1. Introduction
Pancreatic cancer remains one of the most lethal malignancies, with a 5-year relative survival rate of approximately 13% [
1]. Although the survival rate of patients with pancreatic cancer has been improving, the rate of its enhancement has been slower compared with other cancers [
2]. PAAD, a highly malignant and rapidly progressing tumor, shares similar characteristics with other aggressive cancers such as ovarian cancer [
1,
3]. It presents significant challenges for early diagnosis and effective treatment, and its clinical manifestations are often nonspecific in the early stages. PAAD originates from the pancreatic exocrine ducts and constitutes the majority of pancreatic cancer cases [
4]. Obesity, smoking, chronic pancreatitis and late-onset diabetes are several risk factors for pancreatic cancer [
5,
6]. Unfortunately, most patients are diagnosed at advanced stages, where treatment options become limited, and survival rates remain poor.
Despite advances in therapeutic strategies, the molecular mechanisms underlying PAAD’s initiation and progression remain incompletely understood. PAAD is known to progress through precursor lesions, primarily pancreatic intraepithelial neoplasia (PanIN) and intraductal papillary mucinous neoplasm (IPMN), whose genetic evolution involves recurrent driver mutations such as
KRAS,
TP53,
CDKN2A, and
SMAD4 [
7,
8,
9]. However, recent single-cell and spatial multi-omic studies have revealed profound intertumoral heterogeneity and complex evolutionary trajectories that challenge this classical linear model [
10]. Moreover, selective pressures from the tumor microenvironment, along with emerging evidence for epigenetic reprogramming, further complicate the landscape of PAAD progression and therapeutic resistance [
9,
11]. Consequently, while the general framework of PAAD genetic evolution is established, the key molecular mechanisms that sustain its progression and immune evasion remain unresolved. In particular, how tumor cells interact with and remodel their microenvironment through specific signaling mediators is still poorly defined.
Adhesion G-protein-coupled receptor (adhesion GPCR) represents an important subfamily of the GPCR superfamily [
12]. These receptors are unique in that they mediate signaling through interactions with extracellular matrix components, influencing cellular adhesion, migration, and differentiation. The contemporary nomenclature of adhesion GPCRs (with the previous names featured in parentheses) is as follows: Adhesion GPCR D1 (
ADGRD1) (
gpr133),
ADGRF1 (
gpr110),
ADGRG6 (
gpr126), and others. Previous studies have highlighted the critical roles of adhesion GPCRs in tumor biology. For instance,
ADGRD1 (
gpr133) is highly expressed in glioblastoma and plays a role in tumor progression [
13].
ADGRF1 acts as an oncogene in lung and prostate cancers and serves as a prognostic biomarker in osteosarcoma [
14,
15], while
ADGRG1 and
ADGRL4 exert tumor-suppressive functions in melanoma and retinoblastoma, respectively [
16,
17]. Furthermore, adhesion GPCRs have been linked to immune cell infiltration and modulation of the tumor microenvironment (TME), suggesting their potential as therapeutic and immunomodulatory targets [
18].
ADGRG6 (
gpr126), an orphan receptor within this subfamily, plays essential roles in Schwann cell myelination development and disease regulation [
19,
20]. Recent studies have implicated
ADGRG6 in tumor progression and immune regulation, suggesting it as a promising candidate for cancer diagnostics and therapy [
21,
22]. Notably, Wu et al. identified
ADGRG6 as a novel prognostic biomarker for PAAD that promotes tumor progression by stabilizing mutant p53 and activating the EGPR/NF-κB signaling axis [
23]. While their work revealed a tumor cell-intrinsic oncogenic mechanism, the immunological functions of
ADGRG6 and its broader impact on the TME remain poorly defined.
Building upon these findings, the present study aimed to systematically elucidate the oncogenic and immunoregulatory roles of ADGRG6 in PAAD. Through integrative bioinformatics analyses, tissue microarray validation, and functional assays in vitro and in vivo, including 2D and 3D cell cultures, zebrafish xenografts, and murine tumor models, we investigated its expression pattern, prognostic impact, and underlying molecular mechanisms.
We hypothesized that ADGRG6 is overexpressed in PAAD and predicts unfavorable clinical outcomes by promoting tumor cell proliferation and migration through NF-κB/STAT6-mediated signaling and modulation of the TME. The primary endpoints of this study were (i) the association between ADGRG6 expression and patient prognosis and (ii) the functional and mechanistic evidence linking ADGRG6 to tumor progression and immune regulation in PAAD.
2. Materials and Methods
2.1. Tissue Microarray and Immunohistochemistry (IHC)
A PAAD tissue microarray containing 71 tumor and adjacent non-tumorous pancreatic tissues (Shanghai Xinchao Biotechnology, Shanghai, China; Cat no. HPanA120Su02) was subjected to IHC analysis. IHC staining was performed using a non-biotin detection system (ZSGB-Bio, Beijing, China; Cat no. PV-6000) according to the manufacturer’s instructions. Briefly, tissue sections were deparaffinized in xylene, rehydrated through graded ethanol, and subjected to antigen retrieval in citrate buffer (pH 6.0) at 95 °C for 15 min. Endogenous peroxidase activity was quenched using a peroxidase blocking solution for 10 min at room temperature. Sections were then incubated overnight at 4 °C with a rabbit polyclonal anti-ADGRG6 antibody (1:500; Proteintech, Rosemont, IL, USA, Cat. no. 17774-1-AP). Following PBS washes, sections were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG polymer for 20 min at room temperature. The signal was visualized using a freshly prepared 3,3′-diaminobenzidine (DAB) substrate for 5 min, followed by hematoxylin counterstaining, dehydration, clearing, and mounting.
ADGRG6 expression was semi-quantitatively evaluated based on both staining intensity and the percentage of positive tumor cells, following established scoring criteria: Staining intensity: 0 (no staining), 1 (light yellow), 2 (brown-yellow), 3 (dark brown). Positive cell proportion: 0 (<5%), 1 (5~25%), 2 (26~50%), 3 (51~75%), 4 (>75%). A composite score was calculated by multiplying intensity and proportion scores (range: 0–12). The median composite score (6) was used as the cutoff point to define high expression (>6) and low expression (≤6) groups. To minimize subjectivity, two experienced pathologists independently assessed all stained sections in a double-blinded manner, without access to any clinical or outcome information. In cases where their scores differed by ≥2 points, a consensus score was determined after joint re-evaluation and discussion using corresponding H&E-stained sections. Clinicopathological characteristics of the TMA cohort (including age, sex, tumor size, grade, TNM stage, and AJCC stage) are provided in
Supplementary Table S1.
2.2. Cell Culture and Transfection
AsPC-1 and BxPC-3 cell lines were purchased from the Chinese Academy of Sciences Cell Bank and cultured in DMEM + 10% FBS (Meilunbio, Dalian, China, Cat. no. MA0212 and PWL217). Small interfering RNA (siRNA) targeting ADGRG6 (si-ADGRG6, sense strand: 5′-ccaagcaauaaugaaucguautt-3′; antisense strand: 5′-auacgauucauuugcuuggtt-3′) and a negative control (si-NC, sense strand: 5′-uucuccgaacgugucacgutt-3′; antisense strand: 5′-acgugacacguucggagaatt-3′) were synthesized (Sangon Biotech, Shanghai, China) and transfected using LipofectamineTM; 3000 (Invitrogen, Carlsbad, CA, USA, Cat. no. L3000015) per manufacturer’s protocol.
2.3. 3D Spheroid Cultivation Protocol
Transfected AsPC-1 and BxPC-3 cells underwent harvesting and resuspension in complete culture medium to achieve a 5 × 103 cells/mL concentration. Using pipettes operated at reduced dispensing velocity (<50 µL/s), 40 µL aliquots of cell suspension were carefully introduced into individual wells of GravityPLUSTM; Hanging-Drop Plates via SureDropTM; inlet funnels (InSphero, Schlieren, Switzerland, Cat. no. ISP-06-010). Bottom plate reservoirs received humidifier pads soaked in sterile 0.5 × PBS to minimize evaporation. Incubation proceeded at 37 °C within 5% CO2 environment, maintaining >95% relative humidity. After spheroid establishment (2–4 days), formed microtissues underwent transfer to pre-moistened GravityTRAPTM; Tissue Receiver Plates through addition of 70 µL complete medium per well at a controlled dispensing velocity ≤ 10 µL/s. Culture medium underwent replacement at 48-h intervals to sustain appropriate nutritional environments. Spheroid documentation occurred at 3, 7, 14, and 21-day timepoints through inverted microscopy. Diameter measurements were performed using ImageJ analysis software (NIH, Bethesda, MD, USA, version 2.14.0), ensuring a minimum of 6 replicate spheroids per treatment group underwent quantification.
2.4. Cell Proliferation Assay
Transfected cells were seeded in 96-well plates and cultured for 1~4 days. Cell viability was assessed via CCK-8 (Meilunbio, Dalian, China, Cat. no. MA0218) with absorbance measured at 450 nm using a BioTek Synergy2 microplate reader (Winooski, VT, USA).
2.5. Wound-Healing Assay
Cells were grown to 90% confluence, scratched with a pipette tip, washed, and cultured in fresh DMEM. Images were taken at 0 and 24 h to assess wound closure.
2.6. Transwell Migration Assay
Migration was evaluated using Transwell chambers (Corning, NY, USA). Cells (1 × 105) were seeded in serum-free medium in the upper chamber; 10% FBS DMEM was added to the lower chamber. After 24 h, migrated cells were fixed, stained (0.5% crystal violet), imaged, and counted.
2.7. RT-qPCR
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA, Cat. no. 15596026CN) and reverse-transcribed using an M-MLV Kit (Promega, Madison, WI, USA, Cat. no. D1301). qPCR was conducted with SYBR Green (Takara, Tokyo, Japan, Cat. no. RR420L) on an ABI 7500 system. Primer sequences: ADGRG6, F: 5′-TGTCGTTAATATCAGTTTTCACC-3′, R: 5′-TATGTAGCCTCAAGCCTTCA-3′; STAT6, F: 5′-CTTTCCGGAGCCACTACAAG-3′, R: 5′-AGGAAGTGGTTGGTCCCTTT-3′; GATA3, F: 5′-GAACCGGCCCCTCATTAAG-3′, R: 5′-ATTTTTCGGTTTCTGGTCTGGAT-3′; β-actin, F: 5′-ACCGAGCGCGGCTACAG-3′, R: 5′-CTTAATGTCACGCACGATTTCC-3′. PCR conditions: 95 °C for 3 min, then 40 cycles of 95 °C for 10 s, 60 °C for 31 s. Relative expression was calculated using the 2−ΔΔCt method.
2.8. Western Blot
Total protein lysates were prepared from AsPC-1 cells transfected with si-ADGRG6 or si-NC for 48 h. Blots were probed using anti-ADGRG6 (1:1000, Proteintech, Rosemont, IL, USA), anti-phospho-NF-κB p65 (Ser468) (1:1000, Proteintech), anti-NF-κB p65 (1:1000, Proteintech), anti-STAT6 (1:1000, Proteintech), anti-phospho-STAT6 (Tyr641) (1:1000, Proteintech), anti-GATA3 (1:1000, Proteintech), and anti-GAPDH (1:3000, Proteintech) antibodies. Bands were visualized using Enhanced Chemiluminescence (ECL, Meilunbio, Dalian, China) and imaged with a ChemiDocTM; Imaging System (Bio-Rad, Hercules, CA, USA). Densitometric quantification was performed using ImageJ, with ADGRG6 intensity normalized to GAPDH.
2.9. ELISA
To quantify cytokine secretion, supernatants from AsPC-1 and BxPC-3 cells were collected 48 h after siRNA transfection, centrifuged at 1500× g for 10 min, and stored at −80 °C. The concentrations of IL-6 and IL-8 were measured using commercial human ELISA kits (Human IL-6 Quantikine ELISA Kit, Cat. no. MM-0049H2, Meimian, Yancheng, China; Human IL-8 Quantikine ELISA Kit, Cat. no. MM-1558H2, Meimian, Yancheng, China) according to the manufacturer’s instructions. Briefly, 100 µL of standards or samples were added to each well of a pre-coated 96-well plate and incubated for 2 h at room temperature. After washing, detection antibody and streptavidin-HRP were added sequentially, and color development was achieved using TMB substrate. Absorbance was measured at 450 nm using a microplate reader (BioTek, Winooski, VT, USA). Cytokine concentrations were calculated from standard curves and normalized to total protein concentration. All assays were performed in triplicate.
2.10. Zebrafish Xenograft Model
At 48 h post-fertilization (hpf), zebrafish (
Danio rerio) larvae were anesthetized with 0.16 mg/mL tricaine (Sigma-aldrich, MO, USA, Cat. no. E10521) and injected into the yolk sac with 150~200 CM-Dil (Invitrogen, OR, USA, Cat. no. C7000) pre-labeled AsPC-1 cells transfected with either si-ADGRG6 or si-NC per larva. Post-injection, larvae were incubated at 32 °C for 48 h. For each group, at least 30 larvae were injected to ensure a minimum of 10 successful engraftments. More than 10 larvae per group were randomly selected and imaged using a stereomicroscope, Leica. Tumor fluorescence area was quantified using ImageJ software (NIH, Bethesda, MD, USA, version 2.14.0) for further analysis. Euthanasia followed the US Food and Drug Administration (FDA) and the AVMA
Guidelines on Euthanasia via ice water immersion on 5 dpf [
24]. Cardiac arrest was confirmed in a stereomicroscope Leica (Leica, Wetzlar, Germany, M205C) before bleaching in 10% sodium hypochlorite (Aladdin, Shanghai, China, Cat. no. S291945) [
25].
2.11. Animal Experiments
Female athymic BALB/c nude mice aged four to five weeks (body weight: 18–22 g) were procured from Xiamen Fudexin Biotechnology Co., Ltd. (Xiamen, China). The Laboratory Animal Welfare and Ethics Committee of Fujian Provincial Hospital granted approval for all experimental procedures (IACUC-FRH-SL-20250207 [0561]). Animals were maintained within specific pathogen-free (SPF) housing under regulated environmental parameters (temperature: 20–30 °C; humidity: 60–80%) with unrestricted access to SPF rodent diet and sterilized water. Transfected AsPC-1 cells (si-ADGRG6, si-Cont control siRNA, or NC negative control) underwent subcutaneous injection into the right flank region of individual mice at 5 × 106 cells per injection site (n = 6 animals per experimental group). Tumor development was assessed at three-day intervals through caliper measurements of minimum diameter (A) and maximum diameter (B). Volume calculations (V) employed the formula V = (A2 × B)/2. Body weight documentation occurred concurrently with tumor measurements. Following a 30-day experimental period, animals received isoflurane anesthesia (2% via inhalation route) before euthanasia through cervical dislocation, with confirmation via cessation of cardiovascular and respiratory functions. Tumor tissues underwent immediate excision and weight determination post-euthanasia.
2.12. Bioinformatics Databases
For expression analysis, GEPIA (
http://gepia.cancer-pku.cn/index.html, accessed on 12 November 2025) was used to evaluate
ADGRG6 mRNA and protein expression levels and survival in PAAD patients, integrating The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) data. UALCAN (
http://ualcan.path.uab.edu/, accessed on 12 November 2025) is a web-based platform for analyzing cancer OMICS data derived from TCGA, MET500, CPTAC, and CBTTC sources [
26]. It was used to analyze expression in relation to clinicopathological subgroups in this study. “Normal” tissues refer to non-malignant adjacent tissues from the TCGA-CPTAC cohort and are not annotated for patient sex or comorbidity status. TIMER (
https://cistrome.shinyapps.io/timer/, accessed on 12 November 2025) is a comprehensive database used for systematic analyses of immune infiltration in various tumors [
27]. The Kaplan–Meier plotter (
https://kmplot.com/analysis/, accessed on 10 May 2024) was used to explore the prognostic value of ADGRG6 based on immune cell status. TISCH2 (
http://tisch.comp-genomics.org/, accessed on 21 May 2024) is a single-cell RNA-seq database focused on TME landscapes [
28]. From the eight pancreatic cancer datasets available in TISCH, we selected PAAD_CRA001160 and PAAD_GSE154778 for downstream TME analysis due to their comprehensive clinical and molecular profiles and better annotation [
29,
30]. GeneMANIA (
http://www.genemania.org, accessed on 12 November 2025) was used to predict gene function based on co-expression and interaction networks. LinkedOmics (
http://www.linkedomics.org/login.php, accessed on 12 November 2025) provided additional transcriptomic correlation analysis using TCGA data from 32 cancers and 10 CPTAC cohorts [
31]. IHC images of ADGRG6 in PAAD and normal pancreas tissues were randomly obtained from the Human Protein Atlas (HPA) database (
https://www.proteinatlas.org/, accessed on 12 November 2025). The KEGG pathway enrichment of
ADGRG6 co-expressed genes was conducted using the LinkInterpreter module of the Linkedomics database. Pearson correlation analysis between
ADGRG6 and representative signaling genes (
NFKB1,
RELA,
STAT6, and
GATA3) was performed using expression data from 178 TCGA-PAAD clinical samples. Correlation coefficients (R values) and
p values were calculated and visualized using GraphPad Prism 8.
2.13. Statistical Analysis
Data was analyzed using SPSS (version 22.0, IBM, Armonk, NY, USA) and web-based tools. Survival analysis was performed with Kaplan–Meier plots (
https://kmplot.com/analysis/, accessed on 10 May 2024). Association with immune markers were tested via Spearman’s correlation (TIMER2.0 database,
http://timer.cistrome.org/, accessed on 12 November 2025). Univariate and multivariate Cox regression assessed prognostic factors. Student’s
t-test or ANOVA with Bonferroni’s post hoc tests were used. Experiments were repeated ≥3 times.
p < 0.05 was considered statistically significant.
3. Results
3.1. ADGRG6 Is Consistently Upregulated in PAAD Across Clinical and Molecular Subgroups
To investigate the expression pattern of
ADGRG6 in PAAD, we first examined its mRNA levels in tumor and normal pancreatic tissues using the GEPIA database, which integrates RNA-seq data from TCGA and GTEx. The results showed that
ADGRG6 was significantly upregulated in PAAD tissues (Tumor, T,
n = 179) compared with the normal population (Normal, N,
n = 171) (
Figure 1A, *
p < 0.05). To further evaluate whether this elevated expression was consistent across various clinical contexts, we analyzed
ADGRG6 mRNA levels in several patient subgroups using the UALCAN database, which utilizes TCGA-CPTAC data. Subgroups included sex, pancreatitis status, age, drinking habits, diabetes status, tumor grade, lymph node metastasis, and
TP53 mutation status (
Figure 1B–I). It is important to note that the normal cohort in this UALCAN-derived dataset is limited (
n = 4), which warrants caution in interpreting the magnitude of this difference. To assess the validity of differential expression calls within this specific dataset, we performed an internal control analysis using well-established PAAD driver genes within the same platform. As shown in
Supplementary Figure S1, the expression patterns (
KRAS overexpression;
TP53 and
SMAD4 downregulation) align with the canonical molecular landscape of PAAD [
32,
33]. This internal control supports the utility of the dataset for identifying biologically relevant trends, thereby lending credence to the consistent overexpression of
ADGRG6 observed across PAAD subgroups.
In general,
ADGRG6 mRNA expression was significantly elevated in all clinical subgroups compared to normal tissues (all
p < 0.05), with the exception of the two “21~40 Yrs” and “weekly drinker” groups (
p = 0.44 and
p = 0.068, respectively). However, pairwise comparisons among PAAD subgroups themselves (e.g., male vs. female, diabetic vs. non-diabetic, age groups) did not yield statistically significant differences (
p > 0.05 in all cases). A full list of comparisons and
p-values is provided in
Supplementary Table S3.
For the drinking status variable, we further found that metadata was unavailable for 80 of the TCGA-PAAD samples, which reduces the reliability of this clinical subgroup. Although the “non-drinker” group exhibited the second-highest mean ADGRG6 expression, comparisons among drinking groups were not statistically significant, suggesting that drinking habits are unlikely to be a biologically meaningful determinant of ADGRG6 expression in PAAD.
Despite extensive subgroup analyses, ADGRG6 expression showed consistent elevation across clinical and molecular groups, with minimal inter-subgroup variation. This supports its potential utility as a broadly applicable diagnostic or prognostic biomarker in PAAD.
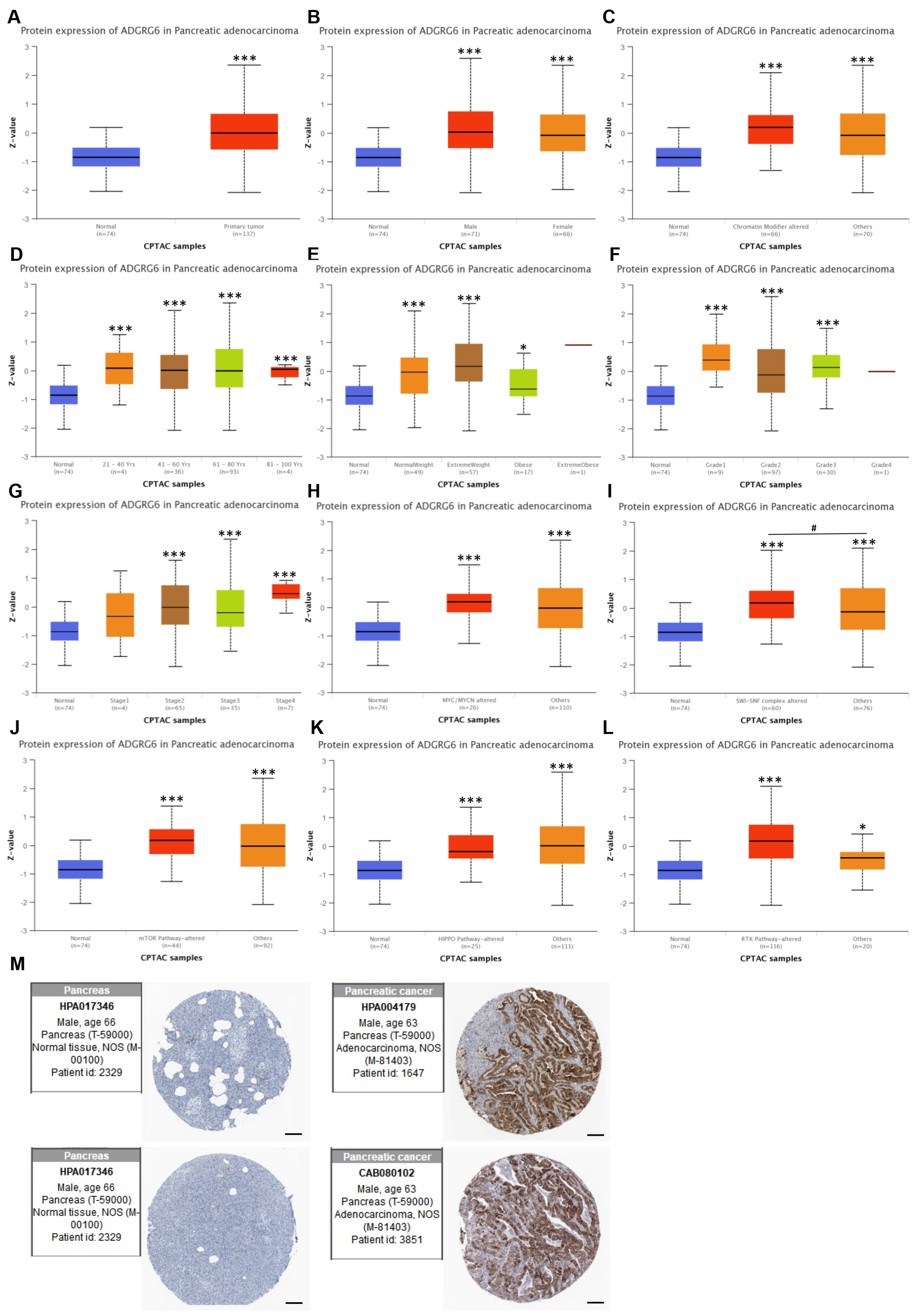
We next analyzed ADGRG6 protein-level expression using the UALCAN proteomics module (based on CPTAC). Consistent with transcriptomic results, ADGRG6 protein levels were significantly elevated in PAAD tissues compared to normal pancreatic tissues (
Figure 2A). Subgroup analyses were further conducted according to clinical and molecular parameters, including sex, chromatin modifier alterations, age, weight, tumor grade,
MYC/MYCN alterations, SWI/SNF complex alterations, and activity status of mTOR, Hippo, and receptor tyrosine kinase (RTK) signaling pathways (
Figure 2B–L), although inter-subgroup differences were not always statistically significant. In subgroup panels such as chromatin modifiers,
MYC/MYCN alterations, the “others” group refers to patients without these mutations or with unannotated genetic status in the CPTAC dataset.
Additionally, IHC data from the HPA database corroborated these findings, showing increased ADGRG6 staining intensity in PAAD tissues compared to normal pancreatic tissues (
Figure 2M). Taken together, these findings demonstrate that ADGRG6 is broadly and consistently upregulated in PAAD tissues at both the mRNA and protein levels, across a variety of clinical and molecular contexts. However, its expression does not appear to be driven by specific clinicopathological features, suggesting it may serve as a general molecular hallmark of PAAD rather than a subgroup-specific marker. These results prompted further evaluation of the clinical significance and biological role of
ADGRG6 in PAAD.
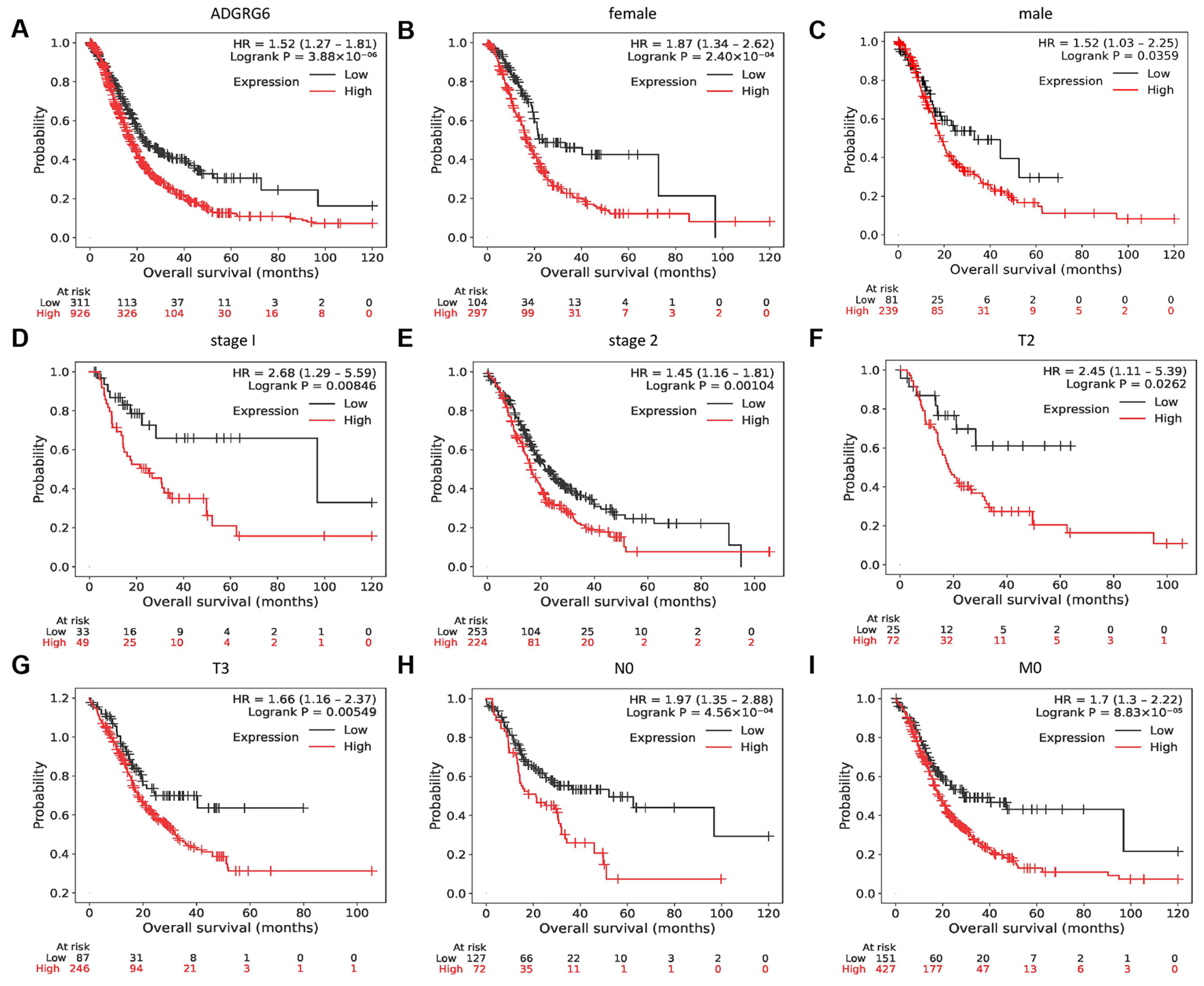
3.2. High ADGRG6 Expression Predicts Poor Overall Survival in Patients with PAAD
Given its robust overexpression in PAAD tissues, we next evaluated whether ADGRG6 expression levels were associated with patient outcomes. Kaplan–Meier survival curve analysis was used to evaluate the correlation between
ADGRG6 expression and the overall survival (OS) of patients with PAAD. High
ADGRG6 expression was associated with a worse OS rate for patients with PAAD (
Figure 3A). Subsequently, the association between
ADGRG6 expression and OS in different subgroups was investigated. Patients with high
ADGRG6 expression had a worse OS in multiple subgroups, including sex, cancer stages 1–2, T2–3 (tumor size >2 cm but ≤4 cm, >4 cm), N0 (no regional lymph node metastasis), and M0 (no distant metastasis) (
Figure 3B–I). Collectively, these findings suggested that
ADGRG6 can be used as a biomarker map to predict the prognosis of patients with PAAD.
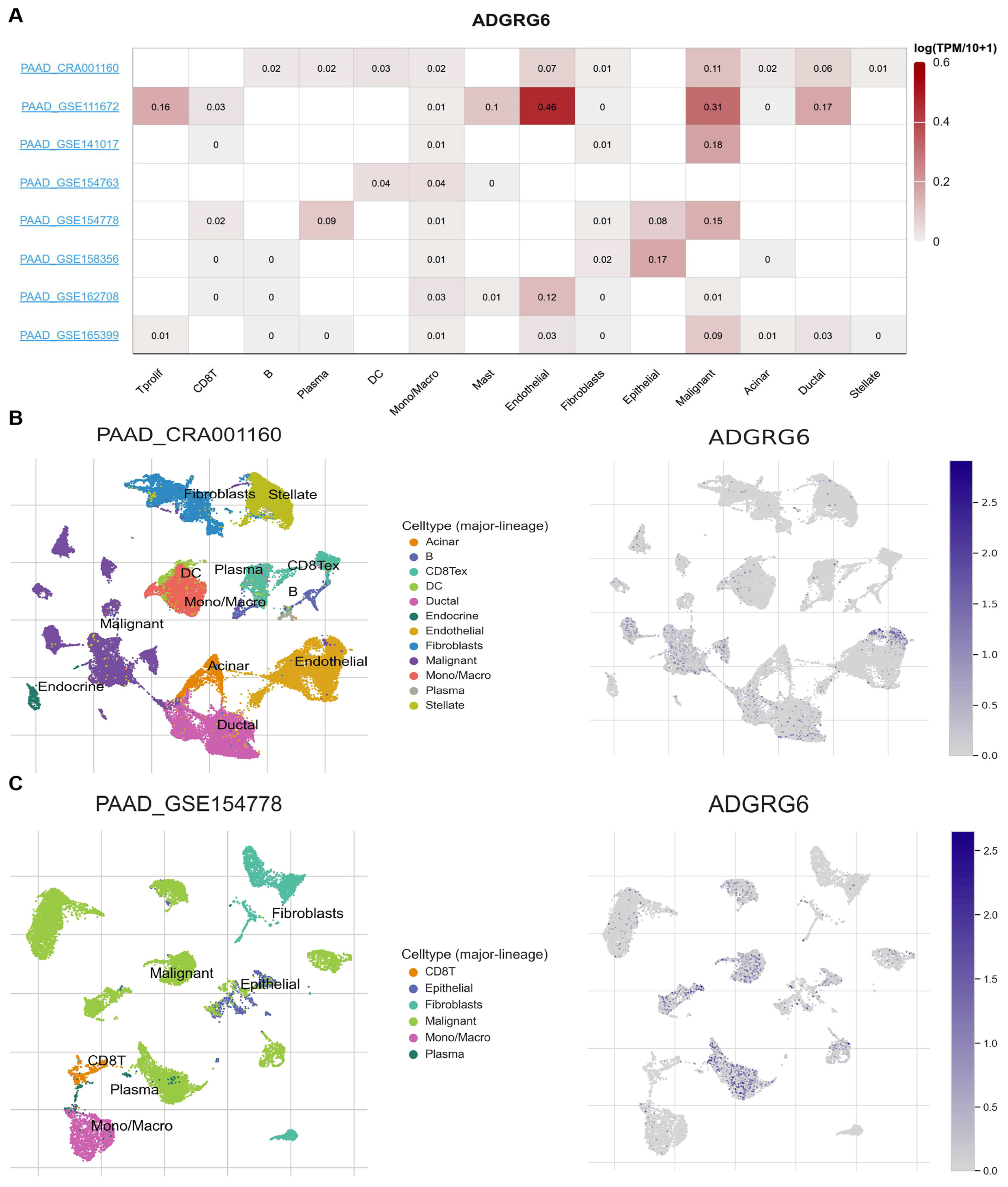
3.3. ADGRG6 Expression Is Enriched in Malignant and Stromal Cell Populations at the Single-Cell Level of the TME
To further investigate the cell-type-specific distribution of
ADGRG6 in the TME of PAAD, we analyzed single-cell RNA sequencing (scRNA-seq) datasets using the TISCH database. The expression of
ADGRG6 across various immune and stromal cell types in different datasets is illustrated in
Figure 4A. Among the eight available PAAD datasets, we specifically focused on PAAD_CRA001160 and PAAD_GSE154778, as these two datasets provide comprehensive clinical and molecular profiles, offering valuable insights into the complexity of the TME, including tumor, immune, and stromal cell populations. Both datasets utilized a high-quality scRNA-seq platform (10x Genomics), enabling a detailed examination of
ADGRG6 expression at the single-cell level.
The PAAD_CRA001160 dataset provides scRNA-seq data from 24 PAAD tumor samples and 11 control pancreases with a total of 41,986 cells from PAAD tumors and 15,544 cells from control tissues. The PAAD_GSE154778 dataset includes 16 patients, with 10 primary tumor tissues and 6 metastatic biopsies (from liver or omentum). A total of 8000 cells were obtained from the 10 primary tumors and 6926 cells from the 6 metastasis samples. To visualize the distribution of
ADGRG6 expression in different immune and stromal cell types, we employed Uniform Manifold Approximation and Projection (UMAP) plots (
Figure 4B,C). In the PAAD_CRA001160 dataset,
ADGRG6 was primarily expressed in malignant cells, endothelial cells, ductal cells, and dendritic cells (DCs) (
Figure 4B). Similarly, in the PAAD_GSE154778 dataset,
ADGRG6 was expressed in malignant cells, plasma cells, epithelial cells, and CD8+ T cells (
Figure 4C). These findings suggested that
ADGRG6 is not only present in malignant cells but also in various immune and stromal cells within the TME, contributing to the heterogeneity observed in PAAD.
3.4. ADGRG6 Expression Correlates with Immune Cell Infiltration and Immune-Related Signatures
To investigate the functional significance of ADGRG6 in the PAAD immune microenvironment, we analyzed the correlation between ADGRG6 expression and molecular signatures of various immune cell subsets using the TIMER and GEPIA database platforms. Spearman’s rank correlation analysis was performed to determine the correlation coefficient (Rho) and statistical significance (p-value).
The results showed that
ADGRG6 was significantly positively correlated with the mast cell marker genes
CPA3 and
KIT, with a relatively strong correlation with
KIT (Rho = 0.285,
p < 0.001 in the TIMER database; Rho = 0.28,
p < 0.001 in the GEPIA database). In M1 macrophages, there was also a significant positive correlation between the expression of
CD86 and
CD80 genes and
ADGRG6 (Rho values were between 0.15 and 0.25,
p < 0.05 for both). For the type I interferon response subset of neutrophils, the marker genes
IFIT1 and
RSAD2 were significantly correlated with
ADGRG6 (Rho was approximately 0.2 to 0.27,
p < 0.01 to
p < 0.001). In addition, the key transcription factors
GATA3,
STAT6,
STAT3, and
BATF of the helper T cell subsets Th2 and Th17 were highly positively correlated with
ADGRG6, especially
STAT6, which had the strongest correlation with
ADGRG6 (Rho > 0.5,
p < 0.001) (
Table 1).
In summary, the expression level of ADGRG6 was significantly positively correlated with the specific gene markers of multiple immune cell types, suggesting that ADGRG6 may play an important role in the regulation of immune cells and the immune microenvironment. Given these associations with immune-related markers, we next explored whether ADGRG6 directly contributes to tumor progression and immune signaling.
3.5. Clinical Validation of ADGRG6 Expression in a PAAD Tissue Microarray Cohort
To validate the clinical significance of ADGRG6 expression, we performed IHC staining on a PAAD TMA containing 71 tumor samples. Elevated ADGRG6 expression was observed in 49 cases (69.01%), while reduced expression was detected in 22 cases (30.99%). Correlation analysis of clinicopathological parameters demonstrated that high ADGRG6 expression was significantly associated with multiple unfavorable tumor parameters, including larger tumor size (
p < 0.05), higher pathological grade (
p < 0.001), presence of organ invasion (
p < 0.05), advanced TNM stage (
p < 0.01), and higher AJCC stage (
p < 0.05). Conversely, no significant correlations were identified between ADGRG6 expression and patient gender, age, or nerve invasion (
Table 2).
To further account for potential confounding among these variables, a multivariate logistic regression analysis was conducted, with ADGRG6 expression status (high vs. low) as the dependent variable, and gender, age, pathological grade, tumor size, organ invasion, nerve invasion, TNM stage, and AJCC stage as independent variables. indicated that pathological grade (OR = 10.026, 95% CI: 1.793–56.047,
p = 0.009) and tumor size (OR = 20.457, 95% CI: 2.133–196.237,
p = 0.009) were independent risk factors associated with high ADGRG6 expression. In contrast, gender, age, organ invasion, nerve invasion, TNM stage, and AJCC stage showed no statistically significant association (all
p > 0.05). TMA-based IHC scoring also revealed that ADGRG6 expression positively correlated with TNM staging, with maximal staining intensity observed in stage III specimens (
Figure 5A). Taken together, these findings suggest that increased ADGRG6 expression is closely associated with higher pathological grade and larger tumor burden, both key indicators of aggressive tumor behavior in PAAD. This supports a potential role for ADGRG6 as a biomarker of malignant progression and provides a rationale for subsequent mechanistic and functional validation.
3.6. ADGRG6 Promotes PAAD Cell Proliferation, Migration, and Invasion In Vitro and In Vivo
To examine
ADGRG6’s functional role in PAAD cellular proliferation, migration, and invasion processes, we conducted targeted silencing of
ADGRG6 mRNA in AsPC-1 and BxPC-3 pancreatic cancer cell lines through specific siRNAs (si-
ADGRG6), utilizing si-NC as negative control. Quantitative verification established effective
ADGRG6 silencing at mRNA levels across both cell lines (
Figure 5B). CCK-8 assays showed that
ADGRG6 silencing markedly inhibited growth in AsPC-1 and BxPC-3 cells throughout a 4-day timeframe (
Figure 5C, **
p < 0.01). Wound-healing experiments indicated that si-
ADGRG6 treatment produced substantially decreased wound closure rates versus si-NC controls across both cell lines within 24 h (
Figure 5D, ***
p < 0.001). Transwell invasion studies additionally revealed that
ADGRG6 silencing generated substantial decreases in invasive potential for both AsPC-1 and BxPC-3 cells (
Figure 5E, ***
p < 0.001). To mimic the physiological 3D microenvironment, we further conducted spheroid formation assays. si-
ADGRG6-transfected cells formed smaller and less compact spheroids than control cells, and quantitative analysis over 21 days verified a significant decrease in spheroid diameter (
Figure 5F, ***
p < 0.001), suggesting impaired 3D proliferative capacity.
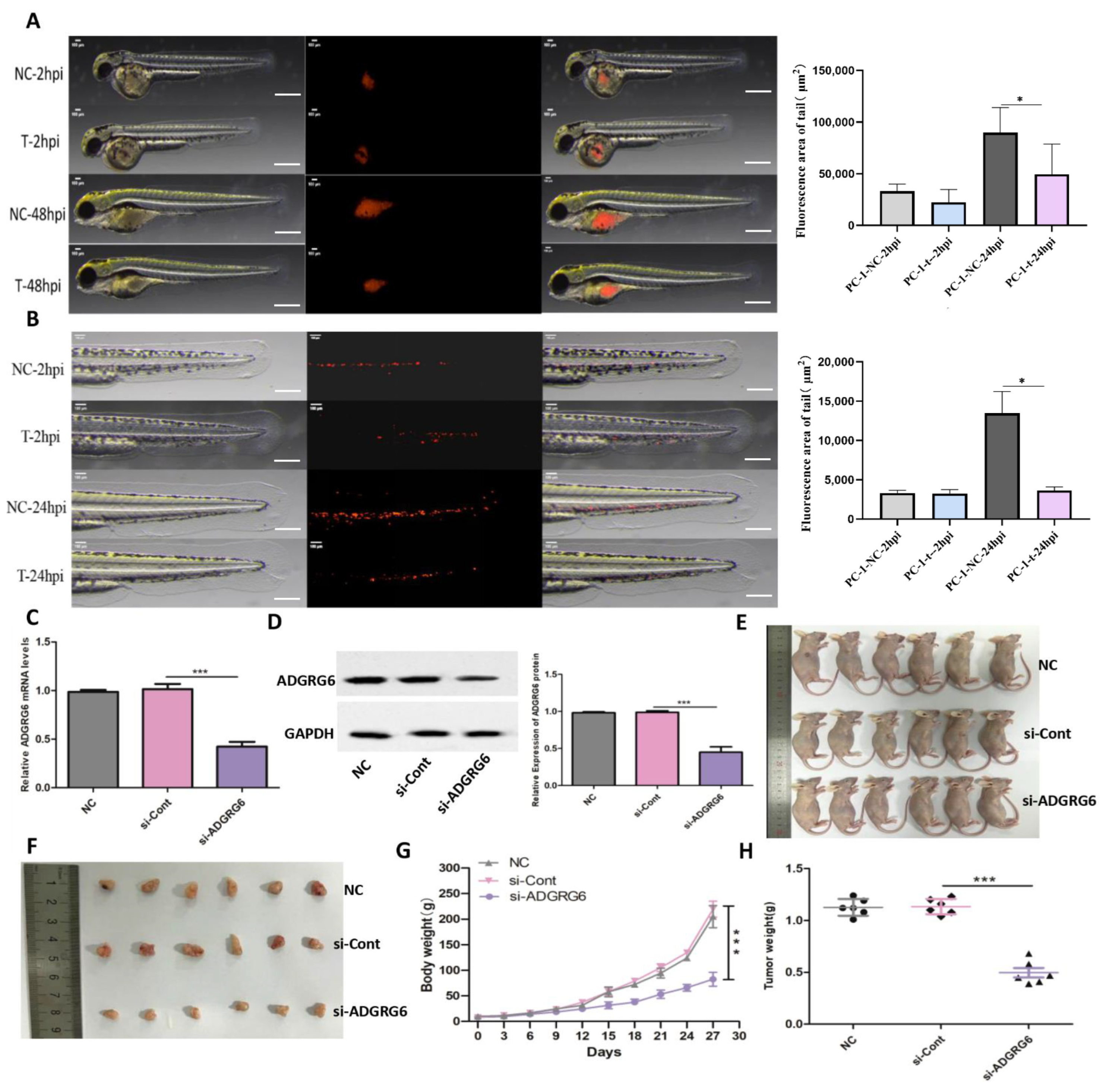
For in vivo validation of these laboratory findings, we implemented a zebrafish xenograft system. CM-DiI-labeled AsPC-1 cells treated with si-
ADGRG6 or si-NC underwent injection into zebrafish, with fluorescence microscopy tracking cellular proliferation and migration. At 48 h post-injection (hpi), fluorescence areas representing cellular proliferation showed significant decreases in si-
ADGRG6 groups versus si-NC controls (
Figure 6A, *
p < 0.05). Migration studies revealed that si-
ADGRG6-treated cells displayed reduced motility within zebrafish at 24 hpi (
Figure 6B, *
p < 0.05). For murine xenograft experiments, si-
ADGRG6-transfected AsPC-1 cells were subcutaneously injected into nude mice. Knockdown efficiency was verified at both mRNA and protein levels prior to injection (
Figure 6C,D, original Western blot images provided in
Supplementary Figure S2). Mice receiving si-
ADGRG6-treated AsPC-1 cells generated substantially smaller tumors versus NC or si-Cont (control siRNA) cohorts (
Figure 6E,F). During the 30-day monitoring period, body weight remained comparable between the NC and si-NC groups. In contrast, mice in the si-
ADGRG6 group exhibited a significant decrease in body weight (
Figure 6G), suggesting potential systemic effects associated with sustained
ADGRG6 knockdown. Final tumor weight measurements showed substantial decreases in si-
ADGRG6 cohorts (
Figure 6H, ***
p < 0.001).
Collectively, these results demonstrate that ADGRG6 exerts potent oncogenic effects in PAAD by promoting cellular proliferation, migration, invasion, and tumor formation, thereby supporting its potential role as a therapeutic target.
3.7. ADGRG6 Modulates the NF-κB→STAT6→GATA3 Signaling Axis
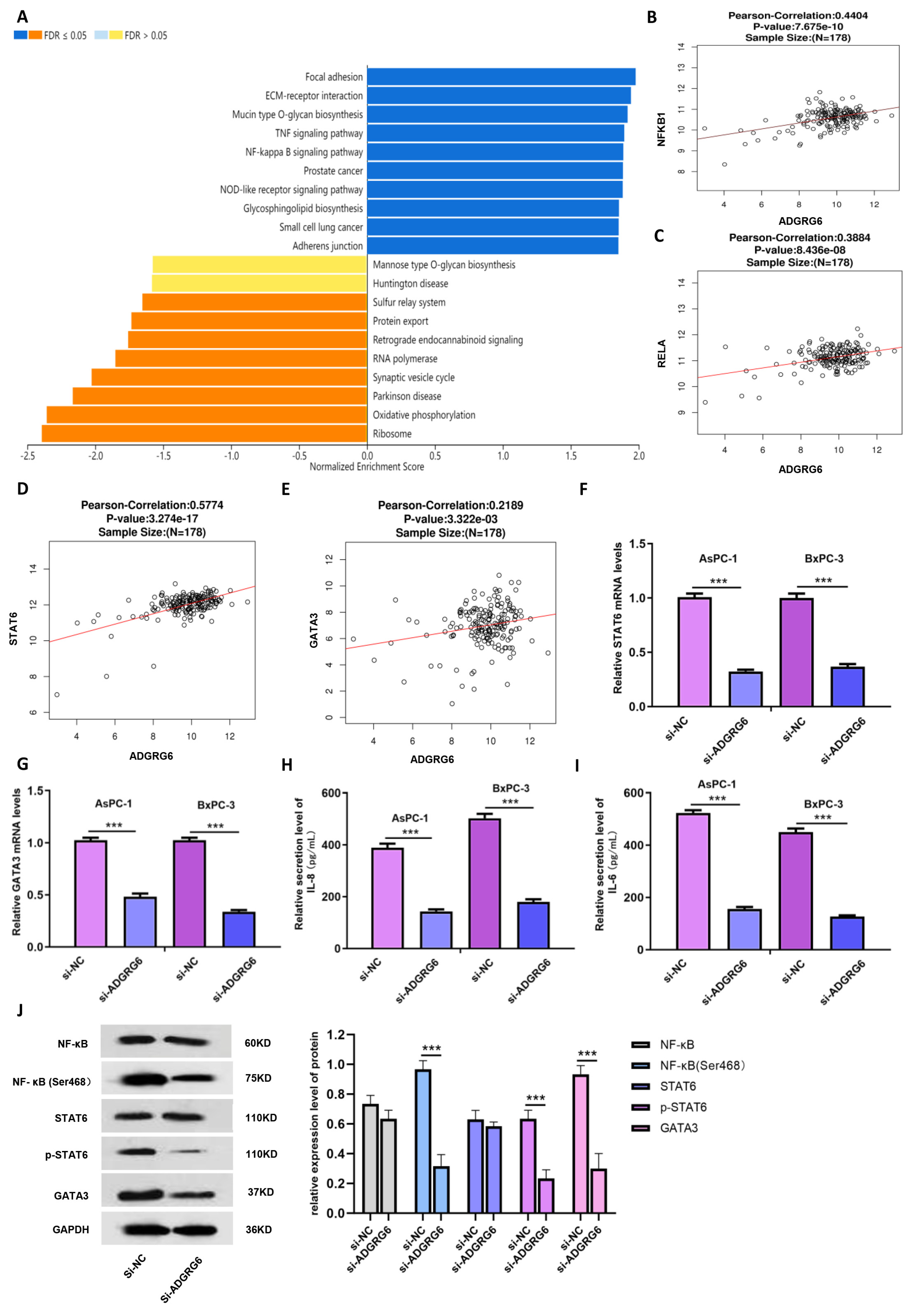
To elucidate the molecular mechanisms through which
ADGRG6 promotes PAAD progression, we conducted pathway enrichment analyses using the LinkInterpreter module. KEGG analysis revealed that
ADGRG6 co-expressed genes were significantly enriched in the NF-κB signaling pathway (
Figure 7A). Given the previously observed positive correlations between
ADGRG6 and immune-related markers such as STAT6 and GATA3, we hypothesized that
ADGRG6 may influence immune-associated transcriptional regulation via the NF-κB→STAT6→GATA3 axis.
Correlation analyses using clinical transcriptomic data (
n = 178) confirmed that ADGRG6 expression was significantly and positively correlated with NF-κB pathway core genes (
NFKB1,
RELA) as well as with
STAT6 and
GATA3 (
Figure 7B–E). To validate these associations experimentally,
ADGRG6 was silenced in AsPC-1 and BxPC-3 cells, and the expression of key pathway genes was quantified by RT-qPCR. Knockdown of
ADGRG6 led to substantial downregulation of
STAT6 and
GATA3 mRNA levels (
Figure 7F,G, ***
p < 0.001). Moreover, ELISA assays revealed that the secretion of downstream proinflammatory cytokines of the NF-κB pathway, IL-6 and IL-8, was markedly reduced in the si-
ADGRG6 group (
Figure 7H,I, ***
p < 0.001).
Western blot analysis further demonstrated that while total NF-κB and STAT6 protein levels remained largely unchanged, their phosphorylated forms (
p-NF-κB at Ser468 and
p-STAT6 at Tyr641) were significantly decreased following
ADGRG6 knockdown (
Figure 7J). GATA3 protein levels were also reduced, consistent with its transcriptional downregulation, suggesting that
ADGRG6 may regulate GATA3 expression by modulating NF-κB and STAT6 activation.
Together, these findings indicate that ADGRG6 promotes PAAD progression by activating the NF-κB→STAT6→GATA3 signaling cascade, enhancing the production of inflammatory cytokines such as IL-6 and IL-8, and potentially contributing to tumor–immune crosstalk within the pancreatic tumor microenvironment.
4. Discussion
In this study, we tested the hypothesis that ADGRG6 is overexpressed in PAAD and drives tumor progression through NF-κB/STAT6-mediated signaling and modulation of the TME. Consistent with this premise, our results demonstrated that ADGRG6 was markedly upregulated in PAAD tissues and cell lines, correlated with aggressive clinicopathological features and poor prognosis, and functionally promoted tumor proliferation, migration, and cytokine secretion across 2D cultures, 3D spheroids, zebrafish xenografts, and murine models. These findings position ADGRG6 as a potential oncogenic regulator linking intracellular signaling activation with immune modulation in PAAD.
Our findings align with and extend prior evidence implicating
ADGRG6 in tumor progression across multiple cancer types. For instance,
ADGRG6 promotes breast cancer growth upon progesterone stimulation [
21], serves as a mutation marker for bladder cancer recurrence and immunotherapy monitoring [
34,
35,
36], and drives colorectal cancer proliferation via HDAC2 and GLI2 signaling [
37]. Its role in angiogenesis, which is mediated through the cAMP-PKA-CREB pathway and VEGFR2 regulation [
38], further underscores its multifaceted oncogenic potential. Importantly, Wu et al. [
23] recently identified
ADGRG6 as a prognostic biomarker that stabilizes mutant p53 and activates the EGFR/NF-κB signaling axis, promoting tumor cell proliferation in a tumor-intrinsic manner. Building upon their findings, our study provides two additional layers of evidence that expand the biological relevance of
ADGRG6.
First, through clinical validation in a TMA cohort, we confirmed that ADGRG6 protein overexpression correlates with multiple aggressive tumor features, reinforcing its translational potential as a prognostic biomarker. Second, we revealed a previously uncharacterized connection between
ADGRG6 and tumor-immune microenvironmental modulation. Transcriptomic correlation analyses indicated that
ADGRG6 expression is positively associated with immune markers of Th2 and Th17 subsets [
39], as well as NF-κB→STAT6→GATA3 signaling components. Single-cell RNA-sequencing data from the TISCH database further showed that
ADGRG6 is expressed not only in malignant epithelial cells but also in endothelial and dendritic cell compartments, suggesting that it may participate in tumor–stroma and tumor–immune crosstalk. Given that endothelial-to-mesenchymal transition (EndMT) [
40,
41,
42,
43] and macrophage/Treg-driven immunosuppression [
44,
45] are central to PAAD progression; the findings raise the possibility that
ADGRG6 contributes to establishing an immunosuppressive niche. Mechanistically, our in vitro data demonstrate that
ADGRG6 knockdown suppresses phosphorylation of NF-κB and STAT6, decreases GATA3 expression, and reduces secretion of IL-6 and IL-8, supporting the existence of an “
ADGRG6→NF-κB→STAT6→GATA3” regulatory axis that may contribute to Th2-skewed immune polarization [
46,
47,
48].
While these findings highlight an intriguing link between ADGRG6 signaling and immunoregulatory pathways, several limitations should be acknowledged. Our in vivo validation relied on zebrafish and murine xenograft models, both of which are immunodeficient systems that lack a fully functional adaptive immune response. Therefore, although these models confirm the tumor-suppressive effects of ADGRG6 silencing on tumor proliferation and invasion, they cannot fully capture the complexity of ADGRG6-mediated immune modulation. Consequently, the proposed role of ADGRG6 in shaping the immunosuppressive PAAD microenvironment should be interpreted as hypothesis-generating rather than conclusive. Future studies employing immunocompetent or humanized mouse models will be essential to delineate the precise mechanisms through which ADGRG6 influences tumor–immune crosstalk and to evaluate its therapeutic potential in immune-relevant contexts.
Furthermore, the small number of normal pancreatic samples available in the TCGA-UALCAN dataset (n = 4) represents an additional constraint that may affect the statistical robustness of differential expression analyses. This limitation is not unique to our study but reflects a broader challenge in pancreatic cancer transcriptomic research, as histologically normal pancreatic tissues are rarely resected and therefore underrepresented in public datasets. Consequently, the apparent magnitude of ADGRG6 upregulation should be viewed as indicative rather than definitive. To partially address this concern, we performed an internal validation using canonical PAAD driver genes (KRAS, TP53, and SMAD4), which displayed the expected expression patterns within the same dataset. This consistency suggests that, despite the small normal cohort, the UALCAN platform captures biologically meaningful expression trends. Future analyses integrating normal pancreatic tissues from GTEx and our institutional cohort will be required to confirm the robustness of ADGRG6 dysregulation under more balanced sample conditions.
Moreover, we observed significant body weight loss in mice bearing ADGRG6-silenced tumors, suggesting possible systemic effects of ADGRG6 inhibition. Although the underlying mechanism remains unclear, this phenomenon may involve altered cytokine production or host–tumor metabolic interactions, warranting further investigation into the systemic safety profile of targeting ADGRG6.
In summary, our findings establish ADGRG6 as a multifaceted oncogenic regulator in PAAD that promotes tumor progression through both cell-autonomous mechanisms and potential immune-associated pathways. By integrating bioinformatic, experimental, and clinical evidence, this study expands the current understanding of ADGRG6 biology in pancreatic cancer and provides a conceptual framework for exploring ADGRG6 as a therapeutic target in future precision oncology and immunomodulation studies.