The Therapeutic Effect of EZH2 Inhibitors in Targeting Human Papillomavirus Associated Cervical Cancer
Abstract
1. Introduction
1.1. High Risk Human Papillomavirus and Cervical Cancer
1.2. HPV Oncoproteins and Tumour Suppressors in Carcinogenesis
1.3. HPV-Driven Transformation via Epithelial–Mesenchymal Transition
1.4. EZH2—A Potential Oncogenic Driver
2. Materials and Methods
2.1. Cell Culture and Maintenance
2.2. Cytotoxicity Assay
2.3. Treatment of Cells
2.4. Apoptosis Detection Assay
2.5. Cell Cycle Assay
2.6. Scratch Wound-Healing Assay
2.7. Immunocytochemical Staining (ICC)
2.8. Western Blot Analysis
2.9. Chorioallantoic Membrane (CAM) Assay
2.10. Histological Examination
2.10.1. Tissue Processing
2.10.2. Immunohistochemistry Staining
2.11. RT-qPCR
2.12. Statistical Analysis
3. Results
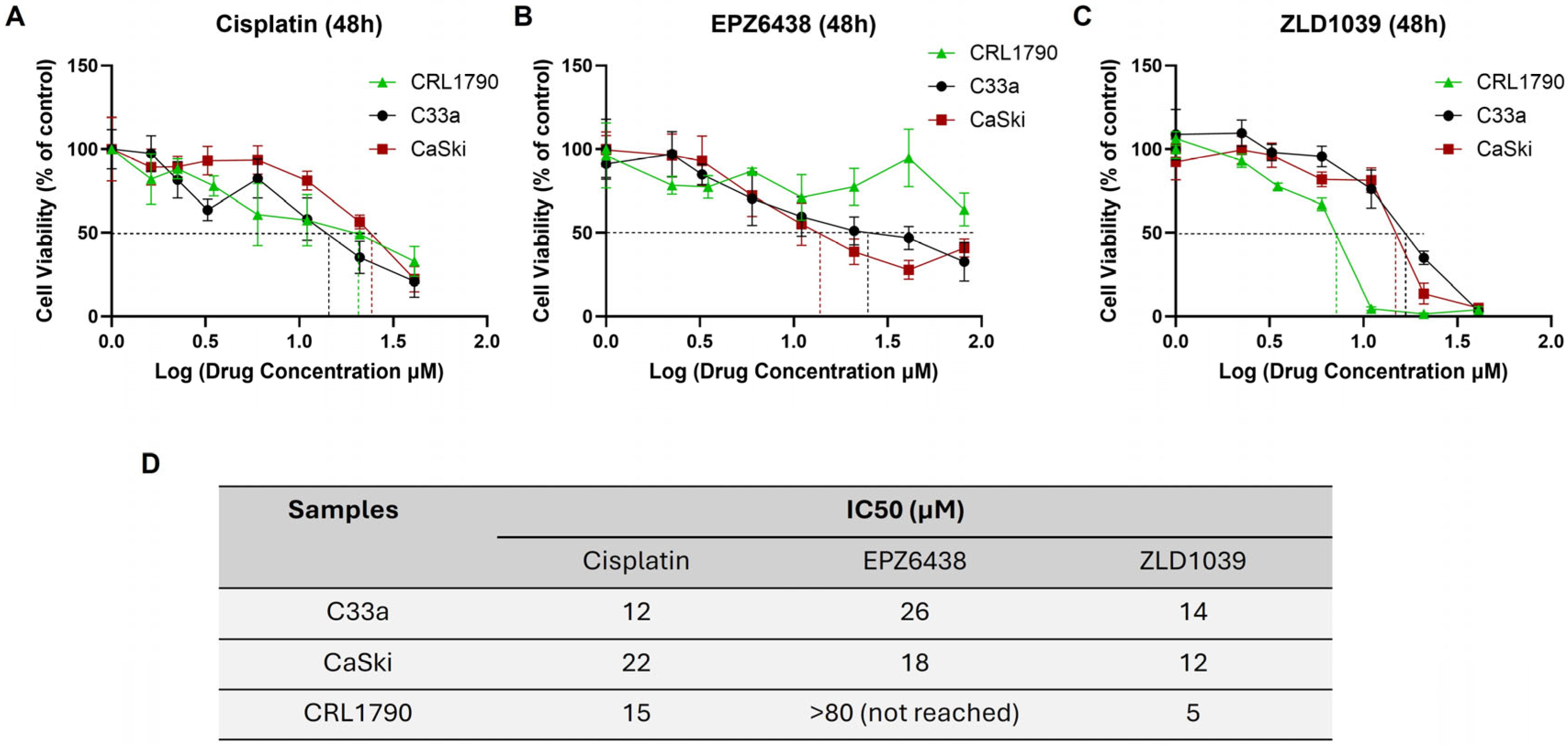
3.1. EZH2 Inhibitors Reduced Viability of Cervical Cancer Cells in a Dose-Dependent Manner
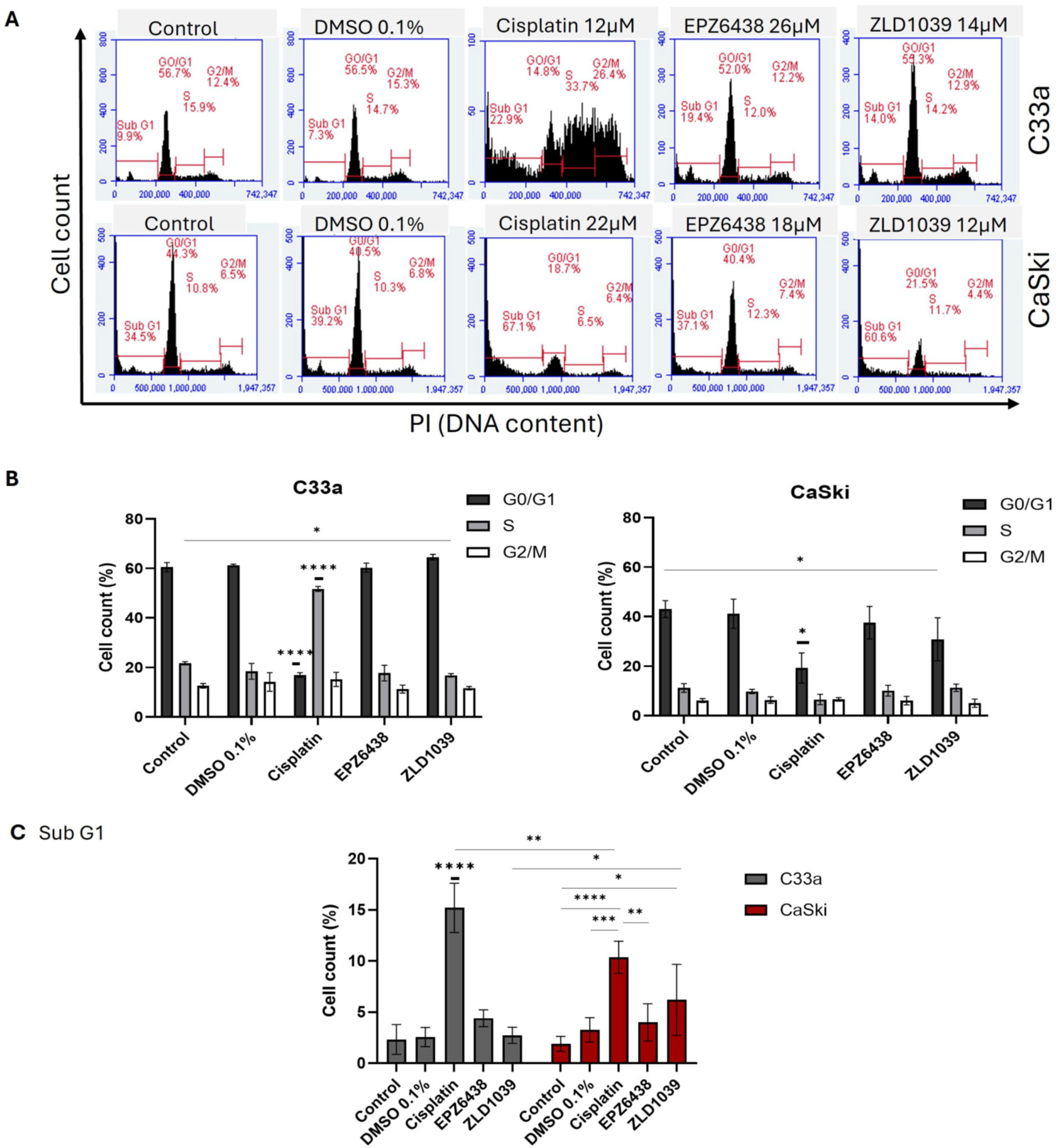
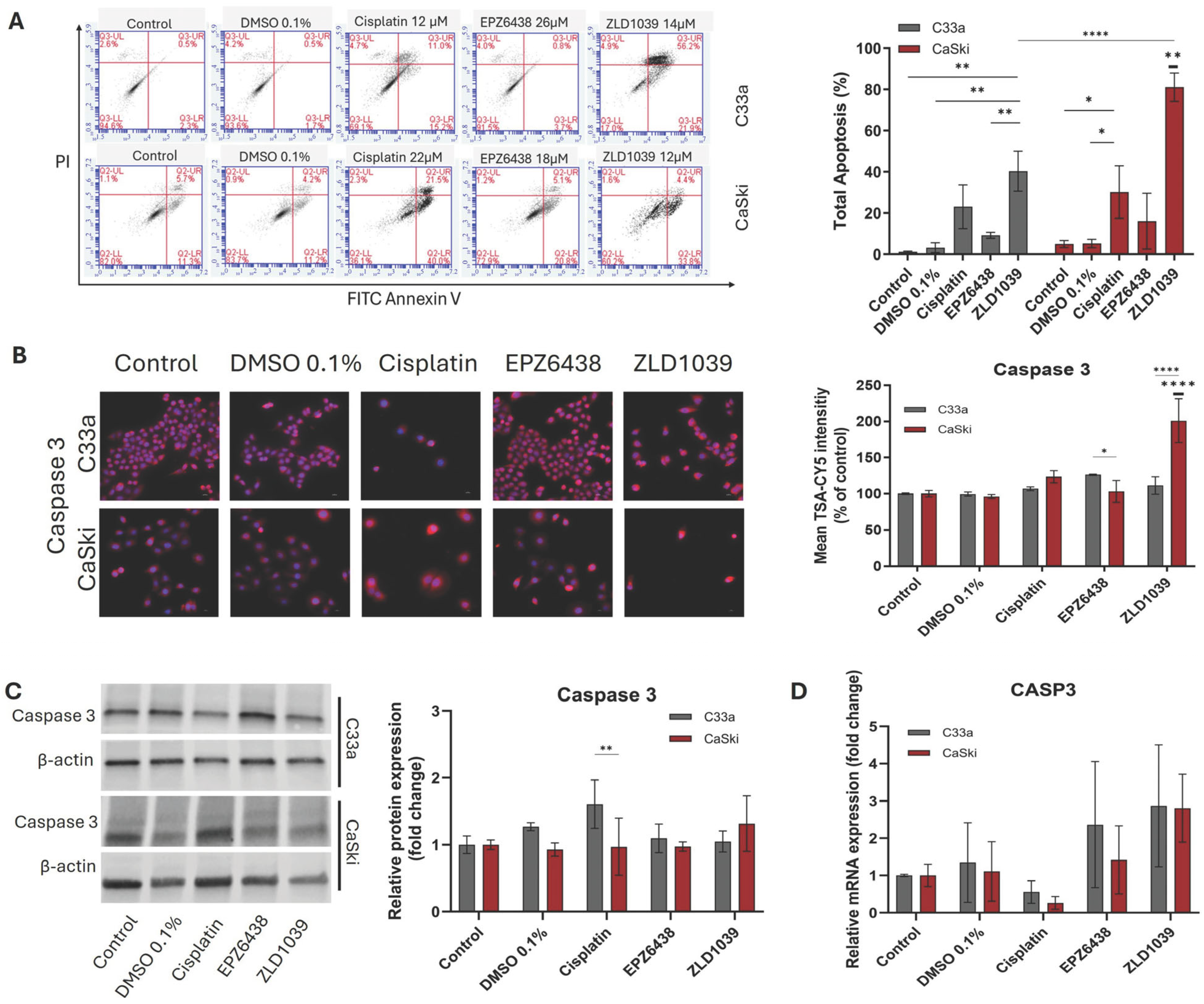
3.2. EZH2 Inhibitors Induce Apoptosis and G0/G1 Arrest in Cervical Cancer Cells
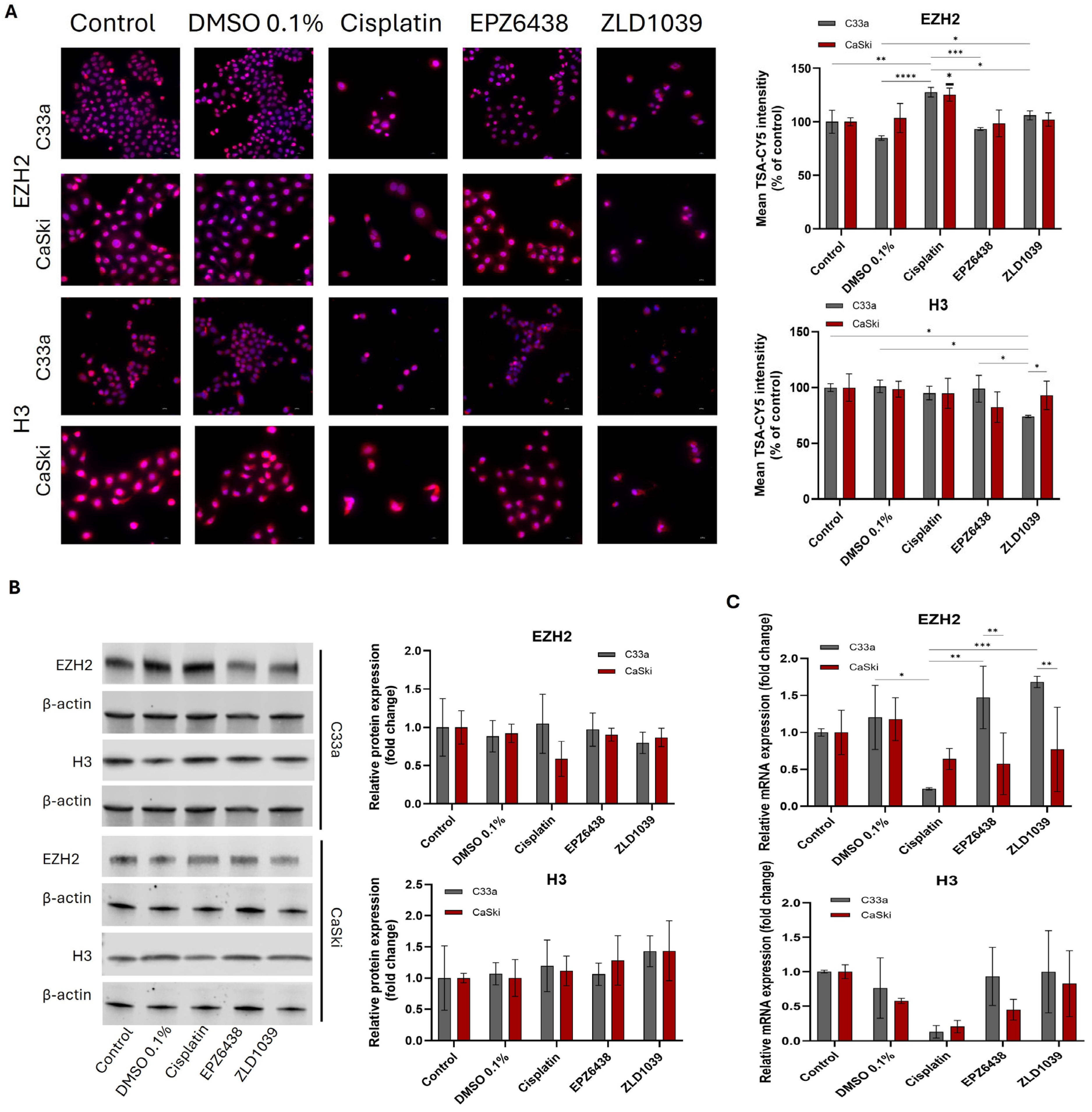
3.3. EZH2 and H3 Protein and mRNA Expression Decreased Following EZH2 Inhibition
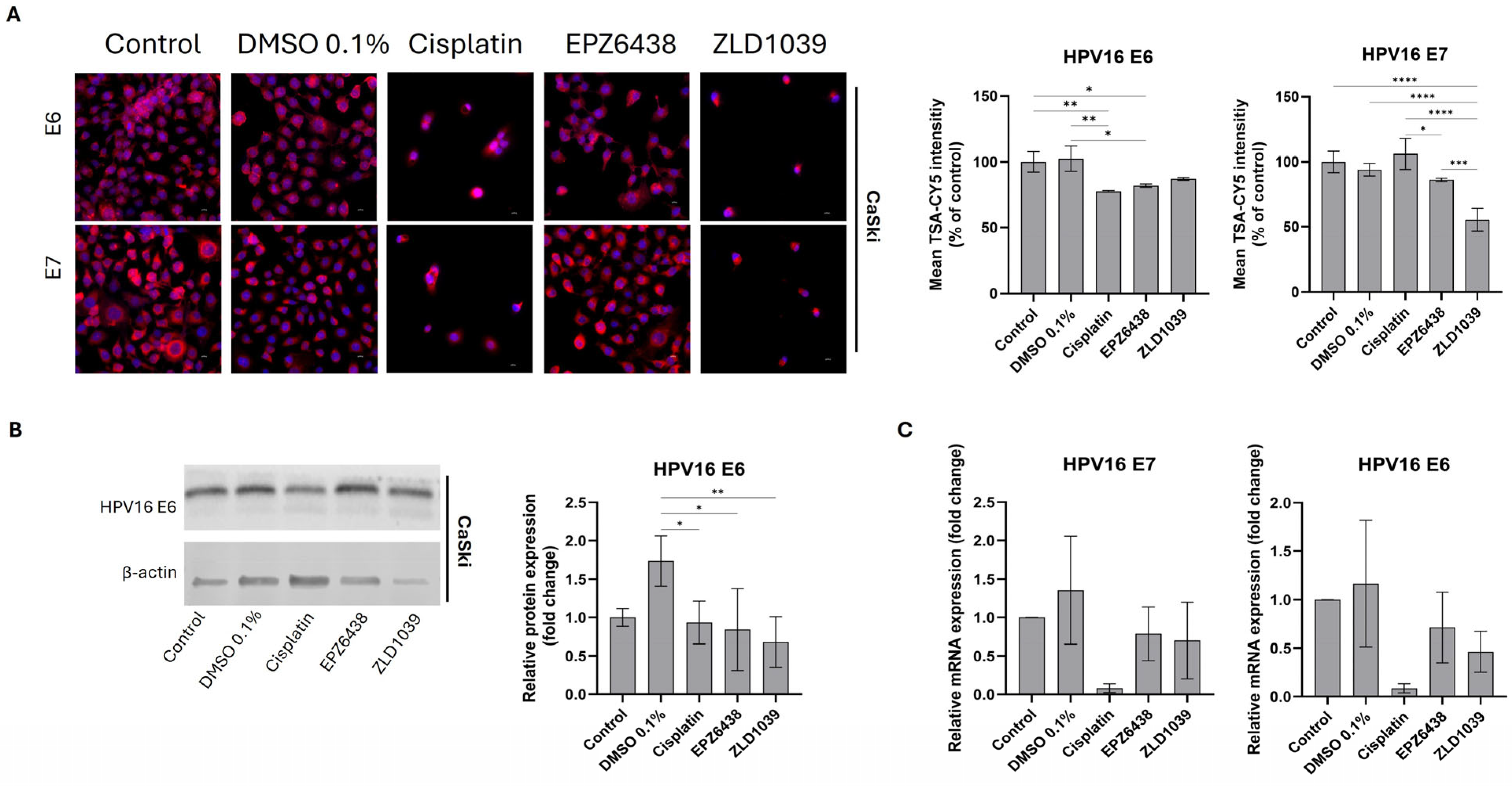
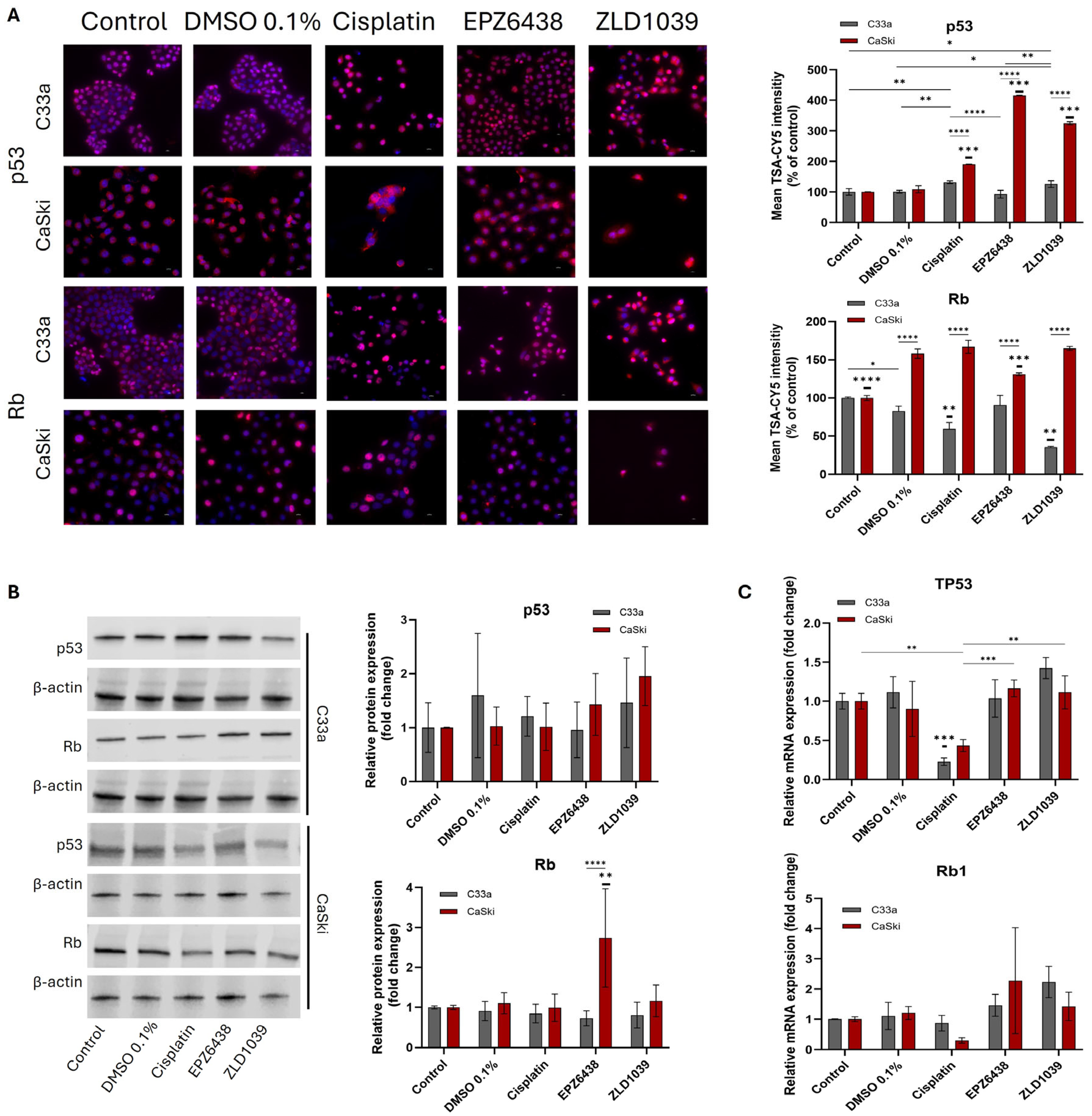
3.4. EZH2 Inhibitors Reduced Expression of HPV Oncogenes E6 and E7 Followed by an Increase in Tumour Suppressors
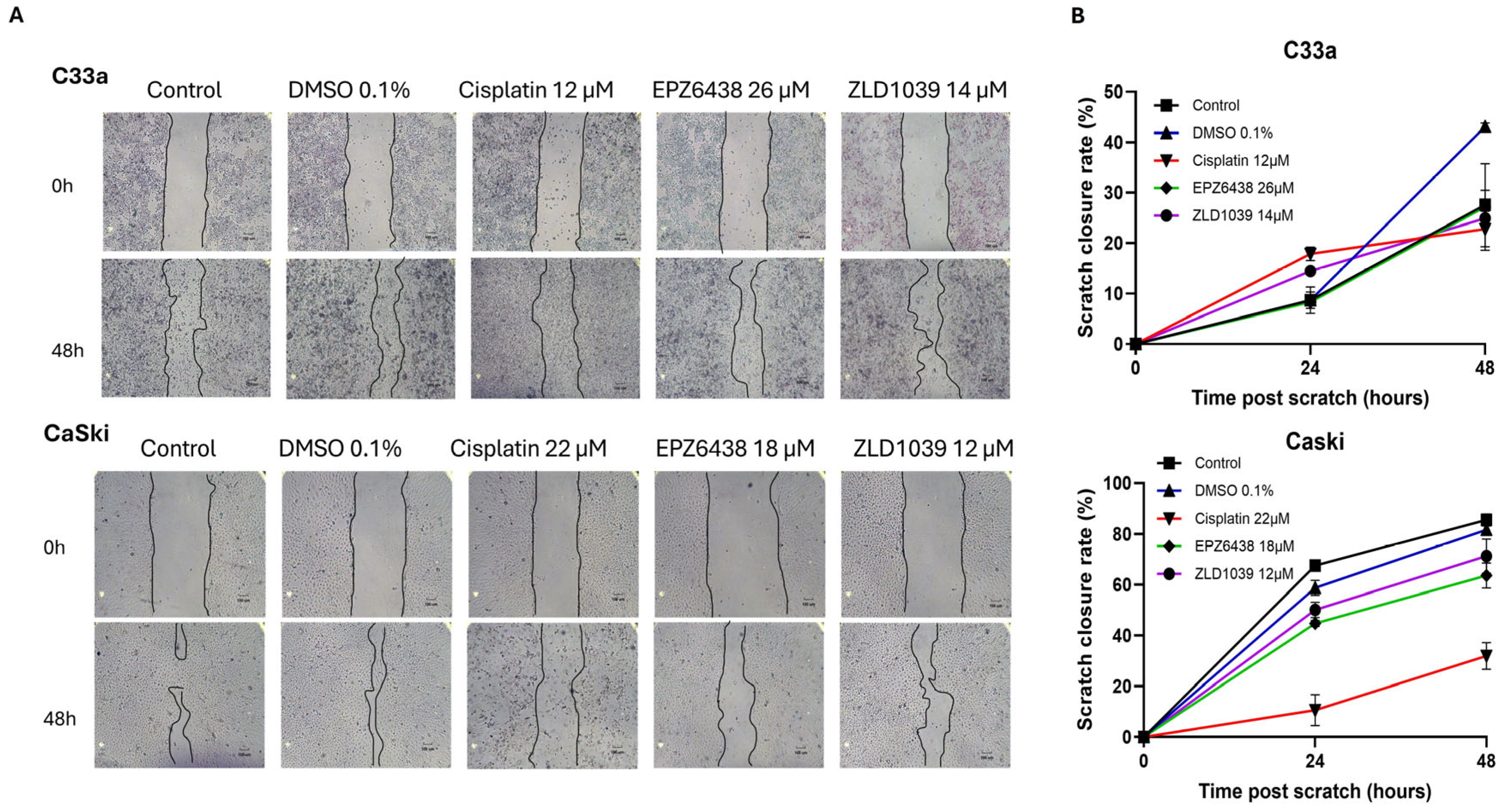
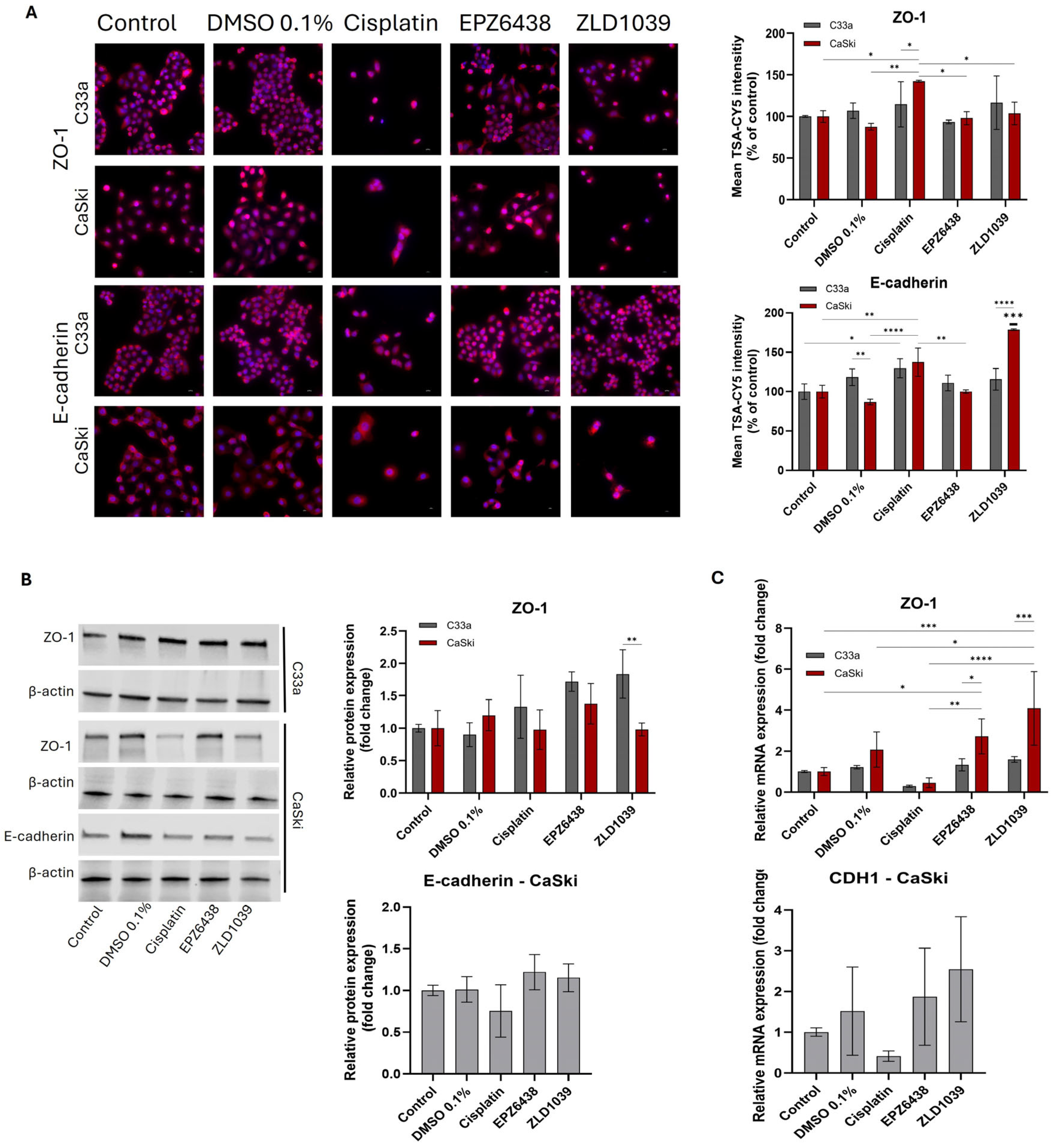
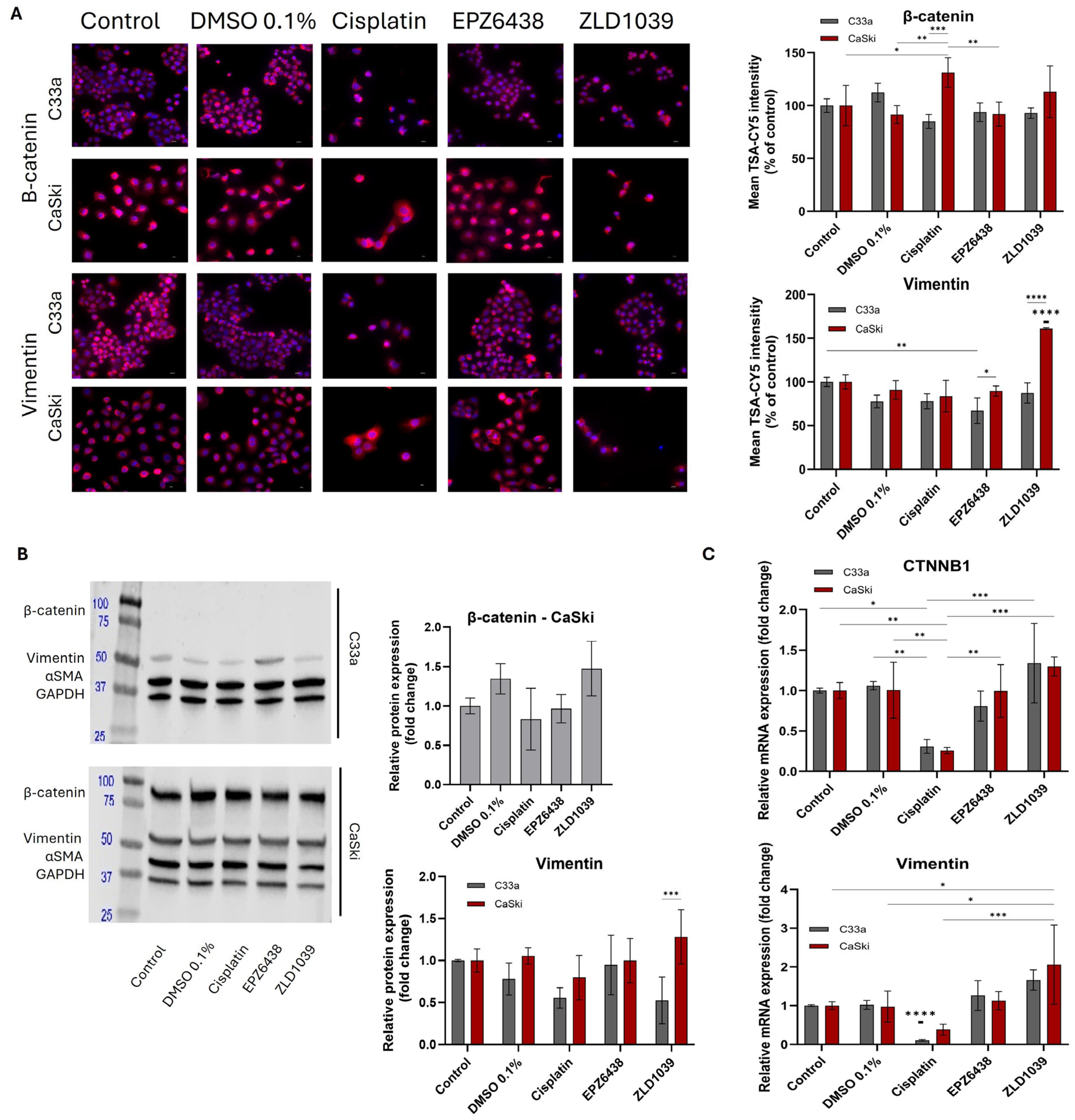
3.5. EMT Involvement in Cervical Cancer and Possible Reversal Following EPZ6438 Treatment
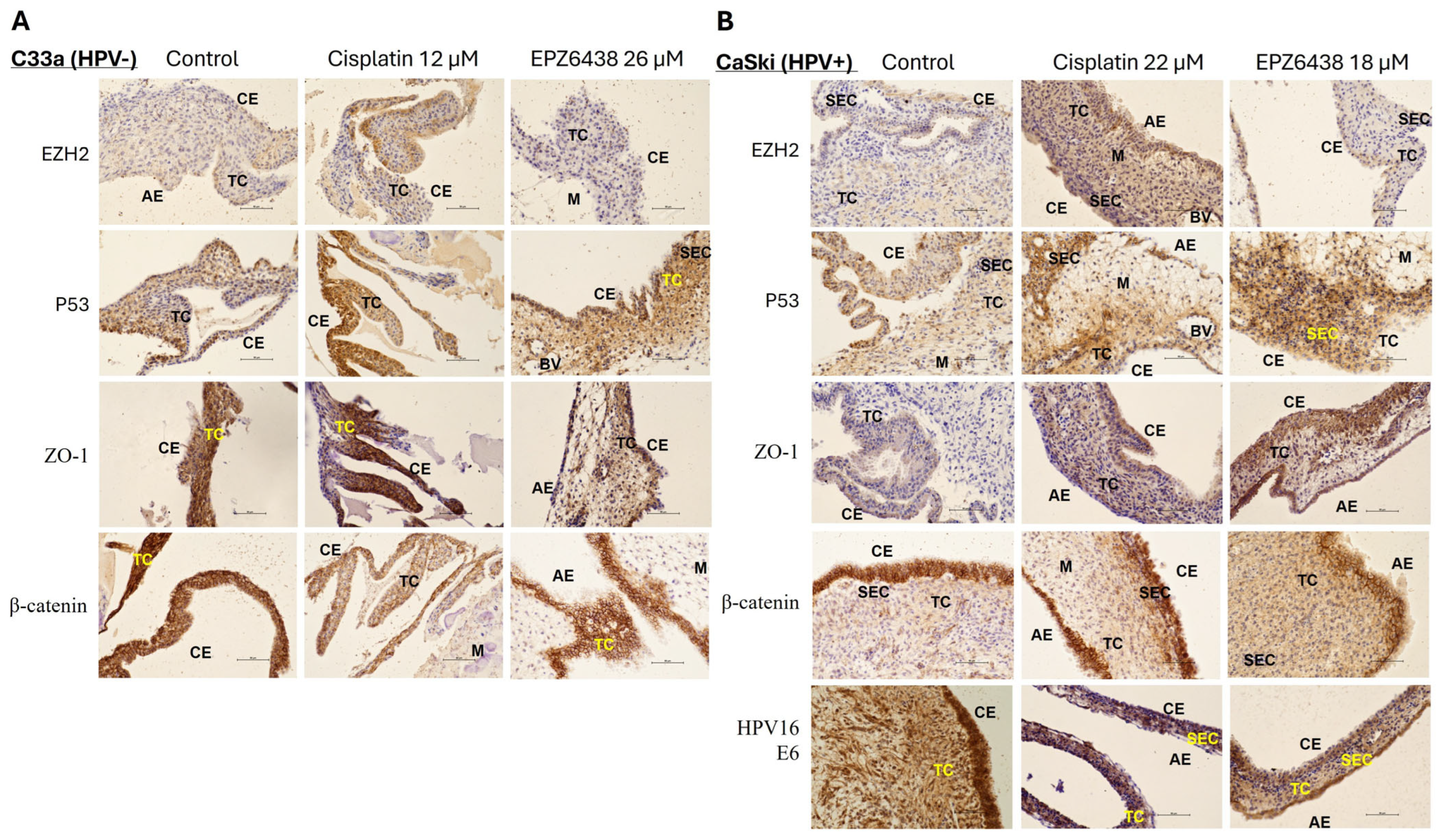
3.6. In Vivo CAM Assay Preliminary Validation of the Result from In Vitro Study Following EPZ6438 Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPV | human papillomavirus |
| EMT | epithelial–mesenchymal transition |
| EZH2 | enhancer of zeste homolog 2 |
| CAM | chorioallantoic membrane assay |
| HNC | head and neck cancer |
| MET | mesenchymal-to-epithelial transition |
| PRC2 | protein core complex 2 |
| H3K27me3 | trimethylation of histone H3 at Lys 27 |
| HNSCC | head and neck squamous cell carcinoma |
References
- Szymonowicz, K.A.; Chen, J. Biological and clinical aspects of HPV-related cancers. Cancer Biol. Med. 2020, 17, 864–878. [Google Scholar] [CrossRef]
- NIH: HPV and Cancer. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer (accessed on 11 November 2024).
- Muñoz, N.; Castellsagué, X.; Berrington de González, A.; Gissmann, L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006, 24, S1–S10. [Google Scholar] [CrossRef]
- WHO: Cervical Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 10 December 2024).
- Allouch, S.; Malki, A.; Allouch, A.; Gupta, I.; Vranic, S.; Al Moustafa, A.-E. High-Risk HPV Oncoproteins and PD-1/PD-L1 Interplay in Human Cervical Cancer: Recent Evidence and Future Directions. Front. Oncol. 2020, 10, 914. [Google Scholar] [CrossRef]
- Estêvão, D.; Costa, N.R.; Gil da Costa, R.M.; Medeiros, R. Hallmarks of HPV carcinogenesis: The role of E6, E7 and E5 oncoproteins in cellular malignancy. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 153–162. [Google Scholar] [CrossRef]
- Subramanian, M.; Jones, M.F.; Lal, A. Long Non-Coding RNAs Embedded in the Rb and p53 Pathways. Cancers 2013, 5, 1655–1675. [Google Scholar] [CrossRef] [PubMed]
- Medda, A.; Duca, D.; Chiocca, S. Human Papillomavirus and Cellular Pathways: Hits and Targets. Pathogens 2021, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Bode, A.M.; Dong, Z.; Cao, Y. The epithelial–mesenchymal transition (EMT) is regulated by oncoviruses in cancer. FASEB J. 2016, 30, 3001–3010, Correction in FASEB J. 2016, 30, 3901. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef]
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic EMT: A multi-tool for tumor progression. EMBO J. 2021, 40, e108647. [Google Scholar] [CrossRef]
- Fukusumi, T.; Guo, T.W.; Sakai, A.; Ando, M.; Ren, S.; Haft, S.; Liu, C.; Amornphimoltham, P.; Gutkind, J.S.; Califano, J.A. The NOTCH4-HEY1 Pathway Induces Epithelial-Mesenchymal Transition in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2018, 24, 619–633. [Google Scholar] [CrossRef]
- Mahmood, M.Q.; Shukla, S.D.; Ward, C.; Walters, E.H. The Underappreciated Role of Epithelial Mesenchymal Transition in Chronic Obstructive Pulmonary Disease and Its Strong Link to Lung Cancer. Biomolecules 2021, 11, 1394. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Barrett, T.F.; Paolini, R.; Parikh, A.; Puram, S.V. Partial EMT in head and neck cancer biology: A spectrum instead of a switch. Oncogene 2021, 40, 5049–5065. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef]
- Kisoda, S.; Shao, W.; Fujiwara, N.; Mouri, Y.; Tsunematsu, T.; Jin, S.; Arakaki, R.; Ishimaru, N.; Kudo, Y. Prognostic value of partial EMT-related genes in head and neck squamous cell carcinoma by a bioinformatic analysis. Oral Dis. 2020, 26, 1149–1156. [Google Scholar] [CrossRef]
- Georgakopoulos-Soares, I.; Chartoumpekis, D.V.; Kyriazopoulou, V.; Zaravinos, A. EMT Factors and Metabolic Pathways in Cancer. Front. Oncol. 2020, 10, 499. [Google Scholar] [CrossRef]
- Lachat, C.; Boyer-Guittaut, M.; Peixoto, P.; Hervouet, E. Epigenetic Regulation of EMT (Epithelial to Mesenchymal Transition) and Tumor Aggressiveness: A View on Paradoxical Roles of KDM6B and EZH2. Epigenomes 2019, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Wang, N.; Ma, T.; Yu, B. Targeting epigenetic regulators to overcome drug resistance in cancers. Signal Transduct. Target. Ther. 2023, 8, 69. [Google Scholar] [CrossRef]
- Duan, R.; Du, W.; Guo, W. EZH2: A novel target for cancer treatment. J. Hematol. Oncol. 2020, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Gwak, S.Y.; Shim, G.A.; Liu, L.; Lim, Y.C.; Kim, J.M.; Jung, M.G.; Koo, B.S. EZH2 is associated with poor prognosis in head-and-neck squamous cell carcinoma via regulating the epithelial-to-mesenchymal transition and chemosensitivity. Oral Oncol. 2016, 52, 66–74. [Google Scholar] [CrossRef]
- Ntziachristos, P.; Tsirigos, A.; Van Vlierberghe, P.; Nedjic, J.; Trimarchi, T.; Flaherty, M.S.; Ferres-Marco, D.; Da Ros, V.G.; Tang, Z.; Siegle, J.; et al. Genetic inactivation of the polycomb repressive complex 2 in T cell acute lymphoblastic leukemia. Nat. Med. 2012, 18, 298–301. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, N.; Cheng, X.; Zhang, J.; Tan, X.; Zhang, C.; Tang, Y.; Teng, Y.; Yang, X. Ezh2 Acts as a Tumor Suppressor in Kras-driven Lung Adenocarcinoma. Int. J. Biol. Sci. 2017, 13, 652–659. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, L. Non-coding RNAs-EZH2 regulatory mechanisms in cervical cancer: The current state of knowledge. Pharmacotherapy 2022, 146, 112123. [Google Scholar] [CrossRef] [PubMed]
- Velcheti, V.; Wong, K.K.; Saunthararajah, Y. EZH2 Inhibitors: Take It EZy, It Is All About Context. Cancer Discov. 2019, 9, 472–475. [Google Scholar] [CrossRef]
- Nienstedt, J.C.; Schroeder, C.; Clauditz, T.; Simon, R.; Sauter, G.; Muenscher, A.; Blessmann, M.; Hanken, H.; Pflug, C. EZH2 overexpression in head and neck cancer is related to lymph node metastasis. J. Oral Pathol. Med. 2018, 47, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Jiang, H.; Li, L.; Zhu, S.; Hou, J.; Yu, Y. Carcinogenic roles and therapeutic effects of EZH2 in gynecological cancers. Bioorg. Med. Chem. 2020, 28, 115379. [Google Scholar] [CrossRef]
- Nakagawa, S.; Okabe, H.; Sakamoto, Y.; Hayashi, H.; Hashimoto, D.; Yokoyama, N.; Sakamoto, K.; Kuroki, H.; Mima, K.; Nitta, H.; et al. Enhancer of zeste homolog 2 (EZH2) promotes progression of cholangiocarcinoma cells by regulating cell cycle and apoptosis. Ann. Surg. Oncol. 2013, 20, 667–675. [Google Scholar] [CrossRef]
- Zingg, D.; Debbache, J.; Schaefer, S.M.; Tuncer, E.; Frommel, S.C.; Cheng, P.; Arenas-Ramirez, N.; Haeusel, J.; Zhang, Y.; Bonalli, M.; et al. The epigenetic modifier EZH2 controls melanoma growth and metastasis through silencing of distinct tumour suppressors. Nat. Commun. 2015, 6, 6051. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zhu, X.; Zhang, L.; Xu, Y.; Chen, G.; Luo, J. EZH2 enhances expression of CCL5 to promote recruitment of macrophages and invasion in lung cancer. Biotechnol. Appl. Biochem. 2020, 67, 1011–1019. [Google Scholar] [CrossRef]
- Zingg, D.; Arenas-Ramirez, N.; Sahin, D.; Rosalia, R.A.; Antunes, A.T.; Haeusel, J.; Sommer, L.; Boyman, O. The Histone Methyltransferase Ezh2 Controls Mechanisms of Adaptive Resistance to Tumor Immunotherapy. Cell Rep. 2017, 20, 854–867. [Google Scholar] [CrossRef]
- Gan, L.; Yang, Y.; Li, Q.; Feng, Y.; Liu, T.; Guo, W. Epigenetic regulation of cancer progression by EZH2: From biological insights to therapeutic potential. Biomark. Res. 2018, 6, 10. [Google Scholar] [CrossRef]
- Clark, J.; Jeffery, C.C.; Zhang, H.; Cooper, T.; O’COnnell, D.A.; Harris, J.; Seikaly, H.; Biron, V.L. Correlation of PET-CT nodal SUVmax with p16 positivity in oropharyngeal squamous cell carcinoma. J. Otolaryngol.-Head Neck Surg. 2015, 44, 37. [Google Scholar] [CrossRef]
- Biron, V.L.; Mohamed, A.; Hendzel, M.J.; Underhill, D.A.; Seikaly, H. Epigenetic differences between human papillomavirus-positive and -negative oropharyngeal squamous cell carcinomas. J. Otolaryngol.-Head Neck Surg. 2012, 41 (Suppl. S1), S65–S70. [Google Scholar]
- Hyland, P.L.; McDade, S.S.; McCloskey, R.; Dickson, G.J.; Arthur, K.; McCance, D.J.; Patel, D. Evidence for Alteration of EZH2, BMI1, and KDM6A and Epigenetic Reprogramming in Human Papillomavirus Type 16 E6/E7-Expressing Keratinocytes. J. Virol. 2011, 85, 10999–11006. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, T.; Bao, X.; He, M.; Li, L.; Yang, X. Increased EZH2 expression is associated with proliferation and progression of cervical cancer and indicates a poor prognosis. Int. J. Gynecol. Pathol. 2014, 33, 218–224. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Q. The roles of EZH2 in cancer and its inhibitors. Med. Oncol. 2023, 40, 167. [Google Scholar] [CrossRef]
- Sarkozy, C.; Morschhauser, F.; Dubois, S.; Molina, T.; Michot, J.M.; Cullières-Dartigues, P.; Suttle, B.; Karlin, L.; Le Gouill, S.; Picquenot, J.M.; et al. A LYSA Phase Ib Study of Tazemetostat (EPZ-6438) plus R-CHOP in Patients with Newly Diagnosed Diffuse Large B-Cell Lymphoma (DLBCL) with Poor Prognosis Features. Clin. Cancer Res. 2020, 26, 3145–3153. [Google Scholar] [CrossRef] [PubMed]
- Knutson, S.K.; Kawano, S.; Minoshima, Y.; Warholic, N.M.; Huang, K.-C.; Xiao, Y.; Kadowaki, T.; Uesugi, M.; Kuznetsov, G.; Kumar, N.; et al. Selective inhibition of EZH2 by EPZ-6438 leads to potent antitumor activity in EZH2-mutant non-Hodgkin lymphoma. Mol. Cancer Ther. 2014, 13, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, K.W.; Keilhack, H. Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc. Natl. Acad. Sci. USA 2013, 110, 7922–7927. [Google Scholar] [CrossRef]
- NIH. Tazemetostat and Pembrolizumab in Patients with Pembrolizumab- or Nivolumab-Resistant, Recurrent or Metastatic Head and Neck Squamous-Cell Carcinoma, Clin. Trials Gov. Available online: https://clinicaltrials.gov/study/NCT04624113 (accessed on 5 December 2024).
- Cheng, Y.; He, C.; Wang, M.; Ma, X.; Mo, F.; Yang, S.; Han, J.; Wei, X. Targeting epigenetic regulators for cancer therapy: Mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 2019, 4, 62. [Google Scholar] [CrossRef]
- Wen, L.; Tao, S.; Guo, F.; Li, L.; Yang, H.; Liang, Y.; Zhang, L.; Ma, L.; Fu, P. Selective EZH2 inhibitor zld1039 alleviates inflammation in cisplatin-induced acute kidney injury partially by enhancing RKIP and suppressing NF-κB p65 pathway. Acta Pharmacol. Sin. 2022, 43, 2067–2080. [Google Scholar] [CrossRef]
- Song, X.; Gao, T.; Wang, N.; Feng, Q.; You, X.; Ye, T.; Lei, Q.; Zhu, Y.; Xiong, M.; Xia, Y.; et al. Selective inhibition of EZH2 by ZLD1039 blocks H3K27methylation and leads to potent anti-tumor activity in breast cancer. Sci. Rep. 2016, 6, 20864. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.; Song, X.; Zhang, Q.; Wang, T.; Xiao, H.; Yu, L. Pharmacological inhibition of EZH2 by ZLD1039 suppresses tumor growth and pulmonary metastasis in melanoma cells in vitro and in vivo. Biochem. Pharmacol. 2023, 210, 115493. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Gong, Y.; Zhang, T.; Huang, J.; Tan, Z.; Xue, L. Finding an easy way to harmonize: A review of advances in clinical research and combination strategies of EZH2 inhibitors. Clin. Epigenet. 2021, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Idris, S.; Lindsay, C.; Kostiuk, M.; Andrews, C.; Côté, D.W.; O’Connell, D.A.; Harris, J.; Seikaly, H.; Biron, V.L. Investigation of EZH2 pathways for novel epigenetic treatment strategies in oropharyngeal cancer. J. Otolaryngol.-Head Neck Surg. 2016, 45, 45–54. [Google Scholar] [CrossRef]
- Lindsay, C.D.; Kostiuk, M.A.; Harris, J.; O’Connell, D.A.; Seikaly, H.; Biron, V.L. Efficacy of EZH2 inhibitory drugs in human papillomavirus-positive and human papillomavirus-negative oropharyngeal squamous cell carcinomas. Clin. Epigenet. 2017, 9, 82–95. [Google Scholar] [CrossRef]
- Khattri, M.; Amako, Y.; Gibbs, J.R.; Collura, J.L.; Arora, R.; Harold, A.; Li, M.Y.; Harms, P.W.; Ezhkova, E.; Shuda, M. Methyltransferase-independent function of enhancer of zeste homologue 2 maintains tumorigenicity induced by human oncogenic papillomavirus and polyomavirus. Tumour Virus Res. 2023, 16, 200264. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Mudianto, T.; Ma, X.; Riley, R.; Uppaluri, R. Targeting EZH2 enhances antigen presentation, antitumor immunity, and circumvents anti–PD-1 resistance in head and neck cancer. Clin. Cancer Res. 2020, 26, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Morschhauser, F.; Tilly, H.; Chaidos, A.; McKay, P.; Phillips, T.; Assouline, S.; Salles, G. Tazemetostat for patients with relapsed or refractory follicular lymphoma: An open-label, single-arm, multicentre, phase 2 trial. Lancet Oncol. 2020, 21, 1433–1442. [Google Scholar] [CrossRef]
- Hoy, S.M. Tazemetostat: First approval. Drugs 2020, 80, 513–521. [Google Scholar] [CrossRef]
- Tang, Q.; Yang, Y.; Sun, J. EZH2 regulates tumor-associated macrophages by the KDM6A-mediated inflammatory response in HPV16-positive cervical cancer. Hum. Cell 2025, 38, 119. [Google Scholar] [CrossRef]
- Liu, M.; Scanlon, C.S.; Banerjee, R.; Russo, N.; Inglehart, R.C.; Willis, A.L.; Weiss, S.J.; D’SIlva, N.J. The histone methyltransferase EZH2 mediates tumor progression on the chick chorioallantoic membrane assay, a novel model of head and neck squamous cell carcinoma. Transl. Oncol. 2013, 6, 273–281. [Google Scholar] [CrossRef]
- Pal, A.; Kundu, R. Human papillomavirus E6 and E7: The cervical cancer hallmarks and targets for therapy. Front. Microbiol. 2020, 10, 3116. [Google Scholar] [CrossRef]
- Vats, A.; Trejo-Cerro, O.; Thomas, M.; Banks, L. Human papillomavirus E6 and E7: What remains? Tumour Virus Res. 2021, 11, 200213. [Google Scholar] [CrossRef]
- Smith, S.P.; Scarpini, C.G.; Groves, I.J.; Odle, R.I.; Coleman, N. Identification of host transcriptional networks showing concentration-dependent regulation by HPV16 E6 and E7 proteins in basal cervical squamous epithelial cells. Sci. Rep. 2016, 6, 29832. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Das, B.C.; Abiha, U.; Sisodiya, S.; Chikara, A.; Nazir, S.U.; Das, A.M.; Rodrigues, A.G.; Passari, A.K.; Tanwar, P.; et al. Insights into the role of complement regulatory proteins in HPV mediated cervical carcinogenesis. Semin. Cancer Biol. 2022, 86, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Q.; Zhang, H.M.; Li, J.B.; Jiang, H.Y.; Fan, L.; Kong, L.Z.; Yao, S.Z. Analyzing simultaneous positive expression of EZH2 and P53 protein to improve predictive value in cervical squamous cell carcinoma. Int. J. Gynecol. Cancer 2014, 24, 1653–1658. [Google Scholar] [CrossRef]
- Nakayama, K.; Takebayashi, Y.; Nakayama, S.; Hata, K.; Fujiwaki, R.; Fukumoto, M.; Miyazaki, K. Prognostic value of overexpression of p53 in human ovarian carcinoma patients receiving cisplatin. Cancer Lett. 2003, 192, 227–235. [Google Scholar] [CrossRef]
- ATCC. HTB-31: Detailed Product Information. Available online: https://www.atcc.org/products/htb-31#detailed-product-information (accessed on 8 September 2025).
- Dirican, E.; Özcan, H.; Uzunçakmak, S.K.; Takım, U. Evaluation expression of the caspase-3 and caspase-9 apoptotic genes in schizophrenia patients. Clin. Psychopharmacol. Neurosci. 2023, 21, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Cregan, I.L.; Dharmarajan, A.M.; Fox, S.A. Mechanisms of cisplatin-induced cell death in malignant mesothelioma cells: Role of inhibitor of apoptosis proteins (IAPs) and caspases. Int. J. Oncol. 2013, 42, 444–452. [Google Scholar] [CrossRef]
- Liu, P.F.; Hu, Y.C.; Kang, B.H.; Tseng, J.K.; Wu, P.C.; Liang, C.C.; Hou, Y.Y.; Fu, T.Y.; Liou, H.H.; Hsieh, I.C.; et al. Expression levels of cleaved caspase-3 and caspase-3 in tumorigenesis and prognosis of oral tongue squamous cell carcinoma. PLoS ONE 2017, 12, e0180620. [Google Scholar] [CrossRef]
- Hougardy, B.M.; van der Zee, A.G.; van den Heuvel, F.A.; Timmer, T.; de Vries, E.G.; de Jong, S. Sensitivity to Fas-mediated apoptosis in high-risk HPV-positive human cervical cancer cells: Relationship with Fas, caspase-8, and Bid. Gynecol. Oncol. 2005, 97, 353–364. [Google Scholar] [CrossRef]
- Eich, M.L.; Athar, M.; Ferguson, J.E., 3rd; Varambally, S. EZH2-targeted therapies in cancer: Hype or a reality. Cancer Res. 2020, 80, 5449–5458. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Eccleston, M.; Clermont, P.L.; Latarani, M.; Kingsley Male, D.; Wang, Y.; Crea, F. EZH2 inhibition: A promising strategy to prevent cancer immune editing. Epigenomics 2020, 12, 1349–1362. [Google Scholar] [CrossRef]
- Ramos, F.S.; Wons, L.; Cavalli, I.J.; Ribeiro, E.M.S.F. Epithelial-mesenchymal transition in cancer: An overview. Integr. Cancer Sci. Ther. 2017, 4, 1–5. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352, Erratum in Nat. Rev. Mol. Cell Biol. 2021, 22, 834. [Google Scholar] [CrossRef] [PubMed]
- Pastushenko, I.; Blanpain, C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef]
- Lüönd, F.; Sugiyama, N.; Bill, R.; Bornes, L.; Hager, C.; Tang, F.; Santacroce, N.; Beisel, C.; Ivanek, R.; Bürglin, T.; et al. Distinct contributions of partial and full EMT to breast cancer malignancy. Dev. Cell 2021, 56, 3203–3221. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Liao, C.; Wang, Q.; An, J.; Long, Q.; Wang, H.; Xiang, M.; Xiang, M.; Zhao, Y.; Liu, Y.; Liu, J.; et al. Partial EMT in squamous cell carcinoma: A snapshot. Int. J. Biol. Sci. 2021, 17, 3036–3047. [Google Scholar] [CrossRef] [PubMed]
- Human Protein Atlas. CDH1 Expression in C-33A Cervical Cancer Cell Line. Available online: https://www.proteinatlas.org/ENSG00000039068-CDH1/cell+line#cervical_cancer (accessed on 8 September 2025).
- Chen, C.-L.; Liu, S.S.; Ip, S.-M.; Wong, L.C.; Ngan, H.Y.S.; Ng, T.Y. E-cadherin expression is silenced by DNA methylation in cervical cancer cell lines and tumours. Eur. J. Cancer 2003, 39, 517–523. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Fang, Y.; Wu, K.; Liu, Y.; Zhang, W. Comprehensive gene and pathway analysis of cervical cancer progression. Oncol. Lett. 2020, 19, 3316–3332. [Google Scholar] [CrossRef] [PubMed]
- Banister, C.E.; Liu, C.; Pirisi, L.; Creek, K.E.; Buckhaults, P.J. Identification and characterization of HPV-independent cervical cancers. Oncotarget 2017, 8, 13375–13386. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Jiang, W.; Ji, H.; Wang, Z.W.; Zhu, X. Discovery of key genes as novel biomarkers specifically associated with HPV-negative cervical cancer. Mol. Ther. Methods Clin. Dev. 2021, 21, 492–506. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, Q.; Zhang, Q. The mechanism of HPV-mediated DNA methylation in the promoter region of long-chain non-coding RNA MAGI2-AS3 in the occurrence and development of cervical cancer. Clin. Exp. Obstet. Gynecol. 2024, 51, 140. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, H.; Tian, M.; Wang, D.; He, J.; Xu, T. DNA methylation and hydroxymethylation in cervical cancer: Diagnosis, prognosis and treatment. Front. Genet. 2020, 11, 347. [Google Scholar] [CrossRef]
- Dovnik, A.; Poljak, M. The role of methylation of host and/or human papillomavirus (HPV) DNA in management of cervical intraepithelial neoplasia grade 2 (CIN2) lesions. Int. J. Mol. Sci. 2023, 24, 6479. [Google Scholar] [CrossRef]
- Chen, R.; Gan, Q.; Zhao, S.; Zhang, D.; Wang, S.; Yao, L.; Yuan, M.; Cheng, J. DNA methylation of miR-138 regulates cell proliferation and EMT in cervical cancer by targeting EZH2. BMC Cancer 2022, 22, 488. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.A.; Wentzensen, N.; Mirabello, L.; Ghosh, A.; Wacholder, S.; Harari, A.; Lorincz, A.; Schiffman, M.; Burk, R.D. Human papillomavirus DNA methylation as a potential biomarker for cervical cancer. Cancer Epidemiol. Biomark. Prev. 2012, 21, 2125–2137. [Google Scholar] [CrossRef]
- Cai, L.Q.; Wang, Z.H.; Liu, D.H. Interference with endogenous EZH2 reverses the chemotherapy drug resistance in cervical cancer cells partly by up-regulating Dicer expression. Tumor Biol. 2016, 37, 6359–6369. [Google Scholar] [CrossRef]
- Dou, D.; Ge, X.; Wang, X.; Xu, X.; Zhang, Z.; Seng, J.; Cao, Z.; Gu, Y.; Han, M. EZH2 contributes to cisplatin resistance in breast cancer by epigenetically suppressing miR-381 expression. OncoTargets Ther. 2019, 12, 9627–9637. [Google Scholar] [CrossRef]
- Samaržija, I.; Tomljanović, M.; Kujundžić, R.N.; Trošelj, K.G. EZH2 inhibition and cisplatin as a combination anticancer therapy: An overview of preclinical studies. Cancers 2022, 14, 4761. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, W.; Li, B.; Cheng, B.; Lin, K.; Bai, J.; Li, H.; Yang, G. Targeting EZH2 could overcome docetaxel resistance in prostate cancer cells. BMC Cancer 2019, 19, 27. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, Y.; Mao, F.; Zhang, Z.; Li, Z.; Wang, R.; Liu, J.; Liu, X. Inhibition of EZH2 enhances the antitumor efficacy of metformin in prostate cancer. Mol. Cancer Ther. 2020, 19, 2490–2501. [Google Scholar] [CrossRef]
- Takashina, T.; Kinoshita, I.; Kikuchi, J.; Shimizu, Y.; Sakakibara-Konishi, J.; Oizumi, S.; Nishimura, M.; Dosaka-Akita, H. Combined inhibition of EZH2 and histone deacetylases as a potential epigenetic therapy for non-small-cell lung cancer cells. Cancer Sci. 2016, 107, 955–962. [Google Scholar] [CrossRef]
- Sun, J.; Cai, X.; Yung, M.M.; Zhou, W.; Li, J.; Zhang, Y.; Li, Z.; Liu, S.S.; Cheung, A.N.Y.; Ngan, H.Y.S.; et al. miR-137 mediates the functional link between c-Myc and EZH2 that regulates cisplatin resistance in ovarian cancer. Oncogene 2019, 38, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Du, Y.; Nakai, K.; Ding, M.; Chang, S.S.; Hsu, J.L.; Yao, J.; Wei, Y.; Nie, L.; Jiao, S.; et al. EZH2 contributes to the response to PARP inhibitors through its PARP-mediated poly-ADP ribosylation in breast cancer. Oncogene 2018, 37, 208–217. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidalina, D.; Ghali, L.; Kassouf, N.; Li, S.; Li, D.; Wen, X. The Therapeutic Effect of EZH2 Inhibitors in Targeting Human Papillomavirus Associated Cervical Cancer. Curr. Issues Mol. Biol. 2025, 47, 990. https://doi.org/10.3390/cimb47120990
Vidalina D, Ghali L, Kassouf N, Li S, Li D, Wen X. The Therapeutic Effect of EZH2 Inhibitors in Targeting Human Papillomavirus Associated Cervical Cancer. Current Issues in Molecular Biology. 2025; 47(12):990. https://doi.org/10.3390/cimb47120990
Chicago/Turabian StyleVidalina, Dora, Lucy Ghali, Nick Kassouf, Shuhan Li, Dong Li, and Xuesong Wen. 2025. "The Therapeutic Effect of EZH2 Inhibitors in Targeting Human Papillomavirus Associated Cervical Cancer" Current Issues in Molecular Biology 47, no. 12: 990. https://doi.org/10.3390/cimb47120990
APA StyleVidalina, D., Ghali, L., Kassouf, N., Li, S., Li, D., & Wen, X. (2025). The Therapeutic Effect of EZH2 Inhibitors in Targeting Human Papillomavirus Associated Cervical Cancer. Current Issues in Molecular Biology, 47(12), 990. https://doi.org/10.3390/cimb47120990






