Antioxidant Naturally Occurring Pleiotropically Acting Bioactive Compounds, as Polymeric Nanotherapeutics Against Autoimmune Diseases Progression
Abstract
1. Introduction
2. Phenolic Antioxidants
3. Folic Acid NPs
4. Carotenoids
5. Miscellaneous
6. Limitations and Concerns
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Pisetsky, D.S. Pathogenesis of autoimmune disease. Nat. Rev. Nephrol. 2023, 19, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Rui, K.; Peng, N.; Xiao, F.; Lu, L. New insights into the functions of MDSCs in autoimmune pathogenesis. Cell Mol. Immunol. 2023, 20, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Mackensen, A.; Mougialakos, D. CAR T-cell therapy in autoimmune diseases. Lancet 2023, 404, 2023–2044. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Kim, W.-U. Targeted Immunotherapy for Autoimmune Disease. Immune Netw. 2022, 22, e9. [Google Scholar] [CrossRef]
- Wojcik, P.; Gegotek, A.; Zarkovic, N.; Skrzydlewska, E. Oxidative Stress and Lipid Mediators Modulate Immune Cell Functions in Autoimmune Diseases. Int. J. Mol. Sci. 2021, 22, 723. [Google Scholar] [CrossRef]
- Papagiouvannis, G.; Theodosis-Nobelos, P.; Kourounakis, P.N.; Rekka, E.A. Multi-target directed compounds with antioxidant and/or anti-inflammatory properties as potent agents for Alzheimer’s disease. Med. Chem. 2021, 17, 1086–1103. [Google Scholar] [CrossRef]
- Papagiouvannis, G.; Theodosis-Nobelos, P.; Rekka, E.A. A Review on Therapeutic Strategies against Parkinson’s Disease: Current Trends and Future Perspectives. Min. Rev. Med. Chem. 2025, 25, 96–111. [Google Scholar] [CrossRef]
- Mannucci, C.; Casciaro, M.; Sorbara, E.E.; Calapai, F.; DiSalvo, E.; Pioggia, G.; Navarra, M.; Calapai, G.; Gangemi, S. Nutraceuticals against Oxidative Stress in Autoimmune Disorders. Antioxidatns 2021, 10, 261. [Google Scholar] [CrossRef]
- Feczko, T. Polymeric nanotherapeutics acting at special regions of body. J. Drug Deliv. Sci. Technol. 2021, 64, 102597. [Google Scholar] [CrossRef]
- Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier Drug delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence. Pharmaceutics 2022, 14, 883. [Google Scholar] [CrossRef]
- De, R.; Mahata, M.K.; Kim, K.-T. Structure-Based Varieties of Polymeric Nanocarriers and Influences of Their Physicochemical Properties on Drug Delivery Profiles. Adv. Sci. 2022, 9, 2105373. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio de Castro, K.; Martins Costa, J.; Nogueira Campos, M.G. Drug-loaded polymeric nanoparticles: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 71, 1–13. [Google Scholar] [CrossRef]
- Su, T.; Feng, X.; Yang, J.; Xu, W.; Liu, T.; Zhang, M.; Ding, J.; Chen, X. Polymer nanotherapeutics to correct autoimmunity. J. Control. Release 2022, 343, 152–174. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Sun, K.; Liu, Y.; Yin, X.; Zhu, H.; Yu, H.; Zhao, W. Resveratrol-loaded macrophage exosomes alleviate multiple sclerosis through targeting microglia. J. Control. Release 2023, 353, 675–684. [Google Scholar] [CrossRef]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M.; Pirzadeh, M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019, 17, 4449–4460. [Google Scholar] [CrossRef]
- Karaka, E.; Yarim, M. Naringenin stimulates aromatase expression and alleviates the clinical and histopathological findings of experimental autoimmune encephalomyelitis in C57bl6 mice. Histochem. Cell. Biol. 2023, 160, 477–490. [Google Scholar] [CrossRef]
- Nagasaki, Y. Design and application of redox polymers for nanomedicine. Polym. J. 2018, 50, 821–826. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, X.; Wu, Y.; Chen, X.; Feng, L.; Xie, N.; Shen, G. Nanotechnology’s frontier in combatting infectious and inflammatory diseases: Prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 34. [Google Scholar] [CrossRef]
- Patel, U.; Rajasingh, S.; Samanta, S.; Cao, T.; Dawn, B.; Rajasingh, J. Macrophage polarization in response to epigenetic modifiers during infection and inflammation. Drug Discov. Today 2017, 22, 186–193. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Singh, C.K.; George, J.; Ahmad, N. Resveratrol-based combinatorial strategies for cancer management. Ann. N. Y. Acad. Sci. 2013, 1290, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Jeong, H.; Lee, M.N.; Koh, A.; Kwon, O.; Yang, Y.R.; Noh, J.; Suh, P.G.; Park, H.; Ryu, S.H. Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition. Sci. Rep. 2016, 6, 21772. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Kent, D.; Botello-Smith, W.M.; Nur, F.; Nur, S.; Alsamarah, A.; Chatterjee, P.; Lambros, M.; Luo, Y. Molecular Mechanism of Resveratrol’s Lipid Membrane Protection. Sci. Rep. 2018, 8, 1587. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, X.; Ma, L.; Lin, A.; Gong, Y.; Yuan, G.; Liu, J. A core-shell structure QRu-PLGA-RES-DS NP nanocomposite with photothermal response-induced M2 macrophage polarization for rheumatoid arthritis therapy. Nanoscale 2019, 11, 18209–18223. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Demetzos, C. Promising Nanotechnology Approaches in Treatment of Autoimmune Diseases of Central Nervous System. Brain Sci. 2020, 10, 338. [Google Scholar] [CrossRef]
- Alizadeh, A.M.; Sadeghizadeh, M.; Najafi, F.; Ardestani, S.K.; Erfani-Moghadam, V.; Khaniki, M.; Rezaei, A.; Zamani, M.; Khodayari, S.; Khodayari, H.; et al. Encapsulation of curcumin in diblock copolymer micelles for cancer therapy. BioMed Res. Int. 2015, 2015, 824746. [Google Scholar] [CrossRef]
- Mohajeri, M.; Sadeghizadeh, M.; Najafi, F.; Javan, M. Polymerized nano-curcumin attenuates neurological symptoms in EAE model of multiple sclerosis through down regulation of inflammatory and oxidative processes and enhancing neuroprotection and myelin repair. Neuropharmacology 2015, 99, 156–167. [Google Scholar] [CrossRef]
- Dolati, S.; Ahmadi, M.; Aghebti-Maleki, L.; Nikmaram, A.; Marofi, F.; Rikhtegar, R.; Ayromlou, H.; Yousefi, M. Nanocurcumin is a potential novel therapy for multiple sclerosis by influencing inflammatory mediators. Pharmacol. Rep. 2018, 70, 1158–1167. [Google Scholar] [CrossRef]
- Huang, Y.; Canup, B.S.B.; Gou, S.; Chen, N.; Dai, F.; Xiao, B.; Li, C. Oral nanotherapeutics with enhanced mucus penetration and ROS-responsive drug release capacities for delivery of curcumin to colitis tissues. J. Mater. Chem. B 2021, 9, 1604–1615. [Google Scholar] [CrossRef]
- Qiao, H.; Fang, D.; Chen, J.; Sun, Y.; Kang, C.; Di, L.; Li, J.; Chen, Z.; Chen, J.; Gao, Y. Orally delivered polycurcumin responsive to bacterial reduction for targeted therapy of inflammatory bowel disease. Drug Deliv. 2017, 24, 233–242. [Google Scholar] [CrossRef]
- Mao, K.L.; Fan, Z.L.; Yuan, J.D.; Chen, P.P.; Yang, J.J.; Xu, J.; ZhuGe, D.L.; Jin, B.H.; Zhu, Q.Y.; Shen, B.X.; et al. Skin-penetrating polymeric nanoparticles incorporated in silk fibroin hydrogel for topical delivery of curcumin to improve its therapeutic effect on psoriasis mouse model. Colloids Surf. B Biointerfaces 2017, 160, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Sim, B.R.; Khang, G. Nature-Derived Aloe Vera Gel Blended Silk Fibroin Film Scaffolds for Cornea Endothelial Cell Regeneration and Transplantation. ACS Appl. Mater. Interfaces 2016, 8, 15160–15168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, Q.; Li, Y.; He, Z.; Li, Z.; Guo, T.; Wu, Z.; Feng, N. CD44 Assists the Topical Anti-Psoriatic Efficacy of Curcumin-Loaded Hyaluronan-Modified Ethosomes: A New Strategy for Clustering Drug in Inflammatory Skin. Theranostics 2019, 9, 48–64. [Google Scholar] [CrossRef]
- Bilia, A.R.; Bergonzi, M.C.; Isacchi, B.; Antiga, E.; Caproni, M. Curcumin nanoparticles potentiate therapeutic effectiveness of acitrein in moderate-to-severe psoriasis patients and control serum cholesterol levels. J. Pharm. Pharmacol. 2018, 70, 919–928. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Rekka, E.A. The Multiple Sclerosis Modulatory Potential of Natural Multi-Targeting Antioxidants. Molecules 2022, 27, 8402. [Google Scholar] [CrossRef]
- Gokhale, J.P.; Mahajan, H.S.; Surana, S.J. Quercetin loaded nanoemulsion-based gel for rheumatoid arthritis: In vivo and in vitro studies. Biomed. Pharmacother. 2019, 112, 108622. [Google Scholar] [CrossRef]
- Jeyadevi, R.; Sivasudha, T.; Rameshkumar, A.; Ananth, D.A.; Aseervatham, G.S.; Kumaresan, K.; Kumar, L.D.; Jagadeeswari, S.; Renganathan, R. Enhancement of anti arthritic effect of quercetin using thioglycolic acid-capped cadmium telluride quantum dots as nanocarrier in adjuvant induced arthritic Wistar rats. Colloids Surf. B Biointerfaces 2013, 112, 255–263. [Google Scholar] [CrossRef]
- Souza, K.S.; Moreira, L.S.; Silva, B.T.; Oliveira, B.P.M.; Carvalho, A.S.; Silva, P.S.; Verri, W.A., Jr.; Sá-Nakanishi, A.B.; Bracht, L.; Zanoni, J.N.; et al. Low dose of quercetin-loaded pectin/casein microparticles reduces the oxidative stress in arthritic rats. Life Sci. 2021, 284, 119910. [Google Scholar] [CrossRef]
- Halake, K.; Lee, J. Functional hyaluronic acid conjugates based on natural polyphenols exhibit antioxidant, adhesive, gelation, and self-healing properties. J. Ind. Eng. Chem. 2017, 54, 44–51. [Google Scholar] [CrossRef]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin Nano-Delivery Systems and Their Therapeutic Applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef]
- Munir, A.; Faqir, M.; Yumna, Z.; Muhammad, A.A.; Mazhar, I.; Mubashar, R.; Muhammad, U.M.; Bushra, A.; Webster, T.J.; Ali, S.; et al. Synthesis of naringenin loaded lipid based nanocarriers and their in-vivo therapeutic potential in a rheumatoid arthritis model. J. Drug Deliv. Sci. Technol. 2021, 66, 102854. [Google Scholar] [CrossRef]
- Mohanty, S.; Konkimalla, V.B.; Pal, A.; Sharma, T.; Si, S.C. Naringin as Sustained Delivery Nanoparticles Ameliorates the Anti-inflammatory Activity in a Freund’s Complete Adjuvant-Induced Arthritis Model. ACS Omega 2021, 6, 28630–28641. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.; Lee, J.; Yoon, S.; Kim, W.J. Tannic acid-based nanogel as an efficient anti-inflammatory agent. Biomater. Sci. 2020, 8, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Dosch, M.; Zindel, J.; Jebbawi, F.; Melin, N.; Sanchez-Taltavull, D.; Stroka, D.; Candinas, D.; Beldi, G. Connexin-43-dependent ATP release mediates macrophage activation during sepsis. Elife 2019, 8, e42670. [Google Scholar] [CrossRef] [PubMed]
- Barra, J.M.; Kozlovskaya, V.; Kharlampieva, E.; Tse, H.M. Localized Immunosuppression With Tannic Acid Encapsulation Delays Islet Allograft and Autoimmune-Mediated Rejection. Diabetes 2020, 69, 1948–1960. [Google Scholar] [CrossRef]
- Tedeschi, E.; Menegazzi, M.; Yao, Y.; Suzuki, H.; Förstermann, U.; Kleinert, H. Green tea inhibits human inducible nitric-oxide synthase expression by down-regulating signal transducer and activator of transcription-1alpha activation. Mol. Pharmacol. 2004, 65, 111–120. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiao, L.; Yu, C.; Jin, P.; Qin, D.; Xu, Y.; Yin, J.; Liu, Z.; Du, Q. Enhanced Antiarthritic Efficacy by Nanoparticles of (-)-Epigallocatechin Gallate-Glucosamine-Casein. J. Agric. Food Chem. 2019, 67, 6476–6486. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Siddiqui, I.A.; Adhami, V.M.; Esnault, S.; Bharali, D.J.; Babatunde, A.S.; Adame, S.; Massey, R.J.; Wood, G.S.; Longley, B.J.; et al. Chitosan-based nanoformulated (-)-epigallocatechin-3-gallate (EGCG) modulates human keratinocyte-induced responses and alleviates imiquimod-induced murine psoriasiform dermatitis. Int. J. Nanomed. 2018, 13, 4189–4206. [Google Scholar] [CrossRef]
- Khan, N.; Bharali, D.J.; Adhami, V.M.; Siddiqui, I.A.; Cui, H.; Shabana, S.M.; Mousa, S.A.; Mukhtar, H. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 2014, 35, 415–423. [Google Scholar] [CrossRef]
- Jabbari, N.; Eftekhari, Z.; Roodbari, N.H.; Parivar, K. Evaluation of Encapsulated Eugenol by Chitosan Nanoparticles on the aggressive model of rheumatoid arthritis. Int. Immunopharmacol. 2020, 85, 106554. [Google Scholar] [CrossRef]
- Chung, C.H.; Jung, W.; Keum, H.; Kim, T.W.; Jon, S. Nanoparticles Derived from the Natural Antioxidant Rosmarinic Acid Ameliorate Acute Inflammatory Bowel Disease. ACS Nano 2020, 14, 6887–6896. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Ghanavati, M.; Ashtary-Larky, D.; Bagheri, R.; Rezaei, K.M.; Nazarian, B.; Nordvall, M.; Wong, A.; Dutheil, F.; Suzuki, K.; et al. Effects of Folic Acid Supplementation on Oxidative Stress Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antioxidants 2021, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.; Eljazzar, S.; Ganji, V. Intended and Unintended Benefits of Folic Acid Fortification—A Narrative Review. Foods 2023, 12, 1612. [Google Scholar] [CrossRef] [PubMed]
- Sijilmassi, O. Folic acid deficiency and vision: A review. Graefes Arch. Clin. Exp. Ophalmol. 2019, 257, 1573–1580. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Rekka, E.A. The Antioxidant Potential of Vitamins and Their Implication in Metabolic Abnormalities. Nutrients 2024, 16, 2740. [Google Scholar] [CrossRef]
- Nogueira, E.; Gomes, A.C.; Preto, A.; Cavaco-Paulo, A. Folate-targeted nanoparticles for rheumatoid arthritis therapy. Nanomedicine 2016, 12, 1113–1126. [Google Scholar] [CrossRef]
- Srivastava, S.; Singh, D.; Singh, M.R. Folate-Conjugated Superoxide Dismutase Adsorbed Over Antioxidant Mimicking Nanomatrix Frameworks for Treatment of Rheumatoid Arthritis. J. Pharm. Sci. 2018, 107, 1530–1539. [Google Scholar] [CrossRef]
- Hu, P.; Tirelli, N. Scavenging ROS: Superoxide dismutase/catalase mimetics by the use of an oxidation-sensitive nanocarrier/enzyme conjugate. Bioconjugate Chem. 2012, 14, 438–449. [Google Scholar] [CrossRef]
- Periyathambi, P.; Sastry, T.P.; Anandasadagopan, S.K.; Manickavasagam, K. Macrophages mediated diagnosis of rheumatoid arthritis using fibrin based magnetic nanoparticles as MRI contrast agents. Biochim. Biophys. Acta Gen. Subj. 2017, 1861 1 Pt A, 2992–3001. [Google Scholar] [CrossRef]
- Schmidt, G.P.; Reiser, M.F.; Baur-Melnyk, A. Whole-body MRI for the staging and follow-up of patients with metastasis. Eur. J. Radiol. 2009, 70, 393–400. [Google Scholar] [CrossRef]
- Pan, H.; Chen, L. Paramagnetic block copolymers: An effective tool for targeted radiography of rheumatoid arthritis. Mater. Express 2021, 11, 1177–1183. [Google Scholar] [CrossRef]
- Wang, X.D. Lycopene metabolism and its biological significance. Am. J. Clin. Nutr. 2012, 96, 1214S–1222S. [Google Scholar] [CrossRef] [PubMed]
- Moia, V.M.; Leal Portilho, F.; Almeida Pádua, T.; Barbosa Corrêa, L.; Ricci-Junior, E.; Cruz Rosas, E.; Magalhaes Rebelo Alencar, L.; Savio Mendes Sinfronio, F.; Sampson, A.; Hussain Iram, S.; et al. Lycopene used as Anti-inflammatory Nanodrug for the Treatment of Rheumathoid Arthritis: Animal assay, Pharmacokinetics, ABC Transporter and Tissue Deposition. Colloids Surf. B Biointerfaces 2020, 188, 110814. [Google Scholar] [CrossRef] [PubMed]
- Malgarim Cordenonsi, L.; Faccendini, A.; Catanzaro, M.; Bonferoni, M.C.; Rossi, S.; Malavasi, L.; Platcheck Raffin, R.; Scherman Schapoval, E.E.; Lanni, C.; Sandri, G.; et al. The role of chitosan as coating material for nanostructured lipid carriers for skin delivery of fucoxanthin. Int. J. Pharm. 2019, 567, 118487. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, S.; Chattopadhyay, S.K. The potential health benefit of polyisoprenylated benzophenones from Garcinia and related genera: Ethnobotanical and therapeutic importance. Fitoterapia 2013, 89, 86–125. [Google Scholar] [CrossRef]
- Miyashita, K.; Nishikawa, S.; Beppu, F.; Tsukui, T.; Abe, M.; Hosokawa, M. The allenic carotenoid fucoxanthin, a novel marine nutraceutical from brown seaweeds. J. Sci. Food Agric. 2011, 91, 1166–1174. [Google Scholar] [CrossRef]
- Dacaranhe, C.D.; Terao, J. A unique antioxidant activity of phosphatidylserine on iron-induced lipid peroxidation of phospholipid bilayers. Lipids 2001, 36, 1105–1110. [Google Scholar] [CrossRef]
- Ma, H.M.; Wu, Z.; Nakanishi, H. Phosphatidylserine-containing liposomes suppress inflammatory bone loss by ameliorating the cytokine imbalance provoked by infiltrated macrophages. Lab. Investig. 2011, 91, 921–931. [Google Scholar] [CrossRef]
- Roberts, R.A.; Eitas, T.K.; Byrne, J.D.; Johnson, B.M.; Short, P.J.; McKinnon, K.P.; Reisdorf, S.; Luft, J.C.; DeSimone, J.M.; Ting, J.P. Towards programming immune tolerance through geometric manipulation of phosphatidylserine. Biomaterials 2015, 72, 1–10. [Google Scholar] [CrossRef]
- Sheng, Z.; Ge, S.; Gao, M.; Jian, R.; Chen, X.; Xu, X.; Li, D.; Zhang, K.; Chen, W.H. Synthesis and Biological Activity of Embelin and its Derivatives: An Overview. Mini Rev. Med. Chem. 2020, 20, 396–407. [Google Scholar] [CrossRef]
- Cui, P.; Qu, F.; Sreeharsha, N.; Sharma, S.; Mishra, A.; Gubbiyappa, S.K. Antiarthritic effect of chitosan nanoparticle loaded with embelin against adjuvant-induced arthritis in Wistar rats. IUBMB Life 2020, 72, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, T.W.; Saleh, M.; Higginson, D.S.; Paul, B.D.; Juluri, K.R.; Snyder, S.H. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc. Natl. Acad. Sci. USA 2009, 106, 5171–5176. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, H.; Kang, S.; Lee, J.; Park, J.; Jon, S. Bilirubin Nanoparticles as a Nanomedicine for Anti-inflammation Therapy. Angew. Chem. Int. Ed. Engl. 2016, 55, 7460–7463. [Google Scholar] [CrossRef]
- Lee, Y.; Sugihara, K.; Gillilland, M.G., 3rd; Jon, S.; Kamada, N.; Moon, J.J. Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat. Mater. 2020, 19, 118–126. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, Y.; Jon, S.; Lee, D.Y. PEGylated bilirubin nanoparticle as an anti-oxidative and anti-inflammatory demulcent in pancreatic islet xenotransplantation. Biomaterials 2017, 133, 242–252. [Google Scholar] [CrossRef]
- Zhu, H.Q.; Gao, Y.; Guo, H.R.; Kong, Q.Z.; Ma, Y.; Wang, J.Z.; Pan, S.H.; Jiang, H.C.; Dai, W.J. Pretreatment with bilirubin protects islet against oxidative injury during isolation and purification. Transplant. Proc. 2011, 43, 1810–1814. [Google Scholar] [CrossRef]
- Wang, Q.; Li, W.; Hu, H.; Lu, X.; Qin, S. Monomeric compounds from traditional Chinese medicine: New hopes for drug discovery in pulmonary fibrosis. Biomed. Pharmacother. 2023, 159, 114226. [Google Scholar] [CrossRef]
- Ren, S.; Liu, H.; Wang, X.; Bi, J.; Lu, S.; Zhu, C.; Li, H.; Kong, W.; Chen, R.; Chen, Z. Acupoint nanocomposite hydrogel for simulation of acupuncture and targeted delivery of triptolide against rheumatoid arthritis. J. Nanobiotechnol. 2021, 19, 409. [Google Scholar] [CrossRef]
- Prasad, S.; Lillicrap, D.; Labelle, A.; Knappe, S.; Keller, T.; Burnett, E.; Powell, S.; Johnson, K.W. Efficacy and safety of a new-class hemostatic drug candidate, AV513, in dogs with hemophilia A. Blood 2008, 111, 672–679. [Google Scholar] [CrossRef]
- Fitton, J.H. Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Ito, M.; Kodama, M.; Tomita, M.; Kimura, S.; Hoyano, M.; Mitsuma, W.; Hirono, S.; Hanawa, H.; Aizawa, Y. Sulfated polysaccharide fucoidan ameliorates experimental autoimmune myocarditis in rats. J. Cardiovasc. Pharmacol. Ther. 2011, 16, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Tissot, B.; Daniel, R. Biological properties of sulfated fucans: The potent inhibiting activity of algal fucoidan against the human compliment system. Glycobiology 2003, 13, 29G–30G. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef]
- Cui, Y.Q.; Zhang, L.J.; Zhang, T.; Luo, D.Z.; Jia, Y.J.; Guo, Z.X.; Zhang, Q.B.; Wang, X.; Wang, X.M. Inhibitory effect of fucoidan on nitric oxide production in lipopolysaccharide-activated primary microglia. Clin. Exp. Pharmacol. Physiol. 2010, 37, 422–428. [Google Scholar] [CrossRef]
- Zahariev, N.; Katsarov, P.; Lukova, P.; Pilicheva, B. Novel Fucoidan Pharmaceutical Formulations and Their Potential Application in Oncology—A Review. Polymers 2023, 15, 3242. [Google Scholar] [CrossRef]
- Baek, Y.; Jeong, E.W.; Lee, H.G. Encapsulation of resveratrol within size-controlled nanoliposomes: Impact on solubility, stability, cellular permeability, and oral bioavailability. Colloids Surf. B Biointerfaces 2023, 224, 113205. [Google Scholar] [CrossRef]
- Chung, J.H.; Lee, J.-S.; Lee, H.G. Resveratrol-loaded chitosan–γ-poly (glutamic acid) nanoparticles: Optimization, solubility, UV stability, and cellular antioxidant activity. Colloids Surf. B Biointerfaces 2020, 186, 110702. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Sandhir, R.; Yadav, A.; Sunkaria, A.; Singhail, N. Nano-antioxidants: An emerging strategy for intervention against neurodegenerative conditions. Neurochem. Int. 2015, 89, 209–226. [Google Scholar] [CrossRef]
- Tanwal, T.; Saifullah, S.; Rehman, J.; Kawish, M.; Razzak, A.; Maharjan, R.; Imran, M.; Ali, I.; Roome, T.; Simjee, S.U.; et al. Design of absorption enhancer containing self-nanoemulsifying drug delivery system (SNEDDS) for curcumin improved anti-cancer activity and oral bioavailability. J. Mol. Liq. 2021, 324, 114774. [Google Scholar]
- Chen, Y.; Lu, Y.; Lee, R.J.; Xiang, G. Nano Encapsulated Curcumin: And Its Potential for Biomedical Applications. Int. J. Nanomed. 2020, 15, 3099–3120. [Google Scholar] [CrossRef]
- Lopresti, A.L. The Problem of Curcumin and Its Bioavailability: Could Its Gastrointestinal Influence Contribute to Its Overall Health-Enhancing Effects? Adv. Nutr. 2018, 9, 41–50. [Google Scholar] [CrossRef]
- Fu, Y.-S.; Chen, T.-H.; Weng, L.; Huang, L.; Lai, D.; Weng, C.-F. Pharmacological properties and underlying mechanisms of curcumin and prospects in medicinal potential. Biomed. Pharmacother. 2021, 141, 111888. [Google Scholar] [CrossRef]
- Gera, M.; Sharma, N.; Ghosh, M.; Huynh, D.L.; Lee, S.J.; Min, T.; Kwon, T.; Jeong, D.K. Nanoformulations of curcumin: An emerging paradigm for improved remedial application. Oncotarget 2017, 8, 66680–66698. [Google Scholar] [CrossRef]
- Rawas-Qalaji, M.; Jagal, J.; Sadik, S.; Said, Z.; Ahmed, I.S.; Haider, M.; Hussain, Z.; Alhalaweh, A. Assessment of enhancing curcumin’s solubility versus uptake on its anti-cancer efficacy. Colloids Surf. B Biointerfaces 2024, 242, 114090. [Google Scholar] [CrossRef]
- Bertoncini-Silva, C.; Vlad, A.; Ricciarelli, R.; Fassini, P.G.; Miguel Suen, V.M.; Zingg, J.-M. Enhancing the Bioavailability and Bioactivity of Curcumin for Disease Prevention and Treatment. Antioxidants 2024, 13, 331. [Google Scholar] [CrossRef]
- Hasnat, H.; Shompa, S.A.; Islam, M.; Alam, S.; Richi, F.T.; Emon, N.U.; Ashrafi, S.; Ahmed, N.U.; Chowdhury, N.R.; Fatema, N.; et al. Flavonoids: A treasure house of prospective pharmacological potentials. Heliyon 2024, 10, e27533. [Google Scholar] [CrossRef]
- Pangeni, R.; Kang, S.-W.; Oak, M.; Park, E.Y.; Park, J.W. Oral delivery of quercetin in oil-in-water nanoemulsion: In vitro characterization and in vivo anti-obesity efficacy in mice. J. Funct. Foods 2017, 38, 571–581. [Google Scholar] [CrossRef]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of quercetin: Problems and promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef]
- De Gaetano, F.; Caridi, F.; Totaro, N.; Celesti, C.; Venuti, V.; Ginestra, G.; Nostro, A.; Tommasini, S.; Ventura, C.A.; Stancanelli, R. Naringenin-Loaded Solid Lipid Nanoparticles: Physical-Chemical Characterization and In Vitro Antibacterial Activity. Pharmaceuticals 2025, 18, 232. [Google Scholar] [CrossRef] [PubMed]
- Duda-Madej, A.; Stecko, J.; Sobieraj, J.; Szymańska, N.; Kozłowska, J. Naringenin and Its Derivatives-Health-Promoting Phytobiotic against Resistant Bacteria and Fungi in Humans. Antibiotics 2022, 11, 1628. [Google Scholar] [CrossRef] [PubMed]
- Peşint, G.B.; Cemek, K.; Zenger, O.; Anar, B.C.; Başeğmez, H.İ.O. Tannic acid purification from pomegranate peel via tannic acid imprinted particle-embedded cryogel column. J. Chromatogr. B 2023, 1229, 123890. [Google Scholar] [CrossRef]
- Ghasemian, M.; Kazeminava, F.; Naseri, A.; Mohebzadeh, S.; Abbaszadeh, M.; Kafil, H.S.; Ahmadian, Z. Recent progress in tannic acid based approaches as a natural polyphenolic biomaterial for cancer therapy: A review. Biomed. Pharmacother. 2023, 166, 115328. [Google Scholar] [CrossRef]
- Jing, W.; Xiaolan, C.; Yu, C.; Feng, Q.; Haifeng, Y. Pharmacological effects and mechanisms of tannic acid. Biomed. Pharmacother. 2022, 154, 113561. [Google Scholar] [CrossRef]
- Pathak, N.M.; Millar, P.J.B.; Pathak, V.; Flatt, P.R.; Gault, V.A. Beneficial metabolic effects of dietary epigallocatechin gallate alone and in combination with exendin-4 in high fat diabetic mice. Mol. Cell. Endocrinol. 2018, 460, 200–208. [Google Scholar] [CrossRef]

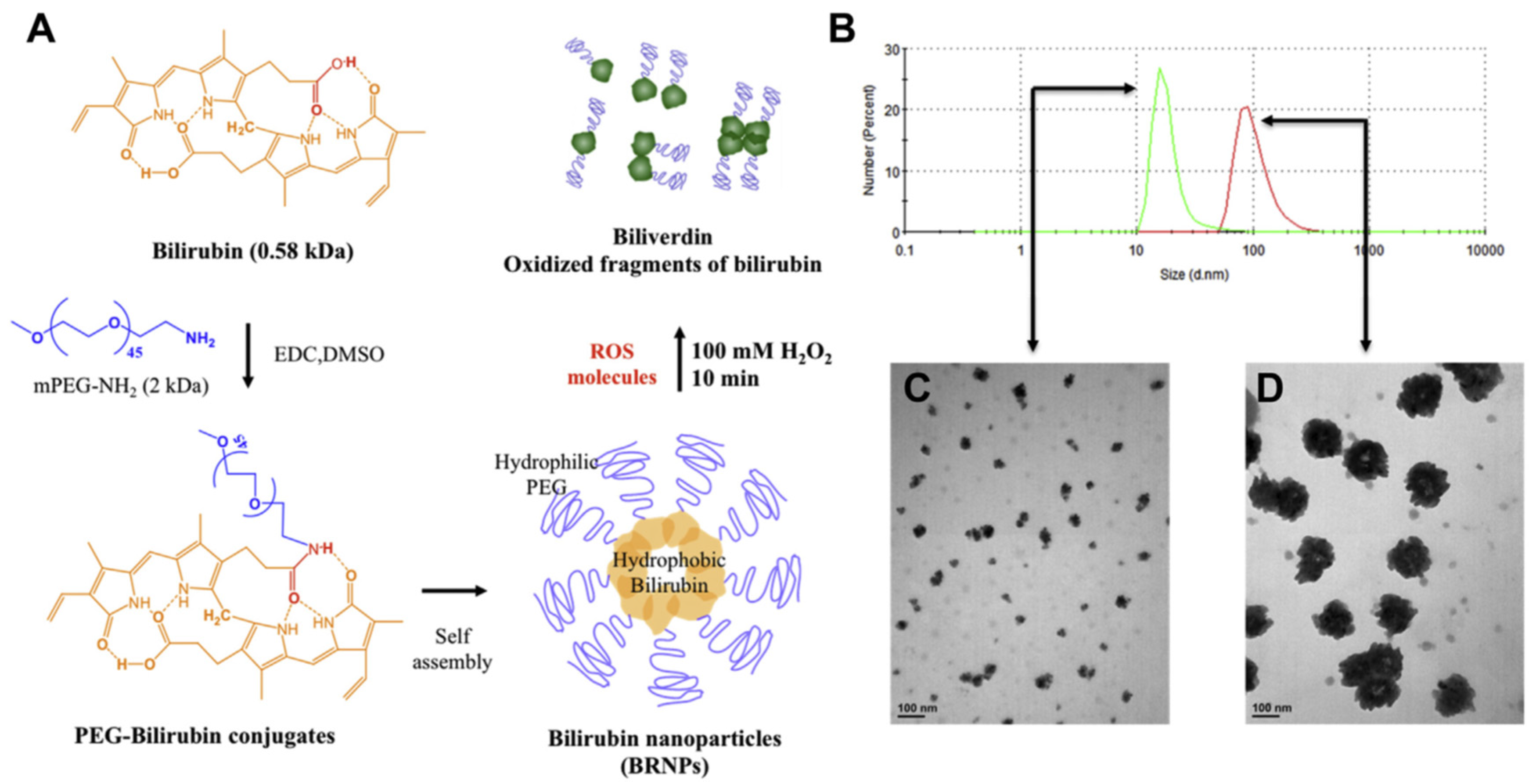
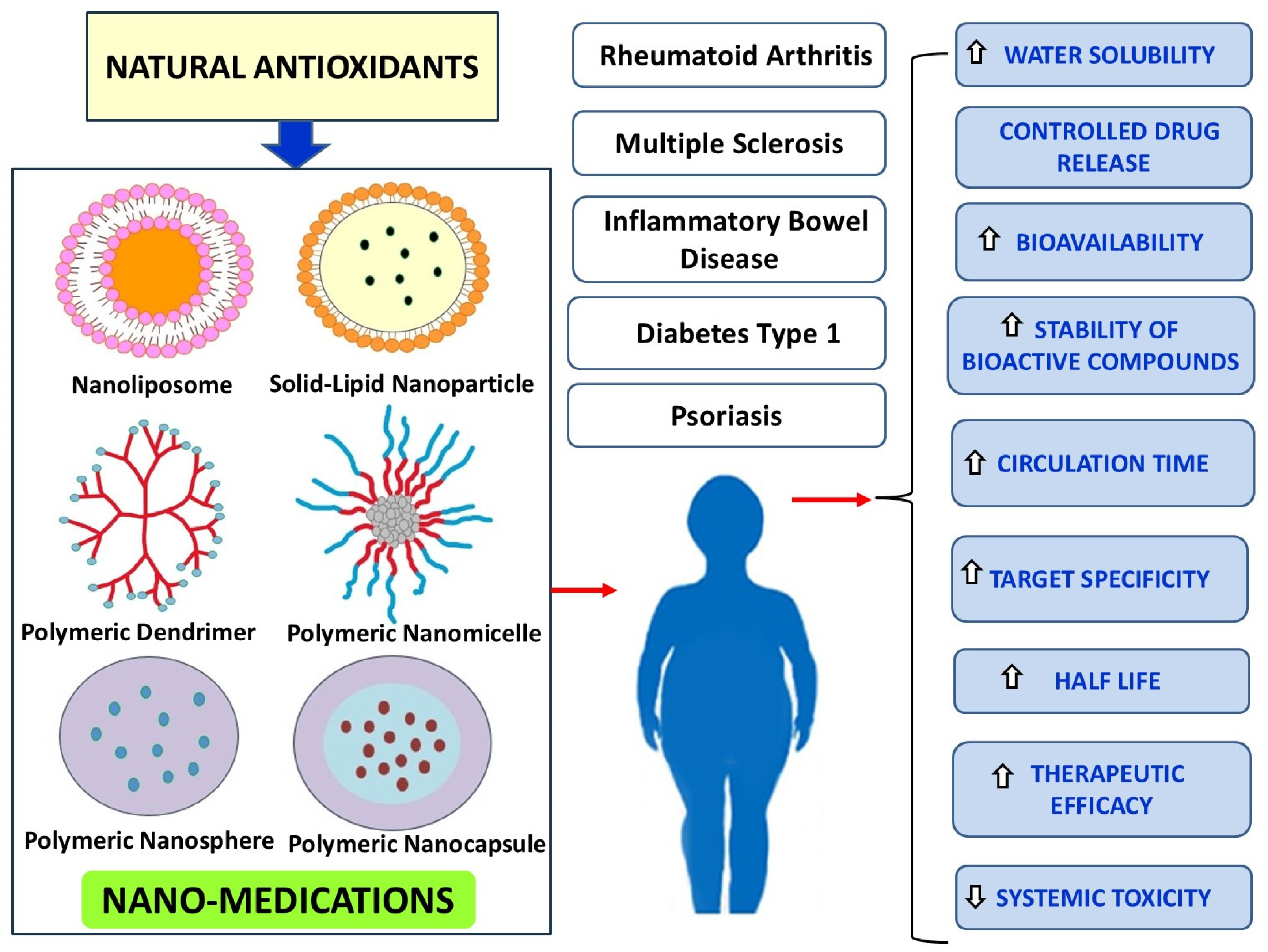
| Antioxidants | Physicochemical Characteristics and Therapeutic Effects of Natural Antioxidants | Nano-Carriers | Methods Preparing Nano-Antioxidants | Physicochemical Characteristics and Outcomes of Nano-Antioxidants |
|---|---|---|---|---|
| Resveratrol (Res) | (a) Natural Res’s molecular weight: 228.25 g/mol [87]. (b) Poor water solubility (20–30 μg/mL) [88]; poor oral bioavailability (<1%) [89]; rapidly metabolized in liver and bowel [89]. (c) It activates SIRT1 and AMPK pathways, modulates intracellular signaling and enhances mitochondrial biogenesis and antioxidant defenses. It inhibits NF-κB and reduces inflammation. It suppresses mTOR pathway mediating autophagy [20,21,22]. | Ruthenium based nano-liposomes [24]. | Hybridation of quadrilateral ruthenium (QRu) nanoparticles with poly lactic-co-glycolic acid (PLGA) nanoparticles [24]. | (a) Significant improvement in water solubility compared to free resveratrol alone [24]. (b) Modulation of macrophage polarization in vitro, reversing the proportion between M1 and M2 cell types and regulation of controlled resveratrol release at the lesion site of MS patients [24]. |
| Curcumin (Cur) | (a) Natural Cur’s Molecular weight: 368.39 g/mol [90]. (b) Poor water solubility (0.0004 mg/mL at pH 7.3) [91]. (c) Poor oral bioavailability [92]; rapidly metabolized and excreted [93]. (d) It inhibits secretions of pro-inflammatory cytokine, such as IL-4, IL-6, IL-8 and TNF-α [94]. It increases anti-inflammatory cytokine production, such as IL-10 and soluble intercellular adhesion molecule 1 (sCAM-1). It serves as ROS scavenger and increases serum GSH and SOD levels [94]. | Nanoliposomes [25]; nanolipid carriers (NLCs) [25]; solid lipid nanoparticles (SLNs) [25]; polymeric dendrimeres [26]; polymeric nano-micelles [27,28,29,30,31,32,33,34]. | Polymerization technique using esterification of oleoyl chloride and methoxyPEG 2000 in the presence of triethylamine and acetone [26]. | (a) Nano-Cur’s size 10–250 nm [32,95]. (b) Increased solubility [30,96]. (c) Increased bioavailability [30,97]; positive Zeta potential (+19.6 mV) [31]; permeability (and targeted pharmacokinetic behavior) up to 12.3 times higher than free Cur [30,34]; prolonged release [32]. (d) It improves OS markers, such as HO-1, NRF2 and iNOS in EAE [27]. It reduces mRNA expression and released pro-inflammatory cytokines such as IL-6, IL-1β, IFN-γ, TNF-α [28,33] and increases anti-inflammatory genes expression in MS patients [28]. It reduces secretion levels of pro-inflammatory cytokines such as TNF-α, IL-6 and IL-12 and increases the anti-inflammatory levels of IL-10 in UC patients [29]. It reduces pro-inflammatory cytokines TNF-α, IL-17A, IL-22 and IL-1β in psoriasis [33]. |
| Quercetin (Quer) | (a) Natural Quer’s molecular weight (302.236 g/mol) [98]. (b) poor water solubility (1 μg/ml) [99,100]; poor gastrointestinal and epidermal absorption (solubility 5.5 μg/mL and 28.9 μg/mL, respectively) [40]; high total polar surface area of 127 Å2; rapid excretion [35,36]. (c) It serves as ROS scavenger and metal chelator and inhibits xanthine oxidase (XO) and nitric oxide synthase (NOS) [35]. | Nanoemulsions [36]; quantum dots (QDs) [37]; biopolymers such as pectin and casein [38]; hyaluronic acid (HA)-based nanoparticles [39]. | Nano-emulsion formulation using spontaneous emulsification [36]. Nano-formulation of thioglycolic acid-capped cadmium telluride quantum dots [37]. Hyaluronic acid (HA) covalent bonded conjugates [39]. | (a) Better permeability and rheological properties than free Quer [36], and efficient encapsulation [38]. (b) It increases the anti-inflammatory effects on lipopolysaccharide induced TNF-α production in RAW 264.7 cells [36]. (c) It inhibits paw oedema formation in RA model [36]. (d) It reduces lipid peroxidation and protein carbonylation, and inflammatory markers, such as CRP, RF, WBC count and ESR and increases GSH, SOD, GPx and CAT in adjuvant induced arthritic Wistar rats [37,38]. (e) HA-Quer shows adhesive, antioxidant, gelation, and self-healing properties in skin autoimmunity disorders [39]. |
| Naringenin (NAR) | (a) Natural NAR’s molecular weight: 580.5 g/mol [40]. (b) Poor water solubility (1 mg/mL at 40 °C). (c) Poor oral bioavailability [101]; rapidly metabolized [40]. (d) It possesses anti-inflammatory properties by inhibiting leucocyte recruitment and preventing macrophages action [102]. It activates Nfr2 resulting in enhanced anti-inflammatory response [102]. It inhibits the activation of NF-κB and the secretion of pro-inflammatory cytokines such as IL-33, TNF-α, IL-1β and IL-6 [102]. Also, it inhibits iNOS [102] and MAPK [102] and suppresses the TLR4 receptor [102]. It possesses antioxidant properties inhibiting ROS production [102], scavenging free radicals [102] and increasing the activity of SOD, CAT, GPx, GST and GSH [102]. | Chitosan-covered liposomes [41]; NAR-PLGA NPs [42]. | Nanoprecipitation technique [41]. Solvent emulsification and evaporation technique [42]. | (a) Nano-NAR’s size < 180–190 nm [41,42]; (b) Increased entrapment efficiency and sustained release [41,42]; (c) Increased stability [42]; positive Zeta potential (+32 mV) [41]; increased entrapment efficiency (74–85%) [41,42]. (d) It decreases the CFA induced rat ankle swelling and the inflammatory markers TNF-α, IL-6 and COX-2 [41]. It reduces paw volume (−22%) on chronic arthritic rat model, CRP, RF, L-6, IL-10, TNF-α and INF-γ levels [42]. |
| Tannic acid (TA) | (a) Natural TA’s molecular weight: 1701.20 g/mol [103]; low bioavailability due to its poor absorption and its low lipid solubility [104]. (b) It has anti-inflammatory effects by inhibiting paw edema through reduction of the activity of myeloperoxidase (MPO) enzyme in a formalin-induced paw edema model [105]. It possesses antidiabetic properties through inhibition of enzymes related to metabolism such as α-glucosidase and α-amylase [105]. It reduces the absorption of monosaccharides in the digestive tract and controls blood sugar levels [105]. It reduces aldose reductase and sorbitol dehydrogenase in the kidneys and attenuates diabetic kidney complications [105]. | Phenylboronic acid (pPBA)-containing polymers [43]; poly(N-vinylpyrrolidone) (PVPON) [45]. | Formation of phenylboronate ester bonds between polymeric phenylboronate and TA [43]. Formation of TA/poly(N-vinylpyrrolidone) (TA/PVPON) hydrogen-bonded multi-layers [45]. | (a) PTNG (polymeric tannic acid-phenylboronic acid nanogel)’s size 250 nm [43]. (b) PTNG possesses anti-inflammatory effects decreasing PMA induced ROS production and TNF-α and IL-6 levels on murine macrophage (RAW264.7) cells [43]. PVPON/TA decreases immune cell infiltration and inflammatory chemokines and increases the anti-inflammatory M2 macrophages on autoimmune type 1 diabetes (T1D) mice model [45]. |
| EGCG | (a) Natural EGCG’s molecular weight: 458.37 g/mol [106]. (b) It possesses antioxidant with anti-inflammatory properties and ROS, RNS scavenging characteristics [46]. | Casein based biopolymer [47]. Chitosan based polymer [48]. | Ultrasound-driven nanoencapsulation of (−)-Epigallocatechin Gallate–Glucosamine–Casein [47]. Ultrasound-driven nano-encapsulation of EGCG loaded chitosan—tripoly Phosphate (CS-TPP) NPs [48,49]. | (a) Nano-EGCG-GA’s mean size: 186 nm [47]; high entrapment efficiency (up to 86.8%) [47]; high stability [47]; preservation of their physicochemical characteristics for a year after freeze-drying process [47]; 3-fold more time for casein degradation in gastric fluids [47]. (b) Nano-EGCG-GA shows 20.8% more profound inhibitory activity on human fibroblast-like synoviocytes-rheumatoid arthritis cells than the free EGCG-GA mixture [47]. Topical application of nano-EGCG reduces the levels of inflammatory cytokines and chemokines such as IL-1α, IL-1β, IL-4, IL-5, IL-6, IL-12, IL-13, and IFN-α and ameliorates the skin thickness, the erythema, the infiltration of inflammatory cells (mast cells, neutrophils, macrophages, and CD4+ T cells) and the angiogenesis in imiquimod induced murine psoriasic model [48]. |
| Eugenol | Natural eugenol has pleiotropic activities, apart from the antioxidant [50]. | Chitosan based polymer [50]. | Nano-encapsulation of eugenol loaded chitosan–tripoly Phosphate (CS-TPP) NPs through centrifugation [50]. | Nano-Eugenol significantly reduces the serum levels of malondialdehyde and Fork head Box O3 (FOXO3) protein and the expression of MCP1/CCL2 and TGF-β on the aggressive model of rheumatoid arthritis [50]. |
| Lycopene (Lyc) | (a) Natural Lyc has low bioavailability due to its low absorption (10–30%) and its metabolic reactions, such as isomerization and oxidation that take place [62]. (b) It possesses anti-inflammatory properties through the inhibition of the NF-κΒ pathway [63]. | Nanoemulsion droplets [63]; nanostructured lipid carriers (NLC) [64]. | Lycopene-loaded emulsions [63]. High shear homogenization method [64]. | (a) NF-Lyc’s mean size: 241 nm [63]; polydispersity index 0.284 [63]; ability to aggregate forming larger nanoparticles [63]; increased bioavailability compared to free Lyc [63]; low excretion rate by the liver and kidneys [63]. (b) NLCs shows increased uptake from psoriatic cells in psoriatic-like cellular model and preserves skin integrity in psoriatic skin [64]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodosis-Nobelos, P.; Varra, F.-N.; Varras, M.; Papagiouvannis, G.; Rekka, E.A. Antioxidant Naturally Occurring Pleiotropically Acting Bioactive Compounds, as Polymeric Nanotherapeutics Against Autoimmune Diseases Progression. Curr. Issues Mol. Biol. 2025, 47, 411. https://doi.org/10.3390/cimb47060411
Theodosis-Nobelos P, Varra F-N, Varras M, Papagiouvannis G, Rekka EA. Antioxidant Naturally Occurring Pleiotropically Acting Bioactive Compounds, as Polymeric Nanotherapeutics Against Autoimmune Diseases Progression. Current Issues in Molecular Biology. 2025; 47(6):411. https://doi.org/10.3390/cimb47060411
Chicago/Turabian StyleTheodosis-Nobelos, Panagiotis, Fani-Niki Varra, Michail Varras, Georgios Papagiouvannis, and Eleni A. Rekka. 2025. "Antioxidant Naturally Occurring Pleiotropically Acting Bioactive Compounds, as Polymeric Nanotherapeutics Against Autoimmune Diseases Progression" Current Issues in Molecular Biology 47, no. 6: 411. https://doi.org/10.3390/cimb47060411
APA StyleTheodosis-Nobelos, P., Varra, F.-N., Varras, M., Papagiouvannis, G., & Rekka, E. A. (2025). Antioxidant Naturally Occurring Pleiotropically Acting Bioactive Compounds, as Polymeric Nanotherapeutics Against Autoimmune Diseases Progression. Current Issues in Molecular Biology, 47(6), 411. https://doi.org/10.3390/cimb47060411







