Anti-Allergic Inflammatory Effect of Agarum cribrosum and Its Phlorotannin Component, Trifuhalol A, against the Ovalbumin-Induced Allergic Asthma Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of A. cribrosum Extract and Trifuhalol A
2.2. Animal Treatments and Induction of Allergic Asthma In Vivo Model
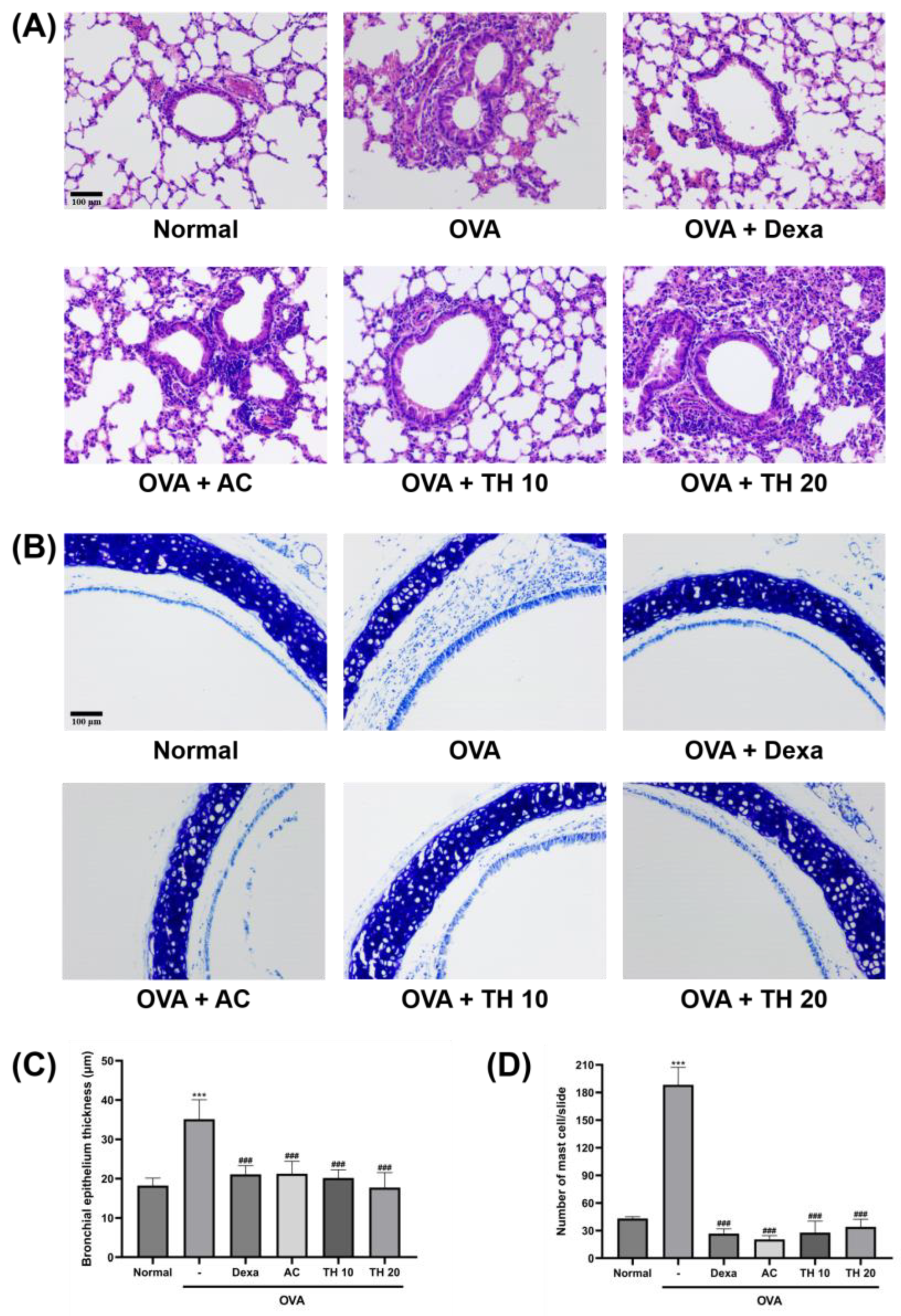
2.3. Histological Examination
2.4. Lung Tissue Homogenate Preparation
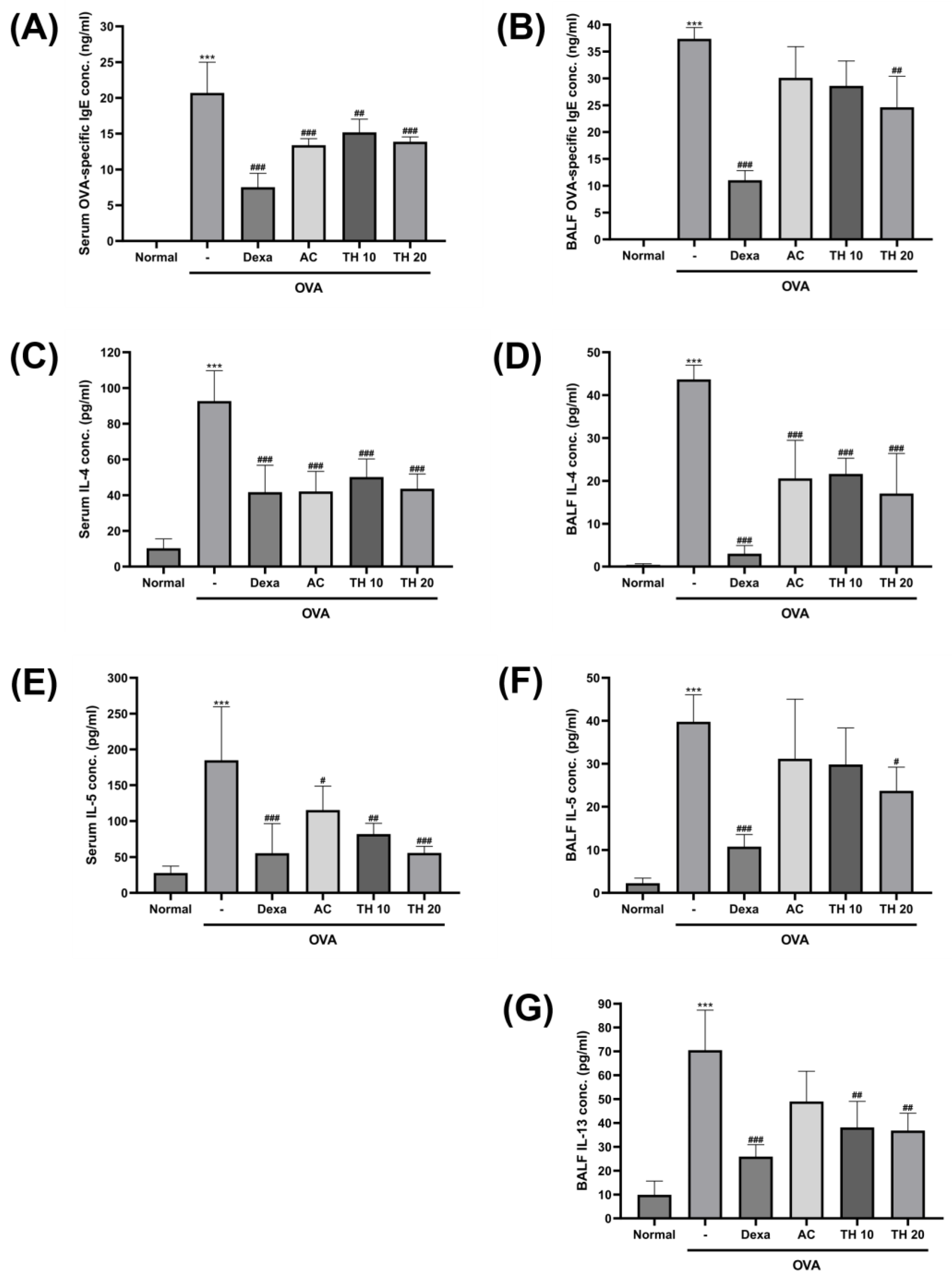
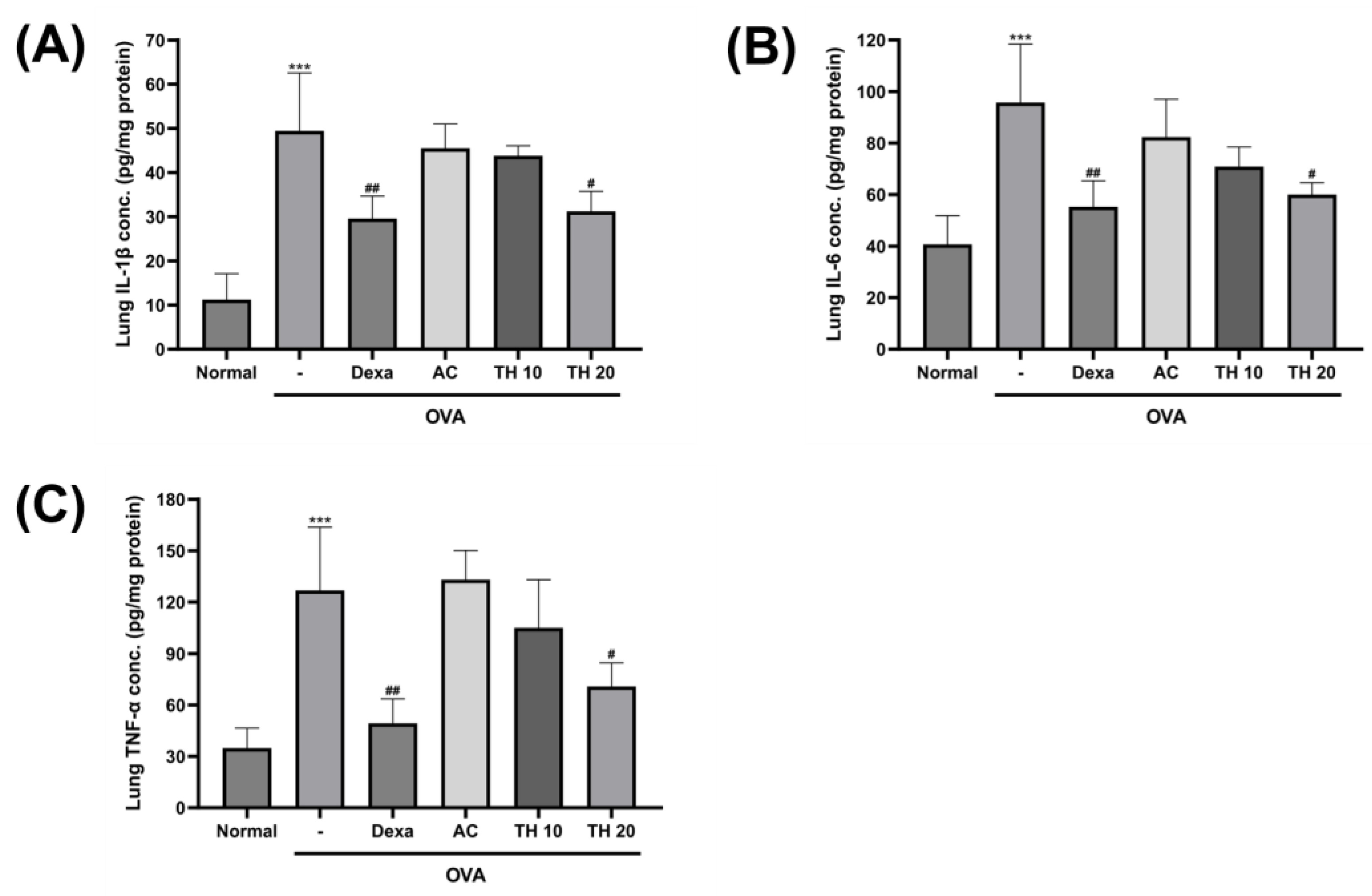
2.5. Measurement of Immunoglobulin and Inflammatory Cytokines
2.6. Statistical Analysis
3. Results
3.1. AC Extract and Trifuhalol A Reduce Histologic Changes in Lung and Trachea
3.2. AC Extract and Trifuhalol A Reduce Levels of OVA-Specific IgE and Allergic Cytokines in Both Serum and BALF
3.3. Trifuhalol A Attenuate IL-1β, IL-6, and TNF-α Levels in the Lung after OVA Inhalation Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Li, Y.; Gao, Y.; Fu, Y.; Lin, J.; Lei, X.; Zheng, J.; Jiang, M. Global, regional, and national burden of asthma and its attributable risk factors from 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Respir. Res. 2023, 24, 169. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.; Love, T.; Harrington, D.; Grape, A. Common allergies in urban adolescents and their relationships with asthma control and healthcare utilization. Allergy Asthma Clin. Immunol. 2018, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T. Innate and adaptive immune responses in asthma. Nat. Med. 2012, 18, 673–683. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Ridhima, W.; Kamal, D.; Ian, M.A.; Jay, C.H.; Richard, Y.K.; Philip, M.H. Cellular mechanisms underlying steroid-resistant asthma. Eur. Respir. Rev. 2019, 28, 190096. [Google Scholar] [CrossRef]
- Yim, R.P.; Koumbourlis, A.C. Steroid-resistant asthma. Paediatr. Respir. Rev. 2012, 13, 172–177. [Google Scholar] [CrossRef]
- Kim, B.M.J.; Jun, J.; Park, Y.B.; Jeong, I.H. Antioxidative activity of methanolic extracts from seaweeds. J. Korean Soc. Food Sci. Nutr. 2006, 35, 1097–1101. [Google Scholar]
- Park, S.-J.; Min, K.-J.; Park, T.-G. Nutritional Characteristics and Screening of Biological Activity of Agarum cribrosum. Korean J. Food Nutr. 2012, 25, 842–849. [Google Scholar] [CrossRef][Green Version]
- Park, S.-J.; Kim, B.; Kim, M.-J.; Kim, Y.-E.; Park, S.-H.; Park, T.-G. Effects of Agarum cribrosum on the Improvements in Treating Constipation and Plasma lipid Profiles. Korean J. Food Cook. Sci. 2013, 29, 185–192. [Google Scholar] [CrossRef][Green Version]
- Park, S.-J.; Lee, S.-A.; Song, H.-N.; Park, T.-G. Effects of Dietary Intake of Agarum cribrosum Ethanol Extract on Lipid Level in Diet-induced Obese Rats. Korean J. Food Cook. Sci. 2013, 29, 361–366. [Google Scholar] [CrossRef][Green Version]
- Shim, J.H. Anti-aging effect of Agarum cribrosum in UVA-irradiated normal human epidermal keratinocytes. Korean J. Pharmacogn. 2021, 52, 228–233. [Google Scholar] [CrossRef]
- Kim, L.; Hong, T.; Ham, J.; Lim, W. Effects of Agarum clathratum extract on cell death and calcium ion levels of ovarian cancer cell. Mol. Cell. Toxicol. 2023, 19, 303–310. [Google Scholar] [CrossRef]
- Kim, I.H.; Yoo, K.Y.; Park, J.H.; Yan, B.C.; Ahn, J.H.; Lee, J.C.; Kwon, H.M.; Kim, J.D.; Kim, Y.M.; You, S.G.; et al. Comparison of neuroprotective effects of extract and fractions from Agarum clathratum against experimentally induced transient cerebral ischemic damage. Pharm. Biol. 2014, 52, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Phasanasophon, K.; Kim, S.M. Antioxidant and Cosmeceutical Activities of Agarum cribrosum Phlorotannin Extracted by Ultrasound Treatment. Nat. Prod. Commun. 2018, 13, 1934578X1801300513. [Google Scholar] [CrossRef]
- Zhang, S.; Taehwan Kim, A.; Liu, X.; Yan, L.; Moo Kim, S. Antioxidant and antidiabetic activities of vanadium-binding protein and trifuhalol A. J. Food Biochem. 2020, 44, e13540. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Alam, M.B.; Naznin, M.; Shafique, I.; Kim, S.; Lee, S.H. Tyrosinase inhibitory activity of Sargassum fusiforme and characterisation of bioactive compounds. Phytochem. Anal. 2023. [Google Scholar] [CrossRef] [PubMed]
- Phasanasophon, K.; Kim, S.M. Anti-Inflammatory Activity of the Phlorotannin Trifuhalol A Using LPS-Stimulated RAW264.7 Cells Through NF-κB and MAPK Main Signaling Pathways. Nat. Prod. Commun. 2019, 14, 1934578X19849798. [Google Scholar] [CrossRef]
- Bong, S.K.; Park, N.J.; Lee, S.H.; Lee, J.W.; Kim, A.T.; Liu, X.; Kim, S.M.; Yang, M.H.; Kim, Y.K.; Kim, S.N. Trifuhalol A Suppresses Allergic Inflammation through Dual Inhibition of TAK1 and MK2 Mediated by IgE and IL-33. Int. J. Mol. Sci. 2022, 23, 10163. [Google Scholar] [CrossRef]
- BAI, T.R.; Knight, D.A. Structural changes in the airways in asthma: Observations and consequences. Clin. Sci. 2005, 108, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Blacquière, M.J.; Hylkema, M.N.; Postma, D.S.; Geerlings, M.; Timens, W.; Melgert, B.N. Airway inflammation and remodeling in two mouse models of asthma: Comparison of males and females. Int. Arch. Allergy Immunol. 2010, 153, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Tanabe, M.; Ito, W.; Ueki, S.; Konnno, Y.; Chihara, M.; Itoga, M.; Kobayashi, Y.; Moritoki, Y.; Kayaba, H.; et al. Gender difference in allergic airway remodelling and immunoglobulin production in mouse model of asthma. Respirology 2013, 18, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Uhm, T.G.; Kim, B.S.; Chung, I.Y. Eosinophil development, regulation of eosinophil-specific genes, and role of eosinophils in the pathogenesis of asthma. Allergy Asthma Immunol. Res. 2012, 4, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sjöstrand, M.; Malmhäll, C.; Rådinger, M.; Jeurink, P.; Lötvall, J.; Bossios, A. New production of eosinophils and the corresponding TH1/TH2 balance in the lungs after allergen exposure in BALB/c and C57BL/6 mice. Scand J. Immunol. 2010, 71, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.B. The role of eosinophils in the pathogenesis of asthma. Trends Mol. Med. 2005, 11, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Watt, A.P.; Schock, B.C.; Ennis, M. Neutrophils and eosinophils: Clinical implications of their appearance, presence and disappearance in asthma and COPD. Curr. Drug Targets Inflamm. Allergy 2005, 4, 415–423. [Google Scholar] [CrossRef]
- Kanda, A.; Driss, V.; Hornez, N.; Abdallah, M.; Roumier, T.; Abboud, G.; Legrand, F.; Staumont-Sallé, D.; Quéant, S.; Bertout, J.; et al. Eosinophil-derived IFN-gamma induces airway hyperresponsiveness and lung inflammation in the absence of lymphocytes. J. Allergy Clin. Immunol. 2009, 124, 573–582, 582.e571–579. [Google Scholar] [CrossRef]
- Banafea, G.H.; Bakhashab, S.; Alshaibi, H.F.; Natesan Pushparaj, P.; Rasool, M. The role of human mast cells in allergy and asthma. Bioengineered 2022, 13, 7049–7064. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.E. Immunoglobulin E: Role in asthma and allergic disease: Lessons from the clinic. Pharmacol. Ther. 2007, 113, 121–133. [Google Scholar] [CrossRef]
- Walsh, G.M. Biologics targeting IL-5, IL-4 or IL-13 for the treatment of asthma—An update. Expert Rev. Clin. Immunol. 2017, 13, 143–149. [Google Scholar] [CrossRef]
- Brown, M.A.; Pierce, J.H.; Watson, C.J.; Falco, J.; Ihle, J.N.; Paul, W.E. B cell stimulatory factor-1/interleukin-4 mRNA is expressed by normal and transformed mast cells. Cell 1987, 50, 809–818. [Google Scholar] [CrossRef]
- Greenfeder, S.; Umland, S.P.; Cuss, F.M.; Chapman, R.W.; Egan, R.W. Th2 cytokines and asthma The role of interleukin-5 in allergic eosinophilic disease. Respir. Res. 2001, 2, 71. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Interleukin-1 Beta as a Target for Atherosclerosis Therapy: Biological Basis of CANTOS and Beyond. J. Am. Coll. Cardiol. 2017, 70, 2278–2289. [Google Scholar] [CrossRef] [PubMed]
- Besnard, A.G.; Guillou, N.; Tschopp, J.; Erard, F.; Couillin, I.; Iwakura, Y.; Quesniaux, V.; Ryffel, B.; Togbe, D. NLRP3 inflammasome is required in murine asthma in the absence of aluminum adjuvant. Allergy 2011, 66, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.; Brightling, C.; Pavord, I.; Wardlaw, A.J. TNF-α in asthma. Curr. Opin. Pharmacol. 2007, 7, 279–282. [Google Scholar] [CrossRef]
- Rincon, M.; Irvin, C.G. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int. J. Biol. Sci. 2012, 8, 1281–1290. [Google Scholar] [CrossRef]
- Marini, M.; Vittori, E.; Hollemborg, J.; Mattoli, S. Expression of the potent inflammatory cytokines, granulocyte-macrophage-colony-stimulating factor and interleukin-6 and interleukin-8, in bronchial epithelial cells of patients with asthma. J. Allergy Clin. Immunol. 1992, 89, 1001–1009. [Google Scholar] [CrossRef]
- Broide, D.H.; Lotz, M.; Cuomo, A.J.; Coburn, D.A.; Federman, E.C.; Wasserman, S.I. Cytokines in symptomatic asthma airways. J. Allergy Clin. Immunol. 1992, 89, 958–967. [Google Scholar] [CrossRef]
- Ying, S.U.N.; Robinson, D.S.; Varney, V.; Meng, Q.I.U.; Tsicopoulos, A.; Moqbel, R.; Durham, S.R.; Kay, A.B.; Hamid, Q. TNFα mRNA expression in allergic inflammation. Clin. Exp. Allergy 1991, 21, 745–750. [Google Scholar] [CrossRef]
- Bradding, P.; Roberts, J.A.; Britten, K.M.; Montefort, S.; Djukanovic, R.; Mueller, R.; Heusser, C.H.; Howarth, P.H.; Holgate, S.T. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: Evidence for the human mast cell as a source of these cytokines. Am. J. Respir. Cell Mol. Biol. 1994, 10, 471–480. [Google Scholar] [CrossRef]
- Van Overveld, F.J.; Jorens Ph, G.; Rampart, M.; De Backer, W.; Vermeire, P.A. Tumour necrosis factor stimulates human skin mast cells to release histamine and tryptase. Clin. Exp. Allergy 1991, 21, 711–714. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, S.H.; Zhang, S.; Bong, S.-K.; Kim, A.T.; Lee, H.; Liu, X.; Kim, S.M.; Kim, S.-N. Anti-Allergic Inflammatory Effect of Agarum cribrosum and Its Phlorotannin Component, Trifuhalol A, against the Ovalbumin-Induced Allergic Asthma Model. Curr. Issues Mol. Biol. 2023, 45, 8882-8893. https://doi.org/10.3390/cimb45110557
Kim J, Lee SH, Zhang S, Bong S-K, Kim AT, Lee H, Liu X, Kim SM, Kim S-N. Anti-Allergic Inflammatory Effect of Agarum cribrosum and Its Phlorotannin Component, Trifuhalol A, against the Ovalbumin-Induced Allergic Asthma Model. Current Issues in Molecular Biology. 2023; 45(11):8882-8893. https://doi.org/10.3390/cimb45110557
Chicago/Turabian StyleKim, Joonki, Sang Heon Lee, Siqi Zhang, Sim-Kyu Bong, Aaron Taehwan Kim, Hara Lee, Xiaoyong Liu, Sang Moo Kim, and Su-Nam Kim. 2023. "Anti-Allergic Inflammatory Effect of Agarum cribrosum and Its Phlorotannin Component, Trifuhalol A, against the Ovalbumin-Induced Allergic Asthma Model" Current Issues in Molecular Biology 45, no. 11: 8882-8893. https://doi.org/10.3390/cimb45110557
APA StyleKim, J., Lee, S. H., Zhang, S., Bong, S.-K., Kim, A. T., Lee, H., Liu, X., Kim, S. M., & Kim, S.-N. (2023). Anti-Allergic Inflammatory Effect of Agarum cribrosum and Its Phlorotannin Component, Trifuhalol A, against the Ovalbumin-Induced Allergic Asthma Model. Current Issues in Molecular Biology, 45(11), 8882-8893. https://doi.org/10.3390/cimb45110557






