Research Progress on the Preparation and Application of Decellularized Tendons
Abstract
1. Introduction
2. Extracellular Matrix Components and Structure of Tendons
2.1. Extracellular Matrix Components of Tendons
2.1.1. Collagen
2.1.2. Proteoglycans
2.1.3. Glycoproteins
| Variety | Type | Function | References |
|---|---|---|---|
| Collagen | Type I Collagen | Provides tensile strength and mechanical deformation ability to tendons | [25] |
| Type III Collagen | Regulates collagen fibril diameter | [26] | |
| Type V Collagen | Involved in tendon development | [27] | |
| Type XI Collagen | Regulates collagen fiber assembly | [28,29] | |
| Type VI Collagen | Absence leads to abnormal fibril alignment and reduced mechanical performance | ||
| Type XII Collagen | Stabilizes collagen fibers | ||
| Type XIV Collagen | Regulates collagen fibril diameter | ||
| Proteoglycan | Glycosaminoglycans | Negatively charged, attracts water molecules | [31] |
| Decorin | Restricts lateral growth of collagen fibers | [33] | |
| Biglycan | Maintains collagen fibril structure and regulates collagen fiber formation and ECM assembly | [34] | |
| Fibromodulin | Involved in collagen synthesis, cell proliferation, and matrix remodeling | ||
| Lumican | Induces increased interfibrillar spacing and reduced fibril diameter | [35] | |
| Aggrecan | Provides compressive resistance to tendons and slows collagen fiber formation | [30,36] | |
| Lubricin | Induces increased interfibrillar spacing and reduced fibril diameter | ||
| Versican | Promotes collagen fiber formation, regulates collagen compaction and reorganization | ||
| Glycoprotein | COMP | Provides mechanical support and stability to tendons | [37] |
| Tenascin-C | Regulates tendon cell adhesion to ECM, fibroblast proliferation, migration, differentiation, and repair | [38] | |
| Fibronectin | Supports and regulates ECM | [39] | |
| Elastin | Provides viscoelasticity to tendons and facilitates collagen fiber sliding | [40] | |
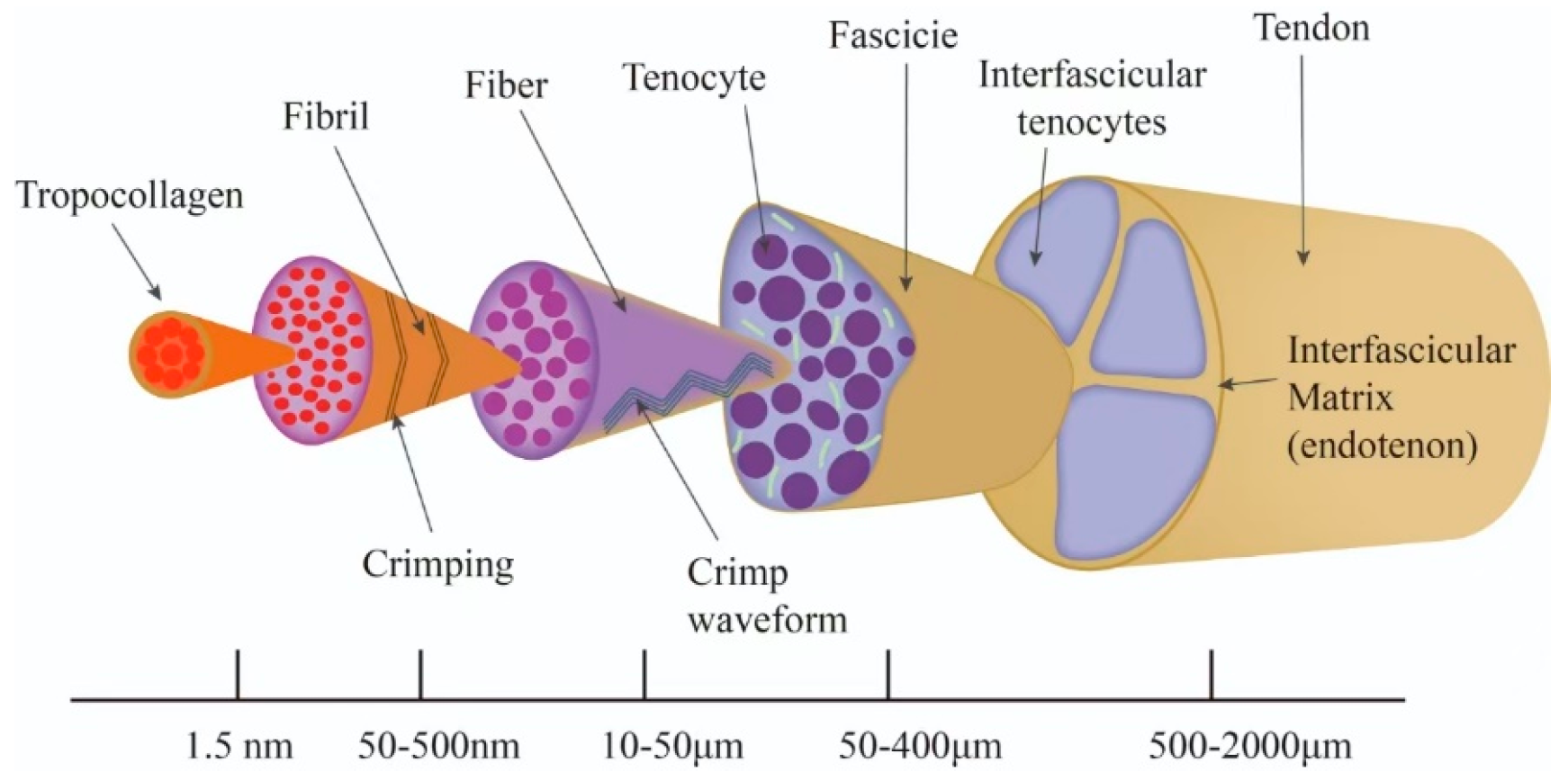
2.2. Hierarchical Structure of Tendons
3. Tendon Decellularization Methods
3.1. Physical Methods
| Decellularized Methods | Specific Method | Decellularization Principle | References |
|---|---|---|---|
| Physical methods | Ultrasound | Loosens tendon structure, facilitating reagent penetration | [8] |
| Repeated freeze–thaw | Ice crystals disrupt cell membranes | [55] | |
| Mechanical stirring | Mechanical forces disrupt cell membranes | [57] | |
| Chemical methods | Acid | Perforates tendon surfaces to facilitate reagent penetration; dissolves cell membranes | [59] |
| Detergents (SDS, Triton X-100, TBP) | Dissolves cell membranes, disrupts DNA-protein interactions | [60] | |
| EDTA | Disrupts adhesion between ECM components | [61] | |
| Hypertonic/Hypotonic solutions | Uses osmotic pressure to disrupt cells | [62] | |
| Biological methods | Trypsin | Digests proteins and peptides, disrupts extracellular matrix around collagen fibers | [63] |
| DNase/RNase | Removes residual DNA and RNA left after cell lysis | [64] |
3.2. Chemical Methods
3.3. Biological Methods
3.4. Combined Methods
| Species | Tendon Type | Decellularization Protocol | Result | References |
|---|---|---|---|---|
| Pig | Patellar tendon | Tris buffer (containing alanine and EDTA), SDS, PBS washing, nuclease solution, PBS washing, ultrasound at different intensities | Under ultrasound conditions (360 W, 1 s pulse, 1 min), the tendon displayed a porous structure with no significant effects on biochemical composition or biomechanics | [66] |
| Horse | Superficial digital flexor tendon | Automated freeze–thaw cycles (freezing machine) and manual freeze-thaw cycles (liquid nitrogen 2 min, 37 °C 10 min, 5 cycles), distilled water, Tris buffer (1% Triton X-100) | No significant difference between automated and manual freeze–thaw cycles; effective for large tendons | [56] |
| Rabbit | Flexor tendon and semitendinosus tendon | Six protocols tested: 1% Triton-X 100, 0.5% SDS, 1% TBP, 1% Triton-X 100 + 0.5% SDS, 1% TBP + 0.5% SDS, 1% TBP + 1% Triton-X 100, followed by distilled water, nuclease, and EDTA | Treatment with 1% TBP + 0.5% SDS achieved complete cell removal, with histology and biomechanics similar to native tendon tissue | [63] |
| Cattle | Achilles tendon | Tissue cut into 0.6 mm slices, freeze–thaw cycles (liquid nitrogen 1 min, 37 °C min, 5 cycles), PBS washing, nuclease solution, α-galactosidase, PBS washing | DNA and α-gal epitopes effectively removed, with good preservation of collagen fibers and chondroitin sulfate characteristics | [67] |
| Beagle | Achilles tendon | Tissue cut into 40 mm slices, PBS washing, repeated freeze–thaw (liquid nitrogen 2 min, 37 °C 10 min), PBS washing, nuclease solution, PBS washing | Repeated freeze–thaw combined with nuclease treatment for twelve hours achieved complete decellularization, with ultrastructure well-preserved | [68] |
| Goat | Flexor tendon | Tissue cut into 2 mm slices, hypotonic solution (4 freeze–thaw cycles), hypertonic solution, SDS, Triton-X 100, ultrapure water washing | DNA content reduced by over 95%. Post-decellularization, fiber morphology was intact, and collagen content showed no significant difference from native tendon tissue | [69] |
| Macaque | Achilles tendon | Tissue cut into 2 cm slices, repeated freeze–thaw, cryosectioned into 300 µm slices, nuclease treatment, PBS washing | H&E staining, DAPI staining, and DNA quantification confirmed the effectiveness of decellularization in macaque Achilles tendons | [16] |
4. Cross-Species Characterization Differences in Tendon Properties
4.1. Species-Specific Differences in ECM Components
4.2. The Dependence of Biomechanical Properties on Species
4.3. Biocompatibility Influencing Factors
4.4. Species Compatibility in Regenerative Function
5. Applications of Decellularized Tendon Biomaterials
5.1. tECM Scaffolds
5.2. Tendon-Derived Hydrogels
5.3. Bioinks
6. Conclusions and Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Steinmann, S.; Pfeifer, C.G.; Brochhausen, C.; Docheva, D. Spectrum of Tendon Pathologies: Triggers, Trails and End-State. Int. J. Mol. Sci. 2020, 21, 844. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, W.; Zhang, T.; Luo, L. Mechanisms of tendon-bone interface healing: Biomechanics, cell mechanics, and tissue engineering approaches. J. Orthop. Surg. Res. 2024, 19, 817. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.M.; Chen, W.S. Conservative Treatment of Tendon Injuries. Am. J. Phys. Med. Rehabil. 2020, 99, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Calfee, R.P. General Principles of Flexor Tendon Repair. Hand Clin. 2023, 39, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, T.; Patel, J.; Choi, J.H.; Fitzpatrick, B.; Begley, B.; Mazzei, C.J.; Harrington, C.J.; Miller, J.M.; Wittig, J.C.; Epstein, D. Mini-Open Achilles Tendon Repair: Improving Outcomes While Decreasing Complications. Foot Ankle Spec. 2023, 16, 363–369. [Google Scholar] [CrossRef]
- Tang, J.B.; Lalonde, D.; Harhaus, L.; Sadek, A.F.; Moriya, K.; Pan, Z.J. Flexor tendon repair: Recent changes and current methods. J. Hand Surg. Eur. Vol. 2022, 47, 31–39. [Google Scholar] [CrossRef]
- Roth, S.P.; Erbe, I.; Burk, J. Decellularization of Large Tendon Specimens: Combination of Manually Performed Freeze-Thaw Cycles and Detergent Treatment. In Decellularized Scaffolds and Organogenesis, Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1577, pp. 227–237. [Google Scholar] [CrossRef]
- Jin, Y.; Sun, Q.; Ma, R.; Li, R.; Qiao, R.; Li, J.; Wang, L.; Hu, Y. The trend of allogeneic tendon decellularization: Literature review. Cell Tissue Bank. 2024, 25, 357–367. [Google Scholar] [CrossRef]
- Perez, M.L.; Castells-Sala, C.; Lopez-Chicon, P.; Nieto-Nicolau, N.; Aiti, A.; Farinas, O.; Casaroli-Marano, R.P.; Porta, O.; Vilarrodona, A. Fast protocol for the processing of split-thickness skin into decellularized human dermal matrix. Tissue Cell 2021, 72, 101572. [Google Scholar] [CrossRef]
- Anjum, S.; Li, T.; Saeed, M.; Ao, Q. Exploring polysaccharide and protein-enriched decellularized matrix scaffolds for tendon and ligament repair: A review. Int. J. Biol. Macromol. 2024, 254, 127891. [Google Scholar] [CrossRef]
- Cui, J.; Ning, L.J.; Wu, F.P.; Hu, R.N.; Li, X.; He, S.K.; Zhang, Y.J.; Luo, J.J.; Luo, J.C.; Qin, T.W. Biomechanically and biochemically functional scaffold for recruitment of endogenous stem cells to promote tendon regeneration. npj Regen. Med. 2022, 7, 26. [Google Scholar] [CrossRef]
- Chen, R.; Chen, F.; Chen, K.; Xu, J. Advances in the application of hydrogel-based scaffolds for tendon repair. Genes Dis. 2024, 11, 101019. [Google Scholar] [CrossRef]
- Dang, R.; Chen, L.; Sefat, F.; Li, X.; Liu, S.; Yuan, X.; Ning, X.; Zhang, Y.S.; Ji, P.; Zhang, X. A Natural Hydrogel with Prohealing Properties Enhances Tendon Regeneration. Small 2022, 18, e2105255. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhu, C.; Ma, X.; Fan, D. Smart hydrogel-based trends in future tendon injury repair: A review. Int. J. Biol. Macromol. 2024, 282, 137092. [Google Scholar] [CrossRef] [PubMed]
- Kent, R.N., III; Huang, A.H.; Baker, B.M. Augmentation of Tendon and Ligament Repair with Fiber-Reinforced Hydrogel Composites. Adv. Healthc. Mater. 2024, 13, e2400668. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.J.; Zhang, Y.J.; Zhang, Y.J.; Zhu, M.; Ding, W.; Jiang, Y.L.; Zhang, Y.; Luo, J.C.; Qin, T.W. Enhancement of Migration and Tenogenic Differentiation of Macaca Mulatta Tendon-Derived Stem Cells by Decellularized Tendon Hydrogel. Front. Cell Dev. Biol. 2021, 9, 651583. [Google Scholar] [CrossRef]
- Tao, M.; Liang, F.; He, J.; Ye, W.; Javed, R.; Wang, W.; Yu, T.; Fan, J.; Tian, X.; Wang, X.; et al. Decellularized tendon matrix membranes prevent post-surgical tendon adhesion and promote functional repair. Acta Biomater. 2021, 134, 160–176. [Google Scholar] [CrossRef]
- Wan, H.; Xiang, J.; Mao, G.; Pan, S.; Li, B.; Lu, Y. Recent Advances in the Application of 3D-Printing Bioinks Based on Decellularized Extracellular Matrix in Tissue Engineering. ACS Omega 2024, 9, 24219–24235. [Google Scholar] [CrossRef]
- Alhaskawi, A.; Zhou, H.; Dong, Y.; Zou, X.; Ezzi, S.H.A.; Kota, V.G.; Abdulla, M.H.A.; Tu, T.; Alenikova, O.; Abdalbary, S.; et al. Advancements in 3D-printed artificial tendon. J. Biomed. Mater. Res. Part B Appl. Biomater. 2024, 112, e35364. [Google Scholar] [CrossRef]
- Kiratitanaporn, W.; Guan, J.; Tang, M.; Xiang, Y.; Lu, T.Y.; Balayan, A.; Lao, A.; Berry, D.B.; Chen, S. 3D Printing of a Biomimetic Myotendinous Junction Assisted by Artificial Intelligence. Biomater. Sci. 2024, 12, 6047–6062. [Google Scholar] [CrossRef]
- Kim, W.; Kwon, D.R.; Lee, H.; Lee, J.; Moon, Y.S.; Lee, S.C.; Kim, G.H. 3D bioprinted multi-layered cell constructs with gradient core-shell interface for tendon-to-bone tissue regeneration. Bioact. Mater. 2025, 43, 471–490. [Google Scholar] [CrossRef]
- Rosset, J.; Olaniyanu, E.; Stein, K.; Almeida, N.D.; França, R. Exploring the Frontier of 3D Bioprinting for Tendon Regeneration: A Review. Eng 2024, 5, 1838–1849. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, D.; Lan, Q.; Zhong, G.; Liu, Y.; Holwell, N.; Wang, X.; Meng, J.; Yao, J.; Amsden, B.G.; et al. Tendon Decellularized Matrix Modified Fibrous Scaffolds with Porous and Crimped Microstructure for Tendon Regeneration. ACS Appl. Bio Mater. 2024, 7, 4747–4759. [Google Scholar] [CrossRef] [PubMed]
- Naba, A. Mechanisms of assembly and remodelling of the extracellular matrix. Nat. Rev. Mol. Cell Biol. 2024, 25, 865–885. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Shi, Y.; Zhou, B.; Wang, X.; Zhang, W.; Zhou, G.; Mo, X.; Wang, W.; Wu, J.; Liu, W. Type I collagen and fibromodulin enhance the tenogenic phenotype of hASCs and their potential for tendon regeneration. npj Regen. Med. 2023, 8, 67. [Google Scholar] [CrossRef]
- Asgari, M.; Latifi, N.; Heris, H.K.; Vali, H.; Mongeau, L. In vitro fibrillogenesis of tropocollagen type III in collagen type I affects its relative fibrillar topology and mechanics. Sci. Rep. 2017, 7, 1392. [Google Scholar] [CrossRef]
- Sun, M.; Luo, E.Y.; Adams, S.M.; Adams, T.; Ye, Y.; Shetye, S.S.; Soslowsky, L.J.; Birk, D.E. Collagen XI regulates the acquisition of collagen fibril structure, organization and functional properties in tendon. Matrix Biol. J. Int. Soc. Matrix Biol. 2020, 94, 77–94. [Google Scholar] [CrossRef]
- Izu, Y.; Adams, S.M.; Connizzo, B.K.; Beason, D.P.; Soslowsky, L.J.; Koch, M.; Birk, D.E. Collagen XII mediated cellular and extracellular mechanisms regulate establishment of tendon structure and function. Matrix Biol. J. Int. Soc. Matrix Biol. 2021, 95, 52–67. [Google Scholar] [CrossRef]
- Antoniel, M.; Traina, F.; Merlini, L.; Andrenacci, D.; Tigani, D.; Santi, S.; Cenni, V.; Sabatelli, P.; Faldini, C.; Squarzoni, S. Tendon Extracellular Matrix Remodeling and Defective Cell Polarization in the Presence of Collagen VI Mutations. Cells 2020, 9, 409. [Google Scholar] [CrossRef]
- Thorpe, C.T.; Birch, H.L.; Clegg, P.D.; Screen, H.R. The role of the non-collagenous matrix in tendon function. Int. J. Exp. Pathol. 2013, 94, 248–259. [Google Scholar] [CrossRef]
- Eisner, L.E.; Rosario, R.; Andarawis-Puri, N.; Arruda, E.M. The Role of the Non-Collagenous Extracellular Matrix in Tendon and Ligament Mechanical Behavior: A Review. J. Biomech. Eng. 2022, 144, 050801. [Google Scholar] [CrossRef]
- Narayanan, N.; Calve, S. Extracellular matrix at the muscle—Tendon interface: Functional roles, techniques to explore and implications for regenerative medicine. Connect. Tissue Res. 2021, 62, 53–71. [Google Scholar] [CrossRef]
- Marqueti, R.C.; Durigan, J.L.Q.; Oliveira, A.J.S.; Mekaro, M.S.; Guzzoni, V.; Aro, A.A.; Pimentel, E.R.; Selistre-de-Araujo, H.S. Effects of aging and resistance training in rat tendon remodeling. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 353–368. [Google Scholar] [CrossRef]
- Beach, Z.M.; Bonilla, K.A.; Dekhne, M.S.; Sun, M.; Adams, T.H.; Adams, S.M.; Weiss, S.N.; Rodriguez, A.B.; Shetye, S.S.; Birk, D.E.; et al. Biglycan has a major role in maintenance of mature tendon mechanics. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2022, 40, 2546–2556. [Google Scholar] [CrossRef]
- Xu, X.; Ha, P.; Yen, E.; Li, C.; Zheng, Z. Small Leucine-Rich Proteoglycans in Tendon Wound Healing. Adv. Wound Care 2022, 11, 202–214. [Google Scholar] [CrossRef]
- Chen, D.; Smith, L.R.; Khandekar, G.; Patel, P.; Yu, C.K.; Zhang, K.; Chen, C.S.; Han, L.; Wells, R.G. Distinct effects of different matrix proteoglycans on collagen fibrillogenesis and cell-mediated collagen reorganization. Sci. Rep. 2020, 10, 19065. [Google Scholar] [CrossRef]
- Smith, R.; Önnerfjord, P.; Holmgren, K.; di Grado, S.; Dudhia, J. Development of a Cartilage Oligomeric Matrix Protein Neo-Epitope Assay for the Detection of Intra-Thecal Tendon Disease. Int. J. Mol. Sci. 2020, 21, 2155. [Google Scholar] [CrossRef] [PubMed]
- Tashjian, R.Z.; Zitnay, J.; Kazmers, N.H.; Veerabhadraiah, S.R.; Zelada, A.C.; Honeggar, M.; Smith, M.C.; Chalmers, P.N.; Henninger, H.B.; Jurynec, M.J. Tenascin C deletion impairs tendon healing and functional recovery after rotator cuff repair. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2024, 43, 483–491. [Google Scholar] [CrossRef]
- Gaffney, L.S.; Davis, Z.G.; Mora-Navarro, C.; Fisher, M.B.; Freytes, D.O. Extracellular Matrix Hydrogels Promote Expression of Muscle-Tendon Junction Proteins. Tissue Eng. Part A 2022, 28, 270–282. [Google Scholar] [CrossRef]
- Ishizaki, Y.; Wang, J.; Kim, J.; Matsumoto, T.; Maeda, E. Contributions of collagen and elastin to elastic behaviours of tendon fascicle. Acta Biomater. 2024, 176, 334–343. [Google Scholar] [CrossRef]
- Grant, T.M.; Thompson, M.S.; Urban, J.; Yu, J. Elastic fibers are broadly distributed in tendon and highly localized around tenocytes. J. Anat. 2013, 222, 573–579. [Google Scholar] [CrossRef]
- Kuniakova, M.; Novakova, Z.V.; Haspinger, D.; Niestrawska, J.A.; Klein, M.; Galfiova, P.; Kovac, J.; Palkovic, M.; Danisovic, L.; Hammer, N.; et al. Effects of Two Decellularization Protocols on the Mechanical Behavior and Structural Properties of the Human Urethra. Int. J. Mol. Sci. 2024, 25, 12361. [Google Scholar] [CrossRef]
- Chen, T.A.; Sharma, D.; Jia, W.; Ha, D.; Man, K.; Zhang, J.; Yang, Y.; Zhou, Y.; Kamp, T.J.; Zhao, F. Detergent-Based Decellularization for Anisotropic Cardiac-Specific Extracellular Matrix Scaffold Generation. Biomimetics 2023, 8, 551. [Google Scholar] [CrossRef]
- Pierantoni, M.; Sharma, K.; Kok, J.; Novak, V.; Eliasson, P.; Isaksson, H. Quantification of 3D microstructures in Achilles tendons during in situ loading reveals anisotropic fiber response. Acta Biomater. 2025, 194, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.B.; Weiss, S.N.; Nuss, C.A.; Darrieutort-Laffite, C.; Eekhoff, J.D.; Birk, D.E.; Soslowsky, L.J. Decorin and/or biglycan knockdown in aged mouse patellar tendon impacts fibril morphology, scar area, and mechanical properties. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2024, 42, 2400–2413. [Google Scholar] [CrossRef]
- Hatami-Marbini, H.; Emu, M.E. Role of sulfated GAGs in shear mechanical properties of human and porcine cornea. Exp. Eye Res. 2025, 251, 110181. [Google Scholar] [CrossRef]
- Fu, Y.; Zhou, Y.; Wang, K.; Li, Z.; Kong, W. Extracellular Matrix Interactome in Modulating Vascular Homeostasis and Remodeling. Circ. Res. 2024, 134, 931–949. [Google Scholar] [CrossRef] [PubMed]
- Ostadi Moghaddam, A.; Arshee, M.R.; Lin, Z.; Sivaguru, M.; Phillips, H.; McFarlin, B.L.; Toussaint, K.C.; Wagoner Johnson, A.J. Orientation-dependent indentation reveals the crosslink-mediated deformation mechanisms of collagen fibrils. Acta Biomater. 2023, 158, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Shama, K.A.; Greenberg, Z.F.; Tammame, C.; He, M.; Taylor, B.L. Diseased Tendon Models Demonstrate Influence of Extracellular Matrix Alterations on Extracellular Vesicle Profile. Bioengineering 2024, 11, 1101019. [Google Scholar] [CrossRef]
- Pei, Y.; Yang, W.; Tang, K.; Kaplan, D.L. Collagen processing with mesoscale aggregates as templates and building blocks. Biotechnol. Adv. 2023, 63, 108099. [Google Scholar] [CrossRef]
- Sun, M.; Li, H.; Hou, Y.; Huang, N.; Xia, X.; Zhu, H.; Xu, Q.; Lin, Y.; Xu, L. Multifunctional tendon-mimetic hydrogels. Sci. Adv. 2023, 9, eade6973. [Google Scholar] [CrossRef]
- Chen, W.; Chen, M.; Chen, S.; Wang, S.; Huang, Z.; Zhang, L.; Wu, J.; Peng, W.; Li, H.; Wen, F. Decellularization of fish tissues for tissue engineering and regenerative medicine applications. Regen. Biomater. 2025, 12, rbae138. [Google Scholar] [CrossRef]
- van Hengel, E.V.A.; van der Laan, L.J.W.; de Jonge, J.; Verstegen, M.M.A. Towards Safety and Regulation Criteria for Clinical Applications of Decellularized Organ-Derived Matrices. Bioengineering 2025, 12, 136. [Google Scholar] [CrossRef]
- Aron, J.; Bual, R.; Alimasag, J.; Arellano, F.; Baclayon, L.; Bantilan, Z.C.; Lumancas, G.; Nisperos, M.J.; Labares, M., Jr.; Valle, K.D.D.; et al. Effects of Various Decellularization Methods for the Development of Decellularized Extracellular Matrix from Tilapia (Oreochromis niloticus) Viscera. Int. J. Biomater. 2024, 2024, 6148496. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Y.; Luo, S.; Dang, H.; Cao, M. Effect of cryoprotectants on rat kidney decellularization by freeze-thaw process. Cryobiology 2022, 105, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.P.; Glauche, S.M.; Plenge, A.; Erbe, I.; Heller, S.; Burk, J. Automated freeze-thaw cycles for decellularization of tendon tissue—A pilot study. BMC Biotechnol. 2017, 17, 13. [Google Scholar] [CrossRef]
- Charras, G.; Yap, A.S. Tensile Forces and Mechanotransduction at Cell-Cell Junctions. Curr. Biol. CB 2018, 28, R445–R457. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.G.; Townsend, J.M.; Detamore, M.S. Automated Decellularization of Musculoskeletal Tissues with High Extracellular Matrix Retention. Tissue Eng. Part C Methods 2022, 28, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef]
- Willemse, J.; Verstegen, M.M.A.; Vermeulen, A.; Schurink, I.J.; Roest, H.P.; van der Laan, L.J.W.; de Jonge, J. Fast, robust and effective decellularization of whole human livers using mild detergents and pressure controlled perfusion. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110200. [Google Scholar] [CrossRef]
- Arumugam, P.; Kaarthikeyan, G.; Eswaramoorthy, R. Comparative Evaluation of Three Different Demineralisation Protocols on the Physicochemical Properties and Biocompatibility of Decellularised Extracellular Matrix for Bone Tissue Engineering Applications. Cureus 2024, 16, e64813. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Bates, S.J.; Morrow, E.; Pham, H.; Pham, B.; Chang, J. Tissue-engineered intrasynovial tendons: Optimization of acellularization and seeding. J. Rehabil. Res. Dev. 2009, 46, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Liu, C.; Xu, B.; Chen, J.; Yin, D.; Zhang, C. Effects of various decellularization methods on histological and biomechanical properties of rabbit tendons. Exp. Ther. Med. 2014, 8, 628–634. [Google Scholar] [CrossRef]
- Youngstrom, D.W.; Barrett, J.G.; Jose, R.R.; Kaplan, D.L. Functional characterization of detergent-decellularized equine tendon extracellular matrix for tissue engineering applications. PLoS ONE 2013, 8, e64151. [Google Scholar] [CrossRef] [PubMed]
- White, L.J.; Taylor, A.J.; Faulk, D.M.; Keane, T.J.; Saldin, L.T.; Reing, J.E.; Swinehart, I.T.; Turner, N.J.; Ratner, B.D.; Badylak, S.F. The impact of detergents on the tissue decellularization process: A ToF-SIMS study. Acta Biomater. 2017, 50, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.H.; Korossis, S.; Howling, G.; Fisher, J.; Ingham, E. The use of ultrasonication to aid recellularization of acellular natural tissue scaffolds for use in anterior cruciate ligament reconstruction. Tissue Eng. 2007, 13, 1561–1572. [Google Scholar] [CrossRef]
- Ning, L.J.; Jiang, Y.L.; Zhang, C.H.; Zhang, Y.; Yang, J.L.; Cui, J.; Zhang, Y.J.; Yao, X.; Luo, J.C.; Qin, T.W. Fabrication and characterization of a decellularized bovine tendon sheet for tendon reconstruction. J. Biomed. Mater. Res. Part A 2017, 105, 2299–2311. [Google Scholar] [CrossRef]
- Ning, L.J.; Zhang, Y.; Chen, X.H.; Luo, J.C.; Li, X.Q.; Yang, Z.M.; Qin, T.W. Preparation and characterization of decellularized tendon slices for tendon tissue engineering. J. Biomed. Mater. Res. Part A 2012, 100, 1448–1456. [Google Scholar] [CrossRef]
- Bhatt, A.; Dhiman, N.; Giri, P.S.; Kasinathan, G.N.; Pati, F.; Rath, S.N. Biocompatibility-on-a-chip: Characterization and evaluation of decellularized tendon extracellular matrix (tdECM) hydrogel for 3D stem cell culture in a microfluidic device. Int. J. Biol. Macromol. 2022, 213, 768–779. [Google Scholar] [CrossRef]
- Chatterjee, M.; Muljadi, P.M.; Andarawis-Puri, N. The role of the tendon ECM in mechanotransduction: Disruption and repair following overuse. Connect. Tissue Res. 2022, 63, 28–42. [Google Scholar] [CrossRef]
- Lal, M.R.; Agrawal, D.K. Chronic Adaptation of Achilles Tendon Tissues upon Injury to Rotator Cuff Tendon in Hyperlipidemic Swine. J. Orthop. Sports Med. 2024, 6, 80–88. [Google Scholar] [CrossRef]
- Castells-Sala, C.; Perez, M.L.; Lopez-Chicon, P.; Lopez-Puerto, L.; Martinez, J.I.R.; Ruiz-Ponsell, L.; Aiti, A.; Madariaga, S.E.; Sastre, S.; Farinas, O.; et al. Development of a full-thickness acellular dermal graft from human skin: Case report of first patient rotator cuff patch augmentation repair. Transpl. Immunol. 2023, 78, 101825. [Google Scholar] [CrossRef] [PubMed]
- Terek, J.C.; Hebb, M.O.; Flynn, L.E. Development of Brain-Derived Bioscaffolds for Neural Progenitor Cell Culture. ACS Pharmacol. Transl. Sci. 2023, 6, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Nagelli, C.V.; Hooke, A.; Quirk, N.; De Padilla, C.L.; Hewett, T.E.; van Griensven, M.; Coenen, M.; Berglund, L.; Evans, C.H.; Muller, S.A. Mechanical and strain behaviour of human Achilles tendon during in vitro testing to failure. Eur. Cells Mater. 2022, 43, 153–161. [Google Scholar] [CrossRef]
- Lal, L.P.M.; Agrawal, D.K. Hyperlipidemia Induced Pathological Changes with no Effect in Biomechanical Properties in the Achilles Tendon of Young Swine. J. Orthop. Sports Med. 2024, 6, 67–72. [Google Scholar] [CrossRef]
- Shi, J.; Yao, H.; Chong, H.; Hu, X.; Yang, J.; Dai, X.; Liu, D.; Wu, Z.; Dang, M.; Fei, W.; et al. Tissue-engineered collagen matrix loaded with rat adipose-derived stem cells/human amniotic mesenchymal stem cells for rotator cuff tendon-bone repair. Int. J. Biol. Macromol. 2024, 282, 137144. [Google Scholar] [CrossRef]
- Gonzalez-Quevedo, D.; Sanchez-Porras, D.; Garcia-Garcia, O.D.; Chato-Astrain, J.; Diaz-Ramos, M.; Campos, A.; Carriel, V.; Campos, F. Nanostructured fibrin-based hydrogel membranes for use as an augmentation strategy in Achilles tendon surgical repair in rats. Eur. Cells Mater. 2022, 43, 162–178. [Google Scholar] [CrossRef]
- Peniche Silva, C.J.; Muller, S.A.; Quirk, N.; De la Vega, R.E.; Coenen, M.J.; Evans, C.H.; Balmayor, E.R.; van Griensven, M. Enthesis: Not the same in each localisation—A molecular, histological and biomechanical study. Eur. Cells Mater. 2022, 44, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Stanczak, M.; Kacprzak, B.; Gawda, P. Tendon Cell Biology: Effect of Mechanical Loading. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2024, 58, 677–701. [Google Scholar] [CrossRef]
- Ellingson, A.J.; Pancheri, N.M.; Schiele, N.R. Regulators of collagen crosslinking in developing and adult tendons. Eur. Cells Mater. 2022, 43, 130–152. [Google Scholar] [CrossRef]
- Salman Hamza, A.; Traef Ali, Q.; Hadi Farman, R. The Healing Effect of Biodegradable Scaffolds Treated with Bone-Marrow Obtained Mesenchymal Stem Cells on Major Tendon Damage in the Dog as a Model. Arch. Razi Inst. 2023, 78, 889–898. [Google Scholar] [CrossRef]
- Ning, L.J.; Cui, J.; He, S.K.; Hu, R.N.; Yao, X.; Zhang, Y.; Ding, W.; Zhang, Y.J.; Luo, J.C.; Qin, T.W. Constructing a highly bioactive tendon-regenerative scaffold by surface modification of tissue-specific stem cell-derived extracellular matrix. Regen. Biomater. 2022, 9, rbac020. [Google Scholar] [CrossRef]
- Itoh, M.; Imasu, H.; Takano, K.; Umezu, M.; Okazaki, K.; Iwasaki, K. Time-series biological responses toward decellularized bovine tendon graft and autograft for 52 consecutive weeks after rat anterior cruciate ligament reconstruction. Sci. Rep. 2022, 12, 6751. [Google Scholar] [CrossRef]
- Zhao, L.L.; Luo, J.J.; Cui, J.; Li, X.; Hu, R.N.; Xie, X.Y.; Zhang, Y.J.; Ding, W.; Ning, L.J.; Luo, J.C.; et al. Tannic Acid-Modified Decellularized Tendon Scaffold with Antioxidant and Anti-Inflammatory Activities for Tendon Regeneration. ACS Appl. Mater. Interfaces 2024, 16, 15879–15892. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Kang, Y.; Gao, H.; Lin, Z.; Huang, D.; Zheng, Z.; Zhao, J.; Wang, L.; Jiang, J. Decellularization-Based Modification Strategy for Bioactive Xenografts Promoting Tendon Repair. Adv. Healthc. Mater. 2024, 13, e2302660. [Google Scholar] [CrossRef]
- Wang, R.M.; Mesfin, J.M.; Karkanitsa, M.; Ungerleider, J.L.; Zelus, E.; Zhang, Y.; Kawakami, Y.; Kawakami, Y.; Kawakami, T.; Christman, K.L. Immunomodulatory contribution of mast cells to the regenerative biomaterial microenvironment. npj Regen. Med. 2023, 8, 53. [Google Scholar] [CrossRef]
- Ma, R.; Gao, X.; Jin, Y.; Wang, X.; Li, R.; Qiao, R.; Wang, X.; Liu, D.; Xie, Z.; Wang, L.; et al. Is there a duration-characteristic relationship for trypsin exposure on tendon? A study on anterior cruciate ligament reconstruction in a rabbit model. Front. Med. 2024, 11, 1417930. [Google Scholar] [CrossRef]
- Liu, Q.W.; Huang, Q.M.; Wu, H.Y.; Zuo, G.S.; Gu, H.C.; Deng, K.Y.; Xin, H.B. Characteristics and Therapeutic Potential of Human Amnion-Derived Stem Cells. Int. J. Mol. Sci. 2021, 22, 970. [Google Scholar] [CrossRef]
- Huang, S.; Rao, Y.; Zhou, M.; Blocki, A.M.; Chen, X.; Wen, C.; Ker, D.F.E.; Tuan, R.S.; Wang, D.M. Engineering an extracellular matrix-functionalized, load-bearing tendon substitute for effective repair of large-to-massive tendon defects. Bioact. Mater. 2024, 36, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Zhu, C.; Suen, H.C.; Huang, S.; Liao, J.; Ker, D.F.E.; Tuan, R.S.; Wang, D. Tenogenic induction of human adipose-derived stem cells by soluble tendon extracellular matrix: Composition and transcriptomic analyses. Stem Cell Res. Ther. 2022, 13, 380. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, M.Z.; Peng, H.; Liu, R.T.; Lim, T.; Zhang, C.Q.; Zhu, Z.Z.; Wei, X.J. Decellularized tilapia fish skin: A novel candidate for tendon tissue engineering. Mater. Today Bio 2022, 17, 100488. [Google Scholar] [CrossRef]
- Ghosh, S.; Pati, F. Decellularized extracellular matrix and silk fibroin-based hybrid biomaterials: A comprehensive review on fabrication techniques and tissue-specific applications. Int. J. Biol. Macromol. 2023, 253, 127410. [Google Scholar] [CrossRef]
- Gao, X.D.; Zhang, X.B.; Zhang, R.H.; Yu, D.C.; Chen, X.Y.; Hu, Y.C.; Chen, L.; Zhou, H.Y. Aggressive strategies for regenerating intervertebral discs: Stimulus-responsive composite hydrogels from single to multiscale delivery systems. J. Mater. Chem. B 2022, 10, 5696–5722. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Qian, Y.; Jin, Y.; Wang, S.; Li, J.; Yuan, W.E.; Fan, C. Biomimetic multilayer polycaprolactone/sodium alginate hydrogel scaffolds loaded with melatonin facilitate tendon regeneration. Carbohydr. Polym. 2022, 277, 118865. [Google Scholar] [CrossRef]
- An, S.; Jeon, E.J.; Han, S.Y.; Jeon, J.; Lee, M.J.; Kim, S.; Shin, M.; Cho, S.W. pH-Universal Catechol-Amine Chemistry for Versatile Hyaluronic Acid Bioadhesives. Small 2022, 18, e2202729. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, S.; He, S.; He, H.; Yuan, G.; Ma, B.; Zhang, Y.; Yuan, C.; Liu, Z.; Deng, Z.; et al. Restoring tendon microenvironment in tendinopathy: Macrophage modulation and tendon regeneration with injectable tendon hydrogel and tendon-derived stem cells exosomes. Bioact. Mater. 2025, 47, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, F.; Zhang, X.; Shang, Y.; Zhao, Y. Living microecological hydrogels for wound healing. Sci. Adv. 2023, 9, eadg3478. [Google Scholar] [CrossRef]
- Alkhilani, M.A.; Hammoodi, O.T.; Emran, H.A.; Alhayani, W.A. Impact of Using Processed Urinary Bladder Submucosa and Hydrogel Fabricated from Tendon on Skin Healing Process in Rabbits. Vet. Med. Int. 2024, 2024, 6641975. [Google Scholar] [CrossRef]
- You, C.; Zhang, Z.; Guo, Y.; Liu, S.; Hu, K.; Zhan, Y.; Aihemaiti, S.; Tao, S.; Chu, Y.; Fan, L. Application of extracellular matrix cross-linked by microbial transglutaminase to promote wound healing. Int. J. Biol. Macromol. 2024, 266, 131384. [Google Scholar] [CrossRef]
- Hu, J.; Liu, S.; Fan, C. Applications of functionally-adapted hydrogels in tendon repair. Front. Bioeng. Biotechnol. 2023, 11, 1135090. [Google Scholar] [CrossRef]
- Salthouse, D.; Novakovic, K.; Hilkens, C.M.U.; Ferreira, A.M. Interplay between biomaterials and the immune system: Challenges and opportunities in regenerative medicine. Acta Biomater. 2023, 155, 1–18. [Google Scholar] [CrossRef]
- Kong, F.; Mehwish, N.; Lee, B.H. Emerging albumin hydrogels as personalized biomaterials. Acta Biomater. 2023, 157, 67–90. [Google Scholar] [CrossRef]
- Zhang, X.; Li, K.; Wang, C.; Rao, Y.; Tuan, R.S.; Wang, D.M.; Ker, D.F.E. Facile and rapid fabrication of a novel 3D-printable, visible light-crosslinkable and bioactive polythiourethane for large-to-massive rotator cuff tendon repair. Bioact. Mater. 2024, 37, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Shi, Q.; Li, M.; Chen, Y.; Zhang, T.; Xu, Y.; Liao, Y.; Ding, S.; Wang, Z.; Li, X.; et al. Engineering an enthesis-like graft for rotator cuff repair: An approach to fabricate highly biomimetic scaffold capable of zone-specifically releasing stem cell differentiation inducers. Bioact. Mater. 2022, 16, 451–471. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.; Sun, Y.; Choi, Y.J.; Ha, D.H.; Jeon, I.; Cho, D.W. 3D cell-printing of tendon-bone interface using tissue-derived extracellular matrix bioinks for chronic rotator cuff repair. Biofabrication 2021, 13, 035005. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, G. Bioprinted hASC-laden cell constructs with mechanically stable and cell alignment cue for tenogenic differentiation. Biofabrication 2023, 15, 045006. [Google Scholar] [CrossRef]
- Han, H.; Kim, M.; Yong, U.; Jo, Y.; Choi, Y.M.; Kim, H.J.; Hwang, D.G.; Kang, D.; Jang, J. Tissue-specific gelatin bioink as a rheology modifier for high printability and adjustable tissue properties. Biomater. Sci. 2024, 12, 2599–2613. [Google Scholar] [CrossRef]
- Monteiro, R.F.; Bakht, S.M.; Gomez-Florit, M.; Stievani, F.C.; Alves, A.L.G.; Reis, R.L.; Gomes, M.E.; Domingues, R.M.A. Writing 3D In Vitro Models of Human Tendon within a Biomimetic Fibrillar Support Platform. ACS Appl. Mater. Interfaces 2023, 15, 50598–50611. [Google Scholar] [CrossRef]
- Wang, B.; Barcelo, X.; Von Euw, S.; Kelly, D.J. 3D printing of mechanically functional meniscal tissue equivalents using high concentration extracellular matrix inks. Mater. Today Bio 2023, 20, 100624. [Google Scholar] [CrossRef]
- Ge, F.; Lu, Y.; Li, Q.; Zhang, X. Decellularized Extracellular Matrices for Tissue Engineering and Regeneration. Adv. Exp. Med. Biol. 2020, 1250, 15–31. [Google Scholar] [CrossRef]
- Amirazad, H.; Dadashpour, M.; Zarghami, N. Application of decellularized bone matrix as a bioscaffold in bone tissue engineering. J. Biol. Eng. 2022, 16, 1. [Google Scholar] [CrossRef]
- Kim, Y.S.; Majid, M.; Melchiorri, A.J.; Mikos, A.G. Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng. Transl. Med. 2019, 4, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.; Elsangeedy, E.; Lee, H.; Atala, A.; Yoo, J.J.; Lee, S.J.; Ju, Y.M. In vitro evaluation of functionalized decellularized muscle scaffold for in situ skeletal muscle regeneration. Biomed. Mater. 2019, 14, 045015. [Google Scholar] [CrossRef] [PubMed]
- Ventura, R.D.; Padalhin, A.R.; Park, C.M.; Lee, B.T. Enhanced decellularization technique of porcine dermal ECM for tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109841. [Google Scholar] [CrossRef]
- Liu, C.; Gao, H.; Sun, G.; Jiang, X.; Song, S.; Zhang, J.; Shen, J. Decellularized Scaffold-Based Artificial Vascular Patch for Porcine Vascular Repair. ACS Appl. Bio Mater. 2023, 6, 1071–1080. [Google Scholar] [CrossRef]
- Ghazanfari, S.; Alberti, K.A.; Xu, Q.; Khademhosseini, A. Evaluation of an elastic decellularized tendon-derived scaffold for the vascular tissue engineering application. J. Biomed. Mater. Res. Part A 2019, 107, 1225–1234. [Google Scholar] [CrossRef]
- Kerr, C.M.; Silver, S.E.; Choi, Y.S.; Floy, M.E.; Bradshaw, A.D.; Cho, S.W.; Palecek, S.P.; Mei, Y. Decellularized heart extracellular matrix alleviates activation of hiPSC-derived cardiac fibroblasts. Bioact. Mater. 2024, 31, 463–474. [Google Scholar] [CrossRef]
- Khajavi, M.; Hashemi, M.; Kalalinia, F. Recent advances in optimization of liver decellularization procedures used for liver regeneration. Life Sci. 2021, 281, 119801. [Google Scholar] [CrossRef]
- Leiby, K.L.; Niklason, L.E. Lung Tissue Engineering: Toward a More Deliberate Approach. ACS Biomater. Sci. Eng. 2022, 8, 4625–4628. [Google Scholar] [CrossRef]
- Mallis, P.; Oikonomidis, C.; Dimou, Z.; Stavropoulos-Giokas, C.; Michalopoulos, E.; Katsimpoulas, M. Optimizing Decellularization Strategies for the Efficient Production of Whole Rat Kidney Scaffolds. Tissue Eng. Regen. Med. 2021, 18, 623–640. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.; Pan, D.; Guo, H.; Jiang, H.; Wang, J.; Feng, H.; He, S.; Du, J.; Zhang, M.; et al. Pig-to-human kidney xenotransplants using genetically modified minipigs. Cell Rep. Med. 2024, 5, 101744. [Google Scholar] [CrossRef] [PubMed]
- Porcine heart xenotransplanation in brain-dead decedents. Nat. Med. 2023, 29, 1918–1919. [CrossRef]
- Lamm, V.; Ekser, B.; Vagefi, P.A.; Cooper, D.K.C. Bridging to Allotransplantation-Is Pig Liver Xenotransplantation the Best Option? Transplantation 2022, 106, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Kollmetz, T.; Castillo-Alcala, F.; Veale, R.W.F.; Taghavi, N.; van Heeswijk, V.M.; Persenaire, M.; May, B.C.H.; Dempsey, S.G. Comparative Analysis of Commercially Available Extracellular Matrix Soft Tissue Bioscaffolds. Tissue Eng. Part A 2024. [Google Scholar] [CrossRef]
- Qiao, S.; Peijie, T.; Nan, J. Crosslinking strategies of decellularized extracellular matrix in tissue regeneration. J. Biomed. Mater. Res. Part A 2024, 112, 640–671. [Google Scholar] [CrossRef]
- Fang, W.; Yang, M.; Jin, Y.; Zhang, K.; Wang, Y.; Liu, M.; Wang, Y.; Yang, R.; Fu, Q. Injectable Decellularized Extracellular Matrix-Based Bio-Ink with Excellent Biocompatibility for Scarless Urethra Repair. Gels 2023, 9, 913. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Jiang, Z.; Li, N.; Wang, X.; Ren, L.; Ye, Y.; Pan, Y.; Yang, G. Insights into the use of genetically modified decellularized biomaterials for tissue engineering and regenerative medicine. Adv. Drug Deliv. Rev. 2022, 188, 114413. [Google Scholar] [CrossRef]
- Han, J.; Rindone, A.N.; Elisseeff, J.H. Immunoengineering Biomaterials for Musculoskeletal Tissue Repair across Lifespan. Adv. Mater. 2024, 36, e2311646. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Ye, W.; Yu, J.; Zhang, X.; Li, Y.; Niu, Y.; Ran, S.; Wang, S.; Luo, Z.; et al. High-throughput CRISPR technology: A novel horizon for solid organ transplantation. Front. Immunol. 2023, 14, 1295523. [Google Scholar] [CrossRef]
- Chen, G.H.; Sia, K.C.; Liu, S.W.; Kao, Y.C.; Yang, P.C.; Ho, C.H.; Huang, S.C.; Lee, P.Y.; Liang, M.Z.; Chen, L.; et al. Implantation of MSC spheroid-derived 3D decellularized ECM enriched with the MSC secretome ameliorates traumatic brain injury and promotes brain repair. Biomaterials 2025, 315, 122941. [Google Scholar] [CrossRef]
- Stone, R.N.; Pu, X.; Oxford, J.T. Proteomic dataset for decellularization of porcine auricular cartilage. BMC Res. Notes 2024, 17, 58. [Google Scholar] [CrossRef]
- Kim, Y.H.; Cidonio, G.; Kanczler, J.M.; Oreffo, R.O.; Dawson, J.I. Human bone tissue-derived ECM hydrogels: Controlling physicochemical, biochemical, and biological properties through processing parameters. Bioact. Mater. 2025, 43, 114–128. [Google Scholar] [CrossRef]
- Li, X.; Shan, J.; Chen, X.; Cui, H.; Wen, G.; Yu, Y. Decellularized diseased tissues: Current state-of-the-art and future directions. MedComm 2023, 4, e399. [Google Scholar] [CrossRef]
- Hurst, D.J.; Cooper, D.K.C. Pressing ethical issues relating to clinical pig organ transplantation studies. Xenotransplantation 2024, 31, e12848. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M. Clinical trials for pig-to-human organ transplants inch closer. Nature 2022, 607, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Garry, D.J.; Weiner, J.I.; Greising, S.M.; Sachs, D.H.; Garry, M.G. Xenotransplantation and exotransplantation: Strategies to expand the number of donor organs. Xenotransplantation 2023, 30, e12786. [Google Scholar] [CrossRef]
- Cooper, D.K.C.; Pierson, R.N., III. Milestones on the path to clinical pig organ xenotransplantation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2023, 23, 326–335. [Google Scholar] [CrossRef]
- Sun, J.; Zhong, H.; Du, L.; Li, X.; Ding, Y.; Cao, H.; Liu, Z.; Ge, L. Gene expression profiles of germ-free and conventional piglets from the same litter. Sci. Rep. 2018, 8, 10745. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, Y.; Yang, G.; Sun, J.; Tang, C.; Liang, H.; Ma, J.; Wu, X.; Cao, H.; Wu, M.; et al. Commensal microbiota modulates phenotypic characteristics and gene expression in piglet Peyer’s patches. Front. Physiol. 2023, 14, 1084332. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wen, M.; Zhang, S.; Du, L.; Fan, X.; Liang, H.; Wang, H.; Sun, J.; Ding, Y.; Ge, L.; et al. Research Progress on the Preparation and Application of Decellularized Tendons. Curr. Issues Mol. Biol. 2025, 47, 251. https://doi.org/10.3390/cimb47040251
Li J, Wen M, Zhang S, Du L, Fan X, Liang H, Wang H, Sun J, Ding Y, Ge L, et al. Research Progress on the Preparation and Application of Decellularized Tendons. Current Issues in Molecular Biology. 2025; 47(4):251. https://doi.org/10.3390/cimb47040251
Chicago/Turabian StyleLi, Jing, Mingxing Wen, Sujuan Zhang, Lingfei Du, Xin Fan, Hao Liang, Hong Wang, Jing Sun, Yuchun Ding, Liangpeng Ge, and et al. 2025. "Research Progress on the Preparation and Application of Decellularized Tendons" Current Issues in Molecular Biology 47, no. 4: 251. https://doi.org/10.3390/cimb47040251
APA StyleLi, J., Wen, M., Zhang, S., Du, L., Fan, X., Liang, H., Wang, H., Sun, J., Ding, Y., Ge, L., Ma, J., & Zhang, J. (2025). Research Progress on the Preparation and Application of Decellularized Tendons. Current Issues in Molecular Biology, 47(4), 251. https://doi.org/10.3390/cimb47040251




