Orthobiologics Revisited: A Concise Perspective on Regenerative Orthopedics
Abstract
1. Introduction
2. Methods
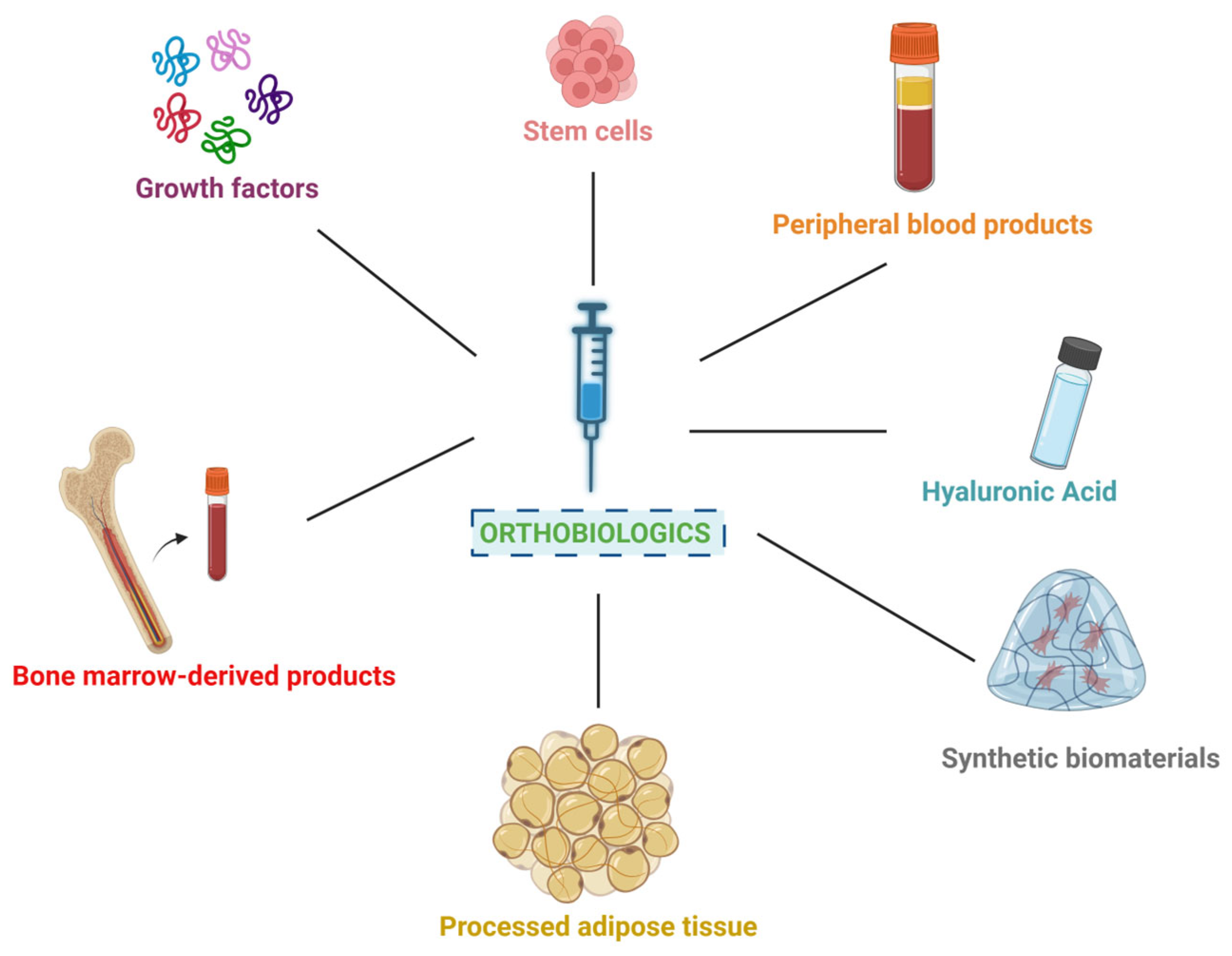
3. Commonly Used Orthobiologics in Regenerative Medicine
- Platelet Derivatives from Peripheral Blood: PRP and PRF are derived from the patient’s own blood through a process of centrifugation. PRP is rich in growth factors that promote healing and tissue regeneration, while PRF includes fibrin to provide a scaffold for cell migration and growth [6,15]. Numerous systematic reviews and meta-analyses have validated the clinical efficacy of PRP and PRF, particularly in the management of osteoarthritis, tendinopathies, and soft tissue repair, confirming their value as evidence-based orthobiologics [25,26,27,28,29].
- Hyaluronic Acid: HA is a naturally occurring substance found in connective tissues and joint fluid. It is commonly used as a viscosupplement to lubricate joints, particularly in the treatment of osteoarthritis, improving joint function and reducing pain [5,22]. Meta-analytical evidence supports the effectiveness of HA in reducing pain and improving function in osteoarthritis, further endorsing its widespread clinical adoption [30,31].
- Bone Marrow-Derived Products: both BMA and BMAC are obtained from the patient’s bone marrow, typically from the posterior superior iliac crest. BMAC is a concentrated form rich in mesenchymal and hematopoietic stem cells (MSCs and HSCs), and growth factors that support tissue repair and regeneration. Despite limitations regarding stem cell count and differentiation capability, bone BMAC still remains a rich source of various regenerative components [32]. In addition to MSCs and HSCs, it also carries megakaryocytes, platelets, growth factors, and cytokines [1,32]. These elements collectively contribute to substantial paracrine effects, which enhance tissue repair and regeneration [1,32]. The presence of these bioactive molecules not only supports cellular proliferation and differentiation, but also modulates the local inflammatory response and promotes angiogenesis, making BMAC a valuable tool in regenerative medicine and orthopedics [32]. Hybrid BMAC may combine BMAC with other orthobiologics, such as PRP, to enhance regenerative potential via synergism [1,23,24]. Systematic reviews have highlighted the therapeutic potential of BMAC in orthopedic applications, including cartilage repair, osteochondral defects, and non-union fractures, with positive clinical outcomes and safety profiles [33,34,35,36].
- Adipose Tissue-Derived Materials: these biologic materials are all obtained through liposuction and processing techniques. Macro-fat and MFAT provide structural support and cushioning, while nano-fat and SVF are rich in various cells, such as adipose-derived stem cells (ADSCs), mesenchymal and endothelial progenitor cells, lymphatic cells, pericytes, leukocyte subtypes, and vascular smooth muscle cells that promote tissue regeneration and healing [7,21,37]. Recent meta-analyses and systematic reviews have demonstrated the clinical utility of SVF and related adipose-derived products in regenerative medicine, particularly in joint preservation and soft tissue reconstruction [38,39,40].
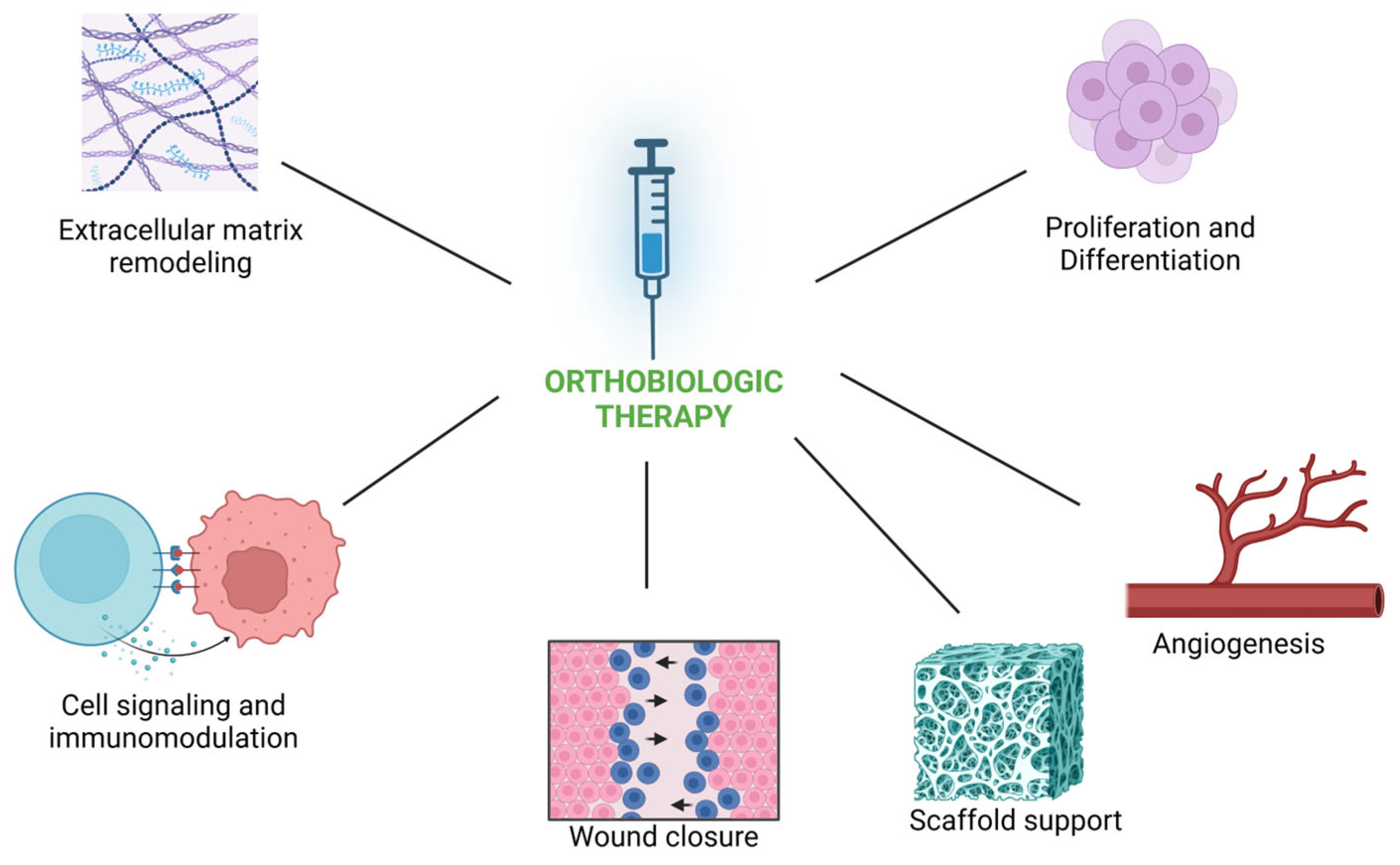
4. Summary of Mechanisms of Action
5. Applications
5.1. Repair of Bone Fractures
5.2. Cartilage Repair and Regeneration
5.3. Tendon and Ligament Injuries
5.4. Soft Tissue Regeneration
6. Delivery Systems
7. Current Limitations of Orthobiologics
8. Future Directions
8.1. Emerging Trends and Developments in Orthobiologics Research
8.2. Advancements in Technology and Treatment Strategies
8.3. Personalized Medicine and Predictive Analytics
8.4. Regulatory and Ethical Considerations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lana, J.F.; da Fonseca, L.F.; Azzini, G.; Santos, G.; Braga, M.; Cardoso Junior, A.M.; Murrell, W.D.; Gobbi, A.; Purita, J.; de Andrade, M.A.P. Bone Marrow Aspirate Matrix: A Convenient Ally in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 2762. [Google Scholar] [CrossRef]
- Panero, A.J.; Everts, P.A.; Nakagawa, H.; Sussman, W.; Qin, X. Basic Science of Allograft Orthobiologics. Phys. Med. Rehabil. Clin. N. Am. 2023, 34, 49–61. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 7, 469. [Google Scholar] [CrossRef] [PubMed]
- Al-Shalawi, F.D.; Mohamed Ariff, A.H.; Jung, D.-W.; Mohd Ariffin, M.K.A.; Seng Kim, C.L.; Brabazon, D.; Al-Osaimi, M.O. Biomaterials as Implants in the Orthopedic Field for Regenerative Medicine: Metal versus Synthetic Polymers. Polymers 2023, 15, 2601. [Google Scholar] [CrossRef]
- Costa, F.R.; Costa Marques, M.R.; Costa, V.C.; Santos, G.S.; Martins, R.A.; Santos, M.d.S.; Santana, M.H.A.; Nallakumarasamy, A.; Jeyaraman, M.; Lana, J.V.B.; et al. Intra-Articular Hyaluronic Acid in Osteoarthritis and Tendinopathies: Molecular and Clinical Approaches. Biomedicines 2023, 11, 1061. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.; Purita, J.; Everts, P.A.; De Mendonça Neto, P.A.T.; de Moraes Ferreira Jorge, D.; Mosaner, T.; Huber, S.C.; Azzini, G.O.M.; da Fonseca, L.F.; Jeyaraman, M.; et al. Platelet-Rich Plasma Power-Mix Gel (Ppm)-An Orthobiologic Optimization Protocol Rich in Growth Factors and Fibrin. Gels 2023, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.S.D.; Lana, A.V.S.D.; da Fonseca, L.F.; Coelho, M.A.; Marques, G.G.; Mosaner, T.; Ribeiro, L.L.; Azzini, G.O.M.; Santos, G.S.; Fonseca, E.; et al. Stromal Vascular Fraction for Knee Osteoarthritis–An Update. J. Stem Cells Regen. Med. 2022, 18, 11–20. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Santos, G.S.; Alkass, N.; Chiesa, T.L.; Azzini, G.O.; da Fonseca, L.F.; dos Santos, A.F.; Rodrigues, B.L.; Mosaner, T.; Lana, J.F. The Regenerative Mechanisms of Platelet-Rich Plasma: A Review. Cytokine 2021, 144, 155560. [Google Scholar] [CrossRef]
- Murray, I.R.; Chahla, J.; Wordie, S.J.; Shapiro, S.A.; Piuzzi, N.S.; Frank, R.M.; Halbrecht, J.; Okada, K.; Nakamura, N.; Mandelbaum, B.; et al. Regulatory and Ethical Aspects of Orthobiologic Therapies. Orthop. J. Sports Med. 2022, 10, 23259671221101626. [Google Scholar] [CrossRef]
- Hafsi, K.; McKay, J.; Li, J.; Lana, J.F.; Macedo, A.; Santos, G.S.; Murrell, W.D. Nutritional, Metabolic and Genetic Considerations to Optimise Regenerative Medicine Outcome for Knee Osteoarthritis. J. Clin. Orthop. Trauma 2019, 10, 2–8. [Google Scholar] [CrossRef]
- Lana, J.F.; Macedo, A.; Ingrao, I.L.G.; Huber, S.C.; Santos, G.S.; Santana, M.H.A. Leukocyte-Rich PRP for Knee Osteoarthritis: Current Concepts. J. Clin. Orthop. Trauma 2019, 10, S179–S182. [Google Scholar] [CrossRef] [PubMed]
- Md Fadilah, N.I.; Mohd Abdul Kader Jailani, M.S.; Badrul Hisham, M.A.I.; Sunthar Raj, N.; Shamsuddin, S.A.; Ng, M.H.; Fauzi, M.B.; Maarof, M. Cell Secretomes for Wound Healing and Tissue Regeneration: Next Generation Acellular Based Tissue Engineered Products. J. Tissue Eng. 2022, 13, 20417314221114273. [Google Scholar] [CrossRef] [PubMed]
- Costela-Ruiz, V.J.; Melguizo-Rodríguez, L.; Bellotti, C.; Illescas-Montes, R.; Stanco, D.; Arciola, C.R.; Lucarelli, E. Different Sources of Mesenchymal Stem Cells for Tissue Regeneration: A Guide to Identifying the Most Favorable One in Orthopedics and Dentistry Applications. Int. J. Mol. Sci. 2022, 23, 6356. [Google Scholar] [CrossRef]
- Azzini, G.O.M.; Santos, G.S.; Visoni, S.B.C.; Azzini, V.O.M.; Santos, R.G.d.; Huber, S.C.; Lana, J.F. Metabolic Syndrome and Subchondral Bone Alterations: The Rise of Osteoarthritis—A Review. J. Clin. Orthop. Trauma 2020, 11, S849–S855. [Google Scholar] [CrossRef]
- Santos, L.C.; Lana, G.L.; Santos, G.S.; Visoni, S.B.C.; Brigagão, R.J.; Santos, N.; Sobreiro, R.; da Cruz Silva Reis, A.; Rodrigues, B.L.; Ferrari, S.; et al. The Biological Role of Platelet Derivatives in Regenerative Aesthetics. Int. J. Mol. Sci. 2024, 25, 5604. [Google Scholar] [CrossRef] [PubMed]
- McKay, J.; Frantzen, K.; Vercruyssen, N.; Hafsi, K.; Opitz, T.; Davis, A.; Murrell, W. Rehabilitation Following Regenerative Medicine Treatment for Knee Osteoarthritis-Current Concept Review. J. Clin. Orthop. Trauma 2019, 10, 59–66. [Google Scholar] [CrossRef]
- Santos Duarte Lana, J.F.; Furtado da Fonseca, L.; Mosaner, T.; Tieppo, C.E.; Marques Azzini, G.O.; Ribeiro, L.L.; Setti, T.; Purita, J. Bone Marrow Aspirate Clot: A Feasible Orthobiologic. J. Clin. Orthop. Trauma 2020, 11, S789–S794. [Google Scholar] [CrossRef]
- Everts, P.A.; Mazzola, T.; Mautner, K.; Randelli, P.S.; Podesta, L. Modifying Orthobiological PRP Therapies Are Imperative for the Advancement of Treatment Outcomes in Musculoskeletal Pathologies. Biomedicines 2022, 10, 2933. [Google Scholar] [CrossRef]
- Huddleston, H.P.; Maheshwer, B.; Wong, S.E.; Chahla, J.; Cole, B.J.; Yanke, A.B. An Update on the Use of Orthobiologics: Use of Biologics for Osteoarthritis. Oper. Tech. Sports Med. 2020, 28, 150759. [Google Scholar] [CrossRef]
- Noback, P.C.; Donnelley, C.A.; Yeatts, N.C.; Parisien, R.L.; Fleischli, J.E.; Ahmad, C.S.; Moorman, C.T.; Trofa, D.P.; Saltzman, B.M. Utilization of Orthobiologics by Sports Medicine Physicians: A Survey-Based Study. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2021, 5, e20.00185. [Google Scholar] [CrossRef]
- Domingues, R.B.; von Rautenfeld, M.; Kavalco, C.M.; Caliari, C.; Dellagiustina, C.; da Fonseca, L.F.; Costa, F.R.; da Cruz Silva Reis, A.; Santos, G.S.; Azzini, G.; et al. The Role of Orthobiologics in Chronic Wound Healing. Int. Wound J. 2024, 21, e14854. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.R.; Santos, M.d.S.; Martins, R.A.; Costa, C.B.; Hamdan, P.C.; Da Silva, M.B.; Azzini, G.O.M.; Pires, L.; Menegassi, Z.; Santos, G.S.; et al. The Synergistic Effects of Hyaluronic Acid and Platelet-Rich Plasma for Patellar Chondropathy. Biomedicines 2023, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.S.D.; da Fonseca, L.F.; Macedo, R.D.R.; Mosaner, T.; Murrell, W.; Kumar, A.; Purita, J.; de sAndrade, M.A.P. Platelet-Rich Plasma vs Bone Marrow Aspirate Concentrate: An Overview of Mechanisms of Action and Orthobiologic Synergistic Effects. World J. Stem Cells 2021, 13, 155–167. [Google Scholar] [CrossRef]
- Purita, J.; Lana, J.F.S.D.; Kolber, M.; Rodrigues, B.L.; Mosaner, T.; Santos, G.S.; Caliari-Oliveira, C.; Huber, S.C. Bone Marrow-Derived Products: A Classification Proposal—Bone Marrow Aspirate, Bone Marrow Aspirate Concentrate or Hybrid? World J. Stem Cells 2020, 12, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Oeding, J.F.; Varady, N.H.; Fearington, F.W.; Pareek, A.; Strickland, S.M.; Nwachukwu, B.U.; Camp, C.L.; Krych, A.J. Platelet-Rich Plasma Versus Alternative Injections for Osteoarthritis of the Knee: A Systematic Review and Statistical Fragility Index-Based Meta-Analysis of Randomized Controlled Trials. Am. J. Sports Med. 2024, 52, 3147–3160. [Google Scholar] [CrossRef]
- Xiong, Y.; Gong, C.; Peng, X.; Liu, X.; Su, X.; Tao, X.; Li, Y.; Wen, Y.; Li, W. Efficacy and Safety of Platelet-Rich Plasma Injections for the Treatment of Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Med. 2023, 10, 1204144. [Google Scholar] [CrossRef]
- Berrigan, W.; Tao, F.; Kopcow, J.; Park, A.L.; Allen, I.; Tahir, P.; Reddy, A.; Bailowitz, Z. The Effect of Platelet Dose on Outcomes after Platelet Rich Plasma Injections for Musculoskeletal Conditions: A Systematic Review and Meta-Analysis. Curr. Rev. Musculoskelet. Med. 2024, 17, 570–588. [Google Scholar] [CrossRef]
- Jawanda, H.; Khan, Z.A.; Warrier, A.A.; Acuña, A.J.; Allahabadi, S.; Kaplan, D.J.; Ritz, E.; Jackson, G.R.; Mameri, E.S.; Batra, A.; et al. Platelet-Rich Plasma, Bone Marrow Aspirate Concentrate, and Hyaluronic Acid Injections Outperform Corticosteroids in Pain and Function Scores at a Minimum of 6 Months as Intra-Articular Injections for Knee Osteoarthritis: A Systematic Review and Network Meta-Analysis. Arthroscopy 2024, 40, 1623–1636.e1. [Google Scholar] [CrossRef]
- Pereira, V.B.S.; Lago, C.A.P.; Almeida, R.d.A.C.; Barbirato, D.d.S.; Vasconcelos, B.C.d.E. Biological and Cellular Properties of Advanced Platelet-Rich Fibrin (A-PRF) Compared to Other Platelet Concentrates: Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 482. [Google Scholar] [CrossRef]
- Salwowska, N.M.; Bebenek, K.A.; Żądło, D.A.; Wcisło-Dziadecka, D.L. Physiochemical Properties and Application of Hyaluronic Acid: A Systematic Review. J. Cosmet. Dermatol. 2016, 15, 520–526. [Google Scholar] [CrossRef]
- Voigt, J.; Driver, V.R. Hyaluronic Acid Derivatives and Their Healing Effect on Burns, Epithelial Surgical Wounds, and Chronic Wounds: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Wound Repair. Regen. 2012, 20, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.J.; Jeon, Y.S.; Park, J.S.; Bae, G.C.; Kim, J.; Kim, M.K. Comparison of Bone Marrow Aspirate Concentrate and Allogenic Human Umbilical Cord Blood Derived Mesenchymal Stem Cell Implantation on Chondral Defect of Knee: Assessment of Clinical and Magnetic Resonance Imaging Outcomes at 2-Year Follow-Up. Cell Transpl. 2020, 29, 0963689720943581. [Google Scholar] [CrossRef]
- Awad, M.E.; Hussein, K.A.; Helwa, I.; Abdelsamid, M.F.; Aguilar-Perez, A.; Mohsen, I.; Hunter, M.; Hamrick, M.W.; Isales, C.M.; Elsalanty, M.; et al. Meta-Analysis and Evidence Base for the Efficacy of Autologous Bone Marrow Mesenchymal Stem Cells in Knee Cartilage Repair: Methodological Guidelines and Quality Assessment. Stem Cells Int. 2019, 2019, 3826054. [Google Scholar] [CrossRef]
- Santinoni, C.d.S.; Levi, Y.L.d.A.S.; Toneto, J.P.P.; Cazuza, J.A.; Maia, L.P.; Verri, F.R. Bone Marrow Aspirate: A Viable Source of Stem Cells for Bone Regeneration. A Systematic Review. Res. Soc. Dev. 2021, 10, e94101119265. [Google Scholar] [CrossRef]
- Bachir, R.M.; Zaia, I.M.; Santos, G.S.; Fonseca, L.F.d.; Boni, G.; Guercia, R.F.; Ferreira, G.F.; Lana, J.F.S.D. Bone Marrow Aspirate Concentrate Improves Outcomes in Adults With Osteochondral Dissecans of the Talus and Achilles Rupture. Arthroscopy 2023, 39, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Imam, M.A.; Mahmoud, S.S.S.; Holton, J.; Abouelmaati, D.; Elsherbini, Y.; Snow, M. A Systematic Review of the Concept and Clinical Applications of Bone Marrow Aspirate Concentrate in Orthopaedics. Sicot-J. 2017, 3, 17. [Google Scholar] [CrossRef]
- Ude, C.C.; Shah, S.; Ogueri, K.S.; Nair, L.S.; Laurencin, C.T. Stromal Vascular Fraction for Osteoarthritis of the Knee Regenerative Engineering. Regen. Eng. Transl. Med. 2022, 8, 210–224. [Google Scholar] [CrossRef]
- Bolia, I.K.; Bougioukli, S.; Hill, W.J.; Trasolini, N.A.; Petrigliano, F.A.; Lieberman, J.R.; Weber, A.E. Clinical Efficacy of Bone Marrow Aspirate Concentrate Versus Stromal Vascular Fraction Injection in Patients With Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Am. J. Sports Med. 2022, 50, 1451–1461. [Google Scholar] [CrossRef]
- Gentile, P.; Sterodimas, A.; Pizzicannella, J.; Dionisi, L.; De Fazio, D.; Calabrese, C.; Garcovich, S. Systematic Review: Allogenic Use of Stromal Vascular Fraction (SVF) and Decellularized Extracellular Matrices (ECM) as Advanced Therapy Medicinal Products (ATMP) in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 4982. [Google Scholar] [CrossRef]
- Lee, H.; Lim, Y.; Lee, S.-H. Rapid-Acting Pain Relief in Knee Osteoarthritis: Autologous-Cultured Adipose-Derived Mesenchymal Stem Cells Outperform Stromal Vascular Fraction: A Systematic Review and Meta-Analysis. Stem Cell Res. Ther. 2024, 15, 446. [Google Scholar] [CrossRef]
- Xu, Q.; Hou, W.; Zhao, B.; Fan, P.; Wang, S.; Wang, L.; Gao, J. Mesenchymal Stem Cells Lineage and Their Role in Disease Development. Mol. Med. 2024, 30, 207. [Google Scholar] [CrossRef] [PubMed]
- Mohyeddin Bonab, M.; Talebian, F.; Borzabadi, A.; Nasr, V.; Abedi Kooshlshahi, A.; Anisie, F.; Haghshenas, R.; Shalbafan, B.; Janzamin, E.; Shahbeigi, S. A Novel Method for Maintaining the Stability of Freshly Cultured Mesenchymal Stem Cells in Clinical Grade Injection Ready State without Cryopreservation. Transl. Med. Commun. 2021, 6, 24. [Google Scholar] [CrossRef]
- Antebi, B.; Asher, A.M.; Rodriguez, L.A.; Moore, R.K.; Mohammadipoor, A.; Cancio, L.C. Cryopreserved Mesenchymal Stem Cells Regain Functional Potency Following a 24-h Acclimation Period. J. Transl. Med. 2019, 17, 297. [Google Scholar] [CrossRef] [PubMed]
- Neo, S.H.; Her, Z.; Othman, R.; Tee, C.A.; Ong, L.C.; Wang, Y.; Tan, I.; Tan, J.; Yang, Y.; Yang, Z.; et al. Expansion of Human Bone Marrow-Derived Mesenchymal Stromal Cells with Enhanced Immunomodulatory Properties. Stem Cell Res. Ther. 2023, 14, 259. [Google Scholar] [CrossRef]
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H.; et al. Clinical Application of Mesenchymal Stem Cell in Regenerative Medicine: A Narrative Review. Stem Cell Res. Ther. 2022, 13, 366. [Google Scholar] [CrossRef]
- Kulebyakin, K.Y.; Nimiritsky, P.P.; Makarevich, P.I. Growth Factors in Regeneration and Regenerative Medicine: “The Cure and the Cause”. Front. Endocrinol. 2020, 11, 384. [Google Scholar] [CrossRef]
- Sun, Y.; Wan, B.; Wang, R.; Zhang, B.; Luo, P.; Wang, D.; Nie, J.-J.; Chen, D.; Wu, X. Mechanical Stimulation on Mesenchymal Stem Cells and Surrounding Microenvironments in Bone Regeneration: Regulations and Applications. Front. Cell Dev. Biol. 2022, 10, 808303. [Google Scholar] [CrossRef]
- Wu, M.; Wu, S.; Chen, W.; Li, Y.-P. The Roles and Regulatory Mechanisms of TGF-β and BMP Signaling in Bone and Cartilage Development, Homeostasis and Disease. Cell Res. 2024, 34, 101–123. [Google Scholar] [CrossRef]
- Lin, H.; Tang, Y.; Lozito, T.P.; Oyster, N.; Wang, B.; Tuan, R.S. Efficient in Vivo Bone Formation by BMP-2 Engineered Human Mesenchymal Stem Cells Encapsulated in a Projection Stereolithographically Fabricated Hydrogel Scaffold. Stem Cell Res. Ther. 2019, 10, 254. [Google Scholar] [CrossRef]
- Coricor, G.; Serra, R. TGF-β Regulates Phosphorylation and Stabilization of Sox9 Protein in Chondrocytes through P38 and Smad Dependent Mechanisms. Sci. Rep. 2016, 6, 38616. [Google Scholar] [CrossRef]
- Ferrai, C.; Schulte, C. Mechanotransduction in Stem Cells. Eur. J. Cell Biol. 2024, 103, 151417. [Google Scholar] [CrossRef]
- Killaars, A.R.; Grim, J.C.; Walker, C.J.; Hushka, E.A.; Brown, T.E.; Anseth, K.S. Extended Exposure to Stiff Microenvironments Leads to Persistent Chromatin Remodeling in Human Mesenchymal Stem Cells. Adv. Sci. 2018, 6, 1801483. [Google Scholar] [CrossRef]
- Park, J.S.; Chu, J.S.; Tsou, A.D.; Diop, R.; Tang, Z.; Wang, A.; Li, S. The Effect of Matrix Stiffness on the Differentiation of Mesenchymal Stem Cells in Response to TGF-β. Biomaterials 2011, 32, 3921–3930. [Google Scholar] [CrossRef]
- Sun, M.; Chi, G.; Xu, J.; Tan, Y.; Xu, J.; Lv, S.; Xu, Z.; Xia, Y.; Li, L.; Li, Y. Extracellular Matrix Stiffness Controls Osteogenic Differentiation of Mesenchymal Stem Cells Mediated by Integrin A5. Stem Cell Res. Ther. 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Vallés, G.; Bensiamar, F.; Maestro-Paramio, L.; García-Rey, E.; Vilaboa, N.; Saldaña, L. Influence of Inflammatory Conditions Provided by Macrophages on Osteogenic Ability of Mesenchymal Stem Cells. Stem Cell Res. Ther. 2020, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, P.; Feldheim, M.; Preusse-Prange, A.; Weitkamp, J.T.; Haake, M.; Eglin, D.; Rolauffs, B.; Fay, J.; Seekamp, A.; Grodzinsky, A.J.; et al. Chondrogenic Potential of IL-10 in Mechanically Injured Cartilage and Cellularized Collagen ACI Grafts. Osteoarthr. Cartil. 2018, 26, 264–275. [Google Scholar] [CrossRef]
- Futrega, K.; Robey, P.G.; Klein, T.J.; Crawford, R.W.; Doran, M.R. A Single Day of TGF-Β1 Exposure Activates Chondrogenic and Hypertrophic Differentiation Pathways in Bone Marrow-Derived Stromal Cells. Commun. Biol. 2021, 4, 29. [Google Scholar] [CrossRef]
- Sonmez Kaplan, S.; Sazak Ovecoglu, H.; Genc, D.; Akkoc, T. TNF-α, IL-1B and IL-6 Affect the Differentiation Ability of Dental Pulp Stem Cells. BMC Oral. Health 2023, 23, 555. [Google Scholar] [CrossRef]
- Pratim Das, P.; Medhi, S. Role of Inflammasomes and Cytokines in Immune Dysfunction of Liver Cirrhosis. Cytokine 2023, 170, 156347. [Google Scholar] [CrossRef]
- Cassano, J.M.; Kennedy, J.G.; Ross, K.A.; Fraser, E.J.; Goodale, M.B.; Fortier, L.A. Bone Marrow Concentrate and Platelet-Rich Plasma Differ in Cell Distribution and Interleukin 1 Receptor Antagonist Protein Concentration. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 333–342. [Google Scholar] [CrossRef]
- Thampatty, B.P.; Li, H.; Im, H.J.; Wang, J.H.C. EP4 Receptor Regulates Collagen Type-I, MMP-1, and MMP-3 Gene Expression in Human Tendon Fibroblasts in Response to IL-1β Treatment. Gene 2007, 386, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.; Lana, J.F.; Onishi, K.; Buford, D.; Peng, J.; Mahmood, A.; Fonseca, L.F.; van Zundert, A.; Podesta, L. Angiogenesis and Tissue Repair Depend on Platelet Dosing and Bioformulation Strategies Following Orthobiological Platelet-Rich Plasma Procedures: A Narrative Review. Biomedicines 2023, 11, 1922. [Google Scholar] [CrossRef]
- Ragni, E.; Perucca Orfei, C.; De Luca, P.; Libonati, F.; de Girolamo, L. Tissue-Protective and Anti-Inflammatory Landmark of PRP-Treated Mesenchymal Stromal Cells Secretome for Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 15908. [Google Scholar] [CrossRef]
- ElHawary, H.; Baradaran, A.; Abi-Rafeh, J.; Vorstenbosch, J.; Xu, L.; Efanov, J.I. Bone Healing and Inflammation: Principles of Fracture and Repair. Semin. Plast. Surg. 2021, 35, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Steppe, L.; Megafu, M.; Tschaffon-Müller, M.E.A.; Ignatius, A.; Haffner-Luntzer, M. Fracture Healing Research: Recent Insights. Bone Rep. 2023, 19, 101686. [Google Scholar] [CrossRef]
- Gharpinde, M.R.; Pundkar, A.; Shrivastava, S.; Patel, H.; Chandanwale, R. A Comprehensive Review of Platelet-Rich Plasma and Its Emerging Role in Accelerating Bone Healing. Cureus 2024, 16, e54122. [Google Scholar] [CrossRef]
- Benshabat, D.; Factor, S.; Maman, E.; Khoury, A.; Krespi, R.; Ashkenazi, I.; Chechik, O.; Dolkart, O. Addition of Bone Marrow Aspirate Concentrate Resulted in High Rate of Healing and Good Functional Outcomes in the Treatment of Clavicle Fracture Nonunion: A Retrospective Case Series. J. Clin. Med. 2021, 10, 4749. [Google Scholar] [CrossRef]
- Blanton, C.M.; Clougherty, C.O. The Role of Bone Marrow Aspirate in Osseous and Soft Tissue Pathology. Clin. Podiatr. Med. Surg. 2021, 38, 1–16. [Google Scholar] [CrossRef]
- Lippi, L.; Ferrillo, M.; Turco, A.; Folli, A.; Moalli, S.; Refati, F.; Perrero, L.; Ammendolia, A.; de Sire, A.; Invernizzi, M. Multidisciplinary Rehabilitation after Hyaluronic Acid Injections for Elderly with Knee, Hip, Shoulder, and Temporomandibular Joint Osteoarthritis. Medicina 2023, 59, 2047. [Google Scholar] [CrossRef]
- Kim, Y.S.; Suh, D.S.; Tak, D.H.; Kwon, Y.B.; Koh, Y.G. Adipose-Derived Stromal Vascular Fractions Are Comparable With Allogenic Human Umbilical Cord Blood–Derived Mesenchymal Stem Cells as a Supplementary Strategy of High Tibial Osteotomy for Varus Knee Osteoarthritis. Arthrosc. Sports Med. Rehabil. 2023, 5, e751–e764. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jones, I.A.; Park, C.; Vangsness, C.T. The Efficacy of Platelet-Rich Plasma on Tendon and Ligament Healing: A Systematic Review and Meta-Analysis With Bias Assessment. Am. J. Sports Med. 2018, 46, 2020–2032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, F.; Augi, T.; Williamson, K.M.; Onishi, K.; Hogan, M.V.; Neal, M.D.; Wang, J.H.-C. Platelet HMGB1 in Platelet-Rich Plasma (PRP) Promotes Tendon Wound Healing. PLoS ONE 2021, 16, e0251166. [Google Scholar] [CrossRef]
- Lu, J.; Li, H.; Zhang, Z.; Xu, R.; Wang, J.; Jin, H. Platelet-Rich Plasma in the Pathologic Processes of Tendinopathy: A Review of Basic Science Studies. Front. Bioeng. Biotechnol. 2023, 11, 1187974. [Google Scholar] [CrossRef]
- Ratajczak, P.; Maciejak, O.; Kopciuch, D.; Paczkowska, A.; Zaprutko, T.; Kus, K. Directions of Hyaluronic Acid Application in Cosmetology. J. Cosmet. Dermatol. 2023, 22, 862–871. [Google Scholar] [CrossRef]
- Glass, G.E.; Ferretti, P. Adipose-Derived Stem Cells in Aesthetic Surgery. Aesthet. Surg. J. 2019, 39, 423–438. [Google Scholar] [CrossRef]
- Naderi, N.; Combellack, E.J.; Griffin, M.; Sedaghati, T.; Javed, M.; Findlay, M.W.; Wallace, C.G.; Mosahebi, A.; Butler, P.E.; Seifalian, A.M.; et al. The Regenerative Role of Adipose-derived Stem Cells (ADSC) in Plastic and Reconstructive Surgery. Int. Wound J. 2016, 14, 112–124. [Google Scholar] [CrossRef]
- Yuan, C.; Song, W.; Jiang, X.; Wang, Y.; Li, C.; Yu, W.; He, Y. Adipose-Derived Stem Cell-Based Optimization Strategies for Musculoskeletal Regeneration: Recent Advances and Perspectives. Stem Cell Res. Ther. 2024, 15, 91. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Chen, Y.; Yuan, L.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020, 2020, 8810813. [Google Scholar] [CrossRef]
- Peçanha, R.; Bagno, L.d.L.E.S.; Ribeiro, M.B.; Robottom Ferreira, A.B.; Moraes, M.O.; Zapata-Sudo, G.; Kasai-Brunswick, T.H.; Campos-de-Carvalho, A.C.; Goldenberg, R.C.d.S.; Saar Werneck-de-Castro, J.P. Adipose-Derived Stem-Cell Treatment of Skeletal Muscle Injury. J. Bone Jt. Surg. Am. 2012, 94, 609–617. [Google Scholar] [CrossRef]
- Yasin, A.; Ren, Y.; Li, J.; Sheng, Y.; Cao, C.; Zhang, K. Advances in Hyaluronic Acid for Biomedical Applications. Front. Bioeng. Biotechnol. 2022, 10, 910290. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Hoing, J.L.; Newman, M.; Simman, R. Role of Hyaluronic Acid Treatment in the Prevention of Keloid Scarring. J. Am. Coll. Clin. Wound Spec. 2013, 4, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Bravo, B.; Correia, P.; Gonçalves Junior, J.E.; Sant’Anna, B.; Kerob, D. Benefits of Topical Hyaluronic Acid for Skin Quality and Signs of Skin Aging: From Literature Review to Clinical Evidence. Dermatol. Ther. 2022, 35, e15903. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic Acid: A Key Molecule in Skin Aging. Dermatoendocrinol 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Longinotti, C. The Use of Hyaluronic Acid Based Dressings to Treat Burns: A Review. Burn. Trauma 2014, 2, 162–168. [Google Scholar] [CrossRef]
- Antoszewska, M.; Sokolewicz, E.M.; Barańska-Rybak, W. Wide Use of Hyaluronic Acid in the Process of Wound Healing—A Rapid Review. Sci. Pharm. 2024, 92, 23. [Google Scholar] [CrossRef]
- Amiryaghoubi, N.; Fathi, M.; Barar, J.; Omidi, Y. Hydrogel-Based Scaffolds for Bone and Cartilage Tissue Engineering and Regeneration. React. Funct. Polym. 2022, 177, 105313. [Google Scholar] [CrossRef]
- Bernuzzi, G.; Petraglia, F.; Pedrini, M.F.; De Filippo, M.; Pogliacomi, F.; Verdano, M.A.; Costantino, C. Use of Platelet-Rich Plasma in the Care of Sports Injuries: Our Experience with Ultrasound-Guided Injection. Blood Transfus. 2014, 12, s229–s234. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Zimmerling, A.; Sunil, C.; Zhou, Y.; Chen, X. Development of a Nanoparticle System for Controlled Release in Bioprinted Respiratory Scaffolds. J. Funct. Biomater. 2024, 15, 20. [Google Scholar] [CrossRef]
- de Oliveira, C.A.A.; Oliveira, B.S.; Theodoro, R.; Wang, J.; Santos, G.S.; Rodrigues, B.L.; Rodrigues, I.J.; Jorge, D.d.M.F.; Jeyaraman, M.; Everts, P.A.; et al. Orthobiologic Management Options for Degenerative Disc Disease. Bioengineering 2024, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Bai, Y.; Qin, X.; Liu, J.; Huang, W.; Lv, Q. Current Understanding of Hydrogel for Drug Release and Tissue Engineering. Gels 2022, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Meissner, S.; Raos, B.; Svirskis, D. Hydrogels Can Control the Presentation of Growth Factors and Thereby Improve Their Efficacy in Tissue Engineering. Eur. J. Pharm. Biopharm. 2022, 181, 1–21. [Google Scholar] [CrossRef]
- Ahmed, A.S.I.; Sheng, M.H.; Wasnik, S.; Baylink, D.J.; Lau, K.-H.W. Effect of Aging on Stem Cells. World J. Exp. Med. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tey, R.V.; Haldankar, P.; Joshi, V.R.; Raj, R.; Maradi, R. Variability in Platelet-Rich Plasma Preparations Used in Regenerative Medicine: A Comparative Analysis. Stem Cells Int. 2022, 2022, 3852898. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Muthu, S.; Amarnath, S.S. Barriers and Solutions Towards Integrating Orthobiologics into Clinical Orthopaedic Practice. Indian J. Orthop. 2024, 58, 987–990. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of Fibrosis: Therapeutic Translation for Fibrotic Disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the Potential of Hydrogels for Advanced Therapeutic Applications: Current Achievements and Future Directions. Sig. Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Matsui, W.H. Cancer Stem Cell Signaling Pathways. Medicine 2016, 95, S8–S19. [Google Scholar] [CrossRef]
- Aderinto, N.; Abdulbasit, M.O.; Olatunji, D. Stem Cell-Based Combinatorial Therapies for Spinal Cord Injury: A Narrative Review of Current Research and Future Directions. Ann. Med. Surg. 2023, 85, 3943–3954. [Google Scholar] [CrossRef]
- Towe, M.; Peta, A.; Saltzman, R.G.; Balaji, N.; Chu, K.; Ramasamy, R. The Use of Combination Regenerative Therapies for Erectile Dysfunction: Rationale and Current Status. Int. J. Impot. Res. 2022, 34, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Dos-Santos, G.; Teixeira-Pinheiro, L.C.; da Silva-Júnior, A.J.; Carvalho, L.R.P.d.; Mesentier-Louro, L.A.; Hauswirth, W.W.; Mendez-Otero, R.; Santiago, M.F.; Petrs-Silva, H. Effects of a Combinatorial Treatment with Gene and Cell Therapy on Retinal Ganglion Cell Survival and Axonal Outgrowth after Optic Nerve Injury. Gene Ther. 2020, 27, 27–39. [Google Scholar] [CrossRef]
- Daneste, H.; Mohammadzadeh Boukani, L.; Ramezani, N.; Asadi, F.; Zaidan, H.K.; Sadeghzade, A.; Ehsannia, M.; Azarashk, A.; Gholizadeh, N. Combination Therapy along with Mesenchymal Stem Cells in Wound Healing; the State of the Art. Adv. Med. Sci. 2023, 68, 441–449. [Google Scholar] [CrossRef]
- Hsu, M.-N.; Chang, Y.-H.; Truong, V.A.; Lai, P.-L.; Nguyen, T.K.N.; Hu, Y.-C. CRISPR Technologies for Stem Cell Engineering and Regenerative Medicine. Biotechnol. Adv. 2019, 37, 107447. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, H.; Domb, A.J.; Sharifzadeh, G.; Nahum, V. Gene Therapy for Regenerative Medicine. Pharmaceutics 2023, 15, 856. [Google Scholar] [CrossRef]
- Han, F.; Wang, J.; Ding, L.; Hu, Y.; Li, W.; Yuan, Z.; Guo, Q.; Zhu, C.; Yu, L.; Wang, H.; et al. Tissue Engineering and Regenerative Medicine: Achievements, Future, and Sustainability in Asia. Front. Bioeng. Biotechnol. 2020, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Liu, Y.; Li, W.; Ma, H.; Li, T.; Ma, X.; Mao, Y.; Liang, Q.; Ma, Z.; Wang, J. Development and Application of 3D Bioprinted Scaffolds Supporting Induced Pluripotent Stem Cells. Biomed. Res. Int. 2021, 2021, 4910816. [Google Scholar] [CrossRef]
- Yazdanpanah, Z.; Johnston, J.D.; Cooper, D.M.L.; Chen, X. 3D Bioprinted Scaffolds for Bone Tissue Engineering: State-Of-The-Art and Emerging Technologies. Front. Bioeng. Biotechnol. 2022, 10, 824156. [Google Scholar] [CrossRef]
- Saini, G.; Segaran, N.; Mayer, J.L.; Saini, A.; Albadawi, H.; Oklu, R. Applications of 3D Bioprinting in Tissue Engineering and Regenerative Medicine. J. Clin. Med. 2021, 10, 4966. [Google Scholar] [CrossRef]
- Valdoz, J.C.; Johnson, B.C.; Jacobs, D.J.; Franks, N.A.; Dodson, E.L.; Sanders, C.; Cribbs, C.G.; Van Ry, P.M. The ECM: To Scaffold, or Not to Scaffold, That Is the Question. Int. J. Mol. Sci. 2021, 22, 12690. [Google Scholar] [CrossRef]
- Budharaju, H.; Sundaramurthi, D.; Sethuraman, S. Embedded 3D Bioprinting—An Emerging Strategy to Fabricate Biomimetic & Large Vascularized Tissue Constructs. Bioact. Mater. 2024, 32, 356–384. [Google Scholar] [CrossRef]
- Ahmad, N.; Bukhari, S.N.A.; Hussain, M.A.; Ejaz, H.; Munir, M.U.; Amjad, M.W. Nanoparticles Incorporated Hydrogels for Delivery of Antimicrobial Agents: Developments and Trends. RSC Adv. 2024, 14, 13535–13564. [Google Scholar] [CrossRef]
- Chabria, Y.; Duffy, G.P.; Lowery, A.J.; Dwyer, R.M. Hydrogels: 3D Drug Delivery Systems for Nanoparticles and Extracellular Vesicles. Biomedicines 2021, 9, 1694. [Google Scholar] [CrossRef] [PubMed]
- Ogay, V.; Mun, E.A.; Kudaibergen, G.; Baidarbekov, M.; Kassymbek, K.; Zharkinbekov, Z.; Saparov, A. Progress and Prospects of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration. Polymers 2020, 12, 2881. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.C.; Wang, Z. Precision Medicine: Disease Subtyping and Tailored Treatment. Cancers 2023, 15, 3837. [Google Scholar] [CrossRef]
- Davenport, T.; Kalakota, R. The Potential for Artificial Intelligence in Healthcare. Future Healthc. J. 2019, 6, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing Healthcare: The Role of Artificial Intelligence in Clinical Practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef]
- Jang, K.; Berrigan, W.A.; Mautner, K. Regulatory Considerations of Orthobiologic Procedures. Phys. Med. Rehabil. Clin. N. Am. 2023, 34, 275–283. [Google Scholar] [CrossRef]
- Thind, M.; Kowey, P.R. The Role of the Food and Drug Administration in Drug Development: On the Subject of Proarrhythmia Risk. J. Innov. Card. Rhythm. Manag. 2020, 11, 3958–3967. [Google Scholar] [CrossRef]
- Goula, A.; Gkioka, V.; Michalopoulos, E.; Katsimpoulas, M.; Noutsias, M.; Sarri, E.F.; Stavropoulos, C.; Kostakis, A. Advanced Therapy Medicinal Products Challenges and Perspectives in Regenerative Medicine. J. Clin. Med. Res. 2020, 12, 780–786. [Google Scholar] [CrossRef]
- Petrini, C. Ethical and Legal Considerations Regarding the Ownership and Commercial Use of Human Biological Materials and Their Derivatives. J. Blood Med. 2012, 3, 87–96. [Google Scholar] [CrossRef] [PubMed]
| Orthobiologic Source | Main Components | Characteristics |
|---|---|---|
| Peripheral Blood | Growth Factors (GFs) | PRP, PPP, PRF; Prepared with or without anticoagulant (ACD) |
| Bone Marrow | Stem Cells (SCs), Growth Factors (GFs) | BMAC, BMA, Hybrid BMAC; May or may not be centrifuged |
| Adipose Tissue | Stem Cells (SCs) | Macro-FAT, MFAT, Nano-FAT, SVF; Derived from fat tissue, varying in cluster size |
| Bioactive Molecule | Biological Role |
|---|---|
| Platelet-Derived Growth Factor (PDGF) | Promotes cell proliferation and angiogenesis |
| Transforming Growth Factor-Beta (TGF-β) | Regulates cell growth, proliferation, differentiation, and apoptosis |
| Vascular Endothelial Growth Factor (VEGF) | Stimulates angiogenesis and increases vascular permeability |
| Epidermal Growth Factor (EGF) | Promotes cell growth, proliferation, and differentiation |
| Insulin-Like Growth Factor (IGF) | Stimulates growth and development of cells |
| Fibroblast Growth Factor (FGF) | Promotes cell growth, proliferation, and differentiation |
| Hepatocyte Growth Factor (HGF) | Stimulates cell growth, motility, and angiogenesis |
| Connective Tissue Growth Factor (CTGF) | Promotes the proliferation and differentiation of fibroblasts |
| Keratinocyte Growth Factor (KGF) | Stimulates epithelial cell growth and differentiation |
| Platelet Factor 4 (PF4) | Modulates inflammation and wound healing |
| Interleukin-1 (IL-1) | Plays a role in inflammation and immune responses |
| Interleukin-8 (IL-8) | Promotes chemotaxis and angiogenesis |
| Orthobiologic | Primary Components | Key Clinical Applications |
|---|---|---|
| Platelet-Rich Plasma (PRP) | Growth factors (PDGF, TGF-β, VEGF, EGF) | Tendinopathies, osteoarthritis, muscle injuries, post-surgical healing |
| Platelet-Rich Fibrin (PRF) | Platelets, fibrin matrix, leukocytes | Wound healing, periodontal regeneration, soft tissue repair |
| Hyaluronic Acid (HA) | Viscous glycosaminoglycan | Osteoarthritis (intra-articular injection), joint lubrication |
| Bone Marrow Aspirate (BMA)/Bone Marrow Aspirate Concentrate (BMAC) | MSCs, hematopoietic cells, growth factors | Cartilage repair, bone defects, non-union fractures |
| Stromal Vascular Fraction (SVF) from Adipose Tissue | MSCs, pericytes, extracellular matrix components | Soft tissue regeneration, wound healing, degenerative joint disease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, F.R.; Pires, L.; Martins, R.A.; Santos, M.; Santos, G.S.; Lana, J.V.; Costa, B.R.; Santos, N.; de Macedo, A.P.; Kruel, A.; et al. Orthobiologics Revisited: A Concise Perspective on Regenerative Orthopedics. Curr. Issues Mol. Biol. 2025, 47, 247. https://doi.org/10.3390/cimb47040247
Costa FR, Pires L, Martins RA, Santos M, Santos GS, Lana JV, Costa BR, Santos N, de Macedo AP, Kruel A, et al. Orthobiologics Revisited: A Concise Perspective on Regenerative Orthopedics. Current Issues in Molecular Biology. 2025; 47(4):247. https://doi.org/10.3390/cimb47040247
Chicago/Turabian StyleCosta, Fábio Ramos, Luyddy Pires, Rubens Andrade Martins, Márcia Santos, Gabriel Silva Santos, João Vitor Lana, Bruno Ramos Costa, Napoliane Santos, Alex Pontes de Macedo, André Kruel, and et al. 2025. "Orthobiologics Revisited: A Concise Perspective on Regenerative Orthopedics" Current Issues in Molecular Biology 47, no. 4: 247. https://doi.org/10.3390/cimb47040247
APA StyleCosta, F. R., Pires, L., Martins, R. A., Santos, M., Santos, G. S., Lana, J. V., Costa, B. R., Santos, N., de Macedo, A. P., Kruel, A., & Lana, J. F. (2025). Orthobiologics Revisited: A Concise Perspective on Regenerative Orthopedics. Current Issues in Molecular Biology, 47(4), 247. https://doi.org/10.3390/cimb47040247


_Kim.png)






