Abstract
The mammalian central nervous system (CNS) coordinates its communication through saltatory conduction, facilitated by myelin-forming oligodendrocytes (OLs). Despite the fact that neurogenesis from stem cell niches has caught the majority of attention in recent years, oligodendrogenesis and, more specifically, the molecular underpinnings behind OL-dependent myelinogenesis, remain largely unknown. In this comprehensive review, we determine the developmental cues and molecular drivers which regulate normal myelination both at the prenatal and postnatal periods. We have indexed the individual stages of myelinogenesis sequentially; from the initiation of oligodendrocyte precursor cells, including migration and proliferation, to first contact with the axon that enlists positive and negative regulators for myelination, until the ultimate maintenance of the axon ensheathment and myelin growth. Here, we highlight multiple developmental pathways that are key to successful myelin formation and define the molecular pathways that can potentially be targets for pharmacological interventions in a variety of neurological disorders that exhibit demyelination.
1. Introduction
As the regulator of all cognitive, sensory, and motor activity, the nervous system is the most complex biological system in humans; the complexities of integrated neural networks are a hot area of intensive research that will require multidisciplinary investigations to address a variable array of neurological disorders that remain an unmet medical need. The main types of cells in the nervous system are neurons and glial cells with the latter performing vital supporting roles [1,2]. The glial/neuronal ratio differs uniformly across brain regions of mammalian species, underlining the pivotal role of interaction between glial cells and neurons for appropriate integration of the central nervous system (CNS) to coordinate neurophysiological and cognitive functions [3]. For propagation of action potentials to ensue in neurons, axonal myelination is crucial [4,5]. The cells responsible for myelination are the oligodendrocytes (OLs) in the CNS and Schwann cells in the peripheral nervous system (PNS). Rudolf Virchow initially designated the term “myelin” in 1854, named after the Greek word “marrow” (myelos), since it is especially plentiful in the brain’s center, or marrow [6]. He posited that neurons produced myelin, but Pío del Río Hortega’s better histological staining processes almost a century ago revealed that myelin is created by specific glial cells, which are OLs [7,8].
The CNS macroglia and neurons have a common embryonic origin from the neuroectoderm, most prominently from neuroepithelial cells of the telencephalic ventricular and subventricular zone (VZ and SVZ), while the spinal cord is supplied with cell derivatives exclusively from the central canal [9,10]. Newborn CNS is radically unmyelinated with a sparse developing pool of unipotent cells, namely oligodendrocyte precursor cells (OPCs), following their birth with gradual widespread functionality in the first few years of childhood [11]. Myelination persists in an asynchronous spatiotemporal pattern through adolescence towards adulthood, coinciding with the establishment and maintenance of correct circuit function and cognitive development [12,13]. Mature myelin sheaths remain stable by and large; however, they maintain the capacity to remodel and reorganize if need be [14]. As expected, aging promulgates limit resources and energy deficiency to sustain such developmental processes, thus cellular senescence is a common event [15]. Consequently, there is a variety in the patterns of myelination, with qualitative- and quantitative-ontogenic checkpoints, throughout human life.
In this review, we focus on the de novo synthesis of myelin referred to herein as myelinogenesis. This is the primordial pattern of myelination, which starts prenatally and predominates during the first two years of human life [16]. In order for myelinogenesis to happen, neural stem cells (NSCs) need to undergo specific developmental stages, with the process of oligodendrogenesis, as well as additional steps for the maintenance of these primary myelin sheaths. Interestingly, it is possible that lifelong myelinogenesis may still occur in specific CNS regions through quiescent, adult OPCs (aOPCs), based on miscellaneous factors, such as unmyelinated space, new OLs turnover, energy balance, and neural circuit activity [17]. Such processes are broadly defined as adaptive myelination or myelin remodelling/plasticity which is under fine regulation and is generally restricted. Lastly, another crucial factor that may trigger myelinogenesis is injury and disease, such as demyelination, and is discussed briefly towards the end, with a process known as remyelination.
2. Myelinogenesis and Myelin Development: A Spatiotemporal Coordination
2.1. Primordium Regions of OPCs
The mammalian CNS emerges from an ectodermal, neuroepithelial lining of the neural tube in the developing embryo [9,18]. Multiple divisions give rise to radial glia (RG), a multipotent neural stem population that colonizes the newly-formed ventricular walls (Figure 1) [18,19]. The VZ is the primary embryonic site for OPCs production through asymmetrical division of the RG cells. In mice, OPCs are firstly detected in the ventral VZ closer to the floor plate on embryonic day 12.5 (E12.5), and in humans at gestational week (GW) 6.5 (~E45) [20]. More specifically, the outer SVZ (oSVZ) is an enlarged cortical germinal zone only generated in humans [21]. In oSVZ, a distinct RG cell population termed as outer RG (oRG) is located peripherally and gives rise to a transit-amplifying population, which is an additional source of OPCs supplying the human cortex [19,21].
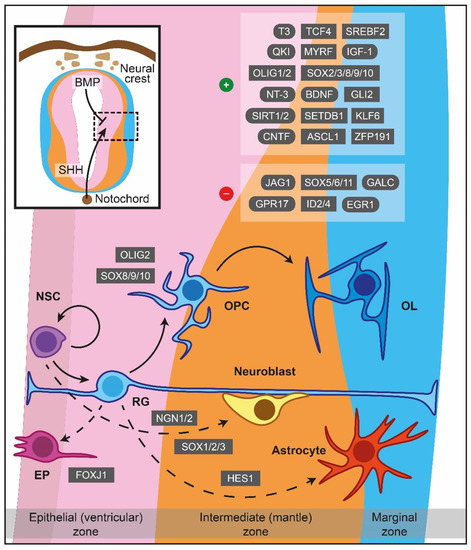
Figure 1.
Major cues of OPCs generation and differentiation during myelinogenesis in the prenatal period. In the neuroepithelium lining the neural tube, NSCs are under the influence of notochord-derived SHH, which drives the cells to become OPCs through OLIG2, SOX8/9/10 or follow neuronal fate (neuroblasts) via NGN1/2 and SOX1/2/3. BMP originated from the neural crest instructing NSCs to become astrocytes, controlled by HES1 as well. FOXJ1 is a crucial transcription factor for the ependymal trajectory. The positive and negative cues controlling OPCs differentiation are displayed in the upper right boxes. The hatched box depicts a representative area around sulcus limitans (between alar and basal plates). Dashed lines showcase naturally occurring processes, albeit not addressed in detail in the current review. ASCL1: Achaete-scute family bHLH transcription factor 1, BDNF: Brain-derived neurotrophic factor, BMP: Bone morphogenetic protein, CNTF: Ciliary neurotrophic factor, EGR1: Early growth response 1, EP: Ependymal cells, FOXJ1: Transcription factor forkhead box J1, GALC: Galactosylceramidase, GLI2: Glioma-associated oncogene family zinc finger 2, GPR17: G protein-coupled receptor 17, HES1: Hes family bHLH transcription factor 1, ID2: Inhibitor of DNA binding 2, ID4: Inhibitor of DNA binding 2, IGF-1: Insulin-like growth factor 1, JAG1: Jagged canonical Notch ligand 1, KLF6: Kruppel-like factor 6, MYRF: Myelin regulatory factor, NGN1: Neurogenin-1, NGN2: Neurogenin-2, NSC: Neural stem cells, NT-3: Neurotrophin 3, OL: Oligodendrocytes, OLIG1: Oligodendrocyte transcription factor 1, OLIG2: Oligodendrocyte transcription factor 2, OPC: Oligodendrocyte precursor cell, QKI: Quaking homolog, KH domain RNA binding, RG: Radial glia, SET domain bifurcated histone lysine methyltransferase 1, SETDB1: SHH: Sonic hedgehog signaling molecule, SIRT1: Sirtuin 1, SIRT2: Sirtuin 2, SOX: Sex-determining region Y-box transcription factor, SREBF2: Sterol regulatory element-binding transcription factor 2, T3: Triiodothyronine, TCF4: Transcription factor 4, ZFP191: Zinc finger protein 191.
In the human forebrain, the first wave of OPCs originates from the medial ganglionic eminence (MGE) and the anterior entopeduncular area (AEP), while a second batch emerges postnatally from the lateral or caudal ganglionic eminences, establishing a sufficient amount of OPCs in the cerebral cortex [22]. OPCs of the human forebrain appear in the SVZ of the MGE at GW7.5, whilst at E12 in mice [19]. In the spinal cord, the majority of the nascent OPCs (about 80% of the total number) complete their formation at the motor neuron progenitor (pMN) domain of the ventral spinal cord, while the pool is enriched later at E15.5 by additional OPCs migrating from dorsal regions [23]. Lastly, the cerebellar OPCs are derived from the metencephalic ventral rhombomere 1 region, manifesting their presence at E16.5 and are reinforced additionally with a secondary population originating from the cerebellar VZ [10].
2.2. Molecular Signals Driving Myelinogenesis
As has been articulated from the experimental evidence, the inauguration of myelinogenesis necessitates the formation of OPCs from multipotent NSCs, which ultimately give rise to mature myelinating OLs through a multistep process (Figure 1) [18,24]. A vital step herein lies in OPCs’ ability to migrate toward miscellaneous sites and proliferate, based predominately on environmental stimuli. These cells become post-mitotic, exiting the cell cycle to express a substantial amount of myelin-associated proteins and differentiate into mature pre-myelinating OLs [23]. Following the proper recognition, targeting and ensheathing specific nerve fibers is the subsequent critical milestone where each pioneer process creates lamellar extensions that stretch and elaborate circumferentially around the target axon [24]. As a new membrane is generated at the leading edge of the forming myelin sheath’s inner tongue, which starts to resemble a spiral cross-sectional shape, the sheath continues to spread along the axonal length. The secured stability and maintenance of a newly-formed myelin sheath is the concluding event. Specific developmental cues and molecular drivers regulate all the aforementioned cellular activities and are enlisted in full capacity in Table A1, Table A2 and Table A3.
2.2.1. Formation of OPCs
OPCs being generated from the ventral VZ are under the influence of the morphogen molecule Sonic hedgehog (SHH) secreted from the notochord, while the dorsal counterparts are SHH-independent [25,26]. SHH signalling drives NSCs into a neuronal or OLs lineage fate superseding the effect of bone morphogenetic proteins (BMPs) which favour astroglial generation (Figure 1) [27,28]. Early secretion of SHH promotes motor neuron lineage formation, while interaction in later time periods promotes OLs differentiation [29]. Interestingly, the concentration of SHH can be controlled by sulfatase 1 expression in the ventral neuroepithelium prior to OPCs specification [30], whereas fibroblast growth factor (FGF) signalling is of paramount importance for further OLs differentiation, especially in the spinal cord [31,32].
Oligodendrocyte transcription factor 2 (OLIG2) is the primary regulator of OPCs generation [33,34], and its gene expression can be potentially repressed throughout the pre to postnatal period by paired box 6 (PAX6), Brahma-related gene-1 (BRG1), Iroquois homeobox 3 (IRX3), histone deacetylase (HDAC) 1, HDAC2, Distal-less homeobox (DLX) 1 and DLX 2 [35,36,37,38,39,40,41]. On the other hand, oligodendrocyte transcription factor 1 (OLIG1) is activated in later stages of OLs development [42]. Interestingly, the Hes family bHLH transcription factor (HES1) can drive RG to an astrocytic phenotype [43], while co-occurrence of OLIG2 with neurogenin-1 or neurogenin-2 supports motor neuron production [38,44,45].
Members of the sex-determining region Y-box transcription factor (SOX) family, such as SOX1, SOX2, and SOX3, can also direct OPCs towards a neuronal fate [33], in contrast to SOX8, SOX9, and SOX10, which favour the turnover of NSCs to OPCs in an autonomous manner [34,35,36]. Additionally, transcription factor forkhead box J1 (FOXJ1) supports the retention of RGs as ependymal cells throughout ventricles. Lastly, glioma-associated oncogene family zinc finger 2 (GLI2), myelin transcription factor 1 (MYT1), NK2 homeobox 6 (NKX2-6), and chromodomain-helicase-DNA-binding protein 8 (CHD8), among others (Table A1), are embryonic cues for OLs specification that vary within CNS regions indicating brain region specificity [37,38,39,40].
2.2.2. Migration
SHH presence is equally catalytic to OPCs migration [41]. Platelet-derived growth factor subunit A (PDGFA) and its cognate receptor, PDGF receptor alpha (PDGFRα), are essential positive drivers for OPCs migration [42]. In line with this, SOX5, SOX6, SOX9, and SOX10 stimulate the migration, ensuring PDGF responsiveness [43,44]. Chondroitin sulfate proteoglycan neuron-glia antigen 2 (NG2) and ephrin-B2/B3 molecules control OPCs polarity and contact abilities, promoting or intercepting migration, respectively [45,46]. Nestin, neural cell adhesion molecule (NCAM), and OLIG1 can also act as chemoattractants, determining cytoskeletal plasticity as well as OPCs motility [47,48,49,50,51]. Other migration chemoattractants are 2′,3′-cyclic nucleotide 3′ phosphodiesterase (CNPase), OLIG2, hepatocyte growth factor (HGF), thrombospondin 1, endothelin 1 (ET-1), oligodendrocyte specific protein (OSP), OSP–associated protein (OAP-1), N-cadherin (NCAD), merosin, fibronectin, and integrin subunit beta 1 (αvβ1 integrin) [52,53,54,55,56,57,58,59,60]. Spassky et al. suggested that netrin-1 is a candidate mediator for chemoattraction during migration [61]. However, other studies considered this molecule as a chemorepellent, antagonizing PDGF [62,63].
More growth factors and associated molecules, such as vascular endothelial growth factor A (VEGF-A) combined with VEFG receptor 2 (VEGFR2), can act as chemoattractant molecules for OPCs migration along with miscellaneous members of the transforming growth factor beta (TGF-β) family (e.g., BMP7 and BMP4), and Gαi-linked sphingosine-1-phosphate receptor (S1PR) 1 and S1PR3 [64,65,66]. In contrast to these specific sphingosine molecules, S1PR2 and S1PR5 negatively regulated migration [66]. Moreover, although C-X-C motif chemokine receptor (CXCR) 4, C-X-C motif chemokine ligand (CXCL) 12 and semaphorin 3F have chemoattractive effects on the OPCs migration, semaphorin 3A, CXCL1, and CXCR2 inhibit migration [61,67,68]. In addition, tenascin-c inhibited OPCs migration, whilst both claudin (CLDN) 1 and CLDN3 supported OPCs relocation, validated also in human specimens [59,69,70].
2.2.3. Proliferation
Specific driver molecules that participate in migration, such as PDGFA and PDGFRα, contribute additionally to the OPC proliferation [47,71]. Interestingly, in the spinal cord, the mitogenic effect of PDGF was enhanced by chemokine CXCL1 and CXCR2 [44,72], while CXCL12 had a proliferative effect on OPCs, mediated by its receptor CXCR4 [73]. More growth factors, such as FGF2, brain-derived neurotrophic factor (BDNF), and epidermal growth factor (EGF) are shown to play a vital role in OPCs proliferation [74,75,76].
Associate developmental pathways are also implicated in this step; PDGF-mediated proliferation depends largely on Wnt/β-catenin and PI3K/AKT/mTOR pathways [77,78]. Furthermore, jagged canonical Notch ligand 1 (JAG1) promotes OPCs proliferation and critically blocks the subsequent differentiation step [79]. Carrying on subcellular, CHD7 and CHD8 regulate gene expression in specific brain regions [80,81]. Another member of the SOX family, SOX9, supports the development of OLs in the cerebellum, regulating the timing of proliferation [82]. MYT1, NCAM, cyclin-dependent kinase inhibitor 1B (p27KIP1), oligodendrocyte myelin glycoprotein (OMgp), and tubulin polymerization promoting protein (TPPP) are negative regulator cues for OPCs proliferation [83,84,85,86,87]. Interestingly, overexpression of inhibitor of DNA binding (ID) 2 and ID4 enhances proliferation [88,89]. Similarly, expression of SHH, HGF, neurotrophin-4 (NT-4), noggin, superoxide dismutase 1 (SOD1), neurotrophin-3 (NT-3), achaete-scute family bHLH transcription factor 1 (ASCL1), PAX6, CLDN1, and CLDN3 promotes the proliferation process [41,54,70,90,91,92,93,94,95].
Integrin-mediated signalling and, more specifically, OSP, OAP-1, αvβ1 integrin, αvβ3 integrin, fibronectin and laminin are pivotal mediators in cytoskeletal remodelling of proliferating OPCs [56,96,97]. Gadea et al. revealed that ET-1 is a candidate molecule for enhancing cell migration without influencing proliferation [60]. Later, Adams and colleagues underscored that loss of ET-1 reduces OPCs proliferation in the developing SVZ via directly binding to endothelin type B receptor (ETBR) [98]. A reduced OPCs proliferation is observed in GS homeobox 1/2 (Gsx1/2) mutant embryos, whereas galectin-4 (GAL-4) treatment increased the proliferation [99,100]. At last, NRG1 and SOX2 induce cell division [101,102]; however, the latest data demonstrate that NRG1 acting via ErbB did not alter the proliferation state of OPCs [103].
2.2.4. Differentiation
OLIG1 and OLIG2 are heavily involved in the post-proliferating step of myelinogenesis, defining the initiation of OPCs differentiation (Figure 1) [51,53,104], while BMPs seem to inhibit this process by downregulating myelin protein expression [105]. The effect can be reversed by using a physiological antagonist of BMP4, such as noggin, which may restore differentiation [91,106,107]. OLIG2 appears to interact with a variety of factors, such as ASCL1, BRG1, transcription factor 4 (TCF4), and SET domain bifurcated histone lysine methyltransferase 1 (SETDB1) to ensure proper OPCs differentiation [108,109,110,111,112]. G protein-coupled receptor 17 (GPR17) can act as a downregulator of OLIG1 that negatively controls the maturation and coordinates the generation of myelinating OLs from pre-myelinating OLs through ID proteins [113]. Although overexpression of ID2 and ID4 both regulate myelin gene expression by inhibiting OLs differentiation [89,114], they are not the major in vivo repressors of differentiation [115]. Moreover, decreased levels of OLIG1 and myelin regulatory factor (MYRF) were observed under early growth response 1 (EGR1) and SOX11 overexpression, delineating the inhibitory action of the latest in OPCs differentiation [116,117]. Intriguingly, MYRF is a unique regulator participating in the late stages of OLs maturation and myelination, while the action of the other OLs’ lineage transcription factors is restricted on OPCs specification or initial differentiation of OLs [118].
SOX family proteins are also participating in the OLs differentiation. In particular, SOX2 and SOX3, through negative regulation of miR145, promote OLs maturation [119], while SOX5 and SOX6 increase PDGFRα expression, maintaining OLs in their immature state [44]. For the terminal differentiation of OLs, SOX8, SOX9, and SOX10 are required [82,120,121,122]. The state of myelinogenesis-associated gene expression is uniformly affected by NKX2-2 and NKX2-6 [40,123,124]. Ji et al. suggested a mechanism regarding NKX2-2-mediated inhibition of OLs differentiation via regulation of sirtuin 2 (SIRT2), which generally is a positive cue for OLs maturation [125]. Similarly, sirtuin 1 (SIRT1) participates in the differentiation of OPCs during development [126] through cytoskeleton-related OLs proteins. The Kruppel-like factor 6 (KLF6) is another transcription factor promoting OPCs differentiation through glycoprotein 130 (GP130)-signal transducer and transcription activator 3 (STAT3) signalling [127]. Growth factor-wise, BDNF is a regulator of OLs differentiation operating via binding to tyrosine receptor kinase B (TrkB) and enhancing the MAPK pathway to upregulate gene expression during OLs maturation [75,77,128]. Evidently, NT-3 is important for the transition of immature OLs to myelin-forming cells by recruiting c-Fos protein-activating protein kinase C (PKC) and tyrosine kinase activities [129,130]. Insulin-like growth factor 1 (IGF-1) is another main factor in assisting the development of OPCs to mature OLs [131]. In accordance with that, GRB2 associated binding protein 1 (GAB1) absence decreased OLs differentiation, acting as a novel target of PDGF [132]. Incidentally, Canoll et al. suggested that NRG1 is a negative regulator of OPCs differentiation [101], while Brinkmann et al. later demonstrated that NRG1 is required for OPCs differentiation [103].
As far as metabolism is concerned, quaking homolog, KH domain RNA binding (QKI)-5 forms a complex with sterol regulatory element-binding transcription factor 2 (SREBF2) that regulates the transcription of genes responsible for cholesterol biosynthesis in OLs during differentiation [133]. Lack of transactive response DNA-binding protein 43 (TDP-43) results in lower SREBF2 and low-density lipoprotein receptor (LDLR) expression and cholesterol levels in vitro and in vivo, indicating the potential role of TDP-43 in cholesterol homeostasis in OLs, which is linked with the proper completion of OLs development [134]. In the same manner, ectonucleotide pyrophosphatase/phosphodiesterase 6 (ENPP6) participates in OLs maturation via a supplement of OLs with choline [135]. Most importantly, triiodothyronine (T3) is a key molecule for blocking OPCs proliferation and promoting their differentiation into mature OLs [136,137]. Thyroid hormone receptor alpha (TRα) is found both in OPCs and mature OLs, whilst thyroid hormone receptor isoform beta 1 (TRβ1) is located only in mature OLs [138]. The OPCs differentiation is mediated by the TRα, while TRβ1 is responsible for promoting myelinogenesis in later stages [77]. Overexpression of HES5 decreases the levels of TRβ1 receptors, while ASCL1 increases them, demonstrating their role in regulating OLs differentiation timing [139]. The neurogenic locus notch homolog protein 1 (NOTCH1) is another receptor that also regulates the differentiation timing [140]. Interestingly, JAG1 is a receptor’s ligand responsible for inhibiting OLs differentiation, while contactin 1 (CNTN1) is another ligand with the opposite function [79,141].
Other membrane molecules which repress OPCs differentiation are NCAM and leucine-rich repeat, and Ig-like domain-containing Nogo receptor interacting protein 1 (LINGO-1) [142,143]. OLs maturation is negatively affected by GAL-4 and galactosylceramidase (GALC), while prominin-1, GLI2, p21-activated kinase 1 (PAK1), myelin-associated glycoprotein (MAG), SOD1, ciliary neurotrophic factor (CNTF), and inward rectifying potassium channel 4.1 (Kir4.1) are crucial for proper differentiation [38,95,100,144,145,146,147,148,149]. On the other hand, proper completion of OLs differentiation requires zinc finger protein 191 (ZFP191) [150]. Microtubule-associated protein 2 (MAP2), microtubule-associated protein tau (MAPT), CNPase, and TPPP may be involved in OLs differentiation by organising the microtubule system, similar to fasciculation and elongation protein zeta 1 (FEZ1), which is responsible for developing OLs processes’ arbour [87,151,152,153]. Additionally, important molecules being involved in the completion of OLs development are OMgp, brain enriched myelin-associated protein 1 (BCAS1) and glutathione (GSH) [154,155,156,157]. Myelin proteolipid protein (PLP) and myelin basic protein (MBP) are the main myelin structural proteins, but it is suggested that they play an additional role in OLs differentiation [158,159]. CLDN1 and CLDN3 control MBP, OLIG2, PLP, and SOX10 expression: these molecules are essential for OLs differentiation, indicating that claudins are needed [70]. Finally, connexin 47 (CX47) and adenosine triphosphate binding cassette subfamily D member 1 (ABCD1) may support OLs during their differentiation, aiding in gap junction coupling and reducing oxidative stress, respectively [160,161,162,163,164].
2.2.5. Ensheathment
Multiple positive cues are important for the inauguration of ensheathment (Figure 2). Amongst the prime ones with a positive effect on axon-glial junction maintenance is NCAD, which regulates the interaction between OLs processes and axons [165]. The L1 cell adhesion molecule (L1-CAM) and laminin expressed in axons bind to contactin and integrin located in OLs [166]. Upon the formation of the first loops/wraps, neurofascin 155 (NF155), located in paranodal loops, forms a well-defined complex with contactin-associated protein (CASPR) and CNTN1, transmembrane proteins which are expressed in axons [167,168,169]. The activation of this complex has a pivotal role in myelin targeting, sheath growth, organisation of paranodal loops and, therefore, supporting the axoglial junction [170,171]. However, CASPR does not participate in myelin targeting [170]. In juxtaparanodes, the axoglial junction is strengthened when transient axonal glycoprotein-1 (TAG-1), a crucial molecule for maintaining enrichment of Kv1.1/Kv1.2 channels [172], interacts with CASPR2. Regarding internodal axoglial adhesion, glial cell adhesion molecule (CADM) 4 binds to axonal CADM2 and CADM3, facilitating myelin targeting, axon wrapping, and myelin sheath growth [173]. Similarly, CADM1b strongly binds to axonal CADM2, positively regulating ensheathment and strengthening the junction [174]. In the same region of the myelin sheath, MAG binds to ganglioside in axons, especially ganglioside GD1a and GT1b, and enforces the junction’s stability [175,176].
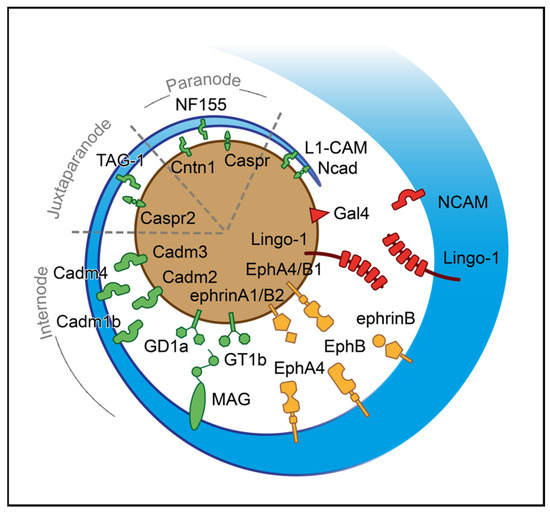
Figure 2.
Axoglial driving cues for the initiation of ensheathment during myelinogenesis. A process of oligodendrocyte (blue) approaches the axon (brown) based on their surfaces’ attractive and repulsive signals. The red-colored shapes represent negative surface molecules; the green ones stand for positive and the yellow for bidirectional signals. For illustrational purposes, the paranode, juxtaparanode, and internode regions are simplified. CADM1b: Cell adhesion molecule 1b, CADM2: Cell adhesion molecule 2, CADM3: Cell adhesion molecule 3, CADM4: Cell adhesion molecule 4, CASPR: Contactin-associated protein, CASPR2: Contactin-associated protein-like 2, CNTN1: Contactin 1, EphA4: Ephrin receptor A4, EphB: Ephrin receptor B, EphB1: Ephrin receptor B1, GAL-4: Galectin-4, GD1a: Ganglioside GD1a, GT1b: Ganglioside GT1b, L1-CAM: L1 cell adhesion molecule, LINGO-1: Leucine-rich repeat and Ig-like domain-containing Nogo receptor interacting protein 1, MAG: Myelin-associated glycoprotein, NCAD: N-cadherin, NCAM: Neural cell adhesion molecule, NF155: Neurofascin 155, TAG-1: Transient axonal glycoprotein-1.
Based on several studies, ephrins (A, B) and cognate receptors (A, B) have dual roles that rely on location and expression. While ephrin receptor (Eph) A4 in OLs is activated by axonal ephrin-A1 ligand, which inhibits the stability of axoglial junctions needed for ensheathment, EphA4, expressed in the axon surface, interacts with ephrin-B, promoting myelin sheet formation [177,178]. In addition, EphB1 of axons is activated through ephrin-B in OLs, which in turn stimulates myelinogenesis [178]. The axonal ephrinB2 via binding with EphB OLs receptor influences integrin activation, reducing myelin sheet formation [178]. The list of negative cues includes LINGO-1, which is located in both axons and OLs, and self-interacts in trans to control the number of targeted axons inhibiting myelinogenesis [143,179]. The NCAM is a cell adhesion molecule negatively regulating myelinogenesis. The downregulation of this protein is essential for promoting myelin formation during development, as myelinogenesis occurs only on NCAM negative axons [180]. A somatodendritic protein, junctional adhesion molecule 2 (JAM2) inhibits oligodendroglial interaction, suppressing myelinogenesis [181]. Apart from the somatodendritic molecules, GAL-4 is expressed only to unmyelinated segments of neurons in hippocampal and cortical regions; this protein is demonstrated as the first identified inhibitor of myelinogenesis in axons [182]. Of particular interest is the possible role of OLIG1 in axonal recognition during myelinogenesis (Table A2) [183].
2.2.6. Myelin Sheath Growth and Preservation
The long-term membrane expansion and maintenance of the newly-formed myelin sheath is the final step in completing myelinogenesis and is utterly controlled by the major myelin proteins (Table A3). The most abundant myelin proteins are PLP (>50%) and MBP (~15%), having a significant role in the stabilization of the myelin structure [24,52,184]. The disruption of PLP gene expression presents impaired membrane compaction [185]. MAG, on the other side, is the third most abundant protein in CNS myelin (~5%), and does not seem to contribute to maintenance as much as it does to the previously described initial interaction between OLs and axons [147,186]. Interestingly, myelin oligodendrocyte glycoprotein (MOG) [187,188], CNPase [52,189], myelin-associated oligodendrocyte basic protein (MOBP) [190], and OMgp [86,191,192], all minor CNS myelin proteins (<1%), need more investigation on how they influence the formation and maintenance of myelin sheaths in compact myelin.
OLs microtubule stability is mediated by MAP2 and MAPT [151], while CX32 and CX47 participate in maintenance [161]. Claudins, such as OSP, CLDN1, and CLDN3, play a pivotal role as well [70,185]. Transcription factors that participate in the lamellar extension process are SOX8, SOX10, NKX2-2, NKX6-2, and MYRF [35,122,193,194]. Transmembrane protein (TMEM) 98, which inhibits the self-cleavage of MYRF, ID4, and OLIG1, could also be involved in the process [114,195], whereas OLIG2 is expressed only until myelin membranes’ production is completed [183,196]. In addition, the ERK1/2 MAP kinase pathway is indispensable in maintaining myelinated axons via FGF–FGF receptor 1 and 2 (FGFR1 and FGFR2) [197,198]. Experiments in Hdac3-mutant optic nerves raised the possibility that HDAC3 is also necessary for myelin integrity [199].
Proper cholesterol biosynthesis is prioritized in myelinogenesis, with QKI regulating this cholesterol production via SREBF2. Specifically, QKI-5 acts synergistically with peroxisome proliferator-activated receptor beta (PPARβ)-retinoid X receptor alpha (RXRα) activating transcription of the response in fatty acid metabolism genes. This operation of QKI-5 is significant for maintaining myelin homeostasis [133]. The ceramide galactosyl transferase (CGT) is a key enzyme for catalyzing GALC synthesis, while ceramide sulfotransferase (CST) is responsible for converting GALC to sulfatide [200,201]. Both CST and CGT mutant animals showed a regionally specific loss of myelin stability [200]. Thus, GALC and sulfatide have a pivotal role in the long-term maintenance of myelin, with the GALC being more crucial for myelin development than its assembly [200,201]. Additionally, peroxisomal metabolism also influences myelin survival [202]. For example, a peroxisomal transmembrane protein responsible for very long-chain fatty metabolism is encoded by the ABCD1 gene and is key in maintaining myelin stability [164,203]. Lastly, the age-dependent changes of TMEM10 might be linked with its action in maintaining CNS myelin [204].
2.3. Myelin Formation after Infancy
Although myelinogenesis has been described in the nascent developmental years, myelination does naturally occur for the duration of a person’s life to promote learning and memory through brain circuit plasticity [205], or as remyelination after an injury [206]. The synaptic plasticity has been studied in depth; however, a newly discovered form of brain plasticity, namely myelin plasticity or myelin remodelling, is under intensive investigation [205]. Extrinsic factors can influence, either positively or negatively, this remodelling in the toddler, adolescent, and adult brain. For example, since myelin formation is sensitive to experience, sensory stimulation may upregulate myelination, while sensory or social deprivation can potentially downregulate axon ensheathment [205,207]. Myelin remodelling initiates when pre-existing OPCs recruit or directly differentiate into newly-formed mature OLs, whereas existing OLs have the ability to engage in plasticity [205]. The principal cues for this “adaptive” myelination should not be different from the ones we scrutinize in this review.
The regenerative process following injury also presents many similarities with specific steps of myelinogenesis [208]. The neonatal OPCs are maintained in a resting, quiescent state through adulthood, and they are referred to as adult aOPCs, constituting ~6% of all cells in the CNS [206]. Interestingly, aOPCs have a transcriptome similar to mature OLs. After injury, the innate immune response activates aOPCs, transforming them into a neonatal-like transcriptome [209]. The activation of aOPCs is followed by their proliferation, migration, and final differentiation into mature OLs. Older literature describes these aOPCs as the primary remyelinating cells [210]. Nevertheless, newer research has suggested that neural progenitors in SVZ, Schwann cells, and surviving mature OLs are also implicated in the remyelinating process [211,212,213].
The myelination efficiency is age-dependent, as the impairment of aged OPCs to recruit and differentiate into mature OLs leads to decreased remodelling and remyelination [214]. The nutrient support of OLs is highly compromised in aging due to the presence of senescent astrocytes, leading to decreased cholesterol biosynthesis which in turn weighs in the impaired OLs membrane development [15,215,216]. This age-related energy depletion that decreases the myelination efficiency is further fed from the accumulation of DNA damage while rendering the neurons vulnerable to oxidative stress through free radicals [15,217]. Additionally, the ineffectiveness of microglia, which translates to aged phagocytes to clear out impaired myelin, is a potential aetiology for the downregulation of remyelination [218]. Taken all together, the detailed investigation of cues that drives de novo myelination could be a crucial point for revisiting them in demyelination and remyelination of the adult CNS, a concept that is discussed briefly in the following section.
3. Myelinogenesis in Disease and Beyond
Although aging is a natural process that leads to a decreased turnover of functional OLs and diminished myelin formation, the integrity of myelinogenesis can be highly compromised in pathological situations such as demyelination, characterized by extensive myelin loss [219]. This condition has to be distinguished from dysmyelination, which is a genetic-based anomaly affecting basic myelin proteins and leads to uneven/not properly compacted myelin sheaths [219]. Demyelinating diseases could be divided into many categories; according to their pathogenesis mechanism, which mostly implicate environmental factors, nutritional deficits, presence of myelinotoxic agents, or virus-mediated impairments. In quite frequent cases, immune system mediators are deregulated, leading to autoimmune inflammatory demyelination [219,220]. Among the three most prevalent inflammatory demyelinating diseases are multiple sclerosis (MS), neuromyelitis optica spectrum disorder (NMOSD), and acute disseminated encephalomyelitis (ADEM).
In this review, we summarized all the potential molecules responsible for the long-term maintenance of myelin along the axoglial junction (see Section 2.2.5 and Section 2.2.6), serving simultaneously as key factors in demyelinating disease sequelae. Recent data revealed that impaired mitochondrial function and oxidative stress are also candidate pathophysiology mechanisms for demyelinating diseases [221]. Berghoff et al. demonstrated that disruption of cholesterol metabolism alters brain lipid metabolism in CNS and is associated with neurological diseases such as autoimmune inflammatory conditions, including MS [222]. Nonetheless, under such circumstances, an autoimmune attack generates myelin debris from damaged myelin [223]. These components impair the CNS remyelination by obstructing OPCs and OLs functionality while triggering additional deleterious immune responses, also known as epitope spreading [213,223,224]. The clearance of myelin debris is crucial for rearrangement since recent studies suggest that the failure of myelin clearance leads to inefficient remyelination [225,226].
Remyelination can be spontaneous or in an experimental setup, achieved by the providence of an exogenous source of neural precursor cells (NPCs) with myelinating potential [227,228]. In various transplantation paradigms, it is shown that these cells can either exert an in situ myelinating effect, as seen and applied successfully in spinal cord injury (SCI) cases [229,230], or by instructing and enhancing the capacity of endogenous cells to remyelinate, documented in experimental autoimmune encephalomyelitis (EAE) [208,231,232] (324). Proposed mechanisms of action also underline immunomodulatory effects rather than direct cell replacement [233,234]. Nevertheless, the scarce population of surviving mature OLs after demyelination is shown to be less effective in comparison to newly created ones [213,235,236,237]. Towards this trajectory, which is a fully functional recruitment of aOPCs to form myelinating OLs [208,238] (324, 325), it is extremely important to comprehend the developmental molecular cues and factors governing the process of myelinogenesis (see Section 2.2.1, Section 2.2.2, Section 2.2.3, Section 2.2.4), since these same molecules can be candidate targets for therapeutic intervention in demyelinating diseases.
4. Conclusions
Through this comprehensive review, we attempt to list and categorize the genes and proteins that act as developmental morphogens to the CNS development and, more specifically, those that are activated in the process of oligodendrogenesis. The fully functional OLs, originating from unspecialized stem cells, are able to identify newly-formed axons which emanate and branch in regions that need fast conduction early in life, completing their task of myelinogenesis. Some of these cells persist in adult life in an intermediate, dormant phenotype scattered or organized around the primordial niches. An argument that was intended to bring into attention is how the powerful dynamics that shape myelination, which is naturally declining as we age, can be sustained, or even re-engaged after an injury or demyelinating disease. In the current era, transcriptomic profiling or metabolomic data can potentially give an answer as to which of the enlisted molecules, drivers, and regulators should be prioritized.
Author Contributions
I.D. and P.T., writing—original draft preparation and visualization; M.E.M., S.M. and D.M., conceptualization, review and editing; E.K., M.B., S.P. and N.G., review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| OPCs | Oligodendrocyte precursor cells |
| OLs | Oligodendrocytes |
| CNS | Central nervous system |
| NSCs | Neural stem cells |
| aOPCs | Adult oligodendrocyte precursor cells |
| E | Embryonic day |
| GW | Gestational week |
| VZ | Ventricular zone |
| SVZ | Subventricular zone |
| RG | Radial glia |
| oSVZ | Outer subventricular zone |
| MGE | Medial ganglionic eminence |
| MS | Multiple sclerosis |
| ABCD1 | Adenosine triphosphate binding cassette subfamily D member 1 |
| ASCL1 | Achaete-scute family basic helix-loop-helix transcription factor 1 |
| BDNF | Brain-derived neurotrophic factor |
| BMP4 | Bone morphogenetic protein 4 |
| BMP7 | Bone morphogenetic protein 7 |
| CADM1b | Cell adhesion molecule 1b |
| CADM2 | Cell adhesion molecule 2 |
| CADM3 | Cell adhesion molecule 3 |
| CADM4 | Cell adhesion molecule 4 |
| CASPR | Contactin-associated protein |
| CASPR2 | Contactin-associated protein-like 2 |
| CGT | Ceramide galactosyl transferase |
| CHD7 | Chromodomain-helicase-DNA-binding protein 7 |
| CHD8 | Chromodomain-helicase-DNA-binding protein 8 |
| CLDN1 | Claudin 1 |
| OSP | Oligodendrocyte specific protein |
| CLDN3 | Claudin 3 |
| CNPase | 2′,3′-cyclic nucleotide 3′ phosphodiesterase |
| CNTN1 | Contactin 1 |
| CST | Ceramide sulfotransferase |
| CX32 | Connexin 32 |
| CX47 | Connexin 47 |
| CXCL1 | C-X-C motif chemokine ligand 1 |
| CXCL12 | C-X-C motif chemokine ligand 12 |
| CXCR2 | C-X-C motif chemokine receptor 2 |
| CXCR4 | C-X-C motif chemokine receptor 4 |
| DLX1 | Distal-less homeobox 1 |
| DLX2 | Distal-less homeobox 2 |
| EphA4 | Ephrin receptor A4 |
| EphB1 | Ephrin receptor B1 |
| EphB2 | Ephrin receptor B2 |
| ET-1 | Endothelin 1 |
| FGF2 | Fibroblast growth factor 2 |
| FGFR1 | Fibroblast growth factor receptor 1 |
| FGFR2 | Fibroblast growth factor receptor 2 |
| GAL-4 | Galectin-4 |
| GALC | Galactosylceramidase |
| GLI2 | Glioma-associated oncogene family zinc finger 2 |
| HDAC1 | Histone deacetylase 1 |
| HDAC2 | Histone deacetylase 2 |
| HDAC3 | Histone deacetylase 3 |
| HES5 | Hairy and enhancer of split family basic helix-loop-helix transcription factor 5 |
| HGF | Hepatocyte growth factor |
| ID2 | Inhibitor of DNA binding 2 |
| ID4 | Inhibitor of DNA binding 4 |
| JAG1 | Jagged canonical Notch ligand 1 |
| LINGO-1 | Leucine-rich repeat and Ig-like domain-containing Nogo receptor interacting protein 1 |
| MAG | Myelin-associated glycoprotein |
| MAP2 | Microtubule-associated protein 2 |
| MAPT | Microtubule-associated protein tau |
| MBP | Myelin basic protein |
| MYRF | Myelin regulatory factor |
| MYT1 | Myelin transcription factor 1 |
| NCAD | N-cadherin |
| NCAM | Neural cell adhesion molecule |
| NKX2-2 | NK2 homeobox 2 |
| NKX2-6 | NK2 homeobox 6 |
| NKX6-2 | NK6 homeobox 2 |
| NRG1 | Neuregulin 1 |
| NT-3 | Neurotrophin 3 |
| OAP-1 | Oligodendrocyte specific protein–associated protein |
| OLIG1 | Oligodendrocyte transcription factor 1 |
| OLIG2 | Oligodendrocyte transcription factor 2 |
| OMgp | Oligodendrocyte myelin glycoprotein |
| PAX6 | Paired box 6 |
| PDGFA | Platelet-derived growth factor subunit A |
| PDGFRα | Platelet-derived growth factor receptor alpha |
| PLP | Myelin proteolipid protein |
| QKI | Quaking homolog, KH domain RNA binding |
| S1PR1 | Sphingosine-1-phosphate receptor 1 |
| S1PR2 | Sphingosine-1-phosphate receptor 2 |
| S1PR3 | Sphingosine-1-phosphate receptor 3 |
| S1PR5 | Sphingosine-1-phosphate receptor 5 |
| SHH | Sonic hedgehog signaling molecule |
| BRG1 | Brahma-Related Gene-1 |
| SOD1 | Superoxide dismutase 1 |
| SOX1 | Sex-determining region Y-box transcription factor 1 |
| SOX10 | Sex-determining region Y-box transcription factor 10 |
| SOX11 | Sex-determining region Y-box transcription factor 11 |
| SOX2 | Sex-determining region Y-box transcription factor 2 |
| SOX3 | Sex-determining region Y-box transcription factor 3 |
| SOX5 | Sex-determining region Y-box transcription factor 5 |
| SOX6 | Sex-determining region Y-box transcription factor 6 |
| SOX8 | Sex-determining region Y-box transcription factor 8 |
| SOX9 | Sex-determining region Y-box transcription factor 9 |
| SREBF2 | Sterol regulatory element-binding transcription factor 2 |
| TRα | Thyroid hormone receptor alpha |
| TRβ1 | Thyroid hormone receptor isoform beta 1 |
| TDP-43 | Transactive response DNA-binding protein 43 |
| TMEM10 | Transmembrane Protein 10 |
| TMEM98 | Transmembrane protein 98 |
| TPPP | Tubulin polymerization promoting protein |
| VEGF-A | Vascular endothelial growth factor A |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
| αvβ1 integrin | Integrin subunit beta 1 |
| αvβ3 integrin | Integrin subunit beta 3 |
Appendix A
The following appendix contains all the genes and relevant chromosomal loci from proteins involved in myelinogenesis in relation to their biological role. This data is supplemental to the main text based on the current knowledge and literature on cues driving oligodendrocyte development, ensheathment and myelin maintenance.

Table A1.
Molecular drivers and morphogens in specification, migration, proliferation, and differentiation of OPCs.
Table A1.
Molecular drivers and morphogens in specification, migration, proliferation, and differentiation of OPCs.
| Gene * | Chromosomal Locus * | Protein * | Biological Role | Reference |
|---|---|---|---|---|
| ABCD1 | Xq28 | ATP binding cassette subfamily D member 1 | Differentiation | [163,164] |
| ADGRG1 | 16q21 | Adhesion G protein-coupled receptor G1 | Proliferation | [239] |
| ANOS1 | Xp22.31 | Anosmin 1 | Migration | [240] |
| ASCL1 | 12q23.2 | Achaete-scute family bHLH transcription factor 1 | Specification; Proliferation; Differentiation | [93,108,139,241] |
| BCAS1 | 20q13.2 | Brain enriched myelin-associated protein 1 | Differentiation | [156,157,242] |
| BDNF | 11p14.1 | Brain-derived neurotrophic factor | Proliferation; Differentiation | [75,128] |
| BMP2 | 20p12.3 | Bone morphogenetic protein 2 | Specification; Differentiation | [27,105] |
| BMP4 | 14q22.2 | Bone morphogenetic protein 4 | Specification; Migration; Differentiation | [28,65,105] |
| BMP7 | 20q13.31 | Bone morphogenetic protein 7 | Migration | [65] |
| CDH2 | 18q12.1 | Cadherin 2 | Migration | [57] |
| CDKN1B | 12p13.1 | Cyclin-dependent kinase inhibitor 1B | Proliferation | [85] |
| CHD7 | 8q12.2 | Chromodomain-helicase-DNA-binding protein 7 | Proliferation; Differentiation | [80,243] |
| CHD8 | 14q11.2 | Chromodomain-helicase-DNA-binding protein 8 | Specification; Proliferation; Differentiation | [80,81] |
| CLDN1 | 3q28 | Claudin 1 | Migration; Proliferation; Differentiation | [70] |
| CLDN3 | 7q11.23 | Claudin 3 | Migration; Proliferation; Differentiation | [70] |
| CLDN11 | 3q26.2 | Claudin 11 | Migration; Proliferation | [56] |
| CNP | 17q21.2 | 2′,3′-cyclic nucleotide 3′ phosphodiesterase | Migration; Differentiation | [52,153] |
| CNTF | 11q12.1 | Ciliary neurotrophic factor | Proliferation; Differentiation | [148,244] |
| CNTFR | 9p13.3 | Ciliary neurotrophic factor receptor | Proliferation | [244] |
| CNTN1 | 12q12 | Contactin 1 | Differentiation | [141] |
| CREB3L2 | 7q33 | CAMP responsive element binding protein 3 like 2 | Differentiation | [243] |
| CSPG4 | 15q24.2 | Chondroitin sulfate proteoglycan 4 | Proliferation | [245] |
| CXCL1 | 4q13.3 | C-X-C motif chemokine ligand 1 | Migration; Proliferation | [67,72] |
| CXCL12 | 10q11.21 | C-X-C motif chemokine ligand 12 | Migration; Proliferation | [68,73] |
| CXCR2 | 2q35 | C-X-C motif chemokine receptor 2 | Migration; Proliferation | [67,72] |
| CXCR4 | 2q22.1 | C-X-C motif chemokine receptor 4 | Migration; Proliferation | [68,73] |
| DLX1 | 2q31.1 | Distal-less homeobox 1 | Specification | [246] |
| DLX2 | 2q31.1 | Distal-less homeobox 2 | Specification | [246] |
| DUSP15 | 20q11.21 | Dual specificity phosphatase 15 | Differentiation | [247] |
| EDN1 | 6p24.1 | Endothelin 1 | Migration; Proliferation | [60,98] |
| EDNRB | 13q22.3 | Endothelin receptor type B | Proliferation | [98] |
| EFNB2 | 13q33.3 | Ephrin B2 | Migration; Proliferation | [46,248] |
| EFNB3 | 17p13.1 | Ephrin B3 | Migration | [46] |
| EGF | 4q25 | Epidermal growth factor | Proliferation | [76] |
| EGR1 | 5q31.2 | Early growth response 1 | Differentiation | [116] |
| ENPP6 | 4q35.1 | Ectonucleotide pyrophosphatase/phosphodiesterase 6 | Differentiation | [135] |
| EPHB2 | 1p36.12 | Ephrin receptor B2 | Migration; Proliferation | [46,248] |
| FEZ1 | 11q24.2 | Fasciculation and elongation protein zeta 1 | Differentiation | [152] |
| FGF2 | 4q28.1 | Fibroblast growth factor 2 | Specification; Migration; Proliferation | [240,249] |
| FGFR1 | 8p11.23 | Fibroblast growth factor receptor 1 | Migration; Proliferation | [240,249] |
| FGFR2 | 10q26.13 | Fibroblast growth factor receptor 2 | Specification | [31,249] |
| FGFR3 | 4p16.3 | Fibroblast growth factor receptor 3 | Proliferation | [249] |
| FLT1 | 13q12.3 | Fms related receptor tyrosine kinase 1 | Proliferation | [250] |
| FN1 | 2q35 | Fibronectin 1 | Migration; Proliferation | [59,96] |
| GAB1 | 4q31.21 | GRB2-associated binding protein 1 | Differentiation | [132] |
| GALC | 14q31.3 | Galactosylceramidase | Differentiation | [144] |
| GDPD2 | Xq13.1 | Glycerophosphodiester phosphodiesterase domain containing 2 | Proliferation | [244] |
| GJC2 | 1q42.13 | Gap junction protein gamma 2 | Differentiation | [160,161,162,251] |
| GLI2 | 2q14.2 | GLI family zinc finger 2 | Specification; Differentiation | [38] |
| GPR17 | 2q14.3 | G protein-coupled receptor 17 | Differentiation | [113] |
| GPR37 | 7q31.33 | G protein-coupled receptor 37 | Differentiation | [252] |
| GSX1 | 13q12.2 | GS homeobox 1 | Proliferation | [99] |
| GSX2 | 4q12 | GS homeobox 2 | Specification; Proliferation | [99,253] |
| HDAC1 | 1p35.2-p35.1 | Histone deacetylase 1 | Specification | [254,255] |
| HDAC2 | 6q21 | Histone deacetylase 2 | Specification | [254] |
| HES1 | 3q29 | Hes family bHLH transcription factor 1 | Specification | [256] |
| HES5 | 1p36.32 | Hes family bHLH transcription factor 5 | Differentiation | [139] |
| HEY1 | 8q21.13 | Hes related family bHLH transcription factor with YRPW motif 1 | Differentiation | [257] |
| HGF | 7q21.11 | Hepatocyte growth factor | Migration; Proliferation | [54] |
| ID2 | 2p25.1 | Inhibitor of DNA binding 2 | Proliferation; Differentiation | [89,113,115] |
| ID4 | 6p22.3 | Inhibitor of DNA binding 4, HLH protein | Proliferation; Differentiation | [88,113,114,115] |
| IGF1 | 12q23.2 | Insulin-like growth factor 1 | Proliferation; Differentiation | [74,131] |
| IRX3 | 16q12.2 | Iroquois homeobox 3 | Specification | [37] |
| ITGB1 | 10p11.22 | Integrin subunit beta 1 | Migration; Proliferation; Differentiation | [56,58,258] |
| ITGB3 | 17q21.32 | Integrin subunit beta 3 | Proliferation | [69,97] |
| JAG1 | 20p12.2 | Jagged canonical Notch ligand 1 | Proliferation; Differentiation | [79] |
| JUN | 1p32.1 | Jun proto-oncogene, AP-1 transcription factor subunit | Proliferation | [259,260] |
| KCNJ10 | 1q23.2 | Potassium inwardly rectifying channel subfamily J member 10 | Differentiation | [149] |
| KDR | 4q12 | Kinase insert domain receptor | Migration; Proliferation | [64,250] |
| KLF6 | 10p15.2 | Kruppel-like factor 6 | Differentiation | [127] |
| LAMA2 | 6q22.33 | Laminin subunit alpha 2 | Migration; Proliferation | [96,261] |
| LAMA4 | 6q21 | Laminin subunit alpha 4 | Migration; Proliferation | [96,261] |
| LAMA5 | 20q13.33 | Laminin subunit alpha 5 | Migration; Proliferation | [96,261] |
| LGALS4 | 19q13.2 | Galectin-4 | Proliferation; Differentiation | [100] |
| LINGO1 | 15q24.3 | Leucine rich repeat and Ig domain containing 1 | Differentiation | [143] |
| MAG | 19q13.12 | Myelin-associated glycoprotein | Differentiation | [147] |
| MAP2 | 2q34 | Microtubule-associated protein 2 | Differentiation | [151] |
| MAPT | 17q21.31 | Microtubule-associated protein tau | Differentiation | [151] |
| MBP | 18q23 | Myelin basic protein | Differentiation | [159] |
| MOBP | 3p22.1 | Myelin associated oligodendrocyte basic protein | Differentiation | [190] |
| MYOC | 1q24.3 | Myocilin | Differentiation | [262] |
| MYRF | 11q12.2 | Myelin regulatory factor | Differentiation | [117] |
| MYT1 | 20q13.33 | Myelin transcription factor 1 | Specification; Proliferation | [39,83] |
| NCAM1 | 11q23.2 | Neural cell adhesion molecule 1 | Migration; Proliferation | [47,48,84,142] |
| NES | 1q23.1 | Nestin | Migration; Proliferation | [49,263] |
| NEUROG1 | 5q31.1 | Neurogenin 1 | Specification | [37,264,265] |
| NEUROG2 | 4q25 | Neurogenin 2 | Specification | [37,264,265] |
| NFIA | 1p31.3 | Nuclear factor I A | Specification | [37,266] |
| NGF | 1p13.2 | Nerve growth factor | Proliferation | [92,267] |
| NKX2-2 | 20p11.22 | NK2 homeobox 2 | Specification; Proliferation; Differentiation | [37,108,123,124,268] |
| NKX2-6 | 8p21.2 | NK2 homeobox 6 | Specification; Differentiation | [40] |
| NKX6-1 | 4q21.23 | NK6 homeobox 1 | Specification | [26] |
| NKX6-2 | 10q26.3 | NK6 homeobox 2 | Specification | [26] |
| NOG | 17q22 | Noggin | Proliferation; Differentiation | [91,106,107] |
| NOTCH1 | 9q34.3 | Notch receptor 1 | Proliferation; Differentiation | [79,140] |
| NRG1 | 8p12 | Neuregulin 1 | Migration; Proliferation; Differentiation | [101,103,269] |
| NTF3 | 12p13.31 | Neurotrophin 3 | Proliferation; Differentiation | [92,129,130] |
| NTF4 | 19q13.33 | Neurotrophin 4 | Proliferation | [90] |
| NTN1 | 17p13.1 | Netrin 1 | Migration | [61,62,63] |
| NTRK2 | 9q21.33 | Neurotrophic receptor tyrosine kinase 2 | Proliferation; Differentiation | [75,270] |
| OLIG1 | 21q22.11 | Oligodendrocyte transcription factor 1 | Migration; Differentiation | [50,51,104,271] |
| OLIG2 | 21q22.11 | Oligodendrocyte transcription factor 2 | Specification; Migration; Differentiation | [51,53,271] |
| OMG | 17q11.2 | Oligodendrocyte myelin glycoprotein | Proliferation; Differentiation | [155] |
| OPALIN | 10q24.1 | Oligodendrocytic myelin paranodal and inner loop protein | Differentiation | [272,273] |
| PAK1 | 11q13.5-q14.1 | P21 (RAC1) activated kinase 1 | Differentiation | [146] |
| PAX6 | 11p13 | Paired box 6 | Specification; Proliferation | [94,274,275] |
| PDE5 | 4q26 | Phosphodiesterase 5A | Differentiation | [276] |
| PDGFA | 7p22.3 | Platelet-derived growth factor subunit A | Migration; Proliferation; Differentiation | [42,277] |
| PDGFRA | 4q12 | Platelet-derived growth factor receptor alpha | Migration; Proliferation; Differentiation | [42,47,71,277] |
| PLP1 | Xq22.2 | Proteolipid protein 1 | Differentiation | [158] |
| PRMT5 | 14q11.2 | Protein arginine methyltransferase 5 | Differentiation | [278] |
| PROM1 | 4p15.32 | Prominin 1 | Differentiation | [145] |
| QKI | 6q26 | QKI, KH domain containing RNA binding | Differentiation | [133,279] |
| RTN4 | 2p16.1 | Reticulon 4 | Migration | [280] |
| S1PR1 | 1p21.2 | Sphingosine-1-phosphate receptor 1 | Migration | [66] |
| S1PR2 | 19p13.2 | Sphingosine-1-phosphate receptor 2 | Migration | [66] |
| S1PR3 | 9q22.1 | Sphingosine-1-phosphate receptor 3 | Migration | [66] |
| S1PR5 | 19p13.2 | Sphingosine-1-phosphate receptor 5 | Migration | [66] |
| SEMA3A | 7q21.11 | Semaphorin 3A | Migration | [61] |
| SEMA3F | 3p21.31 | Semaphorin 3F | Migration; Proliferation | [61] |
| SETDB1 | 1q21.3 | SET domain bifurcated histone lysine methyltransferase 1 | Differentiation | [112] |
| SHH | 7q36.3 | Sonic hedgehog signaling molecule | Specification; Migration; Proliferation | [27,28,29,30,32,41] |
| SIRT1 | 10q21.3 | Sirtuin 1 | Differentiation | [126] |
| SIRT2 | 19q13.2 | Sirtuin 2 | Differentiation | [125] |
| SMARCA4 | 19p13.2 | SWI/SNF related, matrix associated, actin-dependent regulator of chromatin, subfamily a, member 4 | Specification; Differentiation | [109,243] |
| SOD1 | 21q22.11 | Superoxide dismutase 1 | Proliferation; Differentiation | [95] |
| SOX1 | 13q34 | SRY-box transcription factor 1 | Specification | [33] |
| SOX2 | 3q26.33 | SRY-box transcription factor 2 | Specification; Proliferation; Differentiation | [33,102,119] |
| SOX3 | Xq27.1 | SRY-box transcription factor 3 | Specification; Differentiation | [33,119] |
| SOX5 | 12p12.1 | SRY-box transcription factor 5 | Migration; Proliferation; Differentiation | [44] |
| SOX6 | 11p15.2 | SRY-box transcription factor 6 | Migration; Proliferation; Differentiation | [44] |
| SOX8 | 16p13.3 | SRY-box transcription factor 8 | Specification; Differentiation | [34,35,36,120] |
| SOX9 | 17q24.3 | SRY-box transcription factor 9 | Specification; Migration; Proliferation; Differentiation | [36,44,82] |
| SOX10 | 22q13.1 | SRY-box transcription factor 10 | Specification; Migration; Differentiation | [43,44,122,281] |
| SOX11 | 2p25.2 | SRY-box transcription factor 11 | Differentiation | [116] |
| SP7 | 12q13.13 | Sp7 transcription factor | Differentiation | [243] |
| SREBF2 | 22q13.2 | Sterol regulatory element-binding transcription factor 2 | Differentiation | [133] |
| STAT3 | 17q21.2 | Signal transducer and activator of transcription 3 | Differentiation | [282] |
| SULF1 | 8q13.2-q13.3 | Sulfatase 1 | Specification | [30] |
| TARDBP | 1p36.22 | TAR DNA binding protein | Differentiation | [134] |
| TCF4 | 18q21.2 | Transcription factor 4 | Differentiation | [110] |
| TCF7L2 | 10q25.2-q25.3 | Transcription factor 7 like 2 | Differentiation | [111] |
| THBS1 | 15q14 | Thrombospondin 1 | Migration | [55] |
| THRA | 17q21.1 | Thyroid hormone receptor alpha | Differentiation | [136,137,138] |
| TMEM98 | 17q11.2 | Transmembrane protein 98 | Differentiation | [195] |
| TNC | 9q33.1 | Tenascin C | Migration; Proliferation | [59,69] |
| TPPP | 5p15.33 | Tubulin polymerization promoting protein | Proliferation; Differentiation | [87] |
| TSPAN3 | 15q24.3 | Tetraspanin 3 | Migration; Proliferation | [56] |
| VEGFA | 6p21.1 | Vascular endothelial growth factor A | Migration; Proliferation | [64,250] |
| WDR1 | 4p16.1 | WD repeat domain 1 | Differentiation | [279] |
| YY1 | 14q32.2 | YY1 transcription factor | Differentiation | [283] |
| ZBTB33 | Xq24 | Zinc finger and BTB domain containing 33 | Differentiation | [111] |
| ZDHHC5 | 11q12.1 | Zinc finger DHHC-type palmitoyltransferase 5 | Differentiation | [282] |
| ZEB2 | 2q22.3 | Zinc finger E-box binding homeobox 2 | Differentiation | [284] |
| ZNF24 | 18q12.2 | Zinc finger protein 24 | Differentiation | [150] |
* Data are retrieved from “The Human Protein Atlas” [285]. OPCs: Oligodendrocyte precursor cells.

Table A2.
Regulators of axoglial interactions in myelin ensheathment.
Table A2.
Regulators of axoglial interactions in myelin ensheathment.
| Gene * | Chromosomal Locus * | Protein * | Reference |
|---|---|---|---|
| CADM1 | 11q23.3 | Cell adhesion molecule 1 | [174] |
| CADM2 | 3p12.1 | Cell adhesion molecule 2 | [173,174] |
| CADM3 | 1q23.2 | Cell adhesion molecule 3 | [173] |
| CADM4 | 19q13.31 | Cell adhesion molecule 4 | [173] |
| CDH2 | 18q12.1 | Cadherin 2 | [165] |
| CNTN1 | 12q12 | Contactin 1 | [167,168,169,171] |
| CNTN2 | 1q32.1 | Contactin 2 | [172] |
| CNTNAP1 | 17q21.2 | Contactin-associated protein 1 | [167,168,169,170] |
| CNTNAP2 | 7q35–q36.1 | Contactin-associated protein 2 | [172] |
| EFNA1 | 1q22 | Ephrin A1 | [177] |
| EFNB2 | 13q33.3 | Ephrin B2 | [178] |
| EPHA4 | 2q36.1 | Ephrin receptor A4 | [177,178] |
| EPHB1 | 3q22.2 | Ephrin receptor B1 | [178] |
| JAM2 | 21q21.3 | Junctional adhesion molecule 2 | [181] |
| L1CAM | Xq28 | L1 cell adhesion molecule | [166] |
| LGALS4 | 19q13.2 | Galectin-4 | [182] |
| LINGO1 | 15q24.3 | Leucine rich repeat and Ig domain containing 1 | [143,179] |
| MAG | 19q13.12 | Myelin-associated glycoprotein | [176] |
| NCAM1 | 11q23.2 | Neural cell adhesion molecule 1 | [180] |
| NFASC | 1q32.1 | Neurofascin | [167,168,169,170] |
| NRG1 | 8p12 | Neuregulin 1 | [286,287] |
| OLIG1 | 21q22.11 | Oligodendrocyte transcription factor 1 | [183] |
| ST3GAL2 | 16q22.1 | ST3 beta-galactoside alpha-2,3-sialyltransferase 2 | [175,176] |
| ST3GAL3 | 1p34.1 | ST3 beta-galactoside alpha-2,3-sialyltransferase 3 | [175,176] |
| WASL | 7q31.32 | WASP-like actin nucleation promoting factor | [288,289] |
* Data are retrieved from “The Human Protein Atlas” [285].

Table A3.
Molecules implicated in myelin growth and preservation.
Table A3.
Molecules implicated in myelin growth and preservation.
| Gene * | Chromosomal Locus * | Protein * | Reference |
|---|---|---|---|
| ABCD1 | Xq28 | ATP binding cassette subfamily D member 1 | [164,203] |
| AGPS | 2q31.2 | Alkylglycerone phosphate synthase | [24,290] |
| CA2 | 8q21.2 | Carbonic anhydrase 2 | [291] |
| CLDN1 | 3q28 | Claudin 1 | [70] |
| CLDN3 | 7q11.23 | Claudin 3 | [70] |
| CLDN11 | 3q26.2 | Claudin 11 | [185] |
| CNP | 17q21.2 | 2′,3′-cyclic nucleotide 3′ phosphodiesterase | [52,189] |
| DUSP15 | 20q11.21 | Dual specificity phosphatase 15 | [247] |
| FGF1 | 5q31.3 | Fibroblast growth factor 1 | [198] |
| FGF2 | 4q28.1 | Fibroblast growth factor 2 | [198] |
| FGFR1 | 8p11.23 | Fibroblast growth factor receptor 1 | [197,198] |
| FGFR2 | 10q26.13 | Fibroblast growth factor receptor 2 | [197,198] |
| GAL3ST1 | 22q12.2 | Galactose-3-O-sulfotransferase 1 | [200] |
| GALC | 14q31.3 | Galactosylceramidase | [200,201] |
| GFAP | 17q21.31 | Glial fibrillary acidic protein | [292] |
| GJB1 | Xq13.1 | Gap junction protein beta 1 | [161] |
| GJC2 | 1q42.13 | Gap junction protein gamma 2 | [161] |
| GNPAT | 1q42.2 | Glyceronephosphate O-acyltransferase | [290] |
| HDAC3 | 5q31.3 | Histone deacetylase 3 | [199] |
| ID4 | 6p22.3 | Inhibitor of DNA binding 4, HLH protein | [114] |
| MAG | 19q13.12 | Myelin-associated glycoprotein | [147,186] |
| MAL | 2q11.1 | Mal, T cell differentiation protein | [293] |
| MAP2 | 2q34 | Microtubule-associated protein 2 | [151] |
| MAPT | 17q21.31 | Microtubule-associated protein tau | [151] |
| MBP | 18q23 | Myelin basic protein | [52] |
| MOBP | 3p22.1 | Myelin-associated oligodendrocyte basic protein | [190] |
| MOG | 6p22.1 | Myelin oligodendrocyte glycoprotein | [187,188] |
| MYRF | 11q12.2 | Myelin regulatory factor | [193] |
| NCAM1 | 11q23.2 | Neural cell adhesion molecule 1 | [294] |
| NKX2-2 | 20p11.22 | NK2 homeobox 2 | [194] |
| NKX2-6 | 8p21.2 | NK2 homeobox 6 | [194] |
| NPC1 | 18q11.2 | NPC intracellular cholesterol transporter 1 | [295] |
| OLIG1 | 21q22.11 | Oligodendrocyte transcription factor 1 | [183,196] |
| OLIG2 | 21q22.11 | Oligodendrocyte transcription factor 2 | [112,196] |
| OMG | 17q11.2 | Oligodendrocyte myelin glycoprotein | [86,191,192] |
| OPALIN | 10q24.1 | Oligodendrocytic myelin paranodal and inner loop protein | [204] |
| PEX5 | 12p13.31 | Peroxisomal biogenesis factor 5 | [202] |
| PLP1 | Xq22.2 | Proteolipid protein 1 | [184,185] |
| PROM1 | 4p15.32 | Prominin 1 | [296] |
| QKI | 6q26 | QKI, KH domain containing RNA binding | [133] |
| SETDB1 | 1q21.3 | SET domain bifurcated histone lysine methyltransferase 1 | [112] |
| SOX8 | 16p13.3 | SRY-box transcription factor 8 | [35] |
| SOX10 | 22q13.1 | SRY-box transcription factor 10 | [35,122] |
| SREBF2 | 22q13.2 | Sterol regulatory element-binding transcription factor 2 | [133] |
| TMEM98 | 17q11.2 | Transmembrane protein 98 | [195] |
| TPPP | 5p15.33 | Tubulin polymerization promoting protein | [297] |
| UGT8 | 4q26 | UDP glycosyltransferase 8 | [200,201] |
* Data are retrieved from “The Human Protein Atlas” [285].
References
- Allen, N.J.; Barres, B.A. Glia—More than Just Brain Glue. Nature 2009, 457, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Nave, K.-A. Oligodendrocytes: Myelination and Axonal Support. Cold Spring Harb. Perspect. Biol. 2016, 8, a020479. [Google Scholar] [CrossRef]
- Herculano-Houzel, S. The Glia/Neuron Ratio: How It Varies Uniformly across Brain Structures and Species and What That Means for Brain Physiology and Evolution: The Glia/Neuron Ratio. Glia 2014, 62, 1377–1391. [Google Scholar] [CrossRef] [PubMed]
- Bercury, K.K.; Macklin, W.B. Dynamics and Mechanisms of CNS Myelination. Dev. Cell 2015, 32, 447–458. [Google Scholar] [CrossRef]
- Nave, K.-A.; Werner, H.B. Myelination of the Nervous System: Mechanisms and Functions. Annu. Rev. Cell Dev. Biol. 2014, 30, 503–533. [Google Scholar] [CrossRef] [PubMed]
- Boullerne, A.I. The History of Myelin. Exp. Neurol. 2016, 283, 431–445. [Google Scholar] [CrossRef]
- Edgar, J.M.; McGowan, E.; Chapple, K.J.; Möbius, W.; Lemgruber, L.; Insall, R.H.; Nave, K.; Boullerne, A. Río-Hortega’s Drawings Revisited with Fluorescent Protein Defines a Cytoplasm-filled Channel System of CNS Myelin. J. Anat. 2021, 239, 1241–1255. [Google Scholar] [CrossRef]
- Pérez-Cerdá, F.; Sánchez-Gómez, M.V.; Matute, C. Pío Del Río Hortega and the Discovery of the Oligodendrocytes. Front. Neuroanat. 2015, 9, 92. [Google Scholar] [CrossRef]
- Martínez-Cerdeño, V.; Noctor, S.C. Neural Progenitor Cell Terminology. Front. Neuroanat. 2018, 12, 104. [Google Scholar] [CrossRef]
- Van Tilborg, E.; de Theije, C.G.M.; van Hal, M.; Wagenaar, N.; de Vries, L.S.; Benders, M.J.; Rowitch, D.H.; Nijboer, C.H. Origin and Dynamics of Oligodendrocytes in the Developing Brain: Implications for Perinatal White Matter Injury. Glia 2018, 66, 221–238. [Google Scholar] [CrossRef]
- Nishiyama, A.; Shimizu, T.; Sherafat, A.; Richardson, W.D. Life-Long Oligodendrocyte Development and Plasticity. Semin. Cell Dev. Biol. 2021, 116, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Scantlebury, N.; Cunningham, T.; Dockstader, C.; Laughlin, S.; Gaetz, W.; Rockel, C.; Dickson, J.; Mabbott, D. Relations between White Matter Maturation and Reaction Time in Childhood. J. Int. Neuropsychol. Soc. 2014, 20, 99–112. [Google Scholar] [CrossRef]
- Mabbott, D.J.; Noseworthy, M.; Bouffet, E.; Laughlin, S.; Rockel, C. White Matter Growth as a Mechanism of Cognitive Development in Children. NeuroImage 2006, 33, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Young, K.M.; Psachoulia, K.; Tripathi, R.B.; Dunn, S.-J.; Cossell, L.; Attwell, D.; Tohyama, K.; Richardson, W.D. Oligodendrocyte Dynamics in the Healthy Adult CNS: Evidence for Myelin Remodeling. Neuron 2013, 77, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Sams, E.C. Oligodendrocytes in the Aging Brain. Neuronal Signal. 2021, 5, NS20210008. [Google Scholar] [CrossRef] [PubMed]
- Kinney, H.C.; Volpe, J.J. Chapter 8—Myelination Events. In Volpe’s Neurology of the Newborn, 6th ed.; Volpe, J.J., Inder, T.E., Darras, B.T., de Vries, L.S., du Plessis, A.J., Neil, J.J., Perlman, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 176–188. ISBN 978-0-323-42876-7. [Google Scholar]
- Hughes, E.G.; Orthmann-Murphy, J.L.; Langseth, A.J.; Bergles, D.E. Myelin Remodeling through Experience-Dependent Oligodendrogenesis in the Adult Somatosensory Cortex. Nat. Neurosci. 2018, 21, 696–706. [Google Scholar] [CrossRef]
- Grabel, L. Developmental Origin of Neural Stem Cells: The Glial Cell That Could. Stem Cell Rev. Rep. 2012, 8, 577–585. [Google Scholar] [CrossRef]
- Fletcher, J.L.; Makowiecki, K.; Cullen, C.L.; Young, K.M. Oligodendrogenesis and Myelination Regulate Cortical Development, Plasticity and Circuit Function. Semin. Cell Dev. Biol. 2021, 118, 14–23. [Google Scholar] [CrossRef]
- Bergles, D.E.; Richardson, W.D. Oligodendrocyte Development and Plasticity. Cold Spring Harb. Perspect. Biol. 2015, 8, a020453. [Google Scholar] [CrossRef]
- Huang, W.; Bhaduri, A.; Velmeshev, D.; Wang, S.; Wang, L.; Rottkamp, C.A.; Alvarez-Buylla, A.; Rowitch, D.H.; Kriegstein, A.R. Origins and Proliferative States of Human Oligodendrocyte Precursor Cells. Cell 2020, 182, 594–608.e11. [Google Scholar] [CrossRef]
- Kessaris, N.; Fogarty, M.; Iannarelli, P.; Grist, M.; Wegner, M.; Richardson, W.D. Competing Waves of Oligodendrocytes in the Forebrain and Postnatal Elimination of an Embryonic Lineage. Nat. Neurosci. 2006, 9, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Bradl, M.; Lassmann, H. Oligodendrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Baumann, N.; Pham-Dinh, D. Biology of Oligodendrocyte and Myelin in the Mammalian Central Nervous System. Physiol. Rev. 2001, 81, 871–927. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.B.; Clarke, L.E.; Burzomato, V.; Kessaris, N.; Anderson, P.N.; Attwell, D.; Richardson, W.D. Dorsally and Ventrally Derived Oligodendrocytes Have Similar Electrical Properties but Myelinate Preferred Tracts. J. Neurosci. 2011, 31, 6809–6819. [Google Scholar] [CrossRef]
- Cai, J.; Qi, Y.; Hu, X.; Tan, M.; Liu, Z.; Zhang, J.; Li, Q.; Sander, M.; Qiu, M. Generation of Oligodendrocyte Precursor Cells from Mouse Dorsal Spinal Cord Independent of Nkx6 Regulation and Shh Signaling. Neuron 2005, 45, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Mehler, M.F.; Zhao, J.; Yu Yung, S.; Kessler, J.A. Sonic Hedgehog and BMP2 Exert Opposing Actions on Proliferation and Differentiation of Embryonic Neural Progenitor Cells. Dev. Biol. 1999, 215, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Gomes, W.A.; Mehler, M.F.; Kessler, J.A. Transgenic Overexpression of BMP4 Increases Astroglial and Decreases Oligodendroglial Lineage Commitment. Dev. Biol. 2003, 255, 164–177. [Google Scholar] [CrossRef]
- Orentas, D.M.; Hayes, J.E.; Dyer, K.L.; Miller, R.H. Sonic Hedgehog Signaling Is Required during the Appearance of Spinal Cord Oligodendrocyte Precursors. Dev. Camb. Engl. 1999, 126, 2419–2429. [Google Scholar] [CrossRef]
- Danesin, C.; Agius, E.; Escalas, N.; Ai, X.; Emerson, C.; Cochard, P.; Soula, C. Ventral Neural Progenitors Switch toward an Oligodendroglial Fate in Response to Increased Sonic Hedgehog (Shh) Activity: Involvement of Sulfatase 1 in Modulating Shh Signaling in the Ventral Spinal Cord. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 5037–5048. [Google Scholar] [CrossRef]
- Farreny, M.-A.; Agius, E.; Bel-Vialar, S.; Escalas, N.; Khouri-Farah, N.; Soukkarieh, C.; Danesin, C.; Pituello, F.; Cochard, P.; Soula, C. FGF Signaling Controls Shh-Dependent Oligodendroglial Fate Specification in the Ventral Spinal Cord. Neural Develop. 2018, 13, 3. [Google Scholar] [CrossRef]
- Kearns, C.A.; Walker, M.; Ravanelli, A.M.; Scott, K.; Arzbecker, M.R.; Appel, B. Zebrafish Spinal Cord Oligodendrocyte Formation Requires Boc Function. Genetics 2021, 218, iyab082. [Google Scholar] [CrossRef] [PubMed]
- Bylund, M.; Andersson, E.; Novitch, B.G.; Muhr, J. Vertebrate Neurogenesis Is Counteracted by Sox1–3 Activity. Nat. Neurosci. 2003, 6, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Stolt, C.C.; Schmitt, S.; Lommes, P.; Sock, E.; Wegner, M. Impact of Transcription Factor Sox8 on Oligodendrocyte Specification in the Mouse Embryonic Spinal Cord. Dev. Biol. 2005, 281, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Turnescu, T.; Arter, J.; Reiprich, S.; Tamm, E.R.; Waisman, A.; Wegner, M. Sox8 and Sox10 Jointly Maintain Myelin Gene Expression in Oligodendrocytes. Glia 2018, 66, 279–294. [Google Scholar] [CrossRef]
- Pozniak, C.D.; Langseth, A.J.; Dijkgraaf, G.J.P.; Choe, Y.; Werb, Z.; Pleasure, S.J. Sox10 Directs Neural Stem Cells toward the Oligodendrocyte Lineage by Decreasing Suppressor of Fused Expression. Proc. Natl. Acad. Sci. USA 2010, 107, 21795–21800. [Google Scholar] [CrossRef]
- Wegner, M. Specification of Oligodendrocytes. In Patterning and Cell Type Specification in the Developing CNS and PNS; Elsevier: Amsterdam, The Netherlands, 2020; pp. 847–866. ISBN 978-0-12-814405-3. [Google Scholar]
- Qi, Y.; Tan, M.; Hui, C.-C.; Qiu, M. Gli2 Is Required for Normal Shh Signaling and Oligodendrocyte Development in the Spinal Cord. Mol. Cell. Neurosci. 2003, 23, 440–450. [Google Scholar] [CrossRef]
- Hudson, L.D.; Romm, E.; Berndt, J.A.; Nielsen, J.A. A Tool for Examining the Role of the Zinc Finger Myelin Transcription Factor 1 (Myt1) in Neural Development: Myt1 Knock-in Mice. Transgenic Res. 2011, 20, 951–961. [Google Scholar] [CrossRef][Green Version]
- Miron, V.E.; Kuhlmann, T.; Antel, J.P. Cells of the Oligodendroglial Lineage, Myelination, and Remyelination. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2011, 1812, 184–193. [Google Scholar] [CrossRef]
- Merchán, P.; Bribián, A.; Sánchez-Camacho, C.; Lezameta, M.; Bovolenta, P.; de Castro, F. Sonic Hedgehog Promotes the Migration and Proliferation of Optic Nerve Oligodendrocyte Precursors. Mol. Cell. Neurosci. 2007, 36, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Frost, E.E.; Zhou, Z.; Krasnesky, K.; Armstrong, R.C. Initiation of Oligodendrocyte Progenitor Cell Migration by a PDGF-A Activated Extracellular Regulated Kinase (ERK) Signaling Pathway. Neurochem. Res. 2009, 34, 169–181. [Google Scholar] [CrossRef]
- Finzsch, M.; Stolt, C.C.; Lommes, P.; Wegner, M. Sox9 and Sox10 Influence Survival and Migration of Oligodendrocyte Precursors in the Spinal Cord by Regulating PDGF Receptor Aexpression. Development 2008, 135, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Baroti, T.; Zimmermann, Y.; Schillinger, A.; Liu, L.; Lommes, P.; Wegner, M.; Stolt, C.C. Transcription Factors Sox5 and Sox6 Exert Direct and Indirect Influences on Oligodendroglial Migration in Spinal Cord and Forebrain: SoxD Proteins in Oligodendrocyte Progenitors. Glia 2016, 64, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Biname, F.; Sakry, D.; Dimou, L.; Jolivel, V.; Trotter, J. NG2 Regulates Directional Migration of Oligodendrocyte Precursor Cells via Rho GTPases and Polarity Complex Proteins. J. Neurosci. 2013, 33, 10858–10874. [Google Scholar] [CrossRef] [PubMed]
- Prestoz, L.; Chatzopoulou, E.; Lemkine, G.; Spassky, N.; Lebras, B.; Kagawa, T.; Ikenaka, K.; Zalc, B.; Thomas, J.-L. Control of Axonophilic Migration of Oligodendrocyte Precursor Cells by Eph–Ephrin Interaction. Neuron Glia Biol. 2004, 1, 73–83. [Google Scholar] [CrossRef]
- Grinspan, J.B.; Franceschini, B. Platelet-Derived Growth Factor Is a Survival Factor for PSA-NCAM+ Oligodendrocyte Pre-Progenitor Cells. J. Neurosci. Res. 1995, 41, 540–551. [Google Scholar] [CrossRef]
- Decker, L.; Avellana-Adalid, V.; Nait-Oumesmar, B.; Durbec, P.; Baron-Van Evercooren, A. Oligodendrocyte Precursor Migration and Differentiation: Combined Effects of PSA Residues, Growth Factors, and Substrates. Mol. Cell. Neurosci. 2000, 16, 422–439. [Google Scholar] [CrossRef]
- Gallo, V.; Armstrong, R. Developmental and Growth Factor-Induced Regulation of Nestin in Oligodendrocyte Lineage Cells. J. Neurosci. 1995, 15, 394–406. [Google Scholar] [CrossRef]
- Motizuki, M.; Isogaya, K.; Miyake, K.; Ikushima, H.; Kubota, T.; Miyazono, K.; Saitoh, M.; Miyazawa, K. Oligodendrocyte Transcription Factor 1 (Olig1) Is a Smad Cofactor Involved in Cell Motility Induced by Transforming Growth Factor-β. J. Biol. Chem. 2013, 288, 18911–18922. [Google Scholar] [CrossRef]
- Zhou, Q.; Anderson, D.J. The BHLH Transcription Factors OLIG2 and OLIG1 Couple Neuronal and Glial Subtype Specification. Cell 2002, 109, 61–73. [Google Scholar] [CrossRef]
- Yin, X.; Peterson, J.; Gravel, M.; Braun, P.E.; Trapp, B.D. CNP Overexpression Induces Aberrant Oligodendrocyte Membranes and Inhibits MBP Accumulation and Myelin Compaction. J. Neurosci. Res. 1997, 50, 238–247. [Google Scholar] [CrossRef]
- Wegener, A.; Deboux, C.; Bachelin, C.; Frah, M.; Kerninon, C.; Seilhean, D.; Weider, M.; Wegner, M.; Nait-Oumesmar, B. Gain of Olig2 Function in Oligodendrocyte Progenitors Promotes Remyelination. Brain 2015, 138, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Rivkees, S.A. Hepatocyte Growth Factor Stimulates the Proliferation and Migration of Oligodendrocyte Precursor Cells. J. Neurosci. Res. 2002, 69, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Scott-Drew, S.; ffrench-Constant, C. Expression and Function of Thrombospondin-1 in Myelinating Glial Cells of the Central Nervous System. J. Neurosci. Res. 1997, 50, 202–214. [Google Scholar] [CrossRef]
- Tiwari-Woodruff, S.K.; Buznikov, A.G.; Vu, T.Q.; Micevych, P.E.; Chen, K.; Kornblum, H.I.; Bronstein, J.M. OSP/Claudin-11 Forms a Complex with a Novel Member of the Tetraspanin Super Family and Beta1 Integrin and Regulates Proliferation and Migration of Oligodendrocytes. J. Cell Biol. 2001, 153, 295–305. [Google Scholar] [CrossRef]
- Payne, H.R.; Hemperly, J.J.; Lemmon, V. N-Cadherin Expression and Function in Cultured Oligodendrocytes. Dev. Brain Res. 1996, 97, 9–15. [Google Scholar] [CrossRef]
- Milner, R.; Edwards, G.; Streuli, C.; Ffrench-Constant, C. A Role in Migration for the Alpha V Beta 1 Integrin Expressed on Oligodendrocyte Precursors. J. Neurosci. Off. J. Soc. Neurosci. 1996, 16, 7240–7252. [Google Scholar] [CrossRef]
- Frost, E.; Kiernan, B.W.; Faissner, A.; ffrench-Constant, C. Regulation of Oligodendrocyte Precursor Migration by Extracellular Matrix: Evidence for Substrate-Specific Inhibition of Migration by Tenascin-C. Dev. Neurosci. 1996, 18, 266–273. [Google Scholar] [CrossRef]
- Gadea, A.; Aguirre, A.; Haydar, T.F.; Gallo, V. Endothelin-1 Regulates Oligodendrocyte Development. J. Neurosci. 2009, 29, 10047–10062. [Google Scholar] [CrossRef]
- Spassky, N.; de Castro, F.; Le Bras, B.; Heydon, K.; Quéraud-LeSaux, F.; Bloch-Gallego, E.; Chédotal, A.; Zalc, B.; Thomas, J.-L. Directional Guidance of Oligodendroglial Migration by Class 3 Semaphorins and Netrin-1. J. Neurosci. 2002, 22, 5992–6004. [Google Scholar] [CrossRef]
- Jarjour, A.A.; Manitt, C.; Moore, S.W.; Thompson, K.M.; Yuh, S.-J.; Kennedy, T.E. Netrin-1 Is a Chemorepellent for Oligodendrocyte Precursor Cells in the Embryonic Spinal Cord. J. Neurosci. 2003, 23, 3735–3744. [Google Scholar] [CrossRef]
- Tsai, H.-H.; Tessier-Lavigne, M.; Miller, R.H. Netrin 1 Mediates Spinal Cord Oligodendrocyte Precursor Dispersal. Development 2003, 130, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Vutskits, L.; Pepper, M.S.; Kiss, J.Z. VEGF Is a Chemoattractant for FGF-2–Stimulated Neural Progenitors. J. Cell Biol. 2003, 163, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Choe, Y.; Huynh, T.; Pleasure, S.J. Migration of Oligodendrocyte Progenitor Cells Is Controlled by Transforming Growth Factor Family Proteins during Corticogenesis. J. Neurosci. 2014, 34, 14973–14983. [Google Scholar] [CrossRef] [PubMed]
- Novgorodov, A.S.; El-Awani, M.; Bielawski, J.; Obeid, L.M.; Gudz, T.I. Activation of Sphingosine-1-phosphate Receptor S1P5 Inhibits Oligodendrocyte Progenitor Migration. FASEB J. 2007, 21, 1503–1514. [Google Scholar] [CrossRef]
- Tsai, H.-H.; Frost, E.; To, V.; Robinson, S.; Ffrench-Constant, C.; Geertman, R.; Ransohoff, R.M.; Miller, R.H. The Chemokine Receptor CXCR2 Controls Positioning of Oligodendrocyte Precursors in Developing Spinal Cord by Arresting Their Migration. Cell 2002, 110, 373–383. [Google Scholar] [CrossRef]
- Dziembowska, M.; Tham, T.N.; Lau, P.; Vitry, S.; Lazarini, F.; Dubois-Dalcq, M. A Role for CXCR4 Signaling in Survival and Migration of Neural and Oligodendrocyte Precursors. Glia 2005, 50, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Garcion, E.; Faissner, A.; ffrench-Constant, C. Knockout Mice Reveal a Contribution of the Extracellular Matrix Molecule Tenascin-C to Neural Precursor Proliferation and Migration. Dev. Camb. Engl. 2001, 128, 2485–2496. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Z.; Mei, A.; Huang, H.; Lin, F. Claudin-1 and Claudin-3 as Molecular Regulators of Myelination in Leukoaraiosis Patients. Clinics 2021, 76, e2167. [Google Scholar] [CrossRef]
- Calver, A.R.; Hall, A.C.; Yu, W.-P.; Walsh, F.S.; Heath, J.K.; Betsholtz, C.; Richardson, W.D. Oligodendrocyte Population Dynamics and the Role of PDGF In Vivo. Neuron 1998, 20, 869–882. [Google Scholar] [CrossRef]
- Robinson, S.; Tani, M.; Strieter, R.M.; Ransohoff, R.M.; Miller, R.H. The Chemokine Growth-Regulated Oncogene-Alpha Promotes Spinal Cord Oligodendrocyte Precursor Proliferation. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 10457–10463. [Google Scholar] [CrossRef]
- Kadi, L.; Selvaraju, R.; de Lys, P.; Proudfoot, A.E.I.; Wells, T.N.C.; Boschert, U. Differential Effects of Chemokines on Oligodendrocyte Precursor Proliferation and Myelin Formation in Vitro. J. Neuroimmunol. 2006, 174, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Frederick, T.J.; Min, J.; Altieri, S.C.; Mitchell, N.E.; Wood, T.L. Synergistic Induction of Cyclin D1 in Oligodendrocyte Progenitor Cells by IGF-I and FGF-2 Requires Differential Stimulation of Multiple Signaling Pathways. Glia 2007, 55, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Van’t Veer, A.; Du, Y.; Fischer, T.Z.; Boetig, D.R.; Wood, M.R.; Dreyfus, C.F. Brain-Derived Neurotrophic Factor Effects on Oligodendrocyte Progenitors of the Basal Forebrain Are Mediated through TrkB and the MAP Kinase Pathway. J. Neurosci. Res. 2009, 87, 69–78. [Google Scholar] [CrossRef]
- Yang, J.; Cheng, X.; Qi, J.; Xie, B.; Zhao, X.; Zheng, K.; Zhang, Z.; Qiu, M. EGF Enhances Oligodendrogenesis from Glial Progenitor Cells. Front. Mol. Neurosci. 2017, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Pukos, N.; Yoseph, R.; McTigue, D.M. To Be or Not to Be: Environmental Factors That Drive Myelin Formation during Development and after CNS Trauma. Neuroglia 2018, 1, 63–90. [Google Scholar] [CrossRef]
- Hill, R.A.; Patel, K.D.; Medved, J.; Reiss, A.M.; Nishiyama, A. NG2 Cells in White Matter but Not Gray Matter Proliferate in Response to PDGF. J. Neurosci. 2013, 33, 14558–14566. [Google Scholar] [CrossRef]
- Wang, S.; Sdrulla, A.D.; diSibio, G.; Bush, G.; Nofziger, D.; Hicks, C.; Weinmaster, G.; Barres, B.A. Notch Receptor Activation Inhibits Oligodendrocyte Differentiation. Neuron 1998, 21, 63–75. [Google Scholar] [CrossRef]
- Marie, C.; Clavairoly, A.; Frah, M.; Hmidan, H.; Yan, J.; Zhao, C.; Van Steenwinckel, J.; Daveau, R.; Zalc, B.; Hassan, B.; et al. Oligodendrocyte Precursor Survival and Differentiation Requires Chromatin Remodeling by Chd7 and Chd8. Proc. Natl. Acad. Sci. USA 2018, 115, E8246–E8255. [Google Scholar] [CrossRef]
- Zhao, C.; Dong, C.; Frah, M.; Deng, Y.; Marie, C.; Zhang, F.; Xu, L.; Ma, Z.; Dong, X.; Lin, Y.; et al. Dual Requirement of CHD8 for Chromatin Landscape Establishment and Histone Methyltransferase Recruitment to Promote CNS Myelination and Repair. Dev. Cell 2018, 45, 753–768.e8. [Google Scholar] [CrossRef]
- Hashimoto, R.; Hori, K.; Owa, T.; Miyashita, S.; Dewa, K.; Masuyama, N.; Sakai, K.; Hayase, Y.; Seto, Y.; Inoue, Y.U.; et al. Origins of Oligodendrocytes in the Cerebellum, Whose Development Is Controlled by the Transcription Factor, Sox9. Mech. Dev. 2016, 140, 25–40. [Google Scholar] [CrossRef]
- Nielsen, J.A.; Berndt, J.A.; Hudson, L.D.; Armstrong, R.C. Myelin Transcription Factor 1 (Myt1) Modulates the Proliferation and Differentiation of Oligodendrocyte Lineage Cells. Mol. Cell. Neurosci. 2004, 25, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Amoureux, M.-C.; Cunningham, B.A.; Edelman, G.M.; Crossin, K.L. N-CAM Binding Inhibits the Proliferation of Hippocampal Progenitor Cells and Promotes Their Differentiation to a Neuronal Phenotype. J. Neurosci. 2000, 20, 3631–3640. [Google Scholar] [CrossRef] [PubMed]
- Casaccia-Bonnefil, P.; Hardy, R.J.; Teng, K.K.; Levine, J.M.; Koff, A.; Chao, M.V. Loss of P27Kip1 Function Results in Increased Proliferative Capacity of Oligodendrocyte Progenitors but Unaltered Timing of Differentiation. Development 1999, 126, 4027–4037. [Google Scholar] [CrossRef] [PubMed]
- Vourc’h, P.; Dessay, S.; Mbarek, O.; Marouillat Védrine, S.; Müh, J.-P.; Andres, C. The Oligodendrocyte-Myelin Glycoprotein Gene Is Highly Expressed during the Late Stages of Myelination in the Rat Central Nervous System. Brain Res. Dev. Brain Res. 2003, 144, 159–168. [Google Scholar] [CrossRef]
- Lehotzky, A.; Lau, P.; Tokési, N.; Muja, N.; Hudson, L.D.; Ovádi, J. Tubulin Polymerization-Promoting Protein (TPPP/P25) Is Critical for Oligodendrocyte Differentiation. Glia 2010, 58, 157–168. [Google Scholar] [CrossRef]
- Kondo, T. The Id4 HLH Protein and the Timing of Oligodendrocyte Differentiation. EMBO J. 2000, 19, 1998–2007. [Google Scholar] [CrossRef]
- Wang, S.; Sdrulla, A.; Johnson, J.E.; Yokota, Y.; Barres, B.A. A Role for the Helix-Loop-Helix Protein Id2 in the Control of Oligodendrocyte Development. Neuron 2001, 29, 603–614. [Google Scholar] [CrossRef]
- Scarisbrick, I.A.; Asakura, K.; Rodriguez, M. Neurotrophin-4/5 Promotes Proliferation of Oligodendrocyte-Type-2 Astrocytes (O-2A). Dev. Brain Res. 2000, 123, 87–90. [Google Scholar] [CrossRef]
- Kondo, T.; Raff, M.C. A Role for Noggin in the Development of Oligodendrocyte Precursor Cells. Dev. Biol. 2004, 267, 242–251. [Google Scholar] [CrossRef]
- Cohen, R.I.; Marmur, R.; Norton, W.T.; Mehler, M.F.; Kessler, J.A. Nerve Growth Factor and Neurotrophin-3 Differentially Regulate the Proliferation and Survival of Developing Rat Brain Oligodendrocytes. J. Neurosci. 1996, 16, 6433–6442. [Google Scholar] [CrossRef]
- Castro, D.S.; Martynoga, B.; Parras, C.; Ramesh, V.; Pacary, E.; Johnston, C.; Drechsel, D.; Lebel-Potter, M.; Garcia, L.G.; Hunt, C.; et al. A Novel Function of the Proneural Factor Ascl1 in Progenitor Proliferation Identified by Genome-Wide Characterization of Its Targets. Genes Dev. 2011, 25, 930–945. [Google Scholar] [CrossRef]
- Warren, N.; Price, D.J. Roles of Pax-6 in Murine Diencephalic Development. Dev. Camb. Engl. 1997, 124, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Veiga, S.; Ly, J.; Chan, P.H.; Bresnahan, J.C.; Beattie, M.S. SOD1 Overexpression Improves Features of the Oligodendrocyte Precursor Response in Vitro. Neurosci. Lett. 2011, 503, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Deng, L.; Wang, X.; Xu, X.-M. Effects of Extracellular Matrix Molecules on the Growth Properties of Oligodendrocyte Progenitor Cells in Vitro. J. Neurosci. Res. 2009, 87, 2854–2862. [Google Scholar] [CrossRef] [PubMed]
- Blaschuk, K.L.; Frost, E.E.; ffrench-Constant, C. The Regulation of Proliferation and Differentiation in Oligodendrocyte Progenitor Cells by AlphaV Integrins. Dev. Camb. Engl. 2000, 127, 1961–1969. [Google Scholar] [CrossRef]
- Adams, K.L.; Riparini, G.; Banerjee, P.; Breur, M.; Bugiani, M.; Gallo, V. Endothelin-1 Signaling Maintains Glial Progenitor Proliferation in the Postnatal Subventricular Zone. Nat. Commun. 2020, 11, 2138. [Google Scholar] [CrossRef]
- Chapman, H.; Riesenberg, A.; Ehrman, L.A.; Kohli, V.; Nardini, D.; Nakafuku, M.; Campbell, K.; Waclaw, R.R. Gsx Transcription Factors Control Neuronal versus Glial Specification in Ventricular Zone Progenitors of the Mouse Lateral Ganglionic Eminence. Dev. Biol. 2018, 442, 115–126. [Google Scholar] [CrossRef]
- Stancic, M.; Slijepcevic, D.; Nomden, A.; Vos, M.J.; de Jonge, J.C.; Sikkema, A.H.; Gabius, H.-J.; Hoekstra, D.; Baron, W. Galectin-4, a Novel Neuronal Regulator of Myelination. Glia 2012, 60, 919–935. [Google Scholar] [CrossRef]
- Canoll, P.D.; Musacchio, J.M.; Hardy, R.; Reynolds, R.; Marchionni, M.A.; Salzer, J.L. GGF/Neuregulin Is a Neuronal Signal That Promotes the Proliferation and Survival and Inhibits the Differentiation of Oligodendrocyte Progenitors. Neuron 1996, 17, 229–243. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, X.; Gui, X.; Croteau, C.; Song, L.; Xu, J.; Wang, A.; Bannerman, P.; Guo, F. Sox2 Is Essential for Oligodendroglial Proliferation and Differentiation during Postnatal Brain Myelination and CNS Remyelination. J. Neurosci. 2018, 38, 1802–1820. [Google Scholar] [CrossRef]
- Brinkmann, B.G.; Agarwal, A.; Sereda, M.W.; Garratt, A.N.; Müller, T.; Wende, H.; Stassart, R.M.; Nawaz, S.; Humml, C.; Velanac, V.; et al. Neuregulin-1/ErbB Signaling Serves Distinct Functions in Myelination of the Peripheral and Central Nervous System. Neuron 2008, 59, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Bercury, K.K.; Ahrendsen, J.T.; Macklin, W.B. Olig1 Function Is Required for Oligodendrocyte Differentiation in the Mouse Brain. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 4386–4402. [Google Scholar] [CrossRef] [PubMed]
- See, J.; Zhang, X.; Eraydin, N.; Mun, S.-B.; Mamontov, P.; Golden, J.A.; Grinspan, J.B. Oligodendrocyte Maturation Is Inhibited by Bone Morphogenetic Protein. Mol. Cell. Neurosci. 2004, 26, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Harnisch, K.; Teuber-Hanselmann, S.; Macha, N.; Mairinger, F.; Fritsche, L.; Soub, D.; Meinl, E.; Junker, A. Myelination in Multiple Sclerosis Lesions Is Associated with Regulation of Bone Morphogenetic Protein 4 and Its Antagonist Noggin. Int. J. Mol. Sci. 2019, 20, 154. [Google Scholar] [CrossRef]
- Izrael, M.; Zhang, P.; Kaufman, R.; Shinder, V.; Ella, R.; Amit, M.; Itskovitz-Eldor, J.; Chebath, J.; Revel, M. Human Oligodendrocytes Derived from Embryonic Stem Cells: Effect of Noggin on Phenotypic Differentiation in vitro and on Myelination in vivo. Mol. Cell. Neurosci. 2007, 34, 310–323. [Google Scholar] [CrossRef]
- Sugimori, M.; Nagao, M.; Parras, C.M.; Nakatani, H.; Lebel, M.; Guillemot, F.; Nakafuku, M. Ascl1 Is Required for Oligodendrocyte Development in the Spinal Cord. Dev. Camb. Engl. 2008, 135, 1271–1281. [Google Scholar] [CrossRef]
- Matsumoto, S.; Banine, F.; Feistel, K.; Foster, S.; Xing, R.; Struve, J.; Sherman, L.S. Brg1 Directly Regulates Olig2 Transcription and Is Required for Oligodendrocyte Progenitor Cell Specification. Dev. Biol. 2016, 413, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Wedel, M.; Fröb, F.; Elsesser, O.; Wittmann, M.-T.; Lie, D.C.; Reis, A.; Wegner, M. Transcription Factor Tcf4 Is the Preferred Heterodimerization Partner for Olig2 in Oligodendrocytes and Required for Differentiation. Nucleic Acids Res. 2020, 48, 4839–4857. [Google Scholar] [CrossRef]
- Zhao, C.; Deng, Y.; Liu, L.; Yu, K.; Zhang, L.; Wang, H.; He, X.; Wang, J.; Lu, C.; Wu, L.N.; et al. Dual Regulatory Switch through Interactions of Tcf7l2/Tcf4 with Stage-Specific Partners Propels Oligodendroglial Maturation. Nat. Commun. 2016, 7, 10883. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, S.; Yang, Q.; Guo, S.; Chen, Q.; Liu, Z.; Li, L.; Jiang, M.; Li, H.; Hu, J.; et al. The Oligodendrocyte Transcription Factor 2 OLIG2 Regulates Transcriptional Repression during Myelinogenesis in Rodents. Nat. Commun. 2022, 13, 1423. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.; Wang, S.; Koito, H.; Li, J.; Ye, F.; Hoang, J.; Escobar, S.S.; Gow, A.; Arnett, H.A.; et al. The Oligodendrocyte-Specific G Protein–Coupled Receptor GPR17 Is a Cell-Intrinsic Timer of Myelination. Nat. Neurosci. 2009, 12, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Marin-Husstege, M.; He, Y.; Li, J.; Kondo, T.; Sablitzky, F.; Casaccia-Bonnefil, P. Multiple Roles of Id4 in Developmental Myelination: Predicted Outcomes and Unexpected Findings. Glia 2006, 54, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wu, H.; He, W.; Zhou, F.; Yu, X.; Yi, M.; Du, J.; Xie, B.; Qiu, M. Id2 and Id4 Are Not the Major Negative Regulators of Oligodendrocyte Differentiation during Early Central Nervous System Development. Glia 2022, 70, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Swiss, V.A.; Nguyen, T.; Dugas, J.; Ibrahim, A.; Barres, B.; Androulakis, I.P.; Casaccia, P. Identification of a Gene Regulatory Network Necessary for the Initiation of Oligodendrocyte Differentiation. PLoS ONE 2011, 6, e18088. [Google Scholar] [CrossRef] [PubMed]
- Bujalka, H.; Koenning, M.; Jackson, S.; Perreau, V.M.; Pope, B.; Hay, C.M.; Mitew, S.; Hill, A.F.; Lu, Q.R.; Wegner, M.; et al. MYRF Is a Membrane-Associated Transcription Factor That Autoproteolytically Cleaves to Directly Activate Myelin Genes. PLoS Biol. 2013, 11, e1001625. [Google Scholar] [CrossRef]
- Emery, B.; Agalliu, D.; Cahoy, J.D.; Watkins, T.A.; Dugas, J.C.; Mulinyawe, S.B.; Ibrahim, A.; Ligon, K.L.; Rowitch, D.H.; Barres, B.A. Myelin Gene Regulatory Factor Is a Critical Transcriptional Regulator Required for CNS Myelination. Cell 2009, 138, 172–185. [Google Scholar] [CrossRef]
- Hoffmann, S.A.; Hos, D.; Küspert, M.; Lang, R.A.; Lovell-Badge, R.; Wegner, M.; Reiprich, S. Stem Cell Factor Sox2 and Its Close Relative Sox3 Have Differentiation Functions in Oligodendrocytes. Development 2014, 141, 39–50. [Google Scholar] [CrossRef]
- Stolt, C.C.; Lommes, P.; Friedrich, R.P.; Wegner, M. Transcription Factors Sox8 and Sox10 Perform Non-Equivalent Roles during Oligodendrocyte Development despite Functional Redundancy. Development 2004, 131, 2349–2358. [Google Scholar] [CrossRef]
- Stolt, C.C.; Rehberg, S.; Ader, M.; Lommes, P.; Riethmacher, D.; Schachner, M.; Bartsch, U.; Wegner, M. Terminal Differentiation of Myelin-Forming Oligodendrocytes Depends on the Transcription Factor Sox10. Genes Dev. 2002, 16, 165–170. [Google Scholar] [CrossRef]
- Takada, N.; Kucenas, S.; Appel, B. Sox10 Is Necessary for Oligodendrocyte Survival Following Axon Wrapping. Glia 2010, 58, 996–1006. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, H.; Chen, Z.; Zhang, Z.; Lu, W.; Qiu, M. The Transcription Factor NKX2-2 Regulates Oligodendrocyte Differentiation through Domain-Specific Interactions with Transcriptional Corepressors. J. Biol. Chem. 2020, 295, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Cai, J.; Wu, Y.; Wu, R.; Lee, J.; Fu, H.; Rao, M.; Sussel, L.; Rubenstein, J.; Qiu, M. Control of Oligodendrocyte Differentiation by the Nkx2.2 Homeodomain Transcription Factor. Dev. Camb. Engl. 2001, 128, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Doucette, J.R.; Nazarali, A.J. Sirt2 Is a Novel in Vivo Downstream Target of Nkx2.2 and Enhances Oligodendroglial Cell Differentiation. J. Mol. Cell Biol. 2011, 3, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Hisahara, S.; Iwahara, N.; Matsushita, T.; Suzuki, S.; Matsumura, A.; Fujikura, M.; Yokokawa, K.; Saito, T.; Manabe, T.; Kawamata, J.; et al. SIRT1 Decelerates Morphological Processing of Oligodendrocyte Cell Lines and Regulates the Expression of Cytoskeleton-Related Oligodendrocyte Proteins. Biochem. Biophys. Res. Commun. 2021, 546, 7–14. [Google Scholar] [CrossRef]
- Laitman, B.M.; Asp, L.; Mariani, J.N.; Zhang, J.; Liu, J.; Sawai, S.; Chapouly, C.; Horng, S.; Kramer, E.G.; Mitiku, N.; et al. The Transcriptional Activator Krüppel-like Factor-6 Is Required for CNS Myelination. PLOS Biol. 2016, 14, e1002467. [Google Scholar] [CrossRef]
- Vondran, M.W.; Clinton-Luke, P.; Honeywell, J.Z.; Dreyfus, C.F. BDNF+/- Mice Exhibit Deficits in Oligodendrocyte Lineage Cells of the Basal Forebrain. Glia 2010, 58, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Gorath, M.; Richter-Landsberg, C. Neurotrophin-3 (NT-3) Modulates Early Differentiation of Oligodendrocytes in Rat Brain Cortical Cultures. Glia 1999, 28, 244–255. [Google Scholar] [CrossRef]
- Yan, H.; Wood, P.M. NT-3 Weakly Stimulates Proliferation of Adult Rat O1(−)O4(+) Oligodendrocyte-Lineage Cells and Increases Oligodendrocyte Myelination in vitro. J. Neurosci. Res. 2000, 62, 329–335. [Google Scholar] [CrossRef]
- McMorris, F.A.; Dubois-Dalcq, M. Insulin-like Growth Factor I Promotes Cell Proliferation and Oligodendroglial Commitment in Rat Glial Progenitor Cells Developing in vitro. J. Neurosci. Res. 1988, 21, 199–209. [Google Scholar] [CrossRef]
- Zhou, L.; Shao, C.-Y.; Xie, Y.-J.; Wang, N.; Xu, S.-M.; Luo, B.-Y.; Wu, Z.-Y.; Ke, Y.H.; Qiu, M.; Shen, Y. Gab1 Mediates PDGF Signaling and Is Essential to Oligodendrocyte Differentiation and CNS Myelination. eLife 2020, 9, e52056. [Google Scholar] [CrossRef]
- Zhou, X.; Shin, S.; He, C.; Zhang, Q.; Rasband, M.N.; Ren, J.; Dai, C.; Zorrilla-Veloz, R.I.; Shingu, T.; Yuan, L.; et al. Qki Regulates Myelinogenesis through Srebp2-Dependent Cholesterol Biosynthesis. eLife 2021, 10, e60467. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.Y.; Chang, J.-C.; Lim, K.; Cazenave-Gassiot, A.; Nguyen, A.T.; Foo, J.C.; Muralidharan, S.; Viera-Ortiz, A.; Ong, S.J.M.; Hor, J.H.; et al. TDP-43 Mediates SREBF2-Regulated Gene Expression Required for Oligodendrocyte Myelination. J. Cell Biol. 2021, 220, e201910213. [Google Scholar] [CrossRef] [PubMed]
- Morita, J.; Kano, K.; Kato, K.; Takita, H.; Sakagami, H.; Yamamoto, Y.; Mihara, E.; Ueda, H.; Sato, T.; Tokuyama, H.; et al. Structure and Biological Function of ENPP6, a Choline-Specific Glycerophosphodiester-Phosphodiesterase. Sci. Rep. 2016, 6, 20995. [Google Scholar] [CrossRef] [PubMed]
- Baas, D.; Bourbeau, D.; Sarliève, L.L.; Ittel, M.E.; Dussault, J.H.; Puymirat, J. Oligodendrocyte Maturation and Progenitor Cell Proliferation Are Independently Regulated by Thyroid Hormone. Glia 1997, 19, 324–332. [Google Scholar] [CrossRef]
- Barres, B.A.; Lazar, M.A.; Raff, M.C. A Novel Role for Thyroid Hormone, Glucocorticoids and Retinoic Acid in Timing Oligodendrocyte Development. Dev. Camb. Engl. 1994, 120, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Baas, D.; Fressinaud, C.; Ittel, M.E.; Reeber, A.; Dalençon, D.; Puymirat, J.; Sarliève, L.L. Expression of Thyroid Hormone Receptor Isoforms in Rat Oligodendrocyte Cultures. Effect of 3,5,3′-Triiodo-l-Thyronine. Neurosci. Lett. 1994, 176, 47–51. [Google Scholar] [CrossRef]
- Kondo, T.; Raff, M. Basic Helix-Loop-Helix Proteins and the Timing of Oligodendrocyte Differentiation. Dev. Camb. Engl. 2000, 127, 2989–2998. [Google Scholar] [CrossRef]
- Genoud, S.; Lappe-Siefke, C.; Goebbels, S.; Radtke, F.; Aguet, M.; Scherer, S.S.; Suter, U.; Nave, K.-A.; Mantei, N. Notch1 Control of Oligodendrocyte Differentiation in the Spinal Cord. J. Cell Biol. 2002, 158, 709–718. [Google Scholar] [CrossRef]
- Hu, Q.-D.; Ang, B.-T.; Karsak, M.; Hu, W.-P.; Cui, X.-Y.; Duka, T.; Takeda, Y.; Chia, W.; Sankar, N.; Ng, Y.-K.; et al. F3/Contactin Acts as a Functional Ligand for Notch during Oligodendrocyte Maturation. Cell 2003, 115, 163–175. [Google Scholar] [CrossRef]
- Emery, B. Regulation of Oligodendrocyte Differentiation and Myelination. Science 2010, 330, 779–782. [Google Scholar] [CrossRef]
- Jepson, S.; Vought, B.; Gross, C.H.; Gan, L.; Austen, D.; Frantz, J.D.; Zwahlen, J.; Lowe, D.; Markland, W.; Krauss, R. LINGO-1, a Transmembrane Signaling Protein, Inhibits Oligodendrocyte Differentiation and Myelination through Intercellular Self-Interactions. J. Biol. Chem. 2012, 287, 22184–22195. [Google Scholar] [CrossRef] [PubMed]
- Hirahara, Y.; Bansal, R.; Honke, K.; Ikenaka, K.; Wada, Y. Sulfatide Is a Negative Regulator of Oligodendrocyte Differentiation: Development in Sulfatide-Null Mice. Glia 2004, 45, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-H.; Na, J.E.; Yoon, Y.R.; Rhyu, I.J.; Ko, Y.-G.; Baik, J.-H. Hypomyelination and Cognitive Impairment in Mice Lacking CD133 (Prominin-1). Biochem. Biophys. Res. Commun. 2018, 502, 291–298. [Google Scholar] [CrossRef]
- Brown, T.L.; Hashimoto, H.; Finseth, L.T.; Wood, T.L.; Macklin, W.B. PAK1 Positively Regulates Oligodendrocyte Morphology and Myelination. J. Neurosci. 2021, 41, 1864–1877. [Google Scholar] [CrossRef] [PubMed]
- Quarles, R.H. Myelin-Associated Glycoprotein (MAG): Past, Present and Beyond. J. Neurochem. 2007, 100, 1431–1448. [Google Scholar] [CrossRef] [PubMed]
- Stankoff, B.; Aigrot, M.-S.; Noël, F.; Wattilliaux, A.; Zalc, B.; Lubetzki, C. Ciliary Neurotrophic Factor (CNTF) Enhances Myelin Formation: A Novel Role for CNTF and CNTF-Related Molecules. J. Neurosci. 2002, 22, 9221–9227. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, A.S.; Greenwood, K.; Wilkin, G.; Butt, A.M. Kir4.1 Expression by Astrocytes and Oligodendrocytes in CNS White Matter: A Developmental Study in the Rat Optic Nerve. J. Anat. 2004, 204, 475–485. [Google Scholar] [CrossRef]
- Howng, S.Y.B.; Avila, R.L.; Emery, B.; Traka, M.; Lin, W.; Watkins, T.; Cook, S.; Bronson, R.; Davisson, M.; Barres, B.A.; et al. ZFP191 Is Required by Oligodendrocytes for CNS Myelination. Genes Dev. 2010, 24, 301–311. [Google Scholar] [CrossRef]
- Müller, R.; Heinrich, M.; Heck, S.; Blohm, D.; Richter-Landsberg, C. Expression of Microtubule-Associated Proteins MAP2 and Tau in Cultured Rat Brain Oligodendrocytes. Cell Tissue Res. 1997, 288, 239–249. [Google Scholar] [CrossRef]
- Chen, X.; Ku, L.; Mei, R.; Liu, G.; Xu, C.; Wen, Z.; Zhao, X.; Wang, F.; Xiao, L.; Feng, Y. Novel Schizophrenia Risk Factor Pathways Regulate FEZ1 to Advance Oligodendroglia Development. Transl. Psychiatry 2017, 7, 1293. [Google Scholar] [CrossRef]
- Lee, J.; Gravel, M.; Zhang, R.; Thibault, P.; Braun, P.E. Process Outgrowth in Oligodendrocytes Is Mediated by CNP, a Novel Microtubule Assembly Myelin Protein. J. Cell Biol. 2005, 170, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Monin, A.; Baumann, P.S.; Griffa, A.; Xin, L.; Mekle, R.; Fournier, M.; Butticaz, C.; Klaey, M.; Cabungcal, J.H.; Steullet, P.; et al. Glutathione Deficit Impairs Myelin Maturation: Relevance for White Matter Integrity in Schizophrenia Patients. Mol. Psychiatry 2015, 20, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.; Hu, Y.; Zhang, Y.; Yang, Z.; Shao, Z.; Qiu, M.; Pepinsky, B.; Miller, R.H.; Mi, S. Oligodendrocyte Differentiation and Myelination Defects in OMgp Null Mice. Mol. Cell. Neurosci. 2011, 46, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Kaji, S.; Maki, T.; Ueda, J.; Ishimoto, T.; Inoue, Y.; Yasuda, K.; Sawamura, M.; Hikawa, R.; Ayaki, T.; Yamakado, H.; et al. BCAS1-Positive Immature Oligodendrocytes Are Affected by the α-Synuclein-Induced Pathology of Multiple System Atrophy. Acta Neuropathol. Commun. 2020, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Fard, M.K.; van der Meer, F.; Sánchez, P.; Cantuti-Castelvetri, L.; Mandad, S.; Jäkel, S.; Fornasiero, E.F.; Schmitt, S.; Ehrlich, M.; Starost, L.; et al. BCAS1 Expression Defines a Population of Early Myelinating Oligodendrocytes in Multiple Sclerosis Lesions. Sci. Transl. Med. 2017, 9, eaam7816. [Google Scholar] [CrossRef] [PubMed]
- Ikenaka, K.; Kagawa, T.; Mikoshiba, K. Selective Expression of DM-20, an Alternatively Spliced Myelin Proteolipid Protein Gene Product, in Developing Nervous System and in Nonglial Cells. J. Neurochem. 1992, 58, 2248–2253. [Google Scholar] [CrossRef] [PubMed]
- Galiano, M.R.; Andrieux, A.; Deloulme, J.C.; Bosc, C.; Schweitzer, A.; Job, D.; Hallak, M.E. Myelin Basic Protein Functions as a Microtubule Stabilizing Protein in Differentiated Oligodendrocytes. J. Neurosci. Res. 2006, 84, 534–541. [Google Scholar] [CrossRef]
- Uhlenberg, B.; Schuelke, M.; Rüschendorf, F.; Ruf, N.; Kaindl, A.M.; Henneke, M.; Thiele, H.; Stoltenburg-Didinger, G.; Aksu, F.; Topaloğlu, H.; et al. Mutations in the Gene Encoding Gap Junction Protein Alpha 12 (Connexin 46.6) Cause Pelizaeus-Merzbacher-like Disease. Am. J. Hum. Genet. 2004, 75, 251–260. [Google Scholar] [CrossRef]
- Menichella, D.M.; Goodenough, D.A.; Sirkowski, E.; Scherer, S.S.; Paul, D.L. Connexins Are Critical for Normal Myelination in the CNS. J. Neurosci. 2003, 23, 5963–5973. [Google Scholar] [CrossRef]
- Wasseff, S.K.; Scherer, S.S. Cx32 and Cx47 Mediate Oligodendrocyte:Astrocyte and Oligodendrocyte:Oligodendrocyte Gap Junction Coupling. Neurobiol. Dis. 2011, 42, 506–513. [Google Scholar] [CrossRef]
- Mosser, J.; Douar, A.M.; Sarde, C.O.; Kioschis, P.; Feil, R.; Moser, H.; Poustka, A.M.; Mandel, J.L.; Aubourg, P. Putative X-Linked Adrenoleukodystrophy Gene Shares Unexpected Homology with ABC Transporters. Nature 1993, 361, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Strachan, L.R.; Stevenson, T.J.; Freshner, B.; Keefe, M.D.; Miranda Bowles, D.; Bonkowsky, J.L. A Zebrafish Model of X-Linked Adrenoleukodystrophy Recapitulates Key Disease Features and Demonstrates a Developmental Requirement for Abcd1 in Oligodendrocyte Patterning and Myelination. Hum. Mol. Genet. 2017, 26, 3600–3614. [Google Scholar] [CrossRef] [PubMed]
- Schnädelbach, O.; Ozen, I.; Blaschuk, O.W.; Meyer, R.L.; Fawcett, J.W. N-Cadherin Is Involved in Axon-Oligodendrocyte Contact and Myelination. Mol. Cell. Neurosci. 2001, 17, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Laursen, L.S.; Chan, C.W.; ffrench-Constant, C. An Integrin–Contactin Complex Regulates CNS Myelination by Differential Fyn Phosphorylation. J. Neurosci. 2009, 29, 9174–9185. [Google Scholar] [CrossRef]
- Charles, P.; Tait, S.; Faivre-Sarrailh, C.; Barbin, G.; Gunn-Moore, F.; Denisenko-Nehrbass, N.; Guennoc, A.-M.; Girault, J.-A.; Brophy, P.J.; Lubetzki, C. Neurofascin Is a Glial Receptor for the Paranodin/Caspr-Contactin Axonal Complex at the Axoglial Junction. Curr. Biol. 2002, 12, 217–220. [Google Scholar] [CrossRef]
- Thaxton, C.; Pillai, A.M.; Pribisko, A.L.; Labasque, M.; Dupree, J.L.; Faivre-Sarrailh, C.; Bhat, M.A. In Vivo Deletion of Immunoglobulin Domains 5 and 6 in Neurofascin (Nfasc) Reveals Domain-Specific Requirements in Myelinated Axons. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 4868–4876. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Labasque, M.; Hivert, B.; Nogales-Gadea, G.; Querol, L.; Illa, I.; Faivre-Sarrailh, C. Specific Contactin N-Glycans Are Implicated in Neurofascin Binding and Autoimmune Targeting in Peripheral Neuropathies. J. Biol. Chem. 2014, 289, 7907–7918. [Google Scholar] [CrossRef]
- Klingseisen, A.; Ristoiu, A.-M.; Kegel, L.; Sherman, D.L.; Rubio-Brotons, M.; Almeida, R.G.; Koudelka, S.; Benito-Kwiecinski, S.K.; Poole, R.J.; Brophy, P.J.; et al. Oligodendrocyte Neurofascin Independently Regulates Both Myelin Targeting and Sheath Growth in the CNS. Dev. Cell 2019, 51, 730–744.e6. [Google Scholar] [CrossRef]
- Çolakoğlu, G.; Bergstrom-Tyrberg, U.; Berglund, E.O.; Ranscht, B. Contactin-1 Regulates Myelination and Nodal/Paranodal Domain Organization in the Central Nervous System. Proc. Natl. Acad. Sci. USA 2014, 111, E394–E403. [Google Scholar] [CrossRef]
- Traka, M.; Goutebroze, L.; Denisenko, N.; Bessa, M.; Nifli, A.; Havaki, S.; Iwakura, Y.; Fukamauchi, F.; Watanabe, K.; Soliven, B.; et al. Association of TAG-1 with Caspr2 Is Essential for the Molecular Organization of Juxtaparanodal Regions of Myelinated Fibers. J. Cell Biol. 2003, 162, 1161–1172. [Google Scholar] [CrossRef]
- Elazar, N.; Vainshtein, A.; Golan, N.; Vijayaragavan, B.; Schaeren-Wiemers, N.; Eshed-Eisenbach, Y.; Peles, E. Axoglial Adhesion by Cadm4 Regulates CNS Myelination. Neuron 2019, 101, 224–231.e5. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.N.; Appel, B. Oligodendrocytes Express Synaptic Proteins That Modulate Myelin Sheath Formation. Nat. Commun. 2019, 10, 4125. [Google Scholar] [CrossRef] [PubMed]
- Sturgill, E.R.; Aoki, K.; Lopez, P.H.; Colacurcio, D.; Vajn, K.; Lorenzini, I.; Majić, S.; Yang, W.H.; Heffer, M.; Tiemeyer, M.; et al. Biosynthesis of the Major Brain Gangliosides GD1a and GT1b. Glycobiology 2012, 22, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Schnaar, R.L. Brain Gangliosides in Axon-Myelin Stability and Axon Regeneration. FEBS Lett. 2010, 584, 1741–1747. [Google Scholar] [CrossRef]
- Harboe, M.; Torvund-Jensen, J.; Kjaer-Sorensen, K.; Laursen, L.S. Ephrin-A1-EphA4 Signaling Negatively Regulates Myelination in the Central Nervous System. Glia 2018, 66, 934–950. [Google Scholar] [CrossRef]
- Linneberg, C.; Harboe, M.; Laursen, L.S. Axo-Glia Interaction Preceding CNS Myelination Is Regulated by Bidirectional Eph-Ephrin Signaling. ASN Neuro 2015, 7, 175909141560285. [Google Scholar] [CrossRef]
- Almeida, R.G. The Rules of Attraction in Central Nervous System Myelination. Front. Cell. Neurosci. 2018, 12, 367. [Google Scholar] [CrossRef]
- Charles, P.; Hernandez, M.P.; Stankoff, B.; Aigrot, M.S.; Colin, C.; Rougon, G.; Zalc, B.; Lubetzki, C. Negative Regulation of Central Nervous System Myelination by Polysialylated-Neural Cell Adhesion Molecule. Proc. Natl. Acad. Sci. USA 2000, 97, 7585–7590. [Google Scholar] [CrossRef]
- Redmond, S.A.; Mei, F.; Eshed-Eisenbach, Y.; Osso, L.A.; Leshkowitz, D.; Shen, Y.-A.A.; Kay, J.N.; Aurrand-Lions, M.; Lyons, D.A.; Peles, E.; et al. Somatodendritic Expression of JAM2 Inhibits Oligodendrocyte Myelination. Neuron 2016, 91, 824–836. [Google Scholar] [CrossRef]
- Díez-Revuelta, N.; Higuero, A.M.; Velasco, S.; Peñas-de-la-Iglesia, M.; Gabius, H.-J.; Abad-Rodríguez, J. Neurons Define Non-Myelinated Axon Segments by the Regulation of Galectin-4-Containing Axon Membrane Domains. Sci. Rep. 2017, 7, 12246. [Google Scholar] [CrossRef]
- Xin, M.; Yue, T.; Ma, Z.; Wu, F.; Gow, A.; Lu, Q.R. Myelinogenesis and Axonal Recognition by Oligodendrocytes in Brain Are Uncoupled in Olig1-Null Mice. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Lüders, K.A.; Nessler, S.; Kusch, K.; Patzig, J.; Jung, R.B.; Möbius, W.; Nave, K.-A.; Werner, H.B. Maintenance of High Proteolipid Protein Level in Adult Central Nervous System Myelin Is Required to Preserve the Integrity of Myelin and Axons. Glia 2019, 67, 634–649. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.; Mottahedeh, J.; Prins, M.; Ridder, W.; Nusinowitz, S.; Bronstein, J.M. Disrupted Compaction of CNS Myelin in an OSP/Claudin-11 and PLP/DM20 Double Knockout Mouse. Mol. Cell. Neurosci. 2005, 29, 405–413. [Google Scholar] [CrossRef]
- Schachner, M.; Bartsch, U. Multiple Functions of the Myelin-Associated Glycoprotein MAG (Siglec-4a) in Formation and Maintenance of Myelin. Glia 2000, 29, 154–165. [Google Scholar] [CrossRef]
- Clements, C.S.; Reid, H.H.; Beddoe, T.; Tynan, F.E.; Perugini, M.A.; Johns, T.G.; Bernard, C.C.A.; Rossjohn, J. The Crystal Structure of Myelin Oligodendrocyte Glycoprotein, a Key Autoantigen in Multiple Sclerosis. Proc. Natl. Acad. Sci. USA 2003, 100, 11059–11064. [Google Scholar] [CrossRef]
- Johns, T.G.; Bernard, C.C.A. The Structure and Function of Myelin Oligodendrocyte Glycoprotein. J. Neurochem. 1999, 72, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rasband, M.N.; Tayler, J.; Kaga, Y.; Yang, Y.; Lappe-Siefke, C.; Nave, K.-A.; Bansal, R. CNP Is Required for Maintenance of Axon-Glia Interactions at Nodes of Ranvier in the CNS. Glia 2005, 50, 86–90. [Google Scholar] [CrossRef]
- Schäfer, I.; Müller, C.; Luhmann, H.J.; White, R. MOBP Levels Are Regulated by Fyn Kinase and Affect the Morphological Differentiation of Oligodendrocytes. J. Cell Sci. 2016, 129, 930–942. [Google Scholar] [CrossRef]
- Vourc’h, P.; Andres, C. Oligodendrocyte Myelin Glycoprotein (OMgp): Evolution, Structure and Function. Brain Res. Rev. 2004, 45, 115–124. [Google Scholar] [CrossRef]
- Chang, K.-J.; Susuki, K.; Dours-Zimmermann, M.T.; Zimmermann, D.R.; Rasband, M.N. Oligodendrocyte Myelin Glycoprotein Does Not Influence Node of Ranvier Structure or Assembly. J. Neurosci. 2010, 30, 14476–14481. [Google Scholar] [CrossRef]
- Koenning, M.; Jackson, S.; Hay, C.M.; Faux, C.; Kilpatrick, T.J.; Willingham, M.; Emery, B. Myelin Gene Regulatory Factor Is Required for Maintenance of Myelin and Mature Oligodendrocyte Identity in the Adult CNS. J. Neurosci. 2012, 32, 12528–12542. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhu, Q.; Zheng, K.; Li, H.; Qi, Y.; Cao, Q.; Qiu, M. Co-Localization of Nkx6.2 and Nkx2.2 Homeodomain Proteins in Differentiated Myelinating Oligodendrocytes. Glia 2010, 58, 458–468. [Google Scholar] [CrossRef]
- Huang, H.; Teng, P.; Du, J.; Meng, J.; Hu, X.; Tang, T.; Zhang, Z.; Qi, Y.B.; Qiu, M. Interactive Repression of MYRF Self-Cleavage and Activity in Oligodendrocyte Differentiation by TMEM98 Protein. J. Neurosci. 2018, 38, 9829–9839. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.; Frim, D.M.; Polak, P.; Vujicic, S.; Arnason, B.G.W.; Boullerne, A.I. Olig1 Is Expressed in Human Oligodendrocytes during Maturation and Regeneration. Glia 2011, 59, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Ishii, A.; Furusho, M.; Dupree, J.L.; Bansal, R. Role of ERK1/2 MAPK Signaling in the Maintenance of Myelin and Axonal Integrity in the Adult CNS. J. Neurosci. 2014, 34, 16031–16045. [Google Scholar] [CrossRef]
- Furusho, M.; Ishii, A.; Bansal, R. Signaling by FGF Receptor 2, Not FGF Receptor 1, Regulates Myelin Thickness through Activation of ERK1/2–MAPK, Which Promotes MTORC1 Activity in an Akt-Independent Manner. J. Neurosci. 2017, 37, 2931–2946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, X.; Liu, L.; Jiang, M.; Zhao, C.; Wang, H.; He, D.; Zheng, T.; Zhou, X.; Hassan, A.; et al. Hdac3 Interaction with P300 Histone Acetyltransferase Regulates the Oligodendrocyte and Astrocyte Lineage Fate Switch. Dev. Cell 2016, 36, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.; Honigbaum, S.; Shroff, S.; Honke, K.; Rosenbluth, J.; Dupree, J.L. Sulfatide Is Essential for the Maintenance of CNS Myelin and Axon Structure. Glia 2006, 53, 372–381. [Google Scholar] [CrossRef]
- Coetzee, T.; Dupree, J.L.; Popko, B. Demyelination and Altered Expression of Myelin-Associated Glycoprotein Isoforms in the Central Nervous System of Galactolipid-Deficient Mice. J. Neurosci. Res. 1998, 54, 613–622. [Google Scholar] [CrossRef]
- Hulshagen, L.; Krysko, O.; Bottelbergs, A.; Huyghe, S.; Klein, R.; Van Veldhoven, P.P.; De Deyn, P.P.; D’Hooge, R.; Hartmann, D.; Baes, M. Absence of Functional Peroxisomes from Mouse CNS Causes Dysmyelination and Axon Degeneration. J. Neurosci. 2008, 28, 4015–4027. [Google Scholar] [CrossRef]
- Morita, M.; Shinbo, S.; Asahi, A.; Imanaka, T. Very Long Chain Fatty Acid β-Oxidation in Astrocytes: Contribution of the ABCD1-Dependent and -Independent Pathways. Biol. Pharm. Bull. 2012, 35, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yoshikawa, F.; Sadakata, T.; Shinoda, Y.; Koebis, M.; Furuichi, T. Age-Dependent Redistribution and Hypersialylation of the Central Myelin Paranodal Loop Membrane Protein Opalin in the Mouse Brain. Neurosci. Lett. 2014, 581, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Chan, J.R. Myelin Plasticity: Sculpting Circuits in Learning and Memory. Nat. Rev. Neurosci. 2020, 21, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.M.; ffrench-Constant, C. Regenerating CNS Myelin—From Mechanisms to Experimental Medicines. Nat. Rev. Neurosci. 2017, 18, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Craig, G.A.; Yoo, S.; Du, T.Y.; Xiao, J. Plasticity in Oligodendrocyte Lineage Progression: An OPC Puzzle on Our Nerves. Eur. J. Neurosci. 2021, 54, 5747–5761. [Google Scholar] [CrossRef]
- Franklin, R.J.M.; ffrench-Constant, C. Remyelination in the CNS: From Biology to Therapy. Nat. Rev. Neurosci. 2008, 9, 839–855. [Google Scholar] [CrossRef]
- Moyon, S.; Dubessy, A.L.; Aigrot, M.S.; Trotter, M.; Huang, J.K.; Dauphinot, L.; Potier, M.C.; Kerninon, C.; Melik Parsadaniantz, S.; Franklin, R.J.M.; et al. Demyelination Causes Adult CNS Progenitors to Revert to an Immature State and Express Immune Cues That Support Their Migration. J. Neurosci. 2015, 35, 4–20. [Google Scholar] [CrossRef]
- Ffrench-Constant, C.; Raff, M.C. Proliferating Bipotential Glial Progenitor Cells in Adult Rat Optic Nerve. Nature 1986, 319, 499–502. [Google Scholar] [CrossRef]
- Butt, A.M.; Rivera, A.D.; Fulton, D.; Azim, K. Targeting the Subventricular Zone to Promote Myelin Repair in the Aging Brain. Cells 2022, 11, 1809. [Google Scholar] [CrossRef]
- Zawadzka, M.; Rivers, L.E.; Fancy, S.P.J.; Zhao, C.; Tripathi, R.; Jamen, F.; Young, K.; Goncharevich, A.; Pohl, H.; Rizzi, M.; et al. CNS-Resident Glial Progenitor/Stem Cells Produce Schwann Cells as Well as Oligodendrocytes during Repair of CNS Demyelination. Cell Stem Cell 2010, 6, 578–590. [Google Scholar] [CrossRef]
- Duncan, I.D.; Radcliff, A.B.; Heidari, M.; Kidd, G.; August, B.K.; Wierenga, L.A. The Adult Oligodendrocyte Can Participate in Remyelination. Proc. Natl. Acad. Sci. USA 2018, 115, E11807–E11816. [Google Scholar] [CrossRef] [PubMed]
- Sim, F.J.; Zhao, C.; Penderis, J.; Franklin, R.J.M. The Age-Related Decrease in CNS Remyelination Efficiency Is Attributable to an Impairment of Both Oligodendrocyte Progenitor Recruitment and Differentiation. J. Neurosci. 2002, 22, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.L.; Ousman, S.S. Astrocytes and Aging. Front. Aging Neurosci. 2018, 10, 337. [Google Scholar] [CrossRef]
- Thelen, K.M.; Falkai, P.; Bayer, T.A.; Lütjohann, D. Cholesterol Synthesis Rate in Human Hippocampus Declines with Aging. Neurosci. Lett. 2006, 403, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Tse, K.-H.; Herrup, K. DNA Damage in the Oligodendrocyte Lineage and Its Role in Brain Aging. Mech. Ageing Dev. 2017, 161, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Cantuti-Castelvetri, L.; Fitzner, D.; Bosch-Queralt, M.; Weil, M.-T.; Su, M.; Sen, P.; Ruhwedel, T.; Mitkovski, M.; Trendelenburg, G.; Lütjohann, D.; et al. Defective Cholesterol Clearance Limits Remyelination in the Aged Central Nervous System. Science 2018, 359, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Love, S. Demyelinating Diseases. J. Clin. Pathol. 2006, 59, 1151–1159. [Google Scholar] [CrossRef]
- Seil, F.J. Demyelination. In Advances in Cellular Neurobiology; Elsevier: Amsterdam, The Netherlands, 1982; Volume 3, pp. 235–274. ISBN 978-0-12-008303-9. [Google Scholar]
- Zhao, J.-W.; Wang, D.-X.; Ma, X.-R.; Dong, Z.-J.; Wu, J.-B.; Wang, F.; Wu, Y. Impaired Metabolism of Oligodendrocyte Progenitor Cells and Axons in Demyelinated Lesion and in the Aged CNS. Curr. Opin. Pharmacol. 2022, 64, 102205. [Google Scholar] [CrossRef]
- Berghoff, S.A.; Spieth, L.; Saher, G. Local Cholesterol Metabolism Orchestrates Remyelination. Trends Neurosci. 2022, 45, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hammel, G.; Shi, M.; Cheng, Z.; Zivkovic, S.; Wang, X.; Xu, P.; He, X.; Guo, B.; Ren, Y.; et al. Myelin Debris Stimulates NG2/CSPG4 Expression in Bone Marrow-Derived Macrophages in the Injured Spinal Cord. Front. Cell. Neurosci. 2021, 15, 651827. [Google Scholar] [CrossRef] [PubMed]
- Kotter, M.R. Myelin Impairs CNS Remyelination by Inhibiting Oligodendrocyte Precursor Cell Differentiation. J. Neurosci. 2006, 26, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Napoli, I.; Neumann, H. Protective Effects of Microglia in Multiple Sclerosis. Exp. Neurol. 2010, 225, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Lampron, A.; Larochelle, A.; Laflamme, N.; Préfontaine, P.; Plante, M.-M.; Sánchez, M.G.; Yong, V.W.; Stys, P.K.; Tremblay, M.-È.; Rivest, S. Inefficient Clearance of Myelin Debris by Microglia Impairs Remyelinating Processes. J. Exp. Med. 2015, 212, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Ben-Hur, T.; Einstein, O.; Bulte, J.W.M. Stem Cell Therapy for Myelin Diseases. Curr. Drug Targets 2005, 6, 3–19. [Google Scholar] [CrossRef]
- Ottoboni, L.; Merlini, A.; Martino, G. Neural Stem Cell Plasticity: Advantages in Therapy for the Injured Central Nervous System. Front. Cell Dev. Biol. 2017, 5, 52. [Google Scholar] [CrossRef]
- Nagoshi, N.; Khazaei, M.; Ahlfors, J.-E.; Ahuja, C.S.; Nori, S.; Wang, J.; Shibata, S.; Fehlings, M.G. Human Spinal Oligodendrogenic Neural Progenitor Cells Promote Functional Recovery After Spinal Cord Injury by Axonal Remyelination and Tissue Sparing. Stem Cells Transl. Med. 2018, 7, 806–818. [Google Scholar] [CrossRef]
- Sankavaram, S.R.; Hakim, R.; Covacu, R.; Frostell, A.; Neumann, S.; Svensson, M.; Brundin, L. Adult Neural Progenitor Cells Transplanted into Spinal Cord Injury Differentiate into Oligodendrocytes, Enhance Myelination, and Contribute to Recovery. Stem Cell Rep. 2019, 12, 950–966. [Google Scholar] [CrossRef]
- Theotokis, P.; Kesidou, E.; Mitsiadou, D.; Petratos, S.; Damianidou, O.; Boziki, M.; Chatzidimitriou, A.; Grigoriadis, N. Lumbar Spine Intrathecal Transplantation of Neural Precursor Cells Promotes Oligodendrocyte Proliferation in Hot Spots of Chronic Demyelination. Brain Pathol. Zurich Switz. 2021, 32, e13040. [Google Scholar] [CrossRef]
- Theotokis, P.; Kleopa, K.A.; Touloumi, O.; Lagoudaki, R.; Lourbopoulos, A.; Nousiopoulou, E.; Kesidou, E.; Poulatsidou, K.-N.; Dardiotis, E.; Hadjigeorgiou, G.; et al. Connexin43 and Connexin47 Alterations after Neural Precursor Cells Transplantation in Experimental Autoimmune Encephalomyelitis. Glia 2015, 63, 1772–1783. [Google Scholar] [CrossRef]
- Einstein, O.; Grigoriadis, N.; Mizrachi-Kol, R.; Reinhartz, E.; Polyzoidou, E.; Lavon, I.; Milonas, I.; Karussis, D.; Abramsky, O.; Ben-Hur, T. Transplanted Neural Precursor Cells Reduce Brain Inflammation to Attenuate Chronic Experimental Autoimmune Encephalomyelitis. Exp. Neurol. 2006, 198, 275–284. [Google Scholar] [CrossRef]
- Pluchino, S.; Gritti, A.; Blezer, E.; Amadio, S.; Brambilla, E.; Borsellino, G.; Cossetti, C.; Del Carro, U.; Comi, G.; ’t Hart, B.; et al. Human Neural Stem Cells Ameliorate Autoimmune Encephalomyelitis in Non-Human Primates. Ann. Neurol. 2009, 66, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.S.Y.; Djelloul, M.; Steiner, E.; Bernard, S.; Salehpour, M.; Possnert, G.; Brundin, L.; Frisén, J. Dynamics of Oligodendrocyte Generation in Multiple Sclerosis. Nature 2019, 566, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Bacmeister, C.M.; Barr, H.J.; McClain, C.R.; Thornton, M.A.; Nettles, D.; Welle, C.G.; Hughes, E.G. Motor Learning Promotes Remyelination via New and Surviving Oligodendrocytes. Nat. Neurosci. 2020, 23, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Neely, S.A.; Williamson, J.M.; Klingseisen, A.; Zoupi, L.; Early, J.J.; Williams, A.; Lyons, D.A. New Oligodendrocytes Exhibit More Abundant and Accurate Myelin Regeneration than Those That Survive Demyelination. Nat. Neurosci. 2022, 25, 415–420. [Google Scholar] [CrossRef]
- Baaklini, C.S.; Rawji, K.S.; Duncan, G.J.; Ho, M.F.S.; Plemel, J.R. Central Nervous System Remyelination: Roles of Glia and Innate Immune Cells. Front. Mol. Neurosci. 2019, 12, 225. [Google Scholar] [CrossRef]
- Chiou, B.; Gao, C.; Giera, S.; Folts, C.J.; Kishore, P.; Yu, D.; Oak, H.C.; Jiang, R.; Piao, X. Cell Type-Specific Evaluation of ADGRG1/GPR56 Function in Developmental Central Nervous System Myelination. Glia 2021, 69, 413–423. [Google Scholar] [CrossRef]
- Murcia-Belmonte, V.; Medina-Rodríguez, E.M.; Bribián, A.; de Castro, F.; Esteban, P.F. ERK1/2 Signaling Is Essential for the Chemoattraction Exerted by Human FGF2 and Human Anosmin-1 on Newborn Rat and Mouse OPCs via FGFR1: Chemotactic Effect of Anosmin-1 on OPCs. Glia 2014, 62, 374–386. [Google Scholar] [CrossRef]
- Nakatani, H.; Martin, E.; Hassani, H.; Clavairoly, A.; Maire, C.L.; Viadieu, A.; Kerninon, C.; Delmasure, A.; Frah, M.; Weber, M.; et al. Ascl1/Mash1 Promotes Brain Oligodendrogenesis during Myelination and Remyelination. J. Neurosci. 2013, 33, 9752–9768. [Google Scholar] [CrossRef]
- Ulloa-Navas, M.J.; Pérez-Borredá, P.; Morales-Gallel, R.; Saurí-Tamarit, A.; García-Tárraga, P.; Gutiérrez-Martín, A.J.; Herranz-Pérez, V.; García-Verdugo, J.M. Ultrastructural Characterization of Human Oligodendrocytes and Their Progenitor Cells by Pre-Embedding Immunogold. Front. Neuroanat. 2021, 15, 696376. [Google Scholar] [CrossRef]
- He, D.; Marie, C.; Zhao, C.; Kim, B.; Wang, J.; Deng, Y.; Clavairoly, A.; Frah, M.; Wang, H.; He, X.; et al. Chd7 Cooperates with Sox10 and Regulates the Onset of CNS Myelination and Remyelination. Nat. Neurosci. 2016, 19, 678–689. [Google Scholar] [CrossRef]
- Dobrowolski, M.; Cave, C.; Levy-Myers, R.; Lee, C.; Park, S.; Choi, B.-R.; Xiao, B.; Yang, W.; Sockanathan, S. GDE3 Regulates Oligodendrocyte Precursor Proliferation via Release of Soluble CNTFRα. Dev. Camb. Engl. 2020, 147, dev180695. [Google Scholar] [CrossRef]
- Kucharova, K.; Stallcup, W.B. The NG2 Proteoglycan Promotes Oligodendrocyte Progenitor Proliferation and Developmental Myelination. Neuroscience 2010, 166, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Petryniak, M.A.; Potter, G.B.; Rowitch, D.H.; Rubenstein, J.L.R. Dlx1 and Dlx2 Control Neuronal versus Oligodendroglial Cell Fate Acquisition in the Developing Forebrain. Neuron 2007, 55, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Muth, K.N.; Piefke, S.; Weider, M.; Sock, E.; Hermans-Borgmeyer, I.; Wegner, M.; Küspert, M. The Dual-Specificity Phosphatase Dusp15 Is Regulated by Sox10 and Myrf in Myelinating Oligodendrocytes: Dusp15 as a Common Target of Sox10 and Myrf. Glia 2016, 64, 2120–2132. [Google Scholar] [CrossRef]
- Conover, J.C.; Doetsch, F.; Garcia-Verdugo, J.-M.; Gale, N.W.; Yancopoulos, G.D.; Alvarez-Buylla, A. Disruption of Eph/Ephrin Signaling Affects Migration and Proliferation in the Adult Subventricular Zone. Nat. Neurosci. 2000, 3, 1091–1097. [Google Scholar] [CrossRef]
- Azim, K.; Raineteau, O.; Butt, A.M. Intraventricular Injection of FGF-2 Promotes Generation of Oligodendrocyte-Lineage Cells in the Postnatal and Adult Forebrain. Glia 2012, 60, 1977–1990. [Google Scholar] [CrossRef]
- Hayakawa, K.; Pham, L.-D.D.; Som, A.T.; Lee, B.J.; Guo, S.; Lo, E.H.; Arai, K. Vascular Endothelial Growth Factor Regulates the Migration of Oligodendrocyte Precursor Cells. J. Neurosci. 2011, 31, 10666–10670. [Google Scholar] [CrossRef]
- Fasciani, I.; Pluta, P.; González-Nieto, D.; Martínez-Montero, P.; Molano, J.; Paíno, C.L.; Millet, O.; Barrio, L.C. Directional Coupling of Oligodendrocyte Connexin-47 and Astrocyte Connexin-43 Gap Junctions. Glia 2018, 66, 2340–2352. [Google Scholar] [CrossRef]
- Yang, H.-J.; Vainshtein, A.; Maik-Rachline, G.; Peles, E. G Protein-Coupled Receptor 37 Is a Negative Regulator of Oligodendrocyte Differentiation and Myelination. Nat. Commun. 2016, 7, 10884. [Google Scholar] [CrossRef]
- Chapman, H.; Waclaw, R.R.; Pei, Z.; Nakafuku, M.; Campbell, K. The Homeobox Gene Gsx2 Controls the Timing of Oligodendroglial Fate Specification in Mouse Lateral Ganglionic Eminence Progenitors. Dev. Camb. Engl. 2013, 140, 2289–2298. [Google Scholar] [CrossRef]
- Ye, F.; Chen, Y.; Hoang, T.; Montgomery, R.L.; Zhao, X.; Bu, H.; Hu, T.; Taketo, M.M.; van Es, J.H.; Clevers, H.; et al. HDAC1 and HDAC2 Regulate Oligodendrocyte Differentiation by Disrupting the β-Catenin–TCF Interaction. Nat. Neurosci. 2009, 12, 829–838. [Google Scholar] [CrossRef]
- Cunliffe, V.T.; Casaccia-Bonnefil, P. Histone Deacetylase 1 Is Essential for Oligodendrocyte Specification in the Zebrafish CNS. Mech. Dev. 2006, 123, 24–30. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Levine, E.M.; Rao, M.S. Hes1 but Not Hes5 Regulates an Astrocyte versus Oligodendrocyte Fate Choice in Glial Restricted Precursors. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2003, 226, 675–689. [Google Scholar] [CrossRef]
- Havrda, M.C.; Paolella, B.R.; Ran, C.; Jering, K.S.; Wray, C.M.; Sullivan, J.M.; Nailor, A.; Hitoshi, Y.; Israel, M.A. Id2 Mediates Oligodendrocyte Precursor Cell Maturation Arrest and Is Tumorigenic in a PDGF-Rich Microenvironment. Cancer Res. 2014, 74, 1822–1832. [Google Scholar] [CrossRef]
- Malek-Hedayat, S.; Rome, L.H. Expression of a Beta 1-Related Integrin by Oligodendroglia in Primary Culture: Evidence for a Functional Role in Myelination. J. Cell Biol. 1994, 124, 1039–1046. [Google Scholar] [CrossRef]
- Barres, B.A.; Raff, M.C. Control of Oligodendrocyte Number in the Developing Rat Optic Nerve. Neuron 1994, 12, 935–942. [Google Scholar] [CrossRef]
- Miller, R.H. Regulation of Oligodendrocyte Development in the Vertebrate CNS. Prog. Neurobiol. 2002, 67, 451–467. [Google Scholar] [CrossRef]
- Suzuki, N.; Hyodo, M.; Hayashi, C.; Mabuchi, Y.; Sekimoto, K.; Onchi, C.; Sekiguchi, K.; Akazawa, C. Laminin A2, A4, and A5 Chains Positively Regulate Migration and Survival of Oligodendrocyte Precursor Cells. Sci. Rep. 2019, 9, 19882. [Google Scholar] [CrossRef]
- Kwon, H.S.; Nakaya, N.; Abu-Asab, M.; Kim, H.S.; Tomarev, S.I. Myocilin Is Involved in NgR1/Lingo-1-Mediated Oligodendrocyte Differentiation and Myelination of the Optic Nerve. J. Neurosci. 2014, 34, 5539–5551. [Google Scholar] [CrossRef]
- Almazán, G.; Vela, J.M.; Molina-Holgado, E.; Guaza, C. Re-Evaluation of Nestin as a Marker of Oligodendrocyte Lineage Cells: Nestin in Oligodendroglial Lineage. Microsc. Res. Tech. 2001, 52, 753–765. [Google Scholar] [CrossRef]
- Nieto, M.; Schuurmans, C.; Britz, O.; Guillemot, F. Neural BHLH Genes Control the Neuronal versus Glial Fate Decision in Cortical Progenitors. Neuron 2001, 29, 401–413. [Google Scholar] [CrossRef]
- Mizuguchi, R.; Sugimori, M.; Takebayashi, H.; Kosako, H.; Nagao, M.; Yoshida, S.; Nabeshima, Y.; Shimamura, K.; Nakafuku, M. Combinatorial Roles of Olig2 and Neurogenin2 in the Coordinated Induction of Pan-Neuronal and Subtype-Specific Properties of Motoneurons. Neuron 2001, 31, 757–771. [Google Scholar] [CrossRef]
- Deneen, B.; Ho, R.; Lukaszewicz, A.; Hochstim, C.J.; Gronostajski, R.M.; Anderson, D.J. The Transcription Factor NFIA Controls the Onset of Gliogenesis in the Developing Spinal Cord. Neuron 2006, 52, 953–968. [Google Scholar] [CrossRef] [PubMed]
- Althaus, H.H.; Klöppner, S.; Schmidt-Schultz, T.; Schwartz, P. Nerve Growth Factor Induces Proliferation and Enhances Fiber Regeneration in Oligodendrocytes Isolated from Adult Pig Brain. Neurosci. Lett. 1992, 135, 219–223. [Google Scholar] [CrossRef]
- Kucenas, S.; Snell, H.; Appel, B. Nkx2.2a Promotes Specification and Differentiation of a Myelinating Subset of Oligodendrocyte Lineage Cells in Zebrafish. Neuron Glia Biol. 2008, 4, 71–81. [Google Scholar] [CrossRef]
- Ortega, M.C.; Bribián, A.; Peregrín, S.; Gil, M.T.; Marín, O.; de Castro, F. Neuregulin-1/ErbB4 Signaling Controls the Migration of Oligodendrocyte Precursor Cells during Development. Exp. Neurol. 2012, 235, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.W.; Xiao, J.; Kemper, D.; Kilpatrick, T.J.; Murray, S.S. Oligodendroglial Expression of TrkB Independently Regulates Myelination and Progenitor Cell Proliferation. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 4947–4957. [Google Scholar] [CrossRef]
- Liu, Y.; Long, Z.; Yang, C. The Effects of the Olig Family on the Regulation of Spinal Cord Development and Regeneration. Neurochem. Res. 2021, 46, 2776–2782. [Google Scholar] [CrossRef]
- Golan, N.; Adamsky, K.; Kartvelishvily, E.; Brockschnieder, D.; Möbius, W.; Spiegel, I.; Roth, A.D.; Thomson, C.E.; Rechavi, G.; Peles, E. Identification of Tmem10/Opalin as an Oligodendrocyte Enriched Gene Using Expression Profiling Combined with Genetic Cell Ablation. Glia 2008, 56, 1176–1186. [Google Scholar] [CrossRef]
- De Faria, O.; Dhaunchak, A.S.; Kamen, Y.; Roth, A.D.; Kuhlmann, T.; Colman, D.R.; Kennedy, T.E. TMEM10 Promotes Oligodendrocyte Differentiation and Is Expressed by Oligodendrocytes in Human Remyelinating Multiple Sclerosis Plaques. Sci. Rep. 2019, 9, 3606. [Google Scholar] [CrossRef]
- Sun, T.; Pringle, N.P.; Hardy, A.P.; Richardson, W.D.; Smith, H.K. Pax6 Influences the Time and Site of Origin of Glial Precursors in the Ventral Neural Tube. Mol. Cell. Neurosci. 1998, 12, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.S.; Goldman, J.E. Pax6 Expression Is Sufficient to Induce a Neurogenic Fate in Glial Progenitors of the Neonatal Subventricular Zone. PLoS ONE 2011, 6, e20894. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Esquivel, J.; Göttle, P.; Aguirre-Cruz, L.; Flores-Rivera, J.; Corona, T.; Reyes-Terán, G.; Küry, P.; Torres, K.J. Sildenafil Inhibits Myelin Expression and Myelination of Oligodendroglial Precursor Cells. ASN Neuro 2019, 11, 175909141983244. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.M.; Hornby, M.F.; Kirvell, S.; Berry, M. Platelet-Derived Growth Factor Delays Oligodendrocyte Differentiation and Axonal Myelination in Vivo in the Anterior Medullary Velum of the Developing Rat. J. Neurosci. Res. 1997, 48, 588–596. [Google Scholar] [CrossRef]
- Scaglione, A.; Patzig, J.; Liang, J.; Frawley, R.; Bok, J.; Mela, A.; Yattah, C.; Zhang, J.; Teo, S.X.; Zhou, T.; et al. PRMT5-Mediated Regulation of Developmental Myelination. Nat. Commun. 2018, 9, 2840. [Google Scholar] [CrossRef]
- Doukhanine, E.; Gavino, C.; Haines, J.D.; Almazan, G.; Richard, S. The QKI-6 RNA Binding Protein Regulates Actin-Interacting Protein-1 MRNA Stability during Oligodendrocyte Differentiation. Mol. Biol. Cell 2010, 21, 3029–3040. [Google Scholar] [CrossRef]
- Chong, S.Y.C.; Rosenberg, S.S.; Fancy, S.P.J.; Zhao, C.; Shen, Y.-A.A.; Hahn, A.T.; McGee, A.W.; Xu, X.; Zheng, B.; Zhang, L.I.; et al. Neurite Outgrowth Inhibitor Nogo-A Establishes Spatial Segregation and Extent of Oligodendrocyte Myelination. Proc. Natl. Acad. Sci. USA 2012, 109, 1299–1304. [Google Scholar] [CrossRef]
- Hornig, J.; Fröb, F.; Vogl, M.R.; Hermans-Borgmeyer, I.; Tamm, E.R.; Wegner, M. The Transcription Factors Sox10 and Myrf Define an Essential Regulatory Network Module in Differentiating Oligodendrocytes. PLoS Genet. 2013, 9, e1003907. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Ou, Z.; Qi, C.; Xing, R.; Wang, S.; Han, Y.; Zhao, T.-J.; Chen, Y. DHHC5 Facilitates Oligodendrocyte Development by Palmitoylating and Activating STAT3. Glia 2022, 70, 379–392. [Google Scholar] [CrossRef]
- He, Y.; Dupree, J.; Wang, J.; Sandoval, J.; Li, J.; Liu, H.; Shi, Y.; Nave, K.A.; Casaccia-Bonnefil, P. The Transcription Factor Yin Yang1 Is Essential for Oligodendrocyte Progenitor Differentiation. Neuron 2007, 55, 217–230. [Google Scholar] [CrossRef]
- Weng, Q.; Chen, Y.; Wang, H.; Xu, X.; Yang, B.; He, Q.; Shou, W.; Chen, Y.; Higashi, Y.; van den Berghe, V.; et al. Dual-Mode Modulation of Smad Signaling by Smad-Interacting Protein Sip1 Is Required for Myelination in the Central Nervous System. Neuron 2012, 73, 713–728. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 22 June 2022).
- Taveggia, C.; Thaker, P.; Petrylak, A.; Caporaso, G.L.; Toews, A.; Falls, D.L.; Einheber, S.; Salzer, J.L. Type III Neuregulin-1 Promotes Oligodendrocyte Myelination. Glia 2008, 56, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Bare, D.J.; Becker-Catania, S.G.; DeVries, G.H. Differential Localization of Neuregulin-1 Type III in the Central and Peripheral Nervous System. Brain Res. 2011, 1369, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Katanov, C.; Novak, N.; Vainshtein, A.; Golani, O.; Dupree, J.L.; Peles, E. N-Wasp Regulates Oligodendrocyte Myelination. J. Neurosci. 2020, 40, 6103–6111. [Google Scholar] [CrossRef] [PubMed]
- Bacon, C.; Lakics, V.; Machesky, L.; Rumsby, M. N-WASP Regulates Extension of Filopodia and Processes by Oligodendrocyte Progenitors, Oligodendrocytes, and Schwann Cells—Implications for Axon Ensheathment at Myelination. Glia 2007, 55, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Malheiro, A.R.; Correia, B.; Ferreira da Silva, T.; Bessa-Neto, D.; Van Veldhoven, P.P.; Brites, P. Leukodystrophy Caused by Plasmalogen Deficiency Rescued by Glyceryl 1-myristyl Ether Treatment. Brain Pathol. 2019, 29, 622–639. [Google Scholar] [CrossRef]
- Kida, E.; Palminiello, S.; Golabek, A.A.; Walus, M.; Wierzba-Bobrowicz, T.; Rabe, A.; Albertini, G.; Wisniewski, K.E. Carbonic Anhydrase II in the Developing and Adult Human Brain. J. Neuropathol. Exp. Neurol. 2006, 65, 664–674. [Google Scholar] [CrossRef]
- Liedtke, W.; Edelmann, W.; Bieri, P.L.; Chiu, F.-C.; Cowan, N.J.; Kucherlapati, R.; Raine, C.S. GFAP Is Necessary for the Integrity of CNS White Matter Architecture and Long-Term Maintenance of Myelination. Neuron 1996, 17, 607–615. [Google Scholar] [CrossRef]
- Schaeren-Wiemers, N.; Bonnet, A.; Erb, M.; Erne, B.; Bartsch, U.; Kern, F.; Mantei, N.; Sherman, D.; Suter, U. The Raft-Associated Protein MAL Is Required for Maintenance of Proper Axon–Glia Interactions in the Central Nervous System. J. Cell Biol. 2004, 166, 731–742. [Google Scholar] [CrossRef]
- Fewou, S.N.; Ramakrishnan, H.; Büssow, H.; Gieselmann, V.; Eckhardt, M. Down-Regulation of Polysialic Acid Is Required for Efficient Myelin Formation. J. Biol. Chem. 2007, 282, 16700–16711. [Google Scholar] [CrossRef]
- Yu, T.; Lieberman, A.P. Npc1 Acting in Neurons and Glia Is Essential for the Formation and Maintenance of CNS Myelin. PLoS Genet. 2013, 9, e1003462. [Google Scholar] [CrossRef] [PubMed]
- Corbeil, D.; Joester, A.; Fargeas, C.A.; Jászai, J.; Garwood, J.; Hellwig, A.; Werner, H.B.; Huttner, W.B. Expression of Distinct Splice Variants of the Stem Cell Marker Prominin-1 (CD133) in Glial Cells. Glia 2009, 57, 860–874. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.-M.; McAlear, T.S.; Nguyen, H.; Oses-Prieto, J.A.; Valenzuela, A.; Shi, R.D.; Perrino, J.J.; Huang, T.-T.; Burlingame, A.L.; Bechstedt, S.; et al. The Golgi Outpost Protein TPPP Nucleates Microtubules and Is Critical for Myelination. Cell 2019, 179, 132–146.e14. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).