Abstract
To determine predictive biomarkers for prognosis by analyzing the association between tumor mutational burden (TMB) and mutant genes in patients with oral squamous cell carcinoma (OSCC) and to validate PCLO as an OSCC predictive biomarker, OSCC genetic mutation data were downloaded from The Cancer Genome Atlas (TCGA) and the International Cancer Genome Consortium (ICGC) database. Immune cell infiltration analysis and visualization were performed using R software. The relationships between overall survival (OS) and mutant genes or clinicopathological factors were investigated by Kaplan–Meier analysis and Cox regression analysis, respectively. Gene set enrichment analysis (GSEA) was used to explore the associations between mutant genes and functional pathways. Immunohistochemistry was performed to verify the presence of the piccolo protein in OSCC tissues. Finally, 17 mutated genes shared between TCGA and the ICGC database were detected. The TMB in the PCLO-mutated group was found to be significantly greater than that in the PCLO wild-type group, and PCLO mutation was associated with poor OS. Cox regression analysis revealed that PCLO is a significant prognostic factor for OSCC. GSEA and immune cell infiltration analysis revealed that PCLO is associated with the immune system, which suggests that PCLO mutation might affect the immune response. PCLO expression was considerably higher in OSCC tissues with PCLO mutations than in corresponding normal epithelium tissues and OSCC tissues without PCLO mutations (p < 0.05). PCLO mutation could serve as a promising predictive biomarker for prognosis in patients with OSCC.
1. Introduction
Oral squamous cell carcinoma (OSCC) is a malignant neoplasm arising from the mucosal epithelium of the oral cavity and exhibits variable squamous differentiation [1]. It is the 16th most common malignant tumor worldwide [2]. According to the World Health Organization International Classification of Diseases (ICD-11) [1], OSCC includes squamous cell carcinoma arising from any oral mucosal site and squamous cell carcinoma of other or ill-defined sites in the lip, oral cavity, or pharynx. In 2020, ~380,000 new cases of OSCC were diagnosed globally [3]. In high-risk countries such as Sri Lanka and India, OSCC may account for up to 25% of all new cancer cases [4]. The most important etiological factors in OSCC are tobacco use, excess alcohol consumption, and betel quid usage [4]. There are a number of traditional treatment methods for patients with OSCC, including surgery, radiotherapy, and chemotherapy. However, the treatment efficacy of these traditional methods is limited [5]. The emergence of targeted molecular therapies has reduced the side effects of nonspecific cell death and improved survival rates in patients with OSCC [6]. In recent years, researchers in various fields have focused more on the molecular mechanism of tumors to identify effective biomarkers. Numerous biomarkers, such as FAT atypical cadherin 1 (FAT1) [7] and circulating long non-coding RNAs [8], have been identified, and have been suggested to have good potential or diagnostic performance in OSCC treatment.
PCLO encodes piccolo protein, which is part of the presynaptic cytoskeletal matrix, is involved in establishing active synaptic zones and in synaptic vesicle trafficking. Previous studies have shown PCLO is mainly associated with depressive disorder [9]. In recent years, PCLO has been discovered to be frequently mutated in cancers, such as esophageal squamous cell carcinoma (ESCC), gastric cancer and hepatocellular carcinoma [10,11,12].
The tumor mutational burden (TMB), measured as the number of mutations that exist within a megabase of genomic territory, is an emerging and commonly used biomarker for the prediction of immune system therapy response [13]. It has been suggested that a greater frequency of gene mutations in cancers leads to a high TMB, increasing the likelihood of generating immunogenic tumor neoantigens recognized by the host immune system [14,15]. Some evidence suggests that the TMB can serve as a therapeutic biomarker in patients with lung cancer, head and neck cancer, or bladder cancer [16,17,18].
The present study aimed to explore potential biomarkers for clinical prognosis by analyzing the association between TMB and mutant genes in patients with OSCC, and to determine whether PCLO is an OSCC biomarker. Somatic mutation, transcriptome, and clinical data of patients with OSCC were collected from The Cancer Genome Atlas (TCGA) and somatic mutation data were collected from the International Cancer Genome Consortium (ICGC) database. After screening the common high-frequency mutated genes in the two databases, the present study further explored the relationships between these gene mutations and TMB, as well as prognosis. Immunohistochemistry was performed for further exploration.
2. Materials and Methods
2.1. Data Collection
Somatic mutation and transcriptome data for samples from patients with OSCC (n = 372) and Indian patients with OSCC (n = 171) were acquired from TCGA (https://portal.gdc.cancer.gov/ (accessed on 12 September 2022); study abbreviation, HNSC) and the ICGC (https://dcc.icgc.org/ (accessed on 12 September 2022)) database [dataset (ORCA-IN) ORAL CANCER-IN.], respectively. The clinical data associated with 372 OSCC samples were obtained from TCGA. No clinical data were available in the ICGC database. The data were extracted and sorted using Perl software (v5.30.0.1; https://www.perl.org/ (accessed on 11 September 2022)), and analyzed using R (v4.1.3; https://www.r-project.org/ (accessed on 11 September 2022)). Regarding the clinical data, only patients with OSCC whose complete information was available were included, and patients whose survival status, age, sex, grade, or TNM stage data were missing were excluded. To visualize the characteristics of mutations in the two databases, waterfall plots were produced using the R package ‘GenVisR’ (release no. 3.19; https://www.bioconductor.org/packages/release/bioc/html/GenVisR.html (accessed on 12 September 2022)). Using the R package ‘Venn’ (v0.1.10; https://cran.r-project.org/web/packages/ggvenn/index.html (accessed on 14 September 2022)), the mutated genes shared between the two databases were obtained. Sequencing data used to identify patients with PCLO mutations can be found in the Genome Sequence Archive at the BIG Data Centre of the Beijing Institute for Genome Research, Chinese Academy of Sciences, under accession number PRJCA002133 (https://ngdc.cncb.ac.cn/gsa-human/browse/HRA000107 (accessed on 8 February 2023)).
2.2. Calculation of TMB in TCGA Patients with OSCC
TMB was defined as the number of insertions, deletions (indels), replacement mutations, and gene coding errors per megabase in the evaluated coding regions of the genome [19]. Patients with synonymous mutations were removed. To calculate the TMB, the absolute number of somatic mutations was divided by the exome size. Subsequently, the relationship between the TMB and shared mutated genes was obtained via assessment and visualization using the R package ‘ggpub’ (v0.6.0; https://cran.r-project.org/web/packages/ggpubr/index.html (accessed on 14 September 2022)).
2.3. Gene Set Enrichment Analysis (GSEA) in Patients with OSCC from TCGA
GSEA software 4.3.1 (https://www.gsea-msigdb.org/gsea/index.jsp (accessed on 20 September 2022)) was used to identify significantly different functional pathways according to the PCLO mutation level, including the mutated group and the wild-type group. A nominal p-value < 0.05 or a normalized false discovery rate of <0.25 was regarded as the cutoff criterion.
2.4. Immune Cell Infiltration in Patients with OSCC from TCGA
The ‘CIBERSORT’ package (v1.03) of R software (https://cibersortx.stanford.edu/ (accessed on 21 September 2022)) was used to calculate the fractions of each immune cell in each OSCC sample with gene expression data, and p < 0.05 was regarded as the cutoff criterion [20]. A correlation heatmap was used to analyze the correlation of each type of immune cell with the other types in OSCC samples using the ‘corrplot’ package (v0.92; https://cran.r-project.org/web/packages/corrplot/index.html (accessed on 21 September 2022)). The R package ‘vioplot’ (v0.5.0; https://cran.r-project.org/web/packages/vioplot/index.html (accessed on 21 September 2022)) was used to visualize the differences in the infiltration of 22 immune cell types between PCLO-mutated and PCLO-wild-type samples. p < 0.05 was employed as the standard to identify the immune cell populations possibly influenced by the mutation levels of PCLO.
2.5. Immunohistochemistry (IHC) of 5 Patients with OSCC with PCLO Mutations
The exome sequencing data were obtained in our previous study [21]. Patients with OSCC with or without PCLO mutation were identified using the sequencing data. The OSCC tissues were divided into four groups: OSCC without PCLO mutation and its normal epithelium, and OSCC with PCLO mutation and its normal epithelium. The present study was approved by the Institutional Review Board of the Peking University Hospital of Stomatology (approval no. PKUSSIRB-2024104193; Beijing, China). The tissues were fixed with 4% paraformaldehyde overnight at room temperature. Paraffin-embedded tumor tissues were collected from 10 patients with OSCC (date range of recruitment, August 2009–November 2014; 8 male and 2 female patients; mean age, 52.3 years; age range, 30–62 years) who were diagnosed at the Oral Pathology Department of the Peking University Hospital of Stomatology (Beijing, China). The inclusion criteria for the OSCC with PCLO mutation group were: patients with PCLO mutations from the data from Zhang et al. [21]. The normal epithelium group inclusion criteria were: normal mucosa from patients with PCLO mutations. The inclusion criteria for the OSCC without PCLO mutation group were: patients without PCLO mutations and with relatively sufficient tumor tissue remaining for the production of good quality sections. The exclusion criteria were: insufficient tissue remaining to complete the experiment. Tissue sections (3 μm-thick) were deparaffinized with xylene and rehydrated in descending graded ethanol. The sections were subsequently submerged in sodium citrate buffer (pH = 6) and the antigens were repaired at a high temperature (95–100 °C) and high pressure for 2.5 min. The sections were then treated with endogenous peroxidase blockers (undiluted; PV-9005; OriGene Technologies, Inc., Wuxi, China) to quench the endogenous peroxidase activity for 10 min. To block nonspecific binding, the sections were incubated with 10% goat serum albumin (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) for 30 min at room temperature. The sections were incubated with rabbit anti-piccolo (1:200; ab20664; Abcam, Cambridge, UK) at 4 °C overnight. Finally, the sections were treated with goat anti-mouse/rabbit IgG HRP-polymer (undiluted; GK600711A; Gene Tech Co., Ltd., Shanghai, China) for 20 min at room temperature. 3,3′ Diaminobenzidine was used as the chromogen after washing with distilled water for 3 min at room temperature. The sections were then counterstained with hematoxylin for 10 s at room temperature. Tissue staining was observed under a light microscope. Image-Pro Plus 7.0 software (Media Cybernetics, Inc., Rockville, MD, USA) was used to select areas with equal brown and yellow staining as a uniform standard for assessing positive results in all images. IHC results for PCLO were evaluated using the average optical density (AOD) [22].
2.6. H&E Staining
Tissues were fixed with 4% paraformaldehyde overnight at room temperature, and paraffin-embedded tumor tissues were collected from 10 patients with OSCC. Tissue sections (4 μm-thick) were deparaffinized with xylene and rehydrated in descending graded ethanol. Sections were immersed in hematoxylin differentiation solution (undiluted; G1039-500ML; Wuhan Servicebio Technology Co., Ltd., Wuhan, China) for 10 s at room temperature, and then sections were immersed in hematoxylin reblue solution (undiluted; G1040-500ML; Wuhan Servicebio Technology Co., Ltd.) for 10 s at room temperature, then rehydrated in ascending graded ethanol and xylene. Tissue staining was observed under a light microscope.
2.7. Statistical Analysis
The OSCC data were extracted and organized in Perl (v5.30.0.1; https://www.perl.org/ (accessed on 11 September 2022)), so that they could be analyzed in R software (v4.1.3; https://www.r-project.org/ (accessed on 11 September 2022)) to complete all the statistical analyses. Survival analyses were performed using TCGA data. The relationships between overall survival (OS) and 13 common frequently mutated genes were assessed and visualized using Kaplan–Meier curves, and the associations were analyzed by log-rank tests. The genes with no statistically significant association with OS were filtered out. Univariate and multivariate Cox regression analyses were used to investigate the associations between clinicopathologic characteristics (age, sex, grade, stage, TMB, and PCLO status) and OS. In multivariate Cox regression models, we determined whether PCLO could be considered an independent predictor based on whether the confidence interval (CI) for the hazard ratio (HR) of PCLO did not contain 1 and, in conjunction with this, the corresponding p-value reached a level of significance (usually p < 0.05). The Wilcoxon rank-sum test was used to investigate the differences in immune cells between the PCLO wild-type and PCLO-mutant groups. Pearson correlation analysis was used to investigate the correlations between different immune cell types. Comparisons of AOD values between the without PCLO mutation group and the PCLO mutation group were performed using two-way mixed ANOVA with Bonferroni post hoc test. The data are reported as the mean ± standard error of the mean. p < 0.05 was considered to indicate a statistically significant difference.
3. Results
3.1. Overview of Mutated Genes in OSCC
The mutation data of patients with OSCC were downloaded from TCGA and the ICGC database. The top 30 frequently mutated genes with their corresponding mutation profiles in TCGA and the ICGC database were identified (Figure 1a,b). In addition, 17 frequently mutated genes were identified by generating a Venn diagram indicating the intersection of the top 30 most frequently mutated genes in the ICGC and TCGA cohorts, and these were TP53, CASP8, FAT1, TTN, NOTCH1, PIK3CA, MUC16, HRAS, PCLO, OBSCN, XIRP2, FAT3, CDKN2A, USH2A, HUWE1, KMT2D, and PLEC (Figure 1c). In the subsequent analyses, these 17 commonly mutated genes were the focus of the present study.
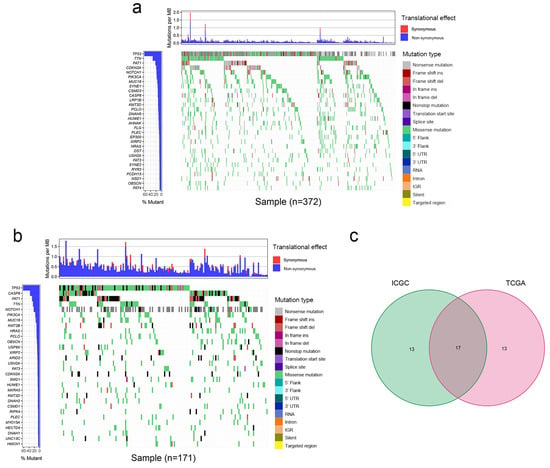
Figure 1.
Mutation profile landscape in patients with OSCC. (a) Frequently mutated genes in OSCC specimens in TCGA are shown in the waterfall plot. Genes ranked by mutation frequency are presented in the left panel. The right panel shows the variety of mutation types. (b) Frequently mutated genes in the OSCC samples from the ICGC dataset are depicted as a waterfall plot. The left panel shows the mutation frequency and corresponding genes. The right panel shows the multiple mutation types of these genes. (c) Common frequently mutated genes in the ICGC and TCGA cohorts are shown in the Venn diagram. Del, deletion; ICGC, International Cancer Genome Consortium; IGR, intergenic region; Ins, insertion; MB, megabase; OSCC, oral squamous cell carcinoma; TCGA, The Cancer Genome Atlas; UTR, untranslated region.
3.2. PCLO Mutation Status Is Associated with TMB and OS
Patients with OSCC were classified into two groups according to the mutation status of the examined genes (wild-type and mutant group). Among the 17 mutated genes analyzed, four were not significantly related to the TMB and were therefore removed. Samples with mutations in the remaining 13 genes (CASP8, FAT1, TTN, PIK3CA, MUC16, PCLO, OBSCN, XIRP2, FAT3, USH2A, HUWE1, KMT2D, and PLEC) exhibited a significantly greater TMB than those in the wild-type group (Figure 2a). A higher TMB frequently indicates a favorable clinical prognosis for patients with OSCC [23]. Consequently, it was assessed whether these gene mutations associated with higher TMB also influenced the survival outcome of patients with OSCC by performing Kaplan–Meier analysis. FAT1 (p = 0.003), PCLO (p = 0.005), and KMT2D (p = 0.019) mutation was associated with OS, whereas the mutations in the other 10 genes appeared not to be significantly associated with OS (p > 0.05) (Figure S1). PCLO is relatively understudied in OSCC, and thus, it was explored further. As shown in Figure 2b, patients with OSCC with PCLO mutations had a worse OS than noncarriers. Thus, PCLO could be a novel biomarker for evaluating the survival prognosis of patients with OSCC. The results of the univariate regression analysis indicated that several clinicopathological factors, including age [hazard ratio (HR), 1.444; 95% CI, 1.036–2.012; p = 0.030], stage (HR, 2.420; 95% CI, 1.542–3.798; p < 0.001), and PCLO mutation status (HR, 1.613; 95% CI, 1.029–2.526; p = 0.037), were significantly associated with OS (Figure 3a). As shown in Figure 3b, PCLO mutation remained a significant prognostic factor after taking age and stage into account; thus, PCLO mutation was regarded to be an independent prognostic factor for OS in patients with OSCC. Age and stage were also significant prognostic factors.
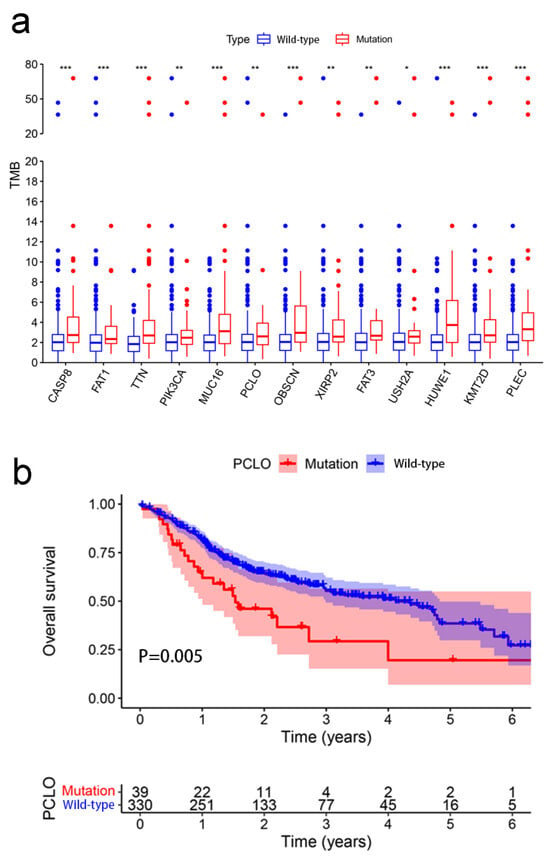
Figure 2.
Association between TMB and mutant genes. (a) Mutations of 13 genes were associated with TMB. The Wilcoxon rank-sum test was used. * p < 0.05; ** p < 0.01; *** p < 0.001. (b) Association between gene mutations of PCLO and overall survival. Kaplan–Meier survival analysis of patients with oral squamous cell carcinoma revealed relationships between PCLO mutations and prognostic outcomes. The p-value is indicated in the plot. TMB, tumor mutational burden.
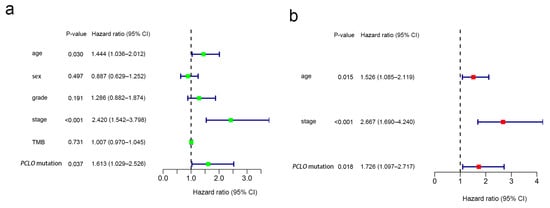
Figure 3.
Cox regression analysis. (a) Univariate and (b) multivariate overall survival analyses of patients with oral squamous cell carcinoma revealed that PCLO mutation was significantly associated with survival status. TMB, tumor mutational burden.
3.3. GSEA for PCLO Mutation
The results of GSEA based on the TMB data showed that the PCLO mutation may be associated with the activation of ‘Alzheimers disease’, ‘Huntingtons disease’, ‘oxidative phosphorylation’, ‘Parkinsons disease’, and the ‘proteasome’ pathway (Figure 4a). GSEA results were also obtained for the PCLO wild-type group, as shown in Figure 4b; pathways such as ‘adherens junction’, ‘aldosterone regulated sodium reabsorption’, ‘axon guidance’, ‘endocytosis’, ‘endometrial cancer’, ‘ERBB signaling pathway’, ‘glioma’, ‘GNRH signaling pathway’, ‘long term depression’, ‘long term potentiation’, ‘type II diabetes mellitus’, and ‘vasopressin regulated water reabsorption’ were significantly enriched.
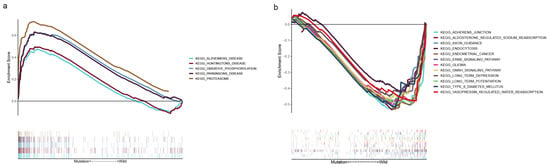
Figure 4.
Gene set enrichment analysis. (a) Multiple gene enrichment plot showing that a series of gene sets were enriched in the PCLO-mutant group. (b) Multiple gene enrichment plot showing that a series of gene sets were enriched in the PCLO wild-type group. KEGG, Kyoto Encyclopedia of Genes and Genomes.
3.4. Relationship Between Immune Cell Infiltration and PCLO Mutation Status
Based on GSEA enrichment analysis, we found that the PCLO mutant group was enriched for immune-related pathways. Therefore, the immune cells of OSCC patients were further analyzed. Using the CIBERSORT algorithm, the proportions of 22 types of immune cells in the tumor microenvironment of patients with OSCC were calculated (Figure 5a). The violin plot showed that the number of M0 macrophages in patients was significantly lower in the PCLO mutation group than in the wild-type group (Figure 5b). The immunocyte correlation matrix showed that M0 macrophages had the strongest negative correlation with CD8 T cells (r = −0.55; Figure 5c), and M0 macrophages were also negatively associated with CD4 memory-activated T cells (r = −0.44) and M1 macrophages (r = −0.41) (Figure 5c).
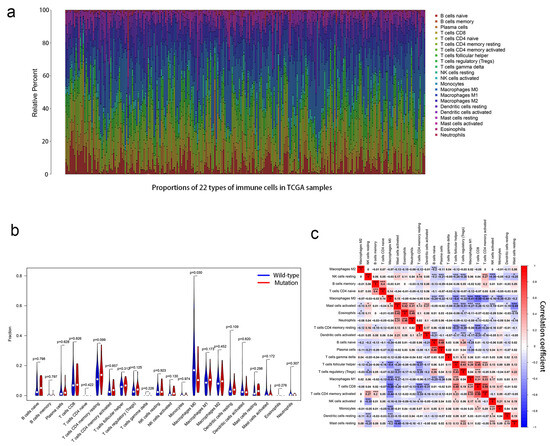
Figure 5.
Association between PCLO mutations and the proportions of 22 types of immune cells. (a) Stacked bar chart showing the proportions of 22 types of immune cells for every sample. (b) Violin plot showing the different proportions of the 22 immune cell types in PCLO-mutant and PCLO wild-type oral squamous cell carcinoma samples. Blue indicates the PCLO wild-type group, and red indicates the PCLO-mutant group. The Wilcoxon rank-sum test was used. (c) Correlations between different immune cell types showing the correlations of 22 immune cell types in OSCC samples from the dataset from TCGA. Red denotes a positive correlation, while blue denotes a negative correlation. Pearson correlation analysis was used. * p < 0.05; *** p < 0.001. NK, natural killer; TCGA, The Cancer Genome Atlas.
3.5. PCLO Expression in OSCC Tissues
Using OSCC tissues from the Peking University School and Hospital of Stomatology (Beijing, China), PCLO expression was detected using IHC, and the corresponding H&E images are shown in Figure 6a. PCLO expression was considerably higher in OSCC tissues with PCLO mutations than in corresponding normal epithelium tissues and OSCC tissues without PCLO mutations (p < 0.01; Figure 6b). Additionally, in patients with OSCC with PCLO mutations, the piccolo protein was mainly distributed in the basal layer of the epithelium (Figure 6b).
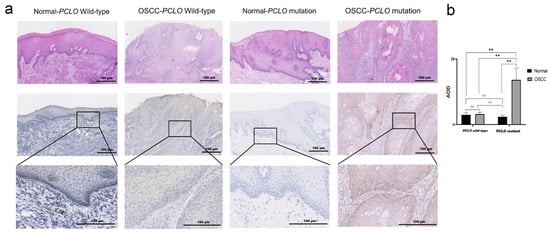
Figure 6.
PCLO expression in tissues. (a) Representative H&E images and corresponding immunohistochemistry images of piccolo expression in adjacent normal epithelium tissues and OSCC tissues from patients with or without PCLO mutation. Scale bar, 100 μm. A total of 5 samples were analyzed in each group, with the normal group being normal tissues from patients in the OSCC group. (b) AOD of piccolo expression in the normal epithelium and OSCC tissues with or without PCLO mutation. Normal group vs. OSCC group, PCLO mutation vs. PCLO wild-type (two-way mixed ANOVA followed by Bonferroni post hoc test). ** p < 0.01; ns, not significant; AOD, average optical density; OSCC, oral squamous cell carcinoma.
4. Discussion
The prognosis of patients with OSCC is still poor, although great strides have been made in screening, diagnosis, and treatment [24,25]. A number of studies have emphasized the efficiency of targeted molecular therapy, which can improve the survival rate of patients with OSCC [25,26]. In recent years, there have been a number of studies on gene mutations in OSCC, such as those in TP53 [27] and Notch1 [28]. The occurrence of OSCC often involves multiple gene mutations [29]. The present study explored potential biomarkers for clinical prognosis by analyzing mutated genes in OSCC.
The present study included 372 patients with OSCC from TCGA and 171 Indian patients with OSCC from the ICGC database, and 17 common frequently mutated genes were identified in the two databases. The present study further investigated the relationship between these 17 mutated genes and the TMB. Based on the mutation status of the 17 overlapping genes, TCGA samples were divided into a wild-type group and a mutated group to further investigate whether these 17 mutated genes were associated with the TMB. The mutation of 13 genes was associated with the TMB. Survival analysis was subsequently performed for these 13 genes. The PCLO mutation group exhibited a worse OS than the wild-type group, while the TMB was significantly greater in patients with mutant PCLO, indicating that PCLO mutation might be an effective factor for determining the TMB. There are few studies related to PCLO in OSCC. Therefore, we continued to explore PCLO. GSEA was subsequently performed on PCLO to investigate whether PCLO might influence immune-related signaling pathways and functions. It revealed that ‘oxidative phosphorylation was activated in the PCLO mutant group, which can have an impact on immune cell function. Immune cell infiltration analysis was performed to investigate whether the PCLO mutation affected immunity, as the mutation caused changes in the TMB, which in turn is associated with immunity [14,15]. The number of M0 macrophages was reduced in PCLO-mutant patients compared with in patients in the PCLO wild-type group. These findings were consistent with a previous study, showing that these immune cells and immune-related pathways serve vital roles in the immune response and tumor microenvironment [30].
PCLO encodes the piccolo protein, which is a short presynaptic cell-matrix protein [10]. It has been reported that piccolo may be involved in regulating membrane transport, exocytosis and endocytosis [31,32,33]. The piccolo protein regulates the release of neurotransmitters and participates in the formation of the presynaptic cytoskeleton matrix [34,35]. Piccolo protein can be found in brain, pancreatic islet, epithelial and cancer cells [36]. PCLO mutations have been found in a number of human tumors, including gastric cancer [37], liver cancer [38], myeloid leukemia [39], diffuse large B-cell lymphoma [34] and colon cancer [40]. It has been reported that silencing PCLO can promote the invasion of liver cancer cell lines [41], and PCLO tends to be mutated in poorly differentiated liver cancer [38]. It has been reported that knockdown of PCLO in esophageal cancer cell lines could inhibit the subcutaneous reproductive capacity of cancer cells in nude mice [10]. Researchers have also injected PCLO-knockdown cells into nude mice, and the lung metastasis rate of cancer cells was substantially lower in PCLO-knockdown mice than in control mice [10]. The study also revealed that piccolo protein expression was upregulated in PCLO-mutant ESCC samples [10]. Two immune subtypes have been reported in HPV+ cervical cancer: Immune-H and immune-L. Compared with the immune-L type, the immune-H type exhibits greater immunity, more stromal content, lower tumor purity, proliferative potential, intra-tumour heterogeneity and stemness, higher tumor mutational load, more neoantigens, lower levels of copy number alterations, lower DNA repair activity, and improved overall survival and prognosis. The mutation rate of PCLO in the immune-L group was greater than that in the immune-H group [35]. Therefore, we hypothesized that these mutations may change the structural stability of PCLO, thus affecting the expression of piccolo. The present study revealed that PCLO might be an immune-related gene since the TMB of the PCLO mutation group was greater than that of the wild-type group. More antigens are exposed under conditions of high TMB, possibly allowing patients in the PCLO mutation group more opportunities to benefit from immunotherapy [42,]. Additionally, the present study showed that piccolo protein expression was upregulated in OSCC with PCLO mutations, which is consistent with the results of ESCC research by Zhang et al. [10]. Based on the IHC results, the piccolo protein was centrally expressed in the stratum basale of the PCLO-mutated OSCC epithelium, which is also called the stratum germinativum and functions as a stem cell with great proliferative capacity [43]. It has been reported that the piccolo protein promotes cell proliferation and other related effects by regulating EGFR signaling [10]. Thus, we hypothesized that there is a similar effect in the stratum basale of the OSCC epithelium, which may shed some light on the poor survival of the PCLO-mutated group. Therefore, the present study provides a novel guide for the pathogenesis and therapeutic targeting of patients with OSCC with PCLO mutations, which may improve the survival rate and prognosis. Meanwhile, based on the upregulation of PCLO protein expression levels in ESCC, perhaps PCLO protein expression levels also have the potential to serve as an OSCC prognostic indicator.
In addition, M0 macrophage levels were significantly lower in the mutant group than in the wild-type group. A previous study has shown that macrophages are crucial in the immune system and that differentiated macrophages are key factors in the immune response [44]. The reason for the decrease in M0 macrophages may be that apoptotic oral cancer cells activate M0 macrophages to differentiate into M2 macrophages, resulting in low infiltration of M0 macrophages in tumor tissue [45,46,47]. Furthermore, according to the immune cell correlation diagram, a decrease in M0 macrophages increased the number of CD8 T cells and CD4 memory-activated cells. Although no significant differences in CD8 T cells and CD4 memory-activated cells were observed, the negative correlation of M0 macrophages with CD4 memory-activated cells and CD8 T cells may provide some clues for the study of the influence of PCLO on OSCC in the future. In the PCLO mutation group, several cancer-related pathways, such as the ‘proteasome’ and ‘oxidative phosphorylation’ pathways, were enriched. The former pathway is related to cell cycle regulation, cell differentiation, signal transduction pathways, and immunity, while the latter pathway affects metabolism in multiple cancer types [48]. Some tumor cells exhibit an enhanced dependence on oxidative phosphorylation [49].
The main limitation of the present study was that the ICGC database lacks corresponding clinical data for patients with OSCC, and thus, the associations of prognosis and immune infiltration with PCLO mutations were explored using clinical data from only TCGA. It was not possible to verify whether PCLO mutations are related to prognosis in Indian patients with OSCC or whether they can cause the same immune response in this population. Although PCLO is a frequently mutated gene in Indian patients with OSCC, its effect may be heterogeneous among different ethnic groups. IHC was conducted to validate the relationship between PCLO mutation and piccolo protein expression, but the sample size was not large enough. Therefore, the relationship between the expression of genes related to PCLO mutation, including genes related to immune infiltration and signaling pathways, and patient prognosis needs to be explored in future experiments. We will also conduct multicenter studies and use in vivo models to overcome these limitations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cimb47060426/s1.
Author Contributions
C.Z. conceived and designed the study; C.Z. edited and reviewed the manuscript; Y.L., L.Z. and H.Z. acquired the data; Y.Z. performed quality control of data and algorithms; Y.L., Y.Z., Y.C. and Z.Z. completed the data analysis and interpretation. Y.L. wrote the main manuscript text; Y.L. and Y.Z. have seen and confirm the authenticity of all the raw data. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was supported by the Beijing Nature Science Foundation (grant no. 7192232).
Institutional Review Board Statement
The present study was approved by the Institutional Review Board of the Peking University Hospital of Stomatology (approval no. PKUSSIRB-2024104193; Beijing, China) with written consent from the ethics committee, and informed consent was obtained from all subjects and/or their legal guardian(s). The guardian was informed and consent was obtained in the event of the patient’s death. The ethics committee agreed with consent being obtained orally. The authors declare that all the permissions or licenses were obtained to collect the data and that the study complies with relevant institutional, national, and international guidelines and legislation for research ethics.
Informed Consent Statement
Informed consent was obtained from all patients involved in the study.
Data Availability Statement
The data generated in the present study are not publicly available due to the protection of patient privacy.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- WHO Classification of Tumours Editorial Board. Head and Neck Tumours, 5th ed.; WHO Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2022; Volume 9, Available online: https://tumourclassification.iarc.who.int/chapters/52/ (accessed on 1 September 2023).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Chen, C.; Zhou, J.; Chen, X.; Cai, K.; Shen, M.; Chen, X.; Jiang, L.; Wang, G. Inhibition of Autophagy Promotes the Anti-Tumor Effect of Metformin in Oral Squamous Cell Carcinoma. Cancers 2022, 14, 4185. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Tseng, Y.-H. The Potential of Phytochemicals in Oral Cancer Prevention and Therapy: A Review of the Evidence. Biomolecules 2020, 10, 1150. [Google Scholar] [CrossRef]
- Kanemaru, A.; Shinriki, S.; Kai, M.; Tsurekawa, K.; Ozeki, K.; Uchino, S.; Suenaga, N.; Yonemaru, K.; Miyake, S.; Masuda, T.; et al. Potential use of EGFR-targeted molecular therapies for tumor suppressor CYLD-negative and poor prognosis oral squamous cell carcinoma with chemoresistance. Cancer Cell Int. 2022, 22, 358. [Google Scholar] [CrossRef]
- Chen, Z.G.; Saba, N.F.; Teng, Y. The diverse functions of FAT1 in cancer progression: Good, bad, or ugly? J. Exp. Clin. Cancer Res. 2022, 41, 248. [Google Scholar] [CrossRef]
- Dey Ghosh, R.; Guha Majumder, S. Circulating Long Non-Coding RNAs Could Be the Potential Prognostic Biomarker for Liquid Biopsy for the Clinical Management of Oral Squamous Cell Carcinoma. Cancers 2022, 14, 5590. [Google Scholar] [CrossRef]
- Igata, R.; Katsuki, A.; Kakeda, S.; Watanabe, K.; Igata, N.; Hori, H.; Konishi , Y.; Atake, K.; Kawasaki, Y.; Korogi, Y.; et al. PCLO rs2522833-mediated gray matter volume reduction in patients with drug-naive, first-episode major depressive disorder. Transl. Psychiatry 2017, 7, e1140. [Google Scholar]
- Zhang, W.; Hong, R.; Xue, L.; Ou, Y.; Liu, X.; Zhao, Z.; Xiao, W.; Dong, D.; Dong, L.; Fu, M.; et al. Piccolo mediates EGFR signaling and acts as a prognostic biomarker in esophageal squamous cell carcinoma. Oncogene 2017, 36, 3890–3902. [Google Scholar] [CrossRef]
- Bernhardt, M.; Behrens, H.-M.; Krüger, S.; Röcken, C. Exploration of the Tumour Biological Significance of PCLO in Gastric Cancer: Results from a Large Central European Cohort. Pathobiol. J. Immunopathol. Mol. Cell. Biol 2024, 91, 187–195. [Google Scholar]
- Gao, J.; Xi, L.; Yu, R.; Xu, H.; Wu, M.; Huang, H. Differential Mutation Detection Capability Through Capture-Based Targeted Sequencing in Plasma Samples in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 596789. [Google Scholar]
- Kim, J.Y.; Kronbichler, A.; Eisenhut, M.; Hong, S.H.; Van, D.V.; Hans, J.; Kang, J.; Shin, J.I.; Gamerith, G. Tumor Mutational Burden and Efficacy of Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 1798. [Google Scholar] [CrossRef]
- Van Rooij, N.; Van Buuren, M.M.; Philips, D.; Velds, A.; Toebes, M.; Heemskerk, B.; Van Dijk, L.J.A.; Behjati, S.; Hilkmann, H.; El Atmioui, D.; et al. Tumor Exome Analysis Reveals Neoantigen-Specific T-Cell Reactivity in an Ipilimumab-Responsive Melanoma. J. Clin. Oncol. 2013, 31, e439–e442. [Google Scholar] [CrossRef]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef]
- Ritterhouse, L.L. Tumor mutational burden. Cancer Cytopathol. 2019, 127, 735–736. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Chen, Y.; Wu, B.; Chen, Y.; Li, Z. Identification of the pyroptosis-related prognostic gene signature and the associated regulation axis in lung adenocarcinoma. Cell Death Discov. 2021, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Du, Y.; Hou, X.; Cheng, W. A prognostic model for oral squamous cell carcinoma using 7 genes related to tumor mutational burden. BMC Oral Health 2022, 22, 152. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Kesmir, C.; van Buuren, M.M. Biomarkers in Cancer Immunotherapy. Cancer Cell 2015, 27, 12–14. [Google Scholar] [CrossRef]
- Chen, B.; Khodadoust, M.S.; Liu, C.L.; Newman, A.M.; Alizadeh, A.A. Profiling Tumor Infiltrating Immune Cells with CIBERSORT. Methods Mol. Biol. 2018, 1711, 243–259. [Google Scholar]
- Zhang, H.; Song, Y.; Du, Z.; Li, X.; Zhang, J.; Chen, S.; Li, T.; Zhan, Q. Exome sequencing identifies new somatic alterations and mutation patterns of tongue squamous cell carcinoma in a Chinese population. J. Pathol. 2020, 251, 353–364. [Google Scholar] [CrossRef]
- Caldwell, N.J.; Li, H.; Bellizzi, A.M.; Luo, J. Altered MANF Expression in Pancreatic Acinar and Ductal Cells in Chronic Alcoholic Pancreatitis: A Cross-Sectional Study. Biomedicines 2023, 11, 434. [Google Scholar] [CrossRef]
- Bi, F.; Chen, Y.; Yang, Q. Significance of tumor mutation burden combined with immune infiltrates in the progression and prognosis of ovarian cancer. Cancer Cell Int. 2020, 20, 373. [Google Scholar] [CrossRef]
- Li, Z.-X.; Zheng, Z.-Q.; Wei, Z.-H.; Zhang, L.-L.; Li, F.; Lin, L.; Liu, R.-Q.; Huang, X.-D.; Lv, J.-W.; Chen, F.-P.; et al. Comprehensive characterization of the alternative splicing landscape in head and neck squamous cell carcinoma reveals novel events associated with tumorigenesis and the immune microenvironment. Theranostics 2019, 9, 7648–7665. [Google Scholar] [CrossRef]
- She, Y.; Kong, X.; Ge, Y.; Yin, P.; Liu, Z.; Chen, J.; Gao, F.; Fang, S. Immune-related gene signature for predicting the prognosis of head and neck squamous cell carcinoma. Cancer Cell Int. 2020, 20, 22. [Google Scholar] [CrossRef]
- Oliva, M.; Spreafico, A.; Taberna, M.; Alemany, L.; Coburn, B.; Mesia, R.; Siu, L.L. Immune biomarkers of response to immune-checkpoint inhibitors in head and neck squamous cell carcinoma. Ann. Oncol. 2019, 30, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Gohara, S.; Yoshida, R.; Kawahara, K.; Sakata, J.; Arita, H.; Nakashima, H.; Kawaguchi, S.; Nagao, Y.; Yamana, K.; Nagata, M.; et al. Re-evaluating the clinical significance of serum p53 antibody levels in patients with oral cancer in Japanese clinical practice. Mol. Clin. Oncol. 2021, 15, 209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, X.; Su, K.; Zheng, Q.; Liu, P.; Xu, Z.; Zhang, Y. A novel mechanism of the lncRNA PTTG3P/miR-142-5p/JAG1 axis modulating tongue cancer cell phenotypes through the Notch1 signaling. Cells Dev. 2022, 169, 203762. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, X.; Xia, K.; Su, T. A novel defined hypoxia-related gene signature to predict the prognosis of oral squamous cell carcinoma. Ann. Transl. Med. 2021, 9, 1565. [Google Scholar] [CrossRef]
- Hwang, W.-T.; Adams, S.F.; Tahirovic, E.; Hagemann, I.S.; Coukos, G. Prognostic Significance of Tumor-infiltrating T-cells in Ovarian Cancer: A Meta-analysis. Gynecol. Oncol. 2012, 124, 192–198. [Google Scholar] [CrossRef]
- Waites, C.L.; Leal-Ortiz, S.A.; Andlauer, T.F.M.; Sigrist, S.J.; Garner, C.C. Piccolo Regulates the Dynamic Assembly of Presynaptic F-Actin. J. Neurosci. 2011, 31, 14250–14263. [Google Scholar] [CrossRef]
- Waites, C.L.; Leal-Ortiz, S.A.; Okerlund, N.; Dalke, H.; Fejtova, A.; Altrock, W.D.; Gundelfinger, E.D.; Garner, C.C. Bassoon and Piccolo maintain synapse integrity by regulating protein ubiquitination and degradation. EMBO J. 2013, 32, 954–969. [Google Scholar] [CrossRef]
- Frattini, M.; Molinari, F.; Epistolio, S. The role of Piccolo in cancer treatment: Relationship with EGFR and related therapies, and a marker for new targeted therapies. J. Thorac. Dis. 2017, 9, 4240–4243. [Google Scholar] [CrossRef]
- Lohr, J.G.; Stojanov, P.; Lawrence, M.S.; Auclair, D.; Chapuy, B.; Sougnez, C.; Cruz-Gordillo, P.; Knoechel, B.; Asmann, Y.W.; Slager, S.L.; et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc. Natl. Acad. Sci. USA 2012, 109, 3879–3884. [Google Scholar] [CrossRef]
- Song, G.; Luo, J.; Zou, S.; Lou Fang Zhang, T.; Zhu, X.; Yang, J.; Wang, X. Molecular classification of human papillomavirus-positive cervical cancers based on immune signature enrichment. Front. Public Health 2022, 10, 979933. [Google Scholar] [CrossRef] [PubMed]
- Garner, C.C.; Ackermann, F. Synaptic logistics: The presynaptic scaffold protein Piccolo a nodal point tuning synaptic vesicle recycling, maintenance and integrity. Mol. Cell. Neurosci. 2023, 124, 103795. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shen, L.; Li, Y.; Lv, J. Integrated characterisation of cancer genes identifies key molecular biomarkers in stomach adenocarcinoma. J. Clin. Pathol. 2020, 73, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qiu, Z.; Wei, L.; Tang, R.; Lian, B.; Zhao, Y.; He, X.; Xie, L. Integrated Analysis of Mutation Data from Various Sources Identifies Key Genes and Signaling Pathways in Hepatocellular Carcinoma. PLoS ONE 2014, 9, e100854. [Google Scholar] [CrossRef]
- Shi, L.; Huang, Y.; Huang, X.; Zhou, W.; Wei, J.; Deng, D.; Lai, Y. Analyzing the key gene expression and prognostics values for acute myeloid leukemia. Transl. Cancer Res. 2020, 9, 7284–7298. [Google Scholar] [CrossRef]
- Yi, T.; Zhang, Y.; Ng, D.M.; Xi, Y.; Ye, M.; Cen, L.; Li, J.; Fan, X.; Li, Y.; Hu, S.; et al. Regulatory Network Analysis of Mutated Genes Based on Multi-Omics Data Reveals the Exclusive Features in Tumor Immune Microenvironment Between Left-Sided and Right-Sided Colon Cancer. Front. Oncol. 2021, 11, 685515. [Google Scholar] [CrossRef]
- Fujimoto, A.; Furuta, M.; Shiraishi, Y.; Gotoh, K.; Kawakami, Y.; Arihiro, K.; Nakamura, T.; Ueno, M.; Ariizumi, S.; Hai Nguyen, H.; et al. Whole-genome mutational landscape of liver cancers displaying biliary phenotype reveals hepatitis impact and molecular diversity. Nat. Commun. 2015, 6, 6120. [Google Scholar] [CrossRef]
- McGranahan, N.; Furness, A.J.S.; Rosenthal, R.; Ramskov, S.; Lyngaa, R.; Saini, S.K.; Jamal-Hanjani, M.; Wilson, G.A.; Birkbak, N.J.; Hiley, C.T.; et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016, 351, 1463–1469. [Google Scholar] [CrossRef]
- Curry, J.M.; Tuluc, M.; Whitaker-Menezes, D.; Ames, J.A.; Anantharaman, A.; Butera, A.; Leiby, B.; Cognetti, D.; Sotgia, F.; Lisanti, M.P.; et al. Cancer metabolism, stemness and tumor recurrence: MCT1 and MCT4 are functional biomarkers of metabolic symbiosis in head and neck cancer. Cell Cycle 2013, 12, 1371–1384. [Google Scholar] [CrossRef]
- Ivanova, E.A.; Orekhov, A.N. Monocyte Activation in Immunopathology: Cellular Test for Development of Diagnostics and Therapy. J. Immunol. Res. 2016, 2016, 4789279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, H.; Mao, Y.; Wang, X.; Zhang, X.H.; Yu, X.; Tian, J.; Lei, Z.; Li, C.; Han, Q.; et al. Apoptotic SKOV3 cells stimulate M0 macrophages to differentiate into M2 macrophages and promote the proliferation and migration of ovarian cancer cells by activating the ERK signaling pathway. Int. J. Mol. Med. 2020, 45, 10–22. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Liu, J.; Xu, M.; Liang, J.; Wang, Y.; Wu, Z.; Xing, Y.; Diao, F. CSMD3 is Associated with Tumor Mutation Burden and Immune Infiltration in Ovarian Cancer Patients. Int. J. Gen. Med. 2021, 14, 7647–7657. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Wang, Y.; Shao, Y.; Xiao, T. Gene Instability-Related lncRNA Prognostic Model of Melanoma Patients via Machine Learning Strategy. J. Oncol. 2021, 2021, 582920. [Google Scholar] [CrossRef]
- Orzechowska-Licari, E.J.; LaComb, J.F.; Mojumdar, A.; Bialkowska, A.B. SP and KLF Transcription Factors in Cancer Metabolism. Int. J. Mol. Sci. 2022, 23, 9956. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).