NGF in Neuropathic Pain: Understanding Its Role and Therapeutic Opportunities
Abstract
1. Introduction
2. Characteristics of NGF
2.1. Structure
2.2. Biosynthesis and Degradation
2.3. Distribution and Biological Functions
3. Neuropathic Pain: A Comprehensive Overview
3.1. Molecular Basis of Neuropathic Pain
- Sensitization of nociceptors: Nociceptors are located at the free nerve endings of unmyelinated C and lightly myelinated Aδ fibers, which constitute the peripheral sensitization in neuropathic pain [96,97]. The action of nociceptors can be triggered by several agents, including inflammatory mediators (such as bradykinin, prostaglandins, neurokinins, calcitonin gene-related peptide (CGRP)) [98] and growth factors (NGF and BDNF) [18].
- 2.
- Abnormal ectopic excitability of afferent neurons: Spontaneous discharges from myelinated Aβ fibers produce paraesthesias and dysesthesias, while altered excitability of myelinated Aδ and unmyelinated C fibers produces burning pain [101,102]. These symptoms have been strongly associated with unusual activity of VGSCs (e.g., Nav1.7, Nav1.8, and Nav1.9) [103], among others.
- 3.
- Pronociceptive facilitation at the spinal cord level: Symptoms such as pin-prick hyperalgesia, cold hyperalgesia, and dynamic allodynia are indicative of central sensitization [104]. The transmission of pain signals involves ionotropic glutamate receptors [105,106], such as AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) and NMDA (N-methyl-D-aspartate), as well as metabotropic glutamate receptors (mGluRs).
- 4.
- Disinhibition of nociception within the spinal dorsal horn: One of the major factors underlying the development and maintenance of neuropathic pain is the disinhibition of nociception in the spinal cord [116]. This occurs under conditions where the inhibitory mechanisms that normally dampen the nociceptive signals, such as GABAergic and glycinergic neurotransmission, are impaired [117]. Disinhibition involves the loss of inhibitory interneurons, disruptions in chloride balance, and altered receptor function, with synaptic plasticity ultimately sustaining persistent hyperexcitability in the spinal cord [118,119]. This hyperexcitability can have profound implications for neural communication and contribute highly to many neurological conditions [120].
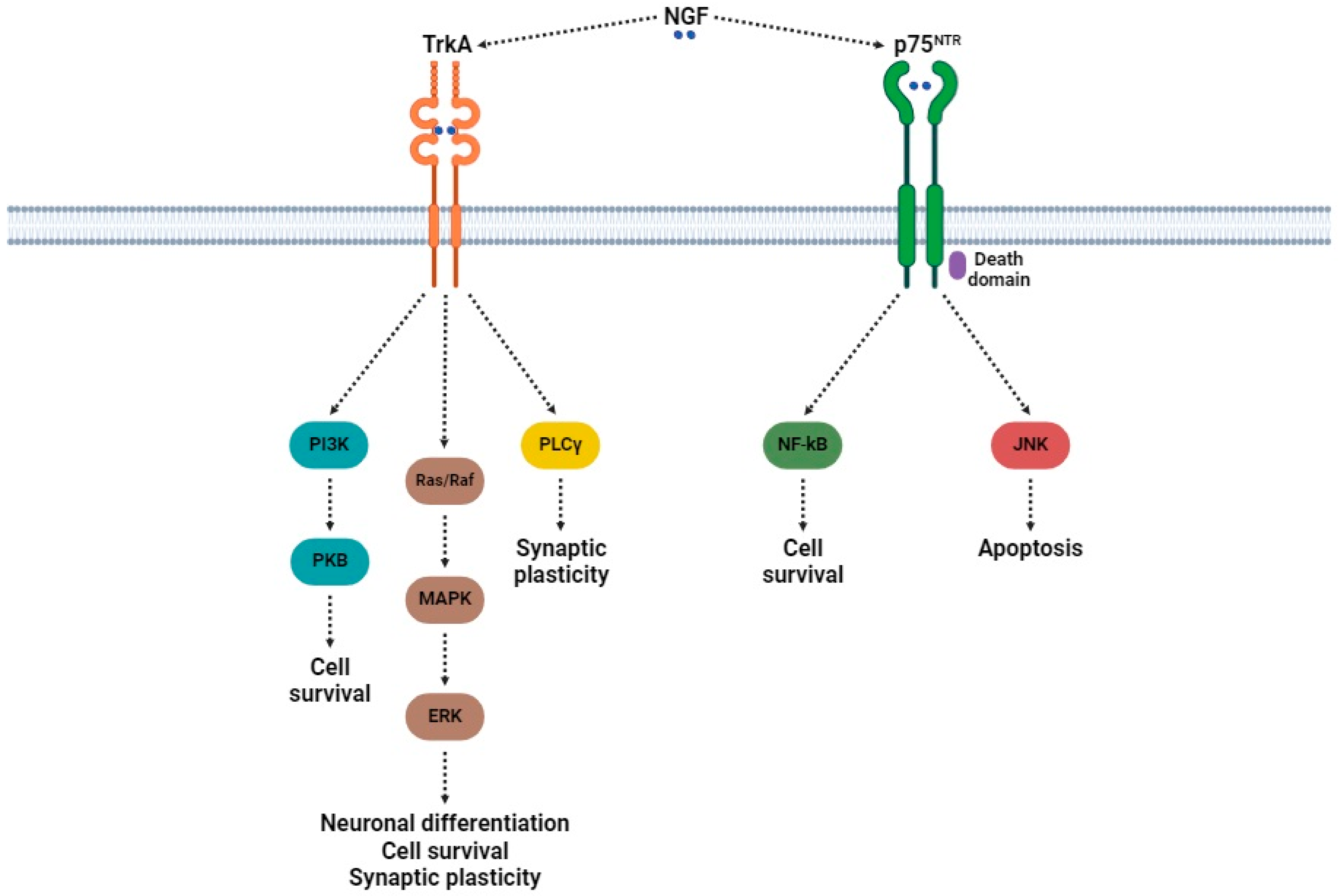
3.2. Role of NGF in Neuropathic Pain
| Cause of Neuropathy | Preclinical/Clinical Research | Role of NGF | References |
|---|---|---|---|
| Chronic constriction injury (CCI) model | Preclinical (rat) | The exogenous administration of NGF has led to a significant decrease in paw withdrawal latency times, highlighting the critical role of NGF in the development of hyperalgesia | [137] |
| Preclinical (rat) | mRNA encoding NGF was present in cells at the site of injury and in the DRG at the lesion’s level. Also, NGF was significantly higher in the ganglia on the ipsilateral side of the CCI | [138] | |
| Preclinical (mouse) | Exogenous NGF exacerbated both mechanical and thermal allodynia induced by CCI. High levels of endogenous NGF also promoted sprouting within the DRG | [139] | |
| Preclinical (rat) | NGF contents were augmented within the spinal cord and the DRG following CCI. This increase in NGF contributed to the long-term reduction in tactile and mechanical thresholds after injury | [140] | |
| Preclinical (rat) | NGF expression was increased in the DRG and sciatic nerve of CCI rats | [141] | |
| Spared nerve injury (SNI) model | Preclinical (rat) | The NGF levels in the red nucleus (RN) of SNI rats were significantly elevated compared to those of sham-operated rats | [142] |
| Preclinical (mouse) | mRNA NGF levels increased in the injured DRG | [143] | |
| Sciatica model induced by intervertebral disc herniation | Preclinical (rat) | This study ablated joint afferents by using the neurotoxin saporin conjugated to a ligand targeted to neurons involved in either peptidergic signaling and investigated the contributions of those neuronal populations to facet-mediated pain. The neurotoxin saporin prevented NGF-induced mechanical and thermal hypersensitivity in the forepaws | [144] |
| Peripheral nerve injury model (transection of lumbar spinal nerve) | Preclinical (rat) | Results confirmed that NGF played a significant role in the development of allodynia following a nerve injury | [145] |
| Sciatic nerve cryoneurolysis (SCN) model | Preclinical (rat) | Increased levels of NGF were found in the spinal dorsal horn of SCN rats manifesting hyperalgesia | [146] |
| Trigeminal neuralgia (TN) | Preclinical (rat) | Increased NGF levels were found in the ipsilateral infraorbital nerve branch at the time point corresponding to the peak of heat hyperalgesia | [147] |
| Multiple sclerosis (MS) | Preclinical (rat) | This study revealed that activated glial cells overexpress NGF mRNA in the CNS of EAE-affected rats. This suggests that elevated NGF levels in EAE rats’ brains are generated by glial cells | [148] |
| Clinical | NGF was increased in the CSF of MS patients with central NP | [149] | |
| Osteoarthritis (OA) | Clinical | NGF expression was induced in chondrocytes by mechanical and inflammatory stimuli | [150] |
| Preclinical (mouse) | NGF expression was increased in the DRG of mice with osteoarthritis | [151] | |
| Preclinical (rat) | During osteoarthritis progression, NGF expression varied by tissue and disease stage. NGF increased in the synovium while continuing to rise in osteochondral channels and bone marrow. This suggests that NGF was a key driver of nerve growth linked to OA pain | [152] | |
| Diabetic polyneuropathy (DPN) | Clinical | These studies have reported significant dose-dependent hyperalgesia at the site of NGF injection | [153,154] |
| Preclinical (rat) | The pronociceptive role of NGF in diabetic rats was evidenced by the increased concentrations of CGRP and substance P found in both the DRG and the spinal dorsal horn | [155] | |
| Preclinical (mouse) | This study hypothesized that NGF participates in the development of mechanical allodynia by enhancing the expression of substance P and CGRP. Indeed, an increase in the expression of NGF, substance P, and CGRP genes at the onset of mechanical allodynia has been demonstrated in the DRG of db/db mice | [156] | |
| HIV-associated neuropathy | Clinical | These studies have reported significant dose-dependent hyperalgesia at the site of NGF injection | [157,158] |
| Chemotherapy-induced peripheral neuropathy (CIPN) | Clinical | Serum NGF levels were elevated in cancer patients with painful CIPN receiving either taxane or platinum. Also, NGF may act as a biomarker of the presence and severity of NP in these populations | [159] |
| Preclinical (rat) | NGF promoted sensory neuritogenesis and sensitized nociceptors. This effect was blocked by the TrkA antagonist GW441756. The administration of this antagonist inhibited TRPV1-mediated nociceptor sensitization induced by cisplatin, thereby preventing the onset of NP associated with this chemotherapeutic agent | [160] |
4. Treatments Against NGF in Neuropathic Pain
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| Akt | AKT serine/threonine kinase |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AP-1 | Activator protein 1 |
| ASIC | Acid-sensing ion channels |
| BDNF | Brain-derived neurotrophic factor |
| cAMP | Cyclic adenosine monophosphate |
| CCI | Chronic constriction injury |
| CCL2 | C-C motif chemokine ligand 2 |
| CGRP | Calcitonin gene-related peptide |
| CIPN | Chemotherapy-induced peripheral neuropathy |
| CNS | Central nervous system |
| CRD | Cysteine-rich domain |
| CRD1 | Cysteine-rich domain 1 |
| CRD2 | Cysteine-rich domain 2 |
| CRD3 | Cysteine-rich domain 3 |
| CRD4 | Cysteine-rich domain 4 |
| CRM | Cysteine-rich motif |
| CSF | Cerebrospinal fluid |
| CX3CL1 | C-X3-C motif chemokine ligand 1 |
| DPN | Diabetic peripheral neuropathy |
| DRG | Dorsal root ganglion |
| EAE | Experimental autoimmune encephalomyelitis |
| ECD | Extracellular domain |
| ERK | Extracellular signal-regulated kinase |
| ERK1/2 | Extracellular signal-regulated kinases 1/2 |
| FAP-1 | Fas-associated phosphatase-1 |
| GABA | Gamma-aminobutyric acid |
| HIV | Human immunodeficiency virus |
| HSAN V | Hereditary sensory neuropathy type V |
| IASP | International Association for the Study of Pain |
| IgG | Immunoglobulin G |
| IL-1β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| JNK | c-Jun N-terminal kinase |
| LBP | Low back pain |
| l-CDL | Levo-corydalmine |
| LRM | Leucine-rich motif |
| LTP | Long-term potentiation |
| mAb | Monoclonal antibody |
| MAPK | Mitogen-activated protein kinase |
| MAPKK | Mitogen-activated protein kinase kinase |
| MEK1/2 | Mitogen-activated protein kinase kinase 1/2 |
| mGluR | Metabotropic glutamate receptors |
| MMP9 | Matrix metalloproteinase-9 |
| mRNA | Ribonucleic acid |
| MS | Multiple sclerosis |
| NADE | p75NTR-associated cell death executor |
| Nav1.7 | Voltage-gated sodium channel, type 1.7 |
| Nav1.8 | Voltage-gated sodium channel, type 1.8 |
| Nav1.9 | Voltage-gated sodium channel, type 1.9 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NGF | Nerve growth factor |
| NMDA | N-methyl-D-aspartate |
| NMR | Nuclear magnetic resonance |
| NO | Nitric oxide |
| NP | Neuropathic pain |
| NRAGE | Neurotrophin receptor-interacting MAGE protein |
| NRIF | Neurotrophin receptor-interacting factor |
| NT-3 | Neurotrophin 3 |
| NT-4 | Neurotrophin 4 |
| OA | Osteoarthritis |
| p65 | NF-kB subunit p65 |
| p75ICD | p75 intracellular domain |
| p75NTR | p75 neurotrophin receptor |
| pAb | Polyclonal antibody |
| PI3K | Phosphoinositide 3-kinase |
| PKA | Protein kinase A |
| PKB | Protein kinase B |
| PKC | Protein kinase C |
| PLCγ | Phospholipase C gamma |
| PNS | Peripheral nervous system |
| proNGF | Pro-nerve growth factor |
| Raf | Rapidly accelerated fibrosarcoma |
| Ras | Rat sarcoma virus protein |
| RIP2 | Receptor-interacting protein kinase 2 |
| RN | Red nucleus |
| SAXS | Small-angle X-ray scattering |
| SCN | Sciatic nerve cryoneurolysis |
| SNI | Spared nerve injury |
| SNRI | Serotonin–norepinephrine reuptake inhibitor |
| SNS | Somatic nervous system |
| Sp1 | Specificity protein 1 |
| TAK1 | TGF-β-activated kinase 1 |
| TCA | Tricyclic antidepressant |
| TN | Trigeminal neuralgia |
| TNFR | Tumor necrosis factor receptor |
| TNF-α | Tumor necrosis factor alpha |
| tPA | Tissue plasminogen activator |
| TRAF6 | TNF receptor-associated factor 6 |
| Trk | Tropomyosin receptor kinase |
| TrkA | Tropomyosin receptor kinase A |
| TRP | Transient receptor potential |
| TRPA1 | Transient receptor potential ankyrin 1 |
| TRPV1 | Transient receptor potential vanilloid 1 |
| UK | United Kingdom |
| VGCC | Voltage-gated calcium channel |
| VGSC | Voltage-gated sodium channel |
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Vader, K.; Bostick, G.P.; Carlesso, L.C.; Hunter, J.; Mesaroli, G.; Perreault, K.; Tousignant-Laflamme, Y.; Tupper, S.; Walton, D.M.; Wideman, T.H.; et al. The Revised IASP Definition of Pain and Accompanying Notes: Considerations for the Physiotherapy Profession. Physiother. Can. 2021, 73, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Bonezzi, C.; Fornasari, D.; Cricelli, C.; Magni, A.; Ventriglia, G. Not All Pain is Created Equal: Basic Definitions and Diagnostic Work-Up. Pain. Ther. 2020, 9, 1–15. [Google Scholar] [CrossRef]
- Petroianu, G.A.; Aloum, L.; Adem, A. Neuropathic pain: Mechanisms and therapeutic strategies. Front. Cell Dev. Biol. 2023, 11, 1072629. [Google Scholar] [CrossRef] [PubMed]
- Rugnath, R.; Orzechowicz, C.; Newell, C.; Carullo, V.; Rugnath, A. A Literature Review: The Mechanisms and Treatment of Neuropathic Pain-A Brief Discussion. Biomedicines 2024, 12, 204. [Google Scholar] [CrossRef]
- Jensen, T.S.; Finnerup, N.B. Allodynia and hyperalgesia in neuropathic pain: Clinical manifestations and mechanisms. Lancet Neurol. 2014, 13, 924–935. [Google Scholar] [CrossRef]
- Campbell, J.N.; Meyer, R.A. Mechanisms of neuropathic pain. Neuron 2006, 52, 77–92. [Google Scholar] [CrossRef]
- Kocot-Kępska, M.; Zajączkowska, R.; Mika, J.; Wordliczek, J.; Dobrogowski, J.; Przeklasa-Muszyńska, A. Peripheral Mechanisms of Neuropathic Pain-the Role of Neuronal and Non-Neuronal Interactions and Their Implications for Topical Treatment of Neuropathic Pain. Pharmaceuticals 2021, 14, 77. [Google Scholar] [CrossRef]
- Ma, Y.C.; Kang, Z.B.; Shi, Y.Q.; Ji, W.Y.; Zhou, W.M.; Nan, W. The Complexity of Neuropathic Pain and Central Sensitization: Exploring Mechanisms and Therapeutic Prospects. J. Integr. Neurosci. 2024, 23, 89. [Google Scholar] [CrossRef] [PubMed]
- Baskozos, G.; Hébert, H.L.; Pascal, M.M.; Themistocleous, A.C.; Macfarlane, G.J.; Wynick, D.; Bennett, D.L.; Smith, B.H. Epidemiology of neuropathic pain: An analysis of prevalence and associated factors in UK Biobank. Pain. Rep. 2023, 8, e1066. [Google Scholar] [CrossRef] [PubMed]
- Bum Chun, S. Etiology and epidemiology of neuropathic pain. J. Korean Med. Assoc. 2021, 64, 461–467. [Google Scholar] [CrossRef]
- Carneado-Ruiz, J.; Morera-Guitart, J.; Alfaro-Sáez, A.; Turpín-Fenoll, L.; Serna-Candel, C.; Matías-Guíu, J. Neuropathic pain as the reason for visiting Neurology: An analysis of its frequency. Rev. Neurol. 2005, 41, 643–648. [Google Scholar] [PubMed]
- Montero Homs, J.; Gutiérrez-Rivas, E.; Pardo Fernández, J.; Navarro Darder, C.; PREVADOL. Epidemiological study of prevalence, incidence and neuropathic pain characterization in neurology units. PREVADOL study. Neurologia 2005, 20, 385–389. [Google Scholar] [PubMed]
- Ekberg, J.; Adams, D.J. Neuronal voltage-gated sodium channel subtypes: Key roles in inflammatory and neuropathic pain. Int. J. Biochem. Cell Biol. 2006, 38, 2005–2010. [Google Scholar] [CrossRef] [PubMed]
- Perret, D.; Luo, Z.D. Targeting voltage-gated calcium channels for neuropathic pain management. Neurotherapeutics 2009, 6, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Basso, L.; Altier, C. Transient Receptor Potential Channels in neuropathic pain. Curr. Opin. Pharmacol. 2017, 32, 9–15. [Google Scholar] [CrossRef]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Siniscalco, D.; Giordano, C.; Rossi, F.; Maione, S.; de Novellis, V. Role of neurotrophins in neuropathic pain. Curr. Neuropharmacol. 2011, 9, 523–529. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, S.; He, L.; Zhou, M.; Guo, J.; Hoke, A.; Zhu, C. Nerve growth factor for neuropathic pain. Cochrane Database Syst. Rev. 2017, 2017, CD012800. [Google Scholar] [CrossRef]
- Lee, S.E.; Shen, H.; Taglialatela, G.; Chung, J.M.; Chung, K. Expression of nerve growth factor in the dorsal root ganglion after peripheral nerve injury. Brain Res. 1998, 796, 99–106. [Google Scholar] [CrossRef]
- Barker, P.A.; Mantyh, P.; Arendt-Nielsen, L.; Viktrup, L.; Tive, L. Nerve Growth Factor Signaling and Its Contribution to Pain. J. Pain Res. 2020, 13, 1223–1241. [Google Scholar] [CrossRef]
- Schaefer, I.; Prato, V.; Arcourt, A.; Taberner, F.J.; Lechner, S.G. Differential modulation of voltage-gated sodium channels by nerve growth factor in three major subsets of TrkA-expressing nociceptors. Mol. Pain 2018, 14, 1744806918814640. [Google Scholar] [CrossRef]
- Cavalli, E.; Mammana, S.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419838383. [Google Scholar] [CrossRef]
- Moisset, X.; Bouhassira, D.; Avez Couturier, J.; Alchaar, H.; Conradi, S.; Delmotte, M.H.; Lanteri-Minet, M.; Lefaucheur, J.P.; Mick, G.; Piano, V.; et al. Pharmacological and non-pharmacological treatments for neuropathic pain: Systematic review and French recommendations. Rev. Neurol. 2020, 176, 325–352. [Google Scholar] [CrossRef]
- Shibata, S.; Tagashira, H.; Nemoto, T.; Kita, S.; Kita, T.; Shinoda, Y.; Akiyoshi, K.; Yamaura, K.; Iwamoto, T. Perineural treatment with anti-TNF-α antibody ameliorates persistent allodynia and edema in novel mouse models with complex regional pain syndrome. J. Pharmacol. Sci. 2023, 153, 1–11. [Google Scholar] [CrossRef]
- Chen, L.H.; Yeh, Y.M.; Chen, Y.F.; Hsu, Y.H.; Wang, H.H.; Lin, P.C.; Chang, L.Y.; Lin, C.K.; Chang, M.S.; Shen, M.R. Targeting interleukin-20 alleviates paclitaxel-induced peripheral neuropathy. Pain 2020, 161, 1237–1254. [Google Scholar] [CrossRef]
- Tonello, R.; Lee, S.H.; Berta, T. Monoclonal Antibody Targeting the Matrix Metalloproteinase 9 Prevents and Reverses Paclitaxel-Induced Peripheral Neuropathy in Mice. J. Pain 2019, 20, 515–527. [Google Scholar] [CrossRef]
- Cheng, H.T.; Dauch, J.R.; Hayes, J.M.; Yanik, B.M.; Feldman, E.L. Nerve growth factor/p38 signaling increases intraepidermal nerve fiber densities in painful neuropathy of type 2 diabetes. Neurobiol. Dis. 2012, 45, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Levi-Montalcini, R. A Nerve Growth-Stimulating Factor Isolated from Snake Venom. Proc. Natl. Acad. Sci. USA 1956, 42, 571–574. [Google Scholar] [CrossRef]
- Rocco, M.L.; Soligo, M.; Manni, L.; Aloe, L. Nerve Growth Factor: Early Studies and Recent Clinical Trials. Curr. Neuropharmacol. 2018, 6, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Meakin, S.O.; Shooter, E.M. The nerve growth factor family of receptors. Trends. Neurosci. 1992, 15, 323–331. [Google Scholar] [CrossRef]
- Aloe, L. Rita Levi-Montalcini and the discovery of NGF, the first nerve cell growth factor. Arch. Ital. Biol. 2011, 149, 175–181. [Google Scholar] [CrossRef]
- Cohen, S. Purification of a Nerve-Growth Promoting Protein from the Mouse Salivary Gland and its Neuro-Cytotoxic Antiserum. Proc. Natl. Acad. Sci. USA 1960, 46, 302–311. [Google Scholar] [CrossRef]
- Godfrey, E.W.; Shooter, E.M. Nerve growth factor receptors on chick embryo sympathetic ganglion cells: Binding characteristics and development. J. Neurosci. 1986, 6, 2543–2550. [Google Scholar] [CrossRef]
- Korsching, S. The role of nerve growth factor in the CNS. Trends. Neurosci. 1986, 9, 570–573. [Google Scholar] [CrossRef]
- Rask, C.A. Biological actions of nerve growth factor in the peripheral nervous system. Eur. Neurol. 1999, 41, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Aloe, L.; Rocco, M.L.; Balzamino, B.O.; Micera, A. Nerve growth factor: Role in growth, differentiation and controlling cancer cell development. J. Exp. Clin. Cancer. Res. 2016, 35, 116. [Google Scholar] [CrossRef]
- Minnone, G.; De Benedetti, F.; Bracci-Laudiero, L. NGF and Its Receptors in the Regulation of Inflammatory Response. Int. J. Mol. Sci. 2017, 18, 1028. [Google Scholar] [CrossRef]
- McDonald, N.Q.; Lapatto, R.; Murray-Rust, J.; Gunning, J.; Wlodawer, A.; Blundell, T.L. New protein fold revealed by a 2.3-A resolution crystal structure of nerve growth factor. Nature 1991, 354, 411–414. [Google Scholar] [CrossRef] [PubMed]
- He, X.L.; Garcia, K.C. Structure of nerve growth factor complexed with the shared neurotrophin receptor p75. Science 2004, 304, 870–875. [Google Scholar] [CrossRef]
- Bradshaw, R.A.; Murray-Rust, J.; Ibáñez, C.F.; McDonald, N.Q.; Lapatto, R.; Blundell, T.L. Nerve growth factor: Structure/function relationships. Protein Sci. 1994, 3, 1901–1913. [Google Scholar] [CrossRef]
- Xie, Y.; Tisi, M.A.; Yeo, T.T.; Longo, F.M. Nerve growth factor (NGF) loop 4 dimeric mimetics activate ERK and AKT and promote NGF-like neurotrophic effects. J. Biol. Chem. 2009, 275, 29868–29874. [Google Scholar] [CrossRef]
- Ebendal, T. Function and evolution in the NGF family and its receptors. J. Neurosci. Res. 1992, 32, 461–470. [Google Scholar] [CrossRef]
- Schwarz, E. Cystine knot growth factors and their functionally versatile proregions. Biol. Chem. 2017, 398, 1295–1308. [Google Scholar] [CrossRef]
- Wehrman, T.; He, X.; Raab, B.; Dukipatti, A.; Blau, H.; Garcia, K.C. Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron 2007, 53, 25–38. [Google Scholar] [CrossRef]
- Paoletti, F.; Covaceuszach, S.; Konarev, P.V.; Gonfloni, S.; Malerba, F.; Schwarz, E.; Svergun, D.I.; Cattaneo, A.; Lamba, D. Intrinsic structural disorder of mouse proNGF. Proteins 2009, 75, 990–1009. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, F.; Malerba, F.; Kelly, G.; Noinville, S.; Lamba, D.; Cattaneo, A.; Pastore, A. Conformational plasticity of proNGF. PLoS ONE 2011, 6, e22615. [Google Scholar] [CrossRef]
- Boldon, L.; Laliberte, F.; Liu, L. Review of the fundamental theories behind small angle X-ray scattering, molecular dynamics simulations, and relevant integrated application. Nano. Rev. 2015, 6, 25661. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C. Review of NMR Spectroscopy: Basic principles, Concepts and Applications in Chemistry. J. Chem. Educ. 2014, 91, 1103–1104. [Google Scholar] [CrossRef]
- Furukawa, Y.; Furukawa, S.; Omae, F.; Awatsuji, H.; Hayashi, K. Alkylcatechols regulate NGF gene expression in astroglial cells via both protein kinase C- and cAMP-independent mechanisms. J. Neurosci. Res. 1993, 35, 522–529. [Google Scholar] [CrossRef]
- Edwards, R.H.; Selby, M.J.; Rutter, W.J. Differential RNA splicing predicts two distinct nerve growth factor precursors. Nature 1986, 319, 784–787. [Google Scholar] [CrossRef]
- Edwards, R.H.; Selby, M.J.; Mobley, W.C.; Weinrich, S.L.; Hruby, D.E.; Rutter, W.J. Processing and secretion of nerve growth factor: Expression in mammalian cells with a vaccinia virus vector. Mol. Cell. Biol. 1988, 8, 2456–2464. [Google Scholar] [CrossRef]
- Seidah, N.G.; Benjannet, S.; Pareek, S.; Savaria, D.; Hamelin, J.; Goulet, B.; Laliberte, J.; Lazure, C.; Chrétien, M.; Murphy, R.A. Cellular processing of the nerve growth factor precursor by the mammalian pro-protein convertases. Biochem. J. 1996, 314, 951–960. [Google Scholar] [CrossRef]
- Rafieva, L.M.; Gasanov, E.V. Neurotrophin Propeptides: Biological Functions and Molecular Mechanisms. Curr. Protein Pept. Sci. 2016, 17, 298–305. [Google Scholar] [CrossRef]
- Rattenholl, A.; Ruoppolo, M.; Flagiello, A.; Monti, M.; Vinci, F.; Marino, G.; Lilie, H.; Schwarz, E.; Rudolph, R. Pro-sequence assisted folding and disulfide bond formation of human nerve growth factor. J. Mol. Biol. 2001, 305, 523–533. [Google Scholar] [CrossRef]
- Bulleid, N.J. Disulfide bond formation in the mammalian endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2012, 4, a013219. [Google Scholar] [CrossRef]
- Bresnahan, P.A.; Leduc, R.; Thomas, L.; Thorner, J.; Gibson, H.L.; Brake, A.J.; Barr, P.J.; Thomas, G. Human fur gene encodes a yeast KEX2-like endoprotease that cleaves pro-beta-NGF in vivo. J. Cell Biol. 1990, 111, 2851–2859. [Google Scholar] [CrossRef]
- Gutiérrez-Fernández, A.; Parmer, R.J.; Miles, L. Plasminogen gene expression is regulated by nerve growth factor. J. Thromb. Haemost. 2007, 5, 1715–1725. [Google Scholar] [CrossRef]
- Stefos, G.C.; Soppa, U.; Dierssen, M.; Becker, W. NGF upregulates the plasminogen activation inhibitor-1 in neurons via the calcineurin/NFAT pathway and the Down syndrome-related proteins DYRK1A and RCAN1 attenuate this effect. PLoS ONE 2013, 8, e67470. [Google Scholar] [CrossRef]
- Allard, S.; Leon, W.C.; Pakavathkumar, P.; Bruno, M.A.; Ribeiro-da-Silva, A.; Cuello, A. Impact of the NGF maturation and degradation pathway on the cortical cholinergic system phenotype. J. Neurosci. 2012, 32, 2002–2012. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Elliott, R.C.; Gall, C.M. Changes in activating protein 1 (AP-1) composition correspond with the biphasic profile of nerve growth factor mRNA expression in rat hippocampus after hilus lesion-induced seizures. J. Neurosci. 2000, 20, 2142–2149. [Google Scholar] [CrossRef]
- Marampon, F.; Casimiro, M.C.; Fu, M.; Powell, M.J.; Popov, V.M.; Lindsay, J.; Zani, B.M.; Ciccarelli, C.; Watanabe, G.; Lee, R.J.; et al. Nerve Growth factor regulation of cyclin D1 in PC12 cells through a p21RAS extracellular signal-regulated kinase pathway requires cooperative interactions between Sp1 and nuclear factor-kappaB. Mol. Biol. Cell 2008, 19, 2566–2578. [Google Scholar] [CrossRef]
- Veenstra, T.D.; Fahnestock, M.; Kumar, R. An AP-1 site in the nerve growth factor promoter is essential for 1,25-dihydroxyvitamin D3-mediated nerve growth factor expression in osteoblasts. Biochemistry 1998, 37, 5988–5994. [Google Scholar] [CrossRef]
- Bouchet, C.; Cardouat, G.; Douard, M.; Coste, F.; Robillard, P.; Delcambre, F.; Ducret, T.; Quignard, J.F.; Vacher, P.; Baudrimont, I.; et al. Inflammation and Oxidative Stress Induce NGF Secretion by Pulmonary Arterial Cells through a TGF-β1-Dependent Mechanism. Cells 2022, 11, 2795. [Google Scholar] [CrossRef]
- Filev, A.D.; Ershova, E.S.; Savinova, E.; Kalakov, A.M.; Veiko, N.N.; Umriukhin, P.; Kostyuk, S. The effect of valproic acid on the transcriptional activity of Ngf and Bdnf genes of in vitro cultured neurons under oxidative stress conditions. Int. J. Biol. Biomed. Eng. 2021, 15, 371–375. [Google Scholar] [CrossRef]
- Xiao, J.; Wong, A.W.; Willingham, M.M.; Kaasinen, S.K.; Hendry, I.A.; Howitt, J.; Putz, U.; Barrett, G.L.; Kilpatrick, T.J.; Murray, S.S. BDNF exerts contrasting effects on peripheral myelination of NGF-dependent and BDNF-dependent DRG neurons. J. Neurosci. 2009, 29, 4016–4022. [Google Scholar] [CrossRef]
- Lad, S.P.; Peterson, D.A.; Bradshaw, R.A.; Neet, K.E. Individual and combined effects of TrkA and p75NTR nerve growth factor receptors. A role for the high affinity receptor site. J. Biol. Chem. 2003, 278, 24808–24817. [Google Scholar] [CrossRef]
- Marlin, M.C.; Li, G. Biogenesis and function of the NGF/TrkA signaling endosome. Int. Rev. Cell Mol. Biol. 2015, 314, 239–257. [Google Scholar] [CrossRef]
- Bruno, F.; Abondio, P.; Montesanto, A.; Luiselli, D.; Bruni, A.C.; Maletta, R. The Nerve Growth Factor Receptor (NGFR/p75NTR): A Major Player in Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 3200. [Google Scholar] [CrossRef] [PubMed]
- Hannestad, J.; García-Suárez, O.; Huerta, J.J.; Esteban, I.; Naves, F.J.; Vega, J.A. TrkA neutrophin receptor protein in the rat and human thymus. Anat. Rec. 1997, 249, 373–379. [Google Scholar] [CrossRef]
- Ultsch, M.H.; Wiesmann, C.; Simmons, L.C.; Henrich, J.; Yang, M.; Reilly, D.; Bass, S.H.; de Vos, A.M. Crystal structures of the neurotrophin-binding domain of TrkA, TrkB and TrkC. J. Mol. Biol. 1999, 290, 149–159. [Google Scholar] [CrossRef]
- Pezet, S.; McMahon, S.B. Neurotrophins: Mediators and modulators of pain. Annu. Rev. Neurosci. 2006, 29, 507–538. [Google Scholar] [CrossRef]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, T.J.; Xiong, Z.Q. NGF-dependent retrograde signaling: Survival versus death. Cell Res. 2009, 19, 525–526. [Google Scholar] [CrossRef] [PubMed]
- Conroy, J.N.; Coulson, E.J. High-affinity TrkA and p75 neurotrophin receptor complexes: A twisted affair. J. Biol. Chem. 2022, 298, 101568. [Google Scholar] [CrossRef]
- Chao, M.; Casaccia-Bonnefil, P.; Carter, B.; Chittka, A.; Kong, H.; Yoon, S.O. Neurotrophin receptors: Mediators of life and death. Brain Res. Rev. 1998, 26, 295–301. [Google Scholar] [CrossRef]
- Ceni, C.; Kommaddi, R.P.; Thomas, R.; Vereker, E.; Liu, X.; McPherson, P.S.; Ritter, B.; Barker, P.A. The p75NTR intracellular domain generated by neurotrophin-induced receptor cleavage potentiates Trk signaling. J. Cell Sci. 2010, 123, 2299–2307. [Google Scholar] [CrossRef]
- Korsching, S.; Auburger, G.; Heumann, R.; Scott, J.; Thoenen, H. Levels of nerve growth factor and its mRNA in the central nervous system of the rat correlate with cholinergic innervation. EMBO J. 1985, 4, 1389–1393. [Google Scholar] [CrossRef]
- Goedert, M.; Fine, A.; Hunt, S.P.; Ullrich, A. Nerve growth factor mRNA in peripheral and central rat tissues and in the human central nervous system: Lesion effects in the rat brain and levels in Alzheimer’s disease. Brain Res. 1986, 387, 85–92. [Google Scholar] [CrossRef]
- Shelton, D.L.; Reichardt, L. Studies on the expression of the beta nerve growth factor (NGF) gene in the central nervous system: Level and regional distribution of NGF mRNA suggest that NGF functions as a trophic factor for several distinct populations of neurons. Proc. Natl. Acad. Sci. USA 1986, 83, 2714–2718. [Google Scholar] [CrossRef]
- Gage, F.H.; Batchelor, P.; Chen, K.S.; Chin, D.; Higgins, G.A.; Koh, S.; Deputy, S.; Rosenberg, M.B.; Fischer, W.; Bjorklund, A. NGF receptor reexpression and NGF-mediated cholinergic neuronal hypertrophy in the damaged adult neostriatum. Neuron 1989, 2, 1177–1184. [Google Scholar] [CrossRef]
- Schatteman, G.C.; Gibbs, L.; Lanahan, A.A.; Claude, P.; Bothwell, M. Expression of NGF receptor in the developing and adult primate central nervous system. J. Neurosci. 1988, 8, 860–873. [Google Scholar] [CrossRef]
- Oliveira, N.K.; Ferreira, R.N.; Lopes, S.D.N.; Chiari, E.; Camargos, E.R.D.S.; Martinelli, P.M. Cardiac autonomic denervation and expression of neurotrophins (NGF and BDNF) and their receptors during experimental Chagas disease. Growth Factors 2017, 35, 161–170. [Google Scholar] [CrossRef]
- Pincelli, C.; Sevignani, C.; Manfredini, R.; Grande, A.; Fantini, F.; Bracci-Laudiero, L.; Aloe, L.; Ferrari, S.; Cossarizza, A.; Giannetti, A. Expression and function of nerve growth factor and nerve growth factor receptor on cultured keratinocytes. J. Invest. Dermatol. 1994, 103, 13–18. [Google Scholar] [CrossRef]
- Yoshida, K.; Gage, F.H. Cooperative regulation of nerve growth factor synthesis and secretion in fibroblasts and astrocytes by fibroblast growth factor and other cytokines. Brain. Res. 1992, 569, 14–25. [Google Scholar] [CrossRef]
- Leon, A.; Buriani, A.; Dal Toso, R.; Fabris, M.; Romanello, S.; Aloe, L.; Levi-Montalcini, R. Mast cells synthesize, store, and release nerve growth factor. Proc. Natl. Acad. Sci. USA 1994, 91, 3739–3743. [Google Scholar] [CrossRef]
- Schenck, K.; Schreurs, O.; Hayashi, K.; Helgeland, K. The Role of Nerve Growth Factor (NGF) and Its Precursor Forms in Oral Wound Healing. Int. J. Mol. Sci. 2017, 18, 386. [Google Scholar] [CrossRef]
- Cunha, G.R.; Ricke, W.; Thomson, A.; Marker, P.C.; Risbridger, G.; Hayward, S.W.; Wang, Y.Z.; Donjacour, A.A.; Kurita, T. Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J. Steroid Biochem. Mol. Biol. 2004, 92, 221–236. [Google Scholar] [CrossRef]
- Dissen, G.A.; Romero, C.; Hirshfield, A.N.; Ojeda, S.R. Nerve growth factor is required for early follicular development in the mammalian ovary. Endocrinology 2001, 142, 2078–2086. [Google Scholar] [CrossRef]
- Aloe, L.; Probert, L.; Kollias, G.; Micera, A.; Tirassa, P. Effect of NGF antibodies on mast cell distribution, histamine and substance P levels in the knee joint of TNF-arthritic transgenic mice. Rheumatol. Int. 1995, 14, 249–252. [Google Scholar] [CrossRef]
- Green, D.P.; Limjunyawong, N.; Gour, N.; Pundir, P.; Dong, X. A Mast-Cell-Specific Receptor Mediates Neurogenic Inflammation and Pain. Neuron 2019, 101, 412–420.e3. [Google Scholar] [CrossRef] [PubMed]
- Pius-Sadowska, E.; Machaliński, B. Pleiotropic activity of nerve growth factor in regulating cardiac functions and counteracting pathogenesis. ESC Heart Fail. 2021, 8, 974–987. [Google Scholar] [CrossRef]
- Liu, S. Neurotrophic factors in enteric physiology and pathophysiology. Neurogastroenterol. Motil. 2018, 30, e13446. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, S.; Tang, L. Nerve Growth Factor: A Potential Therapeutic Target for Lung Diseases. Int. J. Mol. Sci. 2021, 22, 9112. [Google Scholar] [CrossRef]
- Gold, M.S.; Gebhart, G.F. Nociceptor sensitization in pain pathogenesis. Nat. Med. 2010, 16, 1248–1257. [Google Scholar] [CrossRef]
- Cui, C.X.; Liu, H.Y.; Yue, N.; Du, Y.R.; Che, L.M.; Yu, J.S. Research progress on the mechanism of chronic neuropathic pain. IBRO Neurosci. Rep. 2022, 14, 80–85. [Google Scholar] [CrossRef]
- Schomberg, D.; Ahmed, M.; Miranpuri, G.; Olson, J.; Resnick, D.K. Neuropathic pain: Role of inflammation, immune response, and ion channel activity in central injury mechanisms. Ann. Neurosci. 2012, 19, 125–132. [Google Scholar] [CrossRef]
- Hucho, T.; Levine, J.D. Signaling pathways in sensitization: Toward a nociceptor cell biology. Neuron 2007, 55, 365–376. [Google Scholar] [CrossRef]
- Cheng, J.K.; Ji, R.R. Intracellular signaling in primary sensory neurons and persistent pain. Neurochem. Res. 2008, 33, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Beissner, F.; Brandau, A.; Henke, C.; Felden, L.; Baumgärtner, U.; Treede, R.D.; Oertel, B.G.; Lötsch, J. Quick discrimination of A(delta) and C fiber mediated pain based on three verbal descriptors. PLoS ONE 2010, 5, e12944. [Google Scholar] [CrossRef]
- Djouhri, L.; Zeidan, A.; Abd El-Aleem, S.A.; Smith, T. Cutaneous Aβ-Non-nociceptive, but Not C-Nociceptive, Dorsal Root Ganglion Neurons Exhibit Spontaneous Activity in the Streptozotocin Rat Model of Painful Diabetic Neuropathy in vivo. Front. Neurosci. 2020, 14, 530. [Google Scholar] [CrossRef]
- Strickland, I.T.; Martindale, J.C.; Woodhams, P.L.; Reeve, A.J.; Chessell, I.P.; McQueen, D.S. Changes in the expression of NaV1.7, NaV1.8 and NaV1.9 in a distinct population of dorsal root ganglia innervating the rat knee joint in a model of chronic inflammatory joint pain. Eur. J. Pain 2008, 12, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.B.; Wollmuth, L.P.; Bowie, D.; Furukawa, H.; Menniti, F.S.; Sobolevsky, A.I.; Swanson, G.T.; Swanger, S.A.; Greger, I.H.; Nakagawa, T.; et al. Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol. Rev. 2021, 73, 298–487. [Google Scholar] [CrossRef]
- Golubeva, E.A.; Lavrov, M.I.; Radchenko, E.V.; Palyulin, V.A. Diversity of AMPA Receptor Ligands: Chemotypes, Binding Modes, Mechanisms of Action, and Therapeutic Effects. Biomolecules 2022, 13, 56. [Google Scholar] [CrossRef]
- Zhu, S.; Stein, R.A.; Yoshioka, C.; Lee, C.H.; Goehring, A.; Mchaourab, H.S.; Gouaux, E. Mechanism of NMDA Receptor Inhibition and Activation. Cell 2016, 165, 704–714. [Google Scholar] [CrossRef]
- Hansen, K.B.; Yi, F.; Perszyk, R.E.; Menniti, F.S.; Traynelis, S.F. NMDA Receptors in the Central Nervous System. Methods Mol. Biol. 2017, 1677, 1–80. [Google Scholar] [CrossRef]
- Mazzitelli, M.; Presto, P.; Antenucci, N.; Meltan, S.; Neugebauer, V. Recent Advances in the Modulation of Pain by the Metabotropic Glutamate Receptors. Cells 2022, 11, 2608. [Google Scholar] [CrossRef]
- Linden, D.J.; Routtenberg, A. The role of protein kinase C in long-term potentiation: A testable model. Brain Res. Rev. 1989, 14, 279–296. [Google Scholar] [CrossRef]
- Bear, M.F.; Malenka, R.C. Synaptic plasticity: LTP and LTD. Curr. Opin. Neurobiol. 1994, 4, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.P. Synaptic plasticity: A role for nitric oxide in LTP. Curr. Biol. 1997, 7, R141–R143. [Google Scholar] [CrossRef] [PubMed]
- Falcicchia, C.; Tozzi, F.; Arancio, O.; Watterson, D.M.; Origlia, N. Involvement of p38 MAPK in Synaptic Function and Dysfunction. Int. J. Mol. Sci. 2020, 21, 5624. [Google Scholar] [CrossRef]
- Svendsen, F.; Tjølsen, A.; Hole, K. LTP of spinal A beta and C-fibre evoked responses after electrical sciatic nerve stimulation. Neuroreport 1997, 8, 3427–3430. [Google Scholar] [CrossRef]
- Prescott, S.A. Synaptic inhibition and disinhibition in the spinal dorsal horn. Prog. Mol. Biol. Transl. Sci. 2015, 131, 359–383. [Google Scholar] [CrossRef]
- Taylor, B.K. Spinal inhibitory neurotransmission in neuropathic pain. Curr. Pain. Headache Rep. 2009, 13, 208–214. [Google Scholar] [CrossRef]
- Polgár, E.; Hughes, D.I.; Riddell, J.S.; Maxwell, D.J.; Puskár, Z.; Todd, A.J. Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain 2003, 104, 229–239. [Google Scholar] [CrossRef]
- Todd, A.J. Plasticity of inhibition in the spinal cord. Handb. Exp. Pharmacol. 2015, 227, 171–190. [Google Scholar] [CrossRef]
- Telias, M.; Segal, M. Editorial: Pathological hyperactivity and hyperexcitability in the central nervous system. Front. Mol. Neurosci. 2022, 15, 955542. [Google Scholar] [CrossRef]
- Reis, C.; Chambel, S.; Ferreira, A.; Cruz, C.D. Involvement of nerve growth factor (NGF) in chronic neuropathic pain—A systematic review. Rev. Neurosci. 2023, 34, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Mantyh, P.W.; Koltzenburg, M.; Mendell, L.M.; Tive, L.; Shelton, D.L. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology 2011, 115, 189–204. [Google Scholar] [CrossRef]
- Brackenbury, W.J.; Djamgoz, M.B. Nerve growth factor enhances voltage-gated Na+ channel activity and Transwell migration in Mat-LyLu rat prostate cancer cell line. J. Cell. Physiol. 2007, 210, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Homma, K.; Kitamura, Y.; Ogawa, H.; Oka, K. Serotonin induces the increase in intracellular Ca2+ that enhances neurite outgrowth in PC12 cells via activation of 5-HT3 receptors and voltage-gated calcium channels. J. Neurosci. Res. 2006, 84, 316–325. [Google Scholar] [CrossRef]
- Shinoda, M.; Asano, M.; Omagari, D.; Honda, K.; Hitomi, S.; Katagiri, A.; Iwata, K. Nerve growth factor contribution via transient receptor potential vanilloid 1 to ectopic orofacial pain. J. Neurosci. 2011, 31, 7145–7155. [Google Scholar] [CrossRef]
- Munkholm, T.K.; Arendt-Nielsen, L. The interaction between NGF-induced hyperalgesia and acid-provoked pain in the infrapatellar fat pad and tibialis anterior muscle of healthy volunteers. Eur. J. Pain 2017, 21, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Skaper, S.D. Nerve growth factor: A neuroimmune crosstalk mediator for all seasons. Immunology 2017, 151, 1–15. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, J.Y.; Choi, J.Y.; Park, M.J.; Kim, D.S. Nerve growth factor activates brain-derived neurotrophic factor promoter IV via extracellular signal-regulated protein kinase 1/2 in PC12 cells. Mol. Cells 2006, 21, 237–243. [Google Scholar] [CrossRef]
- Jones, M.G.; Munson, J.B.; Thompson, S.W.N. A role for nerve growth factor in sympathetic sprouting in rat dorsal root ganglia. Pain 1999, 79, 21–29. [Google Scholar] [CrossRef]
- Delivanoglou, N.; Boziki, M.; Theotokis, P.; Kesidou, E.; Touloumi, O.; Dafi, N.; Nousiopoulou, E.; Lagoudaki, R.; Grigoriadis, N.; Charalampopoulos, I.; et al. Spatio-temporal expression profile of NGF and the two-receptor system, TrkA and p75NTR, in experimental autoimmune encephalomyelitis. J. Neuroinflamm. 2020, 17, 41. [Google Scholar] [CrossRef]
- Khodorova, A.; Nicol, G.D.; Strichartz, G. The p75NTR signaling cascade mediates mechanical hyperalgesia induced by nerve growth factor injected into the rat hind paw. Neuroscience 2013, 254, 312–323. [Google Scholar] [CrossRef]
- Obata, K.; Katsura, H.; Sakurai, J.; Kobayashi, K.; Yamanaka, H.; Dai, Y.; Fukuoka, T.; Noguchi, K. Suppression of the p75 neurotrophin receptor in uninjured sensory neurons reduces neuropathic pain after nerve injury. J. Neurosci. 2006, 26, 11974–11986. [Google Scholar] [CrossRef] [PubMed]
- Stratiievska, A.; Nelson, S.; Senning, E.N.; Lautz, J.D.; Smith, S.E.; Gordon, S.E. Reciprocal regulation among TRPV1 channels and phosphoinositide 3-kinase in response to nerve growth factor. Elife 2018, 7, e38869. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.; Ferrari, L.F.; Yang, W.; Chung, C.; Zhao, X.; Gu, Y.; Lin, S.; Zhang, K.; Cui, B.; Pearn, M.L.; et al. Swedish Nerve Growth Factor Mutation (NGFR100W) Defines a Role for TrkA and p75NTR in Nociception. J. Neurosci. 2018, 38, 3394–3413. [Google Scholar] [CrossRef]
- Yang, W.; Sung, K.; Zhou, F.; Xu, W.; Rissman, R.A.; Ding, J.; Wu, C. Targeted Mutation (R100W) of the Gene Encoding NGF Leads to Deficits in the Peripheral Sensory Nervous System. Front. Aging Neurosci. 2018, 10, 373. [Google Scholar] [CrossRef]
- Ren, K.; Thomas, D.A.; Dubner, R. Nerve growth factor alleviates a painful peripheral neuropathy in rats. Brain Res. 1995, 699, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, U.; Eliav, E.; Dorsey, J.M.; Gracely, R.H.; Kopin, I.J. NGF involvement in pain induced by chronic constriction injury of the rat sciatic nerve. Neuroreport. 1997, 8, 1613–1618. [Google Scholar] [CrossRef]
- Ramer, M.S.; Kawaja, M.D.; Henderson, J.T.; Roder, J.C.; Bisby, M.A. Glial overexpression of NGF enhances neuropathic pain and adrenergic sprouting into DRG following chronic sciatic constriction in mice. Neurosci. Lett. 1998, 251, 53–56. [Google Scholar] [CrossRef]
- da Silva, J.T.; Evangelista, B.G.; Venega, R.A.G.; Seminowicz, D.A.; Chacur, M. Anti-NGF treatment can reduce chronic neuropathic pain by changing peripheral mediators and brain activity in rats. Behav. Pharmacol. 2019, 30, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.L.; Yan, B.; Bao, Y.N.; Fan, J.F.; Liu, J.H. Suppression of peripheral NGF attenuates neuropathic pain induced by chronic constriction injury through the TAK1-MAPK/NF-κB signaling pathways. Cell Commun. Signal. 2020, 18, 66. [Google Scholar] [CrossRef]
- Jing, Y.Y.; Wang, J.Y.; Li, X.L.; Wang, Z.H.; Pei, L.; Pan, M.M.; Dong, X.P.; Fan, G.X.; Yuan, Y.K. Nerve growth factor of red nucleus involvement in pain induced by spared nerve injury of the rat sciatic nerve. Neurochem. Res. 2009, 34, 1612–1618. [Google Scholar] [CrossRef]
- Terada, Y.; Morita-Takemura, S.; Isonishi, A.; Tanaka, T.; Okuda, H.; Tatsumi, K.; Shinjo, T.; Kawaguchi, M.; Wanaka, A. NGF and BDNF expression in mouse DRG after spared nerve injury. Neurosci. Lett. 2018, 686, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Kras, J.V.; Weisshaar, C.L.; Pall, P.S.; Winkelstein, B.A. Pain from intra-articular NGF or joint injury in the rat requires contributions from peptidergic joint afferents. Neurosci. Lett. 2015, 604, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.F.; Deng, Y.S.; Xian, C.J.; Zhong, J.H. Neurotrophins from dorsal root ganglia trigger allodynia after spinal nerve injury in rats. Eur. J. Neurosci. 2000, 12, 100–105. [Google Scholar] [CrossRef]
- Ju, H.; Feng, Y.; Gao, Z.; Yang, B.X. The potential role of nerve growth factor in cryoneurolysis-induced neuropathic pain in rats. Cryobiology 2012, 65, 132–138. [Google Scholar] [CrossRef]
- Dos Reis, R.C.; Kopruszinski, C.M.; Nones, C.F.; Chichorro, J.G. Nerve growth factor induces facial heat hyperalgesia and plays a role in trigeminal neuropathic pain in rats. Behav. Pharmacol. 2016, 27, 528–535. [Google Scholar] [CrossRef]
- Micera, A.; Vigneti, E.; Aloe, L. Changes of NGF presence in nonneuronal cells in response to experimental allergic encephalomyelitis in Lewis rats. Exp. Neurol. 1998, 154, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, F.; Nicoletti, C.G.; Stampanoni Bassi, M.; Iezzi, E.; Buttari, F.; Furlan, R.; Finardi, A.; Marfia, G.A.; Centonze, D.; Mori, F. Nerve growth factor is elevated in the CSF of patients with multiple sclerosis and central neuropathic pain. J. Neuroimmunol. 2018, 314, 89–93. [Google Scholar] [CrossRef]
- Pecchi, E.; Priam, S.; Gosset, M.; Pigenet, A.; Sudre, L.; Laiguillon, M.C.; Berenbaum, F.; Houard, X. Induction of nerve growth factor expression and release by mechanical and inflammatory stimuli in chondrocytes: Possible involvement in osteoarthritis pain. Arthritis Res. Ther. 2014, 16, R16. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, M.; Ishikawa, T.; Kamoda, H.; Suzuki, M.; Inoue, G.; Sakuma, Y.; Oikawa, Y.; Orita, S.; Uchida, K.; Takahashi, K.; et al. Efficacy of nerve growth factor antibody in a knee osteoarthritis pain model in mice. BMC Musculoskelet. Disord. 2017, 18, 428. [Google Scholar] [CrossRef]
- Aso, K.; Walsh, D.A.; Wada, H.; Izumi, M.; Tomitori, H.; Fujii, K.; Ikeuchi, M. Time course and localization of nerve growth factor expression and sensory nerve growth during progression of knee osteoarthritis in rats. Osteoarthr. Cartil. 2022, 30, 1344–1355. [Google Scholar] [CrossRef]
- Apfel, S.C.; Kessler, J.A.; Adornato, B.T.; Litchy, W.J.; Sanders, C.; Rask, C. Recombinant human nerve growth factor in the treatment of diabetic polyneuropathy. NGF Study Group. Neurology 1998, 51, 695–702. [Google Scholar] [CrossRef]
- Apfel, S.C.; Schwartz, S.; Adornato, B.T.; Freeman, R.; Biton, V.; Rendell, M.; Vinik, A.; Giuliani, M.; Stevens, J.C.; Barbano, R.; et al. Efficacy and safety of recombinant human nerve growth factor in patients with diabetic polyneuropathy: A randomized controlled trial. JAMA 2000, 284, 2215–2221. [Google Scholar] [CrossRef]
- Unger, J.W.; Klitzsch, T.; Pera, S.; Reiter, R. Nerve growth factor (NGF) and diabetic neuropathy in the rat: Morphological investigations of the sural nerve, dorsal root ganglion, and spinal cord. Exp. Neurol. 1998, 153, 23–34. [Google Scholar] [CrossRef]
- Cheng, H.T.; Dauch, J.R.; Hayes, J.M.; Hong, Y.; Feldman, E.L. Nerve growth factor mediates mechanical allodynia in a mouse model of type 2 diabetes. J. Neuropathol. Exp. Neurol. 2009, 68, 1229–1243. [Google Scholar] [CrossRef]
- McArthur, J.C.; Yiannoutsos, C.; Simpson, D.M.; Adornato, B.T.; Singer, E.J.; Hollander, H.; Marra, C.; Rubin, M.; Cohen, B.A.; Tucker, T.; et al. A phase II trial of nerve growth factor for sensory neuropathy associated with HIV infection. AIDS Clinical Trials Group Team 291. Neurology 2000, 54, 1080–1088. [Google Scholar] [CrossRef]
- Schifitto, G.; Yiannoutsos, C.; Simpson, D.M.; Adornato, B.T.; Singer, E.J.; Hollander, H.; Marra, C.M.; Rubin, M.; Cohen, B.A.; Tucker, T.; et al. Long-term treatment with recombinant nerve growth factor for HIV-associated sensory neuropathy. Neurology 2001, 57, 1313–1316. [Google Scholar] [CrossRef]
- Velasco, R.; Navarro, X.; Gil-Gil, M.; Herrando-Grabulosa, M.; Calls, A.; Bruna, J. Neuropathic Pain and Nerve Growth Factor in Chemotherapy-Induced Peripheral Neuropathy: Prospective Clinical-Pathological Study. J. Pain Symptom Manag. 2017, 54, 815–825. [Google Scholar] [CrossRef]
- Hardowar, L.; Valentine, T.; Da Vitoria Lobo, M.; Corbett, J.; Owen, B.; Skeen, O.; Tomblin, L.; Sharma, D.; Elphick-Ross, J.; Hulse, R.P. Cisplatin induced alterations in nociceptor developmental trajectory elicits a TrkA dependent platinum-based chemotherapy induced neuropathic pain. Neuroscience 2024, 559, 39–53. [Google Scholar] [CrossRef]
- Christensen, M.D.; Hulsebosch, C.E. Spinal cord injury and anti-NGF treatment results in changes in CGRP density and distribution in the dorsal horn in the rat. Exp. Neurol. 1997, 147, 463–475. [Google Scholar] [CrossRef]
- Gujar, V.; Pande, R.D.; Hardas, B.M.; Das, S. Nerve Growth Factor Signaling Modulates the Expression of Glutaminase in Dorsal Root Ganglion Neurons during Peripheral Inflammation. Int. J. Mol. Sci. 2024, 25, 6053. [Google Scholar] [CrossRef]
- Nijs, J.; Meeus, M.; Versijpt, J.; Moens, M.; Bos, I.; Knaepen, K.; Meeusen, R. Brain-derived neurotrophic factor as a driving force behind neuroplasticity in neuropathic and central sensitization pain: A new therapeutic target? Expert Opin. Ther. Targets 2015, 19, 565–576. [Google Scholar] [CrossRef]
- Wise, B.L.; Seidel, M.F.; Lane, N.E. The evolution of nerve growth factor inhibition in clinical medicine. Nat. Rev. Rheumatol. 2020, 17, 34–46. [Google Scholar] [CrossRef]
- Ro, L.S.; Chen, S.T.; Tang, L.M.; Chang, H.S. Local application of anti-NGF blocks the collateral sprouting in rats following chronic constriction injury of the sciatic nerve. Neurosci. Lett. 1996, 218, 87–90. [Google Scholar] [CrossRef]
- Ro, L.S.; Chen, S.T.; Tang, L.M.; Jacobs, J.M. Effect of NGF and anti-NGF on neuropathic pain in rats following chronic constriction injury of the sciatic nerve. Pain 1999, 79, 265–274. [Google Scholar] [CrossRef]
- Kryger, G.S.; Kryger, Z.; Zhang, F.; Shelton, D.L.; Lineaweaver, W.C.; Buncke, H.J. Nerve growth factor inhibition prevents traumatic neuroma formation in the rat. J. Hand. Surg. Am. 2001, 26, 635–644. [Google Scholar] [CrossRef]
- Rojewska, E.; Popiolek-Barczyk, K.; Kolosowska, N.; Piotrowska, A.; Zychowska, M.; Makuch, W.; Przewlocka, B.; Mika, J. PD98059 Influences Immune Factors and Enhances Opioid Analgesia in Model of Neuropathy. PLoS ONE 2015, 10, e0138583. [Google Scholar] [CrossRef]
- Tu, Y.; Muley, M.M.; Beggs, S.; Salter, M.W. Microglia-independent peripheral neuropathic pain in male and female mice. Pain 2022, 163, e1129–e1144. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Xu, J.; Jiang, X.; Jing, L.; Tian, Y.; Wang, K.; Zhang, J. Inhibiting the JNK Signaling Pathway Attenuates Hypersensitivity and Anxiety-Like Behavior in a Rat Model of Non-specific Chronic Low Back Pain. J. Mol. Neurosci. 2024, 74, 73. [Google Scholar] [CrossRef]
- Owolabi, J.B.; Rizkalla, G.; Tehim, A.; Ross, G.M.; Riopelle, R.J.; Kamboj, R.; Ossipov, M.; Bian, D.; Wegert, S.; Porreca, F.; et al. Characterization of antiallodynic actions of ALE-0540, a novel nerve growth factor receptor antagonist, in the rat. J. Pharmacol. Exp. Ther. 1999, 289, 1271–1276. [Google Scholar] [CrossRef]
- Sanga, P.; Katz, N.; Polverejan, E.; Wang, S.; Kelly, K.M.; Haeussler, J.; Thipphawong, J. Efficacy, safety, and tolerability of fulranumab, an anti-nerve growth factor antibody, in the treatment of patients with moderate to severe osteoarthritis pain. Pain 2013, 154, 1910–1919. [Google Scholar] [CrossRef]
- Ishikawa, G.; Koya, Y.; Tanaka, H.; Nagakura, Y. Long-term analgesic effect of a single dose of anti-NGF antibody on pain during motion without notable suppression of joint edema and lesion in a rat model of osteoarthritis. Osteoarthr. Cartil. 2015, 23, 925–932. [Google Scholar] [CrossRef]
- Nwosu, L.N.; Mapp, P.I.; Chapman, V.; Walsh, D.A. Blocking the tropomyosin receptor kinase A (TrkA) receptor inhibits pain behaviour in two rat models of osteoarthritis. Ann. Rheum. Dis. 2016, 75, 1246–1254. [Google Scholar] [CrossRef]
- Von Loga, I.S.; El-Turabi, A.; Jostins, L.; Miotla-Zarebska, J.; Mackay-Alderson, J.; Zeltins, A.; Parisi, I.; Bachmann, M.F.; Vincent, T.L. Active immunisation targeting nerve growth factor attenuates chronic pain behaviour in murine osteoarthritis. Ann. Rheum. Dis. 2019, 78, 672–675. [Google Scholar] [CrossRef]
- Tian, Y.; Onodera, T.; Terkawi, M.A.; Iwasaki, K.; Hishimura, R.; Liang, D.; Miyazaki, T.; Iwasaki, N. Local Administration of Low-Dose Nerve Growth Factor Antibody Reduced Pain in a Rat Osteoarthritis Model. Int. J. Mol. Sci. 2021, 22, 2552. [Google Scholar] [CrossRef]
- Wang, H.; Romano, G.; Frustaci, M.E.; Bohidar, N.; Ma, H.; Sanga, P.; Ness, S.; Russell, L.J.; Fedgchin, M.; Kelly, K.M.; et al. Fulranumab for treatment of diabetic peripheral neuropathic pain: A randomized controlled trial. Neurology 2014, 83, 628–637. [Google Scholar] [CrossRef]
- Bramson, C.; Herrmann, D.N.; Carey, W.; Keller, D.; Brown, M.T.; West, C.R.; Verburg, K.M.; Dyck, P.J. Exploring the role of tanezumab as a novel treatment for the relief of neuropathic pain. Pain Med. 2015, 16, 1163–1176. [Google Scholar] [CrossRef]
- Dong, X.; Li, H.; Pei, M.; Tan, J.; Chen, G.; Li, S.; Xie, Z.; Wang, Q.; Wang, G.; Chen, Y.L.; et al. Analgesic effects of nerve growth factor-directed monoclonal antibody on diabetic neuralgia in an animal model. FEBS Open Bio 2022, 12, 1325–1335. [Google Scholar] [CrossRef]
- Liang, Z.J.; Tan, J.; Tang, L.; Xie, Z.B.; Chen, G.J.; Liu, G.J.; Yuan, L.; Wang, K.X.; Ding, H.P.; Qiu, H.; et al. NGF monoclonal antibody DS002 alleviates chemotherapy-induced peripheral neuropathy in rats. Acta Pharmacol. Sin. 2022, 43, 2841–2847. [Google Scholar] [CrossRef]
- Chang, D.S.; Hsu, E.; Hottinger, D.G.; Cohen, S.P. Anti-nerve growth factor in pain management: Current evidence. J. Pain Res. 2016, 9, 373–383. [Google Scholar] [CrossRef]
- Jaffal, S.; Khalil, R. Targeting nerve growth factor for pain relief: Pros and cons. Korean J. Pain 2024, 37, 288–298. [Google Scholar] [CrossRef] [PubMed]

| Cause of Neuropathy | Preclinical/Clinical Research | Treatment Employed | Beneficial Results | References |
|---|---|---|---|---|
| Chronic constriction injury (CCI) model | Preclinical (rat) | Anti-NGF pAb | Inhibition of collateral sprouting by the saphenous nerve into the sciatic nerve’s territory was effectively prevented by the local application of anti-NGF | [165] |
| Preclinical (rat) | Anti-NGF pAb | The application of anti-NGF serum at the injury site delayed the onset of hyperalgesia | [138] | |
| Preclinical (rat) | Anti-NGF mAb | High dosage of anti-NGF completely abolished heat and cold hyperalgesia, induced by CCI | [166] | |
| Preclinical (rat) | TrkA-IgG (inhibitor that comprises the NGF receptor linked to an immunoglobulin) | Inhibition of NGF after peripheral nerve injury reduced neuroma formation and NP while safeguarding the cell bodies of transected neurons | [167] | |
| Preclinical (rat) | PD90859 (inhibitor of the MAPKK family members MEK1/2 and blocks NGF-induced ERK1/2 phosphorylation) | PD98059 reduced pain scores and increased the effectiveness of opioids in neuropathy | [168] | |
| Preclinical (rat) | Anti-NGF mAb | Anti-NGF induced a significant, dose-dependent reduction in mechanical threshold, thermal withdrawal latency, and cold sensitivity | [140] | |
| Preclinical (rat) | Anti-NGF mAb l-CDL (inhibitor of NGF secretion) | Anti-NGF suppressed TAK1 in the periphery, reducing CCI-induced NP by inhibiting downstream MAPK and p65 signaling. Additionally, l-CDL inhibited NGF secretion by macrophages and Schwann cells, as well as downstream TAK1-MAPK/NF-κB signaling in the periphery, to alleviate CCI-induced NP | [141] | |
| Preclinical (mouse) | Y1036 (NGF sequestration agent) | Y1036 prevented NP-induced pain hypersensitivity | [169] | |
| Chronic low back pain (LBP) | Preclinical (rat) | SP600125 (JNK inhibitor) | SP600125 reduced astrocyte and neuronal activation, demonstrating that the hypersensitivity and anxiety-like behaviors induced by NGF in LBP rats can be mitigated by this JNK inhibitor | [170] |
| Spared nerve injury (SNI) model | Preclinical (rat) | Anti-NGF mAb | Anti-NGF antibody was injected into the RN. The anti-NGF antibody attenuated mechanical allodynia | [142] |
| Peripheral nerve injury model (transection of lumbar spinal nerve) | Preclinical (rat) | ALE-0540 (TrkA antagonist) | Administration of ALE-0540 in rats resulted in antiallodynic effects in the L5/L6 spinal nerve ligation model | [171] |
| Preclinical (rat) | Anti-NGF mAb | Direct delivery of anti-NGF antibodies into the injured DRG reduced the percentage of foot withdrawal responses | [145] | |
| Trigeminal neuralgia (TN) | Preclinical (rat) | Anti-NGF mAb | Treatment with anti-NGF significantly alleviated heat hyperalgesia linked to trigeminal neuralgia | [147] |
| Osteoarthritis (OA) | Human | Anti-NGF mAb (Fulranumab) | Primary efficacy results showed that fulranumab significantly reduced the average pain intensity score | [172] |
| Preclinical (rat) | Anti-NGF mAb | Anti-NGF mAb exerted a long-lasting analgesic effect | [173] | |
| Preclinical (rat) | AR786 (selective TrkA antagonist) | AR786 treatment prevented the development of pain behaviors, while therapeutic intervention mitigated established pain behaviors | [174] | |
| Preclinical (mouse) | CuMVttNGF vaccine | NGF vaccine alleviated spontaneous pain behavior in surgically induced OA | [175] | |
| Preclinical (rat) | Anti-NGF mAb | The injection of anti-NGF antibodies reduced pain scores in OA rats, improving their weight-bearing performance; however, it did not alleviate allodynia | [176] | |
| Diabetic polyneuropathy (DPN) | Human | Anti-NGF mAb (Fulranumab) | This study offered evidence that in DPN patients, fulranumab reduces pain scores | [177] |
| Human | Anti-NGF mAb (Tanezumab) | Tanezumab provided effective pain reduction in DPN | [178] | |
| Preclinical (mouse) | Humanized anti-NGF mAb (huAb45) | huAb45, an antibody capable of neutralizing the interaction between NGF and its receptor TrkA, has demonstrated efficacy in alleviating NP associated with DPN | [179] | |
| Chemotherapy-induced peripheral neuropathy (CIPN) | Preclinical (rat) | Humanized anti-NGF mAb (DS002) | In three rat models of CIPN (paclitaxel, cisplatin, and vincristine), subcutaneous administration of DS002 demonstrated a significant prophylactic effect | [180] |
| Preclinical (rat) | GW441756 (selective TrkA antagonist) | TrkA activation by NGF triggered sensory neuritogenesis and nociceptor sensitization, which can be prevented by TrkA inhibition. GW441756 reduced cisplatin-induced TRPV1-related nociceptor sensitization and prevented NP caused by cisplatin | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Domínguez, M. NGF in Neuropathic Pain: Understanding Its Role and Therapeutic Opportunities. Curr. Issues Mol. Biol. 2025, 47, 93. https://doi.org/10.3390/cimb47020093
García-Domínguez M. NGF in Neuropathic Pain: Understanding Its Role and Therapeutic Opportunities. Current Issues in Molecular Biology. 2025; 47(2):93. https://doi.org/10.3390/cimb47020093
Chicago/Turabian StyleGarcía-Domínguez, Mario. 2025. "NGF in Neuropathic Pain: Understanding Its Role and Therapeutic Opportunities" Current Issues in Molecular Biology 47, no. 2: 93. https://doi.org/10.3390/cimb47020093
APA StyleGarcía-Domínguez, M. (2025). NGF in Neuropathic Pain: Understanding Its Role and Therapeutic Opportunities. Current Issues in Molecular Biology, 47(2), 93. https://doi.org/10.3390/cimb47020093




