Pennisetum glaucum (L.) Oral Supplementation Mitigates Multi-Organic Dysfunction Associated with Carcinogenesis in HPV16-Transgenic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Animals
2.3. Pennisetum Glaucum
2.4. Experimental Design
2.5. Body Weight, Food and Water Consumption, and Lee Index
2.6. Animals Sacrifice and Necropsy
2.7. Blood Sample Collection and Analysis
2.8. Oxidative Stress Analysis
2.9. Histological Analysis
2.10. Statistical Analysis
3. Results
3.1. General Results
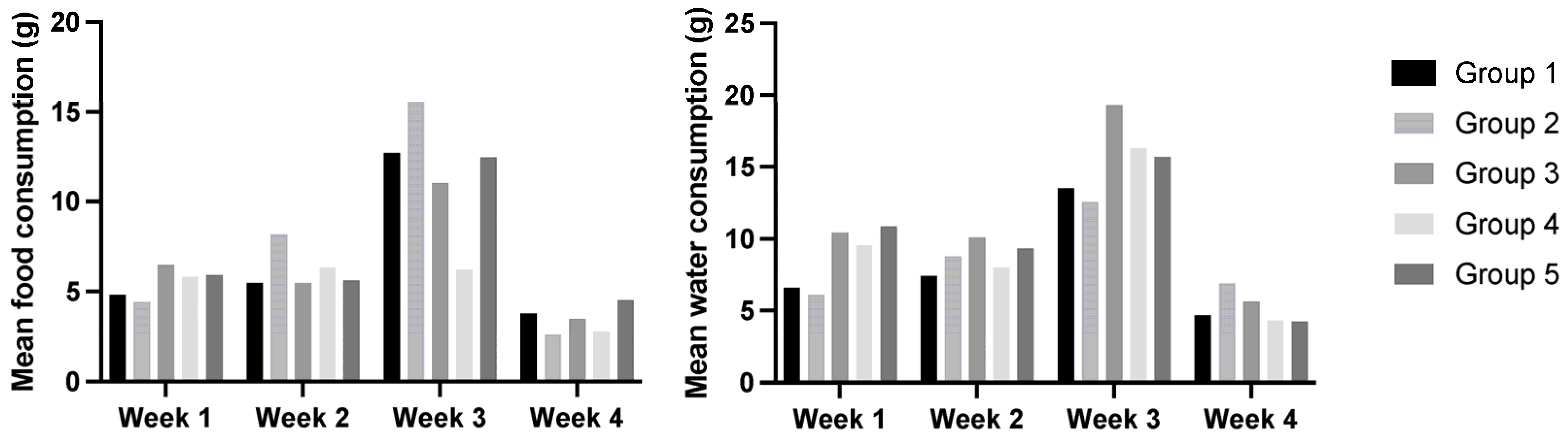
3.2. Food and Water Consumption
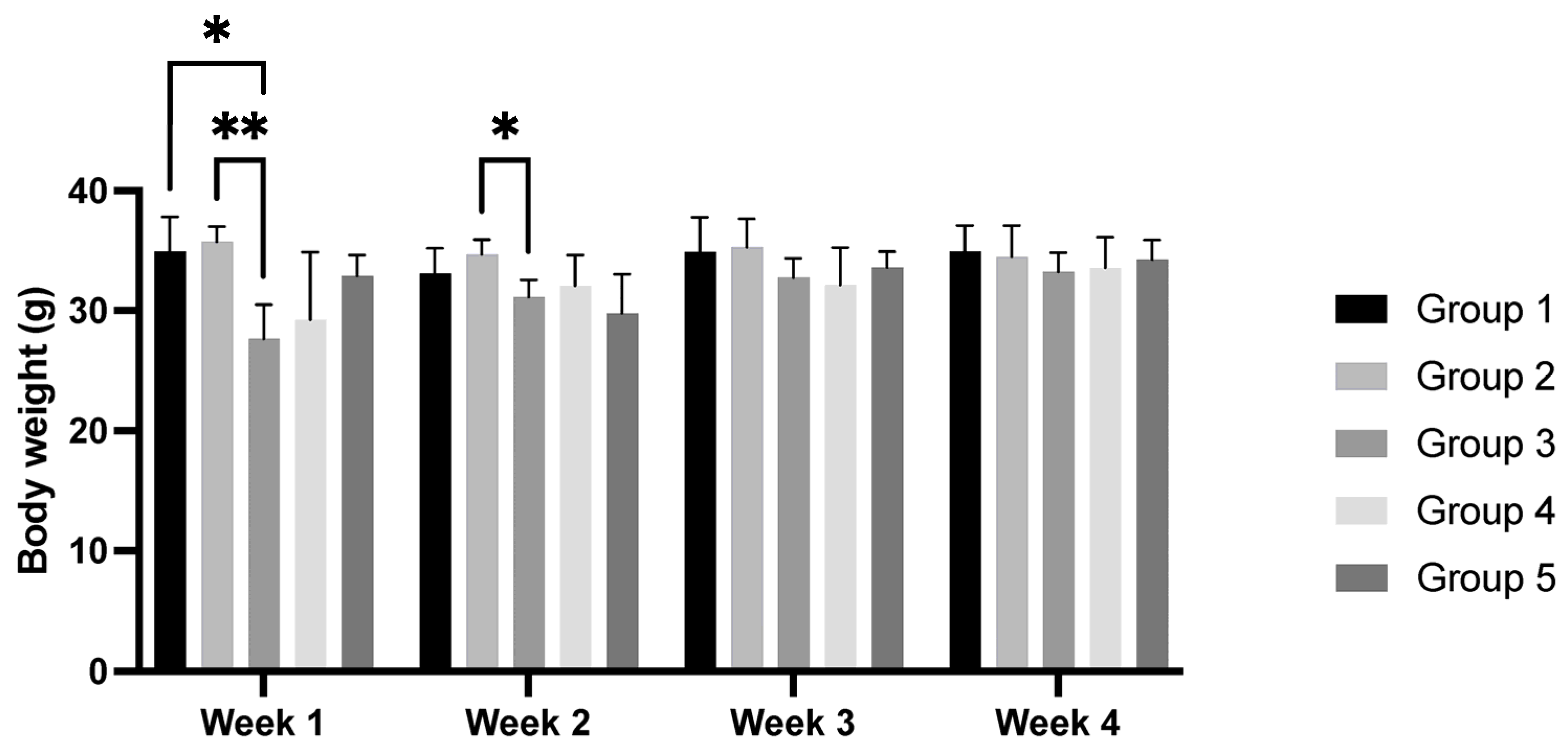
3.3. Body Weight and Murinometric Variables
3.4. Organs Weight
3.5. Hematological Parameters
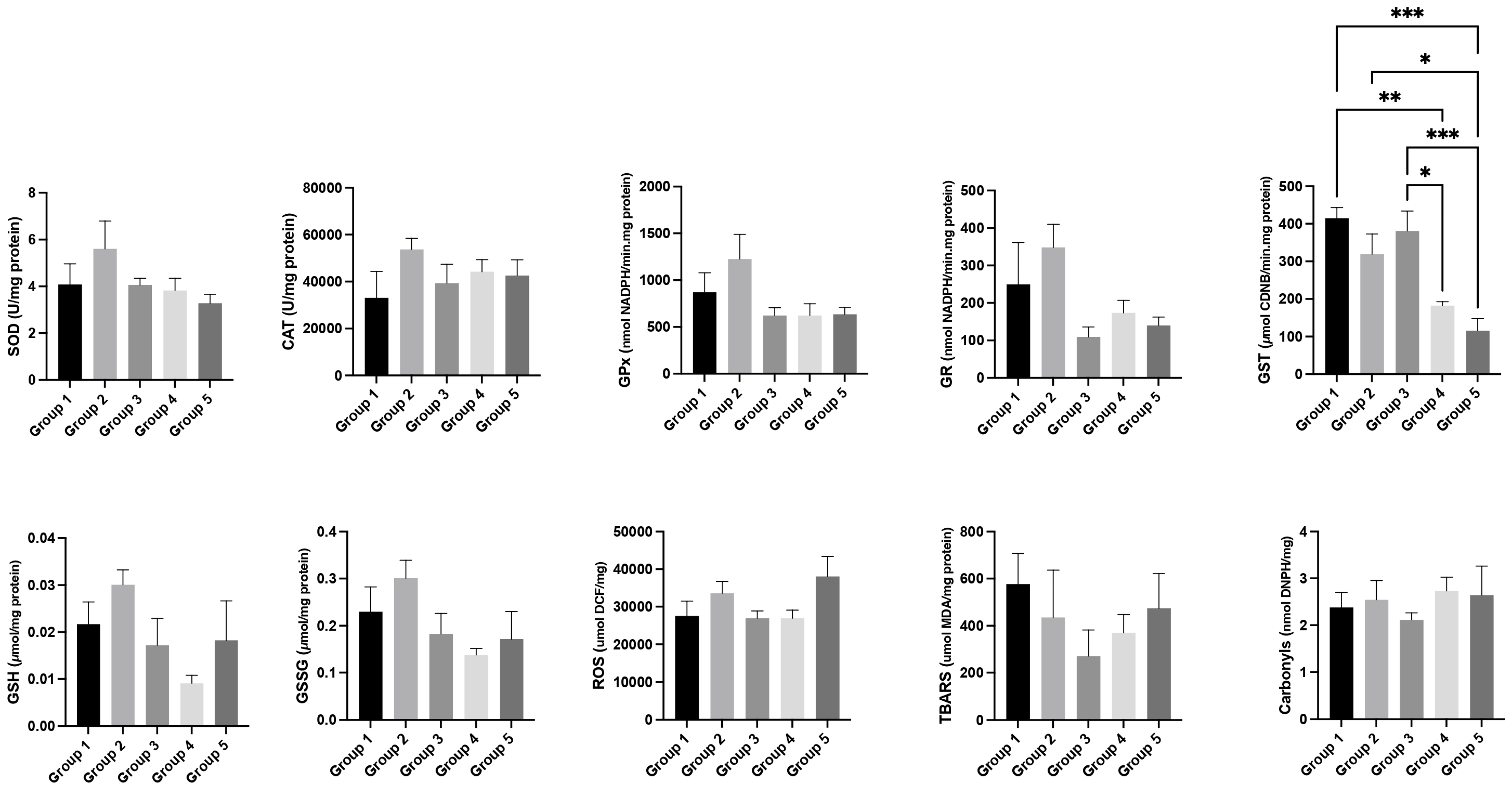
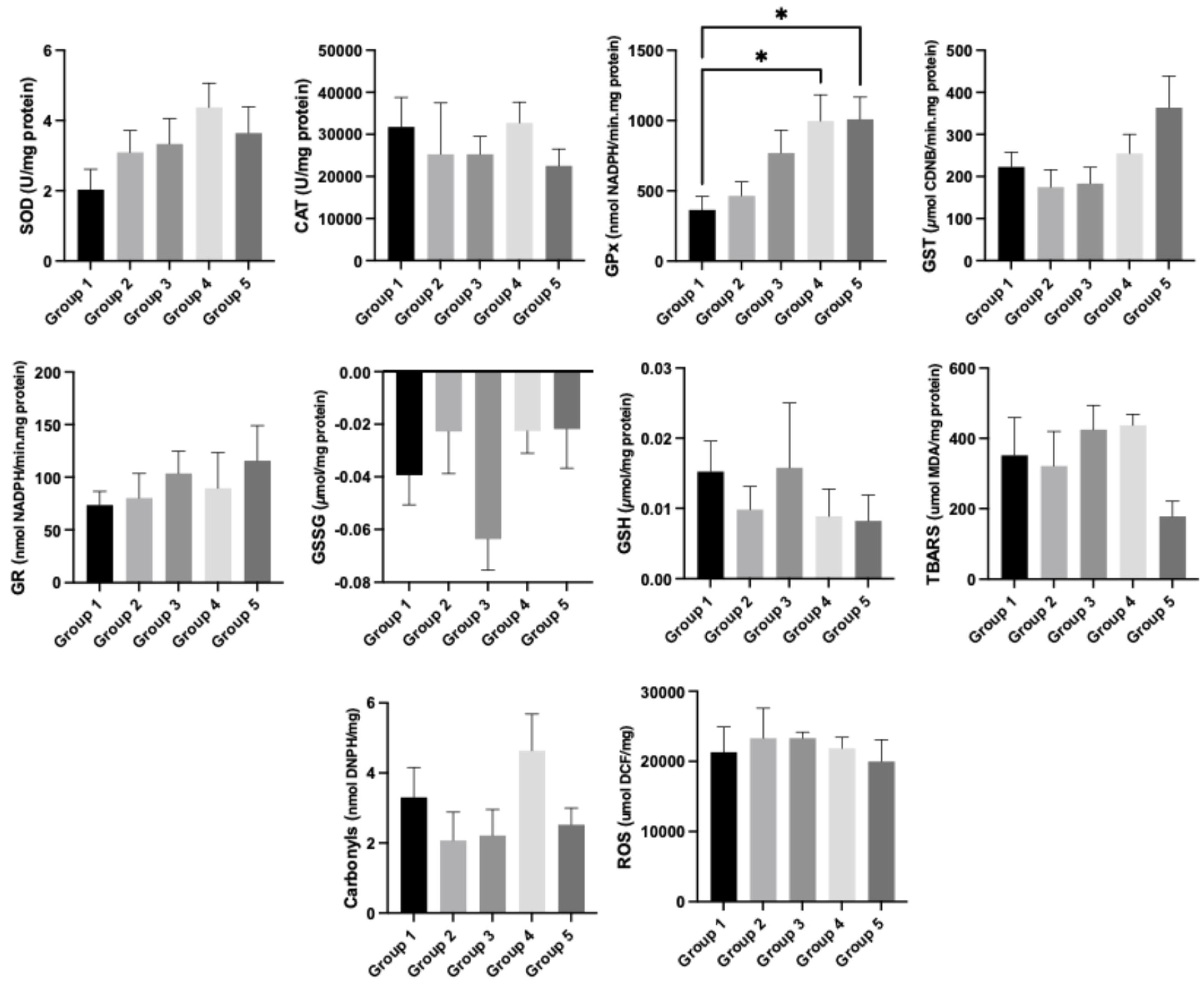
3.6. Oxidative Stress Analysis
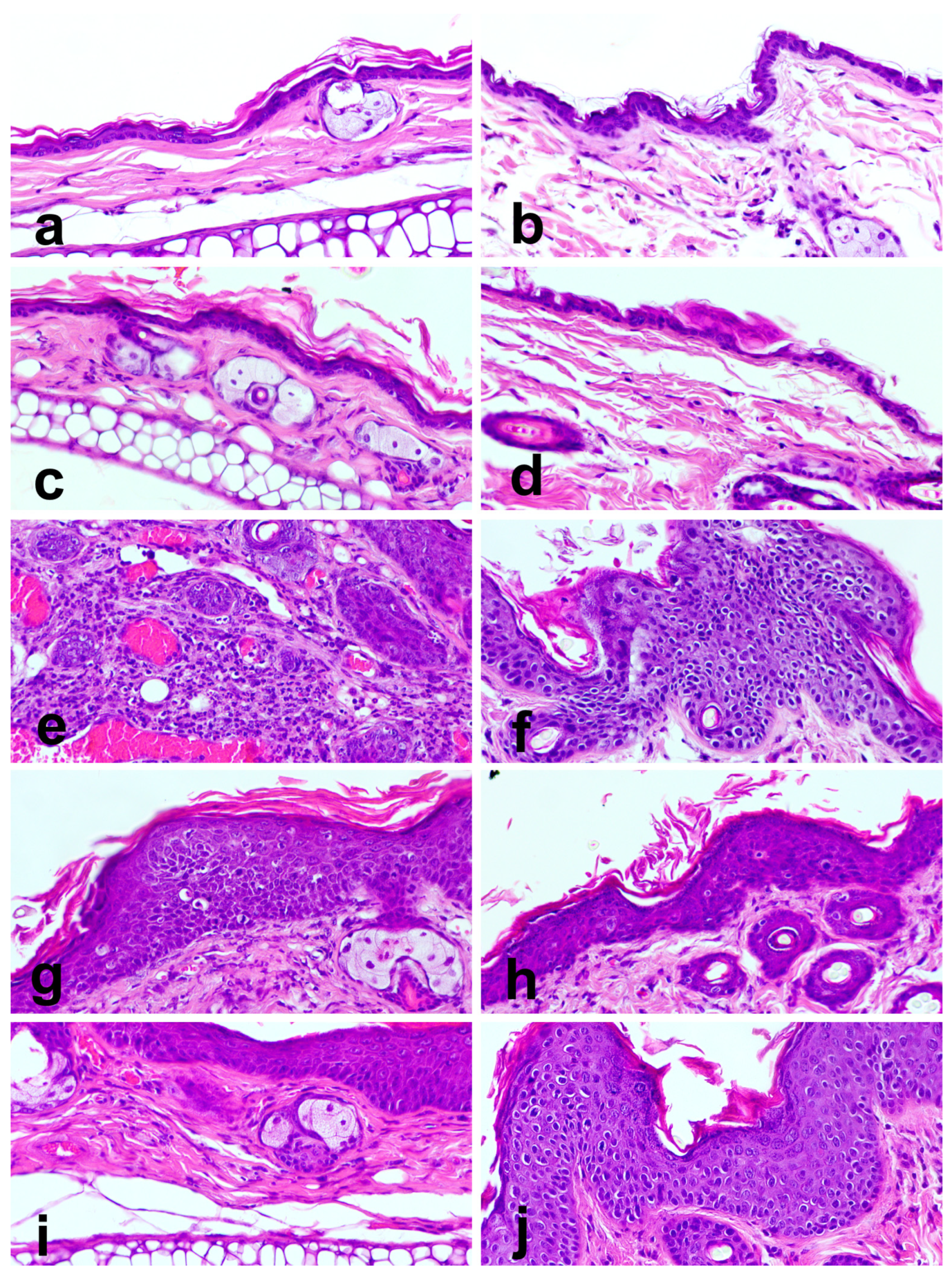
3.7. Epithelial Carcinogenesis
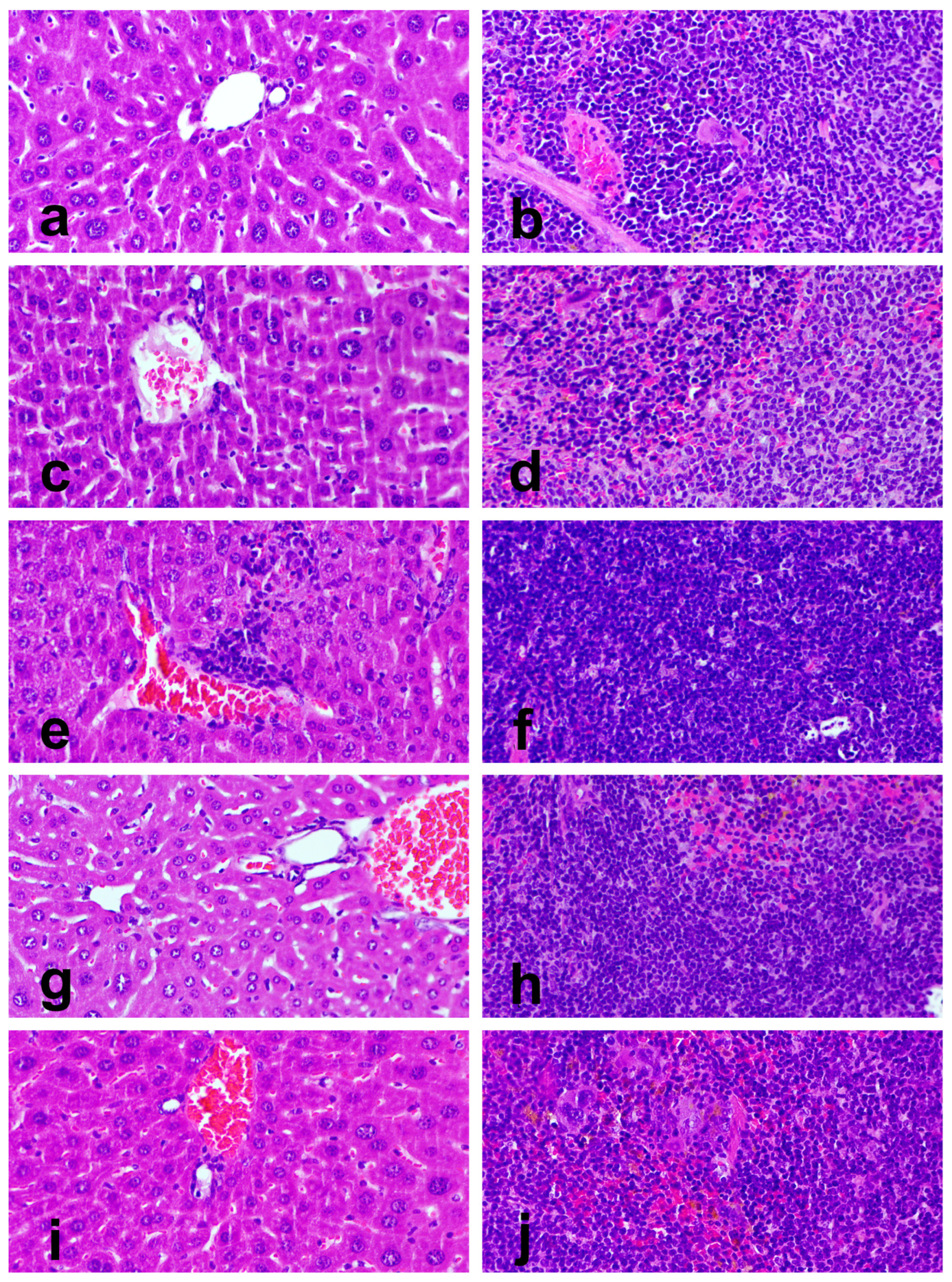
3.8. Organ Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BW | Body weight |
| CAT | Catalase |
| E | Early |
| EDTA | Ethylene diamine tetraacetic acid |
| FELASA | Federation for Laboratory Animal Science Associations |
| GPx | Glutathione peroxidase |
| GR | Glutathione reductase |
| GSH | Glutathione in the reduced state |
| GSSG | Glutathione in the oxidized state |
| GST | Glutathione S-transferase |
| HPV | Human papillomavirus |
| IARC | International Agency for Research on Cancer |
| L | Late |
| LCR | Long control region |
| P. glaucum | Pennisetum glaucum |
| ROS | Oxygen reactive species |
| S.E. | Standard error |
| SOD | Superoxide dismutase |
| TBARS | Thiobarbituric acid reactive substances |
| WT | Wildtype |
References
- Jensen, J.E.; Becker, G.L.; Jackson, J.B.; Rysavy, M.B. Human papillomavirus and associated cancers: A review. Viruses 2024, 16, 680. [Google Scholar] [CrossRef]
- Gil da Costa, R.M.; Medeiros, R. Bovine papillomavirus: Opening new trends for comparative pathology. Arch. Virol. 2014, 159, 191–198. [Google Scholar] [CrossRef]
- McBride, A.A. The Papillomavirus E2 proteins. Virology 2013, 445, 57–79. [Google Scholar] [CrossRef]
- Estêvão, D.; Costa, N.R.; Gil da Costa, R.M.; Medeiros, R. Hallmarks of HPV carcinogenesis: The role of E6, E7 and E5 oncoproteins in celular malignancy. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 153–162. [Google Scholar] [CrossRef]
- Araldi, R.P.; Sant’Ana, T.A.; Módolo, D.G.; de Melo, T.C.; Spadacci-Morena, D.D.; de Cassia Stocco, R.; Cerutti, J.M.; de Souza, E.B. The human papillomavirus (HPV)-related cancer biology: An overview. Biomed. Pharmacother. 2018, 106, 1537–1556. [Google Scholar] [CrossRef]
- Carvalho, N.S.; Silva, R.J.; Val, I.C.; Bazzo, M.L.; Silveira, M.F. Brazilian Protocol for Sexually Transmitted Infections 2020: Human papillomavirus (HPV) infection. Rev. Soc. Bras. Med. Trop. 2021, 54, e2020790. [Google Scholar] [PubMed]
- Leto Mdas, G.P.; Santos Júnior GFdos Porro, A.M.; Tomimori, J. Infecção pelo papilomavírus humano: Etiopatogenia, biologia molecular e manifestações clínicas. An. Bras. Dermatol. 2011, 86, 306–317. [Google Scholar]
- Thobias, A.R.; Patel, M.; Vaghela, C.; Patel, P.S. Attributes of HPV associated cancers. Clin. Transl. Oncol. 2025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Aleezada, Z.N.; Patel, I.; Yusuf, N. Understanding HPV-Induced Cancers and Investigating the Barriers Faced by Low- and Middle-Income Countries in Prevention and Treatment. Int. J. Mol. Sci. 2025, 26, 5581. [Google Scholar] [CrossRef]
- Pantaleão, C.; Luchs, A. Câncer e modelos experimentais de tumores murinos. Rev. Inst. Adolfo Lutz. 2010, 69, 439–445. [Google Scholar]
- Cochicho, D.; Esteves, S.; Rito, M.; Silva, F.; Martins, L.; Montalvão, P.; Cunha, M.; Magalhães, M.; Gil da Costa, R.M.; Felix, A. PIK3CA gene mutations in HNSCC: Systematic review and correlations with HPV status and patient survival. Cancers 2022, 14, 1286. [Google Scholar] [CrossRef]
- Medeiros-Fonseca, B.; Mestre, V.F.; Diogo Estêvão, D.; Sanchez, D.F.; Cañete-Portillo, S.; Fernández-Nestosa, M.J.; Casaca, F.; Silva, S.; Brito, H.; Félix, A.; et al. HPV16 induces penile intraepithelial neoplasia and squamous cell carcinoma in transgenic mice: First mouse model for HPV-related penile cancer. J. Pathol. 2020, 251, 411–419. [Google Scholar] [CrossRef]
- Mestre, V.F.; Medeiros-Fonseca, B.; Estêvão, D.; Casaca, F.; Silva, S.; Félix, A.; Silva, F.; Colaço, B.; Seixas, F.; Bastos, M.M.S.M.; et al. HPV16 is sufficient to induce squamous cell carcinoma specifically in the tongue base in transgenic mice. J. Pathol. 2020, 251, 4–11. [Google Scholar] [CrossRef]
- Gil da Costa, R.M.; Aragão, S.; Moutinho, M.; Alvarado, A.; Carmo, D.; Casaca, F.; Silva, S.; Ribeiro, J.; Sousa, H.; Ferreira, R.; et al. HPV16 induces a wasting syndrome in transgenic mice: Amelioration by dietary polyphenols via NF-κB inhibition. Life Sci. 2017, 169, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Peixoto da Silva, S.; Santos, J.M.O.; Mestre, V.F.; Medeiros-Fonseca, B.; Oliveira, P.A.; MSMBastos, M.; Gil da Costa, R.M.; Medeiros, R. Human Papillomavirus 16-Transgenic Mice as a Model to Study Cancer-Associated Cachexia. Int. J. Mol. Sci. 2020, 21, 5020. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Carvalho, R. Kiwi fruit residues from industry processing: Study for a maximum phenolic recovery yield. J. Food Sci. Technol. 2020, 57, 4265–4276. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhaka, N.; Dhewa, T.; Puniya, A.K. Nutritional and health-promoting attributes of millet: Current and future perspectives. Nutr. Rev. 2022, 81, 684–704. [Google Scholar] [CrossRef]
- Tanwar, R.; Panghal, A.; Kumari, A.; Chhikara, N. Nutritional, Phytochemical and Functional Potential of Pearl Millet: A Review. Chem. Biodivers. 2025, 22, e202402437. [Google Scholar] [CrossRef]
- Iyda, J.H.; Fernandes, Â.; Ferreira, F.D.; Alves, M.J.; Pires, T.C.S.P.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical composition and bioactive properties of the wild edible plant Raphanus raphanistrum L. Food Res. Int. 2019, 121, 714–722. [Google Scholar]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Sumi, S.A.; Siraj, M.A.; Hossain, A.; Mia, M.S.; Afrin, S.; Rahman, M.M. Pharmacological Activities of Ficus racemosa and Analysis of Its Major Bioactive Polyphenols by HPLC-DAD. Highlights Med. Med. Sci. 2021, 5, 68–84. [Google Scholar]
- Vidhyalakshmi, R.; Prabhasankar, P.; Meera, M.S. Ultrasonication assisted pearl millet starch-germ complexing: Evaluation of starch characteristics and its influence on glycaemic index of bread. J. Cereal Sci. 2023, 112, 103686, Correction in J Cereal Sci. 2024, 119, 104000. [Google Scholar] [CrossRef]
- Kumar, R.; Dhiman, M.; Sharma, L.; Dadhich, A.; Kaushik, P.; Sharma, M.M. Nanofertilizers: The targeted nutrient supplier and enhance nutrients uptake by pearl millets (Pennisetum glaucum). Biocatal. Agric. Biotechnol. 2022, 45, 102524. [Google Scholar] [CrossRef]
- Krishnan, V.; Verma, P.; Saha, S.; Singh, B.; Vinutha, T.; Kumar, R.; Kulshreshta, A.; Singh, S.; Sathyavathi, T.; Sachdev, A.; et al. Polyphenol-enriched extract from pearl millet (Pennisetum glaucum) inhibits key enzymes involved in post prandial hyper glycemia (α-amylase, α-glucosidase) and regulates hepatic glucose uptake. Biocatal. Agric. Biotechnol. 2022, 43, 102411. [Google Scholar] [CrossRef]
- Arbeit, J.M.; Olson, D.C.; Hanahan, D. Upregulation of fibroblast growth factors and their receptors during multi-stage epidermal carcinogenesis in K14-HPV16 transgenic mice. Oncogene 1996, 13, 1847–1857. [Google Scholar]
- Paiva, I.; Gil da Costa, R.M.; Ribeiro, J.; Sousa, H.; Bastos, M.M.; Faustino-Rocha, A.; Lopes, C.; Oliveira, P.A.; Medeiros, R. MicroRNA-21 expression and susceptibility to HPV-induced carcinogenesis—Role of microenvironment in K14-HPV16 mice model. Life Sci. 2015, 128, 8–14. [Google Scholar] [CrossRef]
- Oliveira, M.; Nascimento-Gonçalves, E.; Silva, J.; Oliveira, P.A.; Ferreira, R.; Antunes, L.; Arantes-Rodrigues, R.; Faustino-Rocha, A.I. Implementation of Human Endpoints in a Urinary Bladder Carcinogenesis Study in Rats. In Vivo 2017, 31, 1073–1080. [Google Scholar] [CrossRef]
- Fushai, F.; Chitura, T.; Emmanuel, O. Climate-smart livestock nutrition in semi-arid Southern African agricultural systems. Front. Vet. Sci. 2025, 11, 1507152. [Google Scholar] [CrossRef]
- Azevedo, T.; Silva, J.; Peixoto, F.; Silvestre-Ferreira, A.C.; Gama, A.; Seixas, F.; Finimundy, T.C.; Barros, L.; Matos, M.; Oliveira, P.A.; et al. The role of natural compounds in rat mammary cancer: The beneficial effects of Santolina chamaecyparissus L. aqueous extract. Vet. Stanica 2024, 55, 45–61. [Google Scholar] [CrossRef]
- Novelli, E.L.B.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.X.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.H.; Cicogna, A.C.; Novelli Filho, J.L.V.B. Anthropometrical parameters and markers of obesity in rats. Lab Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef]
- Magalhães, T.L.S.; da Silva, B.P.; Grancieri, M.; Lúcio, H.G.; Toledo, R.C.L.; Barra, R.R.S.; de Carvalho, C.W.P.; Martino, H.S.D. Germinated and non-germinated cooked whole millet (Pennisetum glaucum (L.) R. Br.) flours show a promising effect on protein quality, biochemical profile and intestinal health. In Vivo 2023, 14, 5678–5689. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.; Castro-Ribeiro, C.; Lemos, S.; Ferreira, T.; Nascimento-Gonçalves, E.; Rosa, E.; Oliveira, P.A.; Antunes, L.M. Murine Models of Obesity. Obesities 2022, 2, 127–147. [Google Scholar] [CrossRef]
- Ouedraogo, T.-W.C.; Djigma, F.W.; Zohoncon, T.M.; Idani, B.; Ouattara, A.K.; Sorgho, P.A.; Obiri-Yeboah, D.; Bado, P.; Traore, M.A.E.; Diarra, B.; et al. Association between polymorphisms of Glutathione S-Transferase and progression to cervical cancer in women from Burkina Faso and Mali. J. Biosci. Med. 2020, 8, 12–25. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Theodoro, J.M.V.; Grancieri, M.; Gomes, M.J.C.; Toledo, R.C.L.; José, V.P.B.d.S.; Mantovani, H.C.; Carvalho, C.W.P.; da Silva, B.P.; Martino, H.S.D. Germinated Millet (Pennisetum glaucum (L.) R. Br.) Flour Improved the Gut Function and Its Microbiota Composition in Rats Fed with High-Fat High-Fructose Diet. Int. J. Environ. Res. Public Health 2022, 19, 15217. [Google Scholar] [CrossRef]
| Group | 1st Week | 4th Week | |
|---|---|---|---|
| 1 | WT, standard diet | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 2 | WT, 36% Pennisetum glaucum | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 3 | HPV+, standard diet | 0.80 ± 0.20 | 1.20 ± 0.20 |
| 4 | HPV+, 29% Pennisetum glaucum | 0.80 ± 0.20 | 0.40 ± 0.24 |
| 5 | HPV+, 36% Pennisetum glaucum | 0.40 ± 0.24 | 0.60 ± 0.24 |
| Group/Parameter | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
|---|---|---|---|---|---|
| WT Standard Diet | WT 36% P. glaucum | HPV+ Standard Diet | HPV+ 29% P. glaucum | HPV+ 36% P. glaucum | |
| Ponderal weight gain (%) | 0.28 ± 3.10 | −3.47 ± 2.18 * | 21.46 ± 7.06 | 17.56 ± 9.42 | 4.30 ± 2.50 |
| Body mass index | 0.32 ± 0.01 | 0.33 ± 0.01 | 0.34 ± 0.01 | 0.38 ± 0.05 | 0.38 ± 0.02 |
| Naso-anal length (cm) | 10.50 ± 0.27 | 10.30 ± 0.12 | 9.90 ± 0.19 | 9.50 ± 0.45 | 9.60 ± 0.29 |
| Naso-caudal length (cm) | 19.50 ± 0.41 | 20.00 ± 0.20 | 19.10 ± 0.24 | 20.50 ± 1.16 | 19.10 ± 0.24 |
| Abdominal perimeter (cm) | 7.70 ± 0.20 | 7.70 ± 0.12 | 7.10 ± 0.19 | 7.70 ± 0.20 | 7.50 ± 0.16 |
| Lee index | 0.56 ± 0.01 | 0.57 ± 0.01 | 0.58 ± 0.01 | 0.62 ± 0.04 | 0.61 ± 0.02 |
| Group | Heart | Lungs | Liver | Spleen | Right Kidney | Left Kidney | |
|---|---|---|---|---|---|---|---|
| 1 | WT, standard diet | 0.18 ± 0.01 0.005 ± 0.000 | 0.23 ± 0.02 0.007 ± 0.001 | 1.45 ± 0.06 0.042 ± 0.001 | 0.21 ± 0.04 0.006 ± 0.003 | 0.26 ± 0.01 b 0.007 ± 0.000 c | 0.25 ± 0.02 0.007 ± 0.001 |
| 2 | WT, 36% P. glaucum | 0.41 ± 0.23 0.012 ± 0.007 | 0.21 ± 0.01 0.006 ± 0.001 | 1.39 ± 0.06 0.040 ± 0.003 a | 0.17 ± 0.05 0.005 ± 0.003 | 0.25 ± 0.01 0.007 ± 0.001 | 0.25 ± 0.01 0.007 ± 0.000 |
| 3 | HPV+, standard diet | 0.16 ± 0.01 0.005 ± 0.000 | 0.19 ± 0.01 0.006 ± 0.000 | 1.48 ± 0.04 0.044 ± 0.002 | 0.15 ± 0.02 0.004 ± 0.001 | 0.21 ± 0.01 d 0.006 ± 0.001 | 0.22 ± 0.01 0.007 ± 0.000 |
| 4 | HPV+, 29% P. glaucum | 0.15 ± 0.01 0.004 ± 0.000 | 0.20 ±0.01 0.006 ± 0.000 | 1.37 ± 0.05 0.041 ± 0.001 | 0.16 ± 0.01 0.005 ± 0.001 | 0.21 ± 0.01 d 0.006 ± 0.000 | 0.21 ± 0.01 0.006 ± 0.000 |
| 5 | HPV+, 36% P. glaucum | 0.17 ± 0.01 0.005 ± 0.000 | 0.21 ± 0.01 0.006 ± 0.001 | 1.52 ± 0.04 0.044 ± 0.002 | 0.18 ± 0.01 0.005 ± 0.001 | 0.25 ± 0.01 0.007 ± 0.000 | 0.24 ± 0.01 0.007 ± 0.000 |
| Group | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
|---|---|---|---|---|---|
| WT Standard Diet | WT 36% P. glaucum | HPV+ Standard Diet | HPV+ 29% P. glaucum | HPV+ 36% P. glaucum | |
| Red blood cell parameters | |||||
| Erythrocytes (M/μL) | 9.40 ± 0.32 | 9.49 ± 0.39 | 10.01 ± 0.26 | 8.68 ± 0.56 | 8.58 ± 1.45 |
| Hematocrit (%) | 41.25 ± 3.30 | 39.60 ± 2.19 | 43.20 ± 1.04 | 41.20 ± 1.25 | 42.10 ± 2.13 |
| Hemoglobin (g/dL) | 14.24 ± 1.94 | 13.92 ± 0.70 | 14.82 ± 0.27 | 13.34 ± 0.62 | 13.58 ± 0.75 |
| MCV (fL) | 53.30 ± 6.49 | 49.50 ± 2.76 | 46.86 ± 1.01 | 46.04 ± 0.61 | 47.78 ± 1.58 |
| MCH (pg) | 15.92 ± 1.00 | 15.38 ± 0.40 | 14.80 ± 0.24 | 14.66 ± 0.17 | 15.14 ± 0.36 |
| MCHC (g/dL) | 30.05 ± 1.78 | 31.16 ± 1.57 | 31.60 ± 0.35 | 31.86 ± 0.42 | 31.70 ± 0.27 |
| Reticulocytes (K/μL) | 581.16 ± 442.36 | 584.14 ± 580.34 | 407.64 ± 45.78 | 399.54 ± 47.77 | 374.44 ± 49.90 |
| White blood cell parameters | |||||
| Leukocytes (K/μL) | 1.30 ± 0.54 | 1.96 ± 1.57 | 1.94 ± 3.15 | 0.90 ± 0.31 | 2.52 ± 1.85 |
| Neutrophils (K/μL) | 0.63 ± 0.55 | 0.79 ± 1.12 | 1.25 ± 2.23 | 0.21 ± 0.08 | 1.40 ± 1.78 |
| Lymphocytes (K/μL) | 0.64 ± 0.12 | 1.11 ± 0.88 | 0.76 ± 0.74 | 0.64 ± 0.24 | 1.03 ± 0.58 |
| Monocytes (K/μL) | 0.02 ± 0.01 | 0.04 ± 0.04 | 0.06 ± 0.09 | 0.03 ± 0.01 | 0.05 ± 0.02 |
| Eosinophils (K/μL) | 0.0075 ± 0.005 | 0.014 ± 0.009 | 0.012 ± 0.02 | 0.008 ± 0.004 | 0.022 ± 0.019 |
| Basophils (K/μL) | 0.0075± 0.005 | 0.004 ± 0.005 | 0.010 ± 0.012 | 0.010 ± 0.012 | 0.008 ± 0.008 |
| Platelet parameters | |||||
| Platelets (K/μL) | 1108.75 ± 128.32 | 1130.00 ± 117.24 | 1025.80 ± 78.24 | 940.00 ± 53.94 | 1061.40 ± 102.14 |
| MPV (fL) | 7.45 ± 0.24 | 7.62 ± 0.19 | 7.42 ± 0.16 | 7.58 ± 0.04 | 7.70 ± 0.19 |
| PDW (fL) | 6.95 ± 0.37 | 6.90 ± 0.38 | 6.78 ± 0.13 | 6.90 ± 0.23 | 7.00 ± 0.30 |
| Serum parameters | |||||
| Total proteins (g/dL) | 5.50 ± 0.42 | 5.44 ± 0.46 | 5.76 ± 0.30 | 5.28 ± 0.30 | 5.94 ± 0.33 |
| Glucose (mg/dL) | 163.75 ± 36.39 | 184.20 ± 10.10 | 243.40 ± 13.84 a | 207.20 ± 11.80 a | 178.00 ± 21.17 |
| Group (n = 5) | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|---|
| WT Standard Diet | WT 36% P. glaucum | HPV+ Standard Diet | HPV+ 29% P. glaucum | HPV+ 36% P. glaucum | ||
| Ear skin | Normal | 5 (100%) | 5 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hyperplasia | 0 (0%) | 0 (0%) | 0 (0%) | 3 (60%) | 4 (80%) | |
| Dysplasia | 0 (0%) | 0 (0%) | 4 (80%) | 2 (40%) | 1 (20%) | |
| SCC | 0 (0%) | 0 (0%) | 1 (20%) | 0 (0%) | 0 (0%) | |
| Chest skin | Normal | 5 (100%) | 5 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hyperplasia | 0 (0%) | 0 (0%) | 3 (60%) | 5 (100%) | 5 (100%) | |
| Dysplasia | 0 (0%) | 0 (0%) | 2 (40%) | 0 (0%) | 0 (0%) | |
| SCC | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Group (n = 5) | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|---|
| WT Standard Diet | WT 36% P. glaucum | HPV+ Standard Diet | HPV+ 29% P. glaucum | HPV+ 36% P. glaucum | ||
| Liver | Normal | 5 (100%) | 5 (100%) | 0 (0%) | 1 (20%) | 1 (20%) |
| Grade I hepatitis | 0 (0%) | 0 (0%) | 5 (100%) | 4 (80%) | 4 (80%) | |
| Grade II hepatitis | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Spleen | Normal | 5 (100%) | 5 (100%) | 4 (80%) | 2 (40%) | 5 (100%) |
| White pulp hyperplasia | 0 (0%) | 0 (0%) | 1 (20%) | 3 (60%) | 0 (0%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, P.A.; Hajri, L.; Moreno, A.V.P.; Santos, C.E.D.; Brito, H.O.; Bastos, M.M.S.M.; Medeiros, R.; Ghodbane, S.; Ammari, M.; Gil da Costa, R.M.; et al. Pennisetum glaucum (L.) Oral Supplementation Mitigates Multi-Organic Dysfunction Associated with Carcinogenesis in HPV16-Transgenic Mice. Curr. Issues Mol. Biol. 2025, 47, 858. https://doi.org/10.3390/cimb47100858
Oliveira PA, Hajri L, Moreno AVP, Santos CED, Brito HO, Bastos MMSM, Medeiros R, Ghodbane S, Ammari M, Gil da Costa RM, et al. Pennisetum glaucum (L.) Oral Supplementation Mitigates Multi-Organic Dysfunction Associated with Carcinogenesis in HPV16-Transgenic Mice. Current Issues in Molecular Biology. 2025; 47(10):858. https://doi.org/10.3390/cimb47100858
Chicago/Turabian StyleOliveira, Paula A., Latifa Hajri, Armando V. Pinto Moreno, Carlos E. Dias Santos, Haissa O. Brito, Margarida M. S. M. Bastos, Rui Medeiros, Soumaya Ghodbane, Mohamed Ammari, Rui M. Gil da Costa, and et al. 2025. "Pennisetum glaucum (L.) Oral Supplementation Mitigates Multi-Organic Dysfunction Associated with Carcinogenesis in HPV16-Transgenic Mice" Current Issues in Molecular Biology 47, no. 10: 858. https://doi.org/10.3390/cimb47100858
APA StyleOliveira, P. A., Hajri, L., Moreno, A. V. P., Santos, C. E. D., Brito, H. O., Bastos, M. M. S. M., Medeiros, R., Ghodbane, S., Ammari, M., Gil da Costa, R. M., & Faustino-Rocha, A. I. (2025). Pennisetum glaucum (L.) Oral Supplementation Mitigates Multi-Organic Dysfunction Associated with Carcinogenesis in HPV16-Transgenic Mice. Current Issues in Molecular Biology, 47(10), 858. https://doi.org/10.3390/cimb47100858










