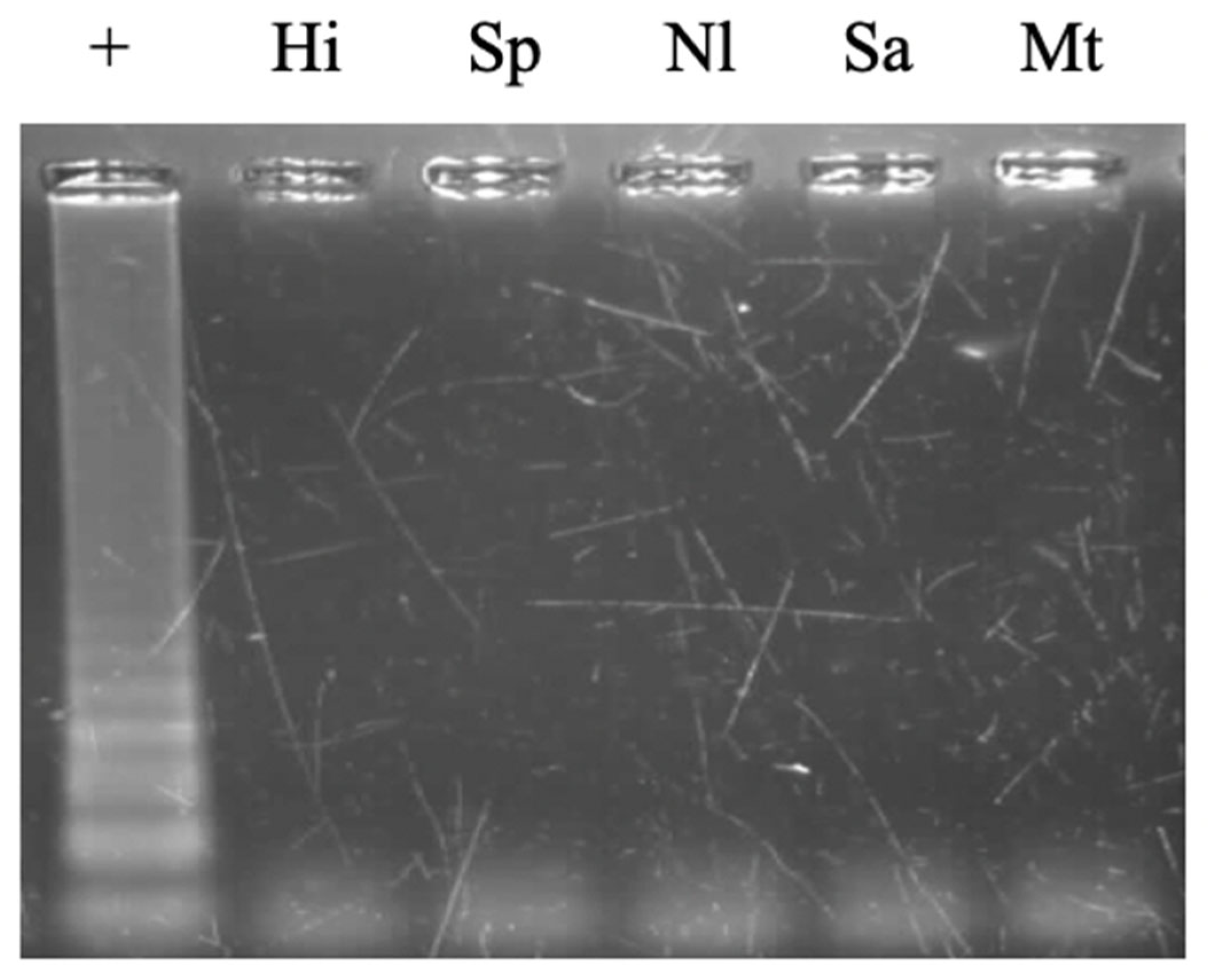
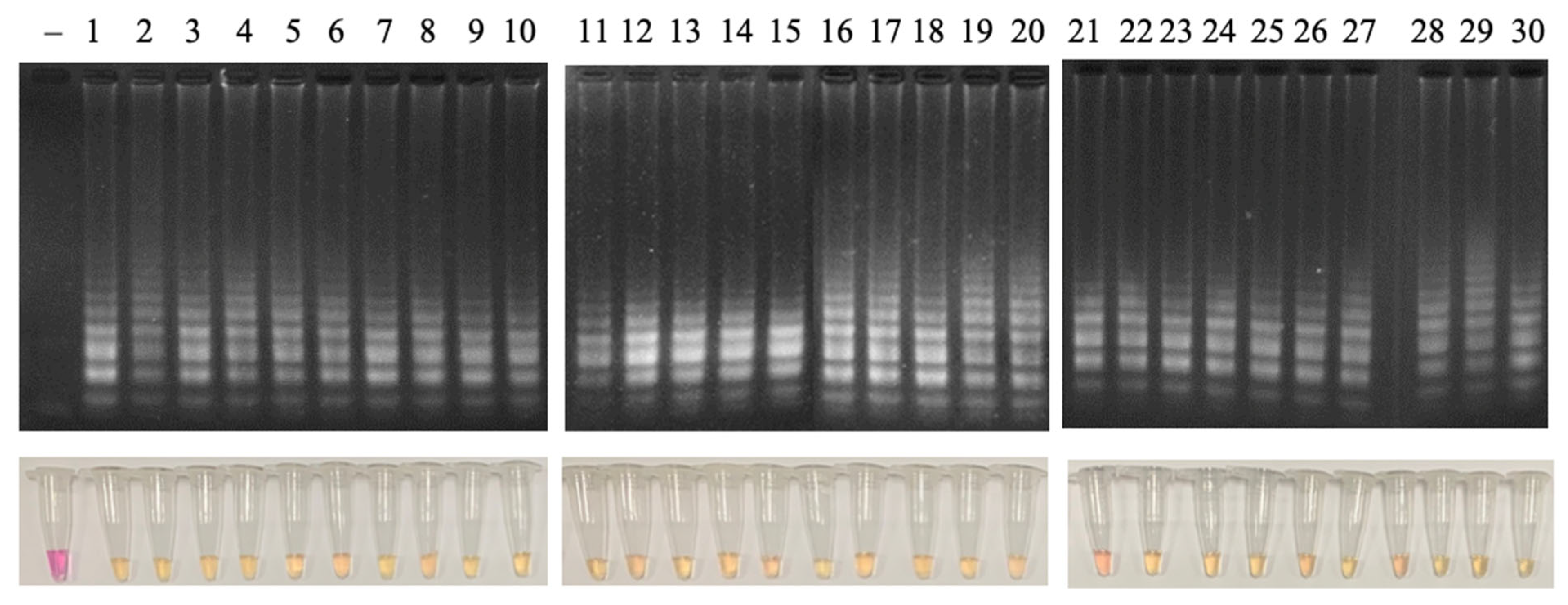
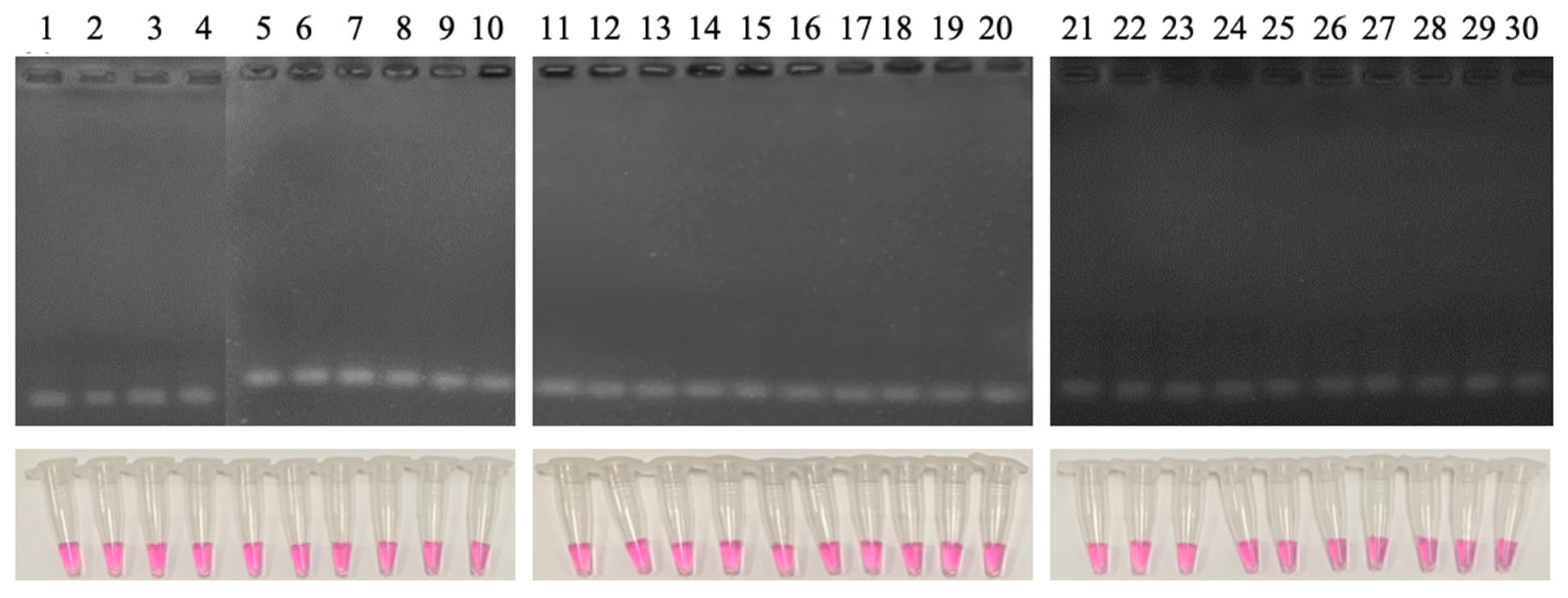
3.4.1. Detectability of Different Genotypes and Cross-Reactivity
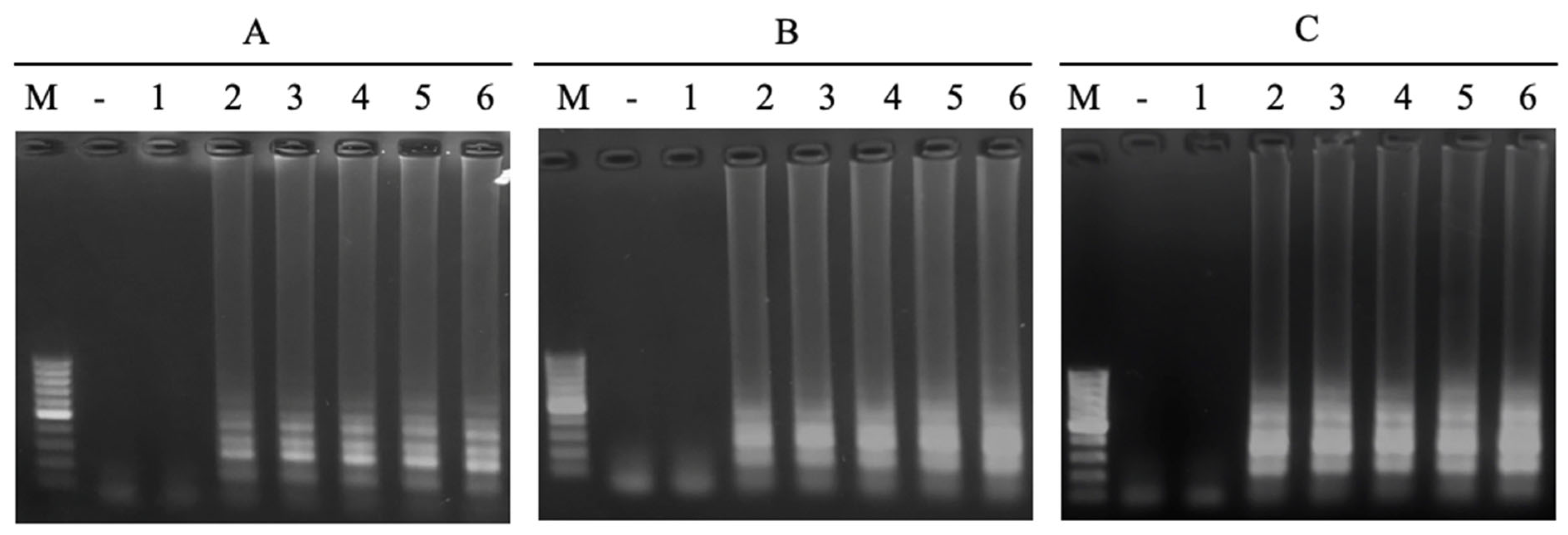
3.4.2. Detection Limit of the LAMP Reaction
3.4.3. Sensitivity and Specificity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hollingshead, S.; Tang, C.M. An Overview of Neisseria meningitidis. In Methods in Molecular Biology; Seib, K.L., Peak, I.R., Eds.; Springer: New York, NY, USA, 2019. [Google Scholar]
- Nguyen, N.; Ashong, D. Meningococcal Disease (Neisseria meningitidis Infection). In StatPearls. Treasure Island (FL): StatPearls Publishing. Available online: http://www.ncbi.nlm.nih.gov/books/NBK549849/ (accessed on 14 January 2025).
- Mazamay, S.; Guégan, J.F.; Diallo, N.; Bompangue, D.; Bokabo, E.; Muyembe, J.J.; Taty, N.; Vita, T.P.; Broutin, H. An overview of bacterial meningitis epidemics in Africa from 1928 to 2018 with a focus on epidemics ‘outside-the-belt’. BMC Infect. Dis. 2021, 21, 1027. [Google Scholar] [CrossRef] [PubMed]
- Mbaeyi, S.A.; Bozio, C.H.; Duffy, J.; Rubin, L.G.; Hariri, S.; Stephens, D.S.; Macneil, J.R. Meningococcal Vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm. Rep. 2020, 69, 1–41. [Google Scholar] [CrossRef]
- Aye, A.M.M.; Bai, X.; Borrow, R.; Bory, S.; Carlos, J.; Caugant, D.A.; Chiou, C.; Dai, V.T.T.; Dinleyici, E.C.; Ghimire, P.; et al. Meningococcal disease surveillance in the Asia–Pacific region (2020): The global meningococcal initiative. J. Infect. 2020, 81, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.N.N.; Hung, N.T.; Mathur, G.; De Jesus Pereira Pinto, T.; Minh, N.H.L. Review of the epidemiology, diagnosis and management of invasive meningococcal disease in Vietnam. Hum. Vaccin. Immunother. 2023, 19, 2172922. [Google Scholar] [CrossRef]
- Van, C.P.; Nguyen, T.T.; Bui, S.T.; Van, N.T.; Chan, H.T.T.; Pham, D.T.; Trieu, L.P.; Nguyen, M.D. Invasive meningococcal disease remains a health threat in Vietnam People’s Army. Infect. Drug. Resist. 2021, 14, 5261–5269. [Google Scholar] [CrossRef] [PubMed]
- Olcén, P.; Fredlund, H. Isolation, Culture, and Identification of Meningococci from Clinical Specimens. Methods Mol. Med. 2001, 67, 9–21. [Google Scholar]
- Vázquez, J.A.; Taha, M.K.; Findlow, J.; Gupta, S.; Borrow, R. Global Meningococcal Initiative: Guidelines for diagnosis and confirmation of invasive meningococcal disease. Epidemiol. Infect. 2016, 144, 3052–3057. [Google Scholar] [CrossRef]
- Borrow, R.; Findlow, J.; Gray, S.; Taylor, S.; Kaczmarski, E. Safe laboratory handling of Neisseria meningitidis. J. Infect. 2014, 68, 305–312. [Google Scholar] [CrossRef]
- Sobanski, M.A.; Barnes, R.A.; Coakley, W.T. Detection of meningococcal antigen by latex agglutination. Methods Mol. Med. 2001, 67, 41–59. [Google Scholar]
- Taha, M.; Alonso, J.; Cafferkey, M.; Caugant, D.A.; Clarke, S.C.; Diggle, M.A.; Fox, A.; Frosch, M.; Gray, S.J.; Guiver, M.; et al. Interlaboratory Comparison of PCR-Based Identification and Genogrouping of Neisseria meningitidis. J. Clin. Microbiol. 2005, 43, 144–149. [Google Scholar] [CrossRef]
- Diallo, K.; Coulibaly, M.D.; Rebbetts, L.S.; Harrison, O.B.; Lucidarme, J.; Gamougam, K.; Tekletsion, Y.K.; Bugri, A.; Toure, A.; Issaka, B.; et al. Development of a PCR algorithm to detect and characterize Neisseria meningitidis carriage isolates in the African meningitis belt. PLoS ONE 2018, 13, e0206453. [Google Scholar] [CrossRef] [PubMed]
- Fraisier, C.; Stor, R.; Tenebray, B.; Sanson, Y.; Nicolas, P. Use of a New Single Multiplex PCR-Based Assay for Direct Simultaneous Characterization of Six Neisseria meningitidis Serogroups. J. Clin. Microbiol. 2009, 47, 2662–2666. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Q.; Wen, L.; Xu, J.; Shao, Z.; Chen, M.; Chen, M.; Reeves, P.R.; Cao, B.; Wang, L. Development of a Multiplex PCR Assay for Detection and Genogrouping of Neisseria meningitidis. J. Clin. Microbiol. 2011, 50, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef]
- McKenna, J.P.; Fairley, D.J.; Shields, M.D.; Cosby, S.L.; Wyatt, D.E.; McCaughey, C.; Coyle, P.V. Development and clinical validation of a loop-mediated isothermal amplification method for the rapid detection of Neisseria meningitidis. Diagn. Microbiol. Infect. Dis. 2011, 69, 137–144. [Google Scholar] [CrossRef]
- Lee, D.; Kim, E.J.; Kilgore, P.E.; Kim, S.A.; Takahashi, H.; Ohnishi, M.; Anh, D.D.; Dong, B.Q.; Kim, J.S.; Tomono, J.; et al. Clinical Evaluation of a Loop-Mediated Isothermal Amplification (LAMP) Assay for Rapid Detection of Neisseria meningitidis in Cerebrospinal Fluid. PLoS ONE 2015, 10, e0122922. [Google Scholar] [CrossRef]
- Lee, D.; Kim, E.J.; Kilgore, P.E.; Takahashi, H.; Ohnishi, M.; Tomono, J.; Miyamoto, S.; Omagari, D.; Kim, D.W.; Seki, M. A Novel Loop-Mediated Isothermal Amplification Assay for Serogroup Identification of Neisseria meningitidis in Cerebrospinal Fluid. Front. Microbiol. 2016, 6, 1548. [Google Scholar] [CrossRef]
- Higgins, O.; Clancy, E.; Cormican, M.; Boo, T.W.; Cunney, R.; Smith, T.J. Evaluation of an internally controlled multiplex TTH endonuclease cleavage Loop-Mediated Isothermal Amplification (TEC-LAMP) assay for the detection of bacterial meningitis pathogens. Int. J. Mol. Sci. 2018, 19, 524. [Google Scholar] [CrossRef] [PubMed]
- Tanner, N.A.; Zhang, Y.; Evans, T.C. Visual detection of isothermal nucleic acid amplification using PH-Sensitive dyes. BioTechniques 2015, 58, 59–68. [Google Scholar] [CrossRef]
- Gadkar, V.J.; Goldfarb, D.M.; Gantt, S.; Tilley, P.A.G. Real-time Detection and Monitoring of Loop Mediated Amplification (LAMP) Reaction Using Self-quenching and De-quenching Fluorogenic Probes. Sci. Rep. 2018, 8, 5548. [Google Scholar] [CrossRef]
- Mwendwa, F.; Mbae, C.K.; Kinyua, J.; Mulinge, E.; Mburugu, G.N.; Njiru, Z.K. Stem loop-mediated isothermal amplification test: Comparative analysis with classical LAMP and PCR in detection of Entamoeba histolytica in Kenya. BMC Res. Notes 2017, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Alhamid, G.; Tombuloglu, H.; Al-Suhaimi, E. Development of loop-mediated isothermal amplification (LAMP) assays using five primers reduces the false-positive rate in COVID-19 diagnosis. Sci. Rep. 2023, 13, 5066. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.L.; Chen, H.M.; Lin, C.Y.; Chen, C.C. Accuracy of antigen tests for meningococcal meningitis in cerebrospinal fluid: A diagnostic meta-analysis. Trop. Med. Int. Health 2023, 8, 797–805. [Google Scholar] [CrossRef]

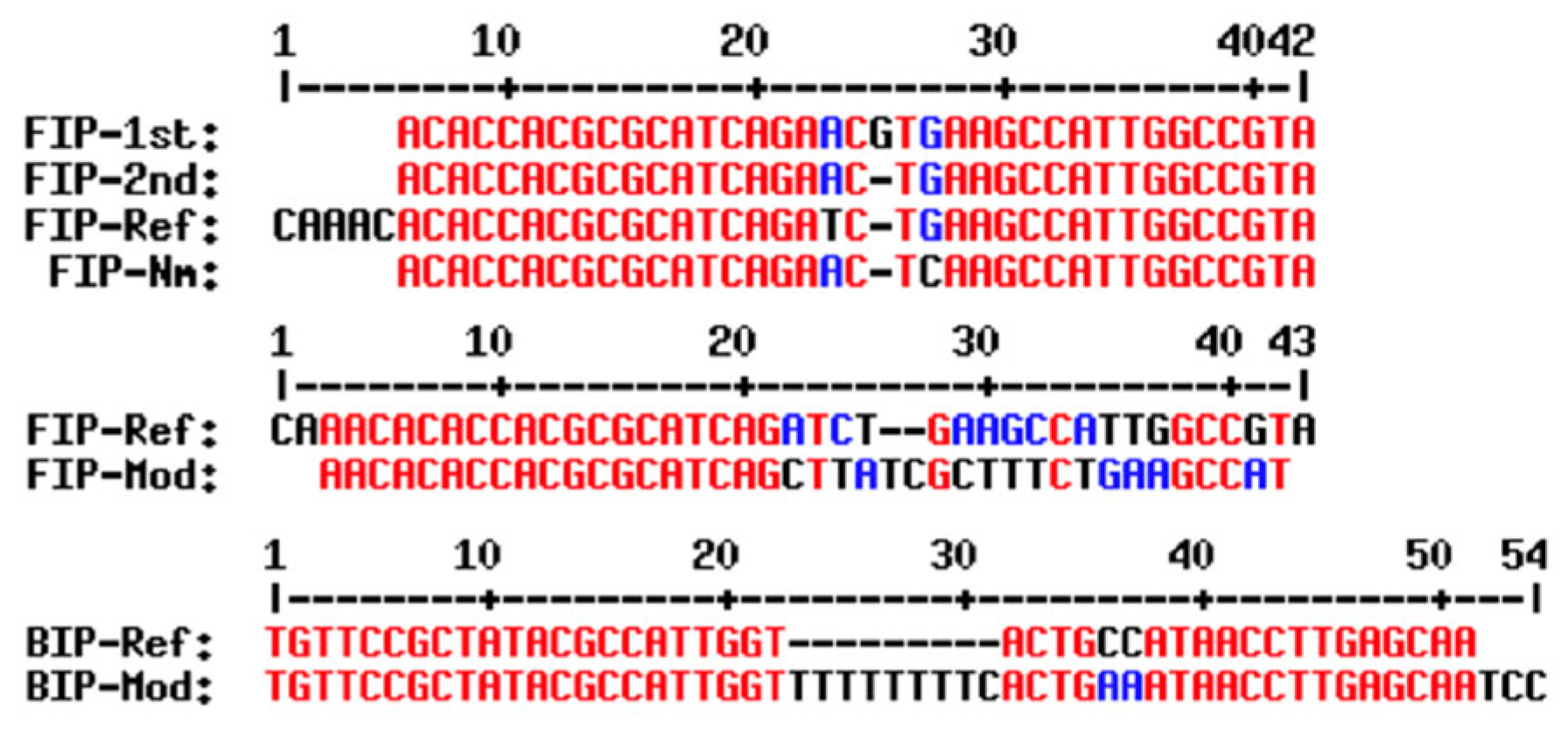





| Primer Set | Primer Name | Sequence | Tm (°C) | GC (%) |
|---|---|---|---|---|
| (1) | FIP-1 | CAAACACACCACGCGCATCAGATCTGAAGCCATTGGCCGTA | 69.7 | 53.7 |
| BIP-1 | TGTTCCGCTATACGCCATTGGTACTGCCATAACCTTGAGCAA | 67.7 | 47.6 | |
| FL-1 | CGATCTTGCAAACCGCCC | 57.3 | 61.1 | |
| BL-1 | GCAGAACGTCAGGATAAATGGA | 54.9 | 45.5 | |
| F3-1 | AGC(C/T)AGAGGCTTATCGCTT | 56.4 | 52.6 | |
| B3-1 | ATACCGTTGGAATCTCTGCC | 54.6 | 50.0 | |
| (2) | FIP-2 | ACGCTCATCAGAACGGCGATCATCGCTTTCTGAAGCCA | 68.9 | 52.6 |
| BIP-2 | TGTTCCGCTATACGCCATTGGTACTGCCATAACCTTGAGCAA | 67.7 | 47.6 | |
| FL-2 | CAAACCGCCCATACGGCC | 59.7 | 66.7 | |
| BL-2 | GCAGAACGTCAGGATAAATGGA | 54.9 | 45.5 | |
| F3-2 | GGTTTTTCAACCAGAGGCTT | 53.5 | 45.0 | |
| B3-2 | ATACCGTTGGAATCTCTGCC | 54.6 | 50.0 | |
| (3) | FIP-3 | GGCCATTTTTTTCAGGCGGCCTTGGCGATATTTCGGTGGTC | 69.4 | 53.7 |
| BIP-3 | CAAGTGATGGTGCG(C/T)TTGGTGCA(G/A)CGGCATACGCA CACTA | 71.1 | 57.5 | |
| FL-3 | ACCTGACCAGGCGTTTTACC | 57.6 | 55.0 | |
| BL-3 | CGGTGATTCGTGCTGGGAA | 57.9 | 57.9 | |
| F3-3 | ACGTGGTACGGTTTCTGTG | 55 | 52.6 | |
| B3-3 | CCACCGCATCCAACACAC | 57 | 61.1 | |
| (4) | FIP-4 | AACACACCACGCGCATCAGCTTATCGCTTTCTGAAGCCAT | 68.8 | 50.0 |
| BIP-4 | TGTTCCGCTATACGCCATTGGTTTTTTTTTCACTGAAATAA CCTTGAGCAATCC | 66.7 | 38.9 | |
| FL-4 | GATCTTGCAAACCGCCCATA | 55.7 | 50.0 | |
| BL-4 | CGGCAGAACGTCAGGATAA | 54.6 | 52.6 | |
| F3-4 | GGTGGGGAGAACACAAGAAAT | 55.1 | 47.6 | |
| B3-4 | AATGCGCATCAGCCATATTCA | 61.4 | 42.9 |
| Sequence | |
|---|---|
| Conserved region (1) | ACGTGGTACGGTTTCTGTGCCGTTTGTTGGCGATATTTCGGTGGTCGGTAAAACGCCTGGTCAGGTTCAGGAAATTATTAAAGGCCGCCTGAAAAAAATGGCCAATCAGCCGCAAGTGATGGTGCGCTTGGTGCAGAATAATGCGGCAAATGTATCGGTGATTCGCGCAGGCAATAGTGTGCGTATGCCGTTGACGGCAGCCGGTGAGCGTGTGTTGGATGCGGTGG |
| Conserved region (2) | GGTGGGGAGAACACAAGAAATCGGTTTTTCAGCTAGAGGCTTATCGCTTTCTGAAGCCATTGGCCGTATGGGCGGTTTGCAAGATCGCCGTTCTGATACGCGTGGTGTGTTTGTGTTCCGCTATACGCCATTGGTGGAATTGCCGGCAGAACGTCAGGATAAATGGATTGCTCAAGGTTATGGCAGTGAGGCAGAGATTCCAACGGTATATCGTGTGAATATGGCTGATGCGCATT |
| LAMP Reaction Components | Concentration/Conditions |
|---|---|
| FIP/BIP primers | 1.5 µM |
| FL/BL primers | 0.8 µM |
| dNTPs | 1.5 mM |
| MgSO4 | 4 mM |
| Betaine | 1.2 M |
| Bst DNA polymerase | 6 IU |
| Reaction temperature | 65 °C |
| Reaction time | 50 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vo, C.V.; Nguyen, T.T.; Ngo, H.T.; Bui, L.A.T.; Trinh, T.V.; Vu, L.T.; Hoang, H.D.; Truong, P.Q. Design of New Primer Sets for the Development of a Loop-Mediated Isothermal Amplification for Rapid Detection of Neisseria meningitidis. Curr. Issues Mol. Biol. 2025, 47, 467. https://doi.org/10.3390/cimb47060467
Vo CV, Nguyen TT, Ngo HT, Bui LAT, Trinh TV, Vu LT, Hoang HD, Truong PQ. Design of New Primer Sets for the Development of a Loop-Mediated Isothermal Amplification for Rapid Detection of Neisseria meningitidis. Current Issues in Molecular Biology. 2025; 47(6):467. https://doi.org/10.3390/cimb47060467
Chicago/Turabian StyleVo, Cuong Viet, Trang Thu Nguyen, Huong Thu Ngo, Lan Anh Thi Bui, Toan Van Trinh, Loan Thi Vu, Hieu Dang Hoang, and Phong Quoc Truong. 2025. "Design of New Primer Sets for the Development of a Loop-Mediated Isothermal Amplification for Rapid Detection of Neisseria meningitidis" Current Issues in Molecular Biology 47, no. 6: 467. https://doi.org/10.3390/cimb47060467
APA StyleVo, C. V., Nguyen, T. T., Ngo, H. T., Bui, L. A. T., Trinh, T. V., Vu, L. T., Hoang, H. D., & Truong, P. Q. (2025). Design of New Primer Sets for the Development of a Loop-Mediated Isothermal Amplification for Rapid Detection of Neisseria meningitidis. Current Issues in Molecular Biology, 47(6), 467. https://doi.org/10.3390/cimb47060467







