Biological Roles and Pathogenic Mechanisms of LncRNA MIR4435-2HG in Cancer: A Comprehensive Review
Abstract
:1. Introduction
2. The Role of MIR4435-2HG in the Advancement and Prediction of Cancer
2.1. MIR4435-2HG and Gastric Cancer
2.2. MIR4435-2HG and Hepatocellular Carcinoma
2.3. MIR4435-2HG and Cholangiocarcinoma
2.4. MIR4435-2HG and Colorectal Cancer
2.5. MIR4435-2HG and Lung Cancer
2.6. MIR4435-2HG and Breast Cancer
2.7. MIR4435-2HG and Glioma
2.8. MIR4435-2HG and Head and Neck Squamous Cell Carcinoma
2.9. MIR4435-2HG and Ovarian Cancer
2.10. MIR4435-2HG and Cervical Cancer
2.11. MIR4435-2HG and Clear Cell Renal Cell Carcinoma
2.12. MIR4435-2HG and Bladder Cancer
2.13. MIR4435-2HG and Prostate Cancer
2.14. MIR4435-2HG and Melanoma
3. MIR4435-2HG-Related Molecular Mechanisms of Carcinogenesis
3.1. Wnt/β-Catenin Pathway
3.2. MAPK Pathway
3.3. PI3K/AKT/mTOR Pathway
3.4. TGF-β Signaling Pathway
3.5. Epigenetic Regulation
3.6. Competitive Endogenous RNAs
3.7. Effects on Immune Cells
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Botta, L.; Gatta, G.; Capocaccia, R.; Stiller, C.; Cañete, A.; Dal Maso, L.; Innos, K.; Mihor, A.; Erdmann, F.; Spix, C.; et al. Long-term survival and cure fraction estimates for childhood cancer in Europe (EUROCARE-6): Results from a population-based study. Lancet Oncol. 2022, 23, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, S.D.; McKinley, M.; McBay, B.; Zylberberg, H.M.; Gomez, S.L.; Hur, C.; Kastrinos, F.; Gupta, S.; Kim, M.K.; Itzkowitz, S.H.; et al. Epidemiology of Gastric Malignancies 2000–2018 according to Histology: A Population-Based Analysis of Incidence and Temporal Trends. Clin. Gastroenterol. Hepatol. 2023, S1542–S3565. [Google Scholar] [CrossRef] [PubMed]
- Poças, J.; Marques, C.; Gomes, C.; Otake, A.H.; Pinto, F.; Ferreira, M.; Silva, T.; Faria-Ramos, I.; Matos, R.; Ribeiro, A.R.; et al. Syndecan-4 is a maestro of gastric cancer cell invasion and communication that underscores poor survival. Proc. Natl. Acad. Sci. USA 2023, 120, e2214853120. [Google Scholar] [CrossRef]
- Kan, R.L.; Chen, J.; Sallam, T. Crosstalk between epitranscriptomic and epigenetic mechanisms in gene regulation. Trends Genet. 2022, 38, 182–193. [Google Scholar] [CrossRef]
- Bergmann, J.H.; Spector, D.L. Long non-coding RNAs: Modulators of nuclear structure and function. Curr. Opin. Cell Biol. 2014, 26, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Shuman, S. Transcriptional interference at tandem lncRNA and protein-coding genes: An emerging theme in regulation of cellular nutrient homeostasis. Nucleic Acids Res. 2020, 48, 8243–8254. [Google Scholar] [CrossRef]
- Ghasemian, M.; Rajabibazl, M.; Sahebi, U.; Sadeghi, S.; Maleki, R.; Hashemnia, V.; Mirfakhraie, R. Long non-coding RNA MIR4435-2HG: A key molecule in progression of cancer and non-cancerous disorders. Cancer Cell Int. 2022, 22, 215. [Google Scholar] [CrossRef]
- Yang, M.; He, X.; Huang, X.; Wang, J.; He, Y.; Wei, L. LncRNA MIR4435-2HG-mediated upregulation of TGF-β1 promotes migration and proliferation of nonsmall cell lung cancer cells. Environ. Toxicol. 2020, 35, 582–590. [Google Scholar] [CrossRef]
- Chen, D.; Tang, P.; Wang, Y.; Wan, F.; Long, J.; Zhou, J.; Zhuang, M.; Chen, X. Downregulation of long non-coding RNA MR4435-2HG suppresses breast cancer progression via the Wnt/β-catenin signaling pathway. Oncol. Lett. 2021, 21, 373. [Google Scholar] [CrossRef]
- Wang, R.; Liu, L.; Jiao, J.; Gao, D. Knockdown of MIR4435-2HG Suppresses the Proliferation, Migration and Invasion of Cervical Cancer Cells via Regulating the miR-128-3p/MSI2 Axis in vitro. Cancer Manag. Res. 2020, 12, 8745–8756. [Google Scholar] [CrossRef]
- Dong, X.; Yang, Z.; Yang, H.; Li, D.; Qiu, X. Long Non-coding RNA MIR4435-2HG Promotes Colorectal Cancer Proliferation and Metastasis through miR-206/YAP1 Axis. Front. Oncol. 2020, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, M.; Lu, Y.; He, K.; Cai, X.; Yu, X.; Lu, J.; Teng, L. LncRNA MIR4435-2HG targets desmoplakin and promotes growth and metastasis of gastric cancer by activating Wnt/β-catenin signaling. Aging 2019, 11, 6657–6673. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Ding, Y.; Lu, F.; Yuan, H.; Luan, W. Long noncoding RNA MIR4435-2HG promotes hepatocellular carcinoma proliferation and metastasis through the miR-22-3p/YWHAZ axis. Am. J. Transl. Res. 2020, 12, 6381–6394. [Google Scholar]
- Kong, Q.; Liang, C.; Jin, Y.; Pan, Y.; Tong, D.; Kong, Q.; Zhou, J. The lncRNA MIR4435-2HG is upregulated in hepatocellular carcinoma and promotes cancer cell proliferation by upregulating miRNA-487a. Cell. Mol. Biol. Lett. 2019, 24, 26. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Yan, D.; He, Q.; Kong, J.; Yang, M.; Ruan, H.; Lin, Q.; Huang, L.; Huang, J.; Lin, T.; et al. LncRNA MIR4435-2HG drives cancer progression by modulating cell cycle regulators and mTOR signaling in stroma-enriched subtypes of urothelial carcinoma of the bladder. Cell. Oncol. 2023, 46, 1509–1527. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.-H.; Huang, T.-W.; Shih, C.-M.; Lee, Y.-T.; Liu, A.-J.; Chen, P.-H.; Chen, K.-C. Glycolysis-associated lncRNAs identify a subgroup of cancer patients with poor prognoses and a high-infiltration immune microenvironment. BMC Med. 2021, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Tan, Y.; Zhou, X.; Zhang, Q.; Zhang, J.; Wu, Y. Landscape of prognosis and immunotherapy responsiveness under tumor glycosylation-related lncRNA patterns in breast cancer. Front. Immunol. 2022, 13, 989928. [Google Scholar] [CrossRef]
- Yu, H.; Chen, C.; Han, F.; Tang, J.; Deng, M.; Niu, Y.; Lai, M.; Zhang, H. Long Noncoding RNA MIR4435-2HG Suppresses Colorectal Cancer Initiation and Progression by Reprogramming Neutrophils. Cancer Immunol. Res. 2022, 10, 1095–1110. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Andresen, J.L.; Manan, R.S.; Langer, R. Nucleic acid delivery for therapeutic applications. Adv. Drug Deliv. Rev. 2021, 178, 113834. [Google Scholar] [CrossRef]
- Nedorezova, D.D.; Dubovichenko, M.V.; Belyaeva, E.P.; Grigorieva, E.D.; Peresadina, A.V.; Kolpashchikov, D.M. Specificity of oligonucleotide gene therapy (OGT) agents. Theranostics 2022, 12, 7132–7157. [Google Scholar] [CrossRef]
- Zhao, R.; Fu, J.; Zhu, L.; Chen, Y.; Liu, B. Designing strategies of small-molecule compounds for modulating non-coding RNAs in cancer therapy. J. Hematol. Oncol. 2022, 15, 14. [Google Scholar] [CrossRef]
- Sheykhhasan, M.; Dermani, F.K.; Gheibi, N.; Datta, I.; Sadeghi, H.; Khoei, S.G. The emerging role of LncRNA AWPPH in multiple cancers: A review study. Curr. Mol. Med. 2023. [Google Scholar] [CrossRef]
- Ke, D.; Li, H.; Zhang, Y.; An, Y.; Fu, H.; Fang, X.; Zheng, X. The combination of circulating long noncoding RNAs AK001058, INHBA-AS1, MIR4435-2HG, and CEBPA-AS1 fragments in plasma serve as diagnostic markers for gastric cancer. Oncotarget 2017, 8, 21516–21525. [Google Scholar] [CrossRef]
- Fu, M.; Huang, Z.; Zang, X.; Pan, L.; Liang, W.; Chen, J.; Qian, H.; Xu, W.; Jiang, P.; Zhang, X. Long noncoding RNA LINC00978 promotes cancer growth and acts as a diagnostic biomarker in gastric cancer. Cell Prolif. 2018, 51, 1. [Google Scholar] [CrossRef]
- Li, C.; Chen, Z.; Gao, J.; Tang, T.; Zhou, L.; Zhang, G.; Zhang, D.; Shen, C.; Guo, L.; Fu, T. MIR4435-2HG in exosomes promotes gastric carcinogenesis by inducing M2 polarization in macrophages. Front. Oncol. 2022, 12, 1017745. [Google Scholar] [CrossRef]
- Gao, L.-F.; Li, W.; Liu, Y.-G.; Zhang, C.; Gao, W.-N.; Wang, L. Inhibition of MIR4435-2HG on Invasion, Migration, and EMT of Gastric Carcinoma Cells by Mediating MiR-138-5p/Sox4 Axis. Front. Oncol. 2021, 11, 661288. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, B.; Xu, G.; Han, C.; Xing, G. lncRNA MIR4435-2HG promotes the progression of liver cancer by upregulating B3GNT5 expression. Mol. Med. Rep. 2022, 25, 38. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Gong, L.; Cai, Q. LncRNA00978 contributes to growth and metastasis of hepatocellular carcinoma cells via mediating microRNA-125b-5p/SOX12 pathway. Bioengineered 2022, 13, 11228–11239. [Google Scholar] [CrossRef] [PubMed]
- Kunadirek, P.; Pinjaroen, N.; Nookaew, I.; Tangkijvanich, P.; Chuaypen, N. Transcriptomic Analyses Reveal Long Non-Coding RNA in Peripheral Blood Mononuclear Cells as a Novel Biomarker for Diagnosis and Prognosis of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2022, 23, 7882. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, J.; Zhang, J.; Xue, H.; Liu, L.; Yang, Z.; Dong, H.; Wang, K. Establishment and validation of lncRNA-related prognostic signatures in cholangiocarcinoma. Genomics 2023, 115, 110621. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, W.; Huang, F.; Sun, J.; Li, K.P.; Shi, J.; Yang, J.; Li, J.; Li, Y.; Hu, N.; et al. Comprehensive Analysis of the Expression Profiles of Long Non-Coding RNAs with Associated ceRNA Network Involved in the Colon Cancer Staging and Progression. Sci. Rep. 2019, 9, 16910. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.Y.; Zhou, G.R.; Zhang, Z.Y. LncRNA MIR4435-2HG contributes into colorectal cancer development and predicts poor prognosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1771–1777. [Google Scholar] [CrossRef]
- Ouyang, W.; Ren, L.; Liu, G.; Chi, X.; Wei, H. LncRNA MIR4435-2HG predicts poor prognosis in patients with colorectal cancer. PeerJ 2019, 7, e6683. [Google Scholar] [CrossRef]
- Zhu, D.; Guo, P.; Jin, X.Y.; Zhang, R.; Ji, G.; Yang, Z.; Hoffman, R.M.; Sheng, W. Long-chain Non-coding RNA MIR4435-2HG Expression Correlates with Size of Intestinal Polyps in Children and with Metastasis of Colon Cancer. Anticancer. Res. 2022, 42, 4707–4714. [Google Scholar] [CrossRef]
- Yu, M.; Yi, Z.; Chen, S.; Chen, X.; Xie, X. MIR4435-2HG, miR-125b-5p, and Sema4D axis affects the aggressiveness of colorectal cancer cells. Folia Histochem. Cytobiol. 2022, 60, 191–202. [Google Scholar] [CrossRef]
- Qian, H.; Chen, L.; Huang, J.; Wang, X.; Ma, S.; Cui, F.; Luo, L.; Ling, L.; Luo, K.; Zheng, G. The lncRNA MIR4435-2HG promotes lung cancer progression by activating β-catenin signalling. J. Mol. Med. 2018, 96, 753–764. [Google Scholar] [CrossRef]
- Deng, L.-L.; Chi, Y.-Y.; Liu, L.; Huang, N.-S.; Wang, L.; Wu, J. LINC00978 predicts poor prognosis in breast cancer patients. Sci. Rep. 2016, 6, 37936. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, J.; Liu, F.; Wu, X.; Wen, Z. An Immune-Related Long Noncoding RNA Pair as a New Biomarker to Predict the Prognosis of Patients in Breast Cancer. Front. Genet. 2022, 13, 895200. [Google Scholar] [CrossRef]
- Ke, J.; Wang, Q.; Zhang, W.; Ni, S.; Mei, H. LncRNA MIR4435-2HG promotes proliferation, migration, invasion and epithelial mesenchymal transition via targeting miR-22-3p/TMEM9B in breast cancer. Am. J. Transl. Res. 2022, 14, 5441–5454. [Google Scholar]
- Meng, M.; Yang, L.; Zhou, H.; Cheng, Q.; Peng, R.; Wang, Z.; Liang, X.; Wen, J.; Nie, J.; Hu, Z.; et al. LINC00978 regulates metabolic rewiring to promote the malignancy of glioblastoma through AKR1B1. Cancer Lett. 2023, 567, 216277. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, B.; Yang, Y.; Li, Z.; Zhao, P.; Wu, W.; Zhang, H.; Mao, J. LncRNA MIR4435-2HG potentiates the proliferation and invasion of glioblastoma cells via modulating miR-1224-5p/TGFBR2 axis. J. Cell. Mol. Med. 2020, 24, 6362–6372. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, J.; Pan, Y.; Jin, Y. LncRNA MIR4435-2HG functions as a ceRNA against miR-125a-5p and promotes neuroglioma development by upregulating TAZ. J. Clin. Lab. Anal. 2021, 35, e24066. [Google Scholar] [CrossRef]
- Li, Z.; Tan, H.; Zhao, W.; Xu, Y.; Zhang, Z.; Wang, M.; Zhou, X. Integrative analysis of DNA methylation and gene expression profiles identifies MIR4435-2HG as an oncogenic lncRNA for glioma progression. Gene 2019, 715, 144012. [Google Scholar] [CrossRef]
- Cai, H.; Liang, J.; Jiang, Y.; Tan, R.; Hou, C.; Hou, J. Integrative Analysis of N6-Methyladenosine-Related Enhancer RNAs Identifies Distinct Prognosis and Tumor Immune Micro-Environment Patterns in Head and Neck Squamous Cell Carcinoma. Cancers 2022, 14, 4657. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Qiao, T. Long non-coding RNA MIR4435-2HG promotes the progression of head and neck squamous cell carcinoma by regulating the miR-383-5p/RBM3 axis. Oncol. Rep. 2021, 45, 99. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Zuo, N.; Wang, L.; Sun, X.; Liang, L.; Ju, M.; Di, X. Necrotic related-lncRNAs: Prediction of prognosis and differentiation between cold and hot tumors in head and neck squamous cell carcinoma. Medicine 2023, 102, e33994. [Google Scholar] [CrossRef] [PubMed]
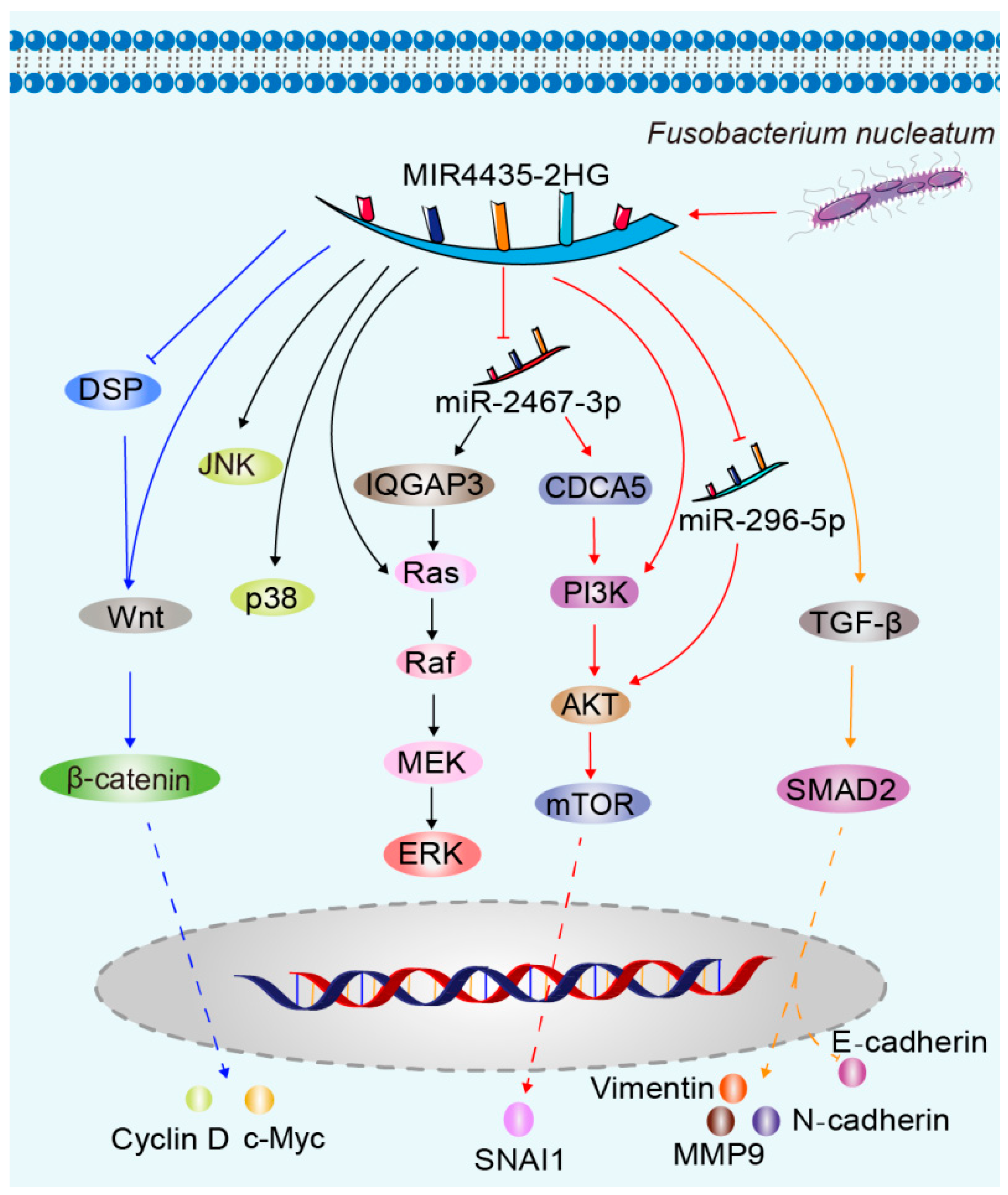
- Shen, H.; Sun, B.; Yang, Y.; Cai, X.; Bi, L.; Deng, L.; Zhang, L. MIR4435-2HG regulates cancer cell behaviors in oral squamous cell carcinoma cell growth by upregulating TGF-β1. Odontology 2020, 108, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, C.; Liu, J.; Geng, F.; Shi, X.; Li, Q.; Lu, Z.; Pan, Y. Fusobacterium nucleatum promotes epithelial-mesenchymal transiton through regulation of the lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 signaling pathway. FEBS J. 2020, 287, 4032–4047. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, Y.; Xie, J.; Zhen, E.; Zhou, X. Integrative profiling analysis identifies the oncogenic long noncoding RNA DUXAP8 in oral cancer. Anti-Cancer Drugs 2020, 31, 792–798. [Google Scholar] [CrossRef]
- Gong, J.; Xu, X.; Zhang, X.; Zhou, Y. LncRNA MIR4435-2HG is a potential early diagnostic marker for ovarian carcinoma. Acta Biochim. Biophys. Sin. 2019, 51, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, A.; Gao, M.; Duan, X.; Li, Z. LncRNA MIR4435-2HG triggers ovarian cancer progression by regulating miR-128-3p/CKD14 axis. Cancer Cell Int. 2020, 20, 145. [Google Scholar] [CrossRef]
- Zhu, K.; Miao, C.; Tian, Y.; Qin, Z.; Xue, J.; Xia, J.; Zhu, S.; Xu, A.; Yang, J.; Wang, Z. lncRNA MIR4435-2HG promoted clear cell renal cell carcinoma malignant progression via miR-513a-5p/KLF6 axis. J. Cell. Mol. Med. 2020, 24, 10013–10026. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Z.; Wang, J.; Chen, R. LINC00978 promotes bladder cancer cell proliferation, migration and invasion by sponging miR-4288. Mol. Med. Rep. 2019, 20, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, Y.; Wang, G.; Guo, L.; Sun, F.; Li, S.; Deng, X.; Liu, J. lncRNA MIR4435-2HG Accelerates the Development of Bladder Cancer through Enhancing IQGAP3 and CDCA5 Expression. BioMed Res. Int. 2022, 2022, 3858249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Meng, H.; Huang, X.; Tong, W.; Liang, X.; Li, J.; Zhang, C.; Chen, M. lncRNA MIR4435-2HG promotes cancer cell migration and invasion in prostate carcinoma by upregulating TGF-β1. Oncol. Lett. 2019, 18, 4016–4021. [Google Scholar] [CrossRef] [PubMed]
- Xing, P.; Wang, Y.; Zhang, L.; Ma, C.; Lu, J. Knockdown of lncRNA MIR4435-2HG and ST8SIA1 expression inhibits the proliferation, invasion and migration of prostate cancer cells in vitro and in vivo by blocking the activation of the FAK/AKT/β-catenin signaling pathway. Int. J. Mol. Med. 2021, 47, 93. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.-M.; Sun, D.; Wang, J.; Jin, D.-H.; Li, Y.; Han, Y.-E. Long non-coding RNA MIR4435-2HG recruits miR-802 from FLOT2 to promote melanoma progression. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2616–2624. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Meltzer, S.J. A Review of the Genomics of Gastric Cancer. Clin. Gastroenterol. Hepatol. 2006, 4, 416–425. [Google Scholar] [CrossRef]
- Miao, Y.; Sui, J.; Xu, S.-Y.; Liang, G.-Y.; Pu, Y.-P.; Yin, L.-H. Comprehensive analysis of a novel four-lncRNA signature as a prognostic biomarker for human gastric cancer. Oncotarget 2017, 8, 75007–75024. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, Y.; Yu, S. Long noncoding RNA AWPPH promotes hepatocellular carcinoma progression through YBX1 and serves as a prognostic biomarker. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1805–1816. [Google Scholar] [CrossRef]
- Xu, X.; Gu, J.; Ding, X.; Ge, G.; Zang, X.; Ji, R.; Shao, M.; Mao, Z.; Zhang, Y.; Zhang, J.; et al. LINC00978 promotes the progression of hepatocellular carcinoma by regulating EZH2-mediated silencing of p21 and E-cadherin expression. Cell Death Dis. 2019, 10, 752. [Google Scholar] [CrossRef]
- Bai, Y.; Lin, H.; Chen, J.; Wu, Y.; Yu, S.a. Identification of Prognostic Glycolysis-Related lncRNA Signature in Tumor Immune Microenvironment of Hepatocellular Carcinoma. Front. Mol. Biosci. 2021, 8, 645084. [Google Scholar] [CrossRef]
- Wu, Z.-H.; Li, Z.-W.; Yang, D.-L.; Liu, J. Development and Validation of a Pyroptosis-Related Long Non-coding RNA Signature for Hepatocellular Carcinoma. Front. Cell Dev. Biol. 2021, 9, 713925. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Bridgewater, J.; Normanno, N. Practical considerations in screening for genetic alterations in cholangiocarcinoma. Ann. Oncol. 2021, 32, 1111–1126. [Google Scholar] [CrossRef]
- Rodrigues, P.M.; Olaizola, P.; Paiva, N.A.; Olaizola, I.; Agirre-Lizaso, A.; Landa, A.; Bujanda, L.; Perugorria, M.J.; Banales, J.M. Pathogenesis of Cholangiocarcinoma. Annu. Rev. Pathol. 2021, 16, 433–463. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Bai, J.; Xu, J.; Zhao, J.; Zhang, R. Downregulation of lncRNA AWPPH inhibits colon cancer cell proliferation by downregulating GLUT-1. Oncol. Lett. 2019, 18, 2007–2012. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Liu, L.; Wu, Y.; Fu, P.; Cao, Y.; Xiong, J.; Tu, Y.; Li, Z.; Liu, Y.; et al. Comprehensive Analysis of Immune Infiltrates of Ferroptosis-Related Long Noncoding RNA and Prediction of Colon Cancer Patient Prognoses. J. Immunol. Res. 2022, 2022, 9480628. [Google Scholar] [CrossRef]
- Luo, P.; Wu, S.; Ji, K.; Yuan, X.; Li, H.; Chen, J.; Tian, Y.; Qiu, Y.; Zhong, X. LncRNA MIR4435-2HG mediates cisplatin resistance in HCT116 cells by regulating Nrf2 and HO-1. PLoS ONE 2020, 15, e0223035. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Yi, M.; Li, T.; Niu, M.; Luo, S.; Chu, Q.; Wu, K. Epidemiological trends of women’s cancers from 1990 to 2019 at the global, regional, and national levels: A population-based study. Biomark. Res. 2021, 9, 55. [Google Scholar] [CrossRef]
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef]
- Denaro, N.; Merlano, M.C.; Russi, E.G. Follow-up in Head and Neck Cancer: Do More Does It Mean Do Better? A Systematic Review and Our Proposal Based on Our Experience. Clin. Exp. Otorhinolaryngol. 2016, 9, 287–297. [Google Scholar] [CrossRef]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar]
- Zhang, Y.; Luo, G.; Li, M.; Guo, P.; Xiao, Y.; Ji, H.; Hao, Y. Global patterns and trends in ovarian cancer incidence: Age, period and birth cohort analysis. BMC Cancer 2019, 19, 984. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Vuong, L.; Kotecha, R.R.; Voss, M.H.; Hakimi, A.A. Tumor Microenvironment Dynamics in Clear-Cell Renal Cell Carcinoma. Cancer Discov. 2019, 9, 1349–1357. [Google Scholar] [CrossRef]
- Zhai, W.; Sun, Y.; Jiang, M.; Wang, M.; Gasiewicz, T.A.; Zheng, J.; Chang, C. Differential regulation of LncRNA-SARCC suppresses VHL-mutant RCC cell proliferation yet promotes VHL-normal RCC cell proliferation via modulating androgen receptor/HIF-2α/C-MYC axis under hypoxia. Oncogene 2017, 36, 4525. [Google Scholar] [CrossRef] [PubMed]
- Bella, L.; Zona, S.; Nestal de Moraes, G.; Lam, E.W.-F. FOXM1: A key oncofoetal transcription factor in health and disease. Semin. Cancer Biol. 2014, 29, 32–39. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M.D. Bladder cancer: A review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, S.; Sun, Y.; Long, G.; Chen, K. Identification of Long Non-Coding RNA MIR4435-2HG as a Prognostic Biomarker in Bladder Cancer. Genes 2022, 13, 1462. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, H.; Subbarayan, K.; Massa, C.; Vaxevanis, C.; Mueller, A.; Seliger, B. Correlation of the tumor escape phenotype with loss of PRELP expression in melanoma. J. Transl. Med. 2023, 21, 643. [Google Scholar] [CrossRef]
- Ang, H.L.; Mohan, C.D.; Shanmugam, M.K.; Leong, H.C.; Makvandi, P.; Rangappa, K.S.; Bishayee, A.; Kumar, A.P.; Sethi, G. Mechanism of epithelial-mesenchymal transition in cancer and its regulation by natural compounds. Med. Res. Rev. 2023, 43, 1141–1200. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct. Target. Ther. 2021, 6, 307. [Google Scholar] [CrossRef]
- Ghasemian, M.; Rajabibazl, M.; Mirfakhraie, R.; Razavi, A.E.; Sadeghi, H. Long noncoding RNA LINC00978 acts as a potential diagnostic biomarker in patients with colorectal cancer. Exp. Mol. Pathol. 2021, 122, 104666. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Du, J.; Zhou, L.; Sun, B. lncRNA AWPPH promotes proliferation and inhibits apoptosis of non-small cell lung cancer cells by activating the Wnt/β-catenin signaling pathway. Mol. Med. Rep. 2019, 19, 4425–4432. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, W.; Deng, J.; Dong, S. LncRNA AWPPH promotes the proliferation, migration and invasion of ovarian carcinoma cells via activation of the Wnt/β-catenin signaling pathway. Mol. Med. Rep. 2019, 19, 3615–3621. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Ronkina, N.; Gaestel, M. MAPK-Activated Protein Kinases: Servant or Partner? Annu. Rev. Biochem. 2022, 91, 505–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cheng, S.; Cao, L.; Yang, J.; Wang, Y.; Chen, Y. LINC00978 promotes hepatocellular carcinoma carcinogenesis partly via activating the MAPK/ERK pathway. Biosci. Rep. 2020, 40, BSR20192790. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Massagué, J.; Sheppard, D. TGF-β signaling in health and disease. Cell 2023, 186, 4007–4037. [Google Scholar] [CrossRef]
- Tang, L.; Wang, T.; Zhang, Y.; Zhang, J.; Zhao, H.; Wang, H.; Wu, Y.; Liu, K. Long Non-Coding RNA AWPPH Promotes Postoperative Distant Recurrence in Resected Non-Small Cell Lung Cancer by Upregulating Transforming Growth Factor beta 1 (TGF-β1). Med. Sci. Monit. 2019, 25, 2535–2541. [Google Scholar] [CrossRef]
- Heldin, C.-H.; Moustakas, A. Signaling Receptors for TGF-β Family Members. Cold Spring Harb. Perspect. Biol. 2016, 8, a022053. [Google Scholar] [CrossRef]
- Li, S.; Hu, X.; Yu, S.; Yi, P.; Chen, R.; Huang, Z.; Huang, Y.; Huang, Y.; Zhou, R.; Fan, X. Hepatic stellate cell-released CXCL1 aggravates HCC malignant behaviors through the MIR4435-2HG/miR-506-3p/TGFB1 axis. Cancer Sci. 2023, 114, 504–520. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, T.; Khalaj-Kondori, M.; Hosseinpour Feizi, M.A.; Asadi, P. Aberrant expression of lncRNAs SNHG6, TRPM2-AS1, MIR4435-2HG, and hypomethylation of TRPM2-AS1 promoter in colorectal cancer. Cell Biol. Int. 2021, 45, 2464–2478. [Google Scholar] [CrossRef]
- Fenton, C.G.; Ray, M.K.; Meng, W.; Paulssen, R.H. Methylation-Regulated Long Non-Coding RNA Expression in Ulcerative Colitis. Int. J. Mol. Sci. 2023, 24, 10500. [Google Scholar] [CrossRef]
- Yang, J.; Xu, J.; Wang, W.; Zhang, B.; Yu, X.; Shi, S. Epigenetic regulation in the tumor microenvironment: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, S.; Chen, J.; Ma, S.; Liu, F.; Liu, C.; Gao, Y. Comprehensive analysis of the MIR4435-2HG/miR-1-3p/MMP9/miR-29-3p/DUXAP8 ceRNA network axis in hepatocellular carcinoma. Discov. Oncol. 2021, 12, 38. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Do, D.N.; Nguyen, T.T.P.; Nguyen, T.L.; Nguyen-Thanh, T.; Nguyen, H.T. Immune–related biomarkers shared by inflammatory bowel disease and liver cancer. PLoS ONE 2022, 17, e0267358. [Google Scholar] [CrossRef]
- Mao, G.; Li, L.; Shan, C.; Liang, B.; Ma, L.; Zhang, S. High expression of RRM2 mediated by non-coding RNAs correlates with poor prognosis and tumor immune infiltration of hepatocellular carcinoma. Front. Med. 2022, 9, 833301. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Shan, C.; Li, W.; Liang, B.; Ma, L.; Zhang, S. High Expression of RRM1 Mediated by ncRNAs Correlates with Poor Prognosis and Tumor Immune Infiltration of Hepatocellular Carcinoma. Int. J. Gen. Med. 2022, 15, 2607–2620. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ren, Y.; Zuo, T. Long noncoding RNA LINC00978 promotes cell proliferation and invasion in non-small cell lung cancer by inhibiting miR-6754-5p. Mol. Med. Rep. 2018, 18, 4725–4732. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.; Li, M.; Zhang, Y.; Lin, T.; Sun, J.; Wang, D.; Liu, Y.; Guo, J.; Yu, W. Comprehensive analysis of ceRNA networks reveals prognostic lncRNAs related to immune infiltration in colorectal cancer. BMC Cancer 2021, 21, 255. [Google Scholar] [CrossRef]
- Xing, X.-L.; Zhang, T.; Yao, Z.-Y.; Xing, C.; Wang, C.; Liu, Y.-W.; Huang, M. Immune-Related Gene Expression Analysis Revealed Three lncRNAs as Prognostic Factors for Colon Cancer. Front. Genet. 2021, 12, 690053. [Google Scholar] [CrossRef]
- Ma, S.; Liu, M.; Xu, Z.; Li, Y.; Guo, H.; Ge, Y.; Liu, Y.; Zheng, D.; Shi, J. A double feedback loop mediated by microRNA-23a/27a/24-2 regulates M1 versus M2 macrophage polarization and thus regulates cancer progression. Oncotarget 2016, 7, 13502–13519. [Google Scholar] [CrossRef] [PubMed]
- Kotzin, J.J.; Spencer, S.P.; McCright, S.J.; Kumar, D.B.U.; Collet, M.A.; Mowel, W.K.; Elliott, E.N.; Uyar, A.; Makiya, M.A.; Dunagin, M.C.; et al. The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature 2016, 537, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Aryankalayil, M.J.; Chopra, S.; Levin, J.; Eke, I.; Makinde, A.; Das, S.; Shankavaram, U.; Vanpouille-Box, C.; Demaria, S.; Coleman, C.N. Radiation-Induced Long Noncoding RNAs in a Mouse Model after Whole-Body Irradiation. Radiat. Res. 2018, 189, 251–263. [Google Scholar] [CrossRef]
- Cai, Z.; Kotzin, J.J.; Ramdas, B.; Chen, S.; Nelanuthala, S.; Palam, L.R.; Pandey, R.; Mali, R.S.; Liu, Y.; Kelley, M.R.; et al. Inhibition of Inflammatory Signaling in Tet2 Mutant Preleukemic Cells Mitigates Stress-Induced Abnormalities and Clonal Hematopoiesis. Cell Stem Cell 2018, 23, 833–849.e5. [Google Scholar] [CrossRef] [PubMed]
- Kotzin, J.J.; Iseka, F.; Wright, J.; Basavappa, M.G.; Clark, M.L.; Ali, M.-A.; Abdel-Hakeem, M.S.; Robertson, T.F.; Mowel, W.K.; Joannas, L.; et al. The long noncoding RNA Morrbid regulates CD8 T cells in response to viral infection. Proc. Natl. Acad. Sci. USA 2019, 116, 11916–11925. [Google Scholar] [CrossRef]
- Ma, C.; Li, F.; Gu, Z.; Yang, Y.; Qi, Y. A novel defined risk signature of cuproptosis-related long non-coding RNA for predicting prognosis, immune infiltration, and immunotherapy response in lung adenocarcinoma. Front. Pharmacol. 2023, 14, 1146840. [Google Scholar] [CrossRef] [PubMed]
- Dang, R.; Jin, M.; Nan, J.; Jiang, X.; He, Z.; Su, F.; Li, D. A Novel Ferroptosis-Related lncRNA Signature for Prognosis Prediction in Patients with Papillary Renal Cell Carcinoma. Int. J. Gen. Med. 2022, 15, 207–222. [Google Scholar] [CrossRef] [PubMed]
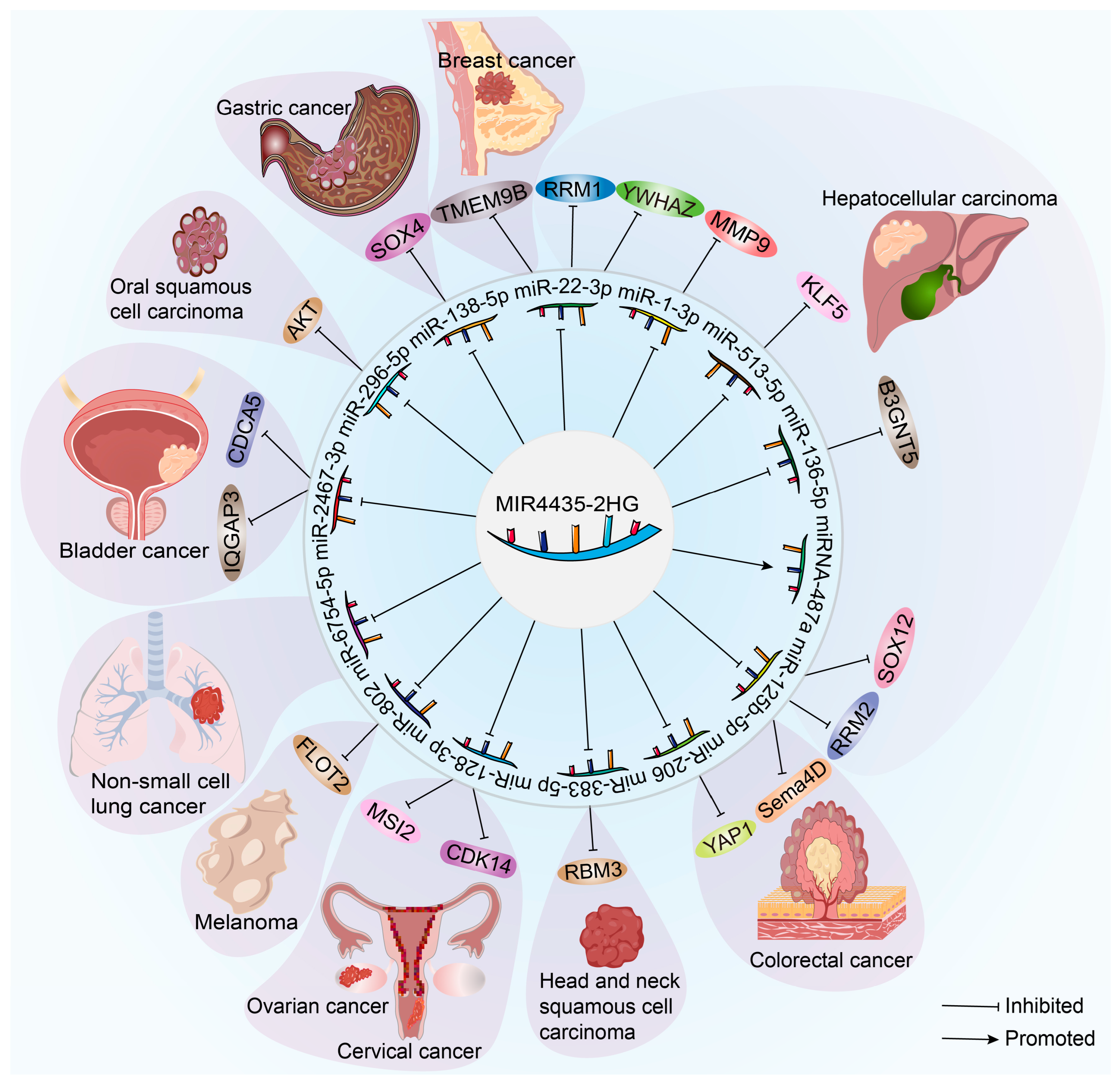
| Cancer Type | MIR4435-2HG Expression | Malignant Behaviors | Prognosis | References |
|---|---|---|---|---|
| Gastric cancer | Up-regulated | Enhanced cancer cell proliferation, invasion and migration. | Poor | [7,23,24,25,26] |
| Hepatocellular carcinoma | Up-regulated | Enhanced cancer cell proliferation, invasion, migration and EMT. | Poor | [14,27,28,29] |
| Cholangiocarcinoma | Up-regulated | Promoted cancer cell proliferation and migration. | Poor | [30] |
| Colorectal cancer | Up-regulated | Promoted cancer cell proliferation, invasion and migration. | Poor | [11,31,32,33,34,35] |
| Lung cancer | Up-regulated | Enhanced cancer cell proliferation, invasion, migration and EMT. | - | [8,36] |
| Breast cancer | Up-regulated | Enhanced cancer cell proliferation, invasion, migration and EMT. | Poor | [9,37,38,39] |
| Glioma | Up-regulated | Enhanced cancer cell proliferation, invasion, migration and EMT. | - | [40,41,42,43] |
| Head and neck squamous cell carcinoma | Up-regulated | Enhanced cancer cell proliferation, invasion, migration and EMT. | Poor | [44,45,46,47,48,49] |
| Ovarian cancer | Up-regulated | Promoted cancer cell proliferation, invasion and migration. | - | [50,51] |
| Cervical cancer | Up-regulated | Promoted cancer cell proliferation, invasion and migration. | - | [10] |
| Clear cell renal cell carcinoma | Up-regulated | Promoted cancer cell proliferation and migration. | - | [52] |
| Bladder cancer | Up-regulated | Promoted cancer cell proliferation, invasion and migration. | - | [53,54] |
| Prostate cancer | Up-regulated | Promoted cancer cell proliferation, invasion and migration. | Poor | [55,56] |
| Melanoma | Up-regulated | Promoted cancer cell proliferation and migration. | - | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Guan, D.; Zhu, Q.; Wang, Z.; Han, F.; Zhou, W. Biological Roles and Pathogenic Mechanisms of LncRNA MIR4435-2HG in Cancer: A Comprehensive Review. Curr. Issues Mol. Biol. 2023, 45, 8864-8881. https://doi.org/10.3390/cimb45110556
Chen Z, Guan D, Zhu Q, Wang Z, Han F, Zhou W. Biological Roles and Pathogenic Mechanisms of LncRNA MIR4435-2HG in Cancer: A Comprehensive Review. Current Issues in Molecular Biology. 2023; 45(11):8864-8881. https://doi.org/10.3390/cimb45110556
Chicago/Turabian StyleChen, Zhou, Defeng Guan, Qiangping Zhu, Zhengfeng Wang, Fangfang Han, and Wence Zhou. 2023. "Biological Roles and Pathogenic Mechanisms of LncRNA MIR4435-2HG in Cancer: A Comprehensive Review" Current Issues in Molecular Biology 45, no. 11: 8864-8881. https://doi.org/10.3390/cimb45110556
APA StyleChen, Z., Guan, D., Zhu, Q., Wang, Z., Han, F., & Zhou, W. (2023). Biological Roles and Pathogenic Mechanisms of LncRNA MIR4435-2HG in Cancer: A Comprehensive Review. Current Issues in Molecular Biology, 45(11), 8864-8881. https://doi.org/10.3390/cimb45110556







