Abstract
Caffeoylquinic acids (CQAs), a class of phenolic acid metabolites widely distributed in plants, encompass 15 positional isomers from mono- to tetra-esters, with 5-O-caffeoylquinic acid (5-CQA) as the predominant form. The biosynthesis of 5-CQA from phenylalanine proceeds through five primary pathways, which are finely regulated by environmental, hormonal, and transcription factors from families such as MYB, WRKY, and bHLH. These regulators control 5-CQA synthesis by binding specifically to the promoter regions of key structural genes, including PAL, 4CL and HCT/HQT. Subsequently, 5-CQA serves as a central precursor for the biosynthesis of other CQAs. In terms of bioactivity, CQAs possess remarkable pharmacological activities, encompassing antioxidant, antimicrobial, anti-diabetic, anti-inflammatory and anti-tumor properties. For instance, anti-inflammatory effects are demonstrated by the ability of 5-CQA to reduce key pro-inflammatory cytokines (e.g., TNF-α and IL-1β) and downregulate the TLR4/NF-κB pathway. The synergistic action of 5-CQA with ultraviolet-A reduced succinate-coenzyme Q reductase activity by approximately 72%, highlighting its potential to disrupt bacterial metabolism and combat antibiotic resistance. Furthermore, 3,4,5-triCQA exhibits potent anti-influenza virus activity, potentially through a mechanism distinct from existing neuraminidase inhibitors. Beyond medicine, CQAs show promise in light industry. They serve as antibiotic alternatives in livestock feed to enhance gut health, extend food shelf life through their antioxidant activity, and function as active ingredients in UV-protective skincare formulations. CQAs also enhance plant stress tolerance to cold, arsenic, and pests by mechanisms such as scavenging reactive oxygen species and inhibiting pest mobility. While this review consolidates progress in the biosynthesis and bioactivity of CQAs specifically with caffeoyl substituents, future efforts should leverage modern biotechnological tools and interdisciplinary approaches to bridge critical knowledge gaps in their biosynthesis, transport, and clinical translation.
1. Introduction
Plant phenolics, as one of the most widespread and diverse classes of natural products, play indispensable roles in human health and plant defense. Among them, Caffeoylquinic acids (CQAs)—esters formed between quinic acid and caffeic acid—stand out for their significant pharmacological value and biological prevalence. This family encompasses mono-, di-, tri-, and tetra- esters, with 5-O-caffeoylquinic acid (5-CQA) being the most abundant and prominent member. It is designated as a key quality control marker in the Chinese Pharmacopoeia for numerous traditional medicinal herbs, including Lonicera japonica Thunb., Prunus mume Siebold & Zucc., Chrysanthemum morifolium Ramat., and Eucommia ulmoides Oliv., as well as for proprietary Chinese medicines such as Yinhuang Oral Liquid, Zhongjiefeng Extract, Yinzhihuang Granules, and Shuanghuanglian Oral Liquid [1]. Plants synthesize 5-CQA as the initial product via the phenylpropanoid pathway. This compound is subsequently converted into other CQAs, including 3-CQA, 4-CQA, and various poly-caffeoylquinic acids. These secondary metabolites exhibit diverse pharmacological properties, including broad-spectrum antimicrobial, anti-inflammatory, antitumor, neuroprotective, and antioxidant activities. They also play significant roles in plant growth, development, and responses to abiotic and biotic stresses [2,3,4,5,6,7,8].
This review is structured to provide a comprehensive overview of CQAs, encompassing literature published up to October 2025, with a dual focus on biosynthesis and bioactivities of CQAs. Research has established five distinct biosynthetic pathways for 5-CQA, originating from phenylalanine, the end product of the shikimate pathway [9]. Numerous studies have identified the structural genes involved and their upstream regulators. For instance, a recent study in L. japonica identified the R2R3-MYB transcription factor LmMYB111, which activates the synthesis of CQAs and luteoloside by binding to the promoters of multiple genes, including PAL, 4CL, and MYB4 [10]. In another study, Luo et al. compared CQAs content and antioxidant activity across various sweet potato (Ipomoea batatas (L.) Lam.) genotypes, identifying the GARP-type transcription factor IbGLK1, which promotes CQAs biosynthesis by directly binding to the promoters of downstream structural genes [11].
Recent studies show that CQAs influence a wide range of applications, such as pharmacology, animal husbandry, food preservation, skin brightening, and plant stress resistance [9,12]. Substantial evidence from animal models confirms CQAs anti-inflammatory, antimicrobial, and neuroprotective effects, with underlying mechanisms increasingly elucidated [2,3]. In animal husbandry, CQAs—as a natural plant-derived compound—has garnered significant research attention as an antibiotic alternative; For instance, dietary supplementation with 5-CQA in broiler chickens effectively ameliorates intestinal health under high-density (HD) stocking stress [13].
Owing to its antioxidant, UV-protective, and skin-conditioning properties, CQAs exemplifies the broader role of polyphenols in dermatological protection [14]. As a potent antioxidant, CQAs mitigates cold stress damage in plants by scavenging reactive oxygen species (ROS) such as H2O2 and O2− [15].
The impetus for this new review stems from the conceptual and methodological breakthroughs witnessed since 2020. The widespread adoption of plant metabolomics and transcriptomics has catalyzed an explosion of research into plant secondary metabolites, unearthing a wealth of new data on CQAs. Key advances include the identification of novel biosynthetic enzymes and regulatory transcription factors in non-model plant species, a deeper understanding of their multifaceted roles in plant–environment interactions, and the discovery of novel pharmacological mechanisms. However, this rapidly expanding body of knowledge has yet to be synthesized into a comprehensive review that critically evaluates these recent discoveries. This review therefore aims to consolidate the latest reports on CQAs biosynthesis and bioactivity, pinpoint critical research gaps, and champion the application of modern biotechnological tools to fully unlock the potential of CQAs in medicine, agriculture, and light industry.
2. Types of CQAs
CQAs have multiple derivatives. Besides caffeic acid, the hydroxyl and carboxyl groups on the carbon ring of quinic acid ((1S,3R,4S,5R)-1,3,4,5-tetrahydroxycyclohexane-1-carboxylic acid) can form esters with other trans-cinnamic acids like ferulic acid, p-coumaric acid, and methyl esters [12]. The carbon ring of quinic acid contains four positions (1, 3, 4, 5) that undergo acylation with caffeoyl groups, as shown in Figure 1.
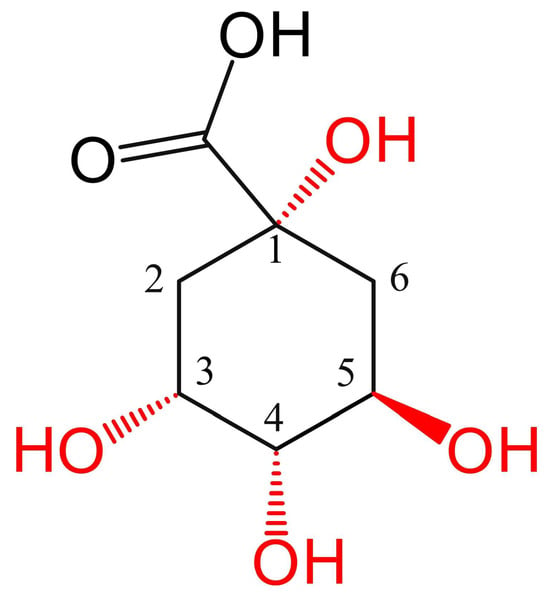
Figure 1.
Chemical structures of quinic acid and four R-base derivatives. The stereochemistry of the natural (3R,5R)-configured quinic acid scaffold is crucial for activity and is retained in all derivatives, as highlighted by the wedged and dashed bonds. The numbers indicate the positional numbering of the substituents on the quinic acid ring, and the red color highlights potential substitution sites.
A critical nomenclature clarification is essential, particularly regarding the distinct isomers 3-CQA and 5-CQA. To clarify these critical distinctions, we provide a direct comparison in Figure 2. Historically, “chlorogenic acid” referred to 3-CQA, but the 1976 IUPAC revision reassigned it to 5-CQA. This outdated usage persists in some literature and supplier catalogs, causing ongoing confusion. To ensure clarity throughout this review, we will exclusively use the systematic name 5-O-Caffeoylquinic acid (5-CQA), which corresponds to CAS number 327-97-9.
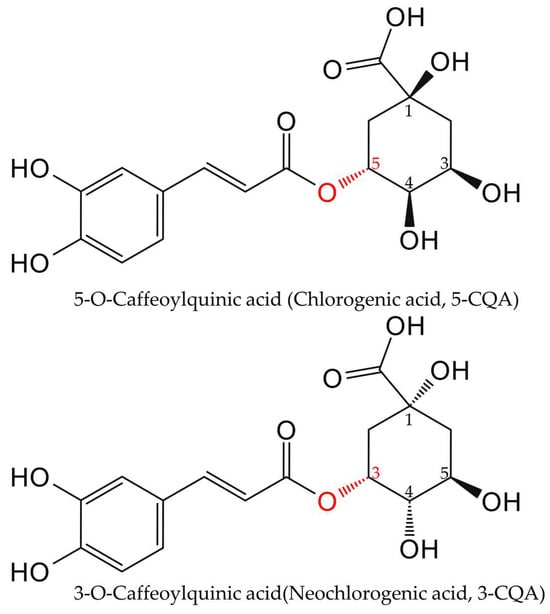
Figure 2.
Key distinctions between 3-CQA (neochlorogenic acid) and 5-CQA (chlorogenic acid). This schematic highlights the critical stereochemical differences arising from their acylation sites, which are essential for resolving the persistent misapplication of the term “chlorogenic acid” in the literature.
This review focuses exclusively on recent advances concerning caffeic acid substituents of the quinic acid backbone. The attachment of one to four caffeoyl groups to different hydroxyl positions of quinic acid generates a well-defined family of fifteen positional isomers. As summarized in Table 1, the CQA family comprises a total of 15 CQAs, ranging from mono- to tetra-caffeoyl substituted forms [16,17]. These CQAs exhibit distinct biological activities that generally correlate with the number of caffeoyl substitutions, following the order of tetra- > tri- > di- > mono-CQAs [9]. The table systematically lists the CAS registry numbers alongside the corresponding systematic names for each compound, thereby helping to mitigate potential confusion arising from historical or conflicting nomenclature in the literature.

Table 1.
CQAs Derivative References.
3. Biosynthesis of CQAs
3.1. Five Biosynthetic Routes
Plants biosynthesize CQAs primarily via the phenylpropanoid pathway, which first yields 5-CQA [9]. This central compound then serves as a precursor for its isomers, such as 3-CQA and 4-CQA, as well as for more complex poly-caffeoylquinic acids. Furthermore, 5-CQA occupies a branch point in the pathway leading to lignin monomer synthesis, a connection that has been extensively documented [18]. Given this central role, the following section reviews five reported biosynthetic routes for 5-CQA. Gene expression within these pathways varies significantly across plant species. Notably, in many species, only one or two of these routes function as the predominant biosynthetic pathways for 5-CQA, while some are unique to particular plant lineages [19,20,21]. 5-CQA synthesis initiates when phenylalanine undergoes deamination by Phenylalanine ammonia-lyase (PAL) to form trans-cinnamic acid [18]. The five distinct biosynthetic routes characterized for 5-CQA are shown in Figure 3, with Pathways 1 and 2 being the most prevalent in plants, having been confirmed in widely studied families such as Asteraceae, Solanaceae, and Rubiaceae [21]. In contrast, Pathways 3 and 4 represent specialized branches derived from enzymes unique to certain plant species. Notably, Pathway 5 operates independently of the others and has thus far been reported exclusively in sweet potato (I. batatas) [9,22,23].
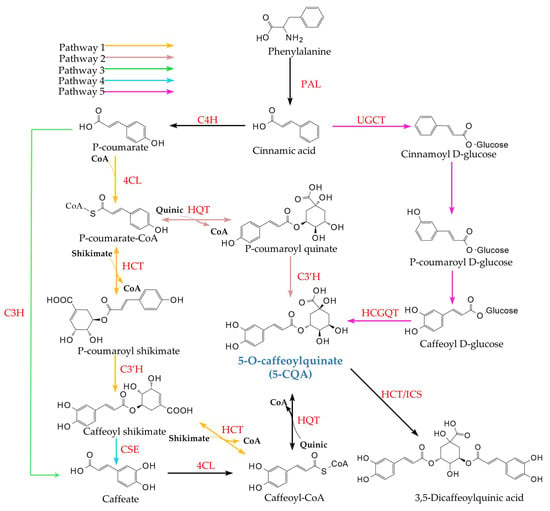
Figure 3.
Different biosynthetic pathways leading to Plant CQAs. The five distinct 5-CQA biosynthetic pathways identified across different plant species are summarized here. Abbreviations: PAL, phenylalanine ammonia-lyase; C4H, cinnamic acid 4-hydroxylase; 4CL, 4-coumaric acid:CoA ligase; C3H, p-coumaric acid 3-hydroxylase; C3’H, p-coumaroyl-shikimic acid/quinic acid 3-hydroxylase; CSE, caffeoyl shikimic acid esterase; HCT, hydroxycinnamoyl-CoA:shikimic acid/quinic acid hydroxycinnamoyltransferase; HQT, hydroxycinnamoyl-CoA:quinic acid hydroxycinnamoyltransferase; UGCT, UDP-Glycosyltransferases; HCGQT, Hydroxycinnamoyl-D-g1ucose:quinate hvdroxvcinnam; ICS, isochlorogenic acid synthase.
5-CQA biosynthesis primarily occurs in the cytoplasm; However, synthesis has also been observed in chloroplasts of L. japonica, with final storage occurring in vacuoles [24]. In both Pathways 1 and 2, trans-cinnamic acid is converted into p-coumaroyl-CoA through the sequential action of cinnamate 4-hydroxylase (C4H) and 4-coumarate: CoA ligase (4CL). Subsequently, Pathways 1 and 2 diverge through the actions of hydroxycinnamoyl-CoA:shikimic acid/quinic acid hydroxycinnamoyltransferase (HCT) and hydroxycinnamoyl-CoA:quinic acid hydroxycinnamoyltransferase (HQT). Pathway 1 involves HCT-catalyzed transesterification of shikimate with Acyl-CoA to form p-coumaroyl shikimate. In contrast, Pathway 2 utilizes HQT to catalyze transesterification of quinate with Acyl-CoA, yielding p-coumaroyl quinate [25]. In Pathway 2, p-coumaroyl quinate is directly hydroxylated by p-coumaroyl-shikimic acid/quinic acid 3-hydroxylase (C3’H) to produce 5-CQA. In Pathway 1, p-coumaroyl shikimate is first hydroxylated by C3’H to caffeoyl shikimate. HCT then transfers the acyl group to form caffeoyl-CoA, followed by HQT-mediated conjugation with quinate to yield 5-CQA. HCT and HQT are structurally similar enzymes that catalyze reversible acyl-transfer reactions between Acyl-CoA and their respective substrates, and HCT exhibits substrate preference for shikimate, whereas HQT favors quinate [25]. Pathway 3 involves the direct hydroxylation of p-coumaric acid to caffeic acid, catalyzed by coumarate 3-hydroxylase (C3H). The resulting caffeic acid is then activated to caffeoyl-CoA by 4CL, after which this pathway converges with Pathway 1 [9,26]. Pathway 4 diverges from Pathway 1 at caffeoyl shikimate, which is hydrolyzed by caffeoyl shikimate esterase (CSE) to form caffeic acid, after which it rejoins Pathway 3 [27]. While seemingly redundant for 5-CQA synthesis, this pathway redirects metabolic flux away from H-lignin toward G/S-lignins in A. thaliana [27]. Similar redirection occurs in Panicum virgatum L., Populus deltoides Marshall, Medicago truncatula Gaertn., and the gymnosperm Larix kaempferi (Lamb.) Carrière [28,29,30]. Notably, CSE homologs are absent in B. distachyon and Zea mays L. [30]. Pathway 5 appears to be unique to I. batatas [22,23]. In its roots, UDP-glucose:cinnamic acid glucosyltransferase (UGCT) first glucosylates trans-cinnamic acid to form cinnamoyl glucose. After hydroxylation at positions 3 and 4 of the aromatic ring to yield caffeoyl glucose, HCGQT transfers the caffeoyl moiety to quinate, synthesizing 5-CQA [9,31].
Beyond pathway 5, which is unique to sweet potatoes, the gene expression profiles of other 5-CQA biosynthetic pathways exhibit significant variation across plant taxa [27]. Substantial evidence indicates that Pathway 4, involving CSE, and the preceding Pathway 1, serve as the primary routes for lignin biosynthesis in plants. Although CSE is absent in some species, this does not negate its crucial role in the conversion of caffeoyl shikimate in numerous plants, including eudicots like Salix psammophila, P. virgatum, P. deltoides, Petunia x hybrida and Medicago truncatula, as well as the gymnosperm L. kaempferi [28,29,30,32,33,34]. Pathway 1 generates caffeoyl-CoA via a reverse reaction of HCT, whereas Pathway 4 bypasses this step through the coordinated actions of CSE and 4CL. Given the central role of CSE in lignin biosynthesis, it can be inferred that Pathway 4 likely represents the major route for 5-CQA synthesis in most plants. Conversely, species lacking CSE (e.g., B. distachyon and Z. mays) likely employ alternative pathways. Pathway 2, catalyzed by C3’H and HQT from p-coumaroyl-CoA, offers the most direct route. However, as this pathway does not align with the metabolic flux of 5-CQA biosynthesis, it is probably only a branch for 5-CQA synthesis and not considered a major route. In Pathway 3, the bifunctional peroxidase C3H, characterized in Arabidopsis and B. distachyon, can directly convert coumaric acid to caffeic acid [28]. This discovery has led to a revision of the traditional lignin biosynthesis model. Although subsequent research on C3H remains limited, it is plausible that plants lacking CSE synthesize 5-CQA primarily through a combination of Pathways 1 and 3.
The prevailing view is that the conversion of 5-CQA to its isomers, 3-CQA and 4-CQA, occurs through a non-enzymatic mechanism. In an alkaline environment, 5-CQA undergoes a spontaneous acyl migration to form these isomers [35]. This is supported by the in vitro enzymatic assays of HCT in tea (Camellia sinensis (L.) Kuntze) conducted by Chen et al., who observed that as the pH of the reaction solution increased from 4 to 10, the level of 5-CQA decreased, while the levels of 3-CQA and 4-CQA correspondingly increased [36].
In contrast to the spontaneous acyl migration forming 3-CQA and 4-CQA, the biosynthesis of diCQAs and triCQAs is now increasingly understood to be enzyme-catalyzed. While diCQAs and triCQAs are widely distributed in plants, their biosynthetic mechanisms remain poorly characterized. Moglia et al. demonstrated that in tomato (Solanum lycopersicum L.), HQT not only synthesizes 5-CQA but also catalyzes the condensation of two 5-CQA molecules (acting as acyl acceptor and donor, respectively) to form 3,5-diCQA and quinate. Through heterologous expression in Escherichia coli, they confirmed HQT in vitro capacity for diCQA biosynthesis. The study further proposed compartment-specific HQT functions: vacuolar conditions—characterized by elevated 5-CQA concentrations and lower pH—favor diCQA synthesis [37]. A recent study on sweet potato (I. batatas) revealed that HCT also exhibits dual functionality [38]. However, in vitro assays showed HCT generated only trace amounts of diCQA, insufficient to account for the high diCQA levels observed in planta. Consequently, the researchers identified IbICS, a GDSL hydrolase that similarly catalyzes 3,5-diCQA formation from two 5-CQA substrates, as shown in Figure 3. Notably, IbICS exhibits significantly higher catalytic efficiency than HCT. Its stable expression in Pichia pastoris offers a promising platform for industrial diCQA production.
3.2. Structural Genes Involved
The biosynthesis of 5-CQA is regulated by multiple structural genes. These genes directly encode functional enzymes in the 5-CQA pathway, thereby governing 5-CQA production [9,18,39]. As previously established, CQAs biosynthesis occurs through the phenylpropanoid pathway [18]. This pathway shares close connections with lignin and flavonoid biosynthesis [39].
Phenylalanine ammonia-lyase (PAL), a pivotal enzyme in the plant phenylpropanoid pathway, catalyzes the deamination of phenylalanine to trans-cinnamic acid. This reaction initiates the phenylpropanoid pathway, bridging primary and secondary metabolism [40]. In rice (Oryza sativa L.), PAL-generated trans-cinnamic acid serves as the precursor for flavonoids, lignins, salicylic acid, and other bioactive compounds. PAL silencing reduces insect resistance [41]. Tobacco (Nicotiana tabacum L.) with silenced PALs shows decreased5-CQA and other phenolic compounds, correlating with diminished disease resistance [42]. Furthermore, overexpression of cloned AmPAL from Astragalus membranaceus (Fisch.) Bunge in Nicotiana benthamiana significantly enhanced saline-alkaline soil adaptation compared to wild-type plants [43].
Cinnamate 4-hydroxylase (C4H), a cytochrome P450 monooxygenase (CYP450), catalyzes the second committed step in the phenylpropanoid pathway [44]. This enzyme mediates the hydroxylation of trans-cinnamic acid to p-coumaric acid. As p-coumaric acid serves as a precursor for diverse secondary metabolites, alterations in C4H expression directly modulate flavonoid and lignin biosynthesis, consequently impacting stress resilience [45]. In tea plant (C. sinensis), C4H transcription responds to environmental stressors. Under hormonal, cold, drought, and high-salinity conditions, CsPAL, CsC4H, and Cs4CL genes exhibit tissue-specific expression patterns [44].
4-Coumarate: CoA ligase (4CL) functions as a critical branch point in the phenylpropanoid pathway. This enzyme activates diverse hydroxylated and methoxylated phenylpropanoid derivatives to their corresponding CoA thioesters, including caffeoyl-CoA, p-coumaroyl-CoA, and feruloyl-CoA [46]. In ‘Crown’ plum (Prunus salicina Lindl.), genome-wide analysis identified eight Ps4CL genes. Among these, Ps4CL1, Ps4CL2, Ps4CL7, and Ps4CL8 likely regulate lignin biosynthesis in fruit mesocarp, with all family members containing hormone-responsive cis-elements [47]. Transgenic tobacco expressing a Populus tomentosa Carr. fusion gene 4CL1-CCR exhibited 18.7% higher total G + S lignin content in stems compared to wild-type controls. Notably, S-lignin levels remained unchanged due to unaltered expression of F5H, which mediates G-to-S lignin conversion [48].
In 2019, Jaime Barros et al. identified p-coumaric acid 3-hydroxylase (C3H) as a bifunctional peroxidase that directly hydroxylates p-coumarate to caffeate. The corresponding gene was cloned in B. distachyon and A. thaliana [26]. Their work established C3H as the only non-membrane-bound hydroxylase in lignin biosynthesis, fundamentally revising accepted phenylpropanoid pathway models [26]. Subsequent C3H knockout in B. distachyon by Shrestha et al. significantly altered phenylpropanoid enzyme profiles in stems and disrupted cellular redox homeostasis [49].
Coumaroyl shikimate/quinate 3′-hydroxylase (C3’H) belongs to the cytochrome P450 CYP98 family. As a key rate-limiting enzyme in the phenylpropanoid pathway, CYP98 cooperates with HCT to metabolize p-coumaroyl-CoA—the central precursor for CQAs and flavonoid biosynthesis [39]. C3’H knockout in the Physcomitrium patens significantly reduced transcription of both upstream and downstream structural genes, ultimately diminishing phenylpropanoid end-product accumulation [50]. It is crucial to distinguish between C3H and C3’H, as they are distinct enzymes with different functions and substrate specificities, playing fundamentally different roles in the phenylpropanoid pathway, and the two should not be conflated.
Caffeoyl shikimate esterase (CSE) was first characterized in 2013, leading to a fundamental revision of established lignin biosynthesis model [27]. In P. x hybrida hort. ex Vilm. ‘Mitchell diploid’ (MD), Kim et al. used RNAi-mediated CSE down-regulated (ir-PhCSE) Petunias. These transgenics exhibited reduced stem lignification and growth impairment. And many floral volatiles benzenoid/phenylpropanoid (FVBP) genes were influenced, including elevated transcripts C3H, HCT and 4CL, alongside significantly decreased phenylalanine and caffeate pools [51]. In L. kaempferi, transcriptome-guided cloning identified LkCSE with confirmed catalytic function. This enzyme exhibits strict tissue-specific expression, showing 50-fold higher transcript levels in stems than roots. This represents the first functional characterization of CSE in gymnosperms [30].
Both HCT and HQT belong to the BAHD acyltransferase family, sharing two conserved motifs: “HXXXD” and “DFGWG” [52]. Their substrate specificities differ: HCT preferentially utilizes shikimate, whereas HQT exclusively accepts quinate. Both enzymes activate p-coumaroyl-CoA to form p-coumaroyl quinate (via the “quinate shunt”) or p-coumaroyl shikimate (via the “shikimate shunt”) [9]. Crucially, these enzymes catalyze reversible reactions. For example, in the quinate shunt, HCT regenerates caffeoyl-CoA from caffeoyl shikimate, which HQT then conjugates with quinate to form 5-CQA. Liu et al. [53] cloned TaHQT1 and TaHQT2 from Taraxacum antungense Kitag., with TaHQT1 showing root-predominant expression and TaHQT2 leaf-predominant expression. Knockout lines showed reduced 5-CQA accumulation, while heterologous expression enhanced production, confirming both isoforms’ essential roles in T. antungense 5-CQA biosynthesis.
The inter-relationships among the key genes (PAL, C4H, 4CL, HCT, HQT, etc.), their encoded enzymes, and associated metabolites are summarized in Figure 3, which depicts the integrated biosynthetic and regulatory network leading to CQAs formation.
3.3. Transcriptional Regulation
Transcription factors (TFs) regulate CQAs accumulation by controlling structural gene expression [11,54]. These TFs bind specifically to promoter regions of target genes, activating or repressing their transcription to modulate CQAs levels [11]. The characterization of numerous transcription factor (TF) regulators in the CQAs biosynthesis pathway is summarized in Figure 4.
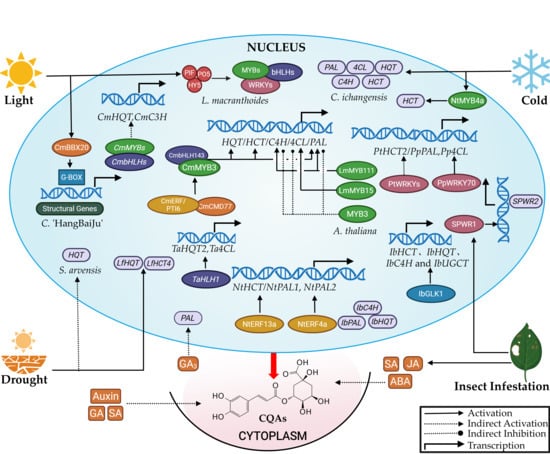
Figure 4.
Regulation of CQAs biosynthesis.
The MYB transcription factor family represents one of the most extensively studied TF groups in plants, playing pivotal roles in growth regulation, secondary metabolism, signal transduction, and biotic/abiotic stress responses. These TFs contain a conserved DNA-binding domain, MYB domain, typically featuring 1–4 tandem imperfect repeats (Rs) at the N-terminus. Based on Rs number and arrangement, MYB TFs are classified into four subfamilies [55]. In C. morifolium ‘HangBaiJu’, Lu et al. [56] identified CmERF/PTI6 and CmCMD77 as upstream modulators of CmMYB3 and CmbHLH143 through integrated metabolomics and transcriptomics. CmMYB3 potentially forms complexes with CmbHLH143 to directly activate structural genes CmPAL1/2, CmCHS1/2, CmFNS, CmHQT and CmHCT, thereby coordinating flavonoid and CQAs biosynthesis. In Lonicera macranthoides, Chen et al. identified an R2R3 MYB transcription factor, LmMYB111, through integrated transcriptomic and metabolomic analysis [11]. They demonstrated that Overexpression of LmMYB111 in both tobacco and honeysuckle resulted in a significant increase in the content of CQAs and luteoloside. Their RNA-seq analysis revealed that this overexpression markedly upregulated the expression of several structural genes, including 10 PALs, 3 C4Hs, 7 4CLs, and 4 HCT/HQTs. Furthermore, through electrophoretic mobility shift assay (EMSA) and dual-luciferase reporter (DLR) assays, they provided evidence that LmMYB111 specifically binds to the promoter regions of LmMYB4, LmPAL1, Lm4CL2, LmCHI, and LmDFR, thereby promoting the excessive accumulation of CQAs, luteoloside, and other phenolic compounds. Previously, this research team confirmed that another R2R3 MYB transcription factor in honeysuckle, LmMYB15, directly binds to the promoter of the key gene 4CL, enhancing CQAs accumulation and boosting phenylpropanoid pathway activity [57]. Luo et al. overexpressed the tobacco-derived NtMYB4a TF. NtMYB4a overexpression upregulated NtPAL and Nt4CL, while knockout downregulated these genes [58]. Interestingly, the nucleus-localized transcription factor MYB3 in A. thaliana has been reported to suppress the excessive accumulation of lignin and anthocyanins under high-salinity stress. When subjected to high salt conditions, myb3 mutant plants accumulated significantly higher levels of lignin and anthocyanins compared to wild-type controls. The expression of related biosynthetic genes, such as PAL1, C4H, and 4CL, was also elevated. These findings indicate that MYB3 acts as a transcriptional repressor under high-salinity stress, downregulating genes associated with the phenylpropanoid pathway, including those involved in CQAs biosynthesis [59]. Guan et al. conducted multi-omics analysis across flowering stages in honeysuckle (L. japonica). MYB, AP2/ERF, and NAC TF families were shown to positively regulate CQAs biosynthesis [60].
The WRKY transcription factor family mediates plant responses to drought, cold, heat, and salinity stresses while regulating signaling networks and structural genes in CQAs biosynthesis [61,62]. Transient overexpression of PtWRKY60, PtWRKY89, PtWRKY93, PtWRKY38, and PtWRKY45 in poplar (P.tomentosa) significantly increased expression levels of PtHCT2, correspondingly elevating 5-CQA accumulation [63]. Wang et al. cloned NtWRKY41a from tobacco, demonstrating that its overexpression enhances CGA and anthocyanin biosynthesis while suppressing scopolin and flavonoid production. Mechanistically, NtWRKY41a activates NtCCoAOMT and NtHST promoters while repressing NtF6’H1 and NtGT3 transcription [64] (Figure 3). In peach (Prunus persica (L.) Batsch), PpWRKY70 binds and activates PpPAL and Pp4CL promoters. Under methyl jasmonate or pathogen induction, this increases total phenolics, flavonoids, and CGA accumulation in fruit [65].
The basic helix-loop-helices (bHLHs) proteins represent one of the largest families of transcription factors found in plants [66]. In T. antungense, the transcription factor TaHLH1 enhances 5-CQA and Cynaroside biosynthesis by directly binding promoters of TaHQT2 and Ta4CL. TaHLH1-overexpressing lines showed increases 5-CQA accumulation, while RNAi-silenced lines exhibited reduction, confirming its regulatory role [67]. Zhao et al. revealed why octoploid Chrysanthemum ‘Gongju’ accumulates more CQAs than tetraploid counterparts through integrated transcriptomics and metabolomics. Elevated CmHQT and CmC3H expression drives enhanced CGA production in octoploids. Co-expression networks identified CmMYB and CmbHLH as key regulators of CmHQT and CmC3H [68]. Vaccinium dunalianum Wight shows developmentally regulated CQAs accumulation, Leaf CQAs accumulation varied significantly across developmental stages. Zhang et al. identified 15 core structural genes: four PAL, two C4H, four 4CL, two HCT, and three C3H homologs—the first comprehensive characterization in this species. Three putative regulators were also discovered: two bHLH and one WRKY transcription factors. This provides novel insights into CQAs regulatory networks [69].
The GARP (Golden2, ARR-B, Psr1) family constitutes a group of plant-specific transcription factors that regulate a diverse array of physiological processes [70]. Luo et al. profiled CQAs across 16 sweet potatoes (I. batatas) genotypes, establishing correlations between CQAs accumulation and antioxidant capacity, and Comparative transcriptomics identified IbGLK1, a GARP-family TF. Yeast one-hybrid and dual-luciferase assays confirmed IbGLK1 directly activates promoters of IbHCT, IbHQT, IbC4H, and IbUGCT, thereby enhancing CQAs biosynthesis [11].
AP2/ERF transcription factors play multifaceted roles in regulating the biosynthesis of various specialized metabolites in response to diverse environmental stresses [71]. Wang et al. identified the NtERF gene, a member of the ERF family’s IXa subfamily, within the tobacco genome [71]. Their research demonstrated that NtERF13a specifically binds to GCC-box or DRE element segments in the promoters of the NtHCT, NtF3’H, and NtANS genes [72]. This binding activates their transcription and promotes the biosynthesis of CQAs, flavonoids, and lignin, this regulatory model is summarized in Figure 4. Furthermore, He et al. identified two NtERF genes involved in the biosynthesis of CQAs and flavonoids in tobacco. Overexpression of NtERF4a significantly enhanced the accumulation of CQAs and flavonoids in tobacco leaves, whereas its silencing had the opposite effect. Chromatin immunoprecipitation and dual-luciferase reporter assays demonstrated that NtERF4a directly binds to the GCC-box element in the promoters of NtPAL1 and NtPAL2, thereby activating their expression.
3.4. Hormonal Regulation
Phytohormones serve as essential endogenous signaling molecules within plant transduction networks, modulating both developmental processes and stress responses, as overviewed in Figure 4 [73]. Salicylic acid (SA)—critically associated with biotic/abiotic stress resistance—demonstrates reciprocal interaction with CQAs. Studies in I. batatas and Z. mays indicate CQAs-mediated SA pathway activation enhances stress tolerance [74,75]. Notably in I. batatas, exogenous application of GA, SA, or ABA to shoot apices consistently elevated CQAs accumulation. Hormone-responsive cis-elements were identified in promoters of multiple CGA biosynthetic genes, confirming tight hormonal coordination of this pathway [76].
Gibberellins (GAs) serve as crucial regulators of plant growth and are increasingly recognized as modulators of CQAs biosynthesis. In Echinacea purpurea (L.) Moench, GA3 application at varying concentrations differentially influenced hairy root growth and metabolite profiles. Optimal GA3 concentrations significantly enhanced 5-CQA accumulation while upregulating PAL gene expression [77]. Similarly in Fagopyrum esculentum Moench, combined GA and IAA treatments maximized production of 5-CQA, caffeic acid, and flavonoids [78]. Sharaf et al. examined GA3 effects on CQAs distribution in Cynara scolymus L., applying treatments to leaves and monitoring receptacle tissues. Transplanted seedlings with controlled development showed substantially increased foliar CQAs following GA3 application. However, receptacle CQA consistently decreased across treatments. GA3 likely redirects phenolic allocation in artichoke, reducing CQA accumulation in receptacles [79].
The role of auxins (IAA) in plant growth regulation is well-established [80]. Emerging evidence indicates auxins significantly influence CQAs biosynthesis. In C. morifolium, Ghimire et al. observed concentration-dependent increases in CQAs accumulation following NAA and IAA treatments, though IBA showed no significant effect [81]. Wang et al. overexpressed MdIAA24, a key auxin biosynthesis gene, in Malus domestica Borkh. Following Glomerella leaf spot pathogen infection, these transgenics exhibited substantially enhanced CQAs accumulation compared to wild-type plants [82].
3.5. Environmental Regulation
Temperature, light, drought, and herbivory differentially regulate CQAs biosynthesis in plants. Under suboptimal growth conditions, plants activate physiological adaptations to mitigate environmental stressors. Secondary metabolites including CQAs and flavonoids serve as critical components of these adaptive metabolic responses [83]. Their specific roles and relationships within this network are summarized in Figure 4.
Light serves dual roles in plants: as the primary energy source for growth and as a key developmental signaling cue. Variations in light intensity, spectral quality, and photoperiod significantly modulate secondary metabolite biosynthesis [84]. In chrysanthemum ‘HangBaiJu’, Lu et al. employed integrated transcriptomic and metabolomic analyses to identify key regulators of flavonoid and CQAs biosynthesis, namely CmMYB3/6/16 and CmBBX20/22.2/22.3. Subsequent transient overexpression assays and Y1H experiments confirmed that CmBBX20 directly binds to the G-Box cis-element in the promoters of downstream target genes, thereby promoting the accumulation of flavonoids and CQAs [85]. Reduced light intensity in Marsdenia tenacissima (Roxb.) Moon suppresses CQAs biosynthesis and downregulates associated pathway genes [86]. Similar light-dependency occurs in L. japonica, where shading treatments substantially decrease foliar 5-CQA accumulation. A co-expression network integrates 5-CQA biosynthetic genes with carbohydrate metabolism, photosynthetic components, light-signaling elements, and transcriptional regulators [87]. Beyond intensity, light spectral quality profoundly influences 5-CQA biosynthesis across species. Blue light upregulates FvHCT expression and enhances 5-CQA production in strawberry (Fragaria × ananassa (Weston) Duchesne ex Rozier). Different light spectra exert multifaceted physiological effects in strawberry fruits [88]. Continuous blue light combined with elevated CO2 increases 5-CQA accumulation in lettuce (Lactuca sativa L.) by activating PAL and downstream enzymes [89]. Blue light coupled with low temperatures maximizes 5-CQA production in Berula erecta (Huds.) Coville [90]. During potato tubers (Solanum tuberosum L.) storage, sodium-vapor and fluorescent lighting enhance 5-CQA accumulation more effectively than high-pressure mercury lamps. Light exposure also induces metabolic conversion of 5-CQA to 3-CQA or 4-CQA isomers [91].
Cold stress significantly impacts plant growth and development [92]. In Citrus ichangensis Swingle, cold exposure upregulates PAL, C4H, 4CL, and HCT/HQT expression, enhancing 5-CQA accumulation. This cold-induced CGA confers frost tolerance through ROS scavenging [15]. Xie et al. identified NtMYB4a as a cold-responsive transcription factor regulating CQAs biosynthesis in tobacco. At ambient temperatures, NtMYB4a-overexpressing and wild-type lines showed comparable CQAs levels. Following cold treatment, transgenics exhibited substantially elevated CQAs accumulation, 4CL/HCT expression, and enzyme activity versus wild type. p-Coumaroyl quinate levels remained similar in wild-type and knockout lines at normal temperatures. Cold exposure increased p-coumaroyl quinate in wild-type but decreased it in knockouts. Overexpressors maintained elevated p-coumaroyl quinate across all conditions. NtMYB4a activates HCT transcription under cold stress, enhancing p-coumaroyl quinate production to boost CQAs biosynthesis [93]. Sim et al. examined phenolic profiles in Aster × chusanensis Ling cultivated at varying temperatures. Optimal elevated temperatures maximized foliar CQAs accumulation, the dominant phenolic acid [94]. Goławska et al. compared cultivated and wild Viburnum dilatatum Thunb., revealing temperature-dependent variations in phenolic/flavonoid profiles. Thermal regimes profoundly influence CQAs biosynthesis across ecotypes [95].
Drought stress is a climatic phenomenon characterized by seasonal occurrence that severely affects crop yields [96]. In field sowthistle (Sonchus arvensis L.), drought stress notably induced the upregulation of genes involved in the CQAs biosynthesis pathway. Under conditions of 50% field capacity, the concentrations of chicoric acid, 5-CQA, and 2,5-dihydroxybenzoic acid reached their highest levels. A strong association was observed between HQT expression and 5-CQA content, indicating that the HQT-dependent mediated pathway serves as the primary route for CQAs in S. arvensis [97]. Khan et al. observed divergent CGA responses in rice (O. sativa) under drought: cultivar PR-115 showed markedly enhanced accumulation, while Super-7 exhibited substantial reduction [98]. Amaranthus L. progressively increased phenolic acids including CQAs with intensifying drought severity [99]. Contrastingly, drought reduced both CQAs and flavonoids in Ligularia fischeri (Ledeb.) Turcz. Downregulation of LfHCT and LfHQT4 directly diminished CQA production, consistent with HCTs established role in Asteraceae CQAs biosynthesis [100]. Ghafari et al. applied partial root-zone drying (PRD) to M. domestica, comparing alternating (APRD) and fixed (FPRD) drying regimes. At 75% crop evapotranspiration (ETc), both PRD methods substantially elevated 5-CQA, caffeate, and catechin accumulation without compromising yield. PRD likely enhances water-use efficiency by activating antioxidant systems, hormone signaling, and drought-responsive transcription factors [101].
Insect infestations severely impact crop yields. Recent studies have reported that insect herbivory can induce the accumulation of CQAs [75,102,103]. In rice (O. sativa), the white-backed planthopper (WBPH, Sogatella furcifera (Horváth, 1899)) is a major migratory pest. Xie et al. conducted integrated transcriptomic and metabolomic analyses of a susceptible cultivar (TN1) and a resistant cultivar (KL35). They identified CQAs as key compounds in rice resistance to WBPH, with significantly higher levels in KL35 than in TN1, both before and after WBPH infestation. The study also pinpointed two critical resistance-related genes, 4CH and C4L, hypothesizing that they are key genes in the CQAs biosynthetic pathway of the highly resistant cultivar KL35 [102]. In sweet potato, the sweetpotato weevil (SPW, Cylas formicarius (Fabricius, 1798)) is one of the most destructive pests. Hou et al. observed that SPW feeding induced the accumulation of CQAs in sweet potato leaves. By investigating the associated hormonal networks and key genes, they demonstrated that SPW herbivory stimulates the production of jasmonic acid, salicylic acid, and abscisic acid. These hormones upregulate key CQA biosynthetic genes, including IbPAL, IbC4H, and IbHQT, thereby enhancing CQAs synthesis. Subsequently, the same team identified two major SPW resistance genes, SPWR1 and SPWR2, from two SPW-resistant sweet potato germplasms. Their research showed that SPW feeding induces the WRKY transcription factor SPWR1, which directly activates the expression of SPWR2. SPWR2, in turn, promotes the accumulation of CQAs and other resistance-related compounds in sweet potato [75,103].
3.6. Metabolic Engineering via Synthetic Biology Strategies
The elucidation of the CQAs biosynthetic pathway has laid a foundation for employing synthetic biology strategies to reconstruct and optimize this pathway in microbial chassis, aiming for the sustainable and scalable production of these high-value compounds. Recent studies have demonstrated significant progress in engineering efficient cellular factories, particularly in E. coli and Saccharomyces cerevisiae. In E. coli, Wang et al. established a de novo pathway from simple carbon sources to CQAs [104]. By crucially enhancing the activities of the cofactor-regenerating enzymes quinate/shikimate 5-dehydrogenase (YdiB) and 4-hydroxyphenylacetate 3-monooxygenase (HpaBC), they achieved a high CQAs titer of 2789.2 mg/L in a 5 L bioreactor. Similarly, Zhang et al. integrated a tyrosine ammonia-lyase (TAL) from Rhodococcus taiwanensis and implemented a multi-level metabolic engineering strategy, constructing the engineered strain CGA13 capable of producing CQAs solely from glucose, with a final titer of 1.53 g/L [105]. To further improve pathway efficiency, Hu et al. applied semi-rational design to the key acyltransferase HQT, significantly enhancing its selectivity for caffeoyl-CoA and p-coumaroyl-CoA, thereby effectively reducing byproduct formation [106]. In the eukaryotic chassis S. cerevisiae, consecutive studies by Zhong et al. realized de novo CQAs synthesis [107,108]. Their strategy involved introducing a TAL from Rhodotorula glutinis (RgTAL), deleting HAM1 and YJL028W to elevate intracellular pools of the precursor coumaric acid, and employing fusion expression of AtC4H and At4CL1 alongside co-expression of CsHQT and AtC3’H. Furthermore, they optimized the P450 enzyme microenvironment by overexpressing the lipid biosynthesis transcription factor INO2 and deleting the heme oxygenase gene HMX1. Through comprehensive fermentation optimization, the CQAs titer was dramatically increased from 234.8 mg/L to 837.2 mg/L in shake flasks. These successful cases signify a transition from proof-of-concept to process development for the synthetic biological production of CQAs, highlighting the immense potential of systematic pathway design, enzyme engineering, and host metabolic network remodeling to achieve efficient and sustainable bio-manufacturing.
4. Bioactivity of CQAs
4.1. In Pharmacological
Compelling evidence from numerous studies demonstrates that CQAs exhibits a broad spectrum of pharmacological activities. These include anti-inflammatory, antimicrobial, antiviral, and anticancer effects, as well as protective roles in the nervous and cardiovascular systems, antidiabetic properties, and hepato-renal protection. Consistent findings indicate that CQAs are a class of polyphenolic compounds with multi-faceted bioactivity. These diverse and potent pharmacological properties have garnered significant and growing research interest in recent years.
Regarding anti-inflammatory effects, Lee et al. demonstrated that administering 5-CQA to STZ-induced diabetic rats significantly reduced cytokine levels, CRP, and CYP1A enzyme activity in a dose-dependent manner. This suggests a potential therapeutic strategy for managing inflammation in diabetes [109]. In an LPS-induced immune stress model in broilers, Tan et al. found that dietary supplementation with 5-CQA alleviated intestinal barrier damage and inflammation. The mechanism involved suppressing pro-inflammatory cytokines, TNF-α and IL-1β, promoting the anti-inflammatory cytokine IL-10, improving intestinal villus morphology, and enhancing the expression of the tight junction protein Claudin-2 in the ileum. Consequently, this led to improved growth performance [110]. In their study of LPS-induced inflammation and oxidative stress in RAW264.7 cells, Han et al. demonstrated that the isomers 5-CQA, 3-CQA, and 4-CQA entered cells via transmembrane transport and differentially inhibited the AKR1B1 enzyme, with potency depending on their hydrogen-bonding sites. Among them, 3-CQA exhibited the strongest AKR1B1 inhibitory effect, which correlated with its superior suppression of pro-inflammatory cytokines [111]. This structure-activity relationship and underlying mechanism are delineated in Figure 5. Jiao et al. treated a mouse model of renal fibrosis with varying concentrations of 5-CQA. Their findings indicate that 5-CQA likely alleviates kidney injury, inflammation, oxidative stress, and fibrosis by modulating the TLR4/NF-κB signaling pathway. This treatment also suppressed the expression of inflammatory factors and oxidative stress in both in vivo and in vitro fibrotic models [112]. Similarly, Xu et al. confirmed in a mouse study that 5-CQA inhibits cellular inflammation by downregulating the expression of TLR4, p-p38, p-p65, and p-IκBα. The anti-inflammatory effect was negated by TLR4 overexpression, which increased inflammatory cytokine levels. This suggests 5-CQA has potential as an anti-inflammatory agent in RAW264.7 cells and allergic rhinitis by regulating the TLR4/MAPK/NF-κB pathway [113]. Wang et al. used a chronic stress model in rats to show that 5-CQA treatment reduced hepatic inflammatory cytokine levels and NF-κB pathway activation, while increasing Resolvin D1 (RvD1) in the serum and liver [114]. They further found that the therapeutic effect was blocked by the inhibitor WRW4, indicating that 5-CQA protective effects are mediated by upregulating RvD1 to suppress the NF-κB pathway. Their work highlights the potential treatment value of 5-CQA for chronic inflammatory diseases. A recent study elucidated the anti-inflammatory mechanism and protein target of dihydrocaffeic acid (DA), a metabolite of 5-CQA, in acute pneumonia. Experiments in in vivo and in vitro inflammatory models confirmed DA’s anti-inflammatory effects. Using a designed DA molecular probe (DA-P) and activity-based protein profiling (ABPP), the protein target was identified as transaldolase 1 (TALDO1). Further analysis proposed that DA interacts with TALDO1 to regulate the PERK-IκBα-NF-κB signaling pathway, thereby inhibiting inflammation [5].
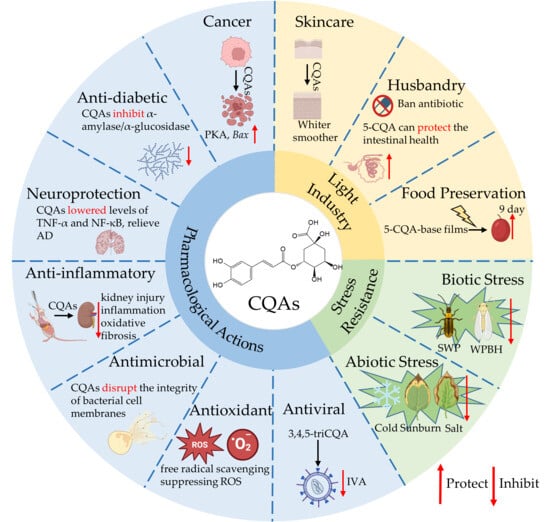
Figure 5.
Bioactivity of CQAs. Abbreviations: PKA, protein kinase A; Bax, Bcl-2-associated x; TNF-α tumor necrosis factor-alpha; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; AD, Alzheimer’s disease; ROS reactive oxygen species; IAV, influenza A virus; WPBH, white-backed planthopper; SWP, sweet potato weevil; •O2− Superoxide anion.
Regarding antioxidant properties, Xu et al. esterified 5-CQA with C18 fatty acids of varying unsaturation (C18:0, C18:1, C18:2, C18:3). They found that the liposolubility of the resulting derivatives decreased as the fatty acid unsaturation increased. Notably, all derivatives exhibited excellent oxidative stability, indicating their potential as highly effective antioxidants in lipid-based food systems [115]. Cejas et al. investigated the role of cholesterol in the protective effect of 5-CQA on model plasma membranes and its antioxidant capacity against lipid peroxidation. Their results showed that 5-CQA delayed lipid peroxidation and preserved the integrity of lipid vesicles [6]. Caruso et al. measured the superoxide antioxidant ability of caffeic and CQAs in several tea-based beverages. Both the plant extracts and the pure compounds exhibited strong antioxidant activity, almost completely eliminating superoxide radicals. The study also elucidated their mechanisms: although caffeic acid and CQAs scavenge radicals differently, both ultimately produce hydrogen peroxide (H2O2) and an [X-acid–η–O2], X = caffeic, 5-CQA, in an acidic environment with sufficient superoxide [116]. Similarly, using bioassay-guided analysis, Gloria et al. identified 5-CQA and 1-CQA as a major component in Avocado (Persea americana Mill.) Fruit Peel, responsible for its significant bioactivities, including free radical scavenging, suppressing ROS and lipid peroxidation, inhibiting nitric oxide production, and modulating antioxidant enzyme activities [117]. Furthermore, their Density Functional Theory analysis suggests that the radical scavenging mechanism of 5-CQA likely involves electron transfer, followed by proton transfer processes, primarily occurring at the 3’OH and 4’OH. This mechanism is schematized in Figure 5.
In neuroprotection, Kang et al. demonstrated that 5-CQA exerts potent antioxidant and neuroprotective effects in a rat model of cerebral artery occlusion. They proposed that it prevents neuronal death after cerebral ischemia by maintaining the binding of thioredoxin (Trx) to ASK1. Under excessive glutamate conditions, 5-CQA mitigated neurotoxicity and reduced ischemia-induced damage by modulating Trx to influence the balance of anti- and pro-apoptotic proteins. In a separate study the same year, Kang et al. also found that 5-CQA protects neurons by regulating the PP2A subunit B. Together, these studies highlight the compound’s potential as a neuroprotective agent [7,118]. In a study using a mouse model of cognitive impairment, Mosalam et al. investigated the combination of the PDE4 inhibitor cilomilast (CILO) with 5-CQA as a potential therapy for Alzheimer’s disease. They discovered that the combination activated the cAMP/PKA/CREB/BDNF signaling pathway, reduced neuronal damage, and lowered levels of neuroinflammatory markers, including TNF-α and NF-κB, demonstrating superior efficacy to individual treatments [119]. This synergistic mechanism and the key findings are summarized in Figure 5. In the same year, Mirzaei et al. also reviewed the protective effects of coffee components, including CQAs, against various neurodegenerative diseases like AD. They noted that these compounds typically mediate neuroprotection by modulating key signaling pathways and inflammatory mediators, such as NF-κB, Nrf2, interleukins, and TNF-α [120]. Furthermore, a study showed that 5-CQA acts as a caloric restriction mimetic. In human neuroblastoma SH-SY5Y cells, it reversed oxidative stress induced by amyloid-β peptide, restored the activity of Na+, K+-ATPase and Ca2+-ATPase pumps, and inhibited the overactivation of acetylcholinesterase. This multi-target synergistic action underpins its neuroprotective effect, positioning 5-CQA as a potential candidate for AD intervention strategies [121].
In cancer therapy, Huang et al. discovered that 5-CQA promotes the expression of p21 by downregulating miR-17 through the SUMOylation of c-Myc. This mechanism induces cancer cell differentiation and attenuates their malignant behavior. There in vivo experiments confirmed that 5-CQA treatment halted tumor growth. Notably, even at very high doses, no toxicity was observed [122]. Furthermore, research revealed that 5-CQA significantly enhances β-lapachone-induced Protein Kinase A activation in cancer cells, leading to cell death [123]. This proposed synergistic mechanism is illustrated in Figure 5. Concurrently, 5-CQA compounds present in mulberry (Morus alba L.) extract were shown to selectively inhibit the growth of MCF-7 breast cancer cells and exhibit strong free radical scavenging activity against DPPH radicals [124]. Moreover, Wang et al. identified mitochondrial Acetyl-CoA acetyltransferase 1 (ACAT1) as a direct target of 5-CQA for inhibiting tumor cell growth. Their study elucidated that 5-CQA, via non-covalent binding, inhibits phosphorylation at the Y407 site of ACAT1. This disrupts its role in regulating tumor cell metabolism and suppresses cancer cell proliferation, providing a mechanistic foundation for developing natural targeted anticancer drugs and the clinical application of 5-CQA [125]. 5-CQA as a cancer differentiation inducer, downregulates PD-L1 expression in tumor cells by inhibiting the STAT1/IRF1 pathway. This enhances the efficacy of anti-PD-1 antibodies, promotes the infiltration of CD8+ T cells into the tumor microenvironment, and augments granzyme B-mediated cytotoxicity. This suggests a novel combinatorial strategy to overcome the low response rates to immune checkpoint inhibitors [2]. Similarly, studies report that 5-CQA and gallic acid can effectively bind to the critical Thr308 phosphorylation site of AKT1. This binding inhibits the proliferation, migration, and invasion of liver cancer cells and promotes apoptosis [126]. Meanwhile, 5-CQA, a key active compound in purple carrot extract (PCE), demonstrates a selective inhibitory effect on MDA-MB-231 triple-negative breast cancer cells. It upregulates the expression of pro-apoptotic genes like Bcl-2-associated x(Bax), revealing its specific suppressive action against breast cancer [127].
In a study on antimicrobial applications, Fu et al. developed supramolecular nan particles from berberine and 5-CQA [4]. They found that these nanoparticles exhibited significantly stronger inhibitory effects against Staphylococcus aureus and methicillin-resistant S. aureus than ampicillin, oxacillin, BBR, 5-CQA, or a simple mixture of BBR and 5-CQA. Furthermore, in a mouse model of wound infection, the nanoparticles markedly promoted wound healing. Furthermore, a study by Kang et al. demonstrated that the bactericidal effect of 5-CQA combined with ultraviolet-A (365 nm) was significantly greater than either treatment alone [128]. Their results indicated that the primary mechanism involves disruption of the bacterial cell membrane, leading to the leakage of intracellular contents and DNA degradation. The combination also reduced succinate-coenzyme Q reductase activity by approximately 72%, enhanced bacterial sensitivity to antibiotics, and inhibited the spread of drug resistance. Based on these findings from this research, we have summarized the proposed mechanism of action in Figure 5. Han et al. reported that 0.5 mg/mL 5-CQA acts as an effective antimicrobial agent against both E. coli and S. aureus when activated by light [129]. By incorporating 5-CQA as a photosensitive antibacterial agent into an agar film, they extended the shelf life of cherries by 9 days, demonstrating its potential for light-based preservation technologies. Similarly, Yang et al. successfully synthesized chitosan-grafted-chlorogenic acid (CS-g-CA) using a carbodiimide method. They confirmed that CS-g-CA significantly inhibits S. aureus growth, with a minimum inhibitory concentration of 0.625 mg/mL. The compound exerts its effects by disrupting cell membrane integrity, inhibiting antioxidant enzyme activity, interfering with DNA metabolism, and reducing extracellular polymeric substance production [130]. Beyond S. aureus, 5-CQA has demonstrated antimicrobial effects against other pathogens. For instance, Liu et al. found that a combined treatment of low-dose 5-CQA and p-coumaric acid drastically increased cell permeability and inhibited biofilm formation in Shigella. This synergistic action significantly inactivated Shigella and, when applied to fresh-cut tomatoes, showed efficacy comparable to chemical disinfectants, supporting the development of potent, low-dose natural preservatives [131]. In the context of COVID-19-associated mucormycosis (CAM), research indicates that 5-CQA and quercetin, derived from the ethanol extract of Thymus vulgaris subsp., showed high binding affinity for key CAM-related proteins, namely EGFR and GRP78. Using an integrated approach of HPLC, molecular docking, ADME analysis, and molecular dynamics simulations, the study proposed 5-CQA and quercetin as promising anti-CAM drug candidates. However, this conclusion requires further validation through in vitro and in vivo experiments to confirm their clinical safety and efficacy [132]. Jiang et al. also reported that mixing or spraying with a 5-CQA solution produces significant antimicrobial effects against S. aureus, E. coli, Candida albicans, and Streptococcus pneumoniae [133].
Regarding antiviral activity, Wang et al. isolated eight CQAs from Ilex pubescens Hook. & Arn leaf extract, all of which exhibited anti-influenza virus activity as neuraminidase (NA) inhibitors [3]. The most potent compound, 3,4,5-triCQA, demonstrated significantly higher inhibition than diCQAs. Its binding site on NA is suggested to be distinct from existing drugs like oseltamivir, marking it as a promising lead for a new anti-influenza class [3]. In a subsequent study, the same group delved into its cellular mechanism, revealing that 3,4,5-triCQA suppresses influenza A virus (IAV)-induced inflammation in host cells through the TLR3/7 signaling pathway [134]. This specific anti-inflammatory mechanism is schematized in Figure 5. Yang et al. reported that 5-CQA inhibits the proliferation of duck enteritis virus (DEV) in duck embryo fibroblast (DEF) cells by modulating the NF-κB signaling pathway. This finding suggests a potential strategy for preventing DEV infection and mitigating the associated economic losses in poultry production [135]. Against porcine deltacoronavirus (PDCoV), 5-CQA effectively suppressed viral self-replication, reducing the synthesis of viral RNA and proteins. It also significantly diminished apoptosis in PDCoV-infected cells and inhibited the release of a progeny virus. As a potent anti-PDCoV agent, 5-CQA provides a valuable reference for the development of related therapeutics [136].
In anti-diabetic research, studies utilizing rat models have demonstrated that 5-CQA effectively manages diabetes and its complications. As summarized in Figure 5, The proposed mechanisms include the inhibition of α-amylase/α-glucosidase, activation of the AMPK pathway, improvement of lipid metabolism, and exertion of antioxidant effects [137]. Furthermore, a recent multidisciplinary study integrating enzyme kinetics, spectroscopy, molecular simulations, and cell models elucidated the mechanism of action. It confirmed that 5-CQA binds to the active pocket of α-glucosidase through a mixed inhibition mechanism involving hydrogen bonding and hydrophobic interactions. This binding induces a conformational change in the enzyme, leading to its inactivation. Using the human liver cell line HepG2 to model glucose metabolism, the study also confirmed that 5-CQA significantly promotes glucose consumption at non-cytotoxic concentrations [138]. Additionally, the flower extract of Sambucus nigra L., rich in phenolic acids such as 5-CQA, has been investigated. Extracts from various elder cultivars demonstrated significant inhibition of α-glucosidase. A strong correlation was observed between high 5-CQA content and the extract’s potent enzyme inhibitory and antioxidant activities, which are crucial for its glucose-regulating effects. This finding provides a valuable reference for developing natural anti-diabetic therapeutics [139]. Similarly, in studies on Cirsium setosum (Willd.) MB., Zhou et al. found that among five different extracts, the ethyl acetate (EA) fraction exhibited the most potent antioxidant and anti-diabetic activities. 5-CQA derivatives and flavonoid glucosides were identified as the primary bioactive constituents. The anti-diabetic effect was primarily attributed to α-glucosidase inhibition, while the antioxidant activity was linked to free radical scavenging [140]. The extract of Artemisia afra Jacq. ex Willd. was shown to possess in vitro anti-diabetic activity via an α-glucosidase inhibition bioassay. The main bioactive compounds identified were 1,5-diCQA and 3,5-diCQA. Notably, the α-glucosidase inhibitory activity of these compounds surpassed that of acarbose, a standard anti-diabetic drug, providing strong support for the development of natural therapies for diabetes [141].
4.2. In Light Industry
Light industry encompasses the production of consumer goods, including food, textiles, leather, paper, and household chemicals. Within these sectors, the application of CQAs has been documented in several areas, most notably in animal husbandry, skin-whitening cosmetics, and food preservation.
In husbandry, the comprehensive ban on antibiotic use in China has spurred significant interest in natural plant extracts, such as CQAs, as potential alternatives. Consequently, research in this area has proliferated in recent years. In a study by Wei et al., dietary supplementation with 5-CQA in weaned piglets significantly enhanced serum concentrations of total protein, albumin, and total cholesterol compared to the control group. Furthermore, it increased the activity of alkaline phosphatase and superoxide dismutase in the ileal mucosa. Notably, 5-CQA also upregulated the expression of key genes involved in intestinal barrier function and antioxidant defense, including ZO-1, SLC7A, and Nrf2, in the duodenum [142]. Zhang et al. demonstrated that 5-CQA alleviates oxidative stress and intestinal inflammation in weaned piglets by suppressing the TLR4/NF-κB signaling pathway and concurrently activating the Nrf2 antioxidant pathway [143]. These findings indicate that 5-CQA enhances intestinal function and promotes growth performance, thereby providing a mechanistic foundation for the development of natural feed additives. The interplay between these two pathways is delineated in Figure 5. Furthermore, 5-CQA has been evaluated as a dietary supplement in broiler chickens. Research indicates that under high-density (HD) stocking stress, 5-CQA supplementation significantly ameliorates intestinal impairment by enhancing serum antioxidant capacity and improving jejunal villus development. These collective effects contribute to the restoration of intestinal barrier integrity [10]. Beyond its role in gut health, emerging research indicates that 5-CQA also protects against cadmium-induced neurotoxicity in chicken cerebral cortical neurons. This neuroprotective effect is mediated through the inhibition of the AMPK-ULK1 signaling pathway, as demonstrated in both in vivo and in vitro models [144]. In laying hens, dietary supplementation with 5-CQA at 250 mg/kg significantly improved key egg quality parameters, including eggshell thickness, egg weight, yolk color intensity, and Haugh units [145].
In the food preservation industry, CQAs have been reported to inhibit the proliferation of Salmonella Enteritidis in chilled chicken, thereby extending its shelf life by preventing microbial spoilage [146]. For cherry (Prunus avium L.), a representative of perishable foods, coating with a 5-CQA-based agar composite film, when activated by light irradiation, extended the postharvest storage period by 9 days [129]. This shelf-life extension is primarily attributed to the light-activated antimicrobial efficacy of the film, which generates ROS upon illumination to effectively inhibit microbial growth (Figure 5). Incorporating 5-CQA into a starch-whey protein-based packaging material significantly enhanced its antioxidant, UV-blocking, and antibacterial properties. In experiments with fresh bananas (Musa acuminata Colla), this 5-CQA-enriched packaging effectively suppressed browning [147]. When embedded into nanofibers for food packaging, 5-CQA demonstrated potent antioxidant activity and favorable release kinetics, highlighting its potential as a natural, non-toxic additive [148]. Furthermore, treatment with 5-CQA reduced the respiration rate and slowed the decline of soluble solids, firmness, and ascorbic acid content in ‘Lvbao melons’ during storage. A concentration of 30 mg/L was identified as the most effective, outperforming both 10 mg/L and 50 mg/L doses [149]. 5-CQA exhibits excellent inhibitory effects against gray mold (Botrytis cinerea). A zein-chitosan-chlorogenic acid (ZCC) nanoparticle delivery system was developed to leverage this property. The ZCC nanoparticles effectively controlled B. cinerea growth on grapes and kiwifruit. Separately, 5-CQA treatment delayed ripening and senescence in peach (P. persica) fruit, enhancing disease resistance and extending its storage life [150]. Song et al. developed an immobilized lipase (CSL@OMS-C18) for the enzymatic synthesis of acylated chlorogenic acid derivatives (ACDs). The immobilized enzyme exhibited excellent conversion efficiency, catalytic stability, and reusability. The study also evaluated the bioactivity of the resulting ACDs, providing a foundation for the sustainable enzymatic production of liposoluble antioxidant compounds for food applications [151]. Zhang et al. found that a composite antioxidant (300 ppm tocopherol + 200 ppm 5-CQA) effectively suppressed oxidation in algal oil emulsions. The mechanism involves 5-CQA facilitating the regeneration of tocopherol and modulating its distribution at the oil-water interface. This composite antioxidant did not compromise the physical stability of the emulsion, thereby supporting the bioaccessibility of the algal oil [152].
Within the skincare industry, several recent reports have highlighted the potential of CQAs. For instance, an anti-pollution film-forming spray (FFS), developed from coffee cherry flesh extract (FFS-CCS) whose key active constituents include 5-CQA, was clinically demonstrated to significantly improve skin hydration, reduce wrinkle appearance, and enhance skin tone, indicating its anti-aging efficacy [153] (Figure 5). Ruscinc et al. evaluated the protective effects of various polyphenols, including 5-CQA, against ultraviolet -induced lipid peroxidation in the stratum corneum. Their findings confirmed that 5-CQA possesses notable antioxidant and anti-inflammatory capacities. However, it is noteworthy that 5-CQA also exhibited pro-oxidant properties under specific conditions. This study underscores the multifaceted and context-dependent roles that polyphenols like 5-CQA play in skin protection [14].
4.3. In Plant Stress Resistance
Throughout their life cycle, plants are exposed to various biotic and abiotic stresses—including low temperature, salinity, drought, and pathogen infection—which can severely inhibit growth [83,154]. In response, plants have evolved sophisticated defense mechanisms to mitigate these adverse effects [154]. These adaptive strategies often involve the orchestrated accumulation of protective metabolites, regulated through complex signal transduction pathways [154,155,156]. Notably, recent studies have frequently reported that CQAs accumulate under stress conditions, enhancing the plant’s ability to resist such adversities [15,102,157].
Under cold stress, Xiao et al. investigated the molecular mechanisms of cold tolerance by comparing two citrus species with markedly different sensitivities [15]. Their metabolomic analysis revealed that 5-CQA and sphinganine specifically accumulated in the cold-hardy citrus species Ichang papeda (C. ichangensis) during cold stress, a phenomenon absent in the cold-sensitive HB pummelo (Citrus grandis (L.) Osbeck “Hirado Buntan”). Subsequent genes, CiSPT and CiHCT2, knockout and exogenous application experiments confirmed that these compounds are crucial for the cold resistance of Ichang papeda. The study concluded that 5-CQA functions as an antioxidant, directly scavenges H2O2 and O2− to mitigate cold-induced cellular damage. They also noted that high accumulation of 5-CQA under cold stress has also been documented in the related species Poncirus trifoliata (L.) Raf. Based on this mechanistic understanding from [15], we have summarized the proposed cold tolerance pathway in Figure 5. In tomatoes (S. lycopersicum), which are susceptible to chilling injury during low-temperature storage, a study demonstrated that treatment with 50 mg/L 5-CQA effectively alleviated chilling injury and reduced weight loss. This treatment also improved cell membrane integrity and lessened oxidative damage, resulting in a significant 30–40% reduction in the incidence of chilling injury [157]. Interestingly, phenolic compounds such as CQAs exhibit a context-dependent dual role in plant physiology. In intact cells, CQAs are stored in the vacuole, while polyphenol oxidase (PPO) is localized in the cytoplasm, plastids, plasma membrane, and mitochondria [158,159]. Upon mechanical damage, phenol-enzyme regional distribution is disrupted, and the protective role of CQAs can reverse and mix with Polyphenol oxidase (PPO); CQAs act as direct substrates for PPO-catalyzed oxidation into highly reactive o-quinones [160,161]. These quinones subsequently undergo non-enzymatic polymerization into dark melanoidins, thereby accelerating browning [159,161]. For instance, contrary to its protective role in other species, CQAs, along with catechin, has been reported to accelerate the browning reaction in fresh-cut potatoes [162]. Conversely, another study on fresh-cut potatoes reported that exogenous application of CQAs delayed browning, reduced PPO activity, and inhibited quality deterioration in potato slices [163]. This seemingly paradoxical finding underscores the complex interactions among phenolic compounds, cellular integrity, and enzymatic activity, highlighting the profoundly context-dependent dual role of CQA. Understanding this duality is essential for comprehending the complex roles of plant phenolics. Furthermore, in Mango (Mangifera indica L.), Perveen et al. used phenolic acid profiling to show that salt stress triggered the significant accumulation of hydroxybenzoic acids, including a 510% increase in 5-CQA, in Mango cultivar ‘Bappakkai’ [164]. This accumulation was strongly associated with enhanced salt tolerance (Figure 5). In apple (M. domestica), Feng et al. reported that overexpression of MdANR, a key gene in the proanthocyanidin (PA) biosynthesis pathway, enhanced tolerance to heat and high-light stress in apple skin and callus tissues [165]. This genetic modification led to increased accumulation of both PAs and 5-CQA, which were found to reduce sunburn damage, a protective relationship modeled in Figure 5.
Plants exposed to metalloid arsenic (AS) often exhibit morphological, physiological, and growth-related abnormalities, collectively leading to reduced productivity [166]. In maize (Z. mays), both hesperidin (HP) and 5-CQA effectively mitigate the detrimental effects of AS stress. This includes alleviating the arsenic-induced loss of dry and fresh biomass, counteracting the negative impact of arsenic toxicity on PSII photochemical quantum efficiency (Fv/Fm), and restoring redox homeostasis within chloroplasts through HP- or CA-mediated increases in ascorbate and glutathione levels. These mechanisms collectively reduce the physiological and biochemical damage caused by AS in maize [166].
CQAs also play a significant role in plant defense against biotic stress. In rice, one study evaluated the resistance of two rice cultivars to WBPH (S. furcifera) by analyzing antixenosis, antibiosis, and tolerance mechanisms. Metabolomic analysis revealed that CQAs are key resistance compounds in the highly resistant cultivar KL35, significantly prolonging WBPH developmental time and reducing its fecundity. The study also demonstrated that exogenous application of CQAs to a susceptible cultivar, TN1, enhanced its resistance to WBPH, markedly reducing the insect’s survival rate and body weight. These findings highlight the considerable potential of plant-derived CQAs for agricultural applications [102] (Figure 4). Similarly, CQAs exhibit notable insecticidal activity in sweet potato. Liao et al. found that treating sweet potato leaves with exogenous CQAs significantly reduced SPW feeding. Further biochemical assays confirmed that compounds containing a 1-hydroxyquinoyl group inhibit SPW intestinal digestive enzymes and activity [75,103] (Figure 4). In eggplant (Solanum melongena L.), Kumar et al. observed significant variation in larval resistance to Spodoptera litura (Fabricius, 1775) among different cultivars. Untargeted metabolomics identified CQAs as the primary resistance compounds. Feeding larvae, a diet supplemented with 5-CQA resulted in a threefold reduction in larval mass, a twofold increase in mortality, and significantly decreased pupation and eclosion rates compared to the control group. Silencing the key CQAs biosynthetic gene SmHQT in the highly resistant cultivar RL22 reduced CQAs levels threefold and diminished its resistance to the armyworm, while exogenous CQAs application restored resistance. These results collectively demonstrate the larvicidal properties of CQAs against S. litura [167]. Furthermore, CQAs have been reported to confer resistance against another lepidopteran pest, Mythimna separata (Walker, 1865). Lin et al. observed a significant concentration-dependent lethal effect when third-instar larvae were fed 5-CQA. The study also assessed the sublethal effects of 5-CQA at the LC20 concentration, noting significantly reduced total survival, pupation rate, eclosion rate, sex ratio, and fecundity compared to the control. These results indicate that CQAs can effectively control M. separata populations [168].
4.4. Bioavailability, Metabolism, and Structure-Activity Relationships (SAR) of CQAs
Although the broad pharmacological profile of CQAs is well-documented, research on their bioavailability, metabolic fate, and structure–activity relationships (SAR) remains comparatively limited. Current evidence indicates that the absorption of CQAs in vivo occurs mainly via two pathways: (I) approximately one-third is absorbed intact in the stomach and upper gastrointestinal tract and enters systemic circulation directly; (II) the remaining portion largely reaches the colon intact, where it is extensively metabolized by gut microbiota, with the resulting metabolites subsequently absorbed [169,170,171,172,173,174]. Only trace amounts of the parent CQAs are ultimately excreted in urine [169,170].
Multiple clinical studies have delineated this intricate metabolic process. For example, Mariana et al. detected several CQAs isomers—including three CQAs and three diCQAs—in human plasma following coffee consumption, along with a range of urinary metabolites [171]. Similarly, Stalmach et al. identified numerous metabolites in plasma and urine, noting that despite considerable inter-individual variability, about one-third of orally administered CQAs are absorbed intact in the gastrointestinal tract [172,173]. The unabsorbed fraction almost completely reaches the colon, where gut microbiota transform it into various metabolites such as dihydrocaffeic acid (DHCA), dihydroferulic acid (DHFA), caffeic acid (CA), 5-CQA, gallic acid (GA), ferulic acid (FA), isoferulic acid (isoFA), vanillic acid (VA), sinapic acid, p-hydroxybenzoic acid, and p-coumaric acid (p-CoA) [171,172,174]. Additionally, some studies suggest that the colon may participate in the reabsorption of CQAs excreted into digestive juices [170]. Although metabolite profiles differ across studies—likely due to variations in analytical methods, dosage, and individual physiology—they consistently support the two primary bioavailability pathways of CQAs in vivo.
Once in systemic circulation, both parent CQAs and their microbial metabolites undergo further hepatic metabolism. The detection of substantial levels of methylated and sulfated conjugates in plasma and urine indicates extensive Phase II metabolism in the liver [173,175].
Regarding structure–activity relationships, several pharmacophores have been identified as critical: the catechol moiety in the caffeoyl group, the acrylic acid double bond, and the acylation pattern of the quinic acid moiety. Supporting this, Cao et al. reported that removing phenolic hydroxyls from the caffeic acid moiety abolished hypolipidemic activity, whereas modifications to the quinic acid portion had minimal impact [176]. Based on SAR analyses of analogs, Wang et al. designed and synthesized a photoaffinity-labeled probe molecule [125,176]. Moreover, dicaffeoylquinic acids often demonstrate enhanced potency, possibly due to a higher density of active caffeoyl groups [9].
In summary, current findings indicate that the in vivo activity of CQAs is closely linked to the caffeoyl group. The overall bioactivity likely results from the combined effects of a small fraction of intact CQAs, colonic microbial metabolites, and potential Phase II hepatic metabolites, with the caffeoyl moiety serving as the essential pharmacophore.
5. Summary and Perspectives
This review systematically consolidates the latest advances in the biosynthesis and multifaceted bioactivities of CQAs. As a class of naturally occurring phenolic acids, CQAs have attracted increasing research interest due to their broad pharmacological properties and industrial applicability. However, the persistent ambiguity in nomenclature—particularly the historical conflation of 3-CQA and 5-CQA as “chlorogenic acid”—remains a barrier to the integration and communication of scientific findings. We therefore emphasize the urgent need for standardized nomenclature and encourage the consistent use of IUPAC-recommended names and CAS numbers in future studies.
The bioactivity profile of CQAs underpins their potential across diverse sectors. Pharmacologically, CQAs exhibit anti-inflammatory, antioxidant, neuroprotective, anticancer, antimicrobial, antiviral, and antidiabetic properties, often mediated through key pathways such as NF-κB, TLR, and Nrf2. In light industry, CQAs serve as antibiotic alternatives in animal feed, natural preservatives in food packaging, and active ingredients in skincare formulations due to their antioxidant and UV-protective properties. Moreover, CQAs contribute to plant resilience against cold, drought, salinity, and insect herbivory, primarily through ROS scavenging and direct antimicrobial or antifeedant actions.
Despite these advances, critical research gaps persist, charting a clear course for future investigations. A primary unresolved area is the biosynthesis of poly-acylated CQAs (e.g., diCQAs, triCQAs), where the specific enzymatic pathways, regulatory logic, and the role of non-enzymatic acyl migration in vivo remain poorly characterized and warrant elucidation. Another significant bottleneck lies in our limited understanding of CQA transport and compartmentalization, particularly the identification of vacuolar transporters responsible for their storage. To construct a comprehensive regulatory network for CQA biosynthesis, future efforts should leverage integrated systems biology approaches, combining genomics, transcriptomics, proteomics, and metabolomics, especially in non-model medicinal plants. Furthermore, despite promising preclinical results, systematic toxicological evaluations, in vivo efficacy studies, and clinical translation of CQAs are still scarce.
Looking ahead, the biotechnological exploitation of CQA pathways holds substantial promise. Metabolic engineering of engineered microbes (e.g., E. coli, S. cerevisiae) or plant systems to express key biosynthetic genes offers a sustainable platform for the large-scale production of specific CQAs, reducing reliance on plant extraction. Synthetic biology strategies, involving the construction of synthetic gene clusters and optimization of chassis cells via modular pathway engineering, could enable the tailored production of rare or structurally complex CQAs with enhanced bioactivities. In agriculture, genome editing techniques like CRISPR/Cas9 applied to transcriptional regulators or structural genes could pave the way for developing stress-resistant crops with elevated CQA levels, combining improved agronomic traits with added health benefits. Finally, incorporating biotechnologically produced CQAs into functional foods, nutraceuticals, and phytopharmaceuticals represents a growing market opportunity, ensuring purity, scalability, and sustainability.
In conclusion, while significant progress has been made in understanding the biosynthesis and bioactivity of CQAs, leveraging modern biotechnological tools and interdisciplinary approaches to address the existing knowledge gaps will unlock their full potential in medicine, agriculture, and light industry. We advocate for interdisciplinary collaborations to translate these promising natural compounds from bench to market.
Author Contributions
Conceptualization, H.C. and C.L.; writing—original draft preparation, H.C.; writing—review and editing, C.L., K.G., D.C., L.W. and T.J.; visualization, H.C., B.P. and S.Z.; supervision, H.C., X.L., B.P. and Y.Z.; funding acquisition, C.H. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Scientific and technological innovation capacity building project of Beijing Academy of Agriculture and Forestry Sciences (KJCX20251011) and by the National Key R&D Program of China of Beijing Engineering Research Center of Functional Floriculture (2024YFD1600905).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We gratefully acknowledge the funding acquisition by C.H. and C.L. We also extend our thanks to all contributing authors for their valuable efforts.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 2020 ed.; China Medical Science Press: Beijing, China, 2020; Volume 1, pp. 221–1677. [Google Scholar]
- Li, R.; Zhan, Y.; Ding, X.; Cui, J.; Han, Y.; Zhang, J.; Zhang, J.; Li, W.; Wang, L.; Jiang, J. Cancer Differentiation Inducer Chlorogenic Acid Suppresses PD-L1 Expression and Boosts Antitumor Immunity of PD-1 Antibody. Int. J. Biol. Sci. 2024, 20, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tang, Y.S.; Cao, F.; Shou, J.W.; Wong, C.K.; Shaw, P.C. 3,4,5-Tri-O-caffeoylquinic acid attenuates influenza A virus induced inflammation through Toll-like receptor 3/7 activated signaling pathway. Phytomedicine 2024, 132, 155896. [Google Scholar] [CrossRef]
- Fu, S.; Yi, X.; Li, Y.; Li, Y.; Qu, X.; Miao, P.; Xu, Y. Berberine and chlorogenic acid-assembled nanoparticles for highly efficient inhibition of multidrug-resistant Staphylococcus aureus. J. Hazard. Mater. 2024, 473, 134680. [Google Scholar] [CrossRef]
- Li, G.; Li, H.; Wang, P.; Zhang, X.; Kuang, W.; Huang, L.; Zhang, Y.; Xiao, W.; Du, Q.; Tang, H.; et al. Chemo-proteomics reveals dihydrocaffeic acid exhibits anti-inflammation effects via Transaldolase 1 mediated PERK-NF-κB pathway. Cell Commun. Signal. 2025, 23, 65. [Google Scholar] [CrossRef]
- Cejas, J.P.; Disalvo, E.A.; Frias, M.A. Effect of cholesterol on the antioxidant action of chlorogenic acid in lipid membranes. Arch. Biochem. Biophys. 2025, 767, 110346. [Google Scholar] [CrossRef]
- Kang, J.B.; Son, H.K.; Park, D.J.; Jin, Y.B.; Koh, P.O. Chlorogenic acid regulates the expression of protein phosphatase 2A subunit B in the cerebral cortex of a rat stroke model and glutamate-exposed neurons. Lab. Anim. Res. 2024, 40, 8. [Google Scholar] [CrossRef]
- Li, Y.M.; Jiang, L.N.; Sun, X.Y.; Li, C.B.; Zhou, M.; Sun, Y.X.; Suo, L.N. Physiological regulation of exogenous chlorogenic acid on chilling tolerance of tomato seedlings. J. Plant Nutr. Fertil. 2024, 30, 315–330. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lou, J.; Liu, G.; Li, Q.; Cao, Z.; Wu, P.; Mashu, H.; Liu, Z.; Deng, J.; Yang, Z.; et al. A R2R3-MYB transcription factor LmMYB111 positively regulates chlorogenic acid and luteoloside biosynthesis in Lonicera macranthoides. Plant Sci. 2025, 358, 112556. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Chen, P.; Zong, J.; Gao, J.; Qin, R.; Wu, C.; Lv, Q.; Xu, Y.; Zhao, T.; Fu, Y. Integrated transcriptomic and CQAs analysis revealed IbGLK1 is a key transcription factor for chlorogenic acid accumulation in sweetpotato (Ipomoea batatas [L.] Lam.) blades. Int. J. Biol. Macromol. 2024, 266, 131045. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Bai, D.; Li, Y.; He, X.; Ito, K.; Liu, K.; Tan, H.; Zhen, W.; Zhang, B.; et al. Dietary Supplementation with Chlorogenic Acid Enhances Antioxidant Capacity, Which Promotes Growth, Jejunum Barrier Function, and Cecum Microbiota in Broilers under High Stocking Density Stress. Animals 2023, 13, 303. [Google Scholar] [CrossRef]
- Ruscinc, N.; Serafim, R.A.M.; Almeida, C.; Rosado, C.; Baby, A.R. Challenging the safety and efficacy of topically applied chlorogenic acid, apigenin, kaempferol, and naringenin by HET-CAM, HPLC-TBARS-EVSC, and laser Doppler flowmetry. Front. Chem. 2024, 12, 1400881. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Qu, J.; Wang, Y.; Fang, T.; Xiao, W.; Wang, Y.; Zhang, Y.; Khan, M.; Chen, Q.; Xu, X.; et al. Transcriptome and metabolome atlas reveals contributions of sphingosine and chlorogenic acid to cold tolerance in Citrus. Plant Physiol. 2024, 196, 634–650. [Google Scholar] [CrossRef]
- Liu, Z.; Bruins, M.E.; de Bruijn, W.J.C.; Vincken, J.-P. A comparison of the phenolic composition of old and young tea leaves reveals a decrease in flavanols and phenolic acids and an increase in flavonols upon tea leaf maturation. J. Food Compos. Anal. 2020, 86, 103385. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current Advances in Naturally Occurring Caffeoylquinic Acids: Structure, Bioactivity, and Synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Feng, K.; Xie, M.; Barros, J.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Chen, J.G. Phylogenetic Occurrence of the Phenylpropanoid Pathway and Lignin Biosynthesis in Plants. Front. Plant Sci. 2021, 12, 704697. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, J.; Lin, Y.; Zhu, H.; Tang, L.; Wang, X.; Wang, X. Comparative transcriptome and weighted correlation network analyses reveal candidate genes involved in chlorogenic acid biosynthesis in sweet potato. Sci. Rep. 2022, 12, 2770. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, X.B.; Wu, Y.; Zhan, W.D.; Guo, Y.F.; Chen, M.; Bai, T.H.; Jiao, J.; Song, C.H.; Song, S.W.; et al. Transcriptome Analysis Identifies Genes Associated with Chlorogenic Acid Biosynthesis during Apple Fruit Development. Horticulturae 2023, 9, 217. [Google Scholar] [CrossRef]
- Cheevarungnapakul, K.; Khaksar, G.; Panpetch, P.; Boonjing, P.; Sirikantaramas, S. Identification and Functional Characterization of Genes Involved in the Biosynthesis of Caffeoylquinic Acids in Sunflower (Helianthus annuus L.). Front. Plant Sci. 2019, 10, 968. [Google Scholar] [CrossRef]
- Kojima, M.; Uritani, I. Studies on chlorogenic acid biosynthesis in sweet potato root tissue using trans-cinnamic acid-2-14C and quinic acid-G-3H1. Plant Cell Physiol. 1972, 13, 311–319. [Google Scholar] [CrossRef]
- Kojima, M.; Uritani, I. Elucidation of the structure of a possible intermediate in chlorogenic acid biosynthesis in sweet potato root tissue1. Plant Cell Physiol. 1972, 13, 1075–1084. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Bai, M.; He, H.; Wang, H.; Wu, H. Correlation of the temporal and spatial expression patterns of HQT with the biosynthesis and accumulation of chlorogenic acid in Lonicera japonica flowers. Hortic. Res. 2019, 6, 73. [Google Scholar] [CrossRef]
- Li, R.L.; Xu, J.; Qi, Z.X.; Zhao, S.W.; Zhao, R.; Ge, Y.R.; Li, R.F.; Kong, X.Y.; Wu, Z.Y.; Zhang, X.; et al. High-resolution genome mapping and functional dissection of chlorogenic acid production in Lonicera maackii. Plant Physiol. 2023, 192, 2902–2922. [Google Scholar] [CrossRef]
- Barros, J.; Escamilla-Trevino, L.; Song, L.; Rao, X.; Serrani-Yarce, J.C.; Palacios, M.D.; Engle, N.; Choudhury, F.K.; Tschaplinski, T.J.; Venables, B.J.; et al. 4-Coumarate 3-hydroxylase in the lignin biosynthesis pathway is a cytosolic ascorbate peroxidase. Nat. Commun. 2019, 10, 1994. [Google Scholar] [CrossRef]
- Vanholme, R.; Cesarino, I.; Rataj, K.; Xiao, Y.; Sundin, L.; Goeminne, G.; Kim, H.; Cross, J.; Morreel, K.; Araujo, P.; et al. Caffeoyl Shikimate Esterase (CSE) Is an Enzyme in the Lignin Biosynthetic Pathway in Arabidopsis. Science 2013, 341, 1103–1106. [Google Scholar] [CrossRef]
- Ha, C.M.; Escamilla-Trevino, L.; Yarce, J.C.S.; Kim, H.; Ralph, J.; Chen, F.; Dixon, R.A. An essential role of caffeoyl shikimate esterase in monolignol biosynthesis in Medicago truncatula. Plant J. 2016, 86, 363–375. [Google Scholar] [CrossRef]
- Escamilla-Treviño, L.L.; Shen, H.; Hernandez, T.; Yin, Y.; Xu, Y.; Dixon, R.A. Early lignin pathway enzymes and routes to chlorogenic acid in switchgrass (Panicum virgatum L.). Plant Mol. Biol. 2014, 84, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chao, N.; Zhang, M.; Jiang, X.; Gai, Y. Functional Characteristics of Caffeoyl Shikimate Esterase in Larix Kaempferi and Monolignol Biosynthesis in Gymnosperms. Int. J. Mol. Sci. 2019, 20, 6071. [Google Scholar] [CrossRef]
- Villegas, R.J.; Kojima, M. Purification and characterization of hydroxycinnamoyl D-glucose. Quinate hydroxycinnamoyl transferase in the root of sweet potato, Ipomoea batatas Lam. J. Biol. Chem. 1986, 261, 8729–8733. [Google Scholar] [CrossRef] [PubMed]
- de Vries, L.; Brouckaert, M.; Chanoca, A.; Kim, H.; Regner, M.R.; Timokhin, V.I.; Sun, Y.; De Meester, B.; Van Doorsselaere, J.; Goeminne, G.; et al. CRISPR-Cas9 editing of CAFFEOYL SHIKIMATE ESTERASE 1 and 2 shows their importance and partial redundancy in lignification in Populus tremula x P. alba. Plant Biotechnol. J. 2021, 19, 2221–2234. [Google Scholar] [CrossRef]
- Saleme, M.D.S.; Cesarino, I.; Vargas, L.; Kim, H.; Vanholme, R.; Goeminne, G.; Van Acker, R.; Fonseca, F.; Pallidis, A.; Voorend, W.; et al. Silencing CAFFEOYL SHIKIMATE ESTERASE Affects Lignification and Improves Saccharification in Poplar. Plant Physiol. 2017, 175, 1040–1057. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.X.; Wang, Y.H.; Shi, L.; Wang, R.P.; Yang, Y.R.; Wei, D.S.; Li, Y.J.; Chao, K.R.; Jia, L.; Liu, G.M.; et al. Insights into Molecular Mechanism of Secondary Xylem Rapid Growth in Salix psammophila. Plants 2025, 14, 459. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, J.; Sun, J.; Qiu, S.; Chu, B.; Fang, R.; Li, L.; Gong, J.; Zheng, F. Degradation kinetics and isomerization of 5-O-caffeoylquinic acid under ultrasound: Influence of epigallocatechin gallate and vitamin C. Food Chem. X 2021, 12, 100147. [Google Scholar] [CrossRef]
- Chen, Y.F.; Yi, N.; Yao, S.B.; Zhuang, J.H.; Fu, Z.P.; Ma, J.; Yin, S.X.; Jiang, X.L.; Liu, Y.J.; Gao, L.P.; et al. CsHCT-Mediated Lignin Synthesis Pathway Involved in the Response of Tea Plants to Biotic and Abiotic Stresses. J. Agric. Food Chem. 2021, 69, 10069–10081. [Google Scholar] [CrossRef]
- Moglia, A.; Lanteri, S.; Comino, C.; Hill, L.; Knevitt, D.; Cagliero, C.; Rubiolo, P.; Bornemann, S.; Martin, C. Dual catalytic activity of hydroxycinnamoyl-coenzyme A quinate transferase from tomato allows it to moonlight in the synthesis of both mono- and dicaffeoylquinic acids. Plant Physiol. 2014, 166, 1777–1787. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.; Legrand, G.; Duriot, L.; Delporte, M.; Menin, B.; Michel, C.; Olry, A.; Chataigné, G.; Salwinski, A.; Bygdell, J.; et al. A GDSL lipase-like from Ipomoea batatas catalyzes efficient production of 3,5-diCQA when expressed in Pichia pastoris. Commun. Biol. 2020, 3, 673, Erratum in Commun. Biol. 2020, 3, 746. https://doi.org/10.1038/s42003-020-01488-x. [Google Scholar] [CrossRef]
- Soviguidi, D.R.J.; Pan, R.; Liu, Y.; Rao, L.P.; Zhang, W.Y.; Yang, X.S. Chlorogenic Acid Metabolism: The Evolution and Roles in Plant Response to Abiotic Stress. Phyton-Int. J. Exp. Bot. 2022, 91, 239–255. [Google Scholar] [CrossRef]
- Barros, J.; Dixon, R.A. Plant Phenylalanine/Tyrosine Ammonia-lyases. Trends Plant Sci. 2020, 25, 66–79. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.; Yuan, D.; Duan, M.; Liu, Y.; Shen, Z.; Yang, C.; Qiu, Z.; Liu, D.; Wen, P.; et al. An R2R3 MYB transcription factor confers brown planthopper resistance by regulating the phenylalanine ammonia-lyase pathway in rice. Proc. Natl. Acad. Sci. USA 2020, 117, 271–277. [Google Scholar] [CrossRef]
- Xu, L.; Xu, Y.; Xiang, H.Y.; Huang, H.T.; Mi, Q.L.; Chen, Y.D.; Jiang, J.R.; Li, X.M.; Gao, X. Cloning and functional analysis of the phenylalanine ammonia-lyase gene NtPAL in tobacco. Acta Tabacaria Sin. 2025, 31, 93–103. [Google Scholar] [CrossRef]
- Fan, L.; Shi, G.; Yang, J.; Liu, G.; Niu, Z.; Ye, W.; Wu, S.; Wang, L.; Guan, Q. A Protective Role of Phenylalanine Ammonia-Lyase from Astragalus membranaceus against Saline-Alkali Stress. Int. J. Mol. Sci. 2022, 23, 15686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, J.; Wang, R. Genome-wide Identification and Expression Analysis of 4CL Gene Family in Camellia sinensis. Acta Tea Sin. 2024, 65, 34–46. [Google Scholar] [CrossRef]
- Khatri, P.; Chen, L.; Rajcan, I.; Dhaubhadel, S. Functional characterization of Cinnamate 4-hydroxylase gene family in soybean (Glycine max). PLoS ONE 2023, 18, e0285698. [Google Scholar] [CrossRef] [PubMed]
- Lavhale, S.G.; Kalunke, R.M.; Giri, A.P. Structural, functional and evolutionary diversity of 4-coumarate-CoA ligase in plants. Planta 2018, 248, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Li, J.K.; Peng, C.; Zhang, Z.Y.; Liang, X.; Wei, M.K.; Yang, Q.; Li, B.Q.; Ali, M.M.; Kayima, V.; Chen, F.X.; et al. Dynamics of Fruit Hollowness and Browning and Associated Lignin Accumulation and Its Genome-Wide Identification of Ps4CL Gene Family in Huangguan Plum. Sci. Agric. Sin. 2025, 58, 759–778. [Google Scholar] [CrossRef]
- Hu, J.Q.; Qi, Q.; Jiang, X.N.; Gai, Y. Effect of Fusion Gene *4CL1-CCR* of Populus tomentosa on Lignin Deposition in Tobacco. Sci. Silvae Sin. 2020, 56, 63–69. [Google Scholar] [CrossRef]
- Shrestha, H.K.; Fichman, Y.; Engle, N.L.; Tschaplinski, T.J.; Mittler, R.; Dixon, R.A.; Hettich, R.L.; Barros, J.; Abraham, P.E. Multi-omic characterization of bifunctional peroxidase 4-coumarate 3-hydroxylase knockdown in Brachypodium distachyon provides insights into lignin modification-associated pleiotropic effects. Front. Plant Sci. 2022, 13, 908649. [Google Scholar] [CrossRef]
- Gao, M. A Preliminary Study on the Gene Function of 4 Coumaruylshikimate/Quinoline 3′-Hydroxylase. Master’s Thesis, Guizhou University, Guiyang, China, 2020. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, K.H.; Keene, S.A.; Colquhoun, T.A. Altered profile of floral volatiles and lignin content by down-regulation of Caffeoyl Shikimate Esterase in Petunia. BMC Plant Biol. 2023, 23, 210. [Google Scholar] [CrossRef]
- Qin, X.; Qiao, J.; Li, Y. Structure, function, and applications of hydroxycinnamoyl transferases. Chin. J. Biochem. Mol. Biol. 2019, 35, 1058–1066. [Google Scholar] [CrossRef]
- Liu, Q.; Yao, L.; Xu, Y.; Cheng, H.; Wang, W.; Liu, Z.; Liu, J.; Cui, X.; Zhou, Y.; Ning, W. In vitro evaluation of hydroxycinnamoyl CoA:quinate hydroxycinnamoyl transferase expression and regulation in Taraxacum antungense in relation to 5-caffeoylquinic acid production. Phytochemistry 2019, 162, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Wu, Q.F.; Chen, G.M.; Zhou, L.X. Multifaceted roles and regulatory mechanisms of MYB transcription factors in plant development, secondary metabolism, and stress adaptation: Current insights and future prospects. Gm Crops Food-Biotechnol. Agric. Food Chain. 2025, 16, 626–655. [Google Scholar] [CrossRef] [PubMed]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Lu, C.; Yan, X.; Zhang, H.; Zhong, T.; Gui, A.; Liu, Y.; Pan, L.; Shao, Q. Integrated metabolomic and transcriptomic analysis reveals biosynthesis mechanism of flavone and caffeoylquinic acid in chrysanthemum. BMC Genom. 2024, 25, 759. [Google Scholar] [CrossRef]
- Tang, N.; Cao, Z.; Yang, C.; Ran, D.; Wu, P.; Gao, H.; He, N.; Liu, G.; Chen, Z. A R2R3-MYB transcriptional activator LmMYB15 regulates chlorogenic acid biosynthesis and phenylpropanoid metabolism in Lonicera macranthoides. Plant Sci. 2021, 308, 110924. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, R.; Zeng, L.; Wu, Y.; Jiang, Y.; Yang, Q.; Nie, Q. Isolation and molecular characterization of NtMYB4a, a putative transcription activation factor involved in anthocyanin synthesis in tobacco. Gene 2020, 760, 144990. [Google Scholar] [CrossRef]
- Kim, D.; Jeon, S.J.; Yanders, S.; Park, S.C.; Kim, H.S.; Kim, S. MYB3 plays an important role in lignin and anthocyanin biosynthesis under salt stress condition in Arabidopsis. Plant Cell Rep. 2022, 41, 1549–1560. [Google Scholar] [CrossRef]
- Guan, R.; Guo, F.; Guo, R.; Wang, S.; Sun, X.; Zhao, Q.; Zhang, C.; Li, S.; Lin, H.; Lin, J. Integrated metabolic profiling and transcriptome analysis of Lonicera japonica flowers for chlorogenic acid, luteolin and endogenous hormone syntheses. Gene 2023, 888, 147739. [Google Scholar] [CrossRef]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Javed, T.; Gao, S.J. WRKY transcription factors in plant defense. Trends Genet. 2023, 39, 787–801. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Zheng, K.; Xie, M.; Feng, K.; Jawdy, S.S.; Gunter, L.E.; Ranjan, P.; Singan, V.R.; Engle, N.; et al. Genome-wide association studies and expression-based quantitative trait loci analyses reveal roles of HCT2 in caffeoylquinic acid biosynthesis and its regulation by defense-responsive transcription factors in Populus. New Phytol. 2018, 220, 502–516. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Liu, P.; Yang, X.; He, X.; Xie, X.; Luo, Z.; Wu, M.; Wang, C.; Yang, J. Molecular cloning and functional characterization of NtWRKY41a in the biosynthesis of phenylpropanoids in Nicotiana tabacum. Plant Sci. 2022, 315, 111154. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Wang, J.; Li, Y.; Li, M.; Jin, P.; Zheng, Y. Involvement of PpWRKY70 in the methyl jasmonate primed disease resistance against Rhizopus stolonifer of peaches via activating phenylpropanoid pathway. Postharvest Biol. Technol. 2021, 174, 111466. [Google Scholar] [CrossRef]
- Gao, F.; Dubos, C. The arabidopsis bHLH transcription factor family. Trends Plant Sci. 2024, 29, 668–680. [Google Scholar] [CrossRef]
- Liu, Q.; Li, L.; Cheng, H.; Yao, L.; Wu, J.; Huang, H.; Ning, W.; Kai, G. The basic helix-loop-helix transcription factor TabHLH1 increases chlorogenic acid and luteolin biosynthesis in Taraxacum antungense Kitag. Hortic. Res. 2021, 8, 195. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, Y.; Shan, G.; Zhou, J.; Li, X.; Liu, P.; Wang, Y.; An, S.; Gao, R. Transcriptome and metabolome profiling unveil the accumulation of chlorogenic acid in autooctoploid Gongju. Front. Plant Sci. 2024, 15, 1461357. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, J.; Li, W.; Yang, L.; Duan, W.; Zhao, P.; Pu, Z.; Ding, Y. Revealing the dynamic changes of metabolites and molecular mechanisms of chlorogenic acid accumulation during the leaf development of Vaccinium dunalianum based on multi-omic analyses. Front. Plant Sci. 2024, 15, 1440589. [Google Scholar] [CrossRef]
- Ohama, N.; Yanagisawa, S. Role of GARP family transcription factors in the regulatory network for nitrogen and phosphorus acquisition. J. Plant Res. 2024, 137, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, J.; Gao, Q.; He, S.; Xu, Y.; Luo, Z.; Liu, P.; Wu, M.; Xu, X.; Ma, L.; et al. The transcription factor NtERF13a enhances abiotic stress tolerance and phenylpropanoid compounds biosynthesis in tobacco. Plant Sci. 2023, 334, 111772. [Google Scholar] [CrossRef]
- He, S.; Xu, X.; Gao, Q.; Huang, C.J.; Luo, Z.P.; Liu, P.P.; Wu, M.Z.; Huang, H.T.; Yang, J.; Zeng, J.M.; et al. NtERF4 promotes the biosynthesis of chlorogenic acid and flavonoids by targeting PAL genes in Nicotiana tabacum. Planta 2024, 259, 31. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Liang, X.; Zhang, Y.; Fernie, A.R. Mass spectrometric exploration of phytohormone profiles and signaling networks. Trends Plant Sci. 2023, 28, 399–414. [Google Scholar] [CrossRef]
- Setotaw, Y.B.; Li, J.; Qi, J.; Ma, C.; Zhang, M.; Huang, C.; Wang, L.; Wu, J. Salicylic acid positively regulates maize defenses against lepidopteran insects. Plant Divers. 2024, 46, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zeng, L.; Rao, S.; Gu, D.; Liu, X.; Wang, Y.; Zhu, H.; Hou, X.; Yang, Z. Induced biosynthesis of chlorogenic acid in sweetpotato leaves confers the resistance against sweetpotato weevil attack. J. Adv. Res. 2020, 24, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Q.; Liu, S.; Ma, P.; Jia, Z.; Xie, Y.; Bian, X. Effects of exogenous phytohormones on chlorogenic acid accumulation and pathway-associated gene expressions in sweetpotato stem tips. Plant Physiol. Biochem. 2021, 164, 21–26. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Stiles, A.R.; Saxena, P.K.; Liu, C.Z. Gibberellic acid increases secondary metabolite production in Echinacea purpurea hairy roots. Appl. Biochem. Biotechnol. 2012, 168, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yeo, H.J.; Park, Y.J.; Morgan, A.M.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.U. Influence of Indole-3-Acetic Acid and Gibberellic Acid on Phenylpropanoid Accumulation in Common Buckwheat (Fagopyrum esculentum Moench) Sprouts. Molecules 2017, 22, 374. [Google Scholar] [CrossRef]
- Sharaf-Eldin, M.A.; Schnitzler, W.H.; Nitz, G.; Razin, A.M.; El-Oksh, I.I. The effect of gibberellic acid (GA3) on some phenolic substances in globe artichoke (Cynara cardunculus var. scolymus (L.) Fiori). Sci. Hortic. 2007, 111, 326–329. [Google Scholar] [CrossRef]
- Casanova-Sáez, R.; Mateo-Bonmatí, E.; Ljung, K. Auxin Metabolism in Plants. Cold Spring Harb. Perspect. Biol. 2021, 13, a039867. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Kim, S.H.; Yu, C.Y.; Chung, I.M. Biochemical and Physiological Changes during Early Adventitious Root Formation in Chrysanthemum indicum Linné Cuttings. Plants 2022, 11, 1440. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, D.; Tu, W.; Ma, F.; Liu, C. Overexpression of auxin/indole-3-acetic acid gene MdIAA24 enhances Glomerella leaf spot resistance in apple (Malus domestica). Hortic. Plant J. 2024, 10, 15–24. [Google Scholar] [CrossRef]
- Sun, Y.M.; Fernie, A.R. Plant secondary metabolism in a fluctuating world: Climate change perspectives. Trends Plant Sci. 2024, 29, 560–571. [Google Scholar] [CrossRef]
- Wu, W.Y.; Chen, L.; Liang, R.T.; Huang, S.P.; Li, X.; Huang, B.L.; Luo, H.M.; Zhang, M.; Wang, X.X.; Zhu, H. The role of light in regulating plant growth, development and sugar metabolism: A review. Front. Plant Sci. 2025, 15, 1507628. [Google Scholar] [CrossRef]
- Lu, C.F.; Liu, Y.C.; Yan, X.Y.; Gui, A.J.; Jiang, Y.L.; Wang, P.; Qiao, Q.; Shao, Q.S. Multiplex Approach of Metabolomic and Transcriptomic Reveals the Biosynthetic Mechanism of Light-induced Flavonoids and CGA in Chrysanthemum. Ind. Crops Prod. 2024, 221, 119420. [Google Scholar] [CrossRef]
- Meng, H.; Li, Y.; Lu, B.; Zhang, W.; Shi, X.; Fu, H.; Long, G. The Effect of Light Intensity on the Chlorogenic Acid Biosynthesis Pathway in Marsdenia tenacissima. Agronomy 2024, 14, 1063. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, N.; Du, L.; Zhang, J.; Chen, R.; Zhu, Q.; Li, W.; Wu, C.; Peng, G.; Rao, L.; et al. Light plays a critical role in the accumulation of chlorogenic acid in Lonicera macranthoides Hand.-Mazz. Plant Physiol. Biochem. 2023, 196, 793–806. [Google Scholar] [CrossRef]
- Chen, X.; Cai, W.; Xia, J.; Yu, H.; Wang, Q.; Pang, F.; Zhao, M. Metabolomic and Transcriptomic Analyses Reveal that Blue Light Promotes Chlorogenic Acid Synthesis in Strawberry. J. Agric. Food Chem. 2020, 68, 12485–12492. [Google Scholar] [CrossRef]
- Fukuda, N.; Yoshida, H.; Kusano, M. Effects of light quality, photoperiod, CO2 concentration, and air temperature on chlorogenic acid and rutin accumulation in young lettuce plants. Plant Physiol. Biochem. 2022, 186, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Senekovič, J.; Ciringer, T.; Ambrožič-Dolinšek, J.; Islamčević Razboršek, M. The Effect of Combined Elicitation with Light and Temperature on the Chlorogenic Acid Content, Total Phenolic Content and Antioxidant Activity of Berula erecta in Tissue Culture. Plants 2024, 13, 1463. [Google Scholar] [CrossRef]
- Percival, G.C.; Baird, L. Influence of storage upon light-induced chlorogenic acid accumulation in potato tubers (Solanum tuberosum L.). J. Agric. Food Chem. 2000, 48, 2476–2482. [Google Scholar] [CrossRef]
- Kidokoro, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant cold-stress responses. Trends Plant Sci. 2022, 27, 922–935. [Google Scholar] [CrossRef]
- Xie, R. Molecular Mechanism of Low Temperature-Induced NtMYB4a Transcription Factor Regulating Chlorogenic Acid Biosynthesis in Tobacco. Master’s Thesis, Guizhou University, Guiyang, China, 2022. [Google Scholar] [CrossRef]
- Sim, H.S.; Kwon, H.J.; Jang, S.N.; Lee, G.O.; Kang, I.J.; Yang, G.S.; Nam, G.H.; Park, J.E.; Byun, H.Y.; You, Y.H.; et al. Aster × chusanensis Growth and Phenolic Acid Composition under Different Cultivation Temperatures. Plants 2024, 13, 1855. [Google Scholar] [CrossRef]
- Goławska, S.; Łukasik, I.; Chojnacki, A.A.; Chrzanowski, G. Flavonoids and Phenolic Acids Content in Cultivation and Wild Collection of European Cranberry Bush Viburnum opulus L. Molecules 2023, 28, 2285. Molecules 2023, 28, 2285. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Zahra, N.; Hafeez, M.B.; Siddique, K.H.M. Recent Advances in Plant Drought Tolerance. J. Plant Growth Regul. 2024, 43, 3337–3369. [Google Scholar] [CrossRef]
- Azizyan, R.; Mandoulakani, B.A. Partial coding sequence identification, gene expression analysis, and content of anticancer phenolic compounds in Sonchus arvensis L. under drought stress conditions. Ind. Crops Prod. 2024, 209, 118030. [Google Scholar] [CrossRef]
- Khan, F.; Upreti, P.; Singh, R.; Shukla, P.K.; Shirke, P.A. Physiological performance of two contrasting rice varieties under water stress. Physiol. Mol. Biol. Plants 2017, 23, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Kwon, D.Y.; Koo, S.Y.; Truong, T.Q.; Hong, S.C.; Choi, J.; Moon, J.; Kim, S.M. Identification of drought-responsive phenolic compounds and their biosynthetic regulation under drought stress in Ligularia fischeri. Front. Plant Sci. 2023, 14, 1140509. [Google Scholar] [CrossRef]
- Ghafari, H.; Hassanpour, H.; Jafari, M.; Besharat, S. Physiological, biochemical and gene-expressional responses to water deficit in apple subjected to partial root-zone drying (PRD). Plant Physiol. Biochem. 2020, 148, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Tao, W.; Zhang, H.; Luo, J.; Deng, X.; Li, D.; Li, Q.; Wang, H.; Yue, Y.; Jiang, S.; et al. Transcriptome and metabolome profiling reveal the chlorogenic acid as a resistance substance for rice against the white-backed planthopper Sogatella furcifera (Horváth). Front. Plant Sci. 2025, 16, 1571893. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Zhu, H.; Mei, G.; Liao, Y.; Rao, S.; Li, S.; Chen, A.; Liu, H.; Zeng, L.; et al. Natural allelic variation confers high resistance to sweet potato weevils in sweet potato. Nat. Plants 2022, 8, 1233–1244. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.J.; Chen, J.B.; Hu, M.L.; Shan, X.Y.; Zhou, J.W. Efficient Production of Chlorogenic Acid in Escherichia coli via Modular Pathway and Cofactor Engineering. J. Agric. Food Chem. 2023, 71, 15204–15212. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Liu, P.F.; Zhang, B.; Shen, J.; Wu, J.Q.; Huang, S.S.; Chu, X.H. De novo Biosynthesis of Caffeic Acid and Chlorogenic Acid in Escherichia coli via Enzyme Engineering and Pathway Engineering. Acs Synth. Biol. 2025, 14, 1581–1593. [Google Scholar] [CrossRef]
- Hu, M.L.; Chen, J.B.; Wang, H.J.; Wang, L.; Gao, S.; Zhou, Z.M.; Zhou, J.W. Efficient Biosynthesis of Chlorogenic Acid in Escherichia coli by Optimization of Precursors Metabolic Flow and Reduction of an Unknown Byproduct. Acs Sustain. Chem. Eng. 2025, 13, 3479–3490. [Google Scholar] [CrossRef]
- Xiao, F.; Lian, J.Z.; Tu, S.; Xie, L.L.; Li, J.; Zhang, F.M.; Linhardt, R.J.; Huang, H.C.; Zhong, W.H. Metabolic Engineering of Saccharomyces cerevisiae for High-Level Production of Chlorogenic Acid from Glucose. Acs Synth. Biol. 2022, 11, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Wang, J.J.; Yang, P.M.; He, Y.; Gong, Z.X.; Zhong, W.H. Enhanced chlorogenic acid production from glucose via systematic metabolic engineering of Saccharomyces cerevisiae. Synth. Syst. Biotechnol. 2025, 10, 707–718. [Google Scholar] [CrossRef]
- Lee, Y.; Bae, C.S.; Ahn, T. Chlorogenic acid attenuates pro-inflammatory response in the blood of streptozotocin-induced diabetic rats. Lab. Anim. Res. 2022, 38, 37. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Zhen, W.; Bai, D.; Liu, K.; He, X.; Ito, K.; Liu, Y.; Li, Y.; Zhang, Y.; Zhang, B.; et al. Effects of dietary chlorogenic acid on intestinal barrier function and the inflammatory response in broilers during lipopolysaccharide-induced immune stress. Poult. Sci. 2023, 102, 102623. [Google Scholar] [CrossRef]
- Han, X.; Wu, X.; Liu, F.; Chen, H.; Hou, H. Inhibition of LPS-induced inflammatory response in RAW264.7 cells by natural Chlorogenic acid isomers involved with AKR1B1 inhibition. Bioorg. Med. Chem. 2024, 114, 117942. [Google Scholar] [CrossRef]
- Jiao, H.; Zhang, M.; Xu, W.; Pan, T.; Luan, J.; Zhao, Y.; Zhang, Z. Chlorogenic acid alleviate kidney fibrosis through regulating TLR4/NF-қB mediated oxidative stress and inflammation. J. Ethnopharmacol. 2024, 335, 118693. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, L.; Wu, G.; Li, X. Therapeutic effects of chlorogenic acid on allergic rhinitis through TLR4/MAPK/NF-κB pathway modulation. Biomol. Biomed. 2025, 25, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, Y.; Zhang, Y.; Yang, T.; Zhao, S.; Sun, N.; Tan, H.; Zhang, H.; Wang, C.; Fan, H. Effect of Chlorogenic Acid via Upregulating Resolvin D1 Inhibiting the NF-κB Pathway on Chronic Restraint Stress-Induced Liver Inflammation. J. Agric. Food Chem. 2022, 70, 10532–10542. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; Pan, T.; Ren, F.; Luo, H.; Zhang, H. Synthesis, characterization and effect of alkyl chain unsaturation on the antioxidant activities of chlorogenic acid derivatives. LWT 2022, 162, 113325. [Google Scholar] [CrossRef]
- Caruso, F.; Sakib, R.; Belli, S.; Caruso, A.; Rossi, M. Antioxidant Scavenging of the Superoxide Radical by Yerba Mate (Ilex paraguariensis) and Black Tea (Camellia sinensis) Plus Caffeic and Chlorogenic Acids, as Shown via DFT and Hydrodynamic Voltammetry. Int. J. Mol. Sci. 2024, 25, 9342. [Google Scholar] [CrossRef]
- Izu, G.O.; Mfotie Njoya, E.; Tabakam, G.T.; Nambooze, J.; Otukile, K.P.; Tsoeu, S.E.; Fasiku, V.O.; Adegoke, A.M.; Erukainure, O.L.; Mashele, S.S.; et al. Unravelling the Influence of Chlorogenic Acid on the Antioxidant Phytochemistry of Avocado (Persea americana Mill.) Fruit Peel. Antioxidants 2024, 13, 456. [Google Scholar] [CrossRef]
- Kang, J.B.; Son, H.K.; Park, D.J.; Jin, Y.B.; Shah, F.A.; Koh, P.O. Modulation of thioredoxin by chlorogenic acid in an ischemic stroke model and glutamate-exposed neurons. Neurosci. Lett. 2024, 825, 137701. [Google Scholar] [CrossRef]
- Heitman, E.; Ingram, D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017, 20, 32–39. [Google Scholar] [CrossRef]
- Mirzaei, F.; Agbaria, L.; Bhatnagar, K.; Sirimanne, N.; Omar A’amar, N.; Jindal, V.; Gerald Thilagendra, A.; Tawfiq Raba, F. Coffee and Alzheimer’s disease. Prog. Brain Res. 2024, 289, 21–55. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, I.; Pai, V. Protective Effects of Chlorogenic Acid Against Amyloid-Beta-Induced Oxidative Stress and Ion Transport Dysfunction in SH-SY5Y Cells. J. Biochem. Mol. Toxicol. 2024, 38, e70046. [Google Scholar] [CrossRef]
- Huang, S.; Wang, L.L.; Xue, N.N.; Li, C.; Guo, H.H.; Ren, T.K.; Zhan, Y.; Li, W.B.; Zhang, J.; Chen, X.G.; et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef] [PubMed]
- Zada, S.; Bahar, M.E.; Kim, W.; Kim, D.R. Chlorogenic Acid Enhances Beta-Lapachone-Induced Cell Death by Suppressing Autophagy in NQO1-Positive Cancer Cells. Cell Biol. Int. 2025, 49, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Srisomsap, C.; Chaisuriya, P.; Liana, D.; Aiyarakanchanakun, P.; Audsasan, T.; Weeraphan, C.; Svasti, J.; Phanumartwiwath, A. Pharmacological Properties of White Mulberry (Morus alba L.) Leaves: Suppressing Migratory and Invasive Activities Against A549 Lung Cancer Cells. Plant Foods Hum. Nutr. 2024, 79, 387–393. [Google Scholar] [CrossRef]
- Wang, Q.; Du, T.; Zhang, Z.; Zhang, Q.; Zhang, J.; Li, W.; Jiang, J.D.; Chen, X.; Hu, H.Y. Target fishing and mechanistic insights of the natural anticancer drug candidate chlorogenic acid. Acta Pharm. Sin. B 2024, 14, 4431–4442. [Google Scholar] [CrossRef]
- Yang, X.; Feng, Y.; Liu, Y.; Ye, X.; Ji, X.; Sun, L.; Gao, F.; Zhang, Q.; Li, Y.; Zhu, B.; et al. Fuzheng Jiedu Xiaoji formulation inhibits hepatocellular carcinoma progression in patients by targeting the AKT/CyclinD1/p21/p27 pathway. Phytomedicine 2021, 87, 153575. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Lee, S.; Kim, S.H.; Park, J.E.; Hwang, Y.S.; Kang, M.H.; Chae, S.Y.; Kim, J.W. Anticancer effects of purple carrot extract via induction of apoptotic genes on human breast cancer cells. Food Sci. Biotechnol. 2025, 34, 1737–1749. [Google Scholar] [CrossRef]
- Kang, M.J.; Kim, D.K. Synergistic antimicrobial action of chlorogenic acid and ultraviolet-A (365 nm) irradiation; mechanisms and effects on DNA integrity. Food Res. Int. 2024, 196, 115132, Corrigendum in Food Res. Int. 2024, 197, 115182. https://doi.org/10.1016/j.foodres.2024.115182. [Google Scholar] [CrossRef]
- Han, X.; Chen, J.; Wang, Q.; Zhang, J.; Mi, J.; Feng, J.; Du, T.; Wang, J.; Zhang, W. Photodynamically activated chlorogenic acid-based antimicrobial packaging films for cherry preservation. Food Chem. 2025, 479, 143857. [Google Scholar] [CrossRef]
- Yang, X.; Lan, W.; Xie, J. Antimicrobial and anti-biofilm activities of chlorogenic acid grafted chitosan against Staphylococcus aureus. Microb. Pathog. 2022, 173, 105748. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guan, L.; Yang, D.; Luo, H.; Zhang, H. Investigating the synergistic antibacterial effects of chlorogenic and p-coumaric acids on Shigella dysenteriae. Food Chem. 2025, 462, 141011. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Saleem, A.; Aziz, T.; Ali, N.; Rajpoot, Z.; Niaz, M.; Khan, A.A.; El Hadi Mohamed, R.A.; Al-Asmari, F.; Al-Joufi, F.A.; et al. Exploring the therapeutic potential of Thymus vulgaris ethanol extract: A computational screening for antimicrobial compounds against COVID-19 induced mucormycosis. Sci. Rep. 2025, 15, 15906. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, F.Q.; Hu, Q.Q.; Yang, F.; Hu, N.; Luo, X.N.; Zhang, Y.; Wu, N.; Li, N. Study on the effect of chlorogenic acid on the anti-microbial effect, physical properties and model accuracy of alginate impression materials. PeerJ 2024, 12, e18228. [Google Scholar] [CrossRef]
- Wang, F.; Kong, B.L.; Tang, Y.S.; Lee, H.K.; Shaw, P.C. Bioassay guided isolation of caffeoylquinic acids from the leaves of Ilex pubescens Hook. et Arn. and investigation of their anti-influenza mechanism. J. Ethnopharmacol. 2023, 309, 116322. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Q.; Cai, H.; Feng, Y.; Wen, A.; Yang, Y.; Wen, M. RNA-seq analysis of chlorogenic acid intervention in duck embryo fibroblasts infected with duck plague virus. Virol. J. 2024, 21, 60. [Google Scholar] [CrossRef]
- Shi, C.; Liang, W.; Guo, M.; Yuan, J.; Zu, S.; Hu, H. Chlorogenic acid inhibits porcine deltacoronavirus release by targeting apoptosis. Int. Immunopharmacol. 2024, 127, 111359. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Rana, H.K.; Singh, V.; Yadav, T.C.; Varadwaj, P.; Pandey, A.K. Evaluation of antidiabetic activity of dietary phenolic compound chlorogenic acid in streptozotocin induced diabetic rats: Molecular docking, molecular dynamics, in silico toxicity, in vitro and in vivo studies. Comput. Biol. Med. 2021, 134, 104462. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Y.; Li, J.; Guo, H.; Wang, L.; Li, J.; Wang, X.; Zhang, Y. Inhibitory mechanism of chlorogenic acid on α-glucosidase and evaluation of its glucose consumption in HepG2 cells. J. Mol. Struct. 2025, 1331, 141607. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Paczkowska-Walendowska, M.; Kledzik, J.; Galanty, A.; Gościniak, A.; Szulc, P.; Korybalska, K.; Cielecka-Piontek, J. Antidiabetic Potential of Black Elderberry Cultivars Flower Extracts: Phytochemical Profile and Enzyme Inhibition. Molecules 2024, 29, 5775. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, H.; Tian, Y.; Lei, J.; Yu, J. Study on antioxidant and antidiabetic components of Cirsium setosum based on molecular networking. Food Biosci. 2024, 61, 104774. [Google Scholar] [CrossRef]
- Stevens, M.R.; van Niekerk, S.E.; Netshimbupfe, M.H.; Hamman, J.H.; Van der Kooy, F. Seasonal Chemical Variation and Antidiabetic Activity of Major Compounds in Artemisia afra Infusions. Rev. Bras. Farmacogn. 2024, 34, 1166–1171. [Google Scholar] [CrossRef]
- Wei, Z.; Yu, B.; Huang, Z.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Yan, H.; He, J. Effect of 3-caffeoylquinic acid on growth performance, nutrient digestibility, and intestinal functions in weaned pigs. J. Anim. Sci. 2023, 101, skad234. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, M.; Wu, J.; Qiu, Y.; Xu, X.; Tian, C.; Hou, J.; Wang, L.; Gao, K.; Yang, X.; et al. Chlorogenic Acid Enhances the Intestinal Health of Weaned Piglets by Inhibiting the TLR4/NF-κB Pathway and Activating the Nrf2 Pathway. Int. J. Mol. Sci. 2024, 25, 9954. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, C.; Memon, M.A.; Shi, Q.; Lu, L.; Tong, X.; Ma, Y.; Zou, H.; Gu, J.; Liu, X.; et al. Chlorogenic acid alleviates cadmium-induced neuronal injury in chicken cerebral cortex by inhibiting incomplete autophagy mediated by AMPK-ULK1 pathway. Poult. Sci. 2025, 104, 104597. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Yang, M.; Liu, X.; Guo, F.; Hu, Z.; Huang, J.; Abbas, W.; Xu, T.; Liu, W.; Wang, Z. Effects of chlorogenic acid on productive and reproductive performances, egg quality, antioxidant functions, and intestinal microenvironment in aged breeder laying hens. Poult. Sci. 2024, 103, 104060. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, X.; Wu, H.; Wang, H.; Bian, H.; Zhu, Y.; Xu, W.; Liu, F.; Wang, D.; Fu, L. Antibacterial activity and action mode of chlorogenic acid against Salmonella Enteritidis, a foodborne pathogen in chilled fresh chicken. World J. Microbiol. Biotechnol. 2020, 36, 24. [Google Scholar] [CrossRef]
- Dai, J.; Dong, F.; Dong, Z.; Bai, Z.; Mao, L. Enhanced antibacterial and antioxidant activities of chlorogenic acid loaded sweet whey/starch active films for edible food packaging. LWT 2024, 199, 116118. [Google Scholar] [CrossRef]
- Estevez-Areco, S.; Goyanes, S.; Garrigós, M.C.; Jiménez, A. Antioxidant water-resistant fish gelatin nanofibers: A comparative analysis of fructose and citric acid crosslinking and investigation of chlorogenic acid release kinetics. Food Hydrocoll. 2024, 150, 109696. [Google Scholar] [CrossRef]
- Hao, X.; Di, J.; Han, Z. Effects of Different Concentrations of Exogenous Chlorogenic Acid Treatments on the Storage Quality of ‘Lvbao Melon’. Storage Process 2025, 25, 59–64. [Google Scholar]
- Dai, B. Inhibitory Mechanism of Chlorogenic Acid on Gray Mold of Postharvest Peach Fruit and the Construction and Application of Its Nanosystems. Master’s Thesis, Zhejiang Gongshang University, Hangzhou, China, 2024. [Google Scholar] [CrossRef]
- Song, S.; Zhang, Y.; Liu, T.; Goh, K.-L.; Zhang, Y.; Zheng, M. Enzymatic synthesis and antioxidation characterization of various acylated chlorogenic acids via regioselective and recyclable immobilized lipases. Food Biosci. 2025, 63, 105678. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Yu, Z.; Xu, Y.; Guo, Y.; Liu, R.; Chang, M.; Wang, X. Enhancing the oxidation stability and bioaccessibility of algal oil emulsion by using tocopherol and chlorogenic acid. Food Biosci. 2024, 61, 104495. [Google Scholar] [CrossRef]
- Preedalikit, W.; Chittasupho, C.; Leelapornpisid, P.; Qi, S.; Kiattisin, K. Development and Evaluation of Anti-Pollution Film-Forming Facial Spray Containing Coffee Cherry Pulp Extract. Pharmaceutics 2025, 17, 360. [Google Scholar] [CrossRef]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Complex plant responses to drought and heat stress under climate change. Plant J. 2024, 117, 1873–1892. [Google Scholar] [CrossRef] [PubMed]
- She, M.; Zheng, D.; Zhang, S.; Ke, Z.; Wu, Z.; Zou, H.; Zhang, Z. Functional analysis of maize GRAS transcription factor gene ZmGRAS72 in response to drought and salt stresses. Agric. Commun. 2024, 2, 100054. [Google Scholar] [CrossRef]
- Kumari, S.; Basu, S.; Kumar, G. A systematic review on the implications of concurrent heat and drought stress in modulating floral development in plants. Plant Sci. 2024, 349, 112248. [Google Scholar] [CrossRef]
- Ilea, M.I.M.; Zapata, P.J.; Fernández-Picazo, C.; Díaz-Mula, H.M.; Castillo, S.; Guillén, F. Chlorogenic Acid as a Promising Tool for Mitigating Chilling Injury: Cold Tolerance and the Ripening Effect on Tomato Fruit (Solanum lycopersicum L.). Plants 2024, 13, 2055. [Google Scholar] [CrossRef]
- Sui, X.; Meng, Z.; Dong, T.T.; Fan, X.T.; Wang, Q.G. Enzymatic browning and polyphenol oxidase control strategies. Curr. Opin. Biotechnol. 2023, 81, 102921. [Google Scholar] [CrossRef]
- Wang, T.T.; Yan, T.; Shi, J.K.; Sun, Y.M.; Wang, Q.G.; Li, Q.Q. The stability of cell structure and antioxidant enzymes are essential for fresh-cut potato browning. Food Res. Int. 2023, 164, 112449. [Google Scholar] [CrossRef]
- Tilley, A.; McHenry, M.P.; McHenry, J.A.; Solah, V.; Bayliss, K. Enzymatic browning: The role of substrates in polyphenol oxidase mediated browning. Curr. Res. Food Sci. 2023, 7, 100623. [Google Scholar] [CrossRef] [PubMed]
- McLarin, M.A.; Leung, I.K.H. Substrate specificity of polyphenol oxidase. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 274–308. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Luo, S.; Shen, J.; Li, C.; Kadeer, W.; Chen, L.; Li, X.; Jiang, Y.; Tang, Y. Synergistic anti-browning effects of short-term high oxygen pre-stimulation and supercooled storage on fresh-cut potatoes by regulating polyphenol biosynthesis and membrane lipid oxidation. Postharvest Biol. Technol. 2025, 219, 113257. [Google Scholar] [CrossRef]
- You, W.L.; Wang, C.F.; Zhang, J.L.; Ru, X.Y.; Xu, F.; Wu, Z.G.; Jin, P.; Zheng, Y.H.; Cao, S.F. Exogenous chlorogenic acid inhibits quality deterioration in fresh-cut potato slices. Food Chem. 2024, 446, 138866. [Google Scholar] [CrossRef]
- Perveen, N.; Dinesh, M.R.; Sankaran, M.; Lakkireddy, V.; Shivashankara, K.S.; Venugopal, R. Phenolics Signatures in Response to Salinity Stress Provide Novel Insights into Physiological Basis of Salt Tolerance in Mango (Mangifera indica L.). J. Plant Growth Regul. 2024, 43, 4866–4885. [Google Scholar] [CrossRef]
- Feng, Y.; Tian, W.; Guo, J.; Fu, J.; Wang, J.; Wang, Y.; Zhao, Z. Regulation of MdANR in Anti-Burning Process of Apple Peel. Int. J. Mol. Sci. 2025, 26, 4656. [Google Scholar] [CrossRef]
- Elbasan, F.; Arikan, B.; Ozfidan-Konakci, C.; Tofan, A.; Yildiztugay, E. Hesperidin and chlorogenic acid mitigate arsenic-induced oxidative stress via redox regulation, photosystems-related gene expression, and antioxidant efficiency in the chloroplasts of Zea mays. Plant Physiol. Biochem. 2024, 208, 108445. [Google Scholar] [CrossRef]
- Kumar, M.; Umesh, K.P.; Pandey, P.P.; Firake, D.M.; Pandit, S.S. Eggplant’s chlorogenic acid provides resistance against the tropical armyworm. bioRxiv 2023. [Google Scholar] [CrossRef]
- Lin, D.J.; Fang, Y.; Li, L.Y.; Zhang, L.Z.; Gao, S.J.; Wang, R.; Wang, J.D. The insecticidal effect of the botanical insecticide chlorogenic acid on Mythimna separata (Walker) is related to changes in MsCYP450 gene expression. Front. Plant Sci. 2022, 13, 1015095. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Katan, M.B.; Hollman, P.C.H. Chlorogenic Acid and Caffeic Acid Are Absorbed in Humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef]
- Farah, A.; de Paula Lima, J. Coffee: Consumption and Health Implications; Farah, A., Farah, A., Eds.; The Royal Society of Chemistry: London, UK, 2019; pp. 364–415. [Google Scholar] [CrossRef]
- Monteiro, M.; Farah, A.; Perrone, D.; Trugo, L.C.; Donangelo, C. Chlorogenic Acid Compounds from Coffee Are Differentially Absorbed and Metabolized in Humans1,2. J. Nutr. 2007, 137, 2196–2201. [Google Scholar] [CrossRef]
- Stalmach, A.; Mullen, W.; Barron, D.; Uchida, K.; Yokota, T.; Cavin, C.; Steiling, H.; Williamson, G.; Crozier, A. Metabolite Profiling of Hydroxycinnamate Derivatives in Plasma and Urine after the Ingestion of Coffee by Humans: Identification of Biomarkers of Coffee Consumption. Drug Metab. Dispos. 2009, 37, 1749–1758. [Google Scholar] [CrossRef]
- Stalmach, A.; Steiling, H.; Williamson, G.; Crozier, A. Bioavailability of chlorogenic acids following acute ingestion of coffee by humans with an ileostomy. Arch. Biochem. Biophys. 2010, 501, 98–105. [Google Scholar] [CrossRef]
- Renouf, M.; Marmet, C.; Giuffrida, F.; Lepage, M.; Barron, D.; Beaumont, M.; Williamson, G.; Dionisi, F. Dose-response plasma appearance of coffee chlorogenic and phenolic acids in adults. Mol. Nutr. Food Res. 2014, 58, 301–309. [Google Scholar] [CrossRef]
- Williamson, G.; Stalmach, A. Absorption and metabolism of dietary chlorogenic acids and procyanidins. In Recent Advances in Polyphenol Research; Cheynier, V., Sarni-Manchado, P., Quideau, S., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2012; Volume 3, pp. 209–222. [Google Scholar] [CrossRef]
- Cao, X.X.; Wu, C.M.; Tian, Y.; Guo, P. The caffeic acid moiety plays an essential role in attenuating lipid accumulation by chlorogenic acid and its analogues. Rsc Adv. 2019, 9, 12247–12254. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).