Salivary BCAA, Glutamate, Glutamine and Urea as Potential Indicators of Nitrogen Metabolism Imbalance in Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Collection and Storage of Saliva Samples
2.3. Determination of the Composition of Saliva
2.4. Statistical Analysis
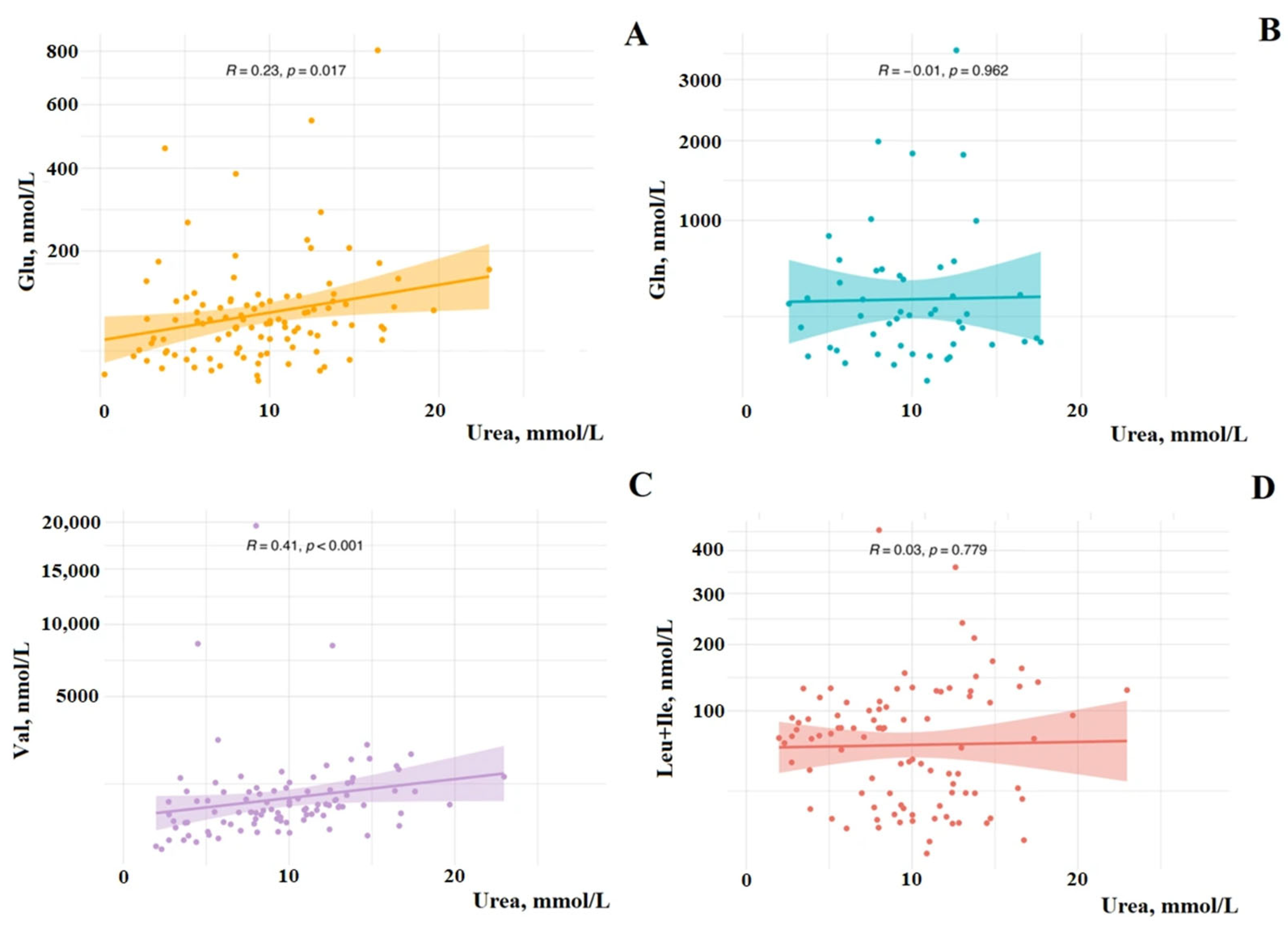
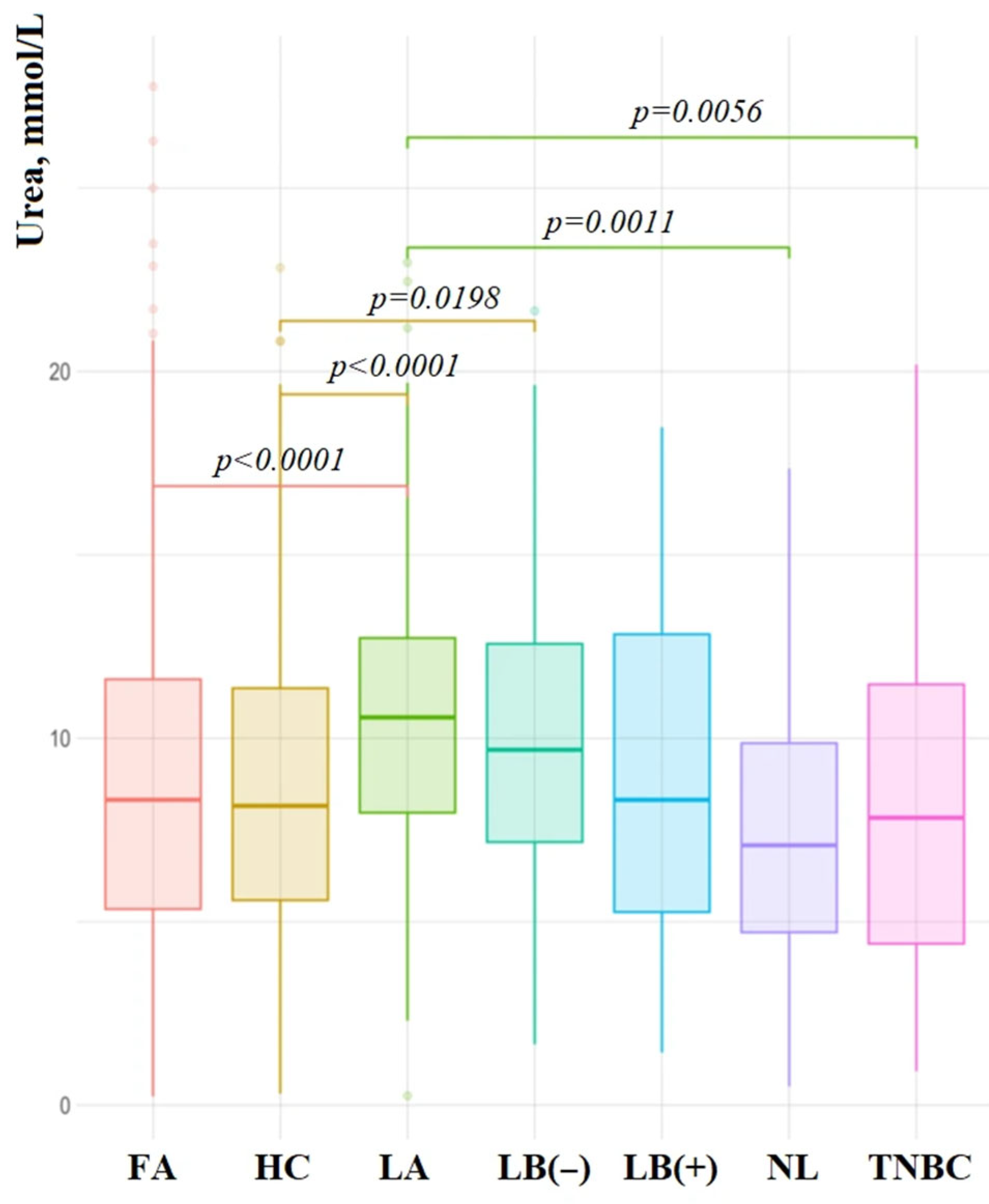
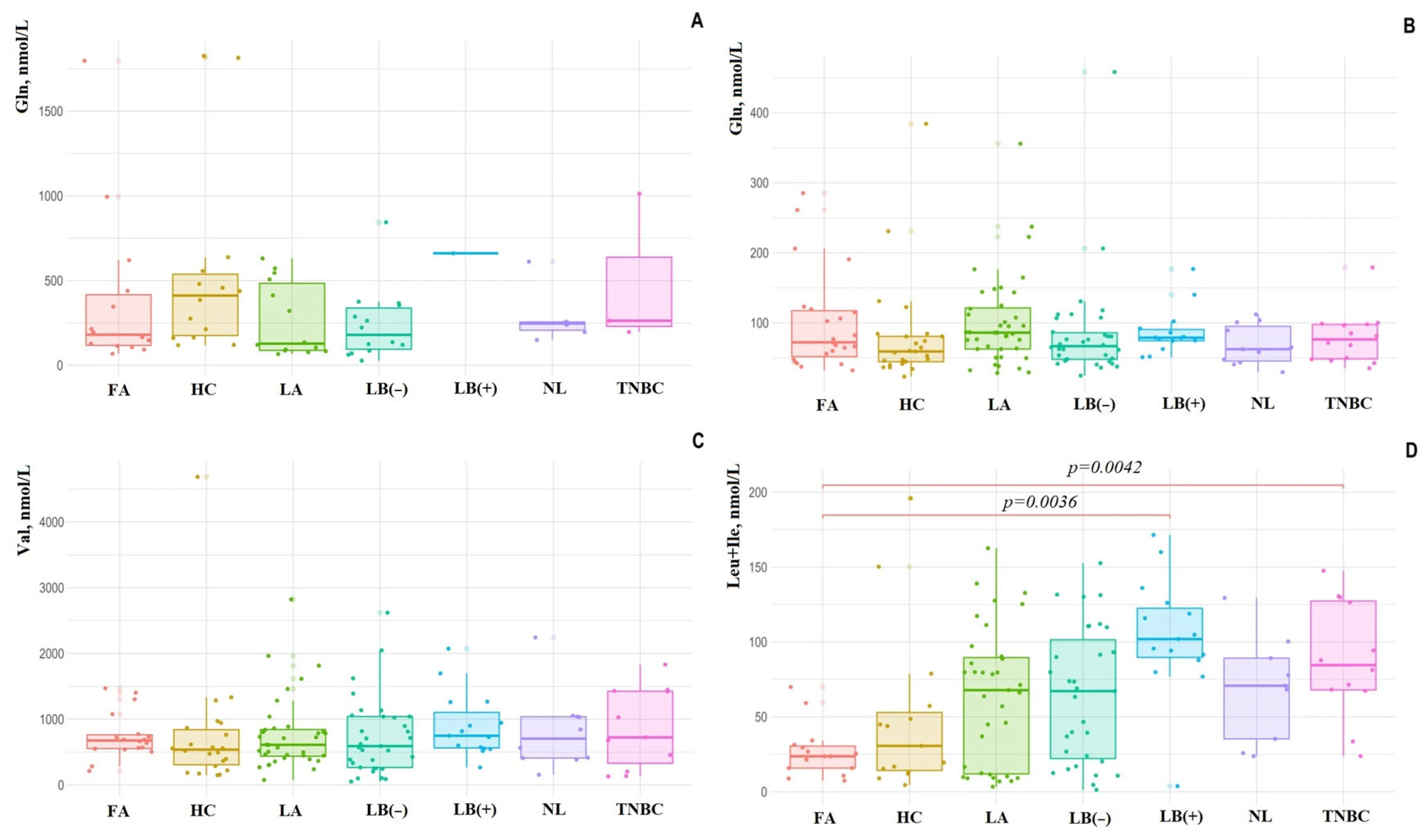
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhagavan, N.V. Metabolic Homeostasis CHAPTER 22. In Medical Biochemistry, 4th ed.; Bhagavan, N.V., Ed.; Academic Press: Cambridge, MA, USA, 2002; pp. 485–519. [Google Scholar] [CrossRef]
- Hoffer, L.J. Human Protein and Amino Acid Requirements. JPEN J. Parenter. Enter. Nutr. 2016, 40, 460–474. [Google Scholar] [CrossRef]
- Carco, P.; Canciullo, D. Urea excretion through the human salivary glands. Ann. Otol. Rhinol. Laryngol. 1958, 67, 1050–1065. [Google Scholar] [CrossRef]
- Cardoso, E.M.; Arregger, A.L.; Tumilasci, O.R.; Elbert, A.; Contreras, L.N. Assessment of salivary urea as a less invasive alternative to serum determinations. Scand. J. Clin. Lab. Investig. 2009, 69, 330–334. [Google Scholar] [CrossRef]
- Lin, T.L.; Evans, R.D.R.; Unwin, R.J.; Norman, J.T.; Rich, P.R. Assessment of Measurement of Salivary Urea by ATR-FTIR Spectroscopy to Screen for CKD. Kidney360 2021, 3, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Keshet, R.; Szlosarek, P.; Carracedo, A.; Erez, A. Rewiring Urea Cycle Metabolism in Cancer to Support Anabolism. Nat. Rev. Cancer 2018, 18, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiahou, Z.; Wu, W.; Song, Y. Nitrogen Metabolism Disorder Accelerates Occurrence and Development of Lung Adenocarcinoma: A Bioinformatic Analysis and In Vitro Experiments. Front. Oncol. 2022, 12, 916777. [Google Scholar] [CrossRef] [PubMed]
- Nan, D.; Yao, W.; Huang, L.; Liu, R.; Chen, X.; Xia, W.; Sheng, H.; Zhang, H.; Liang, X.; Lu, Y. Glutamine and cancer: Metabolism, immune microenvironment, and therapeutic targets. Cell Commun. Signal. 2025, 23, 45. [Google Scholar] [CrossRef]
- Li, T.; Le, A. Glutamine Metabolism in Cancer. In The Heterogeneity of Cancer Metabolism; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2018; Volume 1063, pp. 13–32. [Google Scholar] [CrossRef]
- Watford, M. Glutamine and glutamate: Nonessential or essential amino acids? Anim. Nutr. 2015, 1, 119–122. [Google Scholar] [CrossRef]
- Yudkoff, M. Interactions in the Metabolism of Glutamate and the Branched-Chain Amino Acids and Ketoacids in the CNS. Neurochem. Res. 2017, 42, 10–18. [Google Scholar] [CrossRef]
- Jin, J.; Byun, J.K.; Choi, Y.K.; Park, K.G. Targeting glutamine metabolism as a therapeutic strategy for cancer. Exp. Mol. Med. 2023, 55, 706–715. [Google Scholar] [CrossRef]
- Zhang, L.; Han, J. Branched-chain amino acid transaminase 1 (BCAT1) promotes the growth of breast cancer cells through improving mTOR-mediated mitochondrial biogenesis and function. Biochem. Biophys. Res. Commun. 2017, 486, 224–231. [Google Scholar] [CrossRef]
- Wang, L.; Shi, F.; Cao, Y.; Xie, L. Multiple roles of branched-chain amino acid metabolism in tumour progression. J. Biomed. Sci. 2025, 32, 41. [Google Scholar] [CrossRef]
- Xu, E.; Ji, B.; Jin, K.; Chen, Y. Branched-chain amino acids catabolism and cancer progression: Focus on therapeutic interventions. Front. Oncol. 2023, 13, 1220638. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wang, Y.; Luo, W. Multifaceted role of branched-chain amino acid metabolism in cancer. Oncogene 2020, 39, 6747–6756. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V.; Dyachenko, E.I. Salivary Biomarkers in Breast Cancer: From Salivaomics to Salivaoncoomics. Front. Biosci. (Landmark Ed.) 2024, 29, 253. [Google Scholar] [CrossRef]
- Shah, S. Salivaomics: The current scenario. J. Oral. Maxillofac. Pathol. 2018, 22, 375–381. [Google Scholar] [CrossRef]
- Gardner, A.; Parkes, H.G.; So, P.W.; Carpenter, G.H. Determining bacterial and host contributions to the human salivary metabolome. J. Oral. Microbiol. 2019, 11, 1617014. [Google Scholar] [CrossRef]
- Carpenter, G.H. Salivary Factors that Maintain the Normal Oral Commensal Microflora. J. Dent. Res. 2020, 99, 644–649. [Google Scholar] [CrossRef]
- Lasisi, T.J.; Raji, Y.R.; Salako, B.L. Salivary creatinine and urea analysis in patients with chronic kidney disease: A case control study. BMC Nephrol. 2016, 17, 10. [Google Scholar] [CrossRef]
- Tomás, I.; Marinho, J.S.; Limeres, J.; Santos, M.J.; Araújo, L.; Diz, P. Changes in salivary composition in patients with renal failure. Arch. Oral. Biol. 2008, 53, 528–532. [Google Scholar] [CrossRef]
- Klassen, J.T.; Krasko, B.M. The dental health status of dialysis patients. J. Can. Dent. Assoc. 2002, 68, 34–38. [Google Scholar]
- Huang, L.; Jiang, C.; Yan, M.; Wan, W.; Li, S.; Xiang, Z.; Wu, J. The oral-gut microbiome axis in breast cancer: From basic research to therapeutic applications. Front. Cell Infect. Microbiol. 2024, 14, 1413266. [Google Scholar] [CrossRef]
- Niu, C.; Tu, Y.; Jin, Q.; Chen, Z.; Yuan, K.; Wang, M.; Zhang, P.; Luo, J.; Li, H.; Yang, Y.; et al. Mapping the human oral and gut fungal microbiota in patients with metabolic dysfunction-associated fatty liver disease. Front. Cell. Infect. Microbiol. 2023, 13, 1157368. [Google Scholar] [CrossRef]
- Russo, E.; Gloria, L.D.; Nannini, G.; Meoni, G.; Niccolai, E.; Ringressi, M.N.; Baldi, S.; Fani, R.; Tenori, L.; Taddei, A.; et al. From adenoma to CRC stages: The oral-gut microbiome axis as A source of potential microbial and metabolic biomarkers of Malignancy. Neoplasia 2023, 40, 100901. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Su, D.; Zhang, H.; Lin, H.C.; Zhou, Q.; Cao, B.; Ren, D.L. Clinical implications of the oral−Gut microbiome axis and its association with colorectal cancer (Review). Oncol. Rep. 2022, 48, 192. [Google Scholar] [CrossRef] [PubMed]
- Elghannam, M.T.; Hassanien, M.H.; Ameen, Y.A.; Turky, E.A.; Elattar, G.M.; Elray, A.A.; Eltalkawy, M.D. Oral microbiota and liver diseases. Clin. Nutr. ESPEN 2023, 54, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Ahn, K.; Mun, S.; Yun, K.; Kim, Y.T.; Jung, W.; Lee, K.E.; Kim, M.Y.; Ahn, Y.; Han, K. Understanding the bacterial compositional network associations between oral and gut microbiome within healthy koreans. J. Oral. Microbiol. 2023, 15, 2186591. [Google Scholar] [CrossRef]
- Shinde, D.B.; Mahore, J.G.; Giram, P.S.; Singh, S.L.; Sharda, A.; Choyan, D.; Musale, S. Microbiota of Saliva: A Non-invasive Diagnostic Tool. Indian J. Microbiol. 2024, 64, 328–342. [Google Scholar] [CrossRef]
- Wu, Z.; Byrd, D.A.; Wan, Y.; Ansong, D.; Clegg-Lamptey, J.N.; Wiafe-Addai, B.; Edusei, L.; Adjei, E.; Titiloye, N.; Dedey, F.; et al. The oral microbiome and breast cancer and nonmalignant breast disease, and its relationship with the fecal microbiome in the Ghana breast health study. Int. J. Cancer 2022, 151, 1248–1260. [Google Scholar] [CrossRef]
- Fernández, M.F.; Reina-Pérez, I.; Astorga, J.M.; Rodríguez-Carrillo, A.; Plaza-Díaz, J.; Fontana, L. Breast Cancer and Its Relationship with the Microbiota. Int. J. Environ. Res. Public Health 2018, 15, 1747. [Google Scholar] [CrossRef]
- Thu, M.S.; Chotirosniramit, K.; Nopsopon, T.; Hirankarn, N.; Pongpirul, K. Human gut, breast, and oral microbiome in breast cancer: A systematic review and meta-analysis. Front. Oncol. 2023, 13, 1144021. [Google Scholar]
- Banerjee, S.; Wei, Z.; Tian, T.; Bose, D.; Shih, N.N.C.; Feldman, M.D.; Khoury, T.; De Michele, A.; Robertson, E.S. Prognostic correlations with the microbiome of breast cancer subtypes. Cell Death Dis. 2021, 12, 831. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.; Wei, G.F.; Li, Y.; Du, M.K.; Ni, J.T. Serum urea concentration and risk of 16 site-specific cancers, overall cancer, and cancer mortality in individuals with metabolic syndrome: A cohort study. BMC Med. 2024, 22, 536. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Birsoy, K.; Wang, T.; Chen, W.W.; Freinkman, E.; Abu-Remaileh, M.; Sabatini, D.M. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 2015, 162, 540–551. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Mutlu, G.M. The role of metabolic reprogramming and de novo amino acid synthesis in collagen protein production by myofibroblasts: Implications for organ fibrosis and cancer. Amino Acids 2021, 53, 1851–1862. [Google Scholar] [CrossRef]
- Limón, I.D.; Angulo-Cruz, I.; Sánchez-Abdon, L.; Patricio-Martínez, A. Disturbance of the Glutamate-Glutamine Cycle, Secondary to Hepatic Damage, Compromises Memory Function. Front. Neurosci. 2021, 15, 578922. [Google Scholar] [CrossRef]
- Holecek, M. Relation between glutamine, branched-chain amino acids, and protein metabolism. Nutrition 2002, 18, 130–133. [Google Scholar] [CrossRef]
- Poschke, I.; Mao, Y.; Kiessling, R.; de Boniface, J. Tumor-dependent increase of serum amino acid levels in breast cancer patients has diagnostic potential and correlates with molecular tumor subtypes. J. Transl. Med. 2013, 11, 290. [Google Scholar] [CrossRef]
- Chi, R.; Yao, C.; Chen, S.; Liu, Y.; He, Y.; Zhang, J.; Ellies, L.G.; Wu, X.; Zhao, Q.; Zhou, C.; et al. Elevated BCAA Suppresses the Development and Metastasis of Breast Cancer. Front. Oncol. 2022, 12, 887257. [Google Scholar] [CrossRef]
- Yu, T.J.; Ma, D.; Liu, Y.Y.; Xiao, Y.; Gong, Y.; Jiang, Y.Z.; Shao, Z.M.; Hu, X.; Di, G.H. Bulk and single-cell transcriptome profiling reveal the metabolic heterogeneity in human breast cancers. Mol. Ther. 2021, 29, 2350–2365. [Google Scholar] [CrossRef]
| Indicator | BC, n = 543/116 * (1) | FA, n = 597/24 (2) | HC, n = 298/25 (3) |
|---|---|---|---|
| Age, years | 54.7 ± 12.3 | 48.8 ± 12.8 | 46.3 ± 13.5 |
| p1–2 < 0.0001 p1–3 < 0.0001 | - | - | |
| Urea, mmol/L | 9.77 [6.44; 13.31] | 8.11 [5.15; 11.37] | 6.67 [4.36; 9.13] |
| p1–3 < 0.0001 p1–2 = 0.0001 | p2–3 < 0.0001 p2–3 = 0.0001 | - | |
| Glu, nmol/L | 77.9 [50.9; 102.9] | 74.5 [51.8; 121.3] | 59.2 [44.5; 80.6] |
| p1–3 = 0.0424 | - | - | |
| Gln, nmol/L | 238.8 [104.8; 412.8] | 180.6 [114.5; 439.6] | 438.8 [163.7; 638.4] |
| p1–3 = 0.0050 | - | - | |
| Val, nmol/L | 709.0 [408.9; 1041.0] | 676.2 [551.3; 774.2] | 557.1 [289.6; 944.9] |
| Leu + Ile, nmol/L | 79.02 [34.42; 110.7] | 24.50 [15.76; 32.77] | 37.21 [14.17; 68.03] |
| p1–3 = 0.0397 p1–2 = 0.0016 | p1–2 = 0.0016 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarf, E.A.; Bel’skaya, L.V. Salivary BCAA, Glutamate, Glutamine and Urea as Potential Indicators of Nitrogen Metabolism Imbalance in Breast Cancer. Curr. Issues Mol. Biol. 2025, 47, 837. https://doi.org/10.3390/cimb47100837
Sarf EA, Bel’skaya LV. Salivary BCAA, Glutamate, Glutamine and Urea as Potential Indicators of Nitrogen Metabolism Imbalance in Breast Cancer. Current Issues in Molecular Biology. 2025; 47(10):837. https://doi.org/10.3390/cimb47100837
Chicago/Turabian StyleSarf, Elena A., and Lyudmila V. Bel’skaya. 2025. "Salivary BCAA, Glutamate, Glutamine and Urea as Potential Indicators of Nitrogen Metabolism Imbalance in Breast Cancer" Current Issues in Molecular Biology 47, no. 10: 837. https://doi.org/10.3390/cimb47100837
APA StyleSarf, E. A., & Bel’skaya, L. V. (2025). Salivary BCAA, Glutamate, Glutamine and Urea as Potential Indicators of Nitrogen Metabolism Imbalance in Breast Cancer. Current Issues in Molecular Biology, 47(10), 837. https://doi.org/10.3390/cimb47100837








