Fenbendazole Exhibits Differential Anticancer Effects In Vitro and In Vivo in Models of Mouse Lymphoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Reagents
2.2. Cell Lines
2.3. Measurement of Cellular Metabolic Activity
2.4. Nuclear Staining for Morphological Assessment of Cell Death
2.5. Flow Cytometry Analysis
2.6. In Vivo Study and Drug Treatment
2.7. Histology
2.8. Immunohistochemistry (IHC)
2.9. Statistical Analysis
3. Results
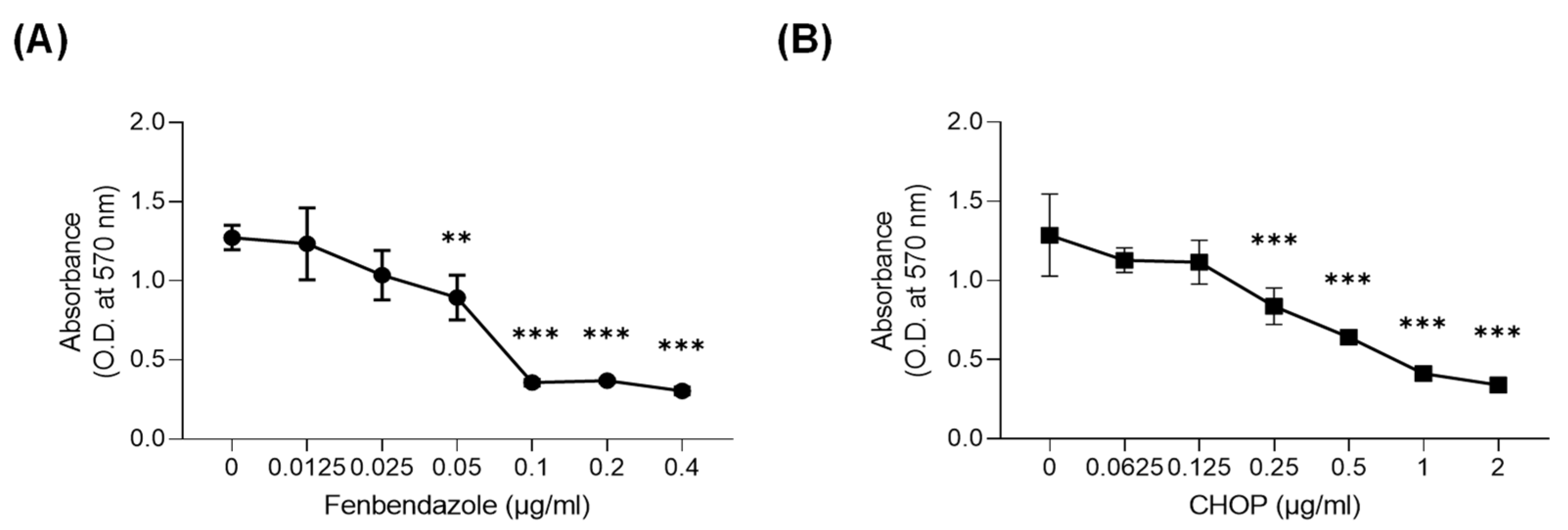
3.1. FBZ and CHOP Decreased the Metabolic Activity of EL-4 Cells
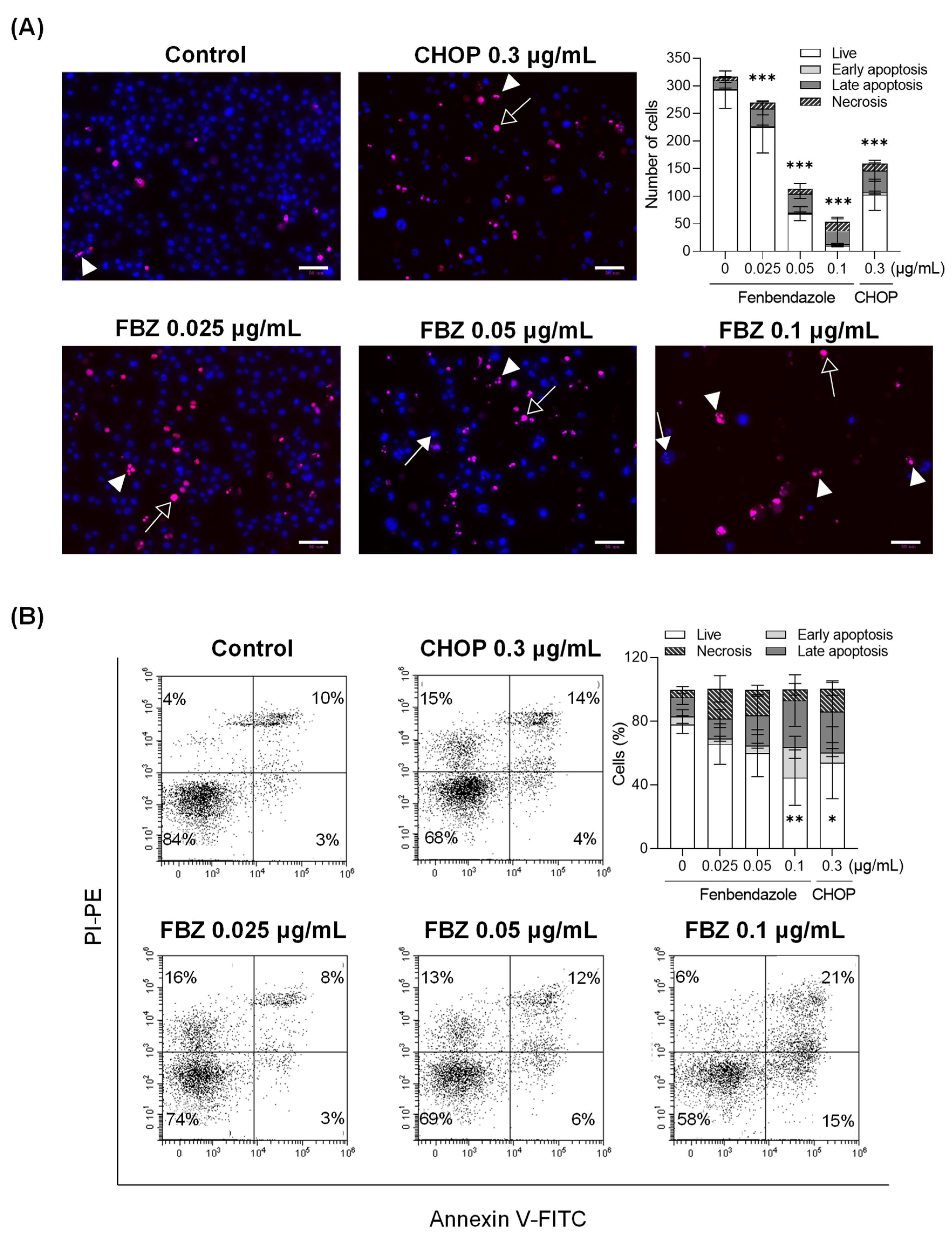
3.2. FBZ and CHOP Induce the Apoptosis and Necrosis of EL-4 Cells
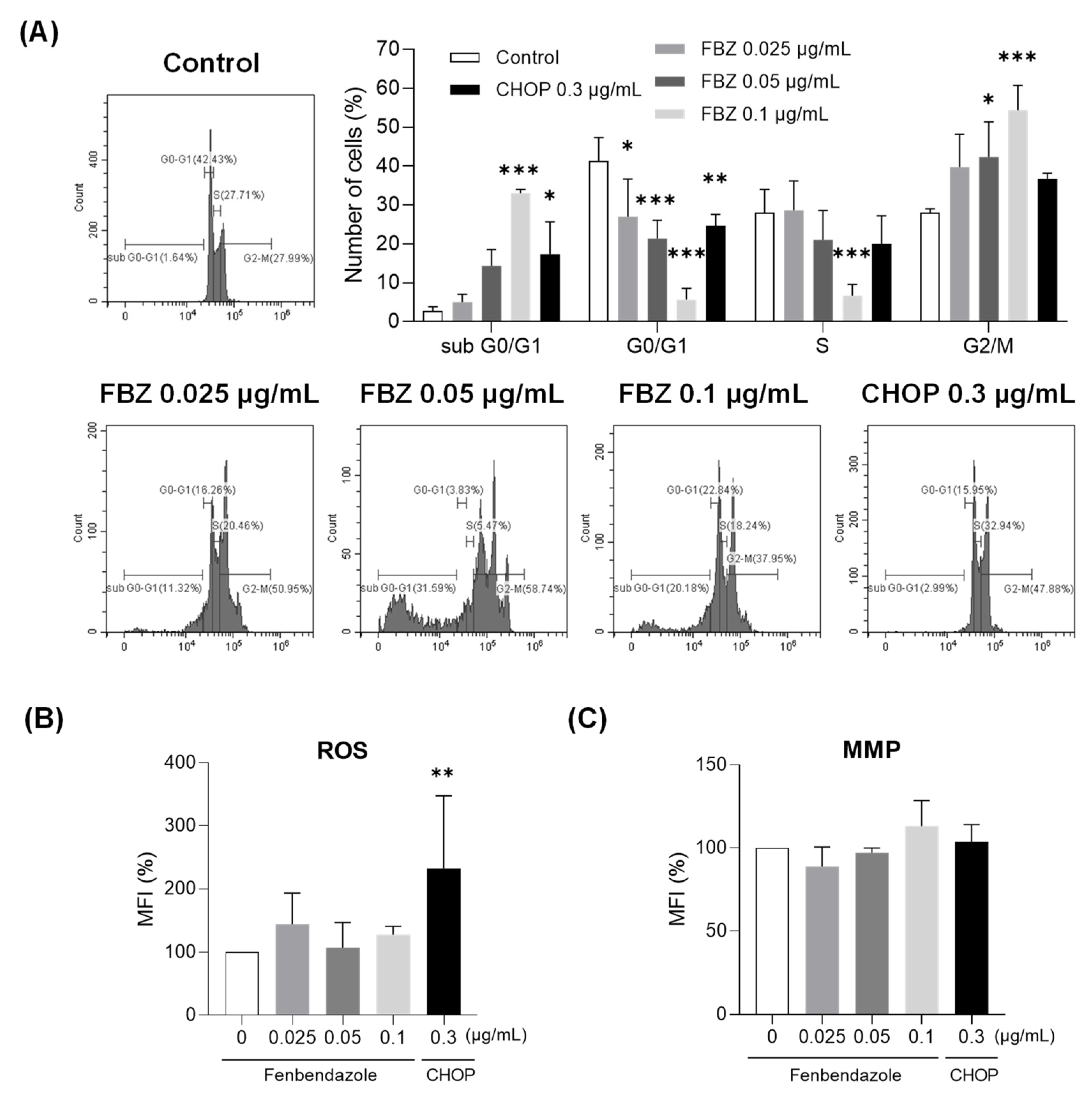
3.3. FBZ Induced G2/M Phase Arrest While CHOP Produced Intracellular ROS in EL-4 Cells
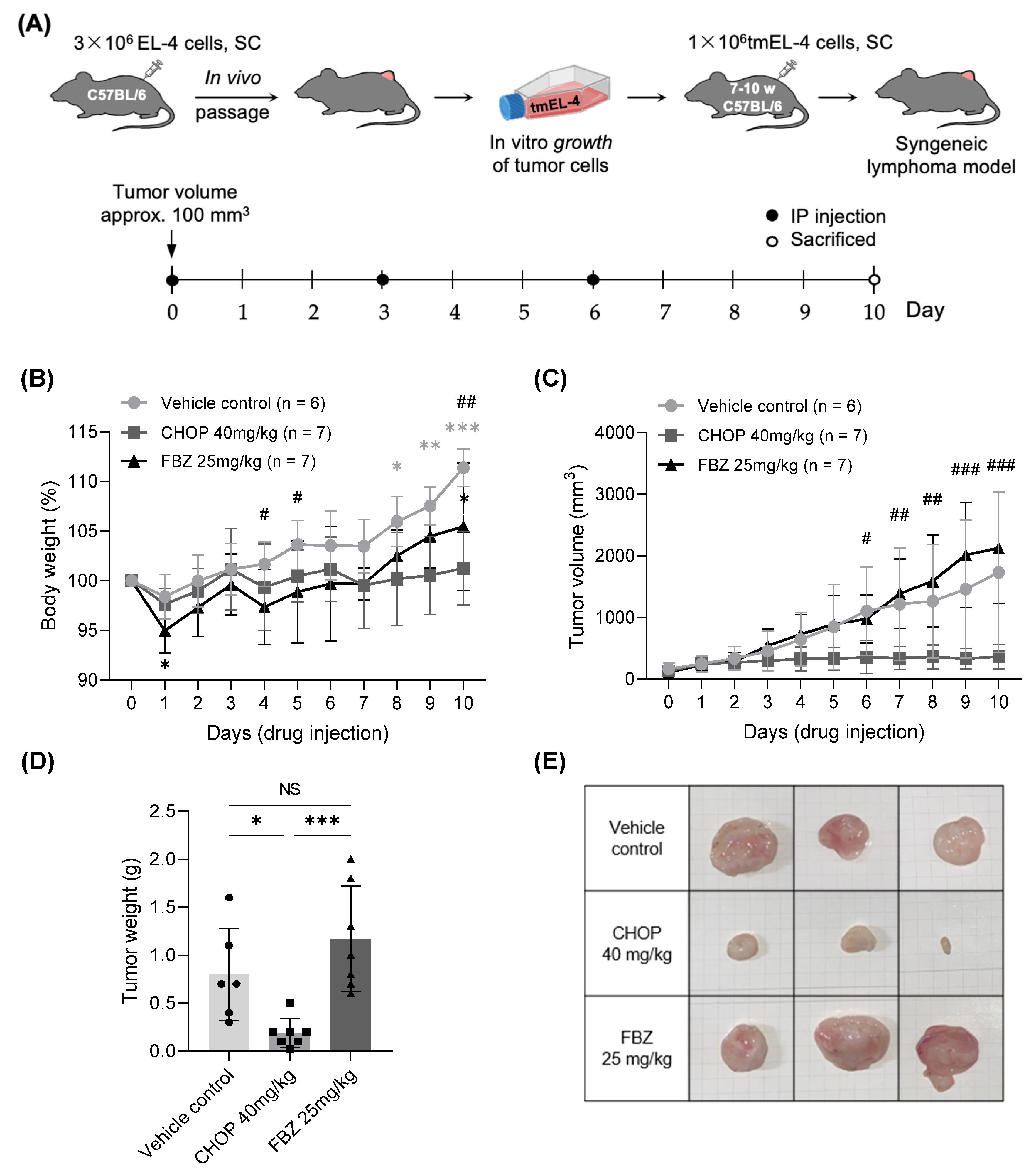
3.4. Effects of FBZ and CHOP in EL-4 Cell-Derived Mouse Lymphoma Model
3.4.1. Changes in Body Weight and Volume of Tumor Mass
3.4.2. Histopathology
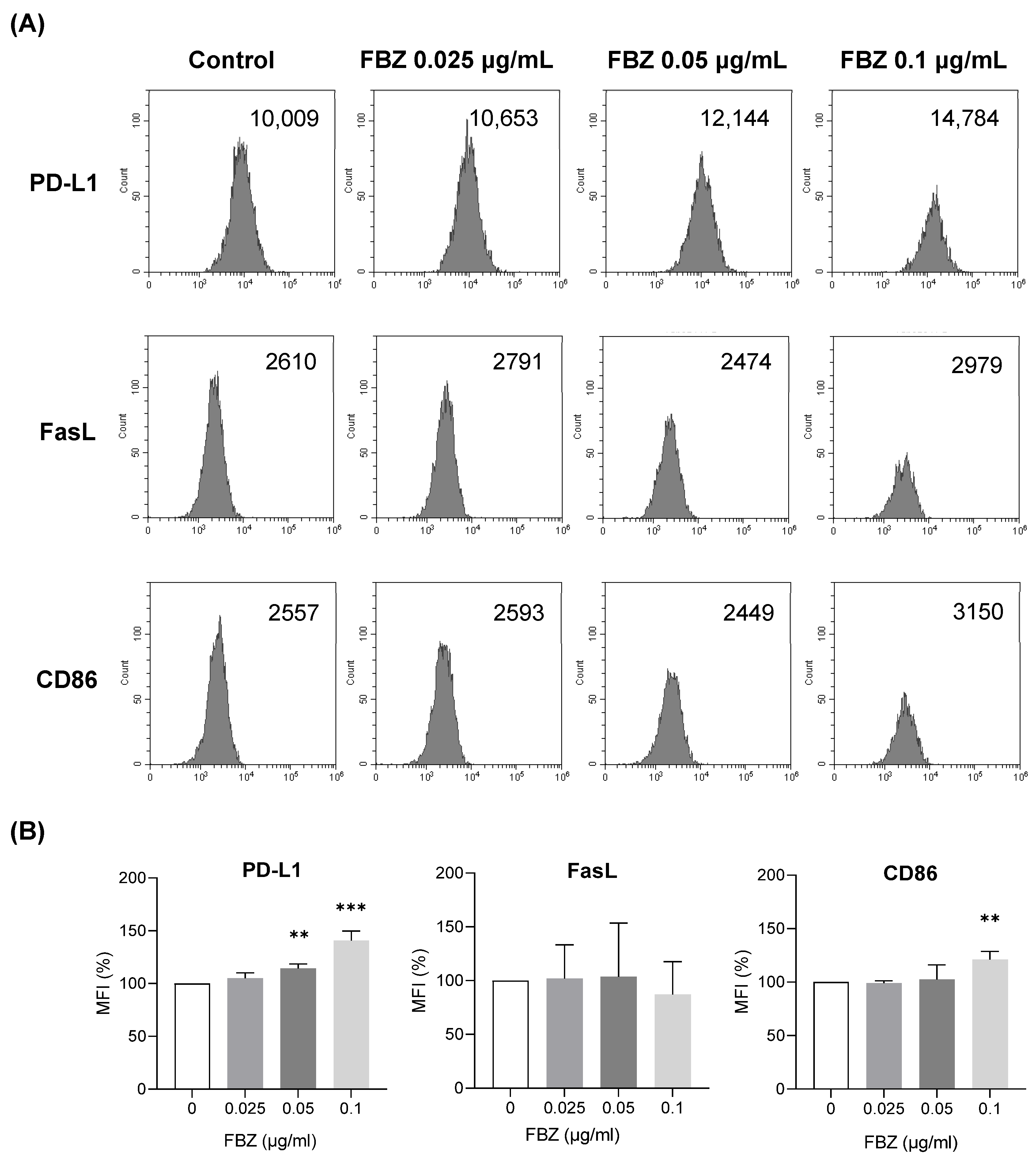
3.5. FBZ Increased the Expression of PD-L1 and CD86 on EL-4 Cells but Not FasL
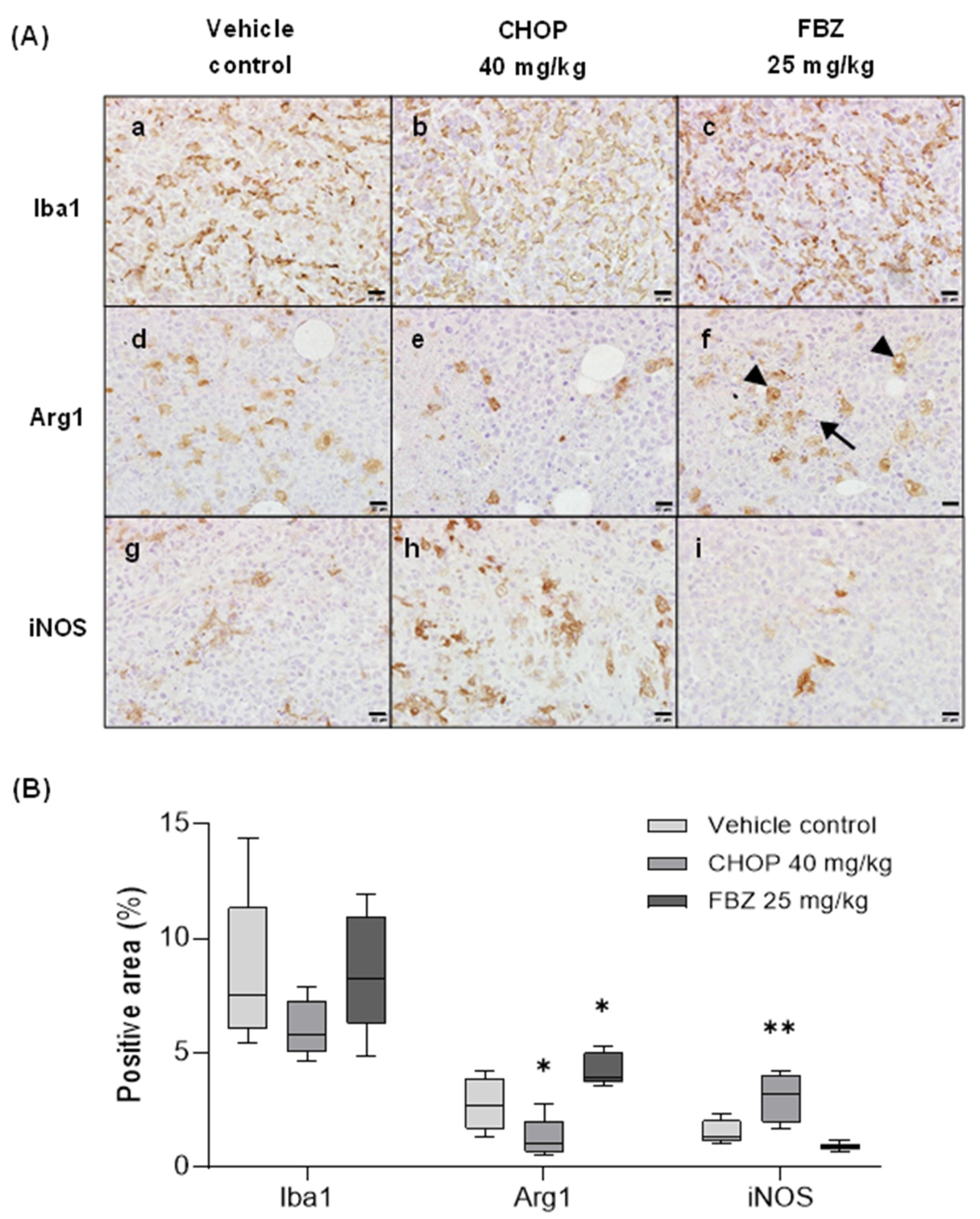
3.6. FBZ Recruited M2 Macrophages in the Tumor
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baeder, C.; Bähr, H.; Christ, O.; Düwel, D.; Kellner, H.M.; Kirsch, R.; Loewe, H.; Schultes, E.; Schütz, E.; Westen, H. Fenbendazole: A new, highly effective anthelmintic. Experientia 1974, 30, 753–754. [Google Scholar] [CrossRef] [PubMed]
- Crotch-Harvey, L.; Thomas, L.-A.; Worgan, H.J.; Douglas, J.-L.; Gilby, D.E.; McEwan, N.R. The effect of administration of fenbendazole on the microbial hindgut population of the horse. J. Equine Sci. 2018, 29, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.D.; Donoghue, A.R.; Baggs, R.B.; Clark, T.; Partington, C. Evaluation of the safety of fenbendazole in cats. Am. J. Vet. Res. 2000, 61, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Villar, D.; Cray, C.; Zaias, J.; Altman, N.H. Biologic Effects of Fenbendazole in Rats and Mice: A Review. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 8–15. [Google Scholar]
- Friedman, P.A.; Platzer, E.G. Interaction of anthelmintic benzimidazoles with AscarisSuum embryonic tubulin. Biochim. Biophys. Acta 1980, 630, 271–278. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef]
- Han, Y.; Joo, H.-G. Involvement of reactive oxygen species in the anti-cancer activity of fenbendazole, a benzimidazole anthelmintic. Korean J. Vet. Res. 2020, 60, 79–83. [Google Scholar] [CrossRef]
- Sung, J.Y.; Joo, H.-G. Anti-cancer effects of Fenbendazole and Paclitaxel combination on HL-60 cells. J. Prev. Vet. Med. 2021, 45, 13–17. [Google Scholar] [CrossRef]
- Park, D. Fenbendazole Suppresses Growth and Induces Apoptosis of Actively Growing H4IIE Hepatocellular Carcinoma Cells via p21-Mediated Cell-Cycle Arrest. Biol. Pharm. Bull. 2022, 45, 184–193. [Google Scholar] [CrossRef]
- Park, D.; Lee, J.H.; Yoon, S.P. Anti-cancer effects of fenbendazole on 5-fluorouracil-resistant colorectal cancer cells. Korean J. Physiol. Pharmacol. 2022, 26, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Pan, J.; Ou, F.; Wang, W.; Hu, H.; Chen, L.; Zeng, S.; Zeng, K.; Yu, L. Fenbendazole and its synthetic analog interfere with HeLa cells’ proliferation and energy metabolism via inducing oxidative stress and modulating MEK3/6-p38-MAPK pathway. Chem. Biol. Interact. 2022, 361, 109983. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, K.H.; Shin, H.Y.; Jun, J.K. How cancer patients get fake cancer information: From TV to YouTube, a qualitative study focusing on fenbendazole scandle. Front. Oncol. 2022, 12, 942045. [Google Scholar] [CrossRef]
- von Keudell, G.; Younes, A. Novel therapeutic agents for relapsed classical Hodgkin lymphoma. Br. J. Haematol. 2019, 184, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Zandvliet, M. Canine lymphoma: A review. Vet. Q. 2016, 36, 76–104. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.I.; Gaynor, E.R.; Dahlberg, S.; Oken, M.M.; Grogan, T.M.; Mize, E.M.; Glick, J.H.; Coltman, C.A., Jr.; Miller, T.P. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N. Engl. J. Med. 1993, 328, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Poletto, S.; Novo, M.; Paruzzo, L.; Frascione, P.M.M.; Vitolo, U. Treatment strategies for patients with diffuse large B-cell lymphoma. Cancer Treat. Rev. 2022, 110, 102443. [Google Scholar] [CrossRef] [PubMed]
- Thamm, D.H. Novel Treatments for Lymphoma. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 903–915. [Google Scholar] [CrossRef] [PubMed]
- LaCasce, A.S. Treating Hodgkin lymphoma in the new millennium: Relapsed and refractory disease. Hematol. Oncol. 2019, 37 (Suppl. S1), 87–91. [Google Scholar] [CrossRef]
- Sultana, T.; Jan, U.; Lee, H.; Lee, H.; Lee, J.I. Exceptional Repositioning of Dog Dewormer: Fenbendazole Fever. Curr. Issues Mol. Biol. 2022, 44, 4977–4986. [Google Scholar] [CrossRef]
- Kurian, R.; Hedrich, W.; Mackowiak, B.; Li, L.; Wang, H. CITCO as an Adjuvant Facilitates CHOP-Based Lymphoma Treatment in hCAR-Transgenic Mice. Cells 2020, 9, 2520. [Google Scholar] [CrossRef]
- Dogra, N.; Kumar, A.; Mukhopadhyay, T. Fenbendazole acts as a moderate microtubule destabilizing agent and causes cancer cell death by modulating multiple cellular pathways. Sci. Rep. 2018, 8, 11926. [Google Scholar] [CrossRef] [PubMed]
- Gokbulut, C.; Bilgili, A.; Hanedan, B.; McKellar, Q.A. Comparative plasma disposition of fenbendazole, oxfendazole and albendazole in dogs. Vet. Parasitol. 2007, 148, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Liu, Y.; Booth, C.J.; Rockwell, S. Use of fenbendazole-containing therapeutic diets for mice in experimental cancer therapy studies. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 224–230. [Google Scholar]
- Gao, P.; Dang, C.V.; Watson, J. Unexpected Antitumorigenic Effect of Fenbendazole when Combined with Supplementary Vitamins. J. Am. Assoc. Lab. Anim. Sci. 2008, 47, 37–40. [Google Scholar]
- Duan, Q.; Liu, Y.; Rockwell, S. Fenbendazole as a potential anticancer drug. Anticancer Res. 2013, 33, 355–362. [Google Scholar]
- Burkholder, T.; Foltz, C.; Karlsson, E.; Linton, C.G.; Smith, J.M. Health Evaluation of Experimental Laboratory Mice. Curr. Protoc. Mouse Biol. 2012, 2, 145–165. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.B.; Choi, J.Y.; Shin, D.H.; Lee, J.W. Anticancer Evaluation of Methoxy Poly(Ethylene Glycol)-b-Poly(Caprolactone) Polymeric Micelles Encapsulating Fenbendazole and Rapamycin in Ovarian Cancer. Int. J. Nanomed. 2023, 18, 2209–2223. [Google Scholar] [CrossRef]
- Chang, C.S.; Ryu, J.Y.; Choi, J.K.; Cho, Y.J.; Choi, J.J.; Hwang, J.R.; Choi, J.Y.; Noh, J.J.; Lee, C.M.; Won, J.E.; et al. Anti-cancer effect of fenbendazole-incorporated PLGA nanoparticles in ovarian cancer. J. Gynecol. Oncol. 2023, 34, e58. [Google Scholar] [CrossRef]
- Ghosh, C.; Luong, G.; Sun, Y. A snapshot of the PD-1/PD-L1 pathway. J. Cancer 2021, 12, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Patsoukis, N.; Wang, Q.; Strauss, L.; Boussiotis, V.A. Revisiting the PD-1 pathway. Sci. Adv. 2020, 6, eabd2712. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef] [PubMed]
- Dy-Ledesma, J.L.; Khoury, J.D.; Agbay, R.L.; Garcia, M.; Miranda, R.N.; Medeiros, L.J. Starry Sky Pattern in Hematopoietic Neoplasms: A Review of Pathophysiology and Differential Diagnosis. Adv. Anat. Pathol. 2016, 23, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.A.; Petrova, S.; Pound, J.D.; Voss, J.J.L.P.; Melville, L.; Paterson, M.; Farnworth, S.L.; Gallimore, A.M.; Cuff, S.; Wheadon, H.; et al. Oncogenic Properties of Apoptotic Tumor Cells in Aggressive B Cell Lymphoma. Curr. Biol. 2015, 25, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Shimizu, J.; Oya, Y.; Horio, Y.; Hida, T. Drug-Induced Liver Injury in a Patient with Nonsmall Cell Lung Cancer after the Self-Administration of Fenbendazole Based on Social Media Information. Case Rep. Oncol. 2021, 14, 886–891. [Google Scholar] [CrossRef] [PubMed]

| Primary Ab | Company | Origin | Dilution |
|---|---|---|---|
| Proliferating cell nuclear antigen (PCNA) | Santa Cruz Biotechnology, Santa Cruz, CA, USA | Rabbit (polyclonal) | 1:400 |
| Ionized calcium-binding adaptor molecule 1 (Iba 1) | FUJIFILM Wako Pure Chemical Co., Tokyo, Japan | 1:1000 | |
| Arginase 1 (Arg 1) | Santa Cruz Biotechnology, Santa Cruz, CA, USA | 1:200 | |
| Inducible nitric oxide synthase (iNOS) | Abcam, Cambridge, UK | 1:800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.; Kim, S.-Y.; Joo, H.-G. Fenbendazole Exhibits Differential Anticancer Effects In Vitro and In Vivo in Models of Mouse Lymphoma. Curr. Issues Mol. Biol. 2023, 45, 8925-8938. https://doi.org/10.3390/cimb45110560
Jung H, Kim S-Y, Joo H-G. Fenbendazole Exhibits Differential Anticancer Effects In Vitro and In Vivo in Models of Mouse Lymphoma. Current Issues in Molecular Biology. 2023; 45(11):8925-8938. https://doi.org/10.3390/cimb45110560
Chicago/Turabian StyleJung, Haebeen, Si-Yeon Kim, and Hong-Gu Joo. 2023. "Fenbendazole Exhibits Differential Anticancer Effects In Vitro and In Vivo in Models of Mouse Lymphoma" Current Issues in Molecular Biology 45, no. 11: 8925-8938. https://doi.org/10.3390/cimb45110560
APA StyleJung, H., Kim, S.-Y., & Joo, H.-G. (2023). Fenbendazole Exhibits Differential Anticancer Effects In Vitro and In Vivo in Models of Mouse Lymphoma. Current Issues in Molecular Biology, 45(11), 8925-8938. https://doi.org/10.3390/cimb45110560






