Competition for Chaperones: A Trade-Off Between Thermotolerance and Antiviral Immunity in Plants
Abstract
1. Introduction
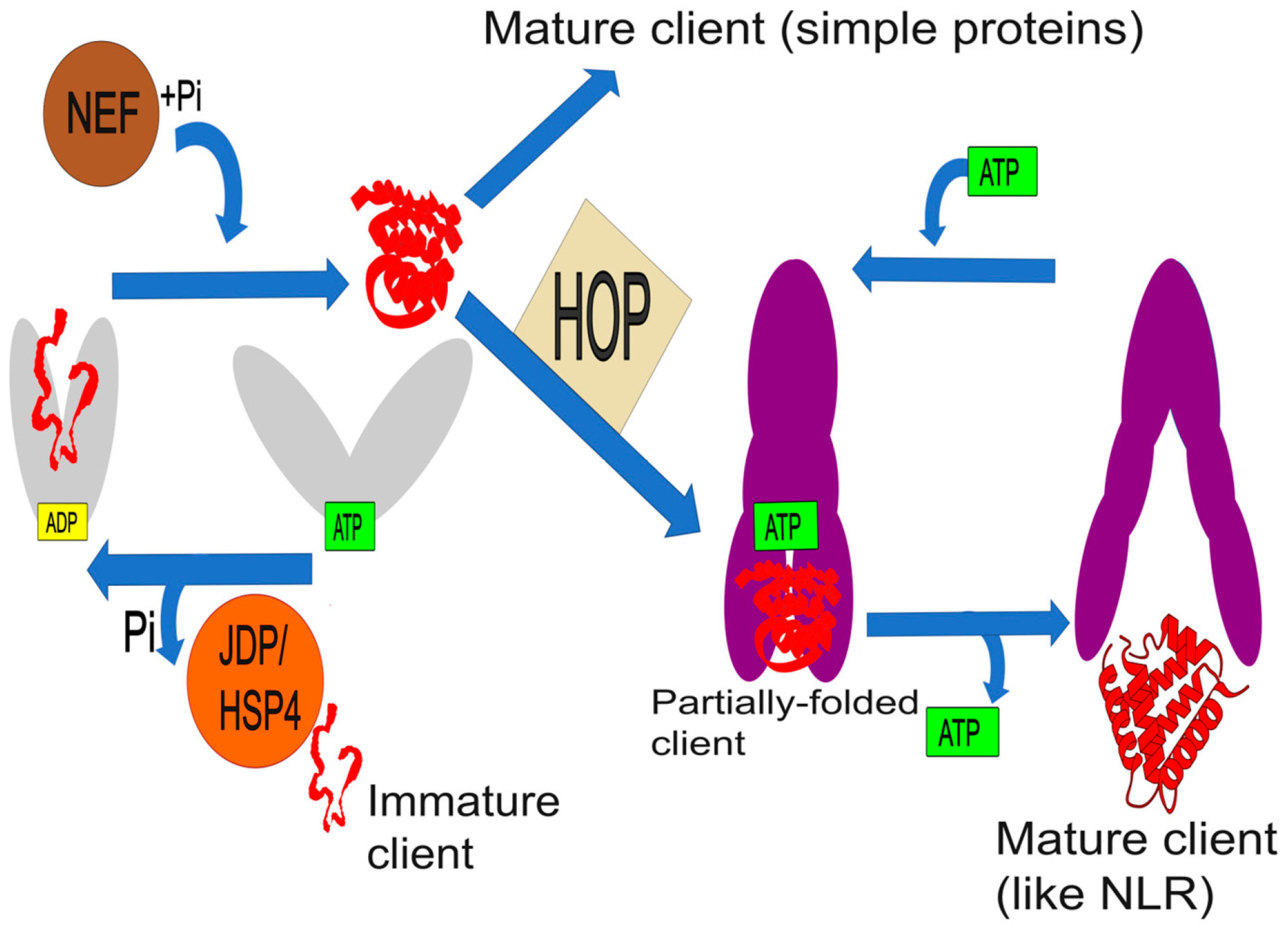
2. The HSP70 and HSP90 Machinery in Plants: Architecture, Mechanisms, and Regulation
3. The Dual Role of Chaperones in Viral Infections
3.1. Pro-Viral Functions: Exploitation of Chaperones for Viral Propagation
3.2. Anti-Viral Functions: HSPs as Sentinels of Cellular Immunity
4. The Impact of Temperature Stress on System Components
4.1. HSPs and Thermotolerance: Maintaining Cellular Proteostasis
4.2. Temperature Effects on the Viral Lifecycle
4.3. Temperature Sensitivity of Plant Immunity
5. Beyond the Chaperone
5.1. Hormonal Crosstalk: SA, JA, and Thermotolerance
5.2. Autophagy: A Critical Hub for Proteostasis and Virophagy
6. An Integrative Model: HSPs at the Nexus of Heat and Viral Stress
6.1. Competition for Chaperones and the Immunity-Thermotolerance Trade-Off
6.2. The Molecular Mechanism of Immunity Collapse: A “Resource Depletion” Hypothesis
6.3. The Influence of Stress Dynamics: Acclimation Versus Shock
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- de Moya-Ruiz, C.; Gómez, P. Thermotolerance Elicits Specific Genes in Cucurbit Plants as a Response to the Combined Effect of Viral Infection and Temperature Stress. J. Exp. Bot. 2025, 76, 5305–5319. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, R. Consideration of Combined Stress: A Crucial Paradigm for Improving Multiple Stress Tolerance in Plants. In Combined Stresses in Plants: Physiological, Molecular, and Biochemical Aspects; Mahalingam, R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–25. ISBN 978-3-319-07899-1. [Google Scholar]
- Shelake, R.M.; Wagh, S.G.; Patil, A.M.; Červený, J.; Waghunde, R.R.; Kim, J.-Y. Heat Stress and Plant–Biotic Interactions: Advances and Perspectives. Plants 2024, 13, 2022. [Google Scholar] [CrossRef]
- Zhang, H.; Sonnewald, U. Differences and Commonalities of Plant Responses to Single and Combined Stresses. Plant J. 2017, 90, 839–855. [Google Scholar] [CrossRef]
- Iksat, N.; Madirov, A.; Artykbayeva, D.; Shevchenko, O.; Zhanassova, K.; Baikarayev, Z.; Masalimov, Z. Heat Stress Induces Partial Resistance to Tomato Bushy Stunt Virus in Nicotiana Benthamiana Via Combined Stress Pathways. Viruses 2025, 17, 1250. [Google Scholar] [CrossRef]
- Ul Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.-X.; Zhang, H.-X.; Wei, A.-M.; Gong, Z.-H. Heat Shock Proteins: Dynamic Biomolecules to Counter Plant Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef]
- Berka, M.; Kopecká, R.; Berková, V.; Brzobohatý, B.; Černý, M. Regulation of Heat Shock Proteins 70 and Their Role in Plant Immunity. J. Exp. Bot. 2022, 73, 1894–1909. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, W. Heat Shock Proteins and Viral Infection. Front. Immunol. 2022, 13, 947789. [Google Scholar] [CrossRef] [PubMed]
- Young, J.C.; Agashe, V.R.; Siegers, K.; Hartl, F.U. Pathways of Chaperone-Mediated Protein Folding in the Cytosol. Nat. Rev. Mol. Cell. Biol. 2004, 5, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Ramegowda, V.; Senthil-Kumar, M. Shared and Unique Responses of Plants to Multiple Individual Stresses and Stress Combinations: Physiological and Molecular Mechanisms. Front. Plant Sci. 2015, 6, 723. [Google Scholar] [CrossRef]
- Liu, J.; Pang, X.; Cheng, Y.; Yin, Y.; Zhang, Q.; Su, W.; Hu, B.; Guo, Q.; Ha, S.; Zhang, J.; et al. The Hsp70 Gene Family in Solanum Tuberosum: Genome-Wide Identification, Phylogeny, and Expression Patterns. Sci. Rep. 2018, 8, 16628. [Google Scholar] [CrossRef]
- Lin, B.-L.; Wang, J.-S.; Liu, H.-C.; Chen, R.-W.; Meyer, Y.; Barakat, A.; Delseny, M. Genomic Analysis of the Hsp70 Superfamily in Arabidopsis Thaliana. Cell Stress Chaperones 2001, 6, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.K.; Kundnani, P.; Grover, A. Functional Analysis of Hsp70 Superfamily Proteins of Rice (Oryza Sativa). Cell Stress Chaperones 2013, 18, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, L.; Ye, T.; Chen, R.; Gao, X.; Xu, Z. Molecular Characterization, Expression Pattern and Function Analysis of the OsHSP90 Family in Rice. Biotechnol. Biotechnol. Equip. 2016, 30, 669–676. [Google Scholar] [CrossRef]
- Marrs, K.A.; Casey, E.S.; Capitant, S.A.; Bouchard, R.A.; Dietrich, P.S.; Mettler, I.J.; Sinibaldi, R.M. Characterization of Two Maize HSP90 Heat Shock Protein Genes: Expression during Heat Shock, Embryogenesis, and Pollen Development. Dev. Genet. 1993, 14, 27–41. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, W.; Qian, Y.; Ren, Q.; Zhang, J. Genome-Wide Identification, Classification and Expression Analysis of the Hsf and Hsp70 Gene Families in Maize. Gene 2021, 770, 145348. [Google Scholar] [CrossRef]
- Lai, D.; Yan, J.; Fan, Y.; Li, Y.; Ruan, J.; Wang, J.; Fan, Y.; Cheng, X.; Cheng, J. Genome-Wide Identification and Phylogenetic Relationships of the Hsp70 Gene Family of Aegilops Tauschii, Wild Emmer Wheat (Triticum Dicoccoides) and Bread Wheat (Triticum Aestivum). 3 Biotech 2021, 11, 301. [Google Scholar] [CrossRef]
- Aparicio, F.; Thomas, C.L.; Lederer, C.; Niu, Y.; Wang, D.; Maule, A.J. Virus Induction of Heat Shock Protein 70 Reflects a General Response to Protein Accumulation in the Plant Cytosol. Plant Physiol. 2005, 138, 529–536. [Google Scholar] [CrossRef]
- Jungkunz, I.; Link, K.; Vogel, F.; Voll, L.M.; Sonnewald, S.; Sonnewald, U. AtHsp70-15-Deficient Arabidopsis Plants Are Characterized by Reduced Growth, a Constitutive Cytosolic Protein Response and Enhanced Resistance to TuMV. Plant J. 2011, 66, 983–995. [Google Scholar] [CrossRef]
- Chen, X.; Shi, L.; Chen, Y.; Zhu, L.; Zhang, D.; Xiao, S.; Aharoni, A.; Shi, J.; Xu, J. Arabidopsis HSP70-16 Is Required for Flower Opening under Normal or Mild Heat Stress Temperatures. Plant Cell Environ. 2019, 42, 1190–1204. [Google Scholar] [CrossRef]
- Fragkostefanakis, S.; Röth, S.; Schleiff, E.; Scharf, K.-D. Prospects of Engineering Thermotolerance in Crops through Modulation of Heat Stress Transcription Factor and Heat Shock Protein Networks. Plant Cell Environ. 2015, 38, 1881–1895. [Google Scholar] [CrossRef]
- Shafikova, T.N.; Omelichkina, Y.V.; Soldatenko, A.S.; Enikeev, A.G.; Kopytina, T.V.; Rusaleva, T.M.; Volkova, O.D. Tobacco Cell Cultures Transformed by the Hsp101 Gene Exhibit an Increased Resistance to Clavibacter Michiganensis Ssp. Sepedonicus. Dokl. Biol. Sci. 2013, 450, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Vierling, E. The Roles of Heat Shock Proteins in Plants. Annu. Rev. Plant Biol. 1991, 42, 579–620. [Google Scholar] [CrossRef]
- Johnson, O.T.; Nadel, C.M.; Carroll, E.C.; Arhar, T.; Gestwicki, J.E. Two Distinct Classes of Cochaperones Compete for the EEVD Motif in Heat Shock Protein 70 to Tune Its Chaperone Activities. J. Biol. Chem. 2022, 298, 101697. [Google Scholar] [CrossRef]
- Mayer, M.P.; Bukau, B. Hsp70 Chaperones: Cellular Functions and Molecular Mechanism. Cell. Mol. Life Sci. 2005, 62, 670–684. [Google Scholar] [CrossRef]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 Chaperone Network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Craig, E.A. The HSP70 Chaperone Machinery: J Proteins as Drivers of Functional Specificity. Nat. Rev. Mol. Cell Biol. 2010, 11, 579–592, Erratum in Nat. Rev. Mol. Cell Biol. 2010, 11, 750. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Tamadaddi, C.; Tak, Y.; Lal, S.S.; Cole, S.J.; Hines, J.K.; Sahi, C. The Expanding World of Plant J-Domain Proteins. CRC Crit. Rev. Plant Sci. 2019, 38, 382–400. [Google Scholar] [CrossRef] [PubMed]
- Rampelt, H.; Mayer, M.P.; Bukau, B. Nucleotide Exchange Factors for Hsp70 Chaperones. Methods Mol. Biol. 2011, 787, 83–91. [Google Scholar] [CrossRef]
- Toribio, R.; Mangano, S.; Fernández-Bautista, N.; Muñoz, A.; Castellano, M.M. HOP, a Co-Chaperone Involved in Response to Stress in Plants. Front. Plant Sci. 2020, 11, 591940. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Fan, H.; Cui, N.; Meng, X.; He, J.; Ran, N.; Yu, Y. Research Progress of Plant Nucleotide-Binding Leucine-Rich Repeat Protein. Horticulturae 2023, 9, 122. [Google Scholar] [CrossRef]
- Guo, M.; Liu, J.-H.; Ma, X.; Luo, D.-X.; Gong, Z.-H.; Lu, M.-H. The Plant Heat Stress Transcription Factors (HSFs): Structure, Regulation, and Function in Response to Abiotic Stresses. Front. Plant Sci. 2016, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Masser, A.E.; Ciccarelli, M.; Andréasson, C. Hsf1 on a Leash—Controlling the Heat Shock Response by Chaperone Titration. Exp. Cell Res. 2020, 396, 112246. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.; Hirt, H.; Bendahmane, A. The Heat—Shock Protein/Chaperone Network and Multiple Stress Resistance. Plant Biotechnol. J. 2017, 15, 405–414. [Google Scholar] [CrossRef]
- Li, C.; Chen, Q.; Gao, X.; Qi, B.; Chen, N.; Xu, S.; Chen, J.; Wang, X. AtHsfA2 Modulates Expression of Stress Responsive Genes and Enhances Tolerance to Heat and Oxidative Stress in Arabidopsis. Sci. China Ser. C Life Sci. 2005, 48, 540–550. [Google Scholar] [CrossRef]
- Schramm, F.; Ganguli, A.; Kiehlmann, E.; Englich, G.; Walch, D.; von Koskull-Döring, P. The Heat Stress Transcription Factor HsfA2 Serves as a Regulatory Amplifier of a Subset of Genes in the Heat Stress Response in Arabidopsis. Plant Mol. Biol. 2006, 60, 759–772. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Chen, J.; Guo, L.; Li, X.; Li, W.; Yu, Z.; Deng, J.; Zhang, P.; Zhang, K.; et al. Arabidopsis Heat Shock Factor HsfA1a Directly Senses Heat Stress, pH Changes, and Hydrogen Peroxide via the Engagement of Redox State. Plant Physiol. Biochem. 2013, 64, 92–98. [Google Scholar] [CrossRef]
- Hervás, R.; Oroz, J. Mechanistic Insights into the Role of Molecular Chaperones in Protein Misfolding Diseases: From Molecular Recognition to Amyloid Disassembly. Int. J. Mol. Sci. 2020, 21, 9186. [Google Scholar] [CrossRef]
- Gunawardene, C.D.; Donaldson, L.W.; White, K.A. Tombusvirus Polymerase: Structure and Function. Virus Res. 2017, 234, 74–86. [Google Scholar] [CrossRef]
- Pogany, J.; Stork, J.; Li, Z.; Nagy, P.D. In Vitro Assembly of the Tomato Bushy Stunt Virus Replicase Requires the Host Heat Shock Protein 70. Proc. Natl. Acad. Sci. USA 2008, 105, 19956–19961. [Google Scholar] [CrossRef]
- Wang, R.Y.-L.; Stork, J.; Nagy, P.D. A Key Role for Heat Shock Protein 70 in the Localization and Insertion of Tombusvirus Replication Proteins to Intracellular Membranes. J. Virol. 2009, 83, 3276–3287. [Google Scholar] [CrossRef] [PubMed]
- Mine, A.; Hyodo, K.; Tajima, Y.; Kusumanegara, K.; Taniguchi, T.; Kaido, M.; Mise, K.; Taniguchi, H.; Okuno, T. Differential Roles of Hsp70 and Hsp90 in the Assembly of the Replicase Complex of a Positive-Strand RNA Plant Virus. J. Virol. 2012, 86, 12091–12104. [Google Scholar] [CrossRef] [PubMed]
- Gorovits, R.; Moshe, A.; Ghanim, M.; Czosnek, H. Recruitment of the Host Plant Heat Shock Protein 70 by Tomato Yellow Leaf Curl Virus Coat Protein Is Required for Virus Infection. PLoS ONE 2013, 8, e70280. [Google Scholar] [CrossRef]
- Whitham, S.; Dinesh-Kumar, S.P.; Choi, D.; Hehl, R.; Corr, C.; Baker, B. The Product of the Tobacco Mosaic Virus Resistance Gene N: Similarity to Toll and the Interleukin-1 Receptor. Cell 1994, 78, 1101–1115. [Google Scholar] [CrossRef]
- Caplan, J.L.; Mamillapalli, P.; Burch-Smith, T.M.; Czymmek, K.; Dinesh-Kumar, S.P. Chloroplastic Protein NRIP1 Mediates Innate Immune Receptor Recognition of a Viral Effector. Cell 2008, 132, 449–462. [Google Scholar] [CrossRef]
- Lu, R.; Malcuit, I.; Moffett, P.; Ruiz, M.T.; Peart, J.; Wu, A.; Rathjen, J.P.; Bendahmane, A.; Day, L.; Baulcombe, D.C. High Throughput Virus—induced Gene Silencing Implicates Heat Shock Protein 90 in Plant Disease Resistance. EMBO J. 2003, 22, 5690–5699. [Google Scholar] [CrossRef]
- Qian, L.; Zhao, J.; Du, Y.; Zhao, X.; Han, M.; Liu, Y. Hsp90 Interacts With Tm-22 and Is Essential for Tm-22-Mediated Resistance to Tobacco Mosaic Virus. Front. Plant Sci. 2018, 9, 411. [Google Scholar] [CrossRef]
- Iki, T.; Yoshikawa, M.; Nishikiori, M.; Jaudal, M.C.; Matsumoto-Yokoyama, E.; Mitsuhara, I.; Meshi, T.; Ishikawa, M. In Vitro Assembly of Plant RNA-Induced Silencing Complexes Facilitated by Molecular Chaperone HSP90. Mol. Cell 2010, 39, 282–291. [Google Scholar] [CrossRef]
- Iwasaki, S.; Kobayashi, M.; Yoda, M.; Sakaguchi, Y.; Katsuma, S.; Suzuki, T.; Tomari, Y. Hsc70/Hsp90 Chaperone Machinery Mediates ATP-Dependent RISC Loading of Small RNA Duplexes. Mol. Cell 2010, 39, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.J.; Ye, Q. RNA Virus Replication Complexes. PLOS Pathog. 2010, 6, e1000943. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Lu, Y.; Li, K.; Lin, L.; Zheng, H.; Yan, F.; Chen, J. Heat Shock Protein 70 Is Necessary for Rice Stripe Virus Infection in Plants. Mol. Plant Pathol. 2014, 15, 907–917. [Google Scholar] [CrossRef]
- Jiang, S.; Wu, B.; Jiang, L.; Zhang, M.; Lu, Y.; Wang, S.; Yan, F.; Xin, X. Triticum Aestivum Heat Shock Protein 23.6 Interacts with the Coat Protein of Wheat Yellow Mosaic Virus. Virus Genes 2019, 55, 209–217. [Google Scholar] [CrossRef]
- Prokhnevsky, A.I.; Peremyslov, V.V.; Napuli, A.J.; Dolja, V.V. Interaction between Long-Distance Transport Factor and Hsp70-Related Movement Protein of Beet Yellows Virus. J. Virol. 2002, 76, 11003–11011. [Google Scholar] [CrossRef] [PubMed]
- Peremyslov, V.V.; Hagiwara, Y.; Dolja, V.V. HSP70 Homolog Functions in Cell-to-Cell Movement of a Plant Virus. Proc. Natl. Acad. Sci. USA 1999, 96, 14771–14776. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.E.; Oh, S.; Seo, E.; Choi, D. HSP70s Enhance a Phytophthora Infestans Effector-Induced Cell Death via an MAPK Cascade in Nicotiana Benthamiana. MPMI 2018, 31, 356–362. [Google Scholar] [CrossRef]
- Yu, J.; Gonzalez, J.M.; Dong, Z.; Shan, Q.; Tan, B.; Koh, J.; Zhang, T.; Zhu, N.; Dufresne, C.; Martin, G.B.; et al. Integrative Proteomic and Phosphoproteomic Analyses of Pattern- and Effector-Triggered Immunity in Tomato. Front. Plant Sci. 2021, 12, 768693. [Google Scholar] [CrossRef]
- Nabi, Z.; Manzoor, S.; Nabi, S.U.; Wani, T.A.; Gulzar, H.; Farooq, M.; Arya, V.M.; Baloch, F.S.; Vlădulescu, C.; Popescu, S.M.; et al. Pattern-Triggered Immunity and Effector-Triggered Immunity: Crosstalk and Cooperation of PRR and NLR-Mediated Plant Defense Pathways during Host–Pathogen Interactions. Physiol. Mol. Biol. Plants 2024, 30, 587–604. [Google Scholar] [CrossRef]
- Chiang, Y.-H.; Coaker, G. Effector Triggered Immunity: NLR Immune Perception and Downstream Defense Responses. Arbo. Book 2015, 2015, e0183. [Google Scholar] [CrossRef][Green Version]
- Seo, Y.-S.; Lee, S.-K.; Song, M.-Y.; Suh, J.-P.; Hahn, T.-R.; Ronald, P.; Jeon, J.-S. The HSP90-SGT1-RAR1 Molecular Chaperone Complex: A Core Modulator in Plant Immunity. J. Plant Biol. 2008, 51, 1–10. [Google Scholar] [CrossRef]
- Takahashi, A.; Casais, C.; Ichimura, K.; Shirasu, K. HSP90 Interacts with RAR1 and SGT1 and Is Essential for RPS2-Mediated Disease Resistance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 11777–11782. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Burch-Smith, T.; Schiff, M.; Feng, S.; Dinesh-Kumar, S.P. Molecular Chaperone Hsp90 Associates with Resistance Protein N and Its Signaling Proteins SGT1 and Rar1 to Modulate an Innate Immune Response in Plants. J. Biol. Chem. 2004, 279, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, T.; Kufer, T.A.; Schulze-Lefert, P. NLR Functions in Plant and Animal Immune Systems: So Far and yet so Close. Nat. Immunol. 2011, 12, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Swiderski, M.R.; Birker, D.; Jones, J.D.G. The TIR Domain of TIR-NB-LRR Resistance Proteins Is a Signaling Domain Involved in Cell Death Induction. MPMI 2009, 22, 157–165. [Google Scholar] [CrossRef]
- Maekawa, T.; Cheng, W.; Spiridon, L.N.; Töller, A.; Lukasik, E.; Saijo, Y.; Liu, P.; Shen, Q.-H.; Micluta, M.A.; Somssich, I.E.; et al. Coiled-Coil Domain-Dependent Homodimerization of Intracellular Barley Immune Receptors Defines a Minimal Functional Module for Triggering Cell Death. Cell Host Microbe 2011, 9, 187–199. [Google Scholar] [CrossRef]
- Contreras, M.P.; Pai, H.; Thompson, R.; Marchal, C.; Claeys, J.; Adachi, H.; Kamoun, S. The Nucleotide-Binding Domain of NRC-Dependent Disease Resistance Proteins Is Sufficient to Activate Downstream Helper NLR Oligomerization and Immune Signaling. New Phytol. 2024, 243, 345–361. [Google Scholar] [CrossRef]
- Shirasu, K. The HSP90-SGT1 Chaperone Complex for NLR Immune Sensors. Annu. Rev. Plant Biol. 2009, 60, 139–164. [Google Scholar] [CrossRef]
- Chen, L.; Shimamoto, K. Emerging Roles of Molecular Chaperones in Plant Innate Immunity. J. Gen. Plant Pathol. 2011, 77, 1–9. [Google Scholar] [CrossRef]
- Boller, T.; He, S.Y. Innate Immunity in Plants: An Arms Race Between Pattern Recognition Receptors in Plants and Effectors in Microbial Pathogens. Science 2009, 324, 742–744. [Google Scholar] [CrossRef]
- Murthy, V.S.; Ravishankar, K.V. Molecular Mechanisms of Heat Shock Proteins and Thermotolerance in Plants. In Abiotic Stress Physiology of Horticultural Crops; Rao, N.K.S., Shivashankara, K.S., Laxman, R.H., Eds.; Springer: New Delhi, India, 2016; pp. 71–83. ISBN 978-81-322-2725-0. [Google Scholar]
- Pirkkala, L.; Nykänen, P.; Sistonen, L. Roles of the Heat Shock Transcription Factors in Regulation of the Heat Shock Response and Beyond. FASEB J. 2001, 15, 1118–1131. [Google Scholar] [CrossRef]
- Queitsch, C.; Hong, S.-W.; Vierling, E.; Lindquist, S. Heat Shock Protein 101 Plays a Crucial Role in Thermotolerance in Arabidopsis. Plant Cell 2000, 12, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-W.; Vierling, E. Hsp101 Is Necessary for Heat Tolerance but Dispensable for Development and Germination in the Absence of Stress. Plant J. 2001, 27, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, U.; Small, I.; des Francs-Small, C.C.; Vierling, E. Mutations in an Arabidopsis Mitochondrial Transcription Termination Factor–Related Protein Enhance Thermotolerance in the Absence of the Major Molecular Chaperone HSP101. Plant Cell 2012, 24, 3349–3365. [Google Scholar] [CrossRef]
- Tiwari, L.D.; Kumar, R.; Sharma, V.; Sahu, A.K.; Sahu, B.; Naithani, S.C.; Grover, A. Stress and Development Phenotyping of Hsp101 and Diverse Other Hsp Mutants of Arabidopsis Thaliana. J. Plant Biochem. Biotechnol. 2021, 30, 889–905. [Google Scholar] [CrossRef]
- Bisht, K.; te Velthuis, A.J.W. Decoding the Role of Temperature in RNA Virus Infections. mBio 2022, 13, e0202122. [Google Scholar] [CrossRef]
- Sharma, V.; Mohammed, S.A.; Devi, N.; Vats, G.; Tuli, H.S.; Saini, A.K.; Dhir, Y.W.; Dhir, S.; Singh, B. Unveiling the Dynamic Relationship of Viruses and/or Symbiotic Bacteria with Plant Resilience in Abiotic Stress. Stress Biol. 2024, 4, 10. [Google Scholar] [CrossRef]
- Prasch, C.M.; Sonnewald, U. Simultaneous Application of Heat, Drought, and Virus to Arabidopsis Plants Reveals Significant Shifts in Signaling Networks. Plant Physiol. 2013, 162, 1849–1866. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, R.; Tu, Z.; Nie, X.; Song, B.; He, C.; Xie, C.; Nie, B. Global Screening and Functional Identification of Major HSPs Involved in PVY Infection in Potato. Genes 2022, 13, 566. [Google Scholar] [CrossRef]
- Makarova, S.; Makhotenko, A.; Spechenkova, N.; Love, A.J.; Kalinina, N.O.; Taliansky, M. Interactive Responses of Potato (Solanum Tuberosum L.) Plants to Heat Stress and Infection With Potato Virus Y. Front. Microbiol. 2018, 9, 2582. [Google Scholar] [CrossRef]
- DeMell, A.; Alvarado, V.; Scholthof, H.B. Molecular Perspectives on Age-Related Resistance of Plants to (Viral) Pathogens. New Phytol. 2023, 240, 80–91. [Google Scholar] [CrossRef]
- Abarca, D.; Martín, M.; Sabater, B. Differential Leaf Stress Responses in Young and Senescent Plants. Physiol. Plant. 2001, 113, 409–415. [Google Scholar] [CrossRef]
- Tsai, W.-A.; Brosnan, C.A.; Mitter, N.; Dietzgen, R.G. Perspectives on Plant Virus Diseases in a Climate Change Scenario of Elevated Temperatures. Stress Biol. 2022, 2, 37. [Google Scholar] [CrossRef] [PubMed]
- Hua, J. Modulation of Plant Immunity by Light, Circadian Rhythm, and Temperature. Curr. Opin. Plant Biol. 2013, 16, 406–413. [Google Scholar] [CrossRef]
- Alcázar, R.; Parker, J.E. The Impact of Temperature on Balancing Immune Responsiveness and Growth in Arabidopsis. Trends Plant Sci. 2011, 16, 666–675. [Google Scholar] [CrossRef]
- Cheng, C.; Gao, X.; Feng, B.; Sheen, J.; Shan, L.; He, P. Plant Immune Response to Pathogens Differs with Changing Temperatures. Nat. Commun. 2013, 4, 2530. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qian, W.; Hua, J. Temperature Modulates Plant Defense Responses through NB-LRR Proteins. PLOS Pathog. 2010, 6, e1000844. [Google Scholar] [CrossRef]
- Király, L.; Hafez, Y.M.; Fodor, J.; Király, Z. Suppression of Tobacco Mosaic Virus-Induced Hypersensitive-Type Necrotization in Tobacco at High Temperature Is Associated with Downregulation of NADPH Oxidase and Superoxide and Stimulation of Dehydroascorbate Reductase. J. Gen. Virol. 2008, 89, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Szittya, G.; Silhavy, D.; Molnár, A.; Havelda, Z.; Lovas, Á.; Lakatos, L.; Bánfalvi, Z.; Burgyán, J. Low Temperature Inhibits RNA Silencing—Mediated Defence by the Control of siRNA Generation. EMBO J. 2003, 22, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, K.; Renovell, A.; Comellas, M.; Serra, P.; García, M.L.; Pina, J.A.; Navarro, L.; Moreno, P.; Guerri, J. Effect of Temperature on RNA Silencing of a Negative-Stranded RNA Plant Virus: Citrus Psorosis Virus. Plant Pathol. 2010, 59, 982–990. [Google Scholar] [CrossRef]
- Zhong, S.-H.; Liu, J.-Z.; Jin, H.; Lin, L.; Li, Q.; Chen, Y.; Yuan, Y.-X.; Wang, Z.-Y.; Huang, H.; Qi, Y.-J.; et al. Warm Temperatures Induce Transgenerational Epigenetic Release of RNA Silencing by Inhibiting siRNA Biogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 9171–9176. [Google Scholar] [CrossRef]
- Mizumoto, H.; Nakamura, I.; Shimomoto, Y.; Sawada, H.; Tomita, R.; Sekine, K.-T.; Kiba, A.; Nishiguchi, M.; Kobayashi, K.; Hikichi, Y. Amino Acids in Tobamovirus Coat Protein Controlling Pepper L1a Gene-Mediated Resistance. Mol. Plant Pathol. 2012, 13, 915–922. [Google Scholar] [CrossRef]
- W, B.I. Resistance to TMV in Capsicum Chacoense Hunz Is Governed by an Allele of the L-Locus. Capsicum Newsl. 1984, 3, 47–48. [Google Scholar]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D.G. Hormone Crosstalk in Plant Disease and Defense: More than Just Jasmonate-Salicylate Antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef]
- Clarke, S.M.; Cristescu, S.M.; Miersch, O.; Harren, F.J.M.; Wasternack, C.; Mur, L.A.J. Jasmonates Act with Salicylic Acid to Confer Basal Thermotolerance in Arabidopsis Thaliana. New Phytol. 2009, 182, 175–187. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Umar, S. Heat Stress Tolerance in Plants: Action of Salicylic Acid. In Salicylic Acid: A Multifaceted Hormone; Nazar, R., Iqbal, N., Khan, N.A., Eds.; Springer: Singapore, 2017; pp. 145–161. ISBN 978-981-10-6068-7. [Google Scholar]
- Caarls, L.; Pieterse, C.M.J.; Van Wees, S.C.M. How Salicylic Acid Takes Transcriptional Control over Jasmonic Acid Signaling. Front. Plant Sci. 2015, 6, 170. [Google Scholar] [CrossRef]
- Thirumalaikumar, V.P.; Kamranfar, I.; Marmagne, A.; Masclaux-Daubresse, C.; Balazadeh, S. A Regulatory Role of Autophagy for Resetting the Memory of Heat Stress in Plants. Plant Cell Environ. 2020, 42, 1054–1064, Erratum in Plant Cell Environ. 2020, 43, 1807–1809. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, Q.; Liu, Y. Multifaceted Roles of Autophagy in Plant–Virus–Insect Interactions. New Phytol. 2025, 248, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, N.K.; Hafrén, A.; Hofius, D. Autophagy–Virus Interplay in Plants: From Antiviral Recognition to Proviral Manipulation. Mol. Plant Pathol. 2019, 20, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Thirumalaikumar, V.P.; Gorka, M.; Schulz, K.; Masclaux-Daubresse, C.; Sampathkumar, A.; Skirycz, A.; Vierstra, R.D.; Balazadeh, S. Selective Autophagy Regulates Heat Stress Memory in Arabidopsis by NBR1-Mediated Targeting of HSP90.1 and ROF1. Autophagy 2021, 17, 2184–2199. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Yu, J.Q.; Chen, Z. Role and Regulation of Autophagy in Heat Stress Responses of Tomato Plants. Front. Plant Sci. 2014, 5, 174. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liao, J.; Chung, K.K.; Feng, L.; Liao, Y.; Yang, Z.; Liu, C.; Zhou, J.; Shen, W.; Li, H.; et al. Stress Granules Sequester Autophagy Proteins to Facilitate Plant Recovery from Heat Stress. Nat. Commun. 2024, 15, 10910. [Google Scholar] [CrossRef]


| Chaperone | Function Type | Mechanism/Role | Key Interacting Partners | Example Viruses | Source |
|---|---|---|---|---|---|
| HSP70 | Pro-viral | VRC Assembly: Localization and membrane insertion of replicase proteins; RdRp activation. | Viral replicase proteins (p33/p92); Phospholipids | Tomato bushy stunt virus (TBSV) | [39,40,41] |
| VRC Assembly: Prevention of replicase protein aggregation. | Viral replicase protein (p27) | Red clover necrotic mosaic virus (RCNMV) | [42] | ||
| Nuclear Import of viral proteins. | Coat Protein (CP) | Tomato yellow leaf curl virus (TYLCV) | [43] | ||
| Anti-viral | Cooperation with the HSP90 complex to modulate NLR-mediated immunity. | HSP90, SGT1, RAR1 | General (e.g., TMV via N protein) | [44,45] | |
| HSP90 | Pro-viral | VRC Assembly: Conformational maturation of replicase protein for binding to viral RNA. | Viral replicase protein (p27); Viral RNA element (YRE) | Red clover necrotic mosaic virus (RCNMV) | [42] |
| Anti-viral | ETI: Stabilization and maintenance of NLR immune receptors in an activation-competent state. | NLR proteins, SGT1, RAR1 | Tobacco mosaic virus (TMV), Potato virus X (PVX) | [46,47] | |
| RNA Silencing: Loading of small RNAs (siRNA/miRNA) into Argonaute (AGO) proteins to form a functional RISC. | Argonaute (AGO) proteins | General antiviral silencing | [48,49] |
| Cellular State | Available HSP70/90 Pool | NLR Receptor Status | Outcome (Susceptibility) |
|---|---|---|---|
| Normal conditions | High (basal) | Stable (HSP90-SGT1-RAR1) | Virus resistance |
| Viral infection only | Slightly reduced (competition with VRC) | Mostly stable | Resistance maintained |
| Heat stress only | Severely reduced (diverted to denatured proteins) | Destabilized (undergoing degradation) | N/A (no virus) |
| Combined stress (Virus + Heat) | Critically reduced (diverted to denatured proteins) | Destabilized and undergoing degradation | ETI collapse, high viral susceptibility |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madirov, A.; Iksat, N.; Turarbekova, Z.; Abzhalelov, B.; Masalimov, Z. Competition for Chaperones: A Trade-Off Between Thermotolerance and Antiviral Immunity in Plants. Curr. Issues Mol. Biol. 2025, 47, 957. https://doi.org/10.3390/cimb47110957
Madirov A, Iksat N, Turarbekova Z, Abzhalelov B, Masalimov Z. Competition for Chaperones: A Trade-Off Between Thermotolerance and Antiviral Immunity in Plants. Current Issues in Molecular Biology. 2025; 47(11):957. https://doi.org/10.3390/cimb47110957
Chicago/Turabian StyleMadirov, Almas, Nurgul Iksat, Zhibek Turarbekova, Bakhytbek Abzhalelov, and Zhaksylyk Masalimov. 2025. "Competition for Chaperones: A Trade-Off Between Thermotolerance and Antiviral Immunity in Plants" Current Issues in Molecular Biology 47, no. 11: 957. https://doi.org/10.3390/cimb47110957
APA StyleMadirov, A., Iksat, N., Turarbekova, Z., Abzhalelov, B., & Masalimov, Z. (2025). Competition for Chaperones: A Trade-Off Between Thermotolerance and Antiviral Immunity in Plants. Current Issues in Molecular Biology, 47(11), 957. https://doi.org/10.3390/cimb47110957






