Screening of Tnfaip1-Interacting Proteins in Zebrafish Embryonic cDNA Libraries Using a Yeast Two-Hybrid System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Zebrafish Husbandry
2.3. Assays of Toxicity and Auto-Activation of the Zebrafish Tnfaip1 Bait Plasmid
2.4. Screening and Identification of Tnfaip1-Interacting Proteins
2.5. Functional Annotation of Potential Tnfaip1-Interacting Proteins
2.6. Yeast Two-Hybrid Spot Analysis
3. Results
3.1. Construction of a Yeast Library of cDNA from Zebrafish Embryos
3.2. Toxicity and Auto-Activation Detection of Zebrafish Tnfaip1 Bait Recombinant Plasmid
3.3. Screening and Sequencing Analysis of Tnfaip1-Interacting Proteins
3.4. Functional Enrichment Analysis of Tnfaip1-Interacting Proteins
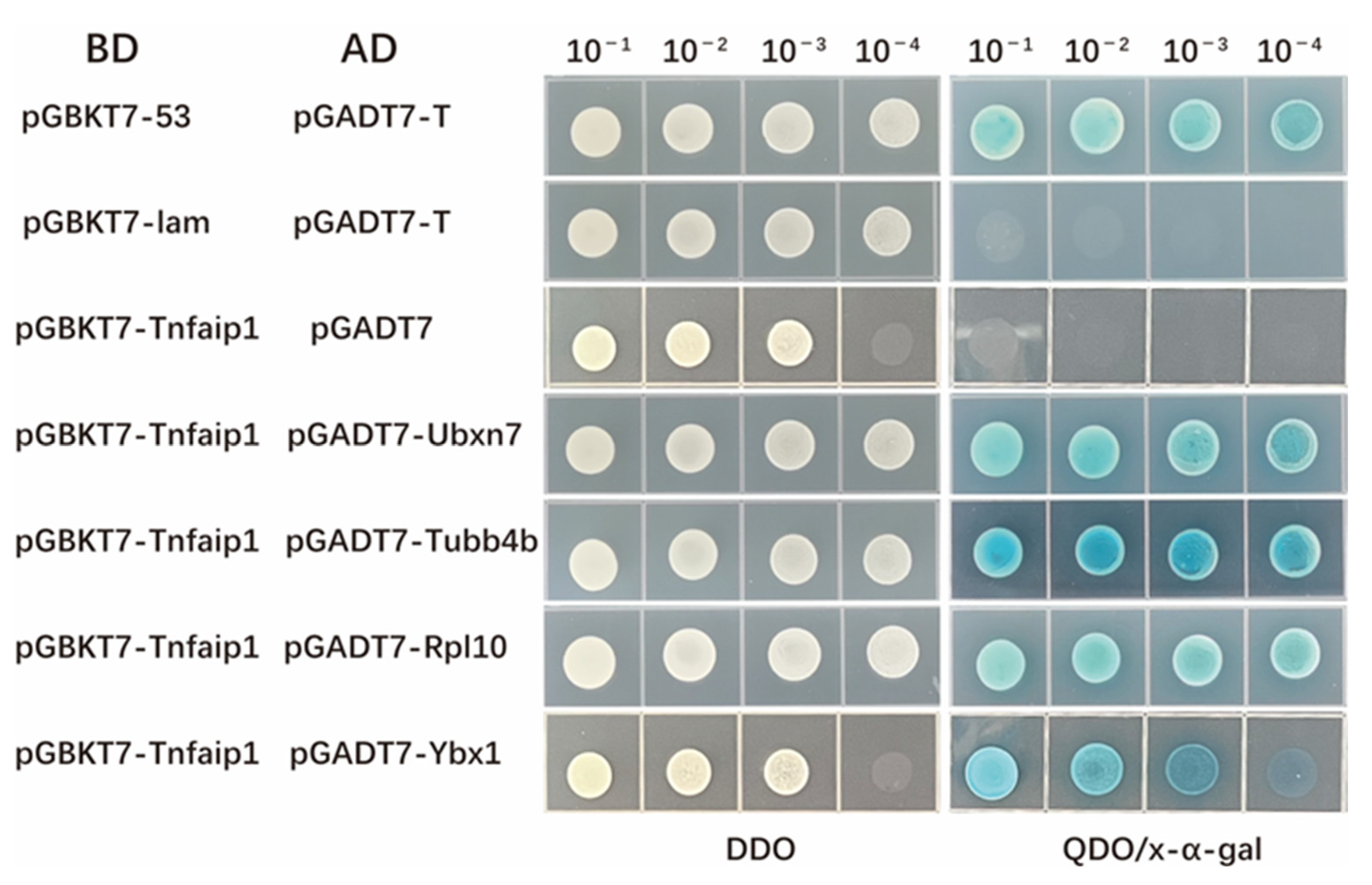
3.5. Yeast Spotting Analysis of the Interaction between Tnfaip1 and Ubxn7, Tubb4b, Rpl10, and Ybx1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolf, F.W.; Marks, R.M.; Sarma, V.; Byers, M.G.; Katz, R.W.; Shows, T.B.; Dixit, V.M. Characterization of a Novel Tumor Necrosis Factor-a-induced Endothelial Primary Response Gene. J. Biol. Chem. 1992, 267, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xiang, Y.; Sun, G. The KCTD family of proteins: Structure, function, disease relevance. Cell Biosci. 2013, 3, 45. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Gan, S.; Xie, G.; Li, L.; Chen, C.; Ding, X.; Han, M.; Xiang, S.; Zhang, J. KCTD10 is critical for heart and blood vessel development of zebrafish. Acta Biochim. Biophys. Sin. 2014, 46, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Zu, Y.; Li, Z.; Li, W.; Ying, L.; Yang, J.; Wang, X.; He, S.; Liu, D.; Zhu, Z.; et al. Kctd10 regulates heart morphogenesis by repressing the transcriptional activity of Tbx5a in zebrafish. Nat. Commun. 2014, 5, 3153. [Google Scholar] [CrossRef]
- Ren, K.; Yuan, J.; Yang, M.; Gao, X.; Ding, X.; Zhou, J.; Hu, X.; Cao, J.; Deng, X.; Xiang, S.; et al. KCTD10 is involved in the cardiovascular system and Notch signaling during early embryonic development. PLoS ONE 2014, 9, e112275. [Google Scholar] [CrossRef]
- Golzio, C.; Willer, J.; Talkowski, M.E.; Oh, E.C.; Taniguchi, Y.; Jacquemont, S.; Reymond, A.; Sun, M.; Sawa, A.; Gusella, J.F.; et al. KCTD13 is a major driver of mirrored neuroanatomical phenotypes of the 16p11.2 copy number variant. Nature 2012, 485, 363–367. [Google Scholar] [CrossRef]
- Lin, G.N.; Corominas, R.; Lemmens, I.; Yang, X.; Tavernier, J.; Hill, D.E.; Vidal, M.; Sebat, J.; Iakoucheva, L.M. Spatiotemporal 16p11.2 protein network implicates cortical late mid-fetal brain development and KCTD13-Cul3-RhoA pathway in psychiatric diseases. Neuron 2015, 85, 742–754. [Google Scholar] [CrossRef]
- Escamilla, C.O.; Filonova, I.; Walker, A.K.; Xuan, Z.X.; Holehonnur, R.; Espinosa, F.; Liu, S.; Thyme, S.B.; Lopez-Garcia, I.A.; Mendoza, D.B.; et al. Kctd13 deletion reduces synaptic transmission via increased RhoA. Nature 2017, 551, 227–231. [Google Scholar] [CrossRef]
- Gladwyn-Ng, I.E.; Li, S.S.; Qu, Z.; Davis, J.M.; Ngo, L.; Haas, M.; Singer, J.; Heng, J.I. Bacurd2 is a novel interacting partner to Rnd2 which controls radial migration within the developing mammalian cerebral cortex. Neural Dev. 2015, 10, 9. [Google Scholar] [CrossRef]
- Gladwyn-Ng, I.; Huang, L.; Ngo, L.; Li, S.S.; Qu, Z.; Vanyai, H.K.; Cullen, H.D.; Davis, J.M.; Heng, J.I. Bacurd1/Kctd13 and Bacurd2/Tnfaip1 are interacting partners to Rnd proteins which influence the long-term positioning and dendritic maturation of cerebral cortical neurons. Neural Dev. 2016, 11, 7. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, X.; Xiong, X.; Liu, X.; Liu, Y.; Ren, K.; Jiang, T.; Hu, X.; Zhang, J. Cloning of two rat PDIP1 related genes and their interactions with proliferating cell nuclear antigen. J. Exp. Zool. Part A Comp. Exp. Biol. 2005, 303, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Chung, K.S.; Choi, S.J.; Jung, Y.J.; Park, S.K.; Han, G.H.; Ha, J.S.; Song, K.B.; Choi, N.S.; Kim, H.M.; et al. RhoB induces apoptosis via direct interaction with TNFAIP1 in HeLa cells. Int. J. Cancer 2009, 125, 2520–2527. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, S.; Qiu, F.; Ding, X.; Sun, Y.; Wei, C.; Hu, X.; Wei, K.; Long, S.; Xie, L.; et al. Tumor necrosis factor alpha-induced protein 1 as a novel tumor suppressor through selective downregulation of CSNK2B blocks nuclear factor-kappaB activation in hepatocellular carcinoma. EBioMedicine 2020, 51, 102603. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, H.; Chen, W.; Su, N.; Yuan, C.; Zhang, J.; Xiang, S.; Hu, X. CRISPR/Cas9-Mediated Knockout of tnfaip1 in Zebrafish Plays a Role in Early Development. Genes 2023, 14, 1005. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Brückner, A.; Polge, C.; Lentze, N.; Auerbach, D.; Schlattner, U. Yeast Two-Hybrid, a Powerful Tool for Systems Biology. Int. J. Mol. Sci. 2009, 10, 2763–2788. [Google Scholar] [CrossRef]
- Fields, S.; Song, C. A novel genetic system to detect protein-protein interactions. Nature 1989, 340, 245–246. [Google Scholar] [CrossRef]
- Paiano, A.; Margiotta, A.; Luca, M.D.; Bucci, C. Yeast Two-Hybrid Assay to Identify Interacting Proteins. Curr. Protoc. Protein Sci. 2019, 95, e70. [Google Scholar] [CrossRef]
- Adhish, M.; Manjubala, I. Effectiveness of zebrafish models in understanding human diseases—A review of models. Heliyon 2023, 9, e14557. [Google Scholar] [CrossRef]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish disease models in drug discovery: From preclinical modelling to clinical trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef]
- Sopel, N.; Müller-Deile, J. The Zebrafish Model to Understand Epigenetics in Renal Diseases. Int. J. Mol. Sci. 2021, 22, 9152. [Google Scholar] [CrossRef] [PubMed]
- Koegl, M.; Uetz, P. Improving yeast two-hybrid screening systems. Brief. Funct. Genom. Proteom. 2008, 6, 302–312. [Google Scholar] [CrossRef]
- Velásquez-Zapata, V.; Elmore, J.M.; Banerjee, S.; Dorman, K.S.; Wise, R.P. Next-generation yeast-two-hybrid analysis with Y2H-SCORES identifies novel interactors of the MLA immune receptor. PLOS Comput. Biol. 2021, 17, e1008890. [Google Scholar] [CrossRef] [PubMed]
- Parrish, J.R.; Gulyas, K.D.; Finley, R.L. Yeast two-hybrid contributions to interactome mapping. Curr. Opin. Biotechnol. 2006, 17, 387–393. [Google Scholar] [CrossRef]
- Hamdi, A.; Colas, P. Yeast two-hybrid methods and their applications in drug discovery. Trends Pharmacol. Sci. 2012, 33, 109–118. [Google Scholar] [CrossRef]
- Ferro, E.; Trabalzini, L. The yeast two-hybrid and related methods as powerful tools to study plant cell signalling. Plant Mol. Biol. 2013, 83, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Raman, M.; Sergeev, M.; Garnaas, M.; Lydeard, J.R.; Huttlin, E.L.; Goessling, W.; Shah, J.V.; Harper, J.W. Systematic proteomics of the VCP–UBXD adaptor network identifies a role for UBXN10 in regulating ciliogenesis. Nat. Cell Biol. 2015, 17, 1356–1369. [Google Scholar] [CrossRef]
- Cilenti, L.; Di Gregorio, J.; Ambivero, C.T.; Andl, T.; Liao, R.; Zervos, A.S. Mitochondrial MUL1 E3 ubiquitin ligase regulates Hypoxia Inducible Factor (HIF-1alpha) and metabolic reprogramming by modulating the UBXN7 cofactor protein. Sci. Rep. 2020, 10, 1609. [Google Scholar] [CrossRef]
- Di Gregorio, J.; Cilenti, L.; Ambivero, C.T.; Andl, T.; Liao, R.; Zervos, A.S. UBXN7 cofactor of CRL3(KEAP1) and CRL2(VHL) ubiquitin ligase complexes mediates reciprocal regulation of NRF2 and HIF-1a proteins. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2021, 1868, 118963. [Google Scholar] [CrossRef]
- Feng, M.; Wang, K.; Fu, S.; Wei, H.; Mu, X.; Li, L.; Zhang, S. Tubulin TUBB4B Is Involved in Spermatogonia Proliferation and Cell Cycle Processes. Genes 2022, 13, 1082. [Google Scholar] [CrossRef]
- Brooks, S.S.; Wall, A.L.; Golzio, C.; Reid, D.W.; Kondyles, A.; Willer, J.R.; Botti, C.; Nicchitta, C.V.; Katsanis, N.; Davis, E.E. A Novel Ribosomopathy Caused by Dysfunction of RPL10 Disrupts Neurodevelopment and Causes X-Linked Microcephaly in Humans. Genetics 2014, 198, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, G.; De Bernardi, M.L.; Morlando, A.; Peduto, C.; Scala, I.; Pinelli, M.; Bellacchio, E.; Gallo, F.G.; Magli, A.; Plaitano, C.; et al. Postnatal microcephaly and retinal involvement expand the phenotype of RPL10-related disorder. Am. J. Med. Genet. Part A 2022, 188, 3032–3040. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, I.A.; Kim, E.R.; Guryanov, S.G.; Ovchinnikov, L.P.; Lyabin, D.N. Y-box-binding protein 1 (YB-1) and its functions. Biochemistry 2012, 76, 1402–1433. [Google Scholar] [CrossRef]
- Evans, M.K.; Matsui, Y.; Xu, B.; Willis, C.; Loome, J.; Milburn, L.; Fan, Y.; Pagala, V.; Peng, J.C. Ybx1 fine-tunes PRC2 activities to control embryonic brain development. Nat. Commun. 2020, 11, 4060. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yan, L.; Shen, W.; Meng, A. Maternal Ybx1 safeguards zebrafish oocyte maturation and maternal-to-zygotic transition by repressing global translation. Development 2018, 145, dev166587. [Google Scholar] [CrossRef] [PubMed]
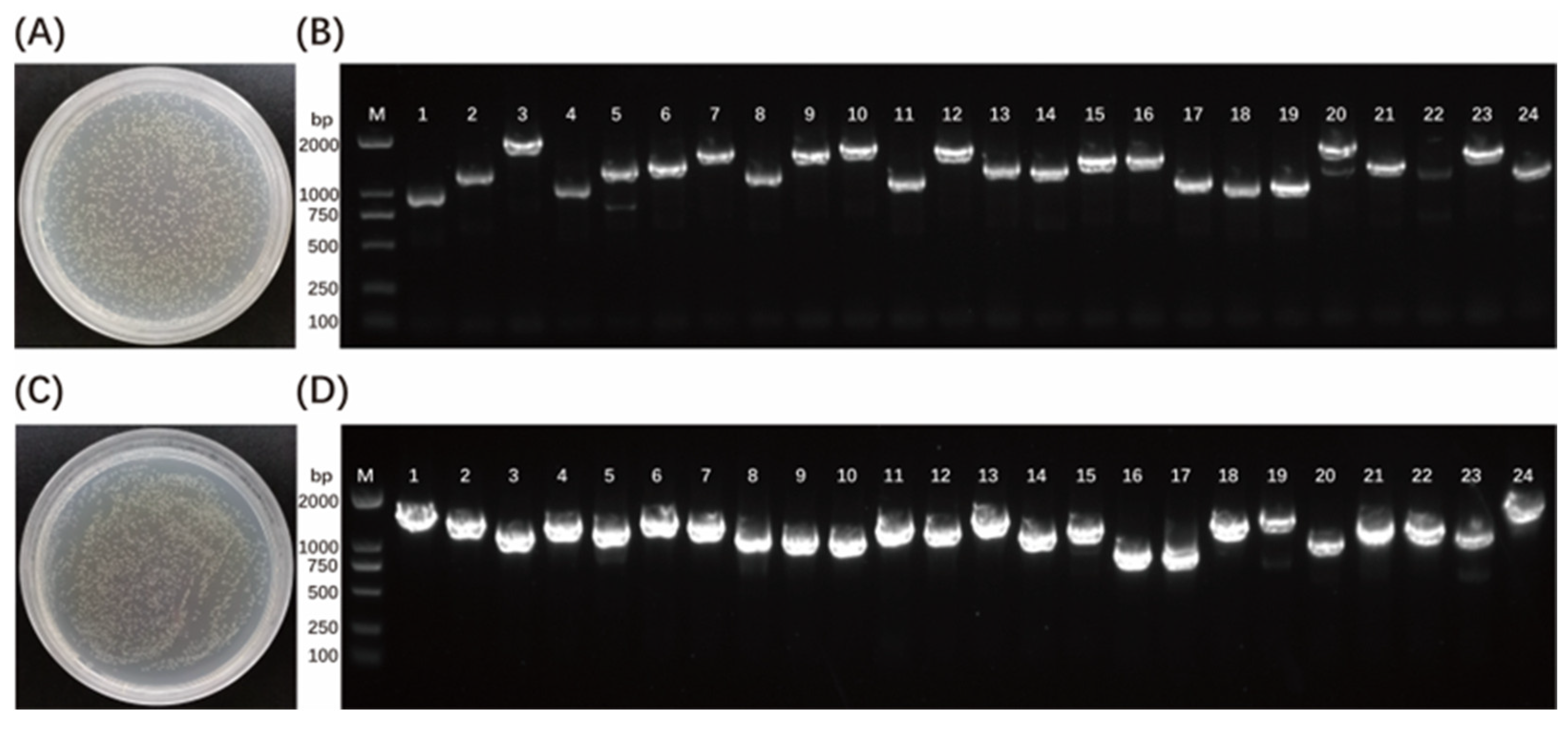
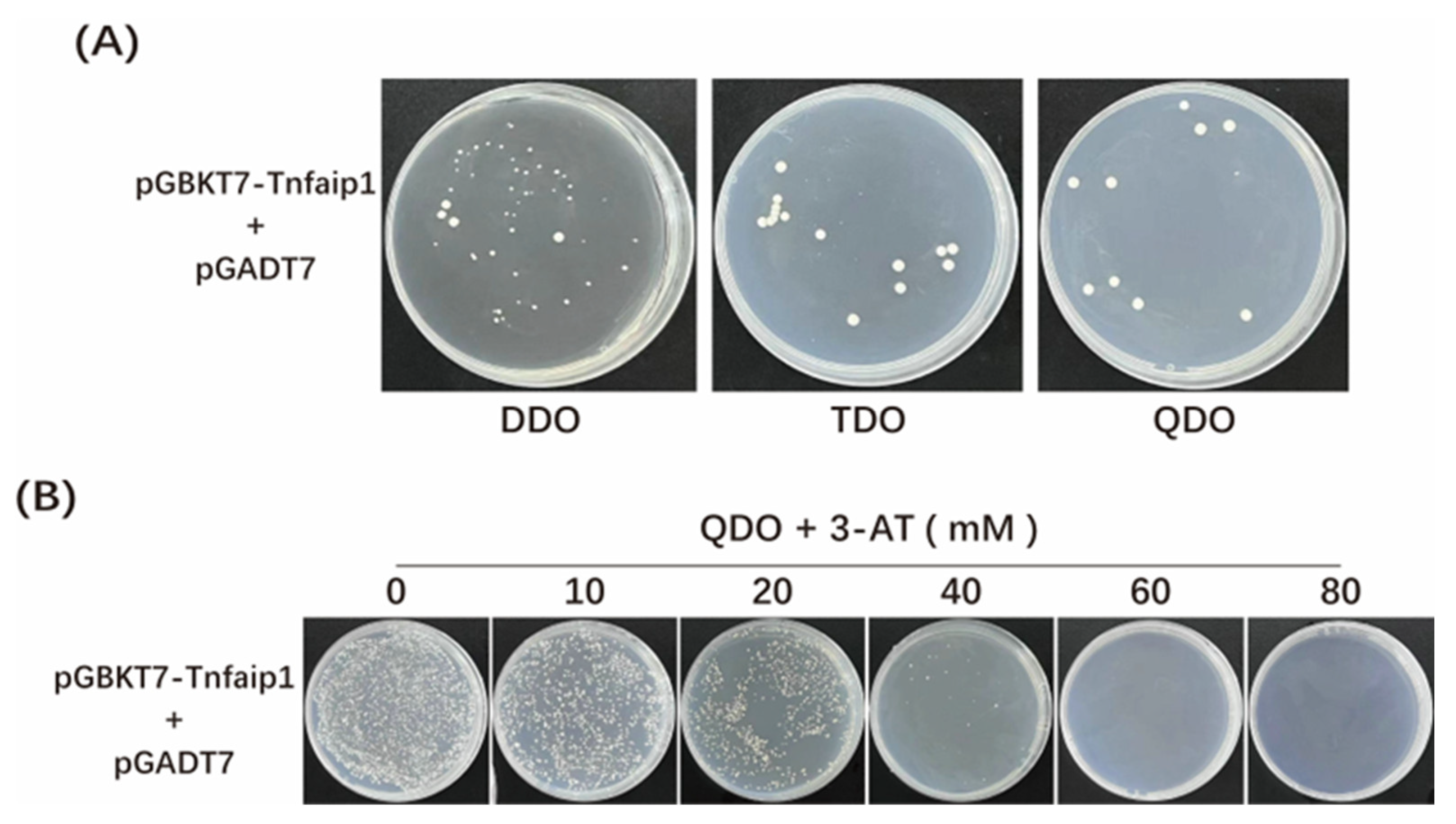
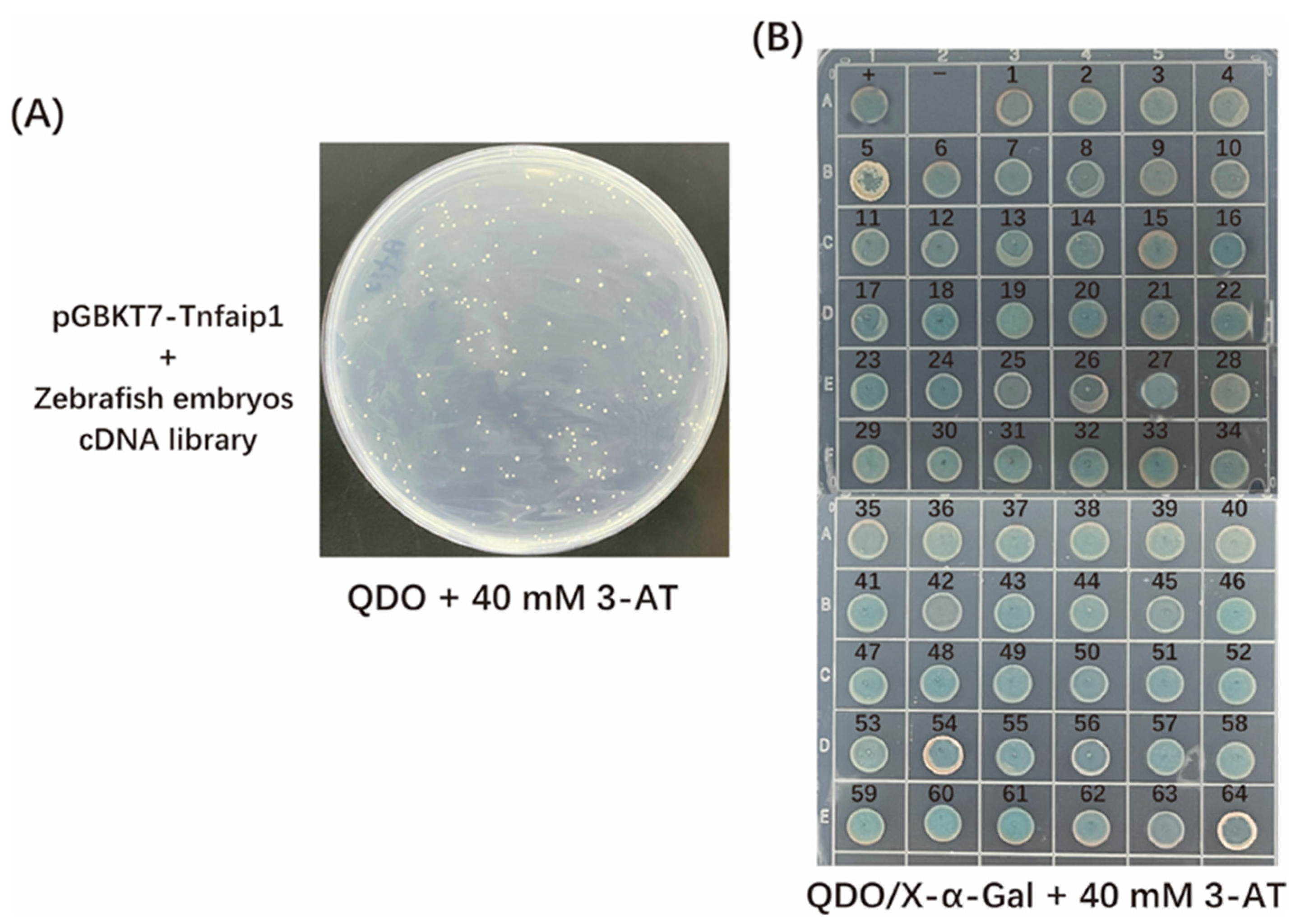
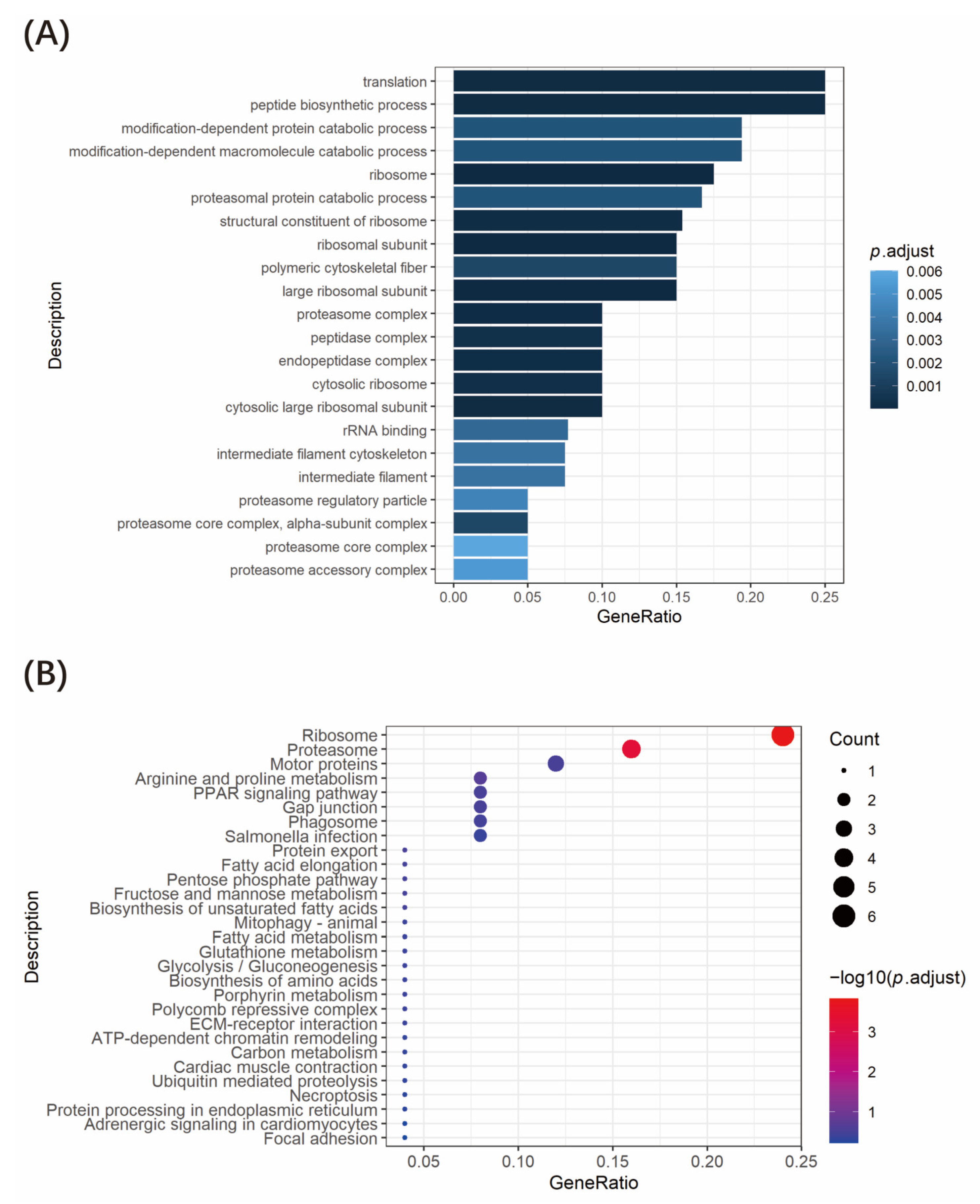
| Clone No. | GenBank Accession No. | Gene Name | Identify |
|---|---|---|---|
| 1 | NP_001001951.1 | UBX domain protein 7 (ubxn7) | 100% |
| 3 | NP_998223.1 | heat shock protein 5 (hspa5) | 98% |
| 4 | AAI63717.1 | Laminin, alpha 4 (lama4) | 100% |
| 6 | NP_942104.2 | tubulin, beta 4B class IVb (tubb4b) | 100% |
| 7 | NP_571519.1 | activated C kinase 1 (rack1) | 100% |
| 8 | AAH71464.1 | eukaryotic translation elongation factor 1 beta 2(eef1b2) | 99% |
| 9 | NP_571182.1 | type I cytokeratin, enveloping layer (cyt1) | 97% |
| 10 | NP_571180.1 | alpha-tropomyosin (tpma) | 100% |
| 12 | AAH45463.1 | proteasome 26S subunit, non-ATPase, 1 (psmd1) | 99% |
| 16 | NP_956269.1 | uncharacterized protein zgc:65894 (zgc:65894) | 99% |
| 17 | NP_705941.2 | proteasome 20S subunit alpha 6a (psma6a) | 99% |
| 18 | NP_001103596.2 | ChaC, cation transport regulator homolog 1 (chac1) | 99% |
| 19 | NP_937783.1 | tumor protein, translationally-controlled 1 (tpt1) | 99% |
| 20 | XP_005170972.1 | myozenin 2b (myoz2b) | 99% |
| 21 | XP_005157650.1 | creatine kinase, muscle b (ckmb) | 100% |
| 22 | AAH67580.1 | ribosomal protein L4 (rpl4) | 100% |
| 23 | XP_002662576.2 | Mov10 RISC complex RNA helicase a (mov10a) | 98% |
| 24 | NP_958456.1 | trans-2,3-enoyl-CoA reductase b (tecrb) | 99% |
| 25 | XP_017209012.1 | ribosomal protein L10 (rpl10) | 100% |
| 26 | NP_571694.1 | myosin, light polypeptide 3, skeletal muscle (mylz3) | 99% |
| 27 | AAH45406.1 | actin, alpha 1, skeletal muscle | 99% |
| 29 | NP_001003861.1 | ribosomal protein L9 (rpl9) | 99% |
| 30 | NP_956324.1 | ribosomal protein L27a (rpl27a) | 100% |
| 32 | AAH65432.1 | ribosomal protein L8 (rpl8) | 100% |
| 33 | AAQ91246.1 | laminin receptor 1 (LAMR1) | 99% |
| 35 | AAH44379.1 | aldolase a, fructose-bisphosphate, a (aldoaa) | 100% |
| 36 | AAH66728.1 | keratin 4 (krt4) | 99% |
| 37 | XP_021332536.1 | matrilin 4 (matn4) | 99% |
| 38 | AAH56706.1 | creatine kinase, muscle a (ckma) | 100% |
| 39 | NP_001077313.1 | collagen, type XI, alpha 1a (col11a1a) | 100% |
| 41 | NP_001373589.1 | dual specificity phosphatase 27 (dusp27) | 100% |
| 42 | XP_005173906.4 | vimentin-like | 99% |
| 43 | NP_001093614.2 | apolipoprotein A-Ib (apoa1b) | 97% |
| 45 | NP_001002498.2 | holocytochrome c synthase a (hccsa.1) | 98% |
| 48 | NP_001071272.2 | ubiquitin C (ubc) | 99% |
| 50 | AAH50156.1 | Y box binding protein 1 (ybx1) | 99% |
| 53 | NP_999862.1 | proteasome 20S subunit alpha 4 (psma4) | 98% |
| 54 | NP_001017824.1 | keratin 92 (krt92) | 99% |
| 55 | NP_001017766.2 | embryonic ectoderm development (eed) | 98% |
| 57 | NP_001002106.1 | ribosomal protein L5b (rpl5b) | 90% |
| 60 | NP_001036788.1 | H2A.Z variant histone 2b (h2az2b) | 99% |
| 62 | NP_938181.1 | translocase of inner mitochondrial membrane 17 homolog A (timm17a) | 98% |
| 63 | NP_956585.1 | proteasome 26S subunit, non-ATPase 6 (psmd6) | 99% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Zhang, H.; Chen, W.; Wang, J.; Wu, Z.; He, M.; Zhang, J.; Hu, X.; Xiang, S. Screening of Tnfaip1-Interacting Proteins in Zebrafish Embryonic cDNA Libraries Using a Yeast Two-Hybrid System. Curr. Issues Mol. Biol. 2023, 45, 8215-8226. https://doi.org/10.3390/cimb45100518
Huang S, Zhang H, Chen W, Wang J, Wu Z, He M, Zhang J, Hu X, Xiang S. Screening of Tnfaip1-Interacting Proteins in Zebrafish Embryonic cDNA Libraries Using a Yeast Two-Hybrid System. Current Issues in Molecular Biology. 2023; 45(10):8215-8226. https://doi.org/10.3390/cimb45100518
Chicago/Turabian StyleHuang, Shulan, Hongning Zhang, Wen Chen, Jiawei Wang, Zhen Wu, Meiqi He, Jian Zhang, Xiang Hu, and Shuanglin Xiang. 2023. "Screening of Tnfaip1-Interacting Proteins in Zebrafish Embryonic cDNA Libraries Using a Yeast Two-Hybrid System" Current Issues in Molecular Biology 45, no. 10: 8215-8226. https://doi.org/10.3390/cimb45100518
APA StyleHuang, S., Zhang, H., Chen, W., Wang, J., Wu, Z., He, M., Zhang, J., Hu, X., & Xiang, S. (2023). Screening of Tnfaip1-Interacting Proteins in Zebrafish Embryonic cDNA Libraries Using a Yeast Two-Hybrid System. Current Issues in Molecular Biology, 45(10), 8215-8226. https://doi.org/10.3390/cimb45100518







