Exploring the Hemolymph of the Pill Millipede Arthrosphaera lutescens (Butler, 1872): Chemical Composition, Bioactive Properties, and Computational Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of A. lutescens and Preparation of Hemolymph
2.2. Chemical Extraction of Hemolymph
2.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
2.4. Liquid Chromatography–Quadrupole Time-of-Flight Mass Spectrometry (LC-MS Q-TOF) Analysis
2.5. Antioxidant Activity Assessment (DPPH Assay)
2.6. Evaluation of Antibacterial Activity
2.7. Cytotoxicity Evaluation (Trypan Blue Exclusion Assay)
Statistical Analysis and Data Representation
2.8. Data Analysis and Compound Selection for Docking Studies
Computational Studies: Mode of Action of DMBP Against Lymphoma
3. Results
3.1. GC-MS Analysis
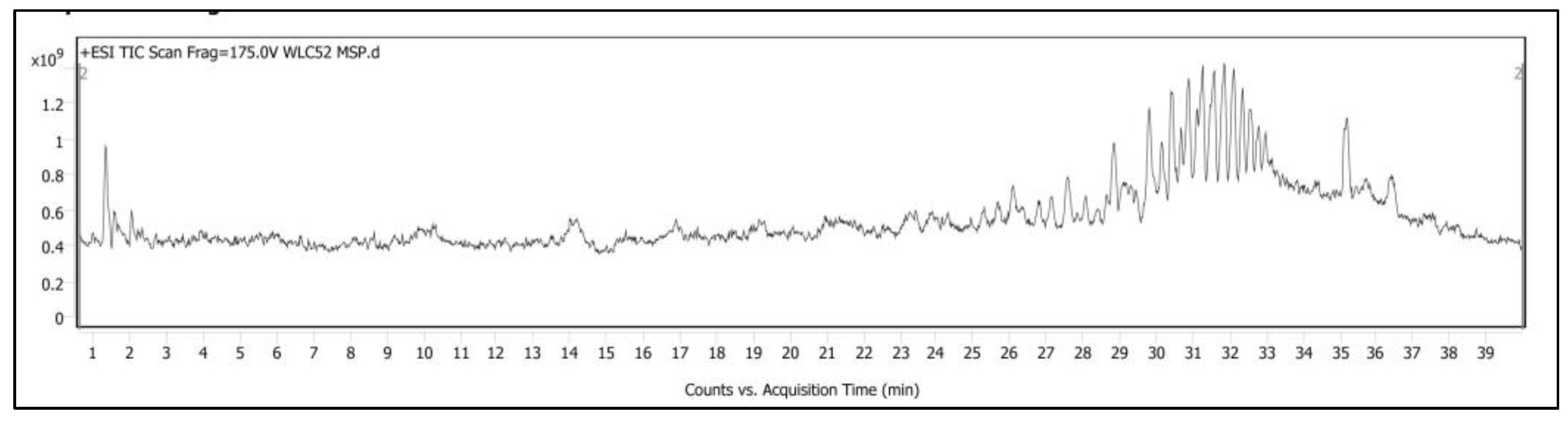
3.2. LC-MS Q-TOF Analysis of Hemolymph
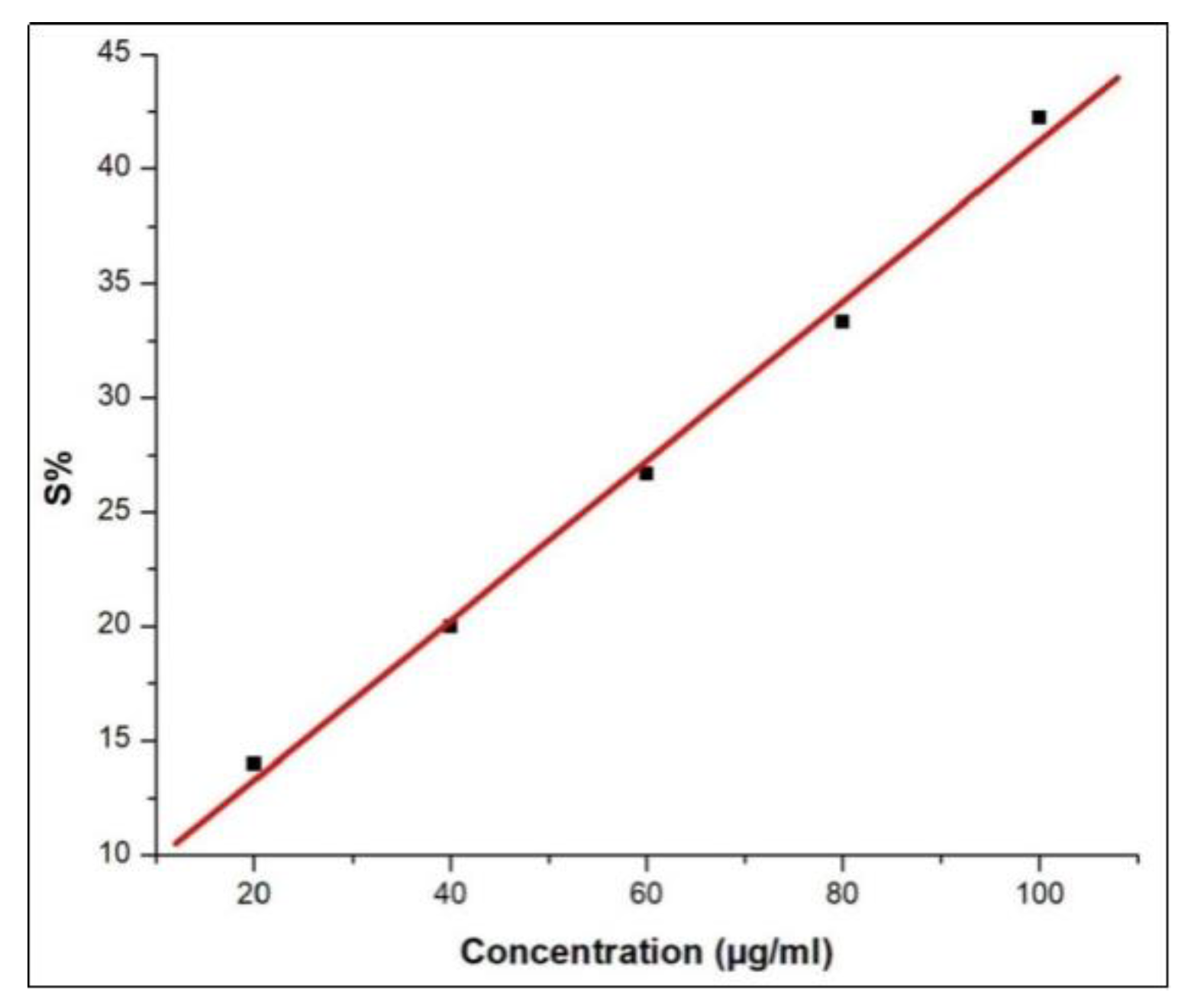
3.3. Antioxidant Activity of Pill Millipede Hemolymph Assessed by DPPH Assay
3.4. Anticancer Activity of Pill Millipede Hemolymph Against DLA Cells
3.5. Antibacterial Activity of Pill Millipede Hemolymph
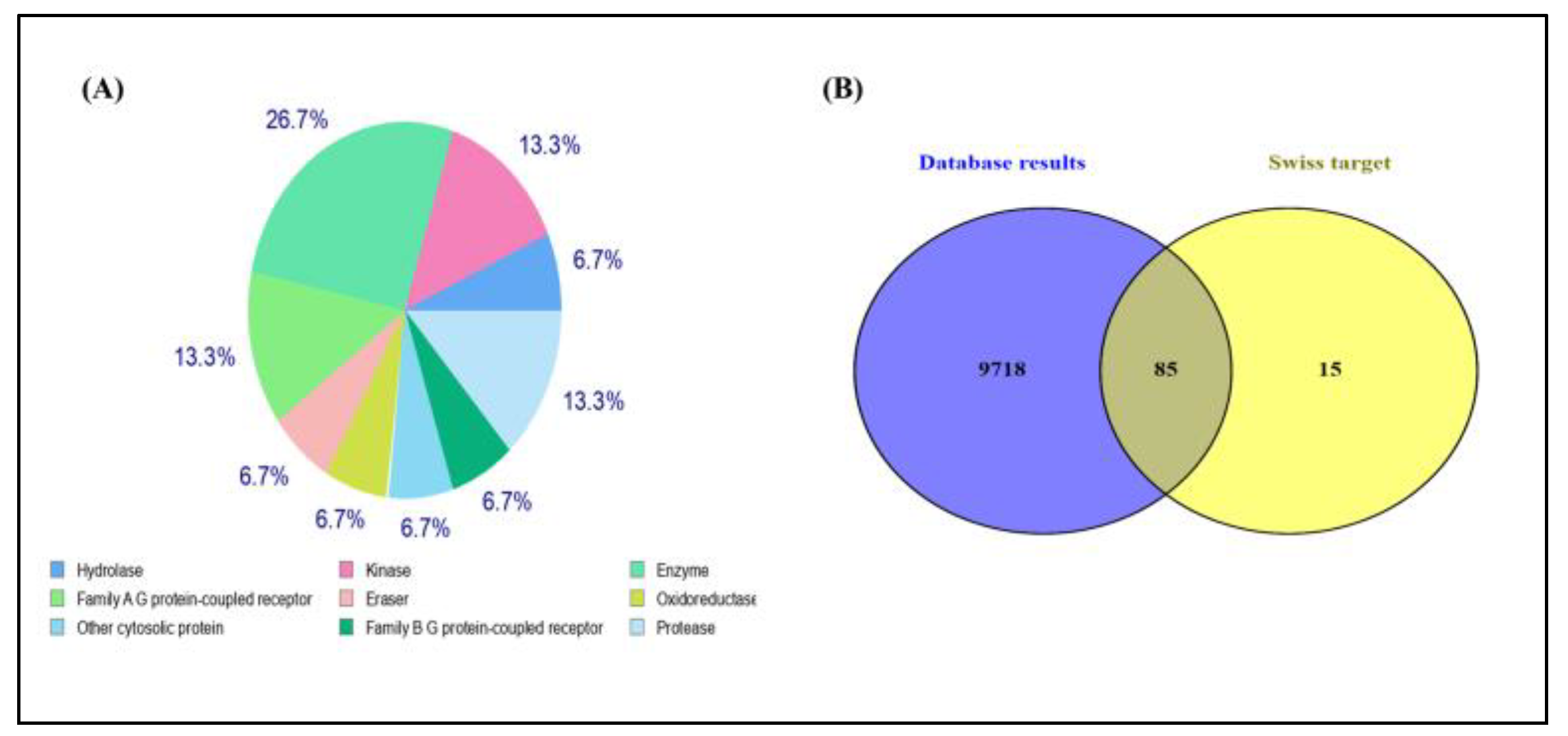
3.6. Identification of Potential Targets
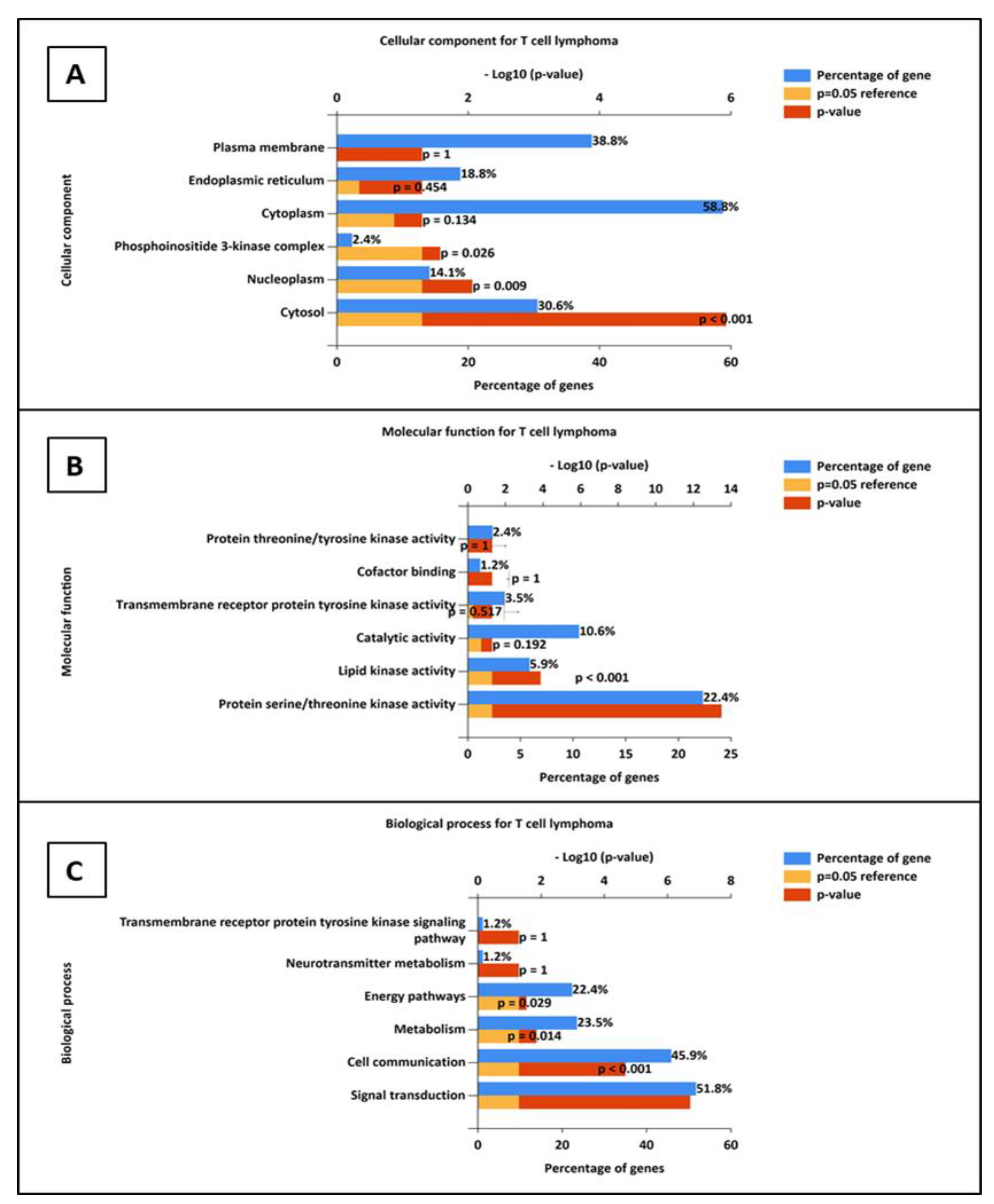
3.6.1. Construction of Protein–Protein Interaction and GO Analysis
3.6.2. Gene Ontology Analysis
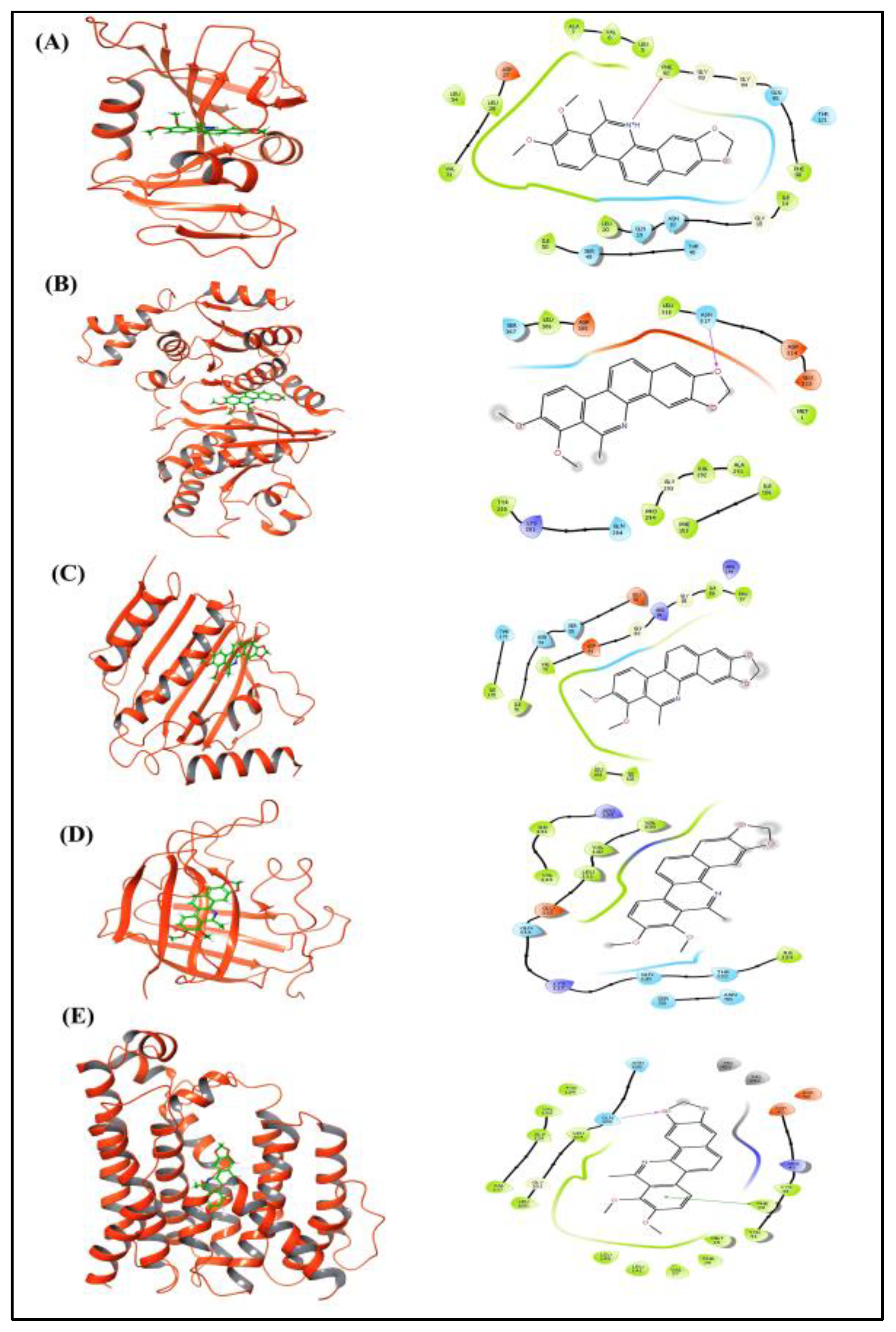
3.6.3. Molecular Docking
3.7. Mode of Action of DMBP Against Staphylococcus aureus (Rosenbach, 1884)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashwini, K.M.; Sridhar, K.R. Distribution of Pill Millipedes (Arthrosphaera) and Associated Soil Fauna in the Western Ghats and West Coast of India. Pedosphere 2008, 18, 749–757. [Google Scholar] [CrossRef]
- Wesener, T. The Giant Pill-Millipedes, Order Sphaerotheriida: An Annotated Species Catalogue with Morphological Atlas and List of Apomorphies (Arthropoda: Diplopoda); Bonn zoological Bulletin—Supplementum; Zoologisches Forschungsmuseum Koenig—Leibniz-Institut für Biodiversität der Tiere: Bonn, Germany, 2016; Available online: https://exuvium.net/wp-content/uploads/2022/12/giant_pill_millipedes-sphaerotheriida_-species_catalogue.pdf (accessed on 22 December 2024).
- Enghoff, H. Phylogeny of Millipedes—A Cladistic Analysis. J. Zool. Syst. Evol. Res. 2009, 22, 8–26. [Google Scholar] [CrossRef]
- Jeekel, C.A.W. The Group Taxonomy and Geography of the Sphaerotheriida (Diplopoda). In Symposia of the Zoological Society of London; Cambridge University Press: Cambridge, UK, 1974; Volume 32, pp. 41–52. [Google Scholar]
- Wesener, T.; Sierwald, P. The Giant Pill-Millipedes of Madagascar. Proc. Calif. Acad. Sci. 2005, 56, 557–599. [Google Scholar]
- Pocock, R.I. A Monograph of the Pill Millipedes Zephroniidae Inhabiting India, Ceylon and Burma. J. Bombay Nat. Hist. Soc. 1899, 12, 269–285. [Google Scholar]
- Kadamannaya, B.S.; Ambarish, C.N.; Sridhar, K.R. Morphological Features of Three Endemic Pill-Millipedes of the Genus Arthrosphaera in the Western Ghats of India. Anim. Biol. 2012, 3, 181–193. [Google Scholar]
- Yashwant, P.; Rajesh, A.; Baisthakur, P.; Ravi, B. Millipedes as Ecosystem Engineers: Their Role in Nutrient Cycling, Soil Health and Biotechnological Significance. Int. J. Entomol. Res. 2024, 9, 168–176. [Google Scholar]
- Klowden, M.J.; Palli, S.R. Klowden, M.J., Palli, S.R., Eds.; Chapter 7—Circulatory Systems. In Physiological Systems in Insects (Fourth Edition); Academic Press: San Diego, CA, USA, 2023; pp. 359–407. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, Y.; Gong, J.; Tian, S.; Li, J.; Dong, Z.; Guo, C.; Peng, L.; Zhao, P.; Xia, Q. Comparative Proteomics Analysis of Silkworm Hemolymph during the Stages of Metamorphosis via Liquid Chromatography and Mass Spectrometry. Proteomics 2016, 16, 1421–1431. [Google Scholar] [CrossRef]
- Riddle, W.A.; Crawford, C.S.; Zeitone, A.M. Patterns of Hemolymph Osmoregulation in Three Desert Arthropods. J. Comp. Physiol. 1976, 112, 295–305. [Google Scholar] [CrossRef]
- Pugach, S.; Crawford, C.S. Seasonal Changes in Hemolymph Amino Acids, Proteins, and Inorganic Ions of a Desert Millipede Orthoporus Ornatus (Girard) (Diplopoda: Spirostreptidae). Can. J. Zool. 1978, 56, 1460–1465. [Google Scholar] [CrossRef]
- Nair, V.K.; Prabhu, V.K.K. On the Free Amino Acids in the Haemolymph of a Millipede. Comp. Biochem. Physiol. B 1971, 38, 1–4. [Google Scholar] [CrossRef]
- Xylander, W.E. Physico-Chemical Properties of Haemolymph of Chilopoda and Diplopoda (Myriapoda, Arthropoda): Protein Content, pH, Osmolarity. Soil Org. 2009, 81, 431–439. [Google Scholar]
- Punzo, F. Composition of the Hemolymph of Mygalomorph Spiders (Orthognatha). Comp. Biochem. Physiol. A 1989, 93, 757–760. [Google Scholar] [CrossRef]
- Mohamed, N.T. Seperation of Bioactive Compounds from Haemolymph of Scarab Beetle Scarabaeus Sacer (Coleoptera: Scarabaeidae) by GC-MS and Determination of Its Antimicrobial Activity. Int. J. Appl. Biol. 2021, 5, 98–116. [Google Scholar] [CrossRef]
- Zhang, Z.-L.; Xu, H.-N.; Gong, C.-M.; Li, Y.-Z.; Song, X.-M.; Li, Y.-M.; Zhang, D.-D.; Wang, R. Microorganism-Derived Bisindole Alkaloids With Anticancer Potential and Their Mechanisms: A Comprehensive Review. Chem. Biodivers. 2024, 22, e202402398. [Google Scholar] [CrossRef] [PubMed]
- Witaicenis, A.; Seito, L.N.; Da Silveira Chagas, A.; De Almeida, L.D.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Di Stasi, L.C. Antioxidant and Intestinal Anti-Inflammatory Effects of Plant-Derived Coumarin Derivatives. Phytomedicine 2014, 21, 240–246. [Google Scholar] [CrossRef]
- Sabira, O.; Vignesh, A.R.; Ajaykumar, A.P.; Varma, S.R.; Jayaraj, K.N.; Sebastin, M.; Nikhila, K.; Babu, A.; Rasheed, V.A.; Binitha, V.S.; et al. The Chemical Composition and Antimitotic, Antioxidant, Antibacterial and Cytotoxic Properties of the Defensive Gland Extract of the Beetle, Luprops Tristis Fabricius. Molecules 2022, 27, 7476. [Google Scholar] [CrossRef]
- SwissTargetPrediction. Available online: http://www.swisstargetprediction.ch/ (accessed on 6 February 2025).
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. CP Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- GeneCards—Human Genes|Gene Database|Gene Search. Available online: https://www.genecards.org/ (accessed on 6 February 2025).
- DISGENET: Genomics Platform for Precision Medicine. Available online: https://www.disgenet.com (accessed on 6 February 2025).
- Hamosh, A.; Scott, A.F.; Amberger, J.S.; Bocchini, C.A.; McKusick, V.A. Online Mendelian Inheritance in Man (OMIM), a Knowledgebase of Human Genes and Genetic Disorders. Nucleic Acids Res. 2005, 33, D514–D517. [Google Scholar] [CrossRef]
- Draw Venn Diagram. Available online: https://bioinformatics.psb.ugent.be/webtools/Venn/ (accessed on 6 February 2025).
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein-Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying Hub Objects and Sub-Networks from Complex Interactome. BMC Syst. Biol. 2014, 8 (Suppl. S4), S11. [Google Scholar] [CrossRef] [PubMed]
- Pathan, M.; Keerthikumar, S.; Ang, C.-S.; Gangoda, L.; Quek, C.Y.J.; Williamson, N.A.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A.; et al. FunRich: An Open Access Standalone Functional Enrichment and Interaction Network Analysis Tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef]
- Haridas, M.; Abdulhameed, S.; Francis, D.; Kumar, S.S. Drugs from Nature: Targets, Assay Systems and Leads; Springer Nature: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Yang, Y.; Yao, K.; Repasky, M.P.; Leswing, K.; Abel, R.; Shoichet, B.K.; Jerome, S.V. Efficient Exploration of Chemical Space with Docking and Deep Learning. J. Chem. Theory Comput. 2021, 17, 7106–7119. [Google Scholar] [CrossRef] [PubMed]
- Abhithaj, J.; Dileep, F.; Sharanya, C.S.; Arun, K.G.; Sadasivan, C.; Jayadevi, V. Repurposing Simeprevir, Calpain Inhibitor IV and a Cathepsin F Inhibitor against SARS-CoV-2 and Insights into Their Interactions with Mpro. J. Biomol. Struct. Dyn. 2022, 40, 325–336. [Google Scholar] [CrossRef]
- Ukwubile, C.A. GC-MS Analysis of Bioactive Compounds from Melastomastrum Capitatum (Vahl) Fern. Leaf Methanol Extract: An Anticancer Plant. Sci. Afr. 2019, 3, e000159. [Google Scholar] [CrossRef]
- (9Z)-octadecen-1-ol (CHEBI:73504). Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=73504 (accessed on 9 April 2025).
- Jin, X.; Zhou, J.; Richey, G.; Wang, M.; Hong, S.M.C.; Hong, S.H. Undecanoic Acid, Lauric Acid, and N-Tridecanoic Acid Inhibit Escherichia Coli Persistence and Biofilm Formation. J. Microbiol. Biotechnol. 2021, 31, 130–136. [Google Scholar] [CrossRef]
- Duke, J.A., Dr. Duke’s Phytochemical and Ethnobotanical Databases. 1994. Available online: https://phytochem.nal.usda.gov/ (accessed on 10 December 2024).
- Krishnan, K.; Mani, A.; Jasmine, S. Cytotoxic Activity of Bioactive Compound 1, 2- Benzene Dicarboxylic Acid, Mono 2- Ethylhexyl Ester Extracted from a Marine Derived Streptomyces Sp. VITSJK8. Int. J. Mol. Cell Med. 2014, 3, 246–254. [Google Scholar] [PubMed]
- Hamsalakshmi; Joghee, S.; Kalarikkal, S.P.; Sundaram, G.M.; Durai Ananda Kumar, T.; Chidambaram, S.B. Chemical Profiling and In-Vitro Anti-Inflammatory Activity of Bioactive Fraction(s) from Trichodesma Indicum (L.) R.Br. against LPS Induced Inflammation in RAW 264.7 Murine Macrophage Cells. J. Ethnopharmacol. 2021, 279, 114235. [Google Scholar] [CrossRef]
- Siswadi, S.; Saragih, G.S. Phytochemical Analysis of Bioactive Compounds in Ethanolic Extract of Sterculia Quadrifida R.Br. AIP Conf. Proc. 2021, 2353, 030098. [Google Scholar] [CrossRef]
- Mizushina, Y.; Tanaka, N.; Yagi, H.; Kurosawa, T.; Onoue, M.; Seto, H.; Horie, T.; Aoyagi, N.; Yamaoka, M.; Matsukage, A.; et al. Fatty Acids Selectively Inhibit Eukaryotic DNA Polymerase Activities in Vitro. Biochim. Biophys. Acta Gene Struct. Express. 1996, 1308, 256–262. [Google Scholar] [CrossRef]
- Boctor, I.Z.; Jwanny, E.W. Fatty Acid Pattern of Haemolymph Lipids in Spodoptera Littoralis Boisduval Larvae. J. Comp. Physiol. B 1974, 93, 151–155. [Google Scholar] [CrossRef]
- Lawal, B.; Shittu, O.K.; AbdulRasheed-Adeleke, T.; Ossai, P.C.; Ibrahim, A.M. GC-MS Determination of Bioactive Constituents of Giant African Snail (Archachatina Maginata) Haemolymph. J. Pharmacol. Biol. Sci. 2015, 10, 59–64. [Google Scholar]
- Giglio, A.; Brandmayr, P.; Dalpozzo, R.; Sindona, G.; Tagarelli, A.; Talarico, F.; Brandmayr, T.Z.; Ferrero, E.A. The Defensive Secretion of Carabus Lefebvrei Dejean 1826 Pupa (Coleoptera, Carabidae): Gland Ultrastructure and Chemical Identification. Microsc. Res. Tech. 2009, 72, 351–361. [Google Scholar] [CrossRef]
- Heil, C.S.; Wehrheim, S.S.; Paithankar, K.S.; Grininger, M. Fatty Acid Biosynthesis: Chain-Length Regulation and Control. ChemBioChem 2019, 20, 2298–2321. [Google Scholar] [CrossRef] [PubMed]
- Leta, M.A.; Gilbert, C.; Morse, R.A. Levels of Hemolymph Sugars and Body Glycogen of Honeybees (Apis mellifera L.) from Colonies Preparing to Swarm. J. Insect Physiol. 1996, 42, 239–245. [Google Scholar] [CrossRef]
- Thompson, S.N. Trehalose—The Insect ‘Blood’ Sugar. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2003; Volume 31, pp. 205–285. [Google Scholar] [CrossRef]
- Pennington, J.E.; Goldstrohm, D.A.; Wells, M.A. The Role of Hemolymph Proline as a Nitrogen Sink during Blood Meal Digestion by the Mosquito Aedes aegypti. J. Insect Physiol. 2003, 49, 115–121. [Google Scholar] [CrossRef]
- Nicolai, A.; Filser, J.; Lenz, R.; Bertrand, C.; Charrier, M. Quantitative Assessment of Hemolymph Metabolites in Two Physiological States and Two Populations of the Land Snail Helix pomatia. Physiol. Biochem. Zool. 2012, 85, 274–284. [Google Scholar] [CrossRef]
- Chen, H.-W.; Wei, B.-J.; He, X.-H.; Liu, Y.; Wang, J. Chemical Components and Cardiovascular Activities of Valeriana spp. Evid. Based Complement. Alternat. Med. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Eisner, T.; Hurst, J.J.; Meinwald, J. Defense Mechanisms of Arthropods. XI. The Structure, Function, and Phenolic Secretions of the Glands of a Chordeumoid Millipede and a Carabid Beetle. Psyche (Camb. Mass.) 1963, 70, 069817. [Google Scholar] [CrossRef]
- Shear, W.A. The Chemical Defenses of Millipedes (Diplopoda): Biochemistry, Physiology and Ecology. Biochem. Syst. Ecol. 2015, 61, 78–117. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Peng, Y.; Zhang, Y.; Liu, Y.; Liu, Y.; Yin, Y. Research Progress on Anti-Stress Nutrition Strategies in Swine. Anim. Nutr. 2023, 13, 342–360. [Google Scholar] [CrossRef] [PubMed]
- Dettner, K. Toxins, Defensive Compounds and Drugs from Insects. In Insect Molecular Biology and Ecology; CRC Press: Boca Raton, FL, USA, 2015; Volume 2. [Google Scholar]
- Mahmoud, S.; Moselhy, W.; El-Khashab, L.A.; Seufi, A.M. Identification and Molecular Characterisation of a Novel Manganese Superoxide Dismutase Gene from Flesh Fly Larvae, Sarcophaga Argyrostoma (Diptera: Sarcophagidae). Afr. Entomol. 2018, 26, 448–457. [Google Scholar] [CrossRef]
- Kaurinovic, B.; Vastag, D. Shalaby, E., Ed.; Flavonoids and Phenolic Acids as Potential Natural Antioxidants. In Antioxidants; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research Progress on Antibacterial Activities and Mechanisms of Natural Alkaloids: A Review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef] [PubMed]
- Azad, I.; Ahmad, R.; Khan, T.; Saquib, M.; Hassan, F.; Akhter, Y.; Khan, A.R.; Nasibullah, M. Phenanthridine Derivatives as Promising New Anticancer Agents: Synthesis, Biological Evaluation and Binding Studies. Future Med. Chem. 2020, 12, 709–739. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Srinivas, K.; Sujini, G.N.; Kumar, G.N.S. Protein-Protein Interaction Detection: Methods and Analysis. Int. J. Proteom. 2014, 2014, 147648. [Google Scholar] [CrossRef]
- Yu, W.; Weng, Y.; Wang, J.; Gao, Y.; Li, Y.; Xie, C.; Jian, Z. Network Pharmacology Approach and Partial Experimental Validation of Aidi Injection Solution for the Treatment of Colorectal Cancer. Nat. Prod. Commun. 2024, 19, 1934578X241239169. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef]
- Shi, D.; Gu, W. Dual Roles of MDM2 in the Regulation of P53. Genes Cancer 2012, 3, 240–248. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, Y.; Jia, Y.; Chen, X.; Niu, T.; Chatterjee, A.; He, P.; Hou, G. Heat Shock Protein 90: Biological Functions, Diseases, and Therapeutic Targets. MedComm 2024, 5, e470. [Google Scholar] [CrossRef]
- He, J.; Qiao, W.; An, Q.; Yang, T.; Luo, Y. Dihydrofolate Reductase Inhibitors for Use as Antimicrobial Agents. Eur. J. Med. Chem. 2020, 195, 112268. [Google Scholar] [CrossRef] [PubMed]
- Reece, R.J.; Maxwell, A. DNA Gyrase: Structure and Function. Crit. Rev. Biochem. Mol. Biol. 1991, 26, 335–375. [Google Scholar] [CrossRef] [PubMed]
- Valliammai, A.; Selvaraj, A.; Muthuramalingam, P.; Priya, A.; Ramesh, M.; Pandian, S.K. Staphyloxanthin Inhibitory Potential of Thymol Impairs Antioxidant Fitness, Enhances Neutrophil Mediated Killing and Alters Membrane Fluidity of Methicillin Resistant Staphylococcus aureus. Biomed. Pharmacother. 2021, 141, 111933. [Google Scholar] [CrossRef] [PubMed]

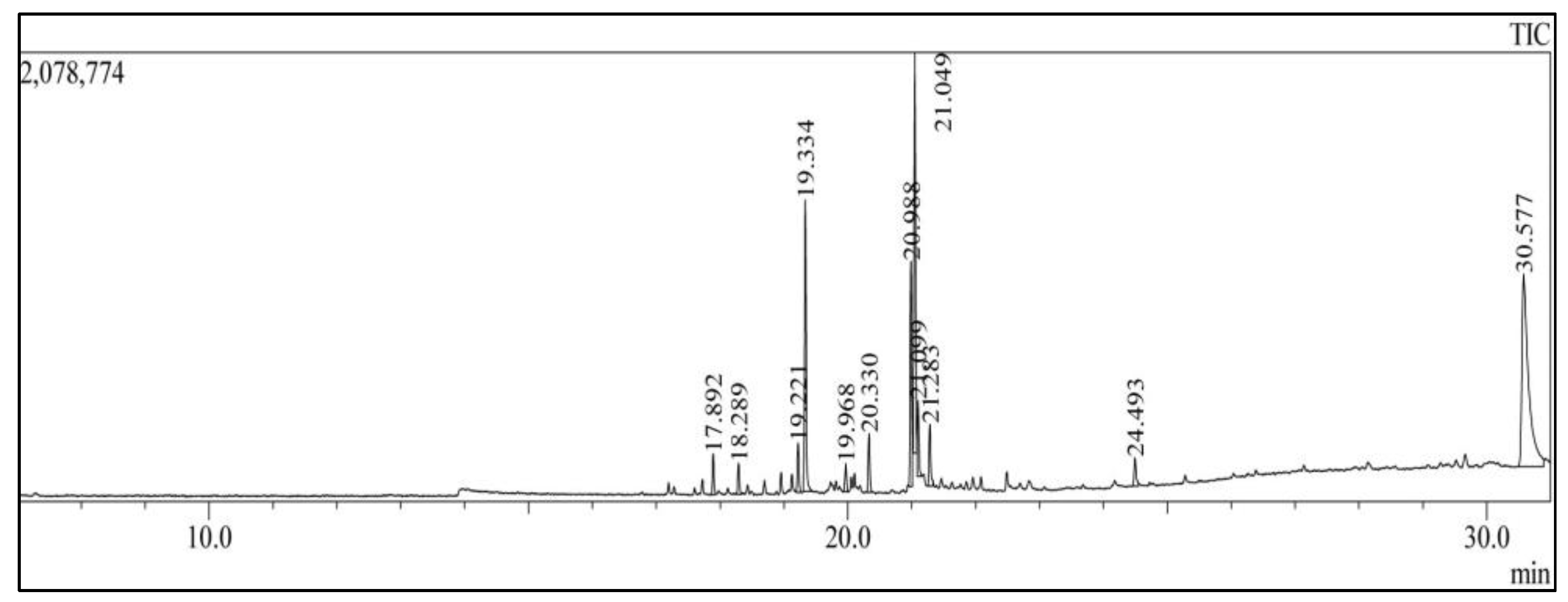




| Compound Name | Molecular Formula | Nature of Compound | Retention Time | Peak Area % |
|---|---|---|---|---|
| Methyl tetradecanoate | C15H30O2 | Ester | 17.195 | 0.48 |
| 9-Octadecen-1-ol | C18H36O | Alcohol | 17.280 | 0.24 |
| Tridecanoic acid | C17H34O2 | Fatty acid | 17.723 | 0.59 |
| Pentadecanoic acid, methyl ester | C16H32O2 | Fatty acid | 17.892 | 1.76 |
| Neophytadiene | C20H38 | Alkene | 18.429 | 0.42 |
| 1,2-Benzenedicarboxylic acid | C16H22O4 | Carboxylic acid | 18.694 | 0.60 |
| Hexadecanoic acid | C17H34O2 | Fatty acids | 18.955 | 1.00 |
| (Z)-Methyl hexadec-11-enoate | C17H32O2 | Ester | 19.221 | 2.17 |
| Tridecanoic acid, 4,8,12-trimethyl-, methyl ester | C17H34O2 | Fatty acids, ester | 19.814 | 0.31 |
| Heptadecanoic acid, methyl ester | C18H36O2 | Fatty acids, ester | 19.968 | 1.32 |
| cis-10-Heptadecanoic acid, methyl ester | C18H34O2 | Fatty acids, ester | 20.104 | 0.55 |
| 9,12-Octadecadienoic acid (Z, Z)-, methyl ester | C19H34O2 | Fatty acids, ester | 20.988 | 9.09 |
| 9-Octadecenoic acid, methyl ester | C19H36O2 | Fatty acids, ester | 21.049 | 18.06 |
| 11-Octadecenoic acid, methyl ester | C19H36O2 | Fatty acids, ester | 21.099 | 3 |
| Methyl stearate | C19H38O2 | Fatty acid ester | 21.283 | 2.72 |
| Eicosatetraenoic acid, methyl ester | C21H34O2 | Fatty acids, ester | 22.488 | 1.11 |
| Docosenoic acid, methyl ester | C23H44O2 | Fatty acids, ester | 24.493 | 1.71 |
| Cholesterol | C27H46O | Alcohol | 30.577 | 35.68 |
| Compound Name | Bioactivity |
|---|---|
| Methyl tetradecanoate | Anticancer activity [33] |
| 9-Octadecen-1-ol | Non-ionic surfactant, thickener, and emulsifier [34] |
| Tridecanoic acid | Antibacterial activity [35] |
| Pentadecanoic acid | Antioxidant properties [36] |
| Neophytadiene | Analgesic, antipyretic, anti-inflammatory, and antimicrobial properties [36] |
| 1,2-Benzenedicarboxylic acid | Cytotoxic activity [37] and anti-inflammatory activity [38] |
| Hexadecanoic acid | Antioxidant, anti-inflammatory, and antibacterial activity [39] |
| 9-Octadecenoic acid | Anticancer activity and antioxidant activity [40] |
| Methyl stearate | Antioxidant and anti-inflammatory effects [36] |
| Class of Compounds | Compound Name | Molecular Formula | Retention Time | Mass-to-Charge Ratio | Scores |
|---|---|---|---|---|---|
| Sugars | Trehalose | C12H22O11 | 1.529 | 342.1156 | 96.38 |
| Lactose | C12H22O11 | 1.529 | 342.1156 | 96.38 | |
| Mannobiose | C12H22O11 | 1.529 | 342.1156 | 96.38 | |
| Maltose | C12H22O11 | 1.529 | 342.1156 | 96.38 | |
| Inulobiose | C12H22O11 | 1.529 | 342.1156 | 96.38 | |
| Gentiobiose | C12H22O11 | 1.529 | 342.1156 | 96.38 | |
| Amino acids/modified amino acids | Phenylalanine | C9H11NO2 | 3.539 | 166.08 | 94.29 |
| N, N-Diethylglycine | C6H13NO2 | 2.193 | 131.0937 | 94.21 | |
| Isoleucin | C6H13NO2 | 2.193 | 131.0937 | 94.21 | |
| Leucine | C6H13NO2 | 2.193 | 131.0937 | 94.21 | |
| Anaserine | C10H16N4O3 | 31.551 | 240.1220 | 93.667 | |
| Na-Hexanoyl-Nb-inosityltryptophan | C23H32N2O8 | 23.244 | 464.2135 | 93.59 | |
| Phenylalanyl-Tyrosine | C18H20N2O4 | 10.666 | 328.143 | 90.11 | |
| Valine | C5H11NO2 | 1.529 | 117.0786 | 90.29 | |
| Fatty acids | Methyl myristic acid | C15H30O2 | 38.097 | 242.2244 | 99 |
| Tetradecanoic acid | C14H28O3 | 30.620 | 244.2028 | 95.33 | |
| Tridecanoic acid | C13H26O2 | 33.063 | 214.1926 | 97.26 | |
| Palmitic acid | C16H32O2 | 34.907 | 256.2400 | 96.61 | |
| Lauric acid | C12H24O2 | 30.521 | 200.1767 | 96.38 | |
| Esters | Butyl dodecanote | C16H32O2 | 34.907 | 256.2400 | 96.61 |
| Pentyl decanoate | C15H30O2 | 38.097 | 242.2244 | 99 | |
| Methyl dodecanote | C13H26O2 | 33.063 | 214.1926 | 97.86 | |
| Ethyl decanoate | C12H24O2 | 30.521 | 200.1767 | 96.38 | |
| Alkaloids | 1,2-Dimethoxy-13-methyl-[1,3]benzodioxolo[5,6-c]phenanthridine | C21H17NO4 | 19.107 | 347.1164 | 92.116 |
| Valerianine | C11H15NO | 35.056 | 177.1152 | 97.79 | |
| 2-(3Phenylpropyl) pyridine | C14H15N | 18.475 | 197.1196 | 95.03 | |
| Phenols | 4-(1-Ethyl-2-ph enyl butyl) phenol | C18H22O | 28.843 | 254.1664 | 92.63 |
| 4-Isopentylphenol | C11H16O | 28.643 | 164.7192 | 92.50 | |
| 4-n-Pentylphenol | C11H16O | 28.643 | 164.1192 | 92.50 | |
| Naphthalene dihydro diol | C10H10O2 | 38.579 | 162.0678 | 98.65 |
| Target Name | Docking Score (kcal/mol) | MMGBSA (kcal/mol) |
|---|---|---|
| AKT1 | −2.091 | −71.0 |
| HSP90AA1 | −4.907 | −35.9 |
| MTOR | −8.5 | −65.6 |
| MAPK1 | −3.529 | −79.50 |
| MDM2 | −4.94 | −51.13 |
| Target Protein | Dock Score (kcal/mol) | MMGBSA(kcal/mol) |
|---|---|---|
| Dihydrofolate reductase | −4.44 | −52.99 |
| Phosphate acetyltransferase | −3.396 | −47.89 |
| DNA gyrase | −5.1 | −65.3 |
| Sortase-A | −3.07 | −51.27 |
| Dehydrosqualene synthase | −5.5 | −9.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palakkaparambil, P.; Venugopal, V.; Vijayan, G.; Alsaegh, M.A.; Thachan Kundil, V.; Gangadharan, A.K.; Sabira, O.; Aswathi; Raghu, A.V.; Narayanan Jayaraj, K.; et al. Exploring the Hemolymph of the Pill Millipede Arthrosphaera lutescens (Butler, 1872): Chemical Composition, Bioactive Properties, and Computational Studies. Curr. Issues Mol. Biol. 2025, 47, 434. https://doi.org/10.3390/cimb47060434
Palakkaparambil P, Venugopal V, Vijayan G, Alsaegh MA, Thachan Kundil V, Gangadharan AK, Sabira O, Aswathi, Raghu AV, Narayanan Jayaraj K, et al. Exploring the Hemolymph of the Pill Millipede Arthrosphaera lutescens (Butler, 1872): Chemical Composition, Bioactive Properties, and Computational Studies. Current Issues in Molecular Biology. 2025; 47(6):434. https://doi.org/10.3390/cimb47060434
Chicago/Turabian StylePalakkaparambil, Priyanka, Veena Venugopal, Gouthami Vijayan, Mohammed Amjed Alsaegh, Varun Thachan Kundil, Arun Kumar Gangadharan, Ovungal Sabira, Aswathi, A. V. Raghu, Kodangattil Narayanan Jayaraj, and et al. 2025. "Exploring the Hemolymph of the Pill Millipede Arthrosphaera lutescens (Butler, 1872): Chemical Composition, Bioactive Properties, and Computational Studies" Current Issues in Molecular Biology 47, no. 6: 434. https://doi.org/10.3390/cimb47060434
APA StylePalakkaparambil, P., Venugopal, V., Vijayan, G., Alsaegh, M. A., Thachan Kundil, V., Gangadharan, A. K., Sabira, O., Aswathi, Raghu, A. V., Narayanan Jayaraj, K., & Ajaykumar, A. P. (2025). Exploring the Hemolymph of the Pill Millipede Arthrosphaera lutescens (Butler, 1872): Chemical Composition, Bioactive Properties, and Computational Studies. Current Issues in Molecular Biology, 47(6), 434. https://doi.org/10.3390/cimb47060434








